Abstract
The receptor tyrosine kinase ERBB4, a member of the epidermal growth factor receptor (EGFR) family, is unusual in that when phosphorylated, ERBB4 can undergo intramembrane proteolysis, releasing a soluble intracellular domain (ICD) that activates transcription in the nucleus. We found that ERBB4 activated the transcriptional coactivator YAP, which promotes organ and tissue growth and is inhibited by the tumor-suppressor Hippo pathway. Overexpressing ERBB4 in cultured mammary epithelial cells or adding the ERBB4 ligand neuregulin 1 (NRG1) to breast cancer cell cultures promoted the expression of genes regulated by YAP, such as CTGF. Knocking down YAP or ERBB4 prevented the induction of CTGF expression by NRG1, as did preventing ERBB4 cleavage by treating cells with the pan-EGFR inhibitors lapatinib or erlotinib. A PPxY motif in the ERBB4 ICD enabled its interaction with WW domains in YAP, similar to the mode of interaction between YAP and the kinase LATS1, which inhibits the transcriptional activity of YAP. The ERBB4 ICD coimmunoprecipitated with YAP and TEAD1, a YAP coactivator, suggesting that the ERBB4 ICD may functionally interact with YAP and TEAD to promote transcriptional activity. NRG1 stimulated YAP activity to an extent comparable to that of EGF or LPA (lysophosphatidic acid), known activators of YAP. NRG1 stimulated YAP-dependent cell migration in breast cancer cell lines. These observations connect the unusual nuclear function of a growth factor receptor with a mechanosensory pathway and suggest that NRG1-ERBB4-YAP signaling may underlie the aggressive behavior of tumor cells.
Introduction
ERBB4 (also known as HER4) is a member of the epidermal growth factor receptor EGFR/ERBB family of receptor tyrosine kinases (RTKs). ERBB4 is essential for normal development and maintenance of the heart, mammary glands, and the nervous system (1–4). ERBB4 is unusual among RTKs in its ability to undergo regulated juxtamembrane and intramembrane proteolysis to release a soluble intracellular domain (ICD) (5). The ERBB4 ICD relocalizes to the nucleus where it regulates transcription through its association with transcriptional co-regulators (such as KAP1, TAB2/N-CoR, and AP2) and sequence-specific DNA binding proteins (such as STAT5A and the estrogen receptor) (6–12). The distinct nuclear functions of the ERBB4 ICD add a dimension to RTK-governed processes and unleash new avenues for signaling.
ERBB4 transcripts undergo tissue-specific alternative splicing (13). ERBB4 CYT-1, but not CYT-2, includes an exon encoding a 16 amino acid peptide distal to the kinase domain with a PPxY motif that is a binding site for p85 PI3K and WW domains (14). This small difference endows CYT-1 with substantially different biological properties: in tissue culture and mouse transgenic models, CYT-1 induces differentiation and survival phenotypes, whereas CYT-2 promotes proliferation (15, 16).
The second splice event affects the extracellular domain. ERBB4 JM-a, but not JM-b, has an extracellular proteolytic cleavage site for TACE [TNF-α (tumor necrosis factor α)-converting enzyme; also known as ADAM17] (14). Activation of TACE by ERBB4 ligands, phorbol esters, or other agonists releases the extracellular domain (ECD) of the receptor, leaving a membrane-embedded 80 kDa isoform (m80) (17). This enables intramembrane proteolysis at a second, γ-secretase, cleavage site, which releases a soluble 80 kDa ICD (s80/ICD) (17). Overall, differential regulation of ERBB4 structure by alternative splicing and proteolysis produces receptors with very different signaling qualities. Full-length (FL) ERBB4 isoforms signal much like other RTKs at the membrane by binding of downstream proteins to the Tyr-phosphorylated receptor. In contrast, s80 isoforms have entirely novel signaling functions in transcriptional regulation. Epithelial tissues and cell lines appear to express only JM-a, whereas neural and mesenchymal tissue express mostly JM-b or both JM-a and JM-b isoforms (13).
Candidate oncogenic mutations or amplification of ERBB4 occur with moderate frequency in medulloblastoma, melanoma, and carcinoma. In fact, at 2.1% incidence, ERBB4 is the fourth most mutated RTK across twelve major cancer types (18), and overexpression of ERBB4 in mouse mammary epithelium can initiate carcinogenesis (15). However, prognostic associations of ERBB4 expression with breast cancer are variable, with favorable (19–23) or unfavorable (24–27) associations reported. Part of this inconsistency is likely due to the failure to discriminate among ERBB4 isoforms.
We have recently compared the signaling associated with expression of full-length ERBB4 and the ICD isoforms through transcriptional and chromatin immunoprecipitation-sequencing (ChIP-Seq) analysis (28). The ERBB4 ICD induced numerous Hippo/YAP pathway-regulated genes. This is consistent with our early transcription profiling studies linking NRG1 (Neuregulin 1) and ERBB4 to the transcription of the YAP-regulated gene CTGF, which encodes connective tissue growth factor (29).
The Hippo pathway has emerged as a critical signaling hub that regulates organ growth and size maintenance (30). Dysregulation of this pathway can promote tumorigenesis (31, 32). Hippo signaling inhibits the transcriptional coactivators YAP and TAZ. Hippo pathway kinases MST1/2 and LATS1/2 operate in a kinase cascade that inhibits cell growth and promotes apoptosis under conditions of high cell density (33). LATS1/2 inactivate YAP and TAZ through inhibitory phosphorylation leading to cytoplasmic retention by 14-3-3 binding and to proteasome-dependent degradation (34). Under growth-permissive conditions, the Hippo kinases are inhibited such that YAP and TAZ are free to translocate to the nucleus and activate transcription of genes that promote growth and migration (such as CTGF, CYR61, ANKRD1, AREG) (34). YAP and TAZ do not have DNA binding domains but interact with sequence-specific DNA binding proteins, including TEAD1-4, p73, SMAD, and RUNX (34).
Several other signaling inputs regulate YAP and TAZ activation. The apical-basal polarity proteins NF2, AMOT, and α-catenin regulate YAP at the membrane (34, 35). Disruption of cell junctions releases YAP from these sequestering proteins, enabling nuclear localization. Both the actin cytoskeleton and microtubules also control YAP activation (36) through mechanical stimulation, facilitating proliferation on stiff substrates (37). Agonists for some G-protein coupled receptors (GPCRs) comprise another major class of upstream Hippo regulators that can either activate or inactivate YAP [as is the case for lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), or glucagon and epinephrine, respectively] (38). YAP is also under metabolic control by the SREBP/mevalonate pathway, which activates YAP by inhibiting its phosphorylation through Rho activation (39).
EGFR signaling converges with the YAP/Hippo pathway, uniting two major growth regulatory systems (40, 41). The ligand EGF activates YAP by relocalizing the kinase PDK1 from its scaffolding position in an inhibitory MST1/2–LATS1/2 complex (41). Additionally, YAP can activate EGFR by inducing the expression of AREG, which encodes the ligand amphiregulin (42). YAP binds ERBB4 through an interaction between PPxY motifs in ERBB4 and the WW domain in YAP (43). Using artificial GAL-4 luciferase assays, YAP was shown to be required for transcriptional co-activation mediated by the ERBB4 C-terminal fragment (amino acids 676-1292) (43, 44). Additionally, YAP and ERBB4 co-localize in the nucleus, where ERBB4 ICD induces genes necessary for lung maturation (45). However, the ability of ERBB4 to activate transcription of YAP/Hippo target genes was not reported, so the functional impact of the ERBB4–YAP interaction is uncertain.
Tissue-specific ERBB4 cleavage enables the integration of cell proliferation and size control through Hippo and RTK-regulated pathways and has broad implications in both development and cancer. Here, we investigated the molecular mechanisms and biological consequences of the ERBB4-YAP interaction.
Results
ERBB4 overexpression enriches for a YAP-target gene signature
Because ERBB4 binds YAP, we investigated whether overexpression of ERBB4 affects YAP target genes. We analyzed the transcription profile induced by ERBB4 ICD CYT-2 (28) in MCF10A mammary epithelial cells through gene set enrichment analysis (GSEA) using the conserved YAP-dependent gene expression signature identified by Cordenonsi et al. (46). Several YAP target genes were strongly enriched in cells expressing ERBB4 ICD CYT-2 (Fig. 1A), including CTGF, suggesting that ERBB4 activates YAP. We focused on CTGF as a canonical YAP/TEAD-regulated gene in subsequent experiments.
FIGURE 1. NRG1-stimulation of ERBB4 activates YAP signaling.
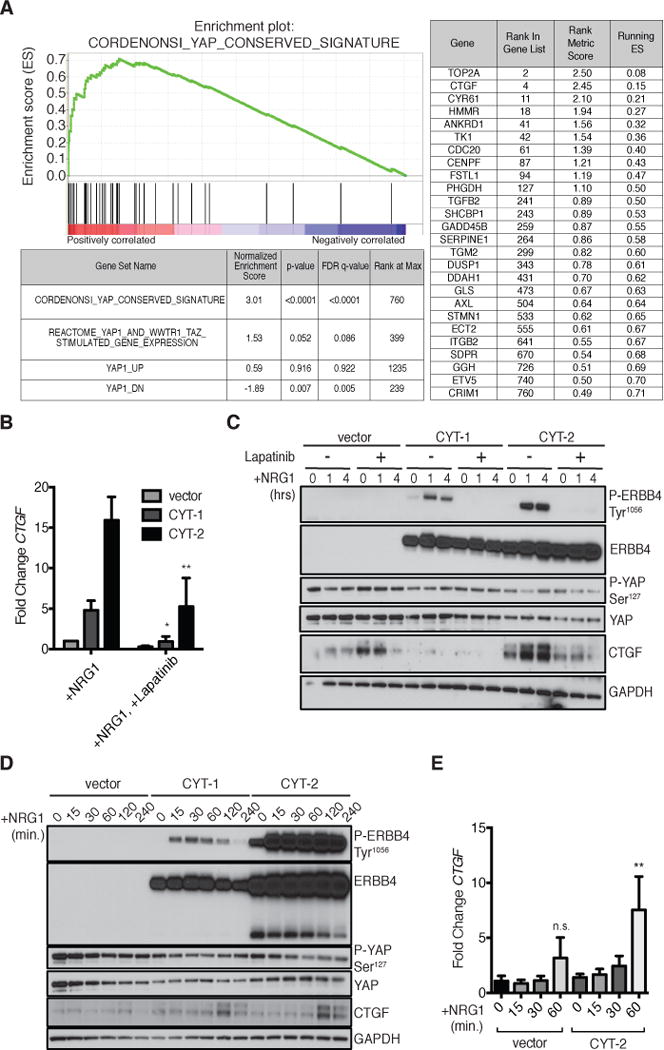
(A) Gene Set Enrichment Analysis (GSEA) in MCF10A cells expressing ERBB4 ICD CYT-2. Enrichment plot shows enrichment of the conserved signature reported by Cordenonsi et al. (46) in the data from cells expressing ERBB4 ICD CYT-2 (Normalized Enrichment Score=3.01, p<0.0001). The table lists genes in the ERBB4 ICD CYT-2 data set with core enrichment for this signature. All enriched YAP target genes are in the top 12.7% of ERBB4 ICD CYT-2-induced genes (1.4 < fold-change < 5.7).
(B) RT-PCR of CTGF in MCF10A cells expressing pINDUCER20 encoding ERBB4 CYT-1, CYT-2, or a vector control and stimulated with NRG1 (100 ng/ml) for 1 hour with or without an hour pre-treatment with lapatinib (2 μM). MCF10A cells were starved overnight in Opti-MEM with simultaneous DOX (50 ng/ml) treatment. Data are means (SD) from 4 experiments.
(C) Immunoblot of phosphorylated YAP at Ser127 and CTGF abundance in MCF10A cells expressing pINDUCER20 encoding ERBB4 CYT-1, CYT-2, or a vector control and treated as in (B). Blots are representative of 3 experiments.
(D) Immunoblot showing the time course of NRG1-induced CTGF abundance and YAP dephosphorylation in MCF10A cells expressing pINDUCER20-encoded ERBB4 and treated as in (B). Blots are representative of 3 experiments.
(E) RT-PCR showing the time course of NRG1-induced CTGF expression in MCF10A cells expressing vector or pINDUCER20-encoded ERBB4 CYT-2 and stimulated with NRG1 (100 ng/ml) for up to 1 hour. Data were normalized to vector-transfected cells at 0 min. Data are means (SD) from 3 experiments. n.s., not significant; *p<0.05; **p<0.01.
Ligand-stimulation of full-length ERBB4 is sufficient for CTGF induction
We next determined if full-length ERBB4 stimulated by its ligand NRG1 is sufficient to induce the expression of CTGF. Doxycycline (DOX)-inducible plasmids encoding JM-a ERBB4 CYT-1 or CYT-2 were introduced into MCF10A cells, a cell devoid of endogenous ERBB4 (Fig. S1). Within one hour, NRG1 increased the abundance of CTGF mRNA 5-fold in MCF10A cells expressing ERBB4 CYT-1 and 15-fold in cells expressing ERBB4 CYT-2 (Fig. 1B). CTGF protein levels increased 1 to 2 hours after stimulation with NRG1 (Fig. 1, C to E). However, the trend for NRG1-induced CTGF mRNA in vector-infected cells was not significant. NRG1 may signal through endogenous ERBB3, which can activate PI3K signaling in collaboration with endogenous EGFR (Fig. 1, D and E). Preincubation with the pan-ERBB inhibitor lapatinib prevented NRG1 induction of CTGF (Fig. 1, B and C). Because the strongest changes in CTGF abundance were observed in cells expressing ERBB4 CYT-2, we chose to focus subsequent experiments on this isoform.
LATS1/2-dependent phosphorylation of YAP at Ser127 reduces the nuclear localization of YAP by enabling its binding to cytoplasmic 14-3-3. Addition of NRG1 to culture medium reduced the phosphorylation of YAP at this site in MCF10A-pINDUCER20 cells (Fig. 1, C and D). Ser127-phosphorylated YAP decreased over the first hour of NRG1 treatment and remained low during periods of high CTGF expression, consistent with the nuclear function of YAP.
YAP mediates NRG1/ERBB4 upregulation of CTGF
Because ERBB4 overexpression strongly enriched for a YAP target gene signature, and addition of NRG1 was sufficient to induce CTGF expression, we investigated whether YAP mediated these effects. Dobutamine, a chemical inhibitor of YAP, suppresses YAP-dependent gene transcription (47). Pre-treating MCF10A cells expressing pINDUCER20-encoded ERBB4 CYT-2 with dobutamine diminished NRG1-induced CTGF expression in at both the protein and mRNA levels (Fig. 2, A and B). We used an inducible YAP shRNA system to ensure YAP expression was only suppressed during experiments and avoid potential selection as cells were passaged (Fig. 2C). YAP knockdown in MCF10A cells expressing DOX-inducible pINDUCER20-encoded ERBB4 CYT-2 and pINDUCER10-encoded YAP shRNA greatly reduced the induction of CTGF mRNA by NRG1 (Fig. 2D). Hence, YAP promotes ERBB4-dependent induction of CTGF by NRG1.
FIGURE 2. YAP mediates NRG1/ERBB4 induction of CTGF.
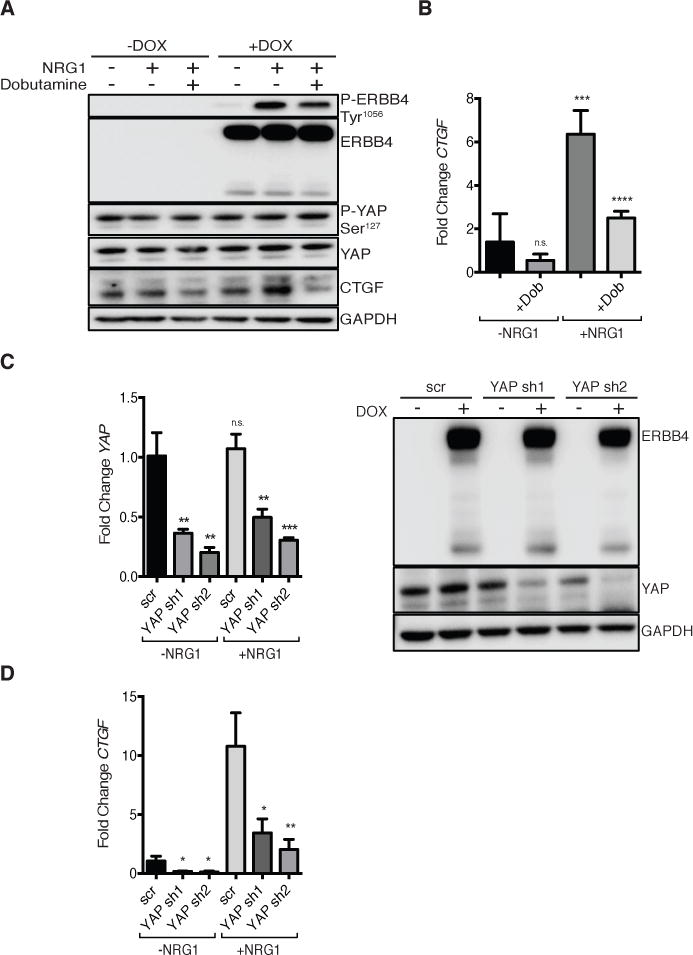
(A) Immunoblot of MCF10A pINDUCER20 ERBB4 CYT-2 cells treated with or without DOX (50 ng/ml, overnight in Opti-MEM) and without or with inclusion of dobutamine (30 μM) for the last four hours. Cells were then incubated with NRG1 (50 ng/ml) for 1 hour. Blots are representative of 2 experiments.
(B) RT-PCR of CTGF in MCF10A cells expressing pINDUCER20 encoding ERBB4 CYT-2 treated with or without dobutamine and NRG1 as in (A). Data are means (SD) from 4 experiments.
(C) RT-PCR (left) and immunoblot (right) of YAP knockdown in MCF10A cells expressing pINDUCER10 encoding YAP shRNA and pINDUCER20 encoding ERBB4 CYT-2. Cells were treated with DOX (1 μg/ml, 72 hours) then starved (Opti-MEM, +DOX) for 3 hours followed by NRG1 (50 ng/ml) for 1 hour. RT-PCR data are means (SD) from 3 experiments. Blots are representative of 3 experiments.
(D) RT-PCR of CTGF in MCF10A cells expressing pINDUCER10 encoding YAP shRNA and pINDUCER20 encoding ERBB4 CYT-2. Cells were treated as in (C). Data are means (SD) from 3 experiments. n.s., not significant; *p<0.05; **p<0.01, ***p<0.001, ****p<0.0001.
We expressed a YAP Ser127Ala mutant that cannot be phosphorylated at the site responsible for 14-3-3 binding and cytoplasmic retention to test the importance of phosphorylation of YAP at Ser127 in the NRG1-induced response. The induction of ERBB4 CYT-2 expression (+DOX) in MCF10A MSCV-vector control cells resulted in a 3-fold increase in CTGF expression, with NRG1 present in all conditions (Fig. S2). As expected, expression of YAP Ser127Ala markedly increased CTGF mRNA production (Fig. S2), because it allows constitutive nuclear localization of YAP. Addition of ERBB4 CYT-2 using DOX in MCF10A YAP Ser127Ala cells did not significantly increase CTGF. These data demonstrate that ERBB4- and YAP-mediated control of CTGF expression are not additive and suggest that NRG1/ERBB4 regulate YAP through phosphorylation at Ser127 and nuclear localization.
ERBB4 promotes NRG1 induction of YAP target genes
To investigate whether the activation of endogenous ERBB4 induces CTGF production, we used T47D mammary carcinoma cells, which express all four ERBB receptors. In this background, NRG1 induced a 300-fold increase in CTGF mRNA (Fig. 3A). As in MCF10A cells, preincubation with the pan-ERBB inhibitor lapatinib prevented the induction of CTGF in response to NRG1 (Fig. 3A).
FIGURE 3. NRG1 activates endogenous ERBB4 to induce YAP target genes.
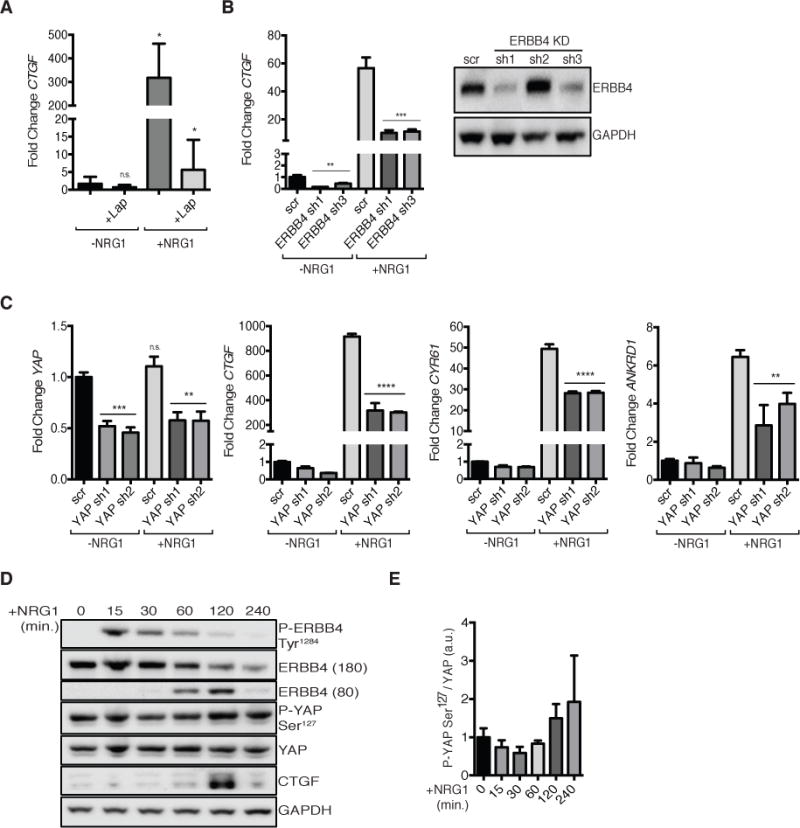
(A) RT-PCR of CTGF in T47D cells starved for 3 hours in Opti-MEM then treated with NRG1 (50 ng/ml) for 1 hour with or without an hour pre-treatment with lapatinib (1 μM). Data are means (SD) from 3 experiments.
(B) RT-PCR (left) of CTGF in T47D cells expressing pLKO encoding ERBB4 shRNA (sh1 or sh3) or scrambled control (scr). Cells were treated as in (A). Immunoblot (right) showing ERBB4 protein knockdown. RT-PCR data are means (SD) from 3 experiments. Blots are representative of 3 experiments.
(C) RT-PCR of (left to right) YAP, CTGF, CYR61, and ANKRD1 in T47D cells expressing pINDUCER10 encoding YAP shRNA. Cells were treated with DOX (1 μg/ml) for 5 days then starved overnight (Opti-MEM, +DOX) followed by 1 hour NRG1 (50 ng/ml) treatment. Data are means (SD) from 3 technical replicates, representative of 3 experiments.
(D) Immunoblot showing time-course of NRG1-induced Tyr1284 phosphorylated ERBB4, ERBB4 ICD, Ser127 phosphorylated YAP, and CTGF abundance in T47D cells. Cells were starved overnight in Opti-MEM then treated with NRG1 (50 ng/ml) for 0, 15, 30, 60, 120, or 240 minutes. Blots are representative of 3 experiments.
(E) Quantification of the ratio of phosphorylated YAP at Ser127 to total YAP protein levels from immunoblots in (D). Data are normalized to t=0 minutes. Data are means (SD) from 3 experiments. n.s., not significant; *p<0.05; **p<0.01, ***p<0.001, ****p<0.0001.
In T47D cells, both ERBB3 and ERBB4 can bind NRG1. We evaluated the importance of ERBB4 in the response to NRG1 with stable shRNA-mediated knockdown of ERBB4 in T47D cells. Partial ERBB4 knockdown greatly diminished both baseline CTGF as well as NRG1-induced CTGF mRNA expression (Fig. 3B). NRG1 did not significantly induce CTGF expression in vector-infected MCF10A cells, which express endogenous ERBB3 but not ERBB4 (Fig. 1, D and E), providing further support for the ERBB4-dependence of NRG1 activation of YAP. Quantitative real-time polymerase chain reaction (qRT-PCR) confirmed both NRG1 responsiveness and YAP-dependence in the expression of the YAP target genes CTGF, CYR61, and ANKRD1 in T47D cells (Fig. 3C). NRG1 induced CTGF with similar kinetics in both MCF10A and T47D cells, with peak protein induction 2 hours after treatment (Fig. 3D). As in MCF10A cells, the phosphorylation of YAP at Ser127 decreased over the first hour of NRG1 treatment, but rebounded 2 hours later (Fig. 3E). Collectively, these data indicate that ERBB4 mediates NRG1-induced activation of YAP in these mammary cell lines.
ERBB4 ICD binds TEAD1 and governs induction of YAP target genes
The ERBB4 ICD (80 kDa), which binds to YAP (43), was most abundant during periods when YAP was dephosphorylated and CTGF expression was increased in T47D cells treated with NRG1 (Fig. 3D). Overexpression of ERBB4 CYT-1 ICD or CYT-2 ICD strongly increased CTGF protein abundance in MCF10A cells cultured at low confluency (Fig. 4A).
FIGURE 4. Density-dependence of ERBB4 ICD induction of CTGF.
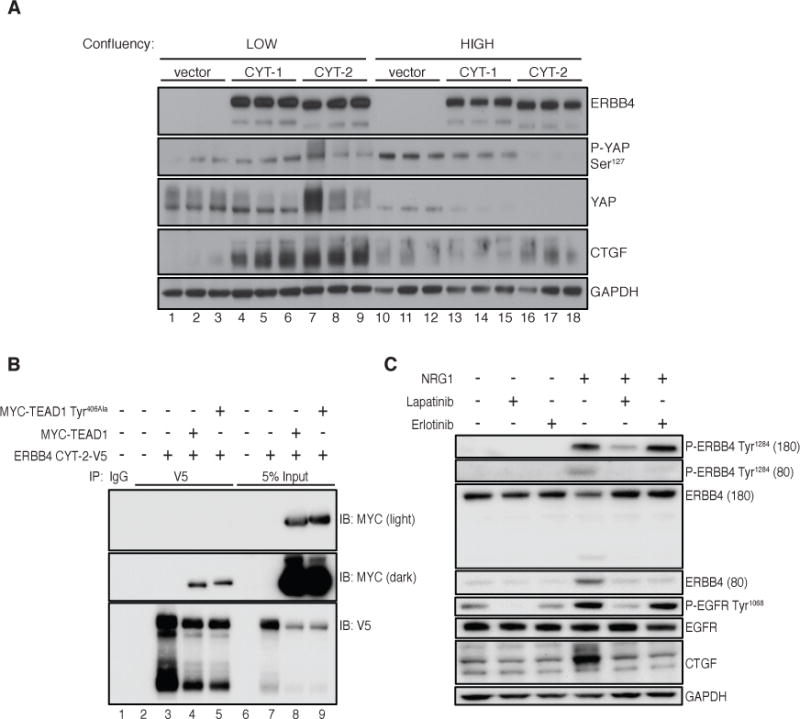
(A) Immunoblot of CTGF protein and YAP phosphorylation in MCF10A cells expressing ERBB4 CYT-1 or CYT-2 ICD seeded to achieve low (30%, lanes 1–9) or high (100%, lanes 10–18) confluency on the day of cell lysis. Samples are shown in technical triplicate. Blots are representative of 3 experiments.
(B) Immunoblot showing co-immunoprecipitation of MYC-TEAD1 with ERBB4 CYT-2-V5 in HEK 293T cells. Cells were transfected with pcDNA3.1-ERBB4 CYT-2-V5 and pRK5-MYC-TEAD1 (or TEAD1 Tyr406Ala) using lipofectamine 2000 and lysed 72 hours post-transfection. Cells were incubated with NRG1 (50 ng/ml) for 1 hour then lysed in NP-40 buffer. 1 μg of anti-V5 antibody or IgG control was used to immunoprecipitate ERBB4 from 1 mg lysate. 5% input (50 μg total lysate) was used as MYC control. Blots are representative of 3 experiments.
(C) Immunoblot of CTGF protein induction in T47D cells incubated with NRG1 (50 ng/ml) for 1 hour following lapatinib (1 μM), erlotinib (1 μM), or DMSO control pretreatment for 30 minutes. Cells were starved in Opti-MEM for 3 hours prior to treatment. Blots are representative of 3 experiments.
Density-dependent growth inhibition functions in part through activation of the Hippo pathway to suppress YAP activity (33). We investigated whether ERBB4 binding to YAP may override YAP inhibition at high density in MCF10A cells expressing ERBB4 ICD. At low confluency (30%, which is permissive for YAP signaling), both ERBB4 ICD isoforms increased the abundance of CTGF (Fig. 4A). In contrast, YAP was much less abundant in confluent cells (likely owing to phosphorylation-dependent proteasomal degradation), and the ERBB4 ICD did not alter CTGF abundance relative to vector-transfected cells. Hence, the ERBB4 ICD does not overcome density-dependent growth inhibition of YAP.
Suppression of the canonical Hippo signaling pathway activates YAP-dependent gene expression through nuclear localization of YAP, leading to the binding of YAP/TEAD complexes to the promoters of genes, including that of CTGF. Because the ERBB4 ICD, YAP, and TEAD1 all localize to the nucleus, and YAP binds both TEAD1 and ERBB4, we investigated whether ERBB4 forms a complex with TEAD1. In human embryonic kidney (HEK) 293T cells, Myc-tagged TEAD1 co-immunoprecipitated with V5-tagged ERBB4 CYT-2 in total cell lysates (Fig. 4B) and in nuclear fractions (Fig. S3). ERBB4 interacts with YAP through the binding of the PPxY domains in ERBB4 to the WW domains in YAP. Distinct sites on YAP mediate its binding to TEAD, thus it is possible that YAP binds simultaneously to both proteins, bridging ERBB4 and TEAD. However, we found no consistent reduction in the abundance of Myc detected in V5 immunoprecipitates in HEK293T cells expressing a YAP binding-deficient mutant of TEAD1 (Tyr406Ala) (Fig. 4B), suggesting that ERBB4 may bind TEAD independently of YAP. These data support the model that NRG1–ERBB4 signaling regulates YAP target genes through interactions with YAP and TEAD1.
Treating T47D cells with the EGFR inhibitor erlotinib blocked the production of ERBB4 ICD (Fig. 4C) despite failing to effectively inhibit EGF- or NRG1-induced phosphorylation of wild-type EGFR (Fig. S4). This coincided with a decrease in the abundance of CTGF (Fig. 4C), suggesting that erlotinib may block the induction of CTGF in response to NRG1 though indirect inhibition of ERBB4 cleavage.
Comparison of NRG1 to other YAP agonists
The ability of ERBB4 JM-a isoforms to couple responses to NRG1 to the expression of YAP-regulated genes means that the expression of ERBB4 JM-a may broadly reprogram the NRG1-induced response to activate YAP (and TAZ) signaling. Hence, we sought to determine how NRG1 compares to canonical agonists for YAP and TAZ. At concentrations that we determined to yield maximal induction of CTGF mRNA, the GPCR-mediated YAP activator lysophosphatidic acid (LPA) induced a 6-fold increase in CTGF mRNA abundance, whereas NRG1 induced a maximum 600-fold increase (Fig. 5A). This finding was recapitulated at the protein level in T47D cells, in which saturating doses of LPA increased the abundance of CTGF very weakly compared with NRG1 (Fig. 5, B and C). Although ERBB proteins transactivate when co-expressed, EGF was approximately three-fold less potent in inducing CTGF abundance than NRG1 in T47D cells (Fig. 5B). Differences in CTGF production induced by NRG1, LPA, and EGF were not affected by timing of induction: EGF induced CTGF maximally after 2 hours of treatment, similar to NRG1 (Fig. 5C), but stimulation with LPA did not significantly increase CTGF protein abundance over 4 hours, despite reducing the phosphorylation of YAP at Ser127 (Fig. 5C).
FIGURE 5. Comparison of NRG1 to other YAP agonists.
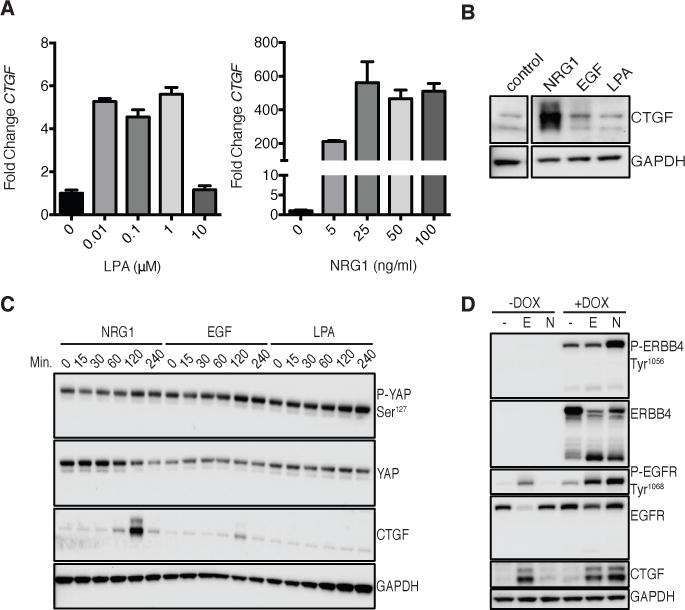
(A) RT-PCR of CTGF in T47D cells starved overnight in Opti-MEM then treated with either LPA (left) or DMSO control or NRG1 (right) at the concentrations indicated for 1 hour. Data are means (SD) from 3 technical replicates, representative of 2 experiments.
(B) Immunoblot of CTGF protein abundance in T47D cells starved overnight in Opti-MEM then treated with NRG1 (50 ng/ml), EGF (50 ng/ml), or LPA (1 μM) for 2 hours. The control was run in same experiment but with no treatment. Blots are representative of 3 experiments.
(C) Immunoblot of CTGF protein and YAP phosphorylation in T47D cells over a time-course of NRG1, EGF, or LPA treatment. Cells were starved overnight in Opti-MEM then incubated with saturating doses of NRG1 (50 ng/ml), EGF (50 ng/ml), or LPA (1 μM) for the times indicated. Blots are representative of 2 experiments.
(D) Immunoblot of CTGF abundance in MCF10A cells expressing pINDUCER20 encoding ERBB4 CYT-2. Cells were incubated overnight in Opti-MEM in the absence or presence of DOX (50 ng/ml) followed by 2 hours EGF (50 ng/ml) or NRG1 (50 ng/ml) treatment. Blots are representative of 3 experiments.
EGF activates YAP in MCF10A cells, which do not express ERBB4, through EGFR–PI3K–PDK1 signaling (41). To compare EGF with NRG1 in the activation of CTGF in this context, we used MCF10A cells that stably expressed pINDUCER20-encoded ERBB4 CYT-2 and cultured them with or without DOX to induce ERBB4 expression. NRG1 induced CTGF abundance as well or slightly better than did EGF, but only when ERBB4 expression was concomitantly induced with DOX (Fig. 5D).
NRG1 regulates YAP independent of the TGF-β and mevalonate pathways
ERBB4 induces the expression of several genes in the mevalonate/cholesterol pathway including HMGCR, HMGCS1, and LDLR (28). Because the mevalonate pathway can activate YAP, this raised the possibility that ERBB4 might activate YAP indirectly through induction of the mevalonate pathway. Independent of LATS1/2, the nuclear localization and transcriptional activity of YAP can be blocked by statins (39). Inhibition of HMG-coA reductase with simvastatin or lovastatin did not prevent the induction of CTGF expression in response to NRG1 (Fig. S5A), so ERBB4 does not appear to regulate YAP signaling via the mevalonate pathway. Knocking down YAP did not block NRG1-induced increases in HMGCR expression (Fig. S5B), suggesting that the mevalonate pathway is upstream from or parallel to ERBB4-dependent activation of YAP.
To determine whether TGF-β signaling is responsible for the induction of CTGF, we evaluated the phosphorylation of SMAD2 (at Ser465/467) in MCF10A cells expressing pINDUCER20-encoded ERBB4 and treated with phorbol 12-myristate 13-acetate (PMA) or NRG1, respectively. NRG1 did not affect SMAD activation (Fig. S5, C and D), suggesting that TGF-β signaling does not mediate the response to NRG1.
NRG1 activates YAP signaling to induce migration in T47D cells
We next evaluated the biological consequences of NRG1–ERBB4-mediated activation of YAP signaling. YAP drives cell proliferation and migration and can bypass signals from cell-cell contact and mechanical stress that constrain cell division in epithelial sheets when activated or overexpressed. ERBB4 expression and activation promote cell migration (28); therefore, we determined whether migration is mediated by YAP. NRG1 induced a 4-fold increase in migration in T47D cells, which was greatly reduced by YAP knockdown (Fig. 6, A and B). Inducible YAP knockdown with DOX was confirmed in the cells used for each migration experiment (Fig. 6C). Inhibition of migration in YAP-deficient cells was not due to reduced cell viability, because cells transfected with either a scrambled control or YAP shRNA were equally viable over the 48 hour experiment (Fig. 6D). Despite high expression of exogenous ERBB4, MCF10A cells transfected with pINDUCER20 did not increase migration when treated with NRG1 (Fig. S6). However, YAP knockdown still reduced migration of these cells, as expected. These findings suggest that NRG1 promotes YAP-mediated biological phenotypes, including migration, in the context of particular cell backgrounds.
FIGURE 6. NRG1 induced cell migration is mediated by YAP.
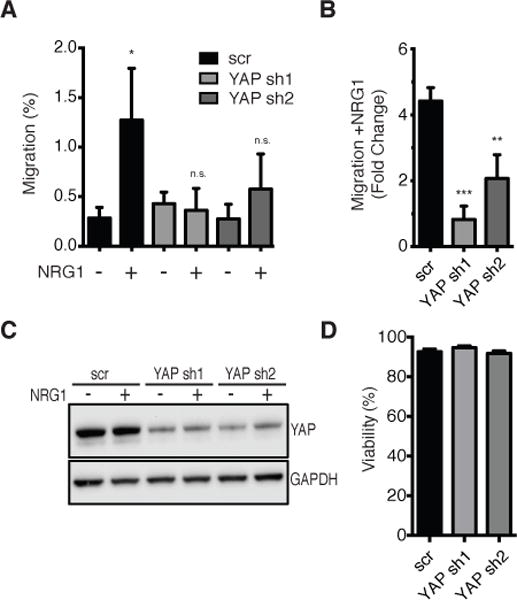
(A) Transwell migration assay of T47D cells expressing pINDUCER10 encoding YAP shRNA or scrambled control. Cells were incubated with DOX (1 μg/ml) for 5 days followed by 3 hours NRG1 (50 ng/ml) pre-treatment then allowed to migrate for 48 hours (from 0.1% to 10% FBS) in the presence of NRG1 and DOX. Percent migration was normalized to scrambled (scr) control without NRG1. Data are means (SD) from 3 experiments.
(B) Migration data from (A) shown as fold-change migration +NRG1/−NRG1. Data are means (SD) from 3 experiments.
(C) Immunoblot of YAP protein knockdown in T47D cells expressing pINDUCER10 encoding YAP shRNA or scrambled control. Cells were treated as in (A). Blots are representative of 3 experiments.
(D) Trypan blue cell viability assay in T47D cells expressing pINDUCER10 encoding YAP shRNA or scrambled control. Cells were treated as in (A). n.s., not significant; *p<0.05; **p<0.01, ***p<0.001.
Discussion
Although the physical binding between ERBB4 and YAP has been known for several years, suggesting a model in which ERBB4 regulates gene expression through this interaction (43–45), the importance of YAP in the Hippo pathway emerged more recently. We found that NRG1 activates YAP-target gene expression through ERBB4 to promote YAP-driven biological phenotypes. Hence, ERBB4 connects the ERBB receptor complex with the Hippo/YAP network.
ERBB4 may affect YAP in the cytosol through indirect modulation of upstream YAP regulators (such as Hippo kinases, GPCRs, etc.). ERBB4 and LATS1, the Hippo pathway kinase that inactivates YAP, both bind to the WW domains of YAP. Binding of YAP to ERBB4 may competitively reduce inhibitory phosphorylation by LATS.
As ERBB4 chaperones STAT5 to the nucleus, another possibility is that ERBB4 diminishes YAP phosphorylation by accelerating translocation of YAP to the nucleus. Mutation of YAP Ser127 to Ala, which would reduce cytoplasmic retention, blocked ERBB4 mediated increases in YAP signaling, consistent with this model, but it has been reported that this mutation also reduces ERBB4/YAP coimmunprecipitation (43).
Direct interaction between nuclear ERBB4 ICD and YAP may modulate transcriptional activation. ERBB4 co-immunoprecipitates with YAP and TEAD, so it is possible that a ternary complex forms. At least with high level expression, this does not require the YAP binding site in TEAD so there may be a direct ERBB4–TEAD interaction. This positions ERBB4 to either aid assembly of the binary YAP–TEAD complex, or participate in a ERBB4/YAP/TEAD ternary complex. In the latter scenario, ERBB4 could modulate transcription at TEAD target sites by recruitment or displacement of transcription factors. This is consistent with transactivation activity of ERBB4, which is enhanced in complexes with YAP (43, 44).
In T47D cells with endogenous ERBB4, NRG1 increased CTGF much more strongly than EGF, and LPA only weakly activated transcription of CTGF. Thus, NRG1 is a bona fide YAP activator and one of only a few known soluble YAP regulators.
Physiological ERBB4 signaling occurs in the presence of other ERBB family members. Because ERBBs efficiently transactivate when co-expressed, the overall scope of ERBB4–YAP signaling must be considered in the context of the other ERBB proteins, particularly ERBB1/EGFR, which activates YAP through an indirect mechanism. It is likely that EGF/EGFR/PDK1 and NRG1/ERBB4 ICD regulation of YAP occur simultaneously (Fig. 7). An additional layer of complexity is that AKT can phosphorylate YAP (48). Therefore, while PI3K activation relocalizes PDK1 and activates YAP, it also activates AKT and leads to YAP inhibition. ERBB3 contains a PPxY motif and may signal in the nucleus (49), raising the possibility that ERBB3-YAP binding also affects Hippo signaling.
FIGURE 7. Model of YAP regulation.
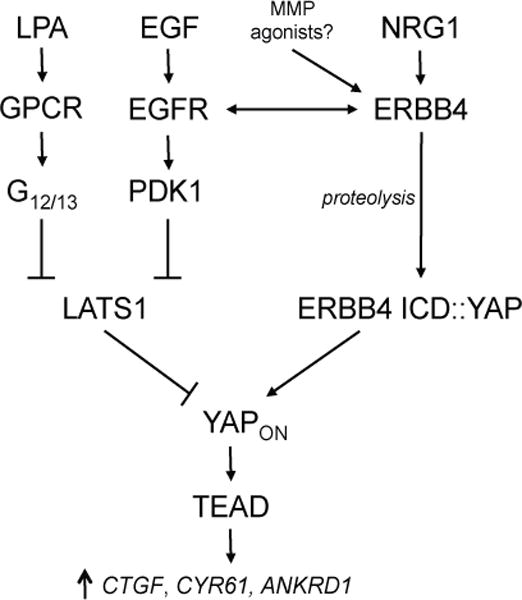
The transcriptional activity of YAP may be induced by NRG1- and proteolysis-mediated activation of ERBB4 signaling. Other pathways, such as G protein-mediated signaling and EGF-induced stimulation of another ERBB family member, may indirectly activate YAP by inhibiting the Hippo kinase LATS1.
The YAP pathway is a major regulator of normal development and cancer. YAP is activated in uveal melanoma through upstream GPCR mutations (50, 51). YAP is dysregulated in breast cancer and other cancers (31, 52). Lapatinib and erlotinib, both US FDA-approved drugs, blocked NRG1 activation of CTGF, pointing to potential therapeutic interventions to suppress ERBB4-driven YAP signaling. The YAP/TEAD inhibitor verteporfin is FDA-approved for treatment of macular degeneration (47).
ERBB4 and YAP/TAZ are important for mammary development, tissue remodeling, and lactational differentiation during pregnancy (3, 46, 53–55). In mice, the Hippo pathway is required during pregnancy, and YAP hyperactivation leads to defects in terminal differentiation (53). TAZ is required for breast cancer stem cell self-renewal and tumor-initiation (46). Furthermore, TAZ can cause lineage switching of mammary epithelial cells from luminal to basal, whereas TAZ depletion in basal cells elicits luminal differentiation (54). In mice lacking mammary ERBB4, lobuloaveoli fail to properly differentiate during pregnancy and lactation is defective (3). ERBB4 is essential during pregnancy-induced mammary differentiation and lactation at least in part through collaboration with STAT5 (55). NRG1 activation of YAP might coordinate cell growth in the mammary gland by sensing substrate stiffness and directing proliferation. Mechanical sensing may be especially important during reorganization of the mammary epithelium late in lactation and at onset of involution triggered by milk stasis at weaning where YAP inactivation might initiate differentiation. A siRNA screen for protein tyrosine kinases mediating rigidity-dependent cell polarization suggested that both ERBB3 and ERBB4 are mechanosensitive in fibroblasts (56). Therefore, the ERBBs might regulate YAP in coordination with the non-canonical Hippo pathway involving F-actin and Rho (36, 57, 58).
The unusual mechanism of ERBB4 intramembrane cleavage and nuclear localization provides new inputs into the YAP/Hippo signaling pathway that broadens the complexity of ERBB and YAP signaling. NRG1 and other ERBB4 agonists can be added to a short list of known YAP activators, which includes EGF, WNTs, and LPA. Expression of ERBB4 JM-a isoforms will enhance coupling of any ERBB agonist to YAP and TAZ signaling if the cognate ERBBs are co-expressed. As metalloproteinase cleavage of ERBB4 JM-a is rate-limiting for formation of s80 isoforms, a number of metalloproteinase agonists, including ligands for unrelated growth factor receptors, may promote YAP signaling through ERBB4 JM-a. This intersection of ERBB4 and YAP/Hippo signaling enables regulation of YAP through alternative ERBB4 mRNA splicing and proteolytic processing of ERBB4, and potentially a spectrum of YAP-driven biological processes.
Materials and Methods
Plasmids
pINDUCER20 and pINDUCER10 plasmids were generously provided by Dr. Stephen Elledge (Harvard Medical School) (59). ERBB4 JM-a CYT-1 or CYT-2 was cloned into pINDUCER20 using pENTR4. YAP shRNA hairpins were cloned into pINDUCER10 from pGIPZ plasmids (Dharmacon, V2LHS_65509 (sh1) and V3LHS_306099 (sh2)). ERBB4 JM-a CYT-2 was cloned into pcDNA3.1-V5/His B to add a C-terminal V5-tag. pRK5-MYC-TEAD1 (Addgene #33109) or pRK5-MYC-TEAD1 Tyr406Ala (Addgene #33047) were used for co-immunoprecipitation (IP) experiments. pcDNA3.1-ERBB4 CYT-2/V5 and MYC-TEAD1 were transiently transfected into 293T cells using Lipofectamine2000 (Life Technologies). ERBB4 pLKO shRNA (Sigma, TRCN0000039688 (sh1), TRCN00000196519 (sh2), TRCN0000314628 (sh3)) or scrambled shRNA control (Addgene #1864) were used to generate lentiviruses for stable knockdown in T47D cells. Murine stem cell virus (MSCV)-YAP Ser127Ala-IRES-Hygro and control MSCV-IRES-Hygro were generous gifts from Dr. Richard Hynes (MIT, (60)) and were used to produce infectious retrovirus.
Reagents
NRG1 (Sigma), EGF (Sigma), lapatinib (Selleck), dobutamine (Sigma), lysophosphatidic acid (LPA, Santa Cruz), doxycycline (Sigma), erlotinib (LC Labratories), simvastatin (Selleck), lovastatin (Selleck), Phorbol 12-myristate 13-acetate (PMA, Sigma).
Cell culture and gene transfer
MCF10A human breast cancer cells [American Type Culture Collection (ATCC)] were maintained in DMEM/F12 (Life Technologies) with 5% horse serum, 1% pen/strep, insulin (10 μg/ml, Gibco), EGF (20ng/ml, Sigma), hydrocortisone (0.5 μg/ml, Sigma), and cholera toxin (100 ng/ml, Sigma). T47D cells were maintained in RPMI (Life Technologies) with 10% FBS, 1% pen/strep, and insulin (5 μg/ml, Gibco). HEK 293T cells were maintained in DMEM (Life Technologies) with 10% FBS and 1% pen/strep.
pINDUCER plasmids were packaged as lentivirus by co-transfecting 293T cells with VSV-G, Tat1b, RaII, and HgPM2 using FuGENE 6 (Promega). Virus was collected in Opti-MEM (Life Technologies) at 48 hrs and 72 hrs, pooled and then concentrated using Centricon plus-20 filters (Millipore). MCF10A cells or T47D cells were infected overnight in 4 μg/ml polybrene, selected in 500 μg/ml G418 (pINDUCER20) or 1 μg/ml puromycin (pINDUCER10) to generate stable polyclonal cell lines. Sub-culture lines were never exposed to DOX prior to experimental use to prevent counter-selection. ERBB4 knockdown lines were generated in the same way, using lentivirus and appropriate pLKO packaging constructs and were selected in 1 ug/ml puromycin.
Quantitative RT-PCR
RNA was isolated using the RNeasy Mini Plus kit with QIAshredder columns (Qiagen). cDNA was prepared using the iScript kit (Bio-Rad). Real-time PCR was performed using Universal TaqMan Master Mix (Applied Biosystems) coupled with TaqMan FAM-labeled probes and ran on a ViiA 7 RT-PCR machine (Life Technologies). Relative mRNA expression was determined using the 2−ΔΔCt method with GAPDH as the reference gene.
Immunoblotting and immunoprecipitation
Cells were lysed in RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate] supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitors (Sigma). Protein was quantified by Bradford assay (Bio-Rad) and diluted with 2× Laemmli sample buffer. Samples were loaded onto 4–12% Bis-Tris gradient gels (NuPAGE, Life Technologies) and run in MOPS buffer. For immunoprecipitation, cells were lysed in NP-40 buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% Igepal]. 1 mg protein was incubated with 1 μg antibody overnight followed by 1 hr incubation with protein A/G Ultralink Resin (Thermo) at 4°C. For isolation of nuclear fractions, cells (10 cm equivalent) were lysed in NP-40 and incubated with agitation for 1 hr at 4°C. Nuclei were isolated by spinning for 3 min at 800 × g then supernatant was removed and nuclei were lysed in two pellet volumes of Nuclear Lysis Buffer C [20 mM HEPES, 0.42M NaCl, 1.5mM MgCl2, 0.2mM EDTA, 1mM DTT] for 1 hr at 4°C. Nuclear IPs were conducted in the presence of 1 mM DTT using 150 μg nuclear lysate and 0.5 μg antibody. IP complexes were washed twice in lysis buffer and twice in Buffer ST [50 mM Tris-HCl (pH 7.4), 150 mM NaCl].
Protein was transferred to PVDF membranes at constant amperage (at 500mA for 1 hour), which worked best for CTGF immunoblotting. Membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies and developed by chemiluminescence (Pierce). Mouse anti-V5 (Invitrogen) was used for immunoprecipitation. Antibodies used for immunoblotting targeted: ERBB4 (Santa Cruz, sc-283, rabbit), phosphorylated ERBB4 at Tyr1056 (Santa Cruz, sc-33040, rabbit) or Tyr1284 (Cell Signaling, cat. no. 4757), phosphorylated YAP at Ser127 (Cell Signaling, cat. no. 4911, rabbit), YAP (Cell Signaling, cat. no. 4912, rabbit), CTGF (Santa Cruz, sc-14939, goat), GAPDH (Santa Cruz, sc-25778, rabbit), MYC (Cell Signaling, cat. no. 2272, rabbit), phosphorylated SMAD2 at Ser465/467 (Cell Signaling, cat. no. 3108, rabbit), SMAD2 (Cell Signaling, #5339, rabbit), phoshorylated EGFR at Tyr1068 (Cell Signaling, cat. no. 3777, rabbit), and EGFR (Santa Cruz, sc-03, rabbit).
Transwell migration assays
T47D pINDUCER10 YAP KD cells or MCF10A pINDUCER20 ERBB4 CYT-2, pINDUCER10 YAP KD cells were treated with DOX (1 μg/ml) for 3 days (MCF10A) or 5 days (T47D). Cells were pre-treated with or without NRG1 (50 ng/ml) for 3 hrs then plated at 1×106 cells/well (T47D) or 1×105 cells/well (MCF10A) in 24-well plates with 8 μm filter inserts (BD Biosciences) in the presence of DOX (1 μg/ml) and with or without NRG1 (50 ng/ml). Cells were allowed to migrate for 48 hrs from 0.1% FBS toward 10% FBS for T47D cells or for 24 hrs from 0.05% horse serum to 5% horse serum for MCF10A. Membranes were fixed and stained and cell number/well was averaged from six fields of view (FOV). Technical duplicates for each trial were averaged within three independent biological replicates. Percent migration was calculated based on seeding density and the surface area of each FOV.
Cell viability assay
T47D cells were treated in the same way as for transwell migration assays except cells were re-plated into 12-well dishes following 5 days of DOX treatment. Cells were left to grow for 48 hrs to mirror the migration assay then were trypsinized and quenched with media supernatant containing any floating cells. Cells were stained with trypan blue dye and counted for viability using a Countess cell counter (Life Technologies). Assays were performed in technical triplicate and biological duplicate.
Gene set enrichment analysis (GSEA)
GSEA was performed on transcription profiles from MCF10A cells overexpressing ERBB4 ICD CYT-2. Genes significantly altered by ERBB4 (adjusted p-value <0.05) compared with empty vector controls were previously reported ((28), GEO GSE57339). YAP pathway gene sets were manually curated from the MSigDB_v4.0 (Broad Institute) (46), (61). ERBB4 CYT-2 genes (n=5965) were first rank ordered by fold-change in expression over vector-transfected cells. This list was evaluated with GSEA using the curated YAP target genes under default settings (GSEAPreranked, 10,000 permutations; (62), http://www.broad.mit.edu/gsea/).
Statistical analysis
Two-tailed Student’s t-tests were performed where appropriate. Error bars represent SD. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Supplementary Material
Fig. S1: Doxycycline-inducible expression of ERBB4
Fig. S2: Impact of YAP Ser127Ala substitution on ERBB4 induction of CTGF
Fig. S3: ERBB4-TEAD1 interaction in the nucleus
Fig. S4: Impact of erlotinib on EGFR phosphorylation induced by EGF or NRG1
Fig. S5: NRG1 regulates YAP independent of TGF-β and mevalonate pathways
Fig. S6: NRG1 does not induce migration in MCF10A cells
Acknowledgments
We thank the Hynes lab (MIT) and Elledge lab (Harvard) for generously providing plasmids, and we are grateful to Shannon Zhang for producing the pcDNA3.1-ERBB4 CYT-2/V5 plasmid.
Funding: This work was supported by USPHS RO1 CA80065 and NIH training grant T32GM07223 (J.W.H.). D.X.N. is supported by NIH/NCI grant 5R01CA166376.
Footnotes
Author contributions: Study was conceived and designed by D.F.S. and J.W.H. Experiments were conducted by J.W.H. Manuscript was written by J.W.H. and D.F.S. Bioinformatics analyses were performed by D.X.N.
Competing interests: Authors declare no competing interests.
Data and materials availability: Reagents and cell lines produced by this laboratory and described here will be made available for research purposes in accordance with Yale University policies.
Editor’s Summary
ERBB4 Signals to YAP
Whereas the Hippo pathway limits cell growth in response to mechanical signals or cell-cell contact, growth factors are soluble signals that stimulate cell proliferation. Kinases in the Hippo pathway phosphorylate and inhibit YAP, sequestering YAP in the cytosol and limiting organ size and tissue growth. Neuregulin stimulates the cleavage of the epidermal growth factor receptor family member ERBB4. Haskins et al. found that the cleaved intracellular domain (ICD) of ERBB4 activated YAP-mediated transcription by interacting with YAP in the same region with which YAP interacts with inhibitory Hippo kinases. In breast cancer cells, knocking down YAP prevented cell migration induced by neuregulin. These results suggest that in addition to receptor tyrosine kinase signaling, ERBB4 can promote tumor aggressiveness by stimulating YAP.
References and Notes
- 1.Junttila TT, Sundvall M, Maatta JA, Elenius K. Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends in cardiovascular medicine. 2000;10:304–310. doi: 10.1016/s1050-1738(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 2.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Current opinion in neurobiology. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 3.Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones FE, Welte T, Fu XY, Stern DF. ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. The Journal of cell biology. 1999;147:77–88. doi: 10.1083/jcb.147.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 6.Vidal GA, Naresh A, Marrero L, Jones FE. Presenilin-dependent gamma-secretase processing regulates multiple ERBB4/HER4 activities. The Journal of biological chemistry. 2005;280:19777–19783. doi: 10.1074/jbc.M412457200. [DOI] [PubMed] [Google Scholar]
- 7.Schlessinger J, Lemmon MA. Nuclear signaling by receptor tyrosine kinases: the first robin of spring. Cell. 2006;127:45–48. doi: 10.1016/j.cell.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore-Hebert M, Ramabhadran R, Stern DF. Interactions of ErbB4 and Kap1 connect the growth factor and DNA damage response pathways. Molecular cancer research: MCR. 2010;8:1388–1398. doi: 10.1158/1541-7786.MCR-10-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Sullivan LL, Nair SS, Williams CC, Pandey AK, Marrero L, Vadlamudi RK, Jones FE. Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer research. 2006;66:7991–7998. doi: 10.1158/0008-5472.CAN-05-4397. [DOI] [PubMed] [Google Scholar]
- 11.Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. The Journal of cell biology. 2004;167:469–478. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundvall M, Veikkolainen V, Kurppa K, Salah Z, Tvorogov D, van Zoelen EJ, Aqeilan R, Elenius K. Cell death or survival promoted by alternative isoforms of ErbB4. Molecular biology of the cell. 2010;21:4275–4286. doi: 10.1091/mbc.E10-04-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veikkolainen V, Vaparanta K, Halkilahti K, Iljin K, Sundvall M, Elenius K. Function of ERBB4 is determined by alternative splicing. Cell cycle. 2011;10:2647–2657. doi: 10.4161/cc.10.16.17194. [DOI] [PubMed] [Google Scholar]
- 14.Sundvall M, Iljin K, Kilpinen S, Sara H, Kallioniemi OP, Elenius K. Role of ErbB4 in breast cancer. Journal of mammary gland biology and neoplasia. 2008;13:259–268. doi: 10.1007/s10911-008-9079-3. [DOI] [PubMed] [Google Scholar]
- 15.Muraoka-Cook RS, Sandahl MA, Strunk KE, Miraglia LC, Husted C, Hunter DM, Elenius K, Chodosh LA, Earp HS., 3rd ErbB4 splice variants Cyt1 and Cyt2 differ by 16 amino acids and exert opposing effects on the mammary epithelium in vivo. Molecular and cellular biology. 2009;29:4935–4948. doi: 10.1128/MCB.01705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muraoka-Cook RS, Sandahl M, Husted C, Hunter D, Miraglia L, Feng SM, Elenius K, Earp HS., 3rd The intracellular domain of ErbB4 induces differentiation of mammary epithelial cells. Molecular biology of the cell. 2006;17:4118–4129. doi: 10.1091/mbc.E06-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, Jung KM, Huang YZ, Bennett LB, Lee JS, Mei L, Kim TW. Presenilin-dependent gamma-secretase-like intramembrane cleavage of ErbB4. The Journal of biological chemistry. 2002;277:6318–6323. doi: 10.1074/jbc.M110371200. [DOI] [PubMed] [Google Scholar]
- 18.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, Leiserson MD, Miller CA, Welch JS, Walter MJ, Wendl MC, Ley TJ, Wilson RK, Raphael BJ, Ding L. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan R, Gillett CE, Barnes DM, Gullick WJ. Nuclear expression of the c-erbB-4/HER-4 growth factor receptor in invasive breast cancers. Cancer research. 2000;60:1483–1487. [PubMed] [Google Scholar]
- 20.Junttila TT, Sundvall M, Lundin M, Lundin J, Tanner M, Harkonen P, Joensuu H, Isola J, Elenius K. Cleavable ErbB4 isoform in estrogen receptor-regulated growth of breast cancer cells. Cancer research. 2005;65:1384–1393. doi: 10.1158/0008-5472.CAN-04-3150. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan R, Poulsom R, Hurst HC, Gullick WJ. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. The Journal of pathology. 1998;185:236–245. doi: 10.1002/(SICI)1096-9896(199807)185:3<236::AID-PATH118>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Thor AD, Edgerton SM, Jones FE. Subcellular localization of the HER4 intracellular domain, 4ICD, identifies distinct prognostic outcomes for breast cancer patients. The American journal of pathology. 2009;175:1802–1809. doi: 10.2353/ajpath.2009.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abd El-Rehim DM, Pinder SE, Paish CE, Bell JA, Rampaul RS, Blamey RW, Robertson JF, Nicholson RI, Ellis IO. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. British journal of cancer. 2004;91:1532–1542. doi: 10.1038/sj.bjc.6602184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang CK, Concepcion XZ, Milan M, Gong X, Montgomery E, Lippman ME. Ribozyme-mediated down-regulation of ErbB-4 in estrogen receptor-positive breast cancer cells inhibits proliferation both in vitro and in vivo. Cancer research. 1999;59:5315–5322. [PubMed] [Google Scholar]
- 25.Lodge AJ, Anderson JJ, Gullick WJ, Haugk B, Leonard RC, Angus B. Type 1 growth factor receptor expression in node positive breast cancer: adverse prognostic significance of c-erbB-4. Journal of clinical pathology. 2003;56:300–304. doi: 10.1136/jcp.56.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bieche I, Onody P, Tozlu S, Driouch K, Vidaud M, Lidereau R. Prognostic value of ERBB family mRNA expression in breast carcinomas. International journal of cancer Journal international du cancer. 2003;106:758–765. doi: 10.1002/ijc.11273. [DOI] [PubMed] [Google Scholar]
- 27.Prickett TD, Agrawal NS, Wei X, Yates KE, Lin JC, Wunderlich JR, Cronin JC, Cruz P, Rosenberg SA, Samuels Y. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nature genetics. 2009;41:1127–1132. doi: 10.1038/ng.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wali VB, Haskins JW, Gilmore-Hebert M, Platt JT, Liu Z, Stern DF. Convergent and Divergent Cellular Responses by ErbB4 Isoforms in Mammary Epithelial Cells. Molecular cancer research: MCR. 2014 doi: 10.1158/1541-7786.MCR-13-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amin DN, Perkins AS, Stern DF. Gene expression profiling of ErbB receptor and ligand-dependent transcription. Oncogene. 2004;23:1428–1438. doi: 10.1038/sj.onc.1207257. [DOI] [PubMed] [Google Scholar]
- 30.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Developmental cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 37.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nature reviews Molecular cell biology. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 38.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, Rosato A, Piccolo S, Del Sal G. Metabolic control of YAP and TAZ by the mevalonate pathway. Nature cell biology. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 40.Reddy BV, Irvine KD. Regulation of Hippo Signaling by EGFR-MAPK Signaling through Ajuba Family Proteins. Developmental cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2569–2574. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nature cell biology. 2009;11:1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. The Journal of biological chemistry. 2003;278:33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 44.Omerovic J, Puggioni EM, Napoletano S, Visco V, Fraioli R, Frati L, Gulino A, Alimandi M. Ligand-regulated association of ErbB-4 to the transcriptional co-activator YAP65 controls transcription at the nuclear level. Experimental cell research. 2004;294:469–479. doi: 10.1016/j.yexcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Hoeing K, Zscheppang K, Mujahid S, Murray S, Volpe MV, Dammann CE, Nielsen HC. Presenilin-1 processing of ErbB4 in fetal type II cells is necessary for control of fetal lung maturation. Biochimica et biophysica acta. 2011;1813:480–491. doi: 10.1016/j.bbamcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 47.Bao Y, Nakagawa K, Yang Z, Ikeda M, Withanage K, Ishigami-Yuasa M, Okuno Y, Hata S, Nishina H, Hata Y. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. Journal of biochemistry. 2011;150:199–208. doi: 10.1093/jb/mvr063. [DOI] [PubMed] [Google Scholar]
- 48.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Molecular cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 49.Adilakshmi T, Ness-Myers J, Madrid-Aliste C, Fiser A, Tapinos N. A nuclear variant of ErbB3 receptor tyrosine kinase regulates ezrin distribution and Schwann cell myelination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:5106–5119. doi: 10.1523/JNEUROSCI.5635-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, Chen Q, Gutkind JS. Hippo-Independent Activation of YAP by the GNAQ Uveal Melanoma Oncogene through a Trio-Regulated Rho GTPase Signaling Circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, Zhao J, Chen X, Zhang L, Wang CY, Bastian BC, Zhang K, Guan KL. Mutant Gq/11 Promote Uveal Melanoma Tumorigenesis by Activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nature reviews Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 53.Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28:432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skibinski A, Breindel JL, Prat A, Galvan P, Smith E, Rolfs A, Gupta PB, Labaer J, Kuperwasser C. The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell reports. 2014;6:1059–1072. doi: 10.1016/j.celrep.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long W, Wagner KU, Lloyd KC, Binart N, Shillingford JM, Hennighausen L, Jones FE. Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development. 2003;130:5257–5268. doi: 10.1242/dev.00715. [DOI] [PubMed] [Google Scholar]
- 56.Prager-Khoutorsky M, Lichtenstein A, Krishnan R, Rajendran K, Mayo A, Kam Z, Geiger B, Bershadsky AD. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nature cell biology. 2011;13:1457–1465. doi: 10.1038/ncb2370. [DOI] [PubMed] [Google Scholar]
- 57.Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS letters. 2014;588:2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 59.Meerbrey KL, Hu G, Kessler JD, Roarty K, Li MZ, Fang JE, Herschkowitz JI, Burrows AE, Ciccia A, Sun T, Schmitt EM, Bernardi RJ, Fu X, Bland CS, Cooper TA, Schiff R, Rosen JM, Westbrook TF, Elledge SJ. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3665–3670. doi: 10.1073/pnas.1019736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2441–2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer research. 2008;68:2789–2794. doi: 10.1158/0008-5472.CAN-07-6205. [DOI] [PubMed] [Google Scholar]
- 62.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Doxycycline-inducible expression of ERBB4
Fig. S2: Impact of YAP Ser127Ala substitution on ERBB4 induction of CTGF
Fig. S3: ERBB4-TEAD1 interaction in the nucleus
Fig. S4: Impact of erlotinib on EGFR phosphorylation induced by EGF or NRG1
Fig. S5: NRG1 regulates YAP independent of TGF-β and mevalonate pathways
Fig. S6: NRG1 does not induce migration in MCF10A cells


