Abstract
OBJECTIVE
We previously reported specific genotypes of polymorphisms in two genes, tumor necrosis factor-α (TNF-α-238G>A) and Apolipoprotein E (ApoE e2), as independent predictors of new intracranial hemorrhage (ICH) in the natural course of untreated brain arteriovenous malformations. We hypothesized that the risk of post-treatment ICH would also be greater in patients with brain arteriovenous malformations with these genotypes.
METHODS
Two hundred fifteen patients undergoing brain arteriovenous malformation treatment (embolization, arteriovenous malformation resection, radiosurgery, or any combination of these) were genotyped and followed longitudinally. Association of genotype with new symptomatic ICH after initiation of treatment was assessed using Cox proportional hazards adjusted for treatment type, demographics, and established ICH risk factors censored at the time of the last follow-up evaluation or death.
RESULTS
The cohort was 48% male and 55% Caucasian, and 52% had an ICH before the initiation of treatment; the mean age ± standard deviation was 36.6 ± 17.2 years. Posttreatment ICH occurred in 34 (16%) patients with a median follow-up period of 1.9 years (interquartile range, 1.6 yr). After adjustment, the risk of posttreatment ICH was greater for TNF-α-238 AG genotype (hazard ratio [HR], 3.5; 95% confidence interval [CI], 1.3–9.8; P = 0.016) and ApoE e2 (HR, 3.2; 95% CI, 1.0–9.7; P = 0.042). Similar trends for the TNF-α-238 AG genotype were seen in surgery (HR, 4.2; 95% CI, 0.6–28.8; P = 0.14) and radiosurgery subsets (HR, 3.8; 95% CI, 0.7–19.4; P = 0.11). An effect of ApoE e2 was seen in radiosurgery subsets (HR, 10.9; 95% CI, 1.3–93.7; P = 0.030), but not in surgery subsets (HR, 1.4; 95% CI, 0.3–7.4; P = 0.67).
CONCLUSION
Despite a variety of different mechanisms for posttreatment hemorrhage, these data suggest that the TNF-α and ApoE genotypes may contribute common phenotypes of enhanced vascular instability that increase the risk of hemorrhagic outcome.
Keywords: Arteriovenous malformation, Cerebral hemorrhage, Genetic epidemiology, Microsurgical resection, Radiosurgery, Risk prediction
Patients with brain arteriovenous malformations (BAVM) are at life-threatening risk of intracranial hemorrhage (ICH) (25). Obliteration or resection of the lesion to prevent future ICH is the primary motivation behind invasive therapy. Effective clinical management requires accurate estimates of natural history risks balanced against the hazards of invasive therapy. Clinical presentation of BAVM with ICH is the most widely demonstrated predictor of future ICH in the natural course of untreated BAVM (11, 12); predictors of future ICH risk in patients with nonhemorrhagic presentations are lacking (34).
The absence of hemorrhagic presentation has recently been reported as an underappreciated risk factor predicting morbidity after microsurgical arteriovenous malformation resection (21), despite the fact that it is not part of major surgical risk prediction tools such as the Spetzler-Martin grading scale (32). Nonhemorrhagic presentation carries a similar risk magnitude for adverse outcome after microsurgical resection as the components of the Spetzler-Martin grade (21), underscoring weaknesses in current risk grading assessments and the need for additional predictors of future morbidity after treatment, particularly in patients with BAVMs with unruptured lesions.
Identification of single nucleotide polymorphisms (SNPs) associated with increased risk of new ICH could facilitate risk stratification and therapeutic decision-making (28). We previously demonstrated that genetic variation might influence the natural course of BAVM. We reported an association between the −238G>A promoter polymorphism of the inflammatory cytokine tumor necrosis factor-alpha (TNF-α) (1) and the epsilon2 (e2) allele of the Apolipoprotein (ApoE) gene (27), and risk of new ICH in the untreated course of the disease.
In the present study, we sought to determine whether or not these genotypes would independently predict posttreatment ICH risks in the clinical course after the initiation of BAVM treatment. Whereas previous reports focused on new ICH in longitudinal follow-up after initial presentation but before any intervening treatment, the present study focused on an entirely different period of observation, i.e., any new ICH in longitudinal follow-up after the initiation of treatment, using similar multivariate analysis with additional adjustment for any risks carried over from natural course events.
MATERIALS AND METHODS
Patient Sample
With Institutional Review Board approval and informed consent, patients with BAVM evaluated at the University of California, San Francisco (UCSF) are entered into a prospective registry, as previously described (13). The database includes information regarding patient demographics, radiographic features of the BAVM, clinical presentation, treatment, follow-up data, and outcomes, including hemorrhages occurring after the initial diagnosis in the natural course as well as after initiation of treatment in the posttreatment course. BAVM characteristics such as size and venous drainage pattern were recorded using standardized guidelines (17).
Genotyping
Patients had blood samples drawn and deoxyribonucleic acid harvested as previously described (1, 27, 28). The TNF-α-38G>A promoter SNP (1) and two SNPs in the ApoE gene, Cys112Arg (T>C) and Arg158Cys (C>T), that determine ApoE e2/e3/e4 genotype (9, 27), were genotyped by template-directed dye-terminator incorporation assays with fluorescence polarization detection as previously described (16, 28). Genotype distributions of the two SNPs that make up the ApoE e2/e3/e4 genotypes and the TNF-α-238G>A promoter SNP were consistent with Hardy-Weinberg equilibrium among all racial and ethnic subgroups (data not shown).
Definitions and Statistical Analyses
We examined the association between the rate of new hemorrhage after the initiation of treatment and genotype in Kaplan-Meier survival analysis and Cox proportional hazards multivariate models. The primary outcome was time to occurrence of new ICH (symptomatic new hemorrhage with intracranial blood on computed tomographic or magnetic resonance imaging scans) after the initiation of BAVM treatment censored at the time of the last follow-up evaluation or death.
Consistent with our previous reports (1, 27, 28), analyses compared risk in carriers of the TNF-α-238 A allele (12% AG, 0% AA) against the homozygote GG genotype reference group (88%), as well as risk in ApoE e2 carriers (ApoE e2+, defined as carrying one or two copies of the ApoE e2 allele; 12%) against the reference group comprising all other ApoE genotypes (ApoE e2−; 88%). Association of genotype with risk of new ICH after treatment was assessed using Kaplan-Meier survival analyses and Cox regression. The period at risk was defined from the date of the first BAVM treatment (surgery, radiosurgery, or embolization) to the date of an event, i.e., onset of new (first subsequent) ICH or censoring attributable to loss to follow-up (using the date of the last available follow-up evaluation).
To adjust for variations in treatment received, patients were grouped by the following primary definitive treatment cohorts: 1) surgery group, patients undergoing arteriovenous malformation resection with or without embolization; 2) radiosurgery group, patients undergoing radiosurgery treatment with or without embolization; and 3) complex treatment, all other combinations, including multiple treatment with both surgery and radiosurgery, with or without embolization, and patients receiving embolization only. To adjust for heterogeneity in intervention, treatment cohort was entered as a predictor in the multivariate model (with the surgery group as the reference group), and a separate predictor was included to indicate whether embolization was received.
Treatment completion status was determined by chart review. For the UCSF patients, postoperative angiography was obtained in all patients after microsurgical resection; those undergoing radiosurgery had follow-up angiography if follow-up magnetic resonance imaging studies suggested nidus obliteration. All cases of posttreatment ICH had treatment completion status verified. Forty-three (20%) of the 215 patients had completion status imputed as follows: for the outside arte-riovenous malformation resection treatments with unconfirmed outcome, treatment was imputed to be “complete” (n = 6); for the radio-surgery patients with unconfirmed outcomes, treatment was imputed to be “partial,” i.e., less than 3 years after radiosurgery treatment date (n = 18), or “complete,” i.e., greater than 3 years after radiosurgery treatment date (n = 19).
In constructing the multivariate model, we began by including all possible variables of clinical importance in a full model, which included genotype, age at first treatment (continuous but measured in decades), race/ethnicity, sex, any pretreatment ICH (ruptured before treatment versus unruptured at first treatment), treatment cohort (radiosurgery, complex treatment versus surgery), embolization (with embolization versus without embolization), treatment completion status (partial versus complete obliteration/removal), large BAVM size (largest dimension ≥3 cm versus <3 cm), exclusively deep venous drainage (only deep versus any superficial), intranidal aneurysm status (any intranidal aneurysm versus none), eloquence, location (subcortical, mixed, posterior fossa versus cortical location), and Spetzler-Martin grade. We then used the backward selection method to arrive at a final model with maximum parsimony by dropping nonstatistically significant predictors one at a time. The predictors that made up the final multivariate model, reported as hazard ratios (HRs) and 95% confidence intervals (CIs), included genotype, pretreatment ICH, race/ethnicity, male sex, age at start, treatment cohort, treatment completion status, and Spetzler-Martin score.
Sensitivity Analyses
The adjustments for treatment cohort and embolization status were dropped from the final model in favor of adjusting for the last treatment immediately preceding the posttreatment ICH, or overall last treatment in censored cases, to explore any possible risk that could have been masked by the categorical grouping and that might have confounded the effect of genotype. An interaction term of genotype with treatment cohorts was entered into the main models to explore whether or not predictive effects of genotype relied in part on an association with treatments received. Ruling out any such interaction, subset analyses were performed within the major treatment cohorts to observe the effect size of genotype in the radiosurgery and surgery treatment cohorts separately. These two groups were chosen because of the homogeneity of the treatment received, either primary surgery or radiosurgery, and because there was a large enough sample size to allow some degree of adjustment for other risk factors or potential confounders.
To reduce potential confounding by race/ethnicity, subset analysis within the major ethnic group (Caucasians) was performed. To assess the impact of unconfirmed treatment outcomes (partial versus complete treatment) on the stability of our estimates of the risks of each genotype, we performed sensitivity analyses in which we repeated the multivariate Cox analyses assuming that all unconfirmed outcomes were “complete” or that all unconfirmed outcomes were “partial.” We reran the multivariate models excluding all intraoperative and perioperative bleeds, defined to be new ICH after initiation of treatment within 7 days of treatment, to explore any possible confounding resulting from procedural complication rates in higher-risk lesions from the natural course.
RESULTS
We genotyped 215 patients undergoing BAVM treatment (48% male; 55% white; 52% with any ICH before initiation of treatment; mean age ± standard deviation, 36.6 ± 17.2 yr) with a total of 138 patients in the surgery cohort, 54 in the radio-surgery cohort, and 23 in the complex treatment cohort (Table 1). The characterization of lesions entering each treatment cohort by Spetzler-Martin surgical grade is displayed in Table 2.
TABLE 1.
| ICH | no ICH | Total | Percent new ICH | P value | |
|---|---|---|---|---|---|
| Total Demographics | 34 | 181 | 215 | 16 | |
| Race | |||||
| Caucasian | 19 | 100 | 119 | 16 | 0.92 |
| Black | 1 | 6 | 7 | 14 | |
| Hispanic | 8 | 47 | 55 | 15 | |
| Asian/Pacific Islander | 6 | 25 | 31 | 19 | |
| Native American | 0 | 3 | 3 | 0 | |
| Total | 34 | 181 | 215 | 16 | |
| Sex | |||||
| Female | 19 | 92 | 111 | 17 | 0.59 |
| Male | 15 | 89 | 104 | 14 | |
| Total | 34 | 181 | 215 | 16 | |
| Age, yrc | |||||
| Mean ± SD | 46.9 ± 15.6 | 34.7 ± 16.9 | 36.6 ± 17.2 | <0.001 | |
| No. | 34 | 181 | 215 | ||
| Follow-up period, yr | |||||
| Median (interquartile range) | 0.63 (3.98) | 1.93 (1.33) | 1.85 (1.62) | 0.018c | |
| No. | 34 | 181 | 215 | ||
| BAVM characteristics | |||||
| Size of BAVM | |||||
| <3 cm | 8 | 107 | 115 | 7 | <0.001 |
| ≥ 3 cm | 24 | 70 | 94 | 26 | |
| Total | 32 | 177 | 209 | 15 | |
| Venous drainage | |||||
| Any superficial | 27 | 138 | 165 | 16 | 0.58 |
| Deep only | 5 | 34 | 39 | 13 | |
| Total | 32 | 172 | 204 | 16 | |
| Eloquence | |||||
| Noneloquent | 7 | 77 | 84 | 8 | 0.025 |
| Eloquent | 25 | 98 | 123 | 20 | |
| Total | 32 | 175 | 207 | 15 | |
| Location | |||||
| Cortical | 16 | 111 | 127 | 13 | 0.45 |
| Subcortical | 5 | 15 | 20 | 25 | |
| Mixed | 8 | 26 | 34 | 24 | |
| Posterior fossa | 3 | 22 | 25 | 12 | |
| Total | 32 | 174 | 206 | 16 | |
| Any intranidal aneurysm | |||||
| Yes | 5 | 31 | 36 | 14 | 0.73 |
| No | 29 | 150 | 179 | 16 | |
| Total | 34 | 181 | 215 | 16 | |
| Spetzler-Martin grade | |||||
| I | 1 | 35 | 36 | 3 | 0.003 |
| II | 5 | 54 | 59 | 8 | |
| III | 12 | 56 | 68 | 18 | |
| IV | 12 | 28 | 40 | 30 | |
| V | 2 | 2 | 4 | 50 | |
| Total | 32 | 175 | 207 | 15 | |
| Initial presentation (full) | |||||
| ICH | 13 | 82 | 95 | 14 | 0.4 |
| Seizure | 6 | 43 | 49 | 12 | |
| Focal neurological deficit | 2 | 12 | 14 | 14 | |
| General neurological deficit | 0 | 7 | 7 | 0 | |
| Aneurysm bleed | 1 | 2 | 3 | 33 | |
| Headache | 6 | 19 | 25 | 24 | |
| Incidental | 6 | 16 | 22 | 27 | |
| Other | 0 | 0 | 0 | — | |
| Total | 34 | 181 | 215 | 16 | |
| Pretreatment ICH | |||||
| Any ICH before treatment | 17 | 95 | 112 | 15 | 0.79 |
| Unruptured at treatment | 17 | 86 | 103 | 17 | |
| Total | 34 | 181 | 215 | 16 | |
| Treatments | |||||
| Surgery only | 2 | 38 | 40 | 5 | <0.001 |
| Embolization + surgery | 9 | 89 | 98 | 9 | |
| Radiosurgery only | 11 | 28 | 39 | 28 | |
| Embolization + radiosurgery | 6 | 9 | 15 | 40 | |
| Complex treatment | 6 | 17 | 23 | 26 | |
| Total | 34 | 181 | 215 | 16 | |
| Treatment completion status | |||||
| Partial treatment | 25 | 27 | 52 | 47 | <0.001 |
| Complete treatment | 9 | 154 | 163 | 7 | |
| Total | 34 | 181 | 215 | 16 | |
| Single nucleotide polymorphisms | |||||
| TNF-α–238G>A | |||||
| GG | 26 | 158 | 184 | 14 | 0.031 |
| AG | 8 | 18 | 26 | 31 | |
| AA | 0 | 0 | 0 | — | |
| Total | 34 | 176 | 210 | 16 | |
| ApoE | |||||
| e2– | 28 | 158 | 186 | 15 | 0.58 |
| e2+ | 5 | 21 | 26 | 19 | |
| Total | 33 | 179 | 212 | 16 | |
BAVM, brain arteriovenous malformation; TNF, tumor necrosis factor; ICH, intracranial hemorrhage; SD, standard deviation; ApoE, Apolipoprotein E.
Some subgroups do not sum to 215 as a result of missing data (P, χ2 test for categorical variables, t test for continuous variables).
Mann-Whitney U test.
TABLE 2.
Characterization by Spetzler-Martin surgical risk grade of genotyped cases included in each treatment cohort (n = 207)a
| Spetzler-Martin surgical risk score | ||||||
|---|---|---|---|---|---|---|
| Total | I | II | III | IV | V | |
| AVM resection | 135 | 31 | 49 | 42 | 12 | 1 |
| AVM resection only | 38 | 13 | 12 | 11 | 2 | 0 |
| Embolization + AVM resection | 97 | 18 | 37 | 31 | 10 | 1 |
| Radiosurgery | 52 | 2 | 7 | 19 | 21 | 3 |
| Radiosurgery only | 38 | 1 | 5 | 16 | 14 | 2 |
| Embolization + radiosurgery | 14 | 1 | 2 | 3 | 7 | 1 |
| Complex treatment | 20 | 3 | 3 | 7 | 7 | 0 |
| Embolization only | 12 | 3 | 3 | 3 | 3 | 0 |
| Radiosurgery + AVM resection | 2 | 0 | 0 | 1 | 1 | 0 |
| Embolization + radiosurgery + AVM resection | 6 | 0 | 0 | 3 | 3 | 0 |
| Total | 207 | 36 | 59 | 68 | 40 | 4 |
AVM, arteriovenous malformation.
Overall, in a median follow-up period of 1.9 years (interquartile range, 1.6), 181 (84%) patients were followed until censoring at the time of their last follow-up evaluation (127 surgery, 37 radiosurgery, 17 complex treatment); new ICH in the clinical course after initiation of treatment occurred in an additional 34 (16%) patients (11 surgery, 17 radiosurgery, and six complex treatment) and was associated with the AG genotype of TNF-α-238G>A (χ2, P = 0.031; Table 1). Of the 11 patients with postsurgical bleeds, nine underwent operation at UCSF and two underwent operation at other institutions and transferred to UCSF with new ICH for further surgery. Of the nine UCSF post-surgical ICH cases, seven were assessed as complete resections with lack of residual nidus seen on follow-up angiography and two were determined to be partial, one of which was attributable to intentional staging. Of the two outside treatments with postsurgical bleeds, one was assessed as complete and one as partial. Of the bleeds in the UCSF group, two were intraoperative, including one in the complete resection group and one in the partial resection group undergoing intentional staging.
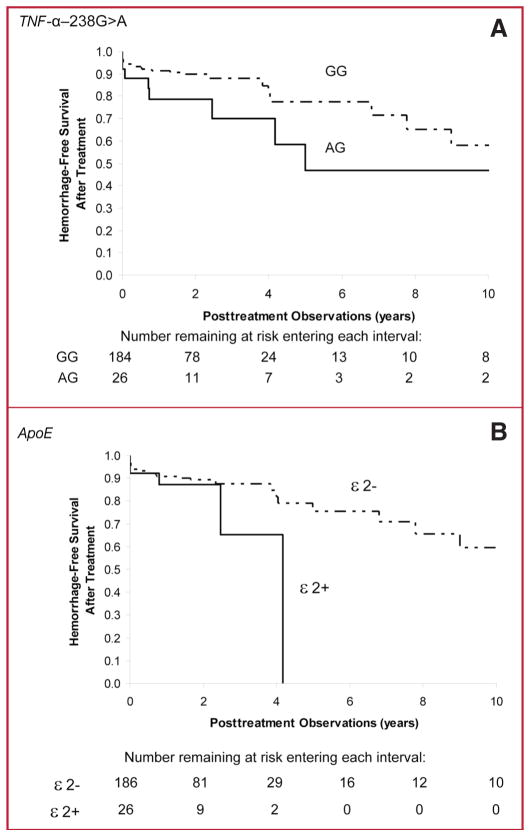
Kaplan-Meier analysis exploring association of genotype with new ICH after the initiation of treatment showed a trend for TNF-α-238 AG genotype (log rank P = 0.15; Fig. 1A) and ApoE e2 carrier status (log rank P = 0.19; Fig. 1B) to be associated with posttreatment ICH. Adjusting for other risk factors in multivariate Cox proportional hazards analysis, risk of post-treatment ICH was greater for TNF-α-238 AG genotype compared with GG genotype (HR, 3.5; 95% CI, 1.3–9.8; P = 0.016; Table 3), as well as for ApoE e2 carriers (HR, 3.2; 95% CI, 1.0–9.7; P = 0.042; Table 3). Among the predictors of posttreatment hemorrhage studied, partial treatment status had the largest effect. Other predictors included advanced age and male sex. Among all predictors studied, genotype was second only to partial treatment status in terms of magnitude of effect.
FIGURE 1.
Kaplan-Meier survival analysis was performed on new ICH during BAVM clinical course after the initiation of treatment by TNF-α-238 AG genotype (A, log rank, P = 0.15) or ApoE e2 carrier status (B, log rank, P = 0.19) on risk of posttreatment ICH.
TABLE 3.
Impact of TNF-α-238 AG genotype and ApoE e2 carrier status on risk of posttreatment ICHa
| No. risk/reference | HR | 95% CI | P value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| TNF-α model | 202 | <0.001 | |||
| TNF-α-238 AG genotype | 24/178 | 3.5 | 1.3 | 9.8 | 0.016 |
| Any pretreatment ICH | 96/106 | 1.8 | 0.8 | 4.2 | 0.16 |
| Race (Caucasian versus) | 112 | 0.30 | |||
| Black | 7 | 0.4 | 0.0 | 3.2 | 0.37 |
| Hispanic | 52 | 2.0 | 0.7 | 5.4 | 0.18 |
| Asian, Pacific Islander, Alaskan/American Native | 31 | 0.8 | 0.2 | 3.0 | 0.79 |
| Male sex | 99/103 | 3.1 | 1.2 | 7.7 | 0.02 |
| Age at first treatment (decades) | 202 | 2.2 | 1.6 | 2.9 | <0.001 |
| Treatment cohort: surgery versus | 130 | 0.06 | |||
| Radiosurgery | 52 | 0.2 | 0.1 | 0.7 | 0.02 |
| Complex treatment | 20 | 0.3 | 0.1 | 1.4 | 0.12 |
| Partial treatment | 50/152 | 25.2 | 5.5 | 116.3 | <0.001 |
| Spetzler-Martin score | 202 | 1.4 | 0.9 | 2.2 | 0.17 |
| ApoE model | 204 | <0.001 | |||
| ApoE e2+ genotype | 26/178 | 3.2 | 1.0 | 9.7 | 0.042 |
| Any pretreatment ICH | 97/107 | 2.0 | 0.9 | 4.8 | 0.11 |
| Race (Caucasian versus) | 113 | 0.32 | |||
| Black | 7 | 0.2 | 0.0 | 1.8 | 0.15 |
| Hispanic | 54 | 1.3 | 0.5 | 3.3 | 0.61 |
| Asian, Pacific Islander, Alaskan/American Native | 30 | 0.6 | 0.2 | 2.1 | 0.43 |
| Male sex | 102/102 | 2.9 | 1.1 | 7.4 | 0.028 |
| Age at first treatment (decades) | 204 | 2.2 | 1.6 | 2.9 | <0.001 |
| Treatment cohort: surgery versus | 134 | 0.10 | |||
| Radiosurgery | 50 | 0.2 | 0.1 | 0.9 | 0.03 |
| Complex treatment | 20 | 0.4 | 0.1 | 2.0 | 0.28 |
| Partial treatment | 48/156 | 25.9 | 5.4 | 123.1 | <0.001 |
| Spetzler-Martin score | 204 | 1.4 | 0.9 | 2.2 | 0.12 |
ICH, intracranial hemorrhage; Risk, risk group sample size; Ref, reference group sample size; HR, hazard ratio; CI, confidence interval; TNF, tumor necrosis factor; ApoE, Apolipoprotein E.
We observed no association of TNF-α-238 AG genotype with Spetzler-Martin grade (odds ratio [OR], 1.1; 95% CI, 0.7–1.7; P = 0.68) or age in decades at the time of the first treatment (OR, 0.9; 95% CI, 0.7–1.2; P = 0.63), and similarly no association of ApoE genotype with Spetzler-Martin grade (OR, 0.8; 95% CI, 0.5–1.2; P = 0.30) or age in decades at the time of the first treatment (OR, 0.9; 95% CI, 0.7–1.1; P = 0.28). There was no association between either of the genotypes and presence of intranidal aneurysms.
In sensitivity analyses, replacing the categorical adjustments for treatment group with a single adjustment for the last treatment immediately preceding posttreatment ICH or overall last treatment in censored cases had minimal impact on the other estimates in the model, and similar findings for risk of TNF-α-238 AG genotype (HR, 3.4; 95% CI, 1.1–10.3, P = 0.028) and ApoE e2 carrier status (HR, 3.3; 95% CI, 1.1–10.2, P = 0.038) were observed. There was no interaction effect between either of the genotypes and the treatments received, and the distribution of genotypes between treatment cohorts was not different (data not shown). In subset analysis, association of genotype with posttreatment ICH rates was assessed in the surgery and radiosurgery treatment cohorts separately; as shown in Figure 2, the postsurgical bleeds were all in the immediate postoperative period. TNF-α-238 AG genotype demonstrated a similar effect size when the multivariate Cox model was run separately within the surgery group (HR, 4.2; 95% CI, 0.6–28.8, P = 0.14) and radiosurgery group (HR, 3.8; 95% CI, 0.7–19.4, P = 0.11). There was no effect of ApoE e2 carrier status observed in the surgery group (HR, 1.4; 95% CI, 0.3–7.4, P = 0.67); however, an effect of ApoE e2 was observed in the radiosurgery group (HR, 10.9; 95% CI, 1.3–93.7; P = 0.030).
FIGURE 2.
New ICH during BAVM clinical course after the initiation of treatment by treatment cohort and TNF-α-238 AG genotype (A) or ApoE e2 carrier status (B) on risk of posttreatment ICH.
In subset analysis within the major ethnic group (Caucasians), risk of posttreatment ICH was consistently increased for both TNF-α-238 AG genotype (HR, 2.9; 95% CI, 0.7–11.3, P = 0.13) and ApoE e2 carriers (HR, 8.4; 95% CI, 1.6–44.0, P = 0.011). The sensitivity analyses, in which we assumed that all unconfirmed outcomes were “complete,” or that all unconfirmed outcomes were “partial,” yielded a range of estimates for the effects of genotypes taking into account the maximum possible variability among unconfirmed outcomes. The estimate for TNF-α-238 AG genotype was HR = 3.7 when assuming all unconfirmed outcomes to be “complete” (95% CI, 1.2–11.2; P = 0.022) and was HR = 6.1 when assuming all unconfirmed outcomes to be “partial” (95% CI, 2.1–17.4; P = 0.001). Similarly, the estimate for ApoE e2 carriers was HR = 3.0 when assuming all unknown cases to be “complete” (95% CI, 0.9–9.4, P = 0.066), and HR = 3.5 when assuming all unknown cases to be “partial” (95% CI, 1.1–10.8, P = 0.033). Therefore, taking into account the maximum possible variability in treatment completion status among our unconfirmed cases suggests consistent estimates of the risks associated with both genotypes.
In further sensitivity analyses, excluding the two cases of intraoperative ICH did not substantially change the effect size or significance for either genotype (data not shown). After excluding all perioperative ICH events that occurred within 7 days of last treatment (n = 14), a similar magnitude of risk was seen for ApoE e2 (HR, 5.5; 95% CI, 0.7–43.3; P = 0.10) and TNF-α-238 AG genotype (HR, 2.4; 95% CI, 0.6–10.1; P = 0.22) in the multivariate Cox model, although there was not a large enough sample size to achieve significance.
DISCUSSION
This study demonstrates the first evidence of an association between genotype and the risk of new ICH after initiation of BAVM treatment in a longitudinal follow-up study. The AG genotype of the TNF-α-238G>A promoter polymorphism was associated with a 3.5-fold increase in risk of new ICH in the posttreatment course of BAVM with a similar 3.2-fold risk in ApoE e2 carriers. Effect sizes were similar to those previously reported for the association of these genotypes with new ICH in the untreated natural course of BAVM after diagnosis but before any treatment (1, 27).
The finding for TNF-α-238G>A genotype further implicates inflammatory processes in the pathogenesis of vessel rupture. Despite a variety of different mechanisms for posttreatment hemorrhage, these data suggest that the TNF-α and ApoE genotypes may contribute common phenotypes of enhanced vascular instability that serve to increase the risk of hemorrhagic outcomes after treatment.
The cytokine TNF-α is a proinflammatory and immunomodulatory pleiotropic polypeptide implicated in inflammatory conditions involving proteolytic processes (14). In particular, TNF-α plays a significant role in brain immune and inflammatory activities and has been implicated in a variety of pathological processes, including ischemia, trauma, and infectious disease (10). TNF-α-238 AG genotype has been associated with a high cytokine expressor phenotype (29). TNF-α is an upstream modulator for many inflammatory cytokines and proteolytic enzymes, including interleukin-6 and matrix metalloproteinases, a family of proteolytic enzymes that degrade extracellular matrix around blood vessels and damage endothelial cells, which could result in destabilization and potential weakening of the vessel wall, passive dilation, and rupture (15). Surgical BAVM specimens display elevated interleukin-6 and matrix metalloproteinase expression (6, 7, 15).
TNF-α promotes inflammation by stimulation of capillary endothelial cell proinflammatory responses and expression of proadhesive molecules on the endothelium, resulting in leukocyte accumulation, adherence, and migration from capillaries into the brain (10). Furthermore, TNF-α activates glial cells, thereby regulating tissue remodeling, gliosis, and scar formation (31). A role for TNF-α in BAVM pathophysiology is, therefore, consistent with the common association of BAVM with intervening and surrounding parenchymal gliosis.
The ApoE genotype has been implicated in many human disease phenotypes, including ICH and subarachnoid hemorrhage (23). The presence of the ApoE e2 variant increases recurrent lobar ICH risk in patients with cerebral amyloid angiopathy (35). ApoE may modify the central nervous system response to acute and chronic injury (20). ApoE functions as an important suppressor of glial cell secretion of TNF-α (19). ApoE also promotes the efflux of lipids from astrocytes and neurons (26).
The order of potency of the ApoE isoforms as lipid acceptors is ApoE e2 > ApoE e3 > ApoE e4 in astrocytes and ApoE e2 > ApoE e3 > ApoE e4 in neurons (26). Because ApoE e2 binds with the highest affinity as a lipid acceptor, this isoform may not be as readily available to suppress glial cell secretion of TNF-α in BAVMs as the ApoE e3 and ApoE e4 isoforms.
ApoE e2 genotype may also confer enhanced proteolytic activity contributing to the pathogenesis of arteriovenous malformation hemorrhage (27). Briefly, the addition of exogenous ApoE e2 can enhance tissue plasminogen activator (tPA)-induced clot lysis (4, 8). tPA and e2 form a tight quaternary structure distinct from a looser tPA–e4 complex and a nonspecific tPA–e3 complex, and these interactions modulate tPA proteolytic activity (2). ApoE e2 may also influence activation of the matrix metalloproteinase cascade (24), consistent with findings in surgical specimens (15). Consistent with our previous findings implicating the ApoE e2 genotype, but not ApoE e3 or e4, as a predictor of future ICH in the natural course of BAVM, neither ApoE e3 nor ApoE e4 demonstrated any effect, protective or otherwise, on the risk of ICH in the posttreatment course of BAVMs. The association of ApoE with vascular instability, both in the natural course and after treatment, appears to be specific to the e2 isoform.
It remains unclear why the ApoE e2 genotype was associated with postradiosurgery but not postresection arteriovenous malformation hemorrhages. The Kaplan-Meier graphs (Fig. 2) demonstrate that posttreatment hemorrhagic outcomes in the surgery subset were relatively acute events occurring soon after the initiation of treatment with no events observed further out in follow-up evaluation in this subset. In contrast, a relatively constant rate of hemorrhagic events was seen in the radiosurgery subset. This may suggest that the acute injury that occurs as a result of surgical extirpation initiates a brief set of proinflammatory stimuli that quickly recedes. On the other hand, there is a long latency period between radiation treatment and obliteration, and during this period, the genetic influences could be driven largely by the same mechanisms that result in hemorrhage during the natural untreated course.
Partial treatment status demonstrated the largest effect among the predictors studied for posttreatment hemorrhage, underscoring the importance of complete obliterative outcome on risk reduction after initiating BAVM treatment. However, it is noteworthy that among all the other predictors of posttreatment hemorrhage studied, genotype was second only to partial treatment status in terms of magnitude of effect.
Older age was also seen to be a factor associated with an increased risk of posttreatment hemorrhage. A recent natural history study (33) found that age was related to hemorrhage in the untreated course of BAVMs, although we could not replicate this finding in the UCSF Bay Area cohort (18). It seems reasonable to speculate that the processes involved in age-related predisposition to hemorrhage might reflect similar mechanisms in natural history and posttreatment hemorrhage.
On the mechanistic level, there is also plausibility, considering that the present study and our previous work (1, 7, 28) have implicated inflammation in the arteriovenous malformation lesional phenotype. Neuroinflammatory disease exhibits age-related prevalence, and normal aging is characterized by increased production of cytokines and an imbalance between pro- and anti-inflammatory cytokines (3, 5, 22, 30).
A limitation of the current study is that the results depend on events in a small subset of our patients, which makes it difficult to fully explore possible confounders and results in wide CIs or loss of power that can make sensitivity analyses difficult to interpret. Ideally, we would obtain follow-up angiograms on all patients to rule out residual arteriovenous malformation. Therefore, any conclusions drawn from our data regarding the relative effect of partial versus complete treatment must be tempered by the possibility that there may be some patients who still harbored residual arteriovenous malformations or who had a recurrence and were analyzed as having had complete treatment. However, our study is not meant to address hemorrhage rates between partially and completely treated lesions; adjusting for completion status, both genotypes were still associated with posttreatment hemorrhage.
Despite these limitations, we believe there are important clinical and genetic risk factors that have been identified for risk of posttreatment ICH and that these findings further support the role of inflammatory cytokines in the pathogenesis of BAVM hemorrhage. Taken together with our previous findings in the untreated course, these new data provide more evidence suggesting that TNF-α and ApoE genotypes may generally confer enhanced vascular instability irrespective of the mechanism of injury. These results warrant further investigation and replication in future studies.
CONCLUSIONS
This study provides the first evidence that genetic variation contributes to the clinical course of BAVMs after treatment. Despite a variety of different mechanisms for posttreatment hemorrhage, TNF-α and ApoE genotype may modulate common phenotypes of enhanced vascular instability that increase the risk of hemorrhagic outcome.
Acknowledgments
The following members of the UCSF BAVM Study Project (http://avm.ucsf.edu) contributed to portions of this work: Nancy J. Quinnine, R.N., Esteban Burchard, M.D., Guo-Yuan Yang, M.D., Ph.D., Yongmei Chen, M.D., Ph.D., Frankye Pang, B.S., Tomoki Hashimoto, M.D., Brad P. Dispensa, B.A., Philippe Jolivalt, M.S., Van Halbach, M.D., Randall T. Higashida, M.D., and Christopher Dowd, M.D. We thank Henry Matallana and the UCSF DNA Bank. This study was supported in part by grants from the National Institutes of Health: R01 NS34949, R01 NS41877, and P01 NS44155.
Contributor Information
Achal S. Achrol, Center for Cerebrovascular Research and Department of Anesthesia and Perioperative Care, University of California, San Francisco, San Francisco, California
Helen Kim, Center for Cerebrovascular Research and Department of Anesthesia and Perioperative Care, University of California, San Francisco, San Francisco, California
Ludmila Pawlikowska, Cardiovascular Research Institute, University of California, San Francisco, San Francisco, California
K.Y. Trudy Poon, Center for Cerebrovascular Research and Department of Anesthesia and Perioperative Care, University of California, San Francisco, San Francisco, California
Charles E. McCulloch, Department of Epidemiology and Biostatistics,University of California, San Francisco, San Francisco, California
Nerissa U. Ko, Department of Neurology, University of California, San Francisco, San Francisco, California
S. Claiborne Johnston, Departments of Epidemiology and Biostatistics and Neurology, University of California, San Francisco, San Francisco, California
Michael W. McDermott, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California
Jonathan G. Zaroff, Department of Medicine, University of California, San Francisco, San Francisco, California
Michael T. Lawton, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California
Pui-Yan Kwok, Cardiovascular Research Institute, University of California, San Francisco, San Francisco, California
William L. Young, Center for Cerebrovascular Research and Departments of Anesthesia and Perioperative Care, Neurology, and Neurological Surgery, University of California, San Francisco, San Francisco, California
References
- 1.Achrol AS, Pawlikowska L, McCulloch CE, Poon KYT, Ha C, Zaroff JG, Johnston SC, Lee C, Lawton MT, Sidney S, Marchuk D, Kwok P-Y, Young WL. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke. 2006;37:231–234. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 2.Biehle SJ, Carrozzella J, Shukla R, Popplewell J, Swann M, Freeman N, Clark JF. Apolipoprotein E isoprotein-specific interactions with tissue plasminogen activator. Biochim Biophys Acta. 2004;1689:244–251. doi: 10.1016/j.bbadis.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Bodles AM, Barger SW. Cytokines and the aging brain—What we don’t know might help us. Trends Neurosci. 2004;27:621–626. doi: 10.1016/j.tins.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Broderick J, Lu M, Jackson C, Pancioli A, Tilley BC, Fagan SC, Kothari R, Levine SR, Marler JR, Lyden PD, Haley EC, Jr, Brott T, Grotta JC. Apolipoprotein E phenotype and the efficacy of intravenous tissue plasminogen activator in acute ischemic stroke. Ann Neurol. 2001;49:736–744. doi: 10.1002/ana.1058. [DOI] [PubMed] [Google Scholar]
- 5.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, McCulloch CE, Hashimoto T, Lee C, Barbaro NM, Bollen AW, Yang GY, Young WL. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–3128. doi: 10.2741/2037. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, Lawton MT, Kwok PY, Yang GY, Young WL. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59:72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- 8.Clark JF, Huri DA, Carrozzella J, Jauch EC, Mehta P, Heaton D, Biehle SJ, Broderick JP. Isoforms of apolipoprotein E can modulate tPA-induced clot lysis in vitro. Front Biosci. 2002;7:a163–168. doi: 10.2741/A750. [DOI] [PubMed] [Google Scholar]
- 9.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: A HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 10.Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: Role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994;6:341–360. [PubMed] [Google Scholar]
- 11.Fullerton HJ, Achrol AS, Johnston SC, McCulloch CE, Higashida RT, Lawton MT, Sidney S, Young WL. Long-term hemorrhage risk in children versus adults with brain arteriovenous malformations. Stroke. 2005;36:2099–2104. doi: 10.1161/01.STR.0000181746.77149.2b. [DOI] [PubMed] [Google Scholar]
- 12.Halim AX, Johnston SC, Singh V, McCulloch CE, Bennett JP, Achrol AS, Sidney S, Young WL. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35:1697–1702. doi: 10.1161/01.STR.0000130988.44824.29. [DOI] [PubMed] [Google Scholar]
- 13.Halim AX, Singh V, Johnston SC, Higashida RT, Dowd CF, Halbach VV, Lawton MT, Gress DR, McCulloch CE, Young WL. Characteristics of brain arteriovenous malformations with coexisting aneurysms: A comparison of two referral centers. Stroke. 2002;33:675–679. doi: 10.1161/hs0302.104104. [DOI] [PubMed] [Google Scholar]
- 14.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, Barbaro NM, Higashida RT, Dowd CF, Halbach VV, Young WL. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34:925–931. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- 16.Hsu TM, Kwok PY. Homogeneous primer extension assay with fluorescence polarization detection. Methods Mol Biol. 2003;212:177–187. doi: 10.1385/1-59259-327-5:177. [DOI] [PubMed] [Google Scholar]
- 17.Joint Writing Group of the Technology Assessment Committee American Society of Interventional and Therapeutic Neuroradiology; Joint Section on Cerebrovascular Neurosurgery, a Section of the American Association of Neurological Surgeons and Congress of Neurological Surgeons. Section of Stroke and the Section of Interventional Neurology of the American Academy of Neurology: Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke. 2001;32:1430–1442. doi: 10.1161/01.str.32.6.1430. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Sidney S, McCulloch CE, Poon KY, Singh V, Johnston SC, Ko NU, Achrol AS, Lawton MT, Higashida RT, Young WL. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients (Abstract) Stroke. 2007;38:468. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- 19.Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J Neuroimmunol. 1997;76:70–74. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 20.Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. J Cereb Blood Flow Metab. 1998;18:465–471. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Lawton MT, Du R, Tran M, Achrol AS, McCulloch CE, Johnston SC, Quinnine NJ, Young WL. Effect of presenting hemorrhage on outcome after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2005;56:485–493. doi: 10.1227/01.neu.0000153924.67360.ea. [DOI] [PubMed] [Google Scholar]
- 22.Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- 23.Leung CH, Poon WS, Yu LM, Wong GK, Ng HK. Apolipoprotein e genotype and outcome in aneurysmal subarachnoid hemorrhage. Stroke. 2002;33:548–552. doi: 10.1161/hs0202.102326. [DOI] [PubMed] [Google Scholar]
- 24.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 25.Mast H, Young WL, Koennecke HC, Sciacca RR, Osipov A, Pile-Spellman J, Hacein-Bey L, Duong H, Stein BM, Mohr JP. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065–1068. doi: 10.1016/s0140-6736(97)05390-7. [DOI] [PubMed] [Google Scholar]
- 26.Michikawa M, Fan QW, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. 2000;74:1008–1016. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- 27.Pawlikowska L, Poon KY, Achrol AS, McCulloch CE, Ha C, Lum K, Zaroff JG, Ko NU, Johnston SC, Sidney S, Marchuk DA, Lawton MT, Kwok PY, Young WL. Apolipoprotein E epsilon 2 is associated with new hemorrhage risk in brain arteriovenous malformation. Neurosurgery. 2006;58:838–843. doi: 10.1227/01.NEU.0000209605.18358.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, Hashimoto T, Zaroff J, Lawton MT, Marchuk DA, Kwok PY, Young WL UCSF BAVM Study Project. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- 29.Reich K, Mössner R, König IR, Westphal G, Ziegler A, Neumann C. Promoter polymorphisms of the genes encoding tumor necrosis factor-alpha and inter-leukin-1beta are associated with different subtypes of psoriasis characterized by early and late disease onset. J Invest Dermatol. 2002;118:155–163. doi: 10.1046/j.0022-202x.2001.01642.x. [DOI] [PubMed] [Google Scholar]
- 30.Saurwein-Teissl M, Blasko I, Zisterer K, Neuman B, Lang B, Grubeck-Loebenstein B. An imbalance between pro- and anti-inflammatory cytokines, a characteristic feature of old age. Cytokine. 2000;12:1160–1161. doi: 10.1006/cyto.2000.0679. [DOI] [PubMed] [Google Scholar]
- 31.Selmaj KW, Farooq M, Norton WT, Raine CS, Brosnan CF. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990;144:129–135. [PubMed] [Google Scholar]
- 32.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 33.Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES, Pile-Spellman J, Mohr JP. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350–1355. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- 34.Stapf C, Mohr JP, Choi JH, Hartmann A, Mast H. Invasive treatment of unruptured brain arteriovenous malformations is experimental therapy. Curr Opin Neurol. 2006;19:63–68. doi: 10.1097/01.wco.0000200546.14668.78. [DOI] [PubMed] [Google Scholar]
- 35.Woo D, Sauerbeck LR, Kissela BM, Khoury JC, Szaflarski JP, Gebel J, Shukla R, Pancioli AM, Jauch EC, Menon AG, Deka R, Carrozzella JA, Moomaw CJ, Fontaine RN, Broderick JP. Genetic and environmental risk factors for intracerebral hemorrhage. Preliminary results of a population-based study. Stroke. 2002;33:1190–1196. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]