Summary
Cytomegalovirus (CMV) is a herpesvirus that persists for life and maintains extremely large numbers of T cells with select specificities in circulation. However, it is unknown how viral persistence impacts T cell populations in mucosal sites. We found that many murine (M)CMV-specific CD8s in mucosal tissues became resident memory T cells (TRM). These cells adopted an intraepithelial localization in the salivary gland that correlated with, but did not depend on, expression of the integrin CD103. MCMV-specific TRM cells formed early after infection and spleen-localized cells had reduced capacities to become TRM at late times. Surprisingly however, small numbers of new TRM cells were formed from the circulating pool throughout infection, favoring populations maintained at high levels in the blood and shifting the immunodominance within the TRM populations over time. These data show that mucosal TRM populations can be dynamically maintained by a persistent infection.
Introduction
Cytomegalovirus (CMV) is a β-herpesvirus that infects the majority of people in the world and establishes an asymptomatic latency, punctuated by periodic reactivation (Crough and Khanna, 2009). Controlling these reactivation events requires constant immune surveillance (Polic et al., 1998; Simon et al., 2006), which induces the accumulation of virus-specific T cells in a unique process called “memory inflation” (Holtappels et al., 2000; Karrer et al., 2003; Komatsu et al., 2003; Munks et al., 2006). This has led to great interest in using CMV as a vaccine vector, with pre-clinical success in a non-human primate model of HIV infection (Hansen et al., 2011; Hansen et al., 2013; Hansen et al., 2009). Like most herpesviruses, CMV displays strict species specificity. Thus we use murine CMV (MCMV), a natural mouse pathogen and the homologue of human (H)CMV. The T cells induced by both viruses are broadly similar in phenotype, function and genetic signature (Crough and Khanna, 2009; Krmpotic et al., 2003; Quinn et al., 2015; Snyder et al., 2011). Using the MCMV model, we found that most of the “inflationary” CD8+ T cells (those that accumulate over time) are confined to the circulation after systemic MCMV infection (Smith et al., 2014). The major exception to this finding was the salivary gland, where MCMV and HCMV both persist and establish latency (Crough and Khanna, 2009; Krmpotic et al., 2003; Polic et al., 1998). It is unknown how CMV-specific T cells develop in this or other mucosal tissues.
It has become clear in recent years that many pathogen-specific T cells within the skin, brain, and mucosal tissues, including the salivary gland, are not in equilibrium with those circulating through the blood and lymphoid organs. These populations have been called tissue resident memory T cells (TRM), and they are thought to form early after infection, persisting in these tissues independently of circulation (reviewed in (Schenkel and Masopust, 2014)). In the small intestine, vagina, skin and lung, pathogen-specific TRM cells localize near or within the epithelial layer, which is thought to enable TRM cells to be “first-responders”: cells that do not require recruitment to rapidly respond to reinfection (Ariotti et al., 2014; Gebhardt et al., 2009; Mackay et al., 2012; Schenkel et al., 2013; Sheridan et al., 2014; Wu et al., 2014; Zhu et al., 2013). For these reasons, establishing TRM in large numbers may be critically important in maintaining immune surveillance in these organs and is a major concern for vaccine design.
Several lines of evidence suggest that TRM cells form independently of local antigen (Casey et al., 2012; Hofmann and Pircher, 2011; Mackay et al., 2012; Wakim et al., 2010). In fact, work with lymphocytic choriomeningitis (LCMV) clone 13, which induces a chronic infection that promotes T cell dysfunction, suggested that antigen may inhibit mucosal TRM populations (Casey et al., 2012). Both MCMV and HCMV undergo prolonged replication in the salivary gland and persist for life in many sites in the body. However, unlike many persistent viruses, neither MCMV, nor HCMV promotes T cell dysfunction. The persistence of low levels of antigen during CMV infection, along with the CMV-driven accumulation of functional CD8+ T cells, raise the possibility that the dynamics of T cell maintenance in the mucosa do not reflect that of cleared infections or chronic infections that drive exhaustion.
We found that many MCMV-specific CD8+ T cells in the salivary gland and other mucosal sites in the body developed a TRM phenotype shortly after infection. Remarkably, our data suggest that persistent antigen stimulation during viral latency promotes the continuous, low-level recruitment of circulating inflationary MCMV-specific T cells to the TRM population in the salivary gland, which resulted in a slow shift in the immunodominance of the MCMV-specific TRM cells over time. These data suggest that mucosal TRM populations driven by persistent infections can be dynamically maintained.
Results
MCMV-specific TRM CD8s are present in large numbers in the salivary gland
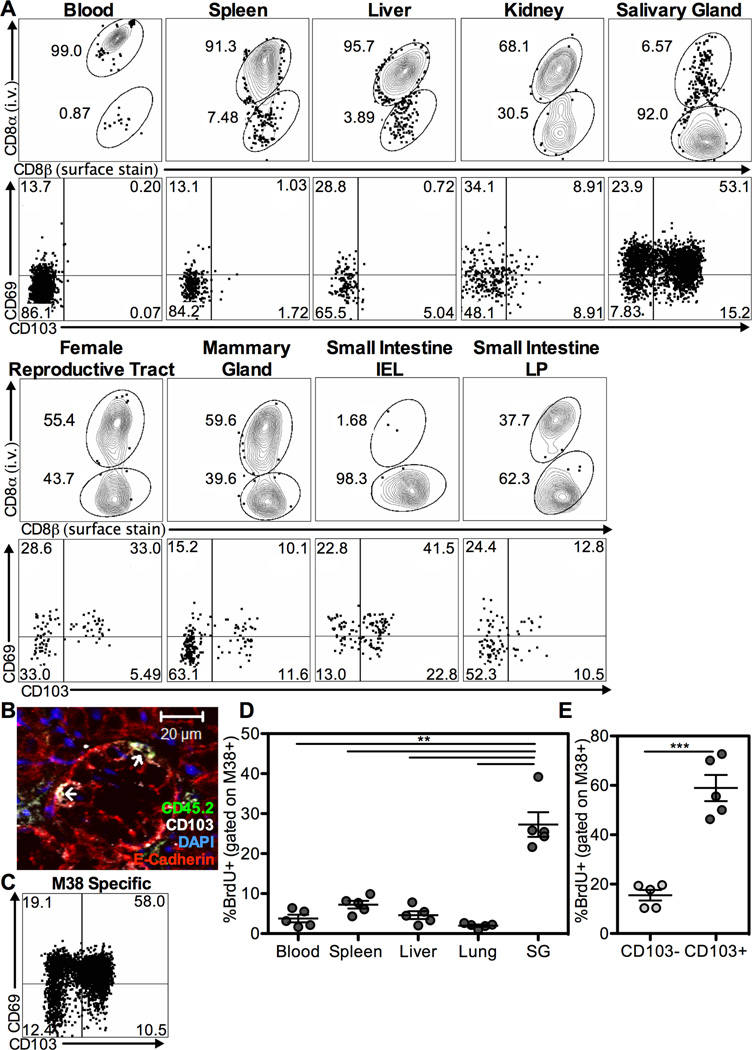
MCMV is a systemic, persistent pathogen that induces the accumulation of virus-specific CD8 T cells. We showed previously that, after an i.p. infection, the vast majority of MCMV specific CD8s stimulated by viral antigen were in the blood or associated with the vasculature during the latent stage of infection (Smith et al., 2014). To investigate MCMV-specific CD8s in mucosal tissues, we used an MCMV virus expressing ovalbumin (MCMV-OVA) and OT-Is. Naive mice were seeded with small numbers of naive congenic OT-Is and infected with MCMV-OVA one day later. This protocol induces robust inflation of OT-Is (Turula et al., 2013), which were largely exposed to an i.v.-injected antibody, suggesting a vascular localization (Figure 1A). When blood-localized CD8s were excluded, the salivary gland harbored the most OT-Is of the organs studied (Figure S1A). Expression of CD103 and CD69, the markers of TRM cells, was most pronounced on T cells within the salivary gland and the intra-epithelial lymphocyte fraction of the small intestine (si-IEL, Figure 1A and S1B). Moreover, cells in both of these sites tended to express low or intermediate amounts of the IL-7Rα (CD127) and lacked KLRG-1 (Figure S1C–D), consistent with the TRM phenotype (Mackay et al., 2013; Sheridan et al., 2014). Importantly, many of the MCMV-driven OT-Is in the salivary gland were IELs located within the acinii and ducts (Figure 1B and Figure S2). Endogenous CD8 T cells specific for MCMV epitopes also upregulated TRM markers in the salivary gland (M38-specific T cells shown in Figure 1C). To test whether these T cells were lodged in the salivary gland, we performed a BrdU pulse during the first 9 weeks of infection, followed by a prolonged chase period. The ongoing antigen-driven memory inflation causes a loss of BrdU-positive inflationary T cells in circulation (Smith et al., 2014). However, inflationary T cells in the salivary gland retained BrdU at a much greater frequency than cells extracted from the spleen, lungs and liver (Figure 1D). The retention of the BrdU label was especially prominent in the CD103+ subset of MCMV specific cells in the salivary gland (Figure 1E). Thus, salivary gland-localized T cells are not in equilibrium with the circulating populations. Together these data show that MCMV infection promotes TRM cells in multiple mucosal tissues and that TRM cells are lodged in the salivary gland where they develop an IEL localization.
Figure 1. MCMV specific CD8s become TRM in the salivary gland and other mucosal sites.
A) OT-Is in congenic B6 mice >12 weeks after infection with MCMV-OVA. Top row: FACS plots of congenic OT-Is labeled with i.v. injected, fluorochrome labeled anti-CD8α antibody to identify blood and tissue localized T cells and surface stained with anti-CD8β in the indicated organs. Bottom row: CD103 and CD69 expression on OT-Is in the i.v. unlabeled fraction of indicated organs (see also Figure S1). Representative of two independent experiments (n=7). B) Immunofluorescent staining of OT-Is in the salivary gland >12 weeks post infection. Shown is an OT-I (identified by CD45.2 - green) expressing CD103 (white) co-localized with the epithelium (identified by E-cadherin- red). DAPI staining of the nucleus shown in blue. Individual images and overlays are in Figure S2. C) FACS plot of CD103 and CD69 on M38-specific T cells in i.v. unlabeled fraction of salivary gland >12 weeks post wild type MCMV infection. D-E) Mice were treated with BrdU in drinking water for the first 9 weeks of MCMV infection then sacrificed 15 weeks after the end of the pulse. The frequency of BrdU labeled cells among M38-specific T cells in the indicated organ (D) and among CD103- or CD103+ M38-specific cells in the salivary gland (E) after the 15 week chase period. Data are representative of two independent experiments.
CD103 expression is not required for maintenance or localization of CD8s within the salivary gland
It has been suggested that expression of CD103 is important for enforcing the residency of lymphocytes in mucosal organs by tethering T cells to the epithelium (Casey et al., 2012; El-Asady et al., 2005). However, recent work has challenged this notion for TRM cells within the si-IEL fraction (Sheridan et al., 2014). For MCMV-driven OT-Is in the salivary gland, CD103 expression was significantly correlated with epithelial localization as assessed by proportion or by distance from the epithelium (Figure 2A and Figure S3B). Surprisingly however, CD103−/− OT-I localization was comparable to wild-type (WT) OT-Is within the salivary gland (Figure 2A–B and Figure S3A–B). These data suggest that CD103 expression marks IELs in the salivary gland, but is not essential for the IEL localization. To determine if CD103 is required for the maintenance of MCMV-specific TRM in the salivary gland, we mixed congenically marked WT and CD103−/− OTIs before transfer and MCMV-OVA infection. Although both populations had reached the salivary gland and small intestine in large numbers after one week, the CD103−/− OT-Is were slightly underrepresented in the salivary gland and the si-IEL relative to the spleen (Figure 2C). In the salivary gland, the ratio of CD103−/− to WT OT-Is was maintained stably over several weeks and only slightly favored WT OT-Is even after 22 weeks (Figure 2D). Importantly, the numbers of CD103−/− OT-Is did not decline over time (Figure S4A). Rather, this shift in ratio reflects a slight increase in the numbers of WT OT-Is in the salivary gland (Figure S4A). This subtle preferential maintenance of WT OT-Is was more pronounced in the si-IEL over time and unexpectedly, in the spleens of the same mice (Figure 2D), reflecting a greater loss of CD103−/− cells after day 7 (Figure S4B–C). The CD103−/− OT-Is in the salivary gland and si-IEL expressed high levels of CD69 from week 2 onwards, mirroring the WT OT-Is in the same mice (Figure 2E–F and Figure S4D–E). These data suggest that CD103−/− OT-Is had a defect in their initial migration to the salivary gland, but were maintained stably thereafter. To test whether persistent antigen was masking a defect in the maintenance of CD103−/− OT-Is, we isolated activated WT and CD103−/− OT-Is from the spleen of adoptive transfer recipients one week after infection and transferred equal numbers into naive mice. Both OT-I populations migrated to the salivary gland and upregulated markers of residency (Figure 2G). Again, CD103−/− OT-Is were underrepresented in the salivary gland 2 weeks after transfer, but the proportion of WT and CD103−/− OT-Is was unchanged over the next 3 weeks indicating that both WT and CD103−/− OT-Is were maintained similarly in the salivary gland, even in the absence of antigen (Figure 2H). Thus, while CD103 marks TRM cells with an IEL localization in the salivary gland, it is not required for their formation, localization or maintenance.
Figure 2. Localization and maintenance of MCMV-specific CD8s in the salivary gland does not depend on CD103 expression.
B6 mice were seeded with wild type or CD 103−/− OT-Is and challenged with MCMV-OVA. A) Localization of OT-Is in the salivary gland was examined histologically by immunofluorescent staining >12 weeks after infection. Left: Distance from the nearest epithelial surface of wild type OT-Is in the salivary gland that did (n=121 cells) or did not (n=87 cells) express CD103. Right: Distance of OT-Is from the nearest epithelial surface for wild type OT-Is (whole population, n=223 cells) compared to CD103−/− OT-Is (n=83 cells). B) CD103−/− OT-Is (identified by CD45.2-green) embedded in the epithelium (identified by E-cadherin-red). CD103 is shown in white and DAPI is shown in blue (see also Figure S3). Data are representative of 2–4 tissue sections per mouse from 4 mice containing wild-type OT-Is and 3 mice containing CD103−/− OT-Is. C to F) Wild type and CD103−/− OT-Is were mixed and co-transferred before MCMV-OVA challenge. Cohorts were sacrificed at different times post infection. C) Ratio of CD103−/− to WT OT-Is in the spleen, salivary gland and intestinal IEL normalized to ratio in the blood within the same mouse at one week post infection. D) Ratio of CD103−/− to WT OT-Is in each organ over time normalized within an experiment to the average ratio in the blood at week 1. E, F) Frequency of indicated phenotype of WT OT-Is (E) and CD103−/− OT-Is (F) in the salivary gland over time. (See also Figure S4) Results are combined from two independent experiments (n=7). G-H) Wild type and CD103−/− OT-Is were mixed and co-transferred before MCMV-OVA infection. One week post infection, WT and CD103−/− OT-Is were sorted from the spleen and equal numbers mixed and transferred into naive congenic recipients. (G) CD103 and CD69 on OT-Is in the salivary gland 2 weeks after transfer. (H) Ratio of CD103/WT OT-Is in the indicated organ of naive recipients at 2 and 5 weeks post transfer. Results are combined from two independent experiments (n=8). Error bars represent the standard error of the mean. Statistical significance was measured by paired (A and C) or unpaired (D and G) student’s t-tests (*p<.05, **p<.01, ***p<.001).
MCMV specific CD8s that undergo memory inflation are maintained at higher numbers than non-inflationary CD8s in the salivary gland
Within the endogenous CD8+ T cell populations, cells responding to different MCMV epitopes display different kinetics of accumulation or contraction. Inflationary populations (represented by M38- and IE3-specific CD8s in B6 mice) are maintained at high levels in the blood by ongoing antigen stimulation, whereas non-inflating populations (represented by M45 and M57 specific CD8s) undergo substantial contraction after the acute phase of infection, likely because their antigen becomes unavailable (Dekhtiarenko et al., 2013), and are maintained homeostatically (Figure S5A–D (Smith et al., 2014; Snyder et al., 2008)). Since TRM cells do not rely on antigen (Casey et al., 2012; Mackay et al., 2012), we did not expect these kinetics to be reflected in the TRM populations. We sacrificed cohorts of B6 mice infected with WT-MCMV (strain K181) at different times post infection. Inflationary M38- and IE3-specific CD8s were present in the salivary gland within 1 to 3 weeks of infection and were maintained stably once they’d reached their peak levels (Figure 3A and B), mirroring their kinetics in the spleen (Figure S5A–B). Surprisingly, non-inflationary populations in the salivary gland declined significantly in a prolonged manner over at least 10 weeks (Figure 3C–D). While this contrasts to the dramatic contraction that occurred in the spleens of the same mice (Figure S5C–D), non-inflationary T cells had become subdominant to inflationary cells by late times after infection (Figure 3E–F). The change in immunodominance was not associated with any major differences in expression of CD69 or CD103 between populations (Figure S5E). Nevertheless, inflationary T cells were far more prevalent in the blood and thus it was possible that these cells were circulating passively through the salivary gland and driving the shift in immunodominance. To test this, we injected CFSE i.v. into latently infected mice, which labeled the vast majority of inflationary cells in the blood, with minimal labeling of T cells in the salivary gland (Figure 3G). Seventy-four days later, CFSE-labeled T cells had not equilibrated in the salivary gland (Figure 3H). Even though migrating cells could lose CFSE by dividing upon salivary gland entry, these data suggest that the shift in immnodominance is not caused by inflationary cells passively circulating through the salivary gland at steady state.
Figure 3. Inflationary CD8s are preferentially maintained in the salivary gland during the latent stages of infection.
A–D) B6 cohorts were infected with K181 MCMV and sacrificed at indicated times post infection. Absolute numbers of CD8s in salivary gland specific for inflationary epitopes M38 (A) and IE3 (B) and non-inflationary epitopes M45 (C) and M57 (D). Dotted lines indicate average number of cells at week 1 for comparison. Asterisks indicate significant differences from week 1, (see also Figure S5). E-F) Ratio of M38 specific to M45 specific cells (E) or IE3 specific to M57 specific cells (F) in the salivary gland and spleen of each mouse calculated from the numbers shown in A to D. Dotted lines indicate a ratio of 1. Data is combined from 7 independent experiments (n=4–16 mice per time point). G) FACS plots of blood and salivary gland from latently infected mice 45 minutes after i.v. injection with CFSE. H) Latently infected mice were given three i.v. injections of CFSE over one week and sacrificed 74 days later. Shown is the frequency of CFSE on inflationary and non-inflationary cells in spleen and salivary gland. Results are combined from two independent experiments (n=6). Error bars represent the standard error of the mean. Statistical significance was measured by unpaired (A–D) and paired (H) student’s t-tests (*p<05, **p<01, ***p<.001, ****p<.0001).
MCMV specific TRM in the salivary gland do not depend on viral replication
Circulating inflationary T cells quickly become immune dominant because they undergo prolonged antigen-driven division (Torti et al., 2011) (Figure S6B–E). Unlike other organs, the salivary gland harbors replicating virus for prolonged periods of time. In our hands, MCMV transcripts encoding the late gene glycoprotein B (gB), were evident in the salivary gland for at least 10 weeks after infection (Figure 4A). The disappearance of gB transcripts correlated with the loss of non-inflationary T cells from the salivary gland (Figure 3C–D). Thus it was possible that the presence of antigen promoted the proliferation of TRM cells in the salivary gland. To test whether viral replication in the salivary gland affects the rate of TRM division, we compared wild type MCMV to a spread defective version of MCMV that lacks the essential glycoprotein L (ΔgL-MCMV). This virus is a single-cycle virus in vivo that cannot spread, but still induced memory inflation (Snyder et al., 2011) and TRM phenotype cells in multiple mucosal tissues after an i.p. infection (Figure S6A). Any TRM division driven by replicating virus in the salivary gland should be absent from mice infected with ΔgL-MCMV. However, there was no difference in salivary gland T cell division (assessed by Ki-67 expression) between mice infected with wild type and ΔgL-MCMV beyond one week post infection (Figure 4B–E). We also adoptively transferred activated OT-Is into these mice on day 5 of infection. Since neither virus expresses OVA, these OT-Is serve as a reference population for antigen-independent division. Remarkably, OT-Is recovered from these salivary glands expressed Ki-67 at an identical rate as endogenous T cells despite the complete absence of the OVA antigen (Figure 4B–C). Collectively, these data suggest that neither viral replication nor antigen in the salivary gland accounts for the preferential maintenance of TRM cells with inflationary specificities. Thus, we hypothesized that a continuous antigen-driven influx of inflationary T cells during latency might explain these data.
Figure 4. Viral replication in the salivary gland does not drive replication of MCMV specific TRM.
A) Nested RT-PCR for MCMV gB performed on cDNA (top) or total DNA (bottom) extracted from salivary glands of mice infected with wild type MCMV. Each lane represents an individual mouse. Lanes labeled H2O contained no template. All samples were positive in a PCR reaction for β-actin and all reverse transcriptase-negative cDNA samples were negative for gB (not shown). B-E) Mice were infected with wild type MCMV or ΔgL-MCMV. At 5 days post infection mice received transfer of OT-Is sorted from spleens of mice infected for 5 days with MCMV-OVA. Shown is the frequency of Ki67 among endogenous antigen specific CD8s and donor OT-Is in the salivary gland at day 7 (B) day 14 (C) day 35 (D) or at latent times (E). (See also Figure S6). Error bars represent the standard error of the mean. Statistical significance was measured by unpaired student’s t-tests (*p<.05, **p<.01, ***p<.001).
Levels of TGF-β in the salivary gland are not markedly altered by MCMV infection or latency
To investigate TRM formation at late times post infection, we first wanted to determine how viral replication and latency in the salivary gland influences the expression of TGF-β, IL-33, and TNF-α, which are critical for TRM differentiation (Casey et al., 2012; El-Asady et al., 2005; Graham et al., 2014; Mackay et al., 2013; Sheridan et al., 2014). Indeed, MCMV-stimulated T cells upregulated CD103 in response to TGF-β, and upregulated CD69 in response to IL-33 and TNF-α (Figure 5A–B), consistent with previous work (Casey et al., 2012; Skon et al., 2013). Transcripts encoding TGF-β, IL-33, and TNF-α were slightly increased at day 7 of infection with wild-type MCMV (Figure 5C). However, levels of these cytokines were not markedly altered after day 7, even at late times, and the increased transcription of TGF-β did not correspond to higher levels of total or active TGF-β in the gland as a whole (Figure 5D–E). Infection with ΔgL-MCMV did not similarly increase TGF-β transcription (Figure 5F). These data suggest that MCMV replication and latency in the salivary gland have a minimal impact on the availability of cytokines responsible for inducing TRM cells. Thus, if MCMV-specific cells were to arrive in the salivary gland at late times after infection, the available levels of cytokine and antigen are unlikely to be a limiting factor in their ability to form new TRM cells.
Figure 5. MCMV in the salivary gland does not alter cytokines important for TRM differentiation.
A-B) Splenocytes isolated 4 days after infection with K181 MCMV were treated in vitro with TGF-β, TNF-α and IL-33 alone or in combination for 40 hours (n=9). Shown is the frequency of CD103 (A) and CD69 (B) on CD8s. Dotted lines indicate marker expression without cytokines. C) Transcription in salivary gland of the indicated cytokines normalized to GAPDH determined by qRT-PCR after infection with WT MCMV. Data shown is the average for 1–4 replicates per sample (n=3–4 mice per group) D-E) Protein levels of total (D) and active (E) TGF-β determined by ELISA (n=3–4 mice per group). F) Transcription of TGF-β in salivary gland one week after infection with WT MCMV or ΔgL-MCMV. Data shown is the average for 1–2 replicates per sample (n=2–4 mice per group). Error bars represent the standard error of the mean. Statistical significance was measured by unpaired student’s t-tests (*p<.05, **p<.01, ***p<.001).
Inflationary cells from the spleen can become TRM with reduced efficiency
Memory inflation promotes the accumulation of T cells with an effector phenotype. To determine whether inflationary cells retain the capacity to become TRM, we mixed naive OT-Is with inflationary OT-Is from the spleens of latently infected mice and co-transferred these cells into naive recipients. Infecting these mice with MCMV-OVA markedly expanded OT-Is from both donors and drove large numbers into the non-lymphoid tissues (Figure 6A). However, even though restimulated and primary OT-Is were approximately equal in the blood, restimulated OT-Is were underrepresented in the parenchyma of all organs tested and markedly so in both the salivary gland and si-IEL (Figure 6B). Importantly, similar results were obtained when naive and latent OT-Is were transferred into separate mice and challenged, ruling out the possibility that competition or an altered environment were affecting the results (not shown). In all cases, restimulated T cells were significantly less likely to express CD69 and CD103 compared to cells undergoing a primary infection (Figure 6C) consistent with previous work (Masopust et al., 2006). Indeed, when restimulated inflationary cells were treated with cytokines in vitro, they were less able to express CD103 in response to TGF-β compared to CD8s from an acute infection (Figure 6D compare to Figure 5A). Together these data show that inflationary T cells from late times after infection can migrate to mucosal tissues and differentiate into TRM upon restimulation, but do so less efficiently than cells undergoing a primary response to infection.
Figure 6. Inflationary CD8s have reduced capacity to become TRM after a new infection.
CD45.1+ inflationary OT-Is from the spleen >12 weeks after infection and CD45.1+/CD45.2+ naive OT-Is were mixed and transferred into naive B6 recipients (CD45.2+), which were then challenged with MCMV-OVA. A) FACS plots show frequency of OT-Is (left) and expression of CD69 and CD103 on restimulated OT-Is (middle) or OT-Is undergoing a primary response (right), in blood, spleen or i.v. unlabeled fraction of the indicated organs 2 weeks after challenge. B) Ratio of restimulated to primary OT-Is in i.v. unlabeled fraction of the indicated organs. C) Frequency of CD103 and CD69 co-expression on restimulated and primary OT-Is in i.v. unlabeled fraction of the indicated organs. Each line connects populations in an individual mouse. Data are representative of two experiments (n=5). D) Expression of CD103 on restimulated inflationary CD8s after treatment with cytokines as in Figure 5A (n=3). Dotted line indicates CD103 expression without cytokines. Error bars represent the standard error of the mean. Statistical significance was measured by paired student’s t-tests (*p<.05, **p<.01, ***p<.001, ****p<.0001).
Circulating inflationary CD8s traffic to and become resident in the salivary gland during latent infection
Inflationary cells were clearly able to respond to a new infection by becoming TRM in the salivary gland. To assess the level at which this occurred in the absence of antigen, inflationary OT-Is were harvested from the spleen 1 week or >12 weeks after infection. These cells were transferred into naive mice to assess their migration to the salivary gland. Transferred OT-Is from latently infected mice infiltrated the salivary glands at a lower rate than OT-Is from 1 week post infection (Figure 7A–B and Figure S7A). Regardless of the donor, KLRG1+ cells failed to traffic to the salivary gland entirely (Figure 7A–B). Interestingly, TRM phenotype cells were rare and almost completely absent when cells were derived from latently infected mice (Figure 7B). These data show that MCMV-specific T cells in the spleen during latency can migrate into the salivary gland at a low level irrespective of viral antigen or infection, but that TRM differentiation is rare without recent exposure to antigen.
Figure 7. Inflationary CD8s can become TRM in the salivary gland during latent infection.
OT-Is were isolated from the spleens of mice at 1 week or >12 weeks after MCMV-OVA infection and transferred into congenic naive mice. A) FACS plots of donor OT-Is (left) and their expression of KLRG1 (right) in the spleen of recipients 2 weeks post transfer. B) donor OT-Is (left), their expression of KLRG1 (middle) or CD69 and CD103 (right) in the salivary gland of recipients 2 weeks post transfer. C-F) Mice latently infected with MCMV-OVA or MCMV-WT received CFSE labeled OT-Is from spleens of mice >12 weeks post infection with MCMV-OVA. C) Ratio of the number of OT-Is in the salivary gland to the spleen of the same mouse 2–4 weeks after transfer into the indicated recipients. D-E) Frequency of CD69 (D) and CD103 (E) on transferred OT-I in the parenchyma of the salivary gland compared to OT-Is in the salivary gland 4 weeks after primary infection (Primary). F) FACS plots of CFSE and CD103 or CD69 on OT-I donors in the salivary glands of latently infected MCMV-OVA recipients. Each plot shows an individual mouse. G–H) OT-Is from spleens of latently infected mice were harvested and stimulated with SIINFEKL in vitro for three hours before transfer into naive recipients. G) Absolute numbers of OT-Is in spleen and salivary gland of naive recipients 2 weeks post transfer. H) FACS plots of CD103 and CD69 on transferred OT-Is 2 weeks post transfer. (See also Figure S7). Error bars represent the standard error of the mean. Statistical significance was measured by unpaired student’s t-tests (*p<.05, **p<.01, ***p<.001, ****p<.0001).
To determine if latent infection with MCMV increased the recruitment or TRM differentiation of inflationary cells, we transferred CFSE labeled OT-Is from the spleens of latently infected mice into mice latently infected with MCMV-OVA (with antigen) or wild-type MCMV (lacking antigen). As above, KLRG1+ OT-Is failed to access the salivary gland in all cases (Figure S7B). Comparing the number of donor OT-Is in the salivary gland to the spleens of the same mice normalized the results for variations in transfer efficiency or the impact of antigen driven expansion. The presence of the OVA antigen slightly improved the rate of recruitment of OT-I T cells to the salivary gland over that of naive or WT infected mice (Figure 7C). However, OT-Is that made it to the salivary gland in MCMV-OVA infected recipients were significantly more likely to have upregulated CD69 and CD103, although expression of both molecules was variable and reduced compared to OT-Is driven by a primary infection (Figure 7D–E). Moreover, OTIs that reached the salivary gland in MCMV-OVA infected recipients were enriched for cells that had fully diluted their CFSE (Figure S7C), and CD103-expressing OT-Is were exclusively CFSE low (Figure 7F left), which is indicative of antigen driven division. Interestingly, CD69 expression did not show a similar restriction and was expressed on both divided and undivided OT-Is in the salivary gland (Figure 7F right). These data are consistent with the idea that antigen promotes TRM differentiation during latency. To directly test the hypothesis that antigen stimulation outside of the salivary gland is sufficient to drive inflationary cells to become TRM, we isolated spleens with inflationary OT-Is from latently infected mice, stimulated the T cells with SIINFEKL peptide in vitro, and adoptively transferred these cells into naive mice. OT-Is that had been stimulated with peptide reached the salivary gland in significantly greater numbers and upregulated TRM markers more efficiently than unstimulated OT-Is, even in the absence of any inflammation or antigen in the recipient (Figure 7G–H and S7D). Together, these data show that MCMV-specific T cells in the spleen during latency could migrate to the salivary gland with or without antigen at steady state, but that the establishment of a TRM phenotype was antigen-dependent. Furthermore, antigen recognition outside of the salivary gland was sufficient to promote new TRM formation and supplement established TRM populations.
To estimate the recruitment of inflationary T cells at steady state, we used the numbers of salivary gland-localized donor cells measured after transfer of inflationary OT-Is into recipients latently infected with MCMV-OVA (Figure 7C). This analysis suggests that for every 1000 OT-Is in the spleen, 8.6 (± 2.3 SEM) OT-Is were recruited to the salivary gland over a 30-day period. While this seems like a small number, it is important to note that, as a result of memory inflation, the average latently infected mouse contains 256,183 (± 21,770) OT-Is in the spleen. Given these numbers, we would expect approximately 2,195 (± 494.1) OT-Is to be recruited to the salivary gland over the course of 30 days, which would represent 5.5% (± 1.47%) of the OT-Is in an average salivary gland. Given the variable expression of TRM markers on transferred OT-Is (Figure 7D–E), we would not expect all of these cells to develop into new TRM cells. Nevertheless, this represents a substantial pool from which new TRM can be generated. Inflationary T cells are present at much higher numbers than non-inflationary populations during latent infection, precisely because they respond to viral antigen during this phase (Seckert et al., 2011; Torti et al., 2011). Thus, recruitment of inflationary populations would be heavily favored over non-inflating CD8s, which is consistent with the different maintenance of inflators and non-inflators in the salivary gland (Figure 3). Collectively, our data indicate that MCMV infections robustly induce intraepithelial TRM populations independently of viral replication in the mucosa and, surprisingly, that these TRM populations can be dynamically supported by continuous TRM formation from the circulating pool.
Discussion
Cytomegalovirus has drawn much interest as a vaccine vector due to its unique ability to induce antigen-driven memory inflation. Most notably, work using Rhesus CMV (RhCMV) as a vaccine vector for simian immunodeficiency virus (SIV) has led to remarkable protection of the vaccinated animals (Hansen et al., 2011; Hansen et al., 2013; Hansen et al., 2009). These authors have speculated that CMV-based vaccines may be so effective because they can sustain large numbers of T cells at the mucosal sites of SIV (and HIV) entry (Masopust and Picker, 2012). Indeed, such tissue resident memory T cells are emerging as critical players in the surveillance of barrier tissues. Using the MCMV model, we have recently shown that most cells undergoing memory inflation are confined to the circulation and are not found within the parenchyma of mucosal or non-mucosal tissues (Smith et al., 2014). In this manuscript, we show that MCMV infection induces the early formation of TRM cells that broadly distribute through the mucosal tissues of the body. Moreover, we found that a single-cycle spread-defective version of MCMV, which could be a useful platform on which to base a vaccine, also induced TRM cells to form in multiple mucosal sites (Figure S6). Most surprisingly, we found that MCMV-driven TRM populations can be dynamically supported by the recruitment of circulating T cells (Figure 7), which heavily favors the inflationary T cells. It is likely that most of these late-arriving T cells fail to differentiate into TRM cells (Figure 7D–E). Indeed, previous work showed that T cells entering mucosal tissues from the circulation did not upregulate CD69 to the degree of cells already resident in the mucosa (Skon et al., 2013). Nevertheless, restimulation of splenic inflationary T cells could clearly drive the formation of new TRM cells, albeit inefficiently, even in latently infected or naive mice (Figures 6 and 7). Thus, the large numbers of T cells stimulated by CMV-based vectors may be uniquely able to promote and sustain TRM cells in multiple mucosal tissues.
The role of sustained local antigen in the formation and maintenance of TRM cells is unclear. This is noteworthy because CMV persists in many sites throughout the body. Antigen is not needed for the formation of TRM in the skin or small intestine after T cell priming (Casey et al., 2012; Mackay et al., 2012) and persistent antigen may antagonize TRM in the small intestine (Casey et al., 2012), although chronic LCMV clone 13 infection may also promote sustained T cell migration to the small intestine (Zhang and Bevan, 2013). In contrast, sustained antigen has been proposed to bolster TRM formation in the lung after influenza infection (Lee et al., 2011). Thus, the impact of persistent antigen on TRM formation may vary by the infection or tissue. CMV infects and replicates for prolonged periods of time in the salivary gland and uses this mucosal barrier tissue as a primary means of transmission, along with breast milk, urine and vaginal secretions (Crough and Khanna, 2009; Krmpotic et al., 2003; Kumar et al., 1984; Wu et al., 2011). We identified MCMV-driven TRM in all of these sites to varying degrees. However, spread-defective ΔgL-MCMV clearly induced a similar pattern of TRM formation, ruling out a direct role for viral replication in the formation of MCMV-specific mucosal TRM cells. Moreover, we could find no evidence that viral antigen in the salivary gland resulted in any increase in the rate of T cell division within the salivary gland (Figure 4). Indeed, viral immune evasion genes are thought to markedly restrict CD8+ T cell recognition of infected cells in the salivary gland (Walton et al., 2011). Nevertheless, the presence of antigen in latently infected mice may have caused a slight improvement in the rate of recruitment of inflationary cells to the salivary gland (Figure 7C) and clearly enhanced TRM differentiation (Figure 7D–E). We have previously demonstrated that circulating MCMV specific T cells undergo a constant low level of antigen stimulation primarily at sites that are accessible to the blood, such as the spleen or liver where the virus is known to persist (Smith et al., 2014). It is clear that restimulated T cells can traffic to the salivary gland and form new TRM (Figures 6 and 7) and (Hofmann and Pircher, 2011), even when they were stimulated prior to infiltration of the salivary gland (Figure 7E–G). Thus, we favor the model that, at late times after infection, the re-stimulation of blood-localized cells drives memory inflation and also promotes the continuous migration of T cells to the mucosa and formation of TRM.
Recent work has indicated that responsiveness to TGF-β is a general requirement for the development of TRM cells in multiple sites (Casey et al., 2012; El-Asady et al., 2005; Lee et al., 2011; Mackay et al., 2013; Sheridan et al., 2014; Skon et al., 2013; Zhang and Bevan, 2013) and it is thought that these TGF-β signals are received within the target tissue. TGF-β signals are particularly important for expression of CD103 on T cells, including MCMV-driven T cells (Figure 5), which is thought to promote the retention of TRM cells within the epithelium of the gut and lungs and the epidermis of the skin (Casey et al., 2012; El-Asady et al., 2005; Lee et al., 2011; Mackay et al., 2013; Zhang and Bevan, 2013). However, after an oral Listeria monocytogenes infection, CD103-deficient T cells were recruited poorly into the si-IEL, but were maintained comparably to wild-type T cells (Sheridan et al., 2014). In agreement with this, our data suggest that CD103 expression correlates with the epithelial-localization of MCMV-driven T cells in the salivary gland (Figure 2A), but that the absence of CD103 does not impair the localization or maintenance of TRM after MCMV infection, even in the absence of viral antigen (Figure 2 and S3–4).
Collectively, our data show that MCMV infection robustly induces mucosal TRM cells. In the salivary gland, these cells adopt an intraepithelial localization and persist in large numbers, in part as a result of continual recruitment from the circulating T cell pool and antigen-driven differentiation. These data show that mucosal TRM cells stimulated by a ubiquitous persistent infection can be maintained dynamically, and support the use of CMV based vaccine vectors to promote long-term mucosal immunity.
Experimental Procedures
Mice and infections
All mice were purchased from Jackson Laboratory and bred in house. C57BL/6 (B6) mice were used for all direct infections and B6 or CD45.1 congenic mice (B6.SJL-Ptprca Pepcb/BoyJ) were used as recipients for adoptive transfer experiments. OT-Is on a B6 background (C57BL/6-Tg(TcraTcrb)1100Mjb/J) were bred to CD45.1 congenic mice (B6.SJL-Ptprca Pepcb/BoyJ) or CD103−/− mice (B6.129S2(C)-Itgaetm1Cmp/J) to generate congenic, or CD103−/− OT-Is. Inflationary OT-Is were generated as described (Turula et al., 2013). For mixed WT and CD103−/− OT-I transfers, 1000 splenocytes from each donor were transferred into naive mice, which were infected with MCMV-OVA one day later. Transfers from latently-infected mice were performed as in (Smith et al., 2014). For in vitro stimulation of OT-Is, splenocytes were harvested from latently infected mice harboring OT-Is and were incubated for 3 hours at 37°C at a concentration of 1x107 splenocytes/ml with either 1 µg/ml SIINFEKL or without peptide before transfer. MCMV K181 (wild type MCMV), MCMV K181-tfr-OVA (MCMV-OVA) and MCMV-ΔgL were produced as described (Snyder et al., 2008; Zurbach et al., 2014). In all cases, mice were infected i.p. with 2 x 105 pfu of virus. All protocols were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee.
Lymphocyte isolation and FACS staining
Lymphocytes from the blood, spleen, liver, salivary glands, mammary glands, kidneys and female reproductive tracts were isolated as described (Smith et al., 2014). Lymphocytes from the small intestine IEL and lamina propria were isolated using the previously described protocol (Lefrançois and Lycke, 1996). Intravascular staining was performed as described recently (Smith et al., 2014). Antigen specific non-transgenic CD8s were identified with tetramers produced at the NIH tetramer core facility (http://tetramer.yerkes.emory.edu/) as previously described (Snyder et al., 2008). Analyses of cellular phenotype and donor cells in adoptive transfers were performed as described previously (Smith et al., 2014) with the additions of antibodies specific for CD103 (clone 2E7) and CD69 (clone H1.2F3). All antibodies were purchased from Biolegend or BD Biosciences. Cells were analyzed on an LSR II flow cytometer (BD Biosciences) and using FlowJo software (TreeStar).
In vivo labeling
For the long-term BrdU pulse (Figure 1), mice were injected i.p. with 1 mg of BrdU (Sigma) on the day of infection and then subsequently provided with 0.8 mg/ml BrdU in their drinking water for the next 9 weeks. BrdU labeling was assayed using the BD Biosciences Flow kit. In vivo CFSE labeling was adapted from previous work (Becker et al., 2004). For the long-term pulse/chase, 45µg of CFSE/mouse was injected retro-orbitally every other day for a total of three injections.
Immunofluorescent Microscopy
Sections of salivary glands were processed as described previously (Smith et al., 2014) and stained with antibodies against CD45.2 (clone 104), E-cadherin (clone DECMA-1), and CD103 (clone 2E7) and co-stained with DAPI (Prolong Gold antifade - Life Technologies). All antibodies were purchased from Biolegend. Images were generated with the LSM 510 Meta (Carl Zeiss) confocal laser scanning microscope and the LSM image browser software (Carl Zeiss) and analyzed with Fiji software (Schindelin et al., 2012).
PCR and ELISA
RNA was isolated from salivary glands with RNeasy Mini kit (Qiagen) and cDNA was generated with the High Capacity cDNA Reverse Transcriptase kit (Applied Biosystems). DNA was isolated using the Gentra Puregene Tissue kit (Qiagen). Nested PCR for gB transcripts and latent gB DNA was performed as described (Cook et al., 2002). Transcript levels of TNF-α, TGF-β and IL-33 were assessed by quantitative PCR with a StepOnePlus system (Applied Biosystems) using SYBR green (Applied Biosystems) for detection. Primers are listed in Supplemental Materials. Protein levels of active and total TGF-β were determined using Legend MAX ELISA kits (Biolegend).
In vitro cytokine assay
Naive mice (Figure 5A–B) or naive mice that received an adoptive transfer of CD8s from latently infected mice (Figure 6D) were infected with MCMV-K181. Four days later, splenocytes were isolated and cultured for 40 hours in the presence of cytokines as described (Casey et al., 2012) and analyzed by FACS.
Supplementary Material
Acknowledgments
This work was supported by the grant AI106810 awarded to C.M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, C.J.S, C.M.S; Investigation, C.J.S., S.C.D, H.T.; Writing, C.J.S., C.M.S.; Funding Acquisition, C.M.S.
The authors have no conflicts of interest to declare.
References
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song J-Y, Jacobs H, Haanen JB, Schumacher TN. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- Becker HM, Chen M, Hay JB, Cybulsky MI. Tracking of leukocyte recruitment into tissues of mice by in situ labeling of blood cells with the fluorescent dye CFDA SE. Journal of Immunological Methods. 2004;286:69–78. doi: 10.1016/j.jim.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CH, Zhang Y, McGuinness BJ, Sedmak DD, Lahm MC, Ferguson RM. Intra-abdominal Bacterial Infection Reactivates Latent Pulmonary Cytomegalovirus in Immunocompetent Mice. J Infect Dis. 2002;185:1395–1400. doi: 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- Crough T, Khanna R. Immunobiology of Human Cytomegalovirus: from Bench to Bedside. CLINICAL MICROBIOLOGY REVIEWS. 2009;22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekhtiarenko I, Jarvis MA, Ruzsics Z, Cicin-Sain L. The Context of Gene Expression Defines the Immunodominance Hierarchy of Cytomegalovirus Antigens. J Immunol. 2013;190:3399–3409. doi: 10.4049/jimmunol.1203173. [DOI] [PubMed] [Google Scholar]
- El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. The Journal of experimental medicine. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature immunology. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Graham JB, Da Costa A, Lund JM. Regulatory T Cells Shape the Resident Memory T cell Response in the Tissues. J Immunol. 2014;192:683–690. doi: 10.4049/jimmunol.1202153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Piatak M, Jr, Ventura AB, Hughes C, Gilbride R, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIVinfection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nature Medicine. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtappels R, Pahl-Seibert M-F, Thomas D, Reddehase MJ. Enrichment of Immediate-Early 1 (m123/pp89) Peptide-Specific CD8 T Cells in a Pulmonary CD62Llo Memory-Effector Cell Pool during Latent Murine Cytomegalovirus Infection of the Lungs. Journal of virology. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory Inflation: Continuous Accumulation of Antiviral CD8+ T Cells Over Time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Sierro SV, Cuero A, Klenerman P. Population analysis of antiviral T cell responses using MHC class I-peptide tetramers. Clin Exp Immunol. 2003;134:9–12. doi: 10.1046/j.1365-2249.2003.02266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. Pathogenesis of murine cytomegalovirus infection. Microb Infect. 2003;5:1263–1277. doi: 10.1016/j.micinf.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Kumar M, Nankervis G, Cooper A, Gold E. Postnatally acquired cytomegalovirus infections in infants of CMV-excreting mothers. J Pediatr. 1984;104:669–673. doi: 10.1016/s0022-3476(84)80941-5. [DOI] [PubMed] [Google Scholar]
- Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. Journal of virology. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançois L, Lycke N. Isolation of Mouse Small Intestinal Intraepithelial Lymphocytes, Peyer’s Patch, and Lamina Propria Cells. Current Protocols in Immunology. 1996:3.19.11–13.19.16. doi: 10.1002/0471142735.im0319s17. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nature immunology. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Picker LJ. Hidden Memories: Frontline Memory T Cellsand Early Pathogen Interception. J Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting Edge: Gut Microenvironment Promotes Differentiation of a Unique Memory CD8 T Cell Population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four Distinct Patterns of Memory CD8 T Cell Responses to Chronic Murine Cytomegalovirus Infection. The Journal of Immunology. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavi I, Lu in P, Jonji S, Koszinowski UH. Hierarchical and Redundant Lymphocyte Subset Control Precludes Cytomegalovirus Replication during Latent Infection. The Journal of experimental medicine. 1998;188:1047–1054. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M, Turula H, Tandon M, Deslouches B, Snyder CM. Memory T cells specific for murine cytomegalovirus re-emerge after multiple challenges and recapitulate immunity in various adoptive transfer scenarios. J Immunol. 2015;194:1726–1736. doi: 10.4049/jimmunol.1402757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nature immunology. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Masopust D. Tissue-Resident Memory T Cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckert CK, Schader SI, Ebert S, Thomas D, Freitag K, Renzaho A, Podlech J, Reddehase MJ, Holtappels R. Antigen-presenting cells of haematopoietic origin prime cytomegalovirus-specific CD8 T-cells but are not sufficient for driving memory inflation during viral latency. The Journal of general virology. 2011;92:1994–2005. doi: 10.1099/vir.0.031815-0. [DOI] [PubMed] [Google Scholar]
- Sheridan BS, Pham Q, Lee YT, Cauley LS, Puddington L, Lefranc¸ois L. Oral Infection Drives a Distinct Population of Intestinal Resident Memory CD8+ TCells with Enhanced Protective Function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CO, Holtappels R, Tervo HM, Bohm V, Daubner T, Oehrlein-Karpi SA, Kuhnapfel B, Renzaho A, Strand D, Podlech J, et al. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. Journal of virology. 2006;80:10436–10456. doi: 10.1128/JVI.01248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nature immunology. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Turula H, Snyder CM. Systemic Hematogenous Maintenance of Memory Inflation by MCMV Infection. PLoS pathogens. 2014;10:e1004233. doi: 10.1371/journal.ppat.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB. Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS pathogens. 2011;7:e1002295. doi: 10.1371/journal.ppat.1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of shortlived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti N, Walton SM, Brocker T, Rulicke T, Oxenius A. Non-hematopoietic cells in lymph nodes drive memory CD8 T cell inflation during murine cytomegalovirus infection. PLoS pathogens. 2011;7:e1002313. doi: 10.1371/journal.ppat.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turula H, Smith CJ, Grey F, Zurbach KA, Snyder CM. Competition between T cells maintains clonal dominance during memory inflation induced by MCMV. European journal of immunology. 2013;43:1252–1263. doi: 10.1002/eji.201242940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. PNAS. 2010;107:8. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton SM, Mandaric S, Torti N, Zimmermann A, Hengel H, Oxenius A. Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4 T cells. PLoS pathogens. 2011;7:e1002214. doi: 10.1371/journal.ppat.1002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CA, Paveglio SA, Lingenheld EG, Zhu L, Lefrancois L, Puddington L. Transmission of murine cytomegalovirus in breast milk: a model of natural infection in neonates. Journal of virology. 2011;85:5115–5124. doi: 10.1128/JVI.01934-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hu Y, Lee Y-T, Bouchard KR, Benechet A, Khanna KM, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. Journal of Leukocyte Biology. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, et al. Immune surveillance by CD8aa1 skin-resident Tcells in human herpes virus infection. Nature. 2013;000:1–4. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbach KA, Moghbeli T, Snyder CM. Resolving the titer of murine cytomegalovirus by plaque assay using the M2-10B4 cell line and low viscosity overlay. Virology Journal. 2014;11:71. doi: 10.1186/1743-422X-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.