Abstract
Background and Aims There is a growing concern about how forests will respond to increased herbivory associated with climate change. Carbon (C) and nitrogen (N) limitation are hypothesized to cause decreasing growth after defoliation, and eventually mortality. This study examines the effects of a natural and massive defoliation by an insect on mature trees’ C and N storage, which have rarely been studied together, particularly in winter-deciduous species.
Methods Survival, growth rate, carbon [C, as non-structural carbohydrate (NSC) concentration] and nitrogen (N) storage, defences (tannins and total polyphenols), and re-foliation traits were examined in naturally defoliated and non-defoliated adult trees of the winter-deciduous temperate species Nothofagus pumilio 1 and 2 years after a massive and complete defoliation caused by the caterpillar of Ormiscodes amphimone (Saturniidae) during summer 2009 in Patagonia.
Key Results Defoliated trees did not die but grew significantly less than non-defoliated trees for at least 2 years after defoliation. One year after defoliation, defoliated trees had similar NSC and N concentrations in woody tissues, higher polyphenol concentrations and lower re-foliation than non-defoliated trees. In the second year, however, NSC concentrations in branches were significantly higher in defoliated trees while differences in polyphenols and re-foliation disappeared and decreased, respectively.
Conclusions The significant reduction in growth following defoliation was not caused by insufficient C or N availability, as frequently assumed; instead, it was probably due to growth limitations due to factors other than C or N, or to preventative C allocation to storage. This study shows an integrative approach to evaluating plant growth limitations in response to disturbance, by examining major resources other than C (e.g. N), and other C sinks besides storage and growth (e.g. defences and re-foliation).
Keywords: Climate change, plant defences, defoliation, herbivory, insect outbreaks, non-structural carbohydrates, nitrogen, Nothofagus pumilio, Nothofagaceae, Ormiscodes amphimone, Patagonia, storage.
INTRODUCTION
In temperate regions, forests are expected to experience more frequent and severe herbivory under future climate warming scenarios (Dale et al., 2001; Bradshaw and Holzapfel, 2010; Paritsis and Veblen, 2010). The defoliation caused by herbivory usually reduces growth of trees and accelerates die-back processes, and may ultimately cause mortality (Rose, 1958; Kulman, 1971; Kosola et al., 2001; Galiano et al., 2011; Saffell et al., 2014), and yet the physiological mechanisms driving these responses remain elusive. A long-standing belief is that reduced photosynthetic area by defoliation causes a carbon (C) shortage (i.e. decreases in non-structural carbohydrates, NSC) which in turn limits growth and survival (Dickson, 1989; Krause et al., 1993) (i.e. C limitation, Hypothesis 1, Fig. 1). Although some studies have reported this response in seedlings and juvenile trees subjected to severe defoliation (Wargo et al., 1972; Parker and Patton, 1975; Tschaplinski and Blake, 1994; Canham et al., 1999), it is largely unknown whether C limitation may explain the growth reductions observed in mature trees after defoliation. Some studies examining responses of mature trees to defoliation have found a positive relationship between C storage and subsequent crown recovery (re-foliation) in evergreen conifers, suggesting that reduced crown recovery could have been driven by limited C storage (Webb, 1981; Galiano et al., 2011). However, this idea is not supported by other studies on conifers. For example, Palacio et al. (2012) found that complete defoliation by the pine processionary moth caused long-term growth decline in Pinus nigra (i.e. over 6 years following defoliation) but only transient decreases in C storage. Similarly, Saffell et al. (2014) reported that reduced leaf area by the Swiss needle cast in Douglas fir led to stronger reductions on growth than on C storage. Evidence for broadleaved winter-deciduous species is scarcer than for evergreen conifers. In a young plantation of poplars, for example, repeated defoliation caused long-term decreases in growth but only transient reductions in C storage (Kosola et al., 2001). Similarly, Anderegg and Callaway (2012) found that repeatedly defoliated ramets of aspen flushed multiple canopies, enduring only moderate drawdown of NSC. To our knowledge, the effects of complete defoliation naturally caused by insects on C storage in mature trees of broadleaved winter-deciduous species have never been examined, which is notable given that this type of defoliation is most expected to result in C limitation (Körner, 2003; Palacio et al., 2014).
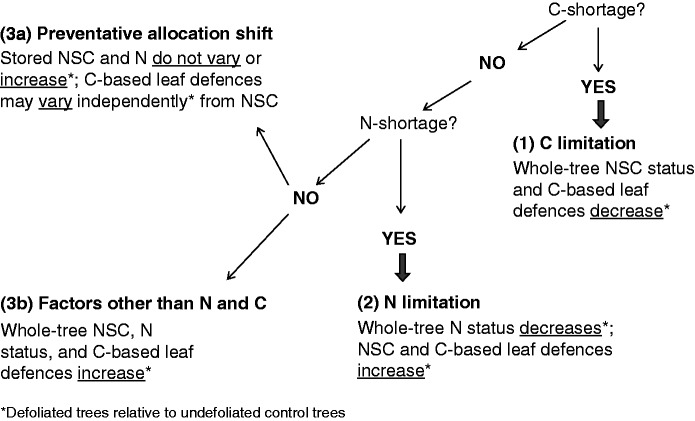
Fig. 1.
Hypothetical alternative mechanisms driving tree growth reductions after complete natural defoliation. A logic tree is constructed on the basis of whether C shortage first, and then N shortage, do occur. If C shortage leads to reduced growth (C limitation), decreases in the tree’s NSC status and C-based leaf defences are expected regardless of the tree’s N status (1). If N shortage leads to reduced growth (N limitation), the tree’s NSC status and leaf C-based defences should increase, at the same time that the tree’s N status decreases (2). Under no C or N shortage two possible explanations emerge: allocation shift (3a) and growth limitation caused by other factors (3b). In the first case, no external factor limits tree growth and whole-tree NSC and N status respond concomitantly, either increasing or reaching control levels; we have no clear expectation for C-based leaf defences although up-regulation could result in independent variation with respect to the other variables. In the second case, growth is limited by factors other than N; whole-tree NSC, N status and C-based leaf defences increase concomitantly.
Another potential cause of reduced growth after severe defoliation relates to impaired nutrient status. Most herbivory usually occurs during the growing season, when stored nutrients are remobilized to the tissues under formation. Herbivory thus leads to a direct loss of nutrients from the tree, especially nitrogen (N) (Lovett et al., 2002). In addition, defoliation often causes root mortality, reduces root metabolism and diminishes nutrient uptake (Tuomi et al., 1990; Kosola et al., 2001). Although nutrients may limit photosynthesis, growth is even more sensitive than photosynthesis to moderate shortages in essential macronutrients (Herms and Mattson, 1992). Because of this, trees under moderate nutrient limitations are predicted to increase their C storage (Hypothesis 2, Fig. 1) (Herms and Mattson, 1992; Kosola et al., 2001; Palacio et al., 2014). Alternatively, it has been suggested that increased C storage and decreased growth in response to stressors, such as defoliation, could be driven not by a C accumulation but rather by a shift in C allocation from growth to storage, to avoid further C losses (i.e. preventative C allocation) (Wiley and Helliker, 2012). Such a C allocation shift would create an internal C limitation because, although the tree would have sufficient C to grow rapidly, changes in allocation priorities driven by defoliation would determine that the C is invested otherwise.
Whether reduced tree growth following defoliation is caused by N limitation or a preventative C allocation (as postulated by Wiley and Helliker, 2012) should be revealed by how trees store and allocate their N and C following defoliation. Because winter-deciduous species can store significant quantities of N in their woody tissues, we would expect a significant drawdown (i.e. remobilization) of woody N reserves following defoliation, if regrowth were primarily N limited (Hypothesis 2, Fig. 1) (Chapin, 1980; Millard et al., 2001; Millard and Grelet, 2010; Piper and Fajardo, 2014). Alternatively, if growth reduction were a consequence of preventative C allocation, as proposed by Wiley and Helliker (2012), we postulate that N storage should not decrease but rather remain invariable or increase concomitantly with C (Hypothesis 3a, Fig. 1). Our rationale is based on the facts that wild trees are often subject to soil N limitation, and that defoliation impairs nutrient root uptake. Hence, allocation of C into storage to prevent further C losses (sensu Wiley and Helliker, 2012) would potentially be evolutionarily unsuccessful if trees could not also acquire required N to re-allocate the C for re-foliation. In fact, high levels of both C and N storage in winter-deciduous species are thought to be an adaptation to tolerate defoliation (Grelet et al., 2001; Millard et al., 2001; Millard and Grelet, 2010; Piper and Fajardo, 2014). Additionally, if tree growth is impaired due to a preferential allocation of C to storage, N pools can be expected to remain high due to a lower demand of nutrients for growth. To date, very few studies have simultaneously examined how plants allocate both C and N into storage in responses to defoliation, leaving substantial uncertainty about how these pools interact.
Another factor that could explain suppressed growth following severe defoliation is an increase in C allocation to the production of secondary metabolites, which could divert C from growth (Herms and Mattson, 1992; Jones and Hartley, 1999; Hamilton et al., 2001). Secondary metabolites are expensive to synthesize, and share (i.e. compete for) common precursors and substrate with primary metabolites (e.g. structural C and NSC invested in growth and storage, respectively) (Herms and Mattson, 1992). Likewise, herbivory or artificial defoliation may induce changes in morphological leaf traits with defensive functions (e.g. higher leaf mass per area, LMA) (Millard et al., 2001; Nabeshima et al., 2001), and thus could increase the total cost of re-foliation. This strategy could be beneficial for species that experience low levels of competition (i.e. where fast growth is not necessary) and strong disturbance pressure caused by defoliation (e.g. herbivory outbreaks). In fact, the production of a well-defended foliage after a season of defoliation (i.e. delayed induced resistance) is a common feature in winter-deciduous species adapted to severe defoliations (e.g. Betula spp.) (Krause et al., 1993). On the other hand, in winter-deciduous species, defoliation-induced synthesis of C-based defences could be a simple consequence of an imbalance between C and N, given that defoliation is suggested to reduce N more than C (Herms and Mattson, 1992; Krause et al., 1993). Although it is difficult to distinguish whether increased allocation to defences along with decreased growth reflects nutrient-limited growth or up-regulation, the former would be supported if reduced growth and increased leaf C-based defences occurred with a concomitant reduction in nutrient concentration across all tissues (Hypothesis 2, Fig. 1). Alternatively, an increase of induced C-based defences under invariable or increasing whole-tree nutrient status could reflect either up-regulation of C-based defences or a C accumulation resulting from growth limitations driven by factors other than nutrients (Hypotheses 3, Fig. 1). Thus, a closer look at the C and N dynamics within a tree during recovery from severe defoliation can help to distinguish among several contrasting mechanisms proposed to explain impaired tree growth.
In this study we analysed the response of adult trees of the winter-deciduous and herbivory-tolerant broadleaved species Nothofagus pumilio to a complete natural defoliation caused by a moth caterpillar outbreak (Ormiscodes amphimone, Saturniidae) that occurred in the southern Andes during summer 2009. This particular outbreak led to the massive and complete natural defoliation of thousands of hectares of forest (Fig. 2). Herbivory in Nothofagus pumilio forests has been shown to be strongly controlled by temperature, and there is hence a growing concern regarding how this tree species will react to a higher herbivory, which is expected to occur under higher temperatures (Garibaldi et al., 2011; Mazía et al., 2012). For two consecutive years following the defoliation event, we monitored tree survival and measured responses of growth, re-foliation, leaf chemical and morphological defences, and C and N storage. A previous study indicated that juvenile trees of the species account for high levels of C and N storage in woody tissues which are re-mobilized to tolerate defoliation (Piper and Fajardo, 2014). Assuming that after defoliation trees effectively grow less and can eventually die, we posited multiple alternative hypotheses that can mechanistically explain such a response (Fig. 1). In brief, we hypothesized that a decrease in growth following defoliation is caused by: (1) a shortage of C (i.e. C limitation hypothesis) (Hypothesis 1, Fig. 1), (2) a shortage of N (e.g. the nutrient limitation hypothesis) (Hypothesis 2, Fig. 1) or (3) trees are not limited by either C or N, but growth is instead reduced by either preventative C allocation to storage (Hypothesis 3a, Fig. 1) or factors other than C or N (Hypothesis 3b, Fig. 1).
Fig. 2.
Nothofagus pumilio forest after a massive defoliation caused by an outbreak of the larvae of Ormiscodes amphimone (Saturniidae) during summer 2009 in the southern Andes of Chile (Aysén Region, Patagonia; left). The left-side photograph was taken in April 2009 (mid-autumn); thus, the reddish canopy at the higher elevation corresponds to the forest which was not defoliated due to the caterpillars' thermal threshold. Note the green (i.e. new) leaves produced after the defoliation event on the tree in the front of the photograph. Larvae of O. amphimone feeding on leaves attached to a branch of N. pumilio (right).
MATERIALS AND METHODS
Species and research site
The study was carried out in the Aysén Region, Patagonia, Chile, specifically in the Reserva Nacional Cerro Castillo conservation area, where the forest comprises primarily Nothofagus pumilio (Nothofagaceae). Mean annual precipitation is approx. 1000 mm and is distributed regularly throughout the year (Dirección General de Aguas, Servicio Meteorológico Nacional); mean temperature for the growing season is 8·6 °C. The soil in the study area is derived from aeolian volcanic ash deposits. Nothofagus pumilio (Poepp. Et Endl.) Krasser (Nothofagaceae) is a broadleaved winter-deciduous tree species endemic to the southern Andes of South America, distributed from 35 to 55 °S. It is one of the most cold-resistant tree species of the region, forming high-elevation forests and being the dominant treeline species (Alberdi et al., 1985; Fajardo et al., 2013). The growing season for N. pumilio typically starts in October and extends to mid-April, although this varies with latitude and elevation (Hevia et al., 1999). In Patagonia, leaf-out occurs in late October, maximum leaf size is reached in early December, leaf senescence (reddish colour) occurs in April and complete leaf shedding is achieved in May (A. Fajardo, unpubl. res.).
We selected four sites in the Cerro Castillo National Reserve that were defoliated during the growing season of 2009. These sites are pure second-growth forest of N. pumilio 50–80 years old, with diameters at breast height (dbh, 1·35 m) of 25–35 cm, heights of 8–15 m and stand densities of 1100–1800 trees ha–1 (A. Fajardo, unpubl. res.). At each site a frontier between trees that were fully defoliated and trees that escaped defoliation was clearly observed. Caterpillars of Ormiscodes amphimone have a very well-defined temperature threshold (Fig. 2), leading to an abrupt boundary between defoliated and non-defoliated trees (i.e. controls). Thus, at each site, we sampled trees 20 m below (defoliated) and 20 m above (non-defoliated) this boundary. The four sites were Estero Parada (46°05′37″S, 72°14′10″W, 805 m a.s.l.), Refugio (46°05′59″S, 72°13′58″W, 754 m a.s.l.), Cerro Castillo (46°06′28″S, 72°05′14″W, 610 m a.s.l.) and La Cuesta (46°06′18″S, 72°03′26″W, 760 m a.s.l.). In April 2009 (the end of the growing season in the austral hemisphere) we photographed these boundaries (Fig. 2) and marked defoliated (no leaves) and non-defoliated trees to simplify a posteriori identification of sampling sites. Non-defoliated trees at this time of the year still had leaves.
Sampling design
With the assistance of the photographs previously taken, we selected 30 defoliated (below the elevational frontier) and 30 non-defoliated N. pumilio adult trees (above the frontier) across four sites (seven trees at two sites, and eight trees at the other two sites). To test our hypotheses we first needed to assess whether defoliated trees survived and if so whether they effectively grew less after defoliation than non-defoliated trees. All trees were monitored for survival and signs of decay (e.g. absence of leaves, branch mortality, colonization by wood decay fungi) in March 2010, 2011 and 2014. For all trees we collected tissue samples for re-foliation measurements, NSC, N and leaf chemical defences, as well as for the determination of LMA (g m–2) and other leaf traits at mid-March 2010 and 2011 (i.e. 1 and 2 years after the defoliation occurred). This time of year represents the end of the growing season in the southern hemisphere, when it is known that C and nutrient replenishment start to occur in winter-deciduous species (Barbaroux and Bréda, 2002; Hoch et al., 2003).
Tree growth determination
Tree growth was assessed retrospectively as an annual basal area increment. In March 2014, we measured on each individual tree dbh (1·35 m above ground), diameter at coring height (dch, approx. 0·2 m above ground) and bark thickness at dch, and extracted two increment cores to the pith at dch. Each tree was cored perpendicular to the slope using a 5·15-mm increment borer (Haglöf, Långsele, Sweden). Cores were prepared following standard dendrochronological techniques (Stokes and Smiley, 1996). For the purposes of this study, we assigned an annual ring to the calendar year in which the radial growth was completed. All samples were dated and visually cross-dated to detect the presence of either false or incomplete rings using marker rings, especially in the defoliated trees; in this case, non-defoliated trees served as chronology references. Following visual cross-dating, tree ring width was measured to the nearest 0·001 mm and assigned to calendar years using a microscope mounted on a dendrochronometer with a Velmex sliding stage (Bloomfield, NY, USA) and Accurite measuring system (St Louis, MO, USA). The annual basal area increment (BAI) was then computed for each of the last 6 years (including the 2 years after defoliation) as:
where R is the radius of the stem without bark at dch and n is the year of the tree ring completion.
Re-foliation measurements
To evaluate the leaf area recovery after defoliation, we estimated re-foliation density as the number, mass and area of leaves per branch in March 2010 and March 2011. We also measured mean leaf area and LMA as traits associated with crown recovery and morphological defences. For each individual tree, we targeted and cut a terminal, fully expanded and sun-exposed branch at 2–3 m height using pruner scissors. Branches were labelled and placed in a cooler for transportation. Selected branches were approx. 4 years old and had a similar diameter for control and defoliated trees (6·5 ± 0·15 and 6·22 ± 0·16 mm, respectively; P > 0·20, Student’s t-test); however, we considered the branch diameter as a co-variable for all response variables used to quantify re-foliation (see Statistical analysis for further details). Tissue samples were collected between 1200 and 1700 h. In the laboratory, all leaves per branch were detached and counted. Some leaves were then separately laid flat on a white paper sheet and photographed with a reference square of known area using a Nikon Coolpix 5000 digital camera (Nikon, Tokyo, Japan), the total projected leaf area was then determined using SIGMAPROC image processing software (Systat Software, Richmond, CA, USA) and mean area per leaf was calculated by dividing total leaf area by the number of leaves. All leaves were then placed to dry in a forced-air stove (Memmert, Schwabach, Germany) at 70°C for 72 h and finally the photographed leaves and the remaining leaves were separately weighted on a scale at 0·0001 g precision to determine LMA and leaf mass per branch. We computed LMA as the oven-dried leaf weight divided by its total foliar surface. Finally, all leaves were ground to a fine powder using a coffee mill; they were then stored at 4°C until chemical analyses were performed.
Leaf C-based defences
For each leaf sample, leaf extracts were created by extracting 0·2 g of ground leaves in 20 mL of 50 % methanol, which were shaken for 1 h, and separated via centrifugation (Gundale et al., 2010; Sundqvist et al., 2012). Extracts were then analysed for total phenolics and condensed tannin concentrations using the Prussian blue technique (Stern et al., 1996) and acid–butanol method (Porter et al., 1985), using catechin (+/–) and procyanidin B2 (Sigma-Aldrich, St Louis, MO, USA) as standards, respectively. Due to potential differences in reactivity of N. pumilio phenolics and tannins with reagents compared with the standards, the total phenolic and tannin masses are reported on a catechin and procyanidin equivalent basis, rather than as absolute masses.
NSC and N analyses
We determined NSC (soluble sugars + starch) and N concentrations in leaves, branches and stems of all trees in mid-March 2010 and mid-March 2011 (i.e. late summer). In addition, we also determined NSC in stems and roots for five trees per treatment on 19 January 2010 (i.e. the middle of the first growing season after defoliation) to examine defoliation effects on C storage at the most active period of growth, and to examine potential differences in NSC trends between stems and roots – the two major C storage sites in winter-deciduous species (Millard et al., 2001), and for N. pumilio in particular (Piper and Fajardo, 2014). The latter helped us to ultimately decide whether an estimation of NSC in major storage sites would be sufficient by sampling only stems, given that root sampling was logistically more complicated. This allowed us to identify that NSC in roots and stems responded similarly, allowing us to exclude roots from the sampling, and focus on leaves, branches and stems to provide an integral view of C storage in the trees.
From each tree we used an increment borer to extract a 10-cm stem core at dbh, and shovels and scissors to remove a coarse piece of superficial root (approx. 0·5–1 cm in diameter, for the first sampling only) and to cut a 5-cm length of branch (approx. 0·5–1 cm in diameter), and hand-collected leaves. Bark and phloem were removed from the pieces of branch and roots in the field with a knife. Plant material was properly labelled and brought to the laboratory in a cooler with ice to reduce tissue respiration during transport (Popp et al., 1996). In the laboratory, all samples were divided into two pieces, one for NSC and one for N analyses. Samples for NSC analyses were heated in a microwave in three 20-s cycles at maximum power to stop enzymatic activity (Popp et al., 1996) and then, along with the samples for N analyses, placed in a forced-air oven at 65°C to dry until constant weight. Branch and stem samples were then ground to a fine powder using a mixer ball mill MM 200 (Retsch, Haan, Germany), and subsequently stored at 4°C until chemical analyses were performed. We determined soluble sugars and starch concentrations in approx. 15 mg of dried powder of every tissue sample. Soluble sugars were extracted with a methanol/chloroform/water solution, separated from pigments and lipids by adding water and chloroform (Rose et al., 1991), and then main sugars (sucrose, glucose, fructose) were determined with the phenol sulphuric method, using 2 % phenol and reading at 490 nm (Chow and Landhäusser, 2004). The residual pellet was dried overnight at 50°C in a forced-air oven and starch was then gelatinized (Rose et al., 1991) and hydrolysed to glucose with amyloglucosidase (Sigma-Aldrich 10115) at 45°C overnight. We determined glucose in a similar way as soluble sugars (Chow and Landhäusser, 2004). Soluble sugars and starch concentrations were expressed as mg per g dry weight. Total NSC concentrations were estimated as the sum of soluble sugars and starch. The N concentration of each tissue sample was determined from 25 mg of dry and ground powder by a combustion analyser (LECO TruSpec Micro CHN, Centro de Investigación en Ecosistemas de la Patagonia, Coyhaique, Chile). N concentrations were expressed on a dry mass basis (as % dry matter). This method quantifies total N (i.e. it does not distinguish between stored and structural N), which is widely used to examine changes in N storage (Chapin, 1980; Millard et al., 2001; Silla and Escudero, 2003), given that most tissue N can be potentially remobilized (Chapin et al., 1990; Millard and Grelet, 2010).
Statistical analyses
The influence of defoliation on BAI, re-foliation (leaf number, area and mass per branch), C and N storage, and leaf morphological and chemical properties (mean leaf area, N, tannins, phenolics and LMA) was analysed fitting linear mixed-effects models (LMMs). In the modelling, we considered defoliation condition (Control and Defoliated) as the fixed factor and sites as the random factor to account for among-site variation. When variables did not meet normality assumptions they were log10 transformed. In all cases, analyses were run separately for each year (2010 and 2011 for all response variables, except for growth, for which analyses were run from 2005 to 2012). For re-foliation variables, we considered branch diameter as a co-variable, and tested the significance of the interaction between branch diameter and defoliation. The lack of significance for the interaction means that our re-foliation measurements were not biased by the diameter of the branches sampled in defoliated and control trees. For C and N storage, analyses were performed by tissue (leaves, branches, stems). Finally, we used Student’s t-tests to compare NSC and N concentrations between control and defoliated trees in January 2010. We found that defoliation did not cause any mortality, and therefore no statistical analysis was performed to evaluate survival. All analyses were performed in JMP Version 8.0 (SAS Institute, Cary, NC, USA).
RESULTS
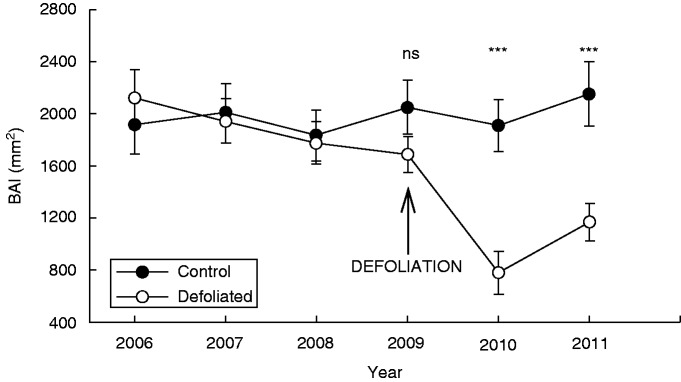
Annual BAI was not significantly different between control and defoliated trees for all years previous to defoliation (P > 0·05). Defoliation occurred between January and March 2009, and thus at this time there was a non-significant growth reduction in defoliated trees when compared with control ones (F = 0·60, P = 0·44). However, 1 year after the defoliation (the growing season period starting in the austral spring of 2009), defoliated trees grew less than half (41 % of BAI) as much as control trees (F = 33·00, P < 0·001). This significant difference remained for the subsequent year 2011 (F = 13·39, P < 0·001, Fig. 3).
Fig. 3.
Mean annual relative tree growth (basal area increment in mm2, BAI) of non-defoliated (i.e. Control) and Defoliated trees of Nothofagus pumilio after a massive and complete defoliation caused by the moth caterpillar Ormiscodes amphimone (Saturniidae) during summer 2009 (i.e. January and February) in the southern Andes of Chile.
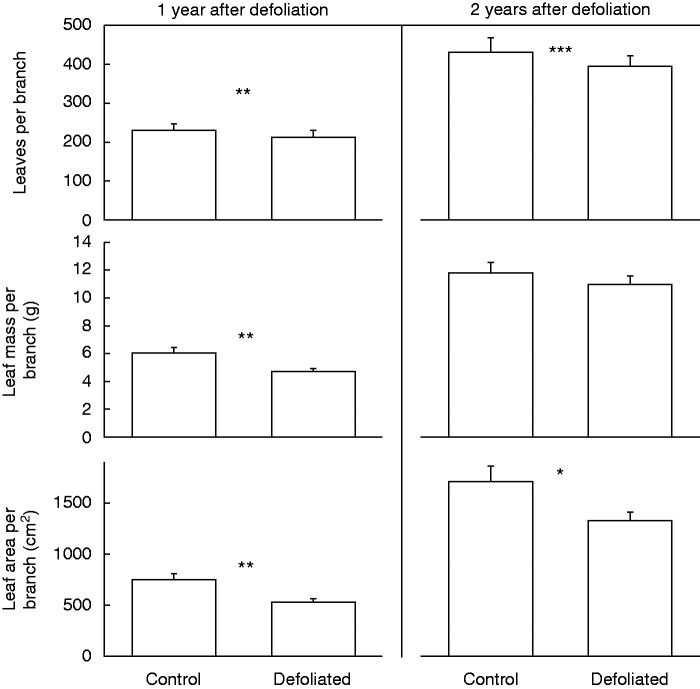
For the 2 years examined after defoliation, defoliated trees had significantly lower re-foliation than control trees (Fig. 4). The former had significantly fewer leaves, and lower leaf mass and leaf area per branch for the first year after the defoliation (2010) than the latter (Table 1). For the second year after defoliation (2011), leaf area per branch remained significantly lower for defoliated than for control trees (Table 1, Fig. 4). Branch diameter had a significant positive effect on leaf number, area and mass per branch, but the interaction term in the analysis between branch diameter and defoliation condition was only significant for leaf area in the second year (Table 1).
Fig. 4.
Number, mass and area of leaves per branch in non-defoliated (i.e. Controls) and Defoliated trees of Nothofagus pumilio 1 and 2 years after a massive and complete natural defoliation caused by the moth caterpillar Ormiscodes amphimone (Saturniidae) during summer 2009 (i.e. January-February) in the southern Andes of Chile. Bars represent mean values, and error bars refer to standard errors (n = 30). Asterisks indicate significant differences between defoliated and control trees at *P < 0·05, **P < 0·01 and ***P < 0·001.
Table 1.
Summary of F-ratios and inference (P-values) for the effects of defoliation (De), branch diameter (Bd), and the interaction of both on leaf number, mass and area per branch of Nothofagus pumilio, 1 and 2 years after a massive and complete defoliation caused by the caterpillar Ormiscodes amphimone (Saturniidae) during summer 2009 in the southern Andes of Chile (46 °04′S, 72 °03′W), based on linear mixed-effect models (LMM)
| Response variable | Model effects | 1 year after defoliation | 2 years after defoliation |
|---|---|---|---|
| Leaf number | De | 0·01 (0·920) | 0·25 (0·619) |
| Bd | 9·58 (0·004) | 7·19 (0·012) | |
| De × Bd | 0·65 (0·424) | 1·15 (0·289) | |
| Leaf mass | De | 8·22 (0·006) | 0·23 (0·637) |
| Bd | 4·68 (0·041) | 17·05 (<0·001) | |
| De × Bd | 0·27 (0·605) | 2·91 (0·094) | |
| Leaf area | De | 12·45 (<0·001) | 4·42 (0·040) |
| Bd | 1·39 (0·244) | 10·64 (0·002) | |
| De × Bd | 0·82 (0·369) | 4·63 (0·036) |
We found a significant difference in leaf morphological and chemical traits between defoliated and control trees, particularly in the first year after defoliation (2010). In particular, defoliated trees displayed smaller leaves (2010: F = 16·53, P < 0·001; 2011: F = 3·77, P = 0·057), lower leaf N concentrations (Table 2) and lower tannin concentrations (2010: F = 10·40, P = 0·002; 2011: F = 34·52, P < 0·001; Fig. 5). Polyphenol leaf concentration, by contrast, was higher for defoliated than for control trees, although this difference was observed only in the first year after defoliation (2010: F = 18·78, P < 0·001; 2011: F = 0·061, P = 0·805). As an exception, LMA was similar between defoliated and control trees for both the first (F = 0·017, P = 0·97) and the second year after the defoliation (F = 1·35, P = 0·250; Fig. 5).
Table 2.
Soluble sugars, starch (on a dry mass basis, mg g–1) and nitrogen (%) concentrations (mean ± 1 s.e.) and statistical inference, for different tissues in adult trees of Nothofagus pumilio growing naturally in Patagonia, Chile, which were or were not affected by a massive defoliation, after 1 and 2 years of an outbreak of Ormiscodes amphimone (Saturniidae); data were analysed using linear mixed-effects models
| 1 year after defoliation |
2 years after defoliation |
|||||
|---|---|---|---|---|---|---|
| Control | Defoliated | F-ratios (P-values) | Control | Defoliated | F-ratios (P-values) | |
| Soluble sugars | ||||||
| Leaf | 148·7 (3·31) | 139·2 (4·05) | 6·36 (0·015) | 122·8 (7·29) | 116·8 (6·56) | 0·68 (0·41) |
| Branch | 25·9 (1·16) | 22·0 (1·29) | 4·88 (0·031) | 27·9 (1·54) | 27·2 (1·89) | 0·13 (0·72) |
| Stem sapwood | 15·6 (0·87) | 16·0 (1·19) | 0·10 (0·75) | 16·2 (1·08) | 13·4 (0·95) | 6·64 (0·013) |
| Starch | ||||||
| Leaf | 41·0 (1·83) | 54·0 (1·81) | 26·3(<0·001) | 35·9 (1·40) | 48·5 (1·77) | 37·12 (<0·001) |
| Branch | 58·4 (2·55) | 63·9 (3·74) | 1·66 (0·202) | 51·8 (2·01) | 62·6 (3·05) | 10·71 (0·002) |
| Stem sapwood | 28·2 (2·80) | 26·0 (2·41) | 0·35 (0·553) | 21·55 (1·04) | 21·1 (1·59) | 0·06 (0·807) |
| Nitrogen | ||||||
| Leaf | 1·84 (0·05) | 1·60 (0·05) | 13·85 (<0·001) | 1·82 (0·04) | 1·60 (0·04) | 14·30 (<0·001) |
| Branch | 0·46 (0·01) | 0·48 (0·02) | 0·95 (0·330) | 0·44 (0·01) | 0·45 (0·02) | 0·06 (0·800) |
| Stem sapwood | 0·29 (0·01) | 0·29 (0·01) | 0·60 (0·440) | 0·30 (0·01) | 0·29 (0·01) | 0·52 (0·470) |
Fig. 5.
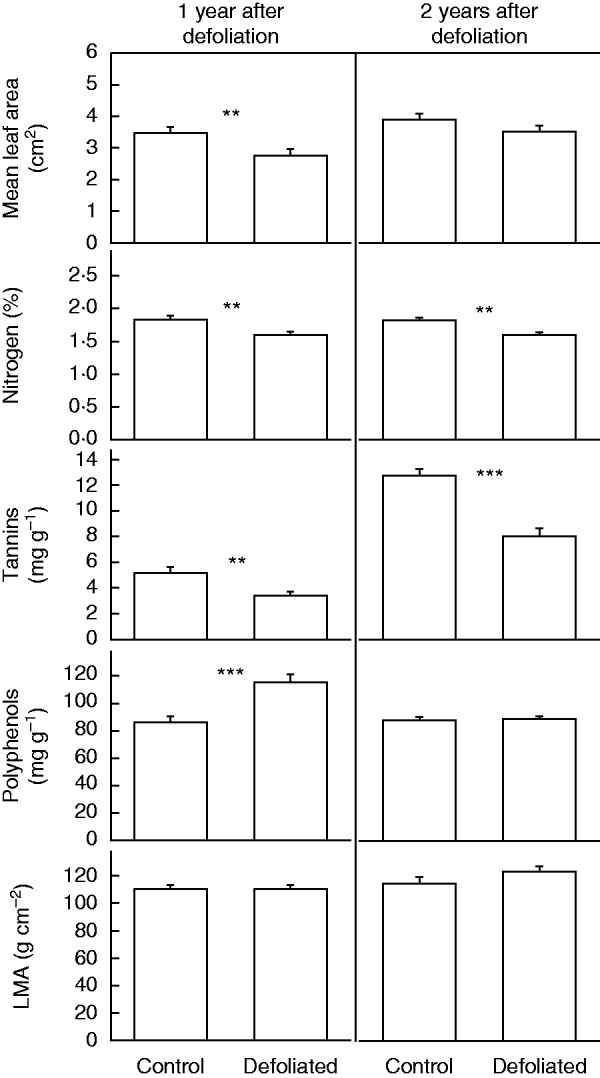
Morphological and chemical characteristics of leaves in non-defoliated (i.e. Controls) and Defoliated trees of Nothofagus pumilio 1 and 2 years after a massive and complete forest natural defoliation caused by the moth caterpillar O. amphimone (Saturniidae) in the southern Andes of Chile during summer 2009 (i.e. January and February 2009). Bars represents mean values, and error bars indicate standard errors (n = 30). Asterisks indicate significant differences between defoliated and control trees at **P < 0·01 and ***P < 0·001.
By January 2010, NSC concentrations in roots and stems were similar between control and defoliated trees (F1,9 = 1·86, P = 0·21 for roots; F1,9 = 2·91, P = 0·13 for stems; Fig. 6 inset). By March 2010, NSC concentrations were also similar between defoliated and control trees for leaves (F = 0·52, P = 0·470), branches (F = 0·12, P = 0·730) and stems (F = 0·15, P = 0·700; Fig. 6). Likewise, after 2 years (March 2011), defoliation had no significant effect on the NSC concentrations of leaves (F = 0·73, P = 0·390) or stems (F = 1·63, P = 0·210; Fig. 6). However, and in contrast to the first year’s results, we found a significant increase in branch NSC concentration of defoliated trees (F = 5·43, P = 0·020; Fig. 6). In more detail, soluble sugars represented the main NSC component of leaves, whilst starch dominated in branches and stems (Table 2). Soluble sugars in leaves and branches experienced a significant reduction after 1 year of defoliation. This effect, however, disappeared in the second year, when soluble sugar concentrations in stems were lower for the defoliated trees (Table 2). In contrast, foliar starch was higher in defoliated than in control trees, for both the first and the second year after defoliation, while branch starch concentration was higher in defoliated trees than in control trees for the second year only (Table 2). Finally, N storage in woody tissues did not vary in response to defoliation. N concentrations in both branches and stems were similar between defoliated and control trees for both of the two years they were measured (Table 2). The same pattern was found for the January 2010 measurements, when stem N concentrations were 0·38 ± 0·03 and 0·37 ± 0·02 % for defoliated and control trees, respectively (F1,9 = 0·02, P = 0·900), and root N concentrations were 0·35 ± 0·03 and 0·35 ± 0·02 % for defoliated and control trees, respectively (F1,9 = 0·01, P = 0·990).
Fig. 6.
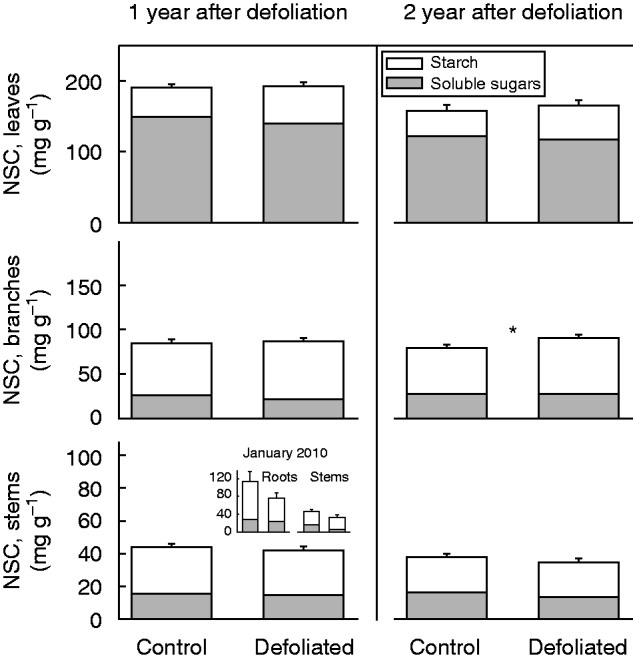
Non-structural carbohydrate (NSC) concentrations per unit of dry mass in leaves, branches and stem sapwood in non-defoliated (i.e. Control) and Defoliated trees of Nothofagus pumilio after 1 and 2 years of a massive outbreak defoliation by the moth caterpillar Ormiscodes amphimone (Saturniidae) during summer 2009 in the southern Andes of Chile. Bars represent mean total NSC values; grey and white sections represent soluble sugars and starch, respectively; error bars refer to standard errors (n = 30). Asterisk indicates a significant difference between control and defoliated trees, at *P < 0·05, according to linear mixed-effect models. Inset: NSC concentrations for roots and stems measured in a subset of control and defoliated trees (n = 5) earlier in the season (January 2010) of the first year after the defoliation.
DISCUSSION
Two years after the widespread complete defoliation event occurred, none of the defoliated trees died or showed evidence of die-back. This highlights the tolerance of Nothofagus pumilio to herbivory, a winter-deciduous species, which is supported by a previous study in which juvenile trees of the same species survived complete and chronic artificial defoliation for 3 years (Piper and Fajardo, 2014). Nonetheless, complete defoliation reduced stem growth and re-foliation for the two subsequent years following the event. Over this period, NSC concentrations in defoliated and in control (non-defoliated) trees were similar or even higher for the former, providing no support that growth reduction of N. pumilio 2 years after complete defoliation was due to C limitation. It has been suggested that C limitation may occur even when high concentrations of NSC are present in storage tissues, if trees were unable to remobilize this C (i.e. C sequestration) (Millard et al., 2007). However, this is unlikely for N. pumilio; juvenile trees of N. pumilio were able to rely strongly (i.e. remobilize) on their woody NSC and N storages to re-foliate after complete artificial defoliation (Piper and Fajardo, 2014). Further evidence that C limitation did not explain the reduced growth is seen in the response of C-based defences. Under C limitation, total polyphenols are expected to decrease because they are C costly (Herms and Mattson, 1992) (Hypothesis 1, Fig. 1). Contrary to this expectation, we found a significant increase in total polyphenol leaf concentrations by the end of the first growing season following defoliation, which is more suggestive of a C surplus than a deficit (Herms and Mattson, 1992).
We think that the lack of C limitation in N. pumilio after defoliation is probably due to its winter-deciduous leaf habit. It has been proposed that evergreen species are more prone than winter-deciduous species to become C limited after defoliation, given that the former store more C in leaves than the latter (Herms and Mattson, 1992; Krause et al., 1993). In fact, significant decreases of C storage and growth, indicative of C limitation, have been found in adult evergreen conifers 1 year after leaf loss caused by disease or defoliation (Li et al., 2002; Galiano et al., 2011; Palacio et al., 2012). In contrast, winter-deciduous trees seem to be less prone to C limitation after defoliation, probably because woody tissues serve as their main location for C storage, which are generally protected from herbivory (Millard et al., 2001). In deciduous species, neither single-season severe defoliations nor moderate chronic defoliation appear to provoke reductions in C storage (Reichenbacker et al., 1996; Kosola et al., 2001; Palacio et al., 2008). Rather, the evidence gathered so far illustrates that only when defoliation is both complete and chronic is C storage reduced in these species (Wargo et al., 1972; Piper and Fajardo, 2014).
In addition to the C limitation hypothesis, our results also do not support our second hypothesis that N limitation could impede growth recovery. Complete defoliations in N. pumilio reduced N concentrations in leaves but not in woody tissues. Woody tissues (i.e. root and trunk sapwood) are known to represent the main pool of N storage in winter-deciduous species (Millard, 1994; Grelet et al., 2001; Silla and Escudero, 2003; Millard and Grelet, 2010). This storage is thought to be an adaptation of deciduous species to severe defoliation, as it can be re-mobilized to meet demands for re-foliation and growth when root nutrient uptake fails (Millard et al., 2001; Millard and Grelet, 2010). Although our approach to estimate N storage (i.e. total N concentration) may not be as precise as others (e.g. isotopes), a previous study showed that juvenile trees of N. pumilio subjected to complete simulated defoliation over three consecutive years exhausted their N storage in woody tissues at the time that they increased their leaf N concentrations (Piper and Fajardo, 2014). In contrast, the mature trees that were naturally defoliated in this study did not show any changes in their N storage (i.e. their levels were comparable to control trees) and did not use their N reserves to re-foliate as predicted by our second hypothesis (Hypothesis 2, Fig. 1). The discrepancy between these studies is probably due to the defoliation regime: in both cases trees were completely defoliated but in the first case it was more frequent and therefore more likely to induce N limitations. Other factors could be also involved. For example, artificial defoliation may elicit different physiological responses from natural herbivory (Quentin et al., 2010; Musser et al., 2012), and the ability to remobilize NSC (and probably also N) and compensate for leaf damage may be lower in mature trees than in saplings (Boege, 2005). Also, whereas in Piper and Fajardo’s (2014) experiment leaf nutrients were exported out of the system, in the natural defoliation examined here the N contained in the insect’s frass was perhaps easily available for immediate uptake (Frost and Hunter, 2008). It may be possible that the access to frass N prevented defoliated trees reducing their leaf N concentrations below the threshold required to induce N mobilization from storage in woody tissues. The lack of support for the N limitation hypothesis as an explanation for growth reduction in defoliated trees of N. pumilio is further supported by the mismatch between trends in leaf C-based defences and trends in NSC concentrations after defoliation. If growth had been reduced as a result of N limitation, we expected that a concomitant increase in NSC and C-based defence concentrations would have occurred, as growth is more sensitive than photosynthesis to nutrient shortages and this in turn determines a C surplus (Tuomi et al., 1990; Herms and Mattson, 1992; Palacio et al., 2014) (Hypothesis 2, Fig. 1). However, we did not observe this trend in our study, suggesting that the increased concentration of total polyphenols for the first year was a result of up-regulation. Likewise, the similar concentration between defoliated and control trees for the second year, when NSC accumulated in the branches of defoliated trees, seems to reflect down-regulation of these defences.
Our scheme of a priori hypotheses leads us to two remaining mechanisms driving growth decrease in defoliated trees of N. pumilio (Fig. 1). First, the remarkable growth reduction in defoliated trees of N. pumilio may relate to a defoliation-driven preventative shift in C allocation from growth to storage (Wiley and Helliker, 2012), and possibly to defences as well (Hamilton et al., 2001) (Hypothesis 3a, Fig. 1). Preventative C allocation has been interpreted as a form of C limitation, i.e. although the tree has enough C to grow, it does not use this C in growth because it would prioritize other physiological functions. Thus, the process of growth is internally C limited. Compatible with this hypothesis, we found similar (or even higher) NSC concentrations in woody tissues of defoliated trees relative to control trees at the end of the first and second growing seasons following defoliation. Our results are also partially consistent with a defoliation-induced shift in C allocation from growth (and perhaps from storage) to defences (Hamilton et al., 2001); leaf polyphenol concentration increased for the first year but not for the second year despite C surplus in branches. Interestingly, condensed tannins actually declined after defoliation for the first year after defoliation, when total polyphenols increased (Fig. 5). This pattern may have been the result of an up-regulation of specific classes of polyphenols other than tannins at deterring the specific herbivore responsible for the outbreak in our study system. Altogether, our results are consistent with the view that tree responses of storage and defences to defoliation are highly regulated and not a mere result of C and/or N imbalances (Chapin et al., 1990; Anderegg and Callaway, 2012; Wiley and Helliker, 2012).
A second possible explanation for the remarkable growth reduction in defoliated trees of N. pumilio is that growth could be directly limited by factors other than N and C (Hypothesis 3b, Fig. 1). For example, limited bud availability has been suggested to constrain re-foliation in another winter-deciduous species, Betula pendula, subjected to browsing (Palacio et al., 2008). Although we are certain that after the defoliation event of 2009 no other pathogen or herbivore fed on trees used in this study, the possibility that the outbreak caterpillars consumed the buds that were forming at the time of defoliation cannot be discarded. Species with an indeterminate growth pattern and with buds capable of neoformed growth are expected to have a greater potential capacity for compensatory growth than those with a fixed growth pattern driven by tissue preformation (Millard et al., 2001). Shoot and foliage expansion in N. pumilio are driven mostly by preformation during the previous season, while neoformation accounts only for a low proportion of leaves and does not occur in all branches (Souza et al., 2000; Guédon et al., 2006). Thus, potential bud herbivory during the outbreak would have reduced shoot growth and re-foliation in the next growing season (i.e. 2010). Furthermore, organogenesis in N. pumilio could depend more on current photoassimilation than on storage, and hence poor re-foliation in 2010 could have limited preformation of tissues expected to expand in the next season (i.e. 2011, second year after defoliation). On the other hand, defoliation may also cause hormonal imbalances that in turn can impede or limit growth (Kulman, 1971; Boege, 2005). Leaves exert a strong hormonal control on budburst, so leaf removal may stimulate renewed growth of buds (i.e. flushing) that otherwise would break in the following season (Collin et al., 2000). This would limit the bud availability in the following season. Indeed, we observed premature budburst in defoliated trees of N. pumilio (e.g. new, green leaves were observed in autumn, Fig. 2). It has also been indicated that the utilization of photosynthates for stem growth is regulated by hormones produced in the foliage (Kulman, 1971). Consistent with this, Palacio et al. (2012) suggested that defoliation in Pinus nigra reduced the levels of indole-3-acetic-acid near the cambial region, leading to decreased import of photoassimilates and eventually to reduced radial growth. Under such conditions of growth limitation, however, defoliated trees should have concomitantly increased their NSC and N storage and C-based defences (Hypothesis 3a, Fig. 1), which was not the case here: among the tissues examined, a slight increase in NSC was found only for branches in the second year, while total polyphenols increased in the first year (i.e. both increases were not concomitant).
CONCLUSIONS
Currently, a growing debate centres on whether extreme disturbances associated with climate change (e.g. defoliation, drought) provoke C or growth limitations in trees (Sala et al., 2010; McDowell et al., 2011; Wiley and Helliker, 2012). The classical approach to distinguish between these two mechanisms has been to assess plant NSC, which has been recently recognized as imperfect and incomplete (Millard and Grelet, 2010; Sala et al., 2012; Wiley and Helliker, 2012; Palacio et al., 2014). Our study provides a more integrative approach to evaluating plant growth limitations in response to disturbance, by examining major resources other than C (e.g. N), and other C sinks besides storage and growth (e.g. defences and re-foliation). In doing so, we show that the significant reduction in growth of N. pumilio in response to herbivory was not caused by insufficient C or N availability, as suggested by several studies. By doing this, we were not only able to discard C limitation (as traditionally defined, i.e. insufficient C availability for growth), but also N limitation, which have been proposed as a major cause of growth limitation in trees affected by defoliation. We propose that the growth reduction in defoliated trees of a deciduous species, such as N. pumilio, may relate to other factors that can limit growth (e.g. hormonal disruption), or, alternatively, to a highly regulated C and nutrient conservation strategy (i.e. preventative allocation) driven by a defoliation-induced shift in allocation priorities that is compatible with C and N limitation in spite of non-reduced levels of C and N storage. We finally suggest that these allocation shifts reduce leaf palatability (lower leaf N concentration, higher polyphenol concentrations) over the seasons following the defoliation to repel potential new defoliators and allow trees to more quickly replenish NSC and nutrient stores. Large-scale severe defoliation events, such as the one we describe in our study, are increasingly reported in ecosystems around the world, with many of them associated with global warming or other environmental change factors (van Mantgem et al., 2009). Our study highlights that a more integrative plant physiological approach is needed to understand how tree growth is regulated in response to disturbance or environmental change.
ACKNOWLEDGEMENTS
This study was supported by the Dirección de Investigación y Desarrollo, Universidad Austral de Chile, through the project DID S-2010-67. Additional support came from the Chilean Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) Grant 11121175 to F.I.P. and Grant 1120171 to A.F., and also from ICM P05-002. We thank Professor John Marshall for valuable comments on the manuscript, Juan Llancabure, Pablo Bravo, Beth Roskilly and Jonathan Riquelme for their help in the field and the lab, and Soraya Villagrán (CIEP) for the analysis of N concentrations.
LITERATURE CITED
- Alberdi M, Romero M, Ríos D, Wenzel H. 1985. Altitudinal gradients of seasonal frost resistance in Nothofagus communities of southern Chile. Acta Oecologica 6: 21–30. [Google Scholar]
- Anderegg WRL, Callaway ES. 2012. Infestation and hydraulic consequences of induced carbon starvation. Plant Physiology 159: 1866–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaroux C, Bréda N. 2002. Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiology 22: 1201–1210. [DOI] [PubMed] [Google Scholar]
- Boege K. 2005. Influence of plant ontogeny on compensation to leaf damage. American Journal of Botany 92: 1632–1640. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. 2010. Insects at not so low temperature: Climate change in the temperate zone and its biotic consequences . In Denlinger DL, Lee RE, eds. Low temperature biology of insects. Cambridge: Cambridge University Press, 242–275. [Google Scholar]
- Canham CD, Kobe RK, Latty EF, Chazdon RL. 1999. Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121: 1–11. [DOI] [PubMed] [Google Scholar]
- Chapin FS. 1980. Nutrient allocation and responses to defoliation in tundra plants. Arctic and Alpine Research 12: 553–563. [Google Scholar]
- Chapin FS, Schulze ED, Mooney HA. 1990. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21: 423–447. [Google Scholar]
- Chow PS, Landhäusser SM. 2004. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiology 24: 1129–1136. [DOI] [PubMed] [Google Scholar]
- Collin P, Epron D, Alaoui-Sossé B, Badot PM. 2000. Growth responses of common ash seedlings (Fraxinus excelsior L.) to total and partial defoliation. Annals of Botany 85: 317–323. [Google Scholar]
- Dale VH, Joyce LA, McNulty S, et al. 2001. Climate change and forest disturbances. BioScience 51: 723–734. [Google Scholar]
- Dickson RE. 1989. Carbon and nitrogen allocation in tree. Annals of Forest Science 46: 631–647. [Google Scholar]
- Fajardo A, Piper FI, Hoch G. 2013. Similar variation in carbon storage between deciduous and evergreen treeline species across elevational gradients. Annals of Botany 112: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost CJ, Hunter MD. 2008. Insect herbivores and their frass affect Quercus rubra leaf quality and initial stages of subsequent litter decomposition. Oikos 117: 13–22. [Google Scholar]
- Galiano L, Martínez-Vilalta J, Lloret F. 2011. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytologist 190: 750–759. [DOI] [PubMed] [Google Scholar]
- Garibaldi LA, Kitzberger T, Ruggiero A. 2011. Latitudinal decrease in folivory within Nothofagus pumilio forests: dual effect of climate on insect density and leaf traits? Global Ecology and Biogeography 20: 609–619. [Google Scholar]
- Grelet GA, Alexander IJ, Proe MF, Frossard JS, Millard P. 2001. Leaf habit influences nitrogen remobilization in Vaccinium species. Journal of Experimental Botany 52: 993–1002. [DOI] [PubMed] [Google Scholar]
- Guédon Y, Puntieri JG, Sabatier S, Barthélémy D. 2006. Relative extents of preformation and neoformation in tree shoots: analysis by a deconvolution method. Annals of Botany 98: 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundale M, Sverker J, Albrectsen B, Nilsson M-C, Wardle D. 2010. Variation in protein complexation capacity among and within six plant species across a boreal forest chronosequence. Plant Ecology 211: 253–266. [Google Scholar]
- Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR. 2001. The carbon–nutrient balance hypothesis: its rise and fall. Ecology Letters 4: 86–95. [Google Scholar]
- Herms DA, Mattson WJ. 1992. The dilemma of plants: to grow or defend. The Quarterly Review of Biology 67: 283–335. [Google Scholar]
- Hevia F, Minoletti OML, Decker KL, Boerner RE. 1999. Foliar nitrogen and phosphorus dynamics of three Chilean Nothofagus (Fagaceae) species in relation to leaf lifespan. American Journal of Botany 86: 447–455. [PubMed] [Google Scholar]
- Hoch G, Richter A, Körner C. 2003. Non-structural carbon compounds in temperate forest trees. Plant, Cell & Environment 26: 1067–1081. [Google Scholar]
- Jones CG, Hartley SE. 1999. A protein competition model of phenolic allocation. Oikos 86: 27–44. [Google Scholar]
- Körner C. 2003. Carbon limitation in trees. Journal of Ecology 91: 4–17. [Google Scholar]
- Kosola KR, Dickmann DI, Paul EA, Parry D. 2001. Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia 129: 65–74. [DOI] [PubMed] [Google Scholar]
- Krause S, Raffa K, Wagner M. 1993. Tree responses to stress: a role in sawfly outbreaks? In: Wagner M, Raffa K. eds. Sawfly life history adaptations to woody plants . San Diego: Academic Press, 211–227. [Google Scholar]
- Kulman HM. 1971. Effects of insect defoliation on growth and mortality of trees. Annual Review of Entomology 16: 289–324. [Google Scholar]
- Li M, Hoch G, Körner C. 2002. Source/sink removal affects mobile carbohydrates in Pinus cembra at the Swiss treeline. Trees – Structure and Function 16: 331–337. [Google Scholar]
- Lovett GM, Christenson LM, Groffman PM, Jones CG, Hart JE, Mitchell MJ. 2002. Insect defoliation and nitrogen cycling in forests. BioScience 52: 335–341. [Google Scholar]
- Mazía N, Chaneton EJ, Dellacanonica C, Dipaolo L, Kitzberger T. 2012. Seasonal patterns of herbivory, leaf traits and productivity consumption in dry and wet Patagonian forests. Ecological Entomology 37: 193–203. [Google Scholar]
- McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M. 2011. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends in Ecology & Evolution 26: 523–532. [DOI] [PubMed] [Google Scholar]
- Millard P. 1994. Measurement of the remobilization of nitrogen for spring leaf growth of trees under field conditions. Tree Physiology 14: 1049–1054. [DOI] [PubMed] [Google Scholar]
- Millard P, Grelet GA. 2010. Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiology 30: 1083–1095. [DOI] [PubMed] [Google Scholar]
- Millard P, Hester A, Wendler R, Baillie G. 2001. Interspecific defoliation responses of trees depend on sites of winter nitrogen storage. Functional Ecology 15: 535–543. [Google Scholar]
- Millard P, Sommerkorn M, Grelet GA. 2007. Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytologist 175: 11–28. [DOI] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Lee HK, DesRochers BL, Williams SA, Vogel H. 2012. Caterpillar labial saliva alters tomato plant gene expression. Journal of Chemical Ecology 38: 1387–1401. [DOI] [PubMed] [Google Scholar]
- Nabeshima E, Murakami M, Hiura T. 2001. Effects of herbivory and light conditions on induced defense in Quercus crispula. Journal of Plant Research 114: 403–409. [Google Scholar]
- Palacio S, Hester AJ, Maestro M, Millard P. 2008. Browsed Betula pubescens trees are not carbon-limited. Functional Ecology 22: 808–815. [Google Scholar]
- Palacio S, Hernández R, Maestro-Martínez M, Camarero JJ. 2012. Fast replenishment of initial carbon stores after defoliation by the pine processionary moth and its relationship to the re-growth ability of trees. Trees 26: 1627–1640. [Google Scholar]
- Palacio S, Hoch G, Sala A, Körner C, Millard P. 2014. Does carbon storage limit tree growth? New Phytologist 201: 1096–1100. [DOI] [PubMed] [Google Scholar]
- Paritsis J, Veblen TT. 2010. Dendroecological analysis of defoliator outbreaks on Nothofagus pumilio and their relation to climate variability in the Patagonian Andes. Global Change Biology 17: 239–253. [Google Scholar]
- Parker J, Patton RL. 1975. Effects of drought and defoliation on some metabolites in roots of black oak seedlings. Canadian Journal of Forest Research 5: 457–463. [Google Scholar]
- Piper FI, Fajardo A. 2014. Foliar habit, tolerance to defoliation and their link to carbon and nitrogen storage. Journal of Ecology 102: 1101–1111. [Google Scholar]
- Popp M, Lied W, Meyer AJ, Richter A, Schiller P, Schwitte H. 1996. Sample preservation for determination of organic compounds: microwave versus freeze-drying. Journal of Experimental Botany 47: 1469–1473. [Google Scholar]
- Porter LJ, Hrstich LN, Chan BG. 1985. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25: 223–230. [Google Scholar]
- Quentin A, Pinkard E, Beadle C, et al. 2010. Do artificial and natural defoliation have similar effects on physiology of Eucalyptus globulus Labill. seedlings? Annals of Forest Science 67: 203. [Google Scholar]
- Reichenbacker RR, Schultz RC, Hart ER. 1996. Artificial defoliation effect on Populus growth, biomass production, and total nonstructural carbohydrate concentration. Environmental Entomology 25: 632–642. [Google Scholar]
- Rose AH. 1958. The effect of defoliation on foliage production and radial growth of quaking aspen. Forest Science 4: 335–342. [Google Scholar]
- Rose R, Rose CL, Omi SK, Forry KR, Durall DM, Bigg WL. 1991. Starch determination by perchloric acid vs enzymes: evaluating the accuracy and precision of six colorimetric methods. Journal of Agricultural and Food Chemistry 39: 2–11. [Google Scholar]
- Saffell BJ, Meinzer FC, Woodruff DR, et al. 2014. Seasonal carbohydrate dynamics and growth in Douglas-fir trees experiencing chronic, fungal-mediated reduction in functional leaf area. Tree Physiology 34: 218–228. [DOI] [PubMed] [Google Scholar]
- Sala A, Piper FI, Hoch G. 2010. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytologist 186: 274–281. [DOI] [PubMed] [Google Scholar]
- Sala A, Woodruff DR, Meinzer FC. 2012. Carbon dynamics in trees: feast or famine? Tree Physiology 32: 764–775. [DOI] [PubMed] [Google Scholar]
- Silla F, Escudero A. 2003. Uptake, demand and internal cycling of nitrogen in saplings of Mediterranean Quercus species. Oecologia 136: 28–36. [DOI] [PubMed] [Google Scholar]
- Souza MS, Puntieri JG, Barthélémy D, Brion C. 2000. Bud content and its relation to shoot size and structure in Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae). Annals of Botany 85: 547–555. [Google Scholar]
- Stern JL, Hagerman A, Steinberg P, Winter F, Estes J. 1996. A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. Journal of Chemical Ecology 22: 1273–1293. [DOI] [PubMed] [Google Scholar]
- Stokes MA, Smiley TL. 1996. An introduction to tree-ring dating. Tucson, AZ: University of Arizona Press. [Google Scholar]
- Sundqvist MK, Wardle DA, Olofsson E, Giesler R, Gundale MJ. 2012. Chemical properties of plant litter in response to elevation: subarctic vegetation challenges phenolic allocation theories. Functional Ecology 26: 1090–1099. [Google Scholar]
- Tschaplinski TJ, Blake TJ. 1994. Carbohydrate mobilization following shoot defoliation and decapitation in hybrid poplar. Tree Physiology 14: 141–151. [DOI] [PubMed] [Google Scholar]
- Tuomi J, Niemelä P, Sirén S. 1990. The panglossian paradigm and delayed inducible accumulation of foliar phenolics in mountain birch. Oikos 59: 399–410. [Google Scholar]
- van Mantgem PJ, Stephenson NL, Byrne JC, et al. 2009. Widespread increase of tree mortality rates in the western United States. Science 323: 521–524. [DOI] [PubMed] [Google Scholar]
- Wargo PM, Parker J, Houston DR. 1972. Notes: starch content in roots of defoliated sugar maple. Forest Science 18: 203–204. [Google Scholar]
- Webb WL. 1981. Relation of starch content to conifer mortality and growth loss after defoliation by the Douglas-fir Tussock moth. Forest Science 27: 224–232. [Google Scholar]
- Wiley E, Helliker B. 2012. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytologist 195: 285–289. [DOI] [PubMed] [Google Scholar]