Abstract
Background and Aims With the exception of angiosperms, the main euphyllophyte lineages (i.e. ferns sensu lato, progymnosperms and gymnosperms) had evolved laminate leaves by the Late Devonian. The evolution of laminate leaves, however, remains unclear for early-diverging ferns, largely represented by fern-like plants. This study presents a novel fern-like taxon with pinnules, which provides new insights into the early evolution of laminate leaves in early-diverging ferns.
Methods Macrofossil specimens were collected from the Upper Devonian (Famennian) Wutong Formation of Anhui and Jiangsu Provinces, South China. A standard degagement technique was employed to uncover compressed plant portions within the rock matrix.
Key Results A new fern-like taxon, Shougangia bella gen. et sp. nov., is described and represents an early-diverging fern with highly derived features. It has a partially creeping stem with adventitious roots only on one side, upright primary and secondary branches arranged in helices, tertiary branches borne alternately or (sub)oppositely, laminate and usually lobed leaves with divergent veins, and complex fertile organs terminating tertiary branches and possessing multiple divisions and numerous terminal sporangia.
Conclusions Shougangia bella provides unequivocal fossil evidence for laminate leaves in early-diverging ferns. It suggests that fern-like plants, along with other euphyllophyte lineages, had independently evolved megaphylls by the Late Devonian, possibly in response to a significant decline in atmospheric CO2 concentration. Among fern-like plants, planate ultimate appendages are homologous with laminate pinnules, and in the evolution of megaphylls, fertile organs tend to become complex.
Keywords: Adventitious root, atmospheric CO2, euphyllophytes, fern-like plants, ferns sensu lato, fertile organ, growth habit, Late Devonian, leaf evolution, monilophyte, Shougangia bella, stem, Wutong Formation
INTRODUCTION
Euphyllophytina, the main clade of vascular plants, includes several extinct stem groups, moniliformopses and radiatopses (Kenrick and Crane, 1997). Moniliformopses or monilophytes (Pryer et al., 2004) refer to ferns, sphenopsids and their fossil relatives (i.e. ferns sensu lato), while radiatopses represent progymnosperms and spermatophytes (seed plants). The terms moniliformopses and monilophytes have been commonly used but essentially can be regarded as simply a synonym for ‘fern’ (Christenhusz and Chase, 2014). Early-diverging ferns include Middle Devonian–Early Carboniferous iridopteridaleans, pseudosporochnaleans, non-pseudosporochnaleans and rhacophytaleans, Late Devonian–Carboniferous stauropteridaleans, and sphenophyllaleans (sphenopsids traced to the Famennian of Late Devonian). Except for sphenophyllaleans, these groups without foliar-borne sporangia are called fern-like plants (Soria and Meyer-Berthaud, 2005; Taylor et al., 2009; Galtier, 2010).
Early Devonian Eophyllophyton has been placed at the most basal position within the euphyllophytes (Kenrick and Crane, 1997; Hao and Xue, 2013a, b), and its lobed megaphyll has been interpreted as a rare example of early adaptation to high atmospheric CO2 concentration (Hao et al., 2003; Osborne et al., 2004). After a long delay, the rapid drop of CO2 levels during the Middle to Late Devonian is proposed to have led to the initial widespread occurrence of laminate leaves of euphyllophytes in the Late Devonian and Early Carboniferous (Beerling et al., 2001). In the process of evolving megaphylls, the planation (i.e. transformation from three- to two-dimensional ultimate appendages) is followed by lamination (i.e. webbing in planate appendages) (Zimmermann, 1952). Leaf evolution of ferns with foliar-borne sporangia, and of radiatopses, has been studied or summarized in detail (Boyce and Knoll, 2002; Osborne et al., 2004; Phillips and Galtier, 2005; Sanders et al., 2009; Galtier, 2010; Corvez et al., 2012). Early-diverging ferns with planate appendages are central to our understanding of the evolution of laminate leaves (Corvez et al., 2012). However, fern-like plants, usually with terminal sporangia on branches, rarely bear laminate leaves. In this plant group, the homology between laminate leaves and planate appendages remains unclear, and the evolution of fertile organs is poorly known.
Here, we report a new fern-like plant, Shougangia bella gen. et sp. nov., from the Late Devonian in China. Known from vegetative and fertile morphology and growth habit, Shougangia provides definite evidence of laminate leaves for fern-like plants. The evolution of planate ultimate appendages toward laminate pinnules within fern-like plants is discussed in the background of CO2 and fertile organs.
MATERIAL AND METHODS
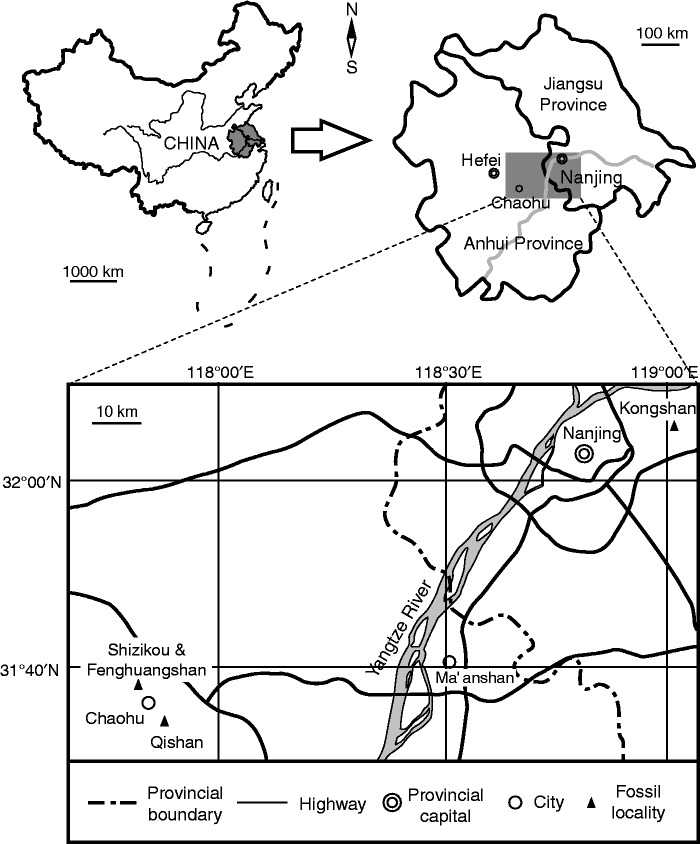
The fossils of Shougangia bella gen. et sp. nov. were recovered from two localities in the Upper Devonian Wutong (Wutung) Formation of China. Over 300 specimens were collected from a hill named Qishan (GPS data: 31°35′04′′N, 117°54′23′′E), Chaohu City, Anhui Province (Fig. 1). About 80 specimens were obtained from Kongshan (32°4′37′′N, 119°1′24′′E), Jiangning County, Nanjing City, Jiangsu Province (Fig. 1). At both the Qishan and the Kongshan sections, the Wutong Formation includes the Guanshan Member lacking fossils and overlying fossiliferous Leigutai Member. At these localities, the Guanshan and Leigutai Members are characterized, respectively, by quartzose sandstone and by inter-beds of sandstone and silty mudstone (Cai et al., 1988; Hou and Qi, 2006). In the Chaohu area are several sections, such as Shizikou, Fenghuangshan and Qishan, which yield Late Devonian plants (Fig. 1). The Qishan section at Yafu County is represented in several quarries, where locals have excavated mudstone or clay for manufacturing pottery. Since 2006, we have visited this locality more than 15 times, keeping pace with the progress of clay exposure. Fieldwork at the Kongshan section has been systematically conducted by many geologists since the 1960s, with numerous plants including this new taxon continuously obtained.
Fig. 1.
Maps showing localities of Qishan and Kongshan, where the fossil plant Shougangia bella gen. et sp. nov. was collected.
At the Qishan section, the Guanshan Member is mainly covered by Quaternary sediments, and the exposed lower part of the Leigutai Member is 14 m thick. Here, Shougangia bella occurs at the lower to middle part of the Leigutai Member and near the horizon bearing two sphenopsids, Hamatophyton verticillatum (Wang and Guo, 2009) and Eviostachya sp. (Fig. 2). The plant bed is about 3·0 m thick and extends laterally for about 10 m.
Fig. 2.
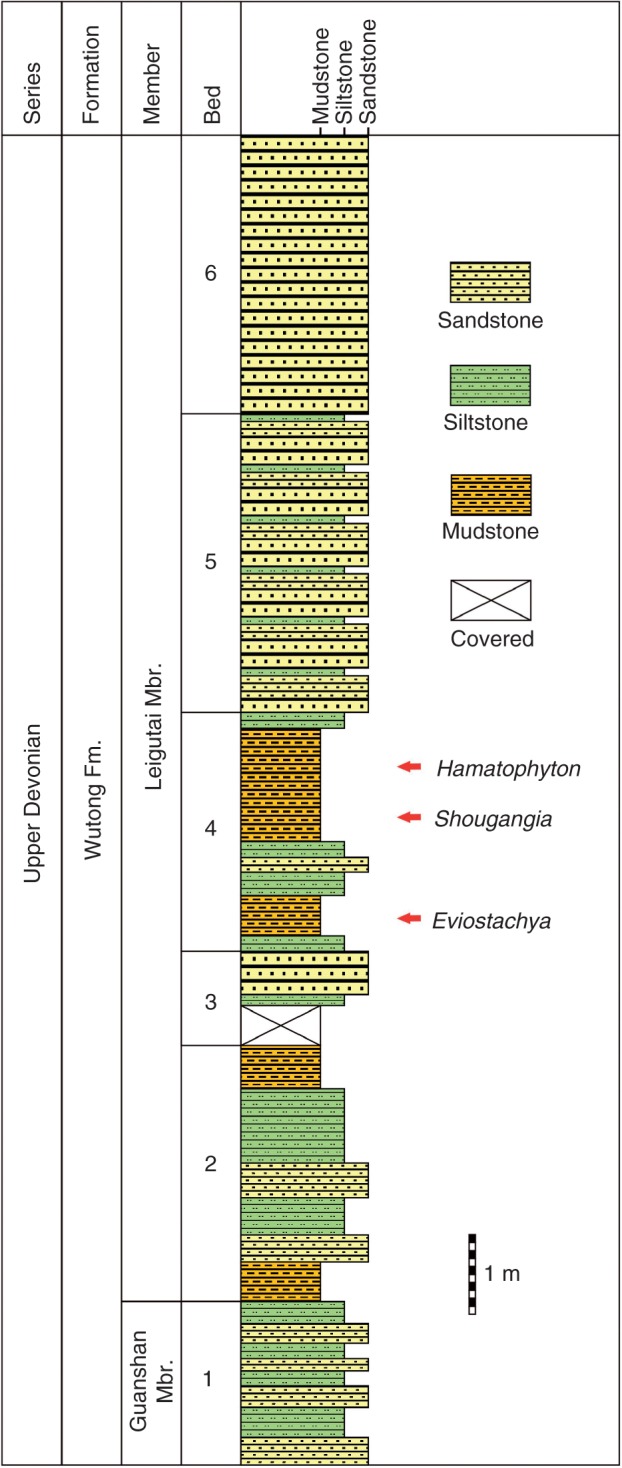
Stratigraphic column at Qishan section, Chaohu City, Anhui Province, China, showing the bed within the Upper Devonian (Famennian) Leigutai Member that preserves plants including Shougangia bella gen. et sp. nov.
At the Kongshan section, the Guanshan Member and lower to middle part of the Leigutai Member are 81·7 and 84 m thick, respectively (see stratigraphic column in Cai et al., 1988). At this section, the lower to middle part of the Leigutai Member contains seven plant beds of various thicknesses, in ascending order: (1) progymnosperm Archaeopteris sp., Eviostachya hoegii, fern-like plant Rhacophyton? sp. and sphenopsid Sphenophyllum lungtanense (2·7 m thick); (2) Hamatophyton verticillatum (5·2 m); (3) lycopsid Sublepidodendron? sp. (6·0 m); (4) Hamatophyton verticillatum (20 m); (5) lycopsid Leptophloeum rhombicum (3·4 m); (6) Hamatophyton verticillatum, and two lycopsids – Sublepidodendron sp. and Stigmaria sp. (9·2 m); (7) Archaeopteris (3·7 m). Shougangia occurs at the basal part (the first plant bed) of the Leigutai Member where Eviostachya hoegii (Wang, 1993) and the seed plant Kongshania synangioides (Wang, 2000) have been described. Cai et al. (1988) tentatively recognized limited fertile remains as ‘Rhacophyton? sp.’ in the first plant bed, but morphological comparison leads us to conclude that these remains represent Shougangia bella.
Among the plants from the Qishan and Kongshan sections, Hamatophyton is widespread in Famennian deposits of South China (Wang and Guo, 2009) and Eviostachya is also present in coeval flora of Belgium (Leclercq, 1957). Leptophloeum rhombicum represents a Late Devonian element that is distributed worldwide (Cai et al., 1988; Wang et al., 2005). The Wutong Formation with Guanshan and Leigutai Members occurs in many localities of Anhui, Jiangsu and Zhejiang Provinces. In the Chaohu area of Anhui, the middle part of the Leigutai Member contains the LH spore assemblage (Retispora lepidophyta var. minor and Apiculiretusispora hunanensis) (Hou and Qi, 2006). Retispora lepidophyta has also been found in the middle part of the Leigutai Member, Kongshan section (Cai et al., 1988). On the basis of data from several localities, this spore assemblage at the lower to middle part of the Leigutai Member is interpreted as Famennian in age (Fa2d) (Chen and Ouyang, 1987; Ouyang, 2000).
Sedimentary and geochemical characteristics indicate that the Upper Devonian Wutong Formation in the above three provinces represents littoral rather than terrestrial deposits (Zhu et al., 1999). Plants, fish, conchostracans and trace fossils from the Leigutai Member in the Chaohu area and at the Kongshan section support this conclusion (Cai et al., 1988; Hou and Qi, 2006).
Shougangia is preserved as impressions and compressions in yellow–grey or red–purple silty mudstone. Many axes, leaves and fertile organs are coloured red, giving great contrast to the rock matrix. Under a microscope, the plant morphology was exposed with steel needles. A serial dégagement technique was used in some cases to show the complex branches within the fertile organs.
SYSTEMATICS
Order and Family: Incertae sedis
Genus: Shougangia D.M. Wang et al. gen. nov.
Type: Shougangia bella D.M. Wang et al. sp. nov.
Generic diagnosis.
Adventitious roots occurring on only one side of horizontal portions of slender stem, with upright primary branches borne helically. Primary branches bearing secondary branches in irregular helices. Tertiary branches arranged alternately or (sub)oppositely, possessing basal fan-shaped pinnules in pairs, lateral alternate or subopposite Sphenopteris-like pinnules with three to six lobes, and either a terminal pinnule with two to four lobes or a terminal fertile organ. Fertile secondary branches also bearing basal fan-shaped pinnules. Pinnules laminate, with veins proximally parallel and then dichotomous. Fertile organs complex, three-dimensionally dichotomous up to ten times and terminating in numerous sporangia. Sporangia paired, elongate and distally tapered.
Etymology.
The generic name is dedicated to Professor Shou-Gang Hao (Peking University, Beijing) for his outstanding contributions to the study of early vascular plants.
Shougangia bella D.M. Wang et al. sp. nov.
(Figs 3A–D, 4A–J, 5A–E, 6A–F, 7A–G, 8A–I, 9A–C, 10A–G, 11A–E)
Fig. 3.
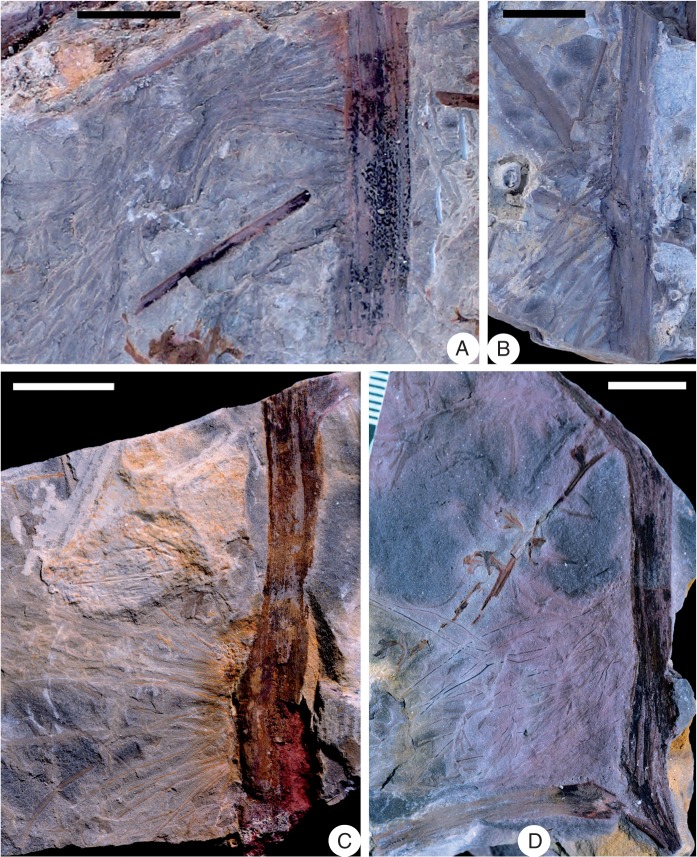
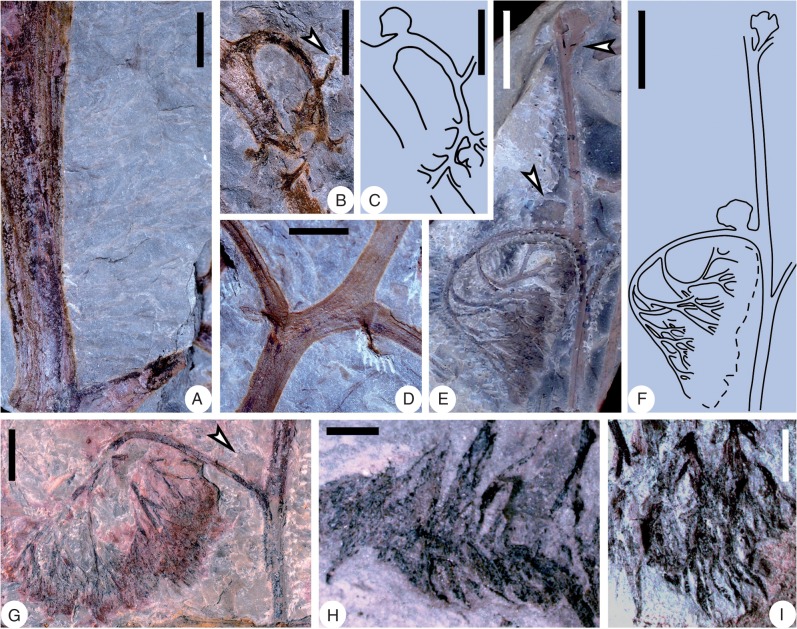
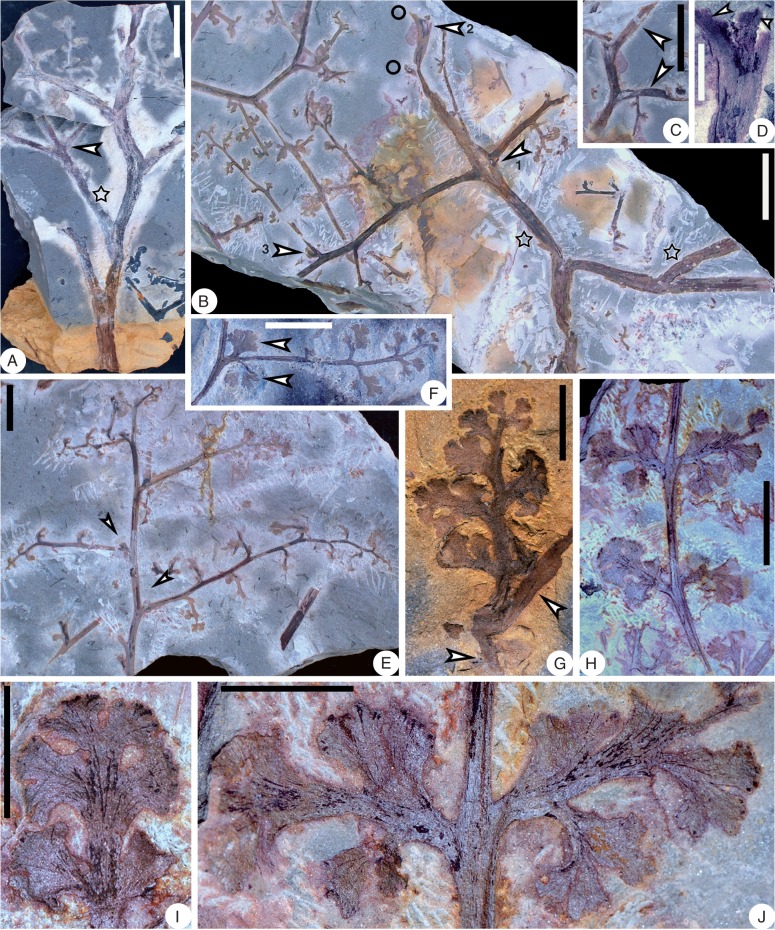
Shougangia bella gen. et sp. nov. from Chaohu City, Anhui Province, China. (A–D) Stems with one side bearing adventitious roots, and showing several longitudinal grooves and ridges (PKUB14248, PKUB14247, PKUB14310, PKUB14313). (D) Primary branch and roots appearing to depart from the stem in the same direction, due to compression during fossilization. Scale bars = 1 cm.
Fig. 5.
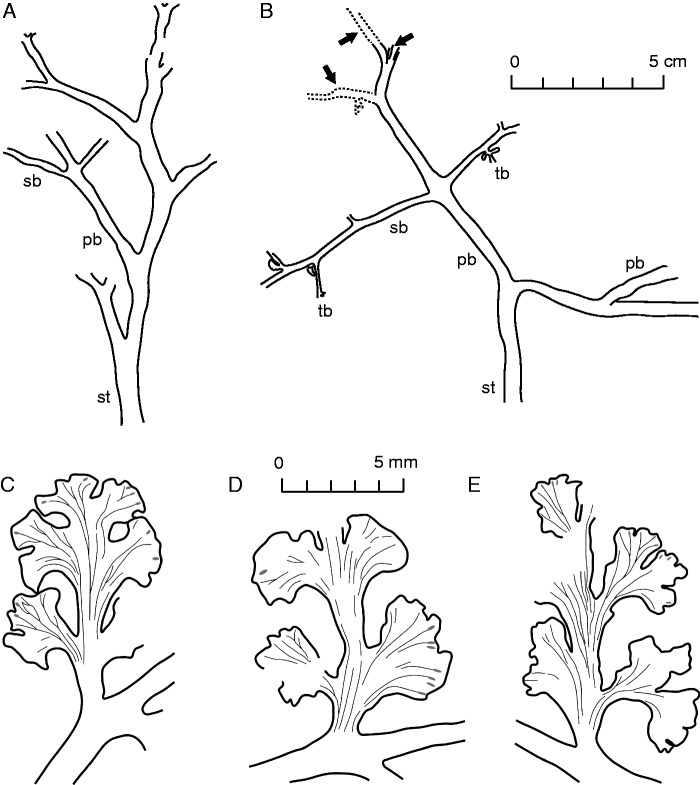
Shougangia bella gen. et sp. nov. (A) Line drawing of Fig. 4A. (B) Combined line drawing of Fig. 4B–D showing three secondary branches (arrows). Dotted lines represent portions in Fig. 4C. (C–E) Line drawings of Fig. 4I and J, showing three pinnules with veins. st, stem; pb, primary branch; sb, secondary branch; tb, tertiary branch. Scale bars = (A, B) 5 cm, (C–E) 5 mm.
Fig. 6.
Shougangia bella gen. et sp. nov. from Chaohu City, Anhui Province (A, B) and from Jiangning County, Nanjing City, Jiangsu Province (C–F), China. (A–D) Secondary branches with tertiary branches bearing pinnules (PKUB14307, PKUB14301b, PB21950, PB21951). (A) Paired pinnules at base of tertiary branch (arrow). (D) Single pinnule at base of tertiary branch (arrow). (E) Enlargement of D (arrow). (F) Line drawing of E. Scale bars = (A) 1 cm, (B–D) 5 mm, (E, F) 2 mm.
Fig. 7.
Shougangia bella gen. et sp. nov. from Chaohu City, Anhui Province, China. (A, B) Part and counterpart of holotype showing stem, primary (stars), secondary and tertiary branches, and fertile organs (PKUB14229a, b). (A) One primary branch (arrow 1), tertiary branch with terminal fertile organ (arrow 2), tertiary branch incomplete distally (arrow 3), secondary branch (arrow 4), and pinnules at base of secondary branches (arrows 5 and 6). (B) Two tertiary branches with terminal fertile organs (arrows 1 and 2), secondary branch (arrow 3), and pinnule at base of secondary branch (arrow 4). (C) Enlargement of A (arrow 4) showing basal and lateral pinnules (arrows). (D) Enlargement of B (arrow 4) showing pinnule (arrow). (E–G) Enlargement of two tertiary branches in A (arrow 2) and B (arrows 1 and 2), respectively, showing pinnules and fertile organs. (E, F) Part and counterpart. (E, G) Lateral (upper arrows) and basal (lower arrows) pinnules partly covered by fertile organ. Scale bars = (A, B) 2 cm, (C–G) 5 mm.
Fig. 8.
Shougangia bella gen. et sp. nov. from Chaohu City, Anhui Province, China. (A) Enlargement of lower part of Fig. 7B showing adventitious roots on one side of stem. (B) Enlargement of Fig. 7B (arrow 3), arrow indicating base of a probable tertiary branch. (C) Line drawing of B. (D) Enlargement of Fig. 7A (arrows 5 and 6). (E) Secondary branch bearing three tertiary branches. A tertiary branch with basal pinnule (lower arrow) and terminal fertile organ. Pinnule at base of another tertiary branch (upper arrow) (PKUB14213). (F) Line drawing of E. (G) Tertiary branch with a basal pinnule (arrow) and terminal fertile organ (PKUB14206). (H, I) Sporangia within fertile organs (PKUB14210, PKUB14211). Scale bars = (A–D, G) 5 mm, (E, F) 1 cm, (H, I) 2 mm.
Fig. 9.
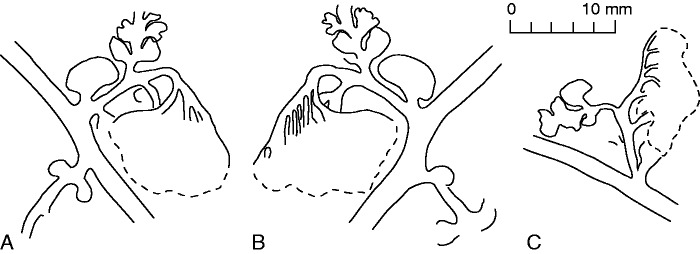
Shougangia bella gen. et sp. nov. (A–C) Line drawings of Fig. 7E–G, respectively.
Fig. 10.
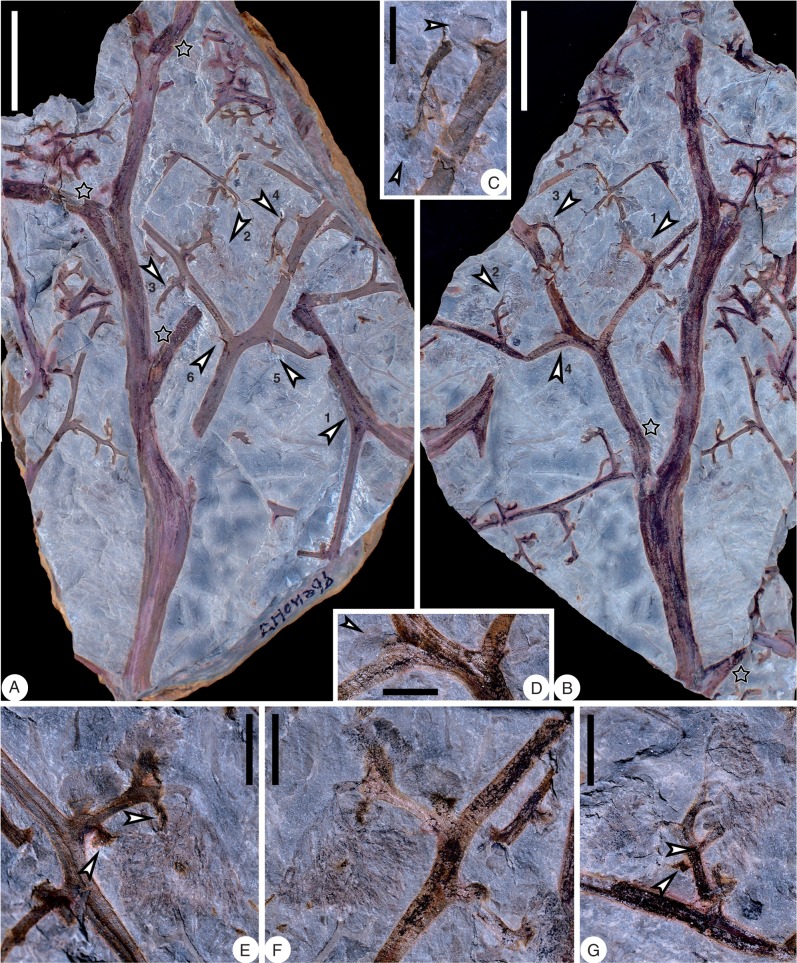
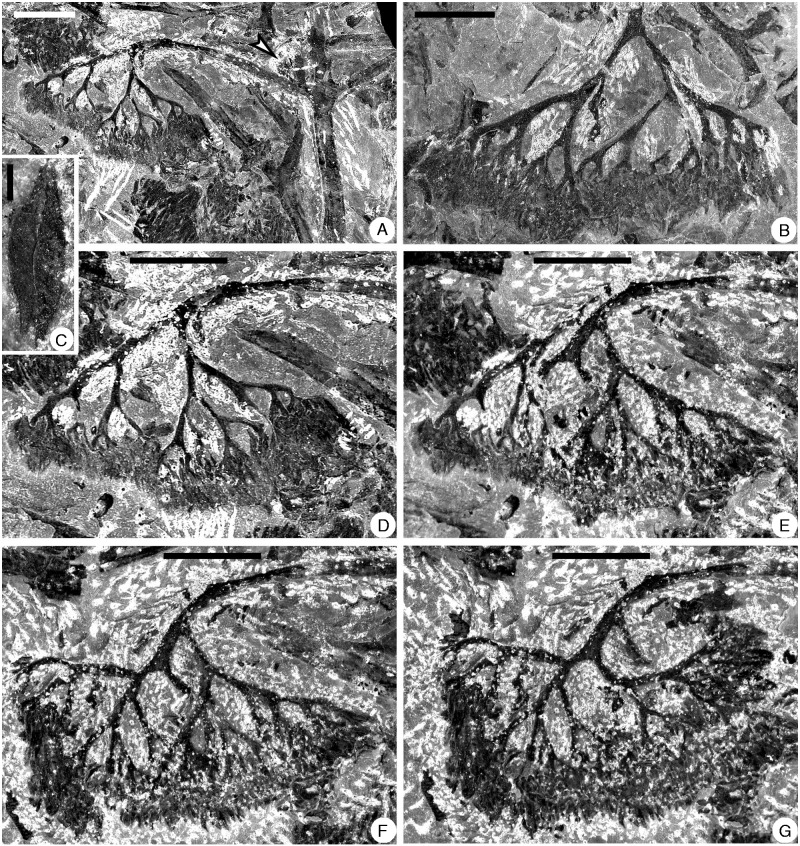
Shougangia bella gen. et sp. nov. from Jiangning County, Nanjing City, Jiangsu Province, China. (A) Secondary branch with two tertiary branches, one of which bears a basal pinnule (arrow) and terminal fertile organ (PB21952). (B) Detached fertile organ (PB21953). (C) Sporangia in a pair (PB21954). (D–G) Serial dégagement of the fertile organ in A. Scale bars = (A, B, D–G) 5 mm, (C) 0·5 mm.
Fig. 11.
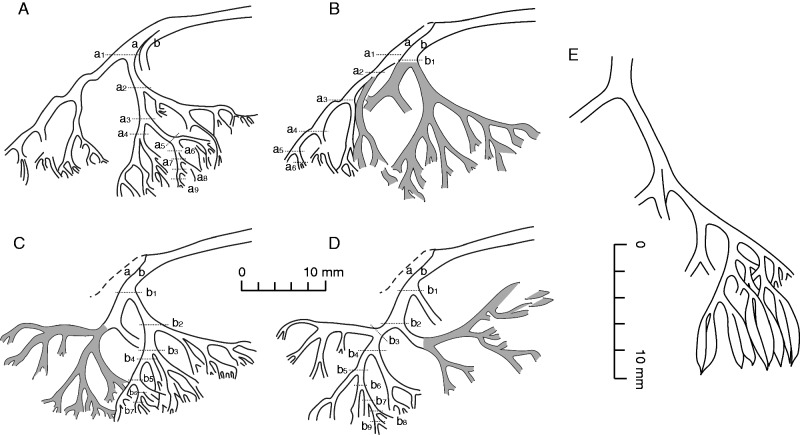
Shougangia bella gen. et sp. nov. (A–D) Line drawings of Fig. 10D–G, respectively. a, b, two major axes within fertile organ; a1–9, b1–9, numbers of dichotomies of two major axes, respectively; shaded area, recovered axes following the former stage of dégagement. (E) Line drawing of right part of fertile organ in Fig. 10B, showing dichotomy and terminal elongate sporangia in pairs.
Specific diagnosis.
As in the generic diagnosis. Stems up to 152 mm long and 4·5–7·8 mm wide, with adventitious roots departing at 60–90°. Primary branches up to 83 mm long and 2·6–4·8 mm wide; secondary branches up to 65 mm long and 1·0–4·0 mm wide; and tertiary branches 8·8–65 mm long and 0·4–1·6 mm wide. Basal pinnules 2·6–4·8 mm long and 2·8–4·8 mm wide and uneven at margin. Lateral pinnules of tertiary branches 2·8–14 mm long and 2·3–9·2 mm wide, with number of lobes decreasing acropetally. Terminal pinnules 1·5–2·6 mm long and 2·8–3·8 mm wide. Fertile organs pendulous, 7·5–16 mm long and 12–22·8 mm wide; axes comprising fertile organs 0·1–0·5 mm wide. Sporangia 2·7–3·8 mm long and 0·4–0·7 mm wide.
Etymology.
The specific epithet is from the Latin bellus, beautiful, for the beautifully preserved laminate pinnules that characterize this plant.
Holotype.
PKUB14229a, b (designated here; part and counterpart of a specimen; Fig. 7A, B), from Qishan section, Chaohu City, Anhui Province, China.
Paratypes.
PKUB14213, PKUB14227, PKUB14232a, b, PKUB14234, PKUB14248, PB21952, PB21953 (designated here; Figs 3A, 4B, C, E, H, 8E, 10A, B).
Fig. 4.
Shougangia bella gen. et sp. nov. from Chaohu City, Anhui Province, China. (A) Stem with one primary branch (star) bearing two secondary branches (arrow) (PKUB14233a). (B) Markedly curved stem with primary, secondary and tertiary branches. One primary branch (right star) on stem. Another primary branch (left star) bearing two secondary branches (arrow 1) and bases of three additional secondary branches (arrow 2, circles). A pinnule at the base of a tertiary branch (arrow 3). Arrow 2 indicates the part enlarged in D (PKUB14232a). (C) Counterpart of portion in B (arrow 2, circles) showing primary branch bearing two secondary branches (arrows) which correspond to branch bases in B (circles) (PKUB14232b). (D) Enlargement of part in B (arrow 2) showing base of a secondary branch (left arrow) and base of another secondary branch extending into the rock matrix (right arrow). (E) Secondary branch with tertiary branches bearing basal (arrows), lateral and terminal pinnules (PKUB14234). (F) Tertiary branch with a pair of basal pinnules (arrows), and lateral and terminal pinnules (PKUB14231). (G) Secondary branch (upper arrow), base of a tertiary branch (lower arrow) and another tertiary branch above with laminate and lobed pinnules (PKUB14203). (H) Tertiary branch with laminate and lobed pinnules (PKUB14227). (I, J) Enlargement of lower left and upper parts of H, respectively, showing pinnules with veins. Scale bars = (A–C) 2 cm, (E, F, H) 1 cm, (D, G, I, J) 5 mm.
Localities and horizon.
Qishan in Chaohu City, Anhui Province, China; and Kongshan in Nanjing City, Jiangsu Province, China; Wutong Formation, Upper Devonian (Famennian).
Repository.
The holotype and other specimens prefixed ‘PKUB’ (Figs 3, 4, 6A, B, 7, 8A, B, D, E, G–I) are deposited at Peking University, Beijing, China; specimens prefixed ‘PB’ (Figs 6C–E, 10) are housed at Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China.
RESULTS
Measurements of vegetative and fertile morphology are given in Table 1.
Table 1.
Measurements of morphology of Shougangia bella gen. et sp. nov.
| Plant organ/tissue | Length (mm) | Width (mm) |
|---|---|---|
| Stems and roots | ||
| Stems | up to 152 | 4·5–7·8 |
| Root mats | up to 60 | 13·2–33 |
| Individual root | 0·4–1·0 | |
| Vascular bundle in root | approx. 0·1 | |
| Vegetative branches, laminate pinnules on vegetative tertiary branches | ||
| Primary branches | up to 83 | 3·2–4·8 |
| Secondary branches | up to 65 | 1·0–2·5 |
| Tertiary branches | 13·2–65 | 0·4–1·0 |
| Basal pinnules | 2·6–4·8 | 2·8–4·8 |
| Lateral pinnules | 2·8–14 | 2·3–9·2 |
| Terminal pinnules | 1·5–2·6 | 2·8–3·8 |
| Pinnule veins | approx. 0·04 | |
| Glands terminating pinnule veins | 0·2–0·3 | 0·1–0·2 |
| Fertile branches and organs, laminate pinnules on fertile tertiary branches | ||
| Primary branches | up to 68 | 2·6–3·8 |
| Secondary branches | up to 50 | 1·5–4·0 |
| Tertiary branches | 8·8–18·8 | 0·8–1·6 |
| Basal pinnules | approx. 3·0 | approx. 4·7 |
| Lateral pinnules | 5·3–7·0 | 4·2–5·0 |
| Fertile organs | 7·5–16 | 12–22·8 |
| Axes within fertile organs | 0·1–0·5 | |
| Sporangia | 2·7–3.8 | 0·4–0.7 |
Stems and roots
Stems bear roots (Fig. 3), primary branches (Figs 4A, B, 5A, B) or both (Figs 7A, B, 8A). Adventitious roots depart from one side of the rhizomatous stem at 60–90° to form a dense mat (Figs 3, 7A, B, 8A). The stems lack spines or other ornamentation, have a few wide grooves and narrow ridges extending longitudinal, and sometimes curve (Figs 3D, 4B, 5B) or present a zigzag shape (Figs 4A, 5A). Probably owing to preservation, the roots sometimes curve (Fig. 3A) or extend in different directions (Fig. 3C). They are not branched and lack root hairs. An individual root contains a single vascular bundle (Fig. 3C, D).
Vegetative branches and laminate pinnules
A primary branch and roots occur on one side of the stem (Fig. 3D). Primary branches are borne helically at 35–90° on the stem (Figs 4A, B, 5A, B). The primary branch (Fig. 4A, star) is beneath the overlying stem and other three primary branches, and is attached at 45–90° by two opposite secondary branches (Fig. 4A, arrow). One primary branch departs from the front side of the stem (Fig. 4B, right star), below which occurs another primary branch (Fig. 4B, left star). On the latter primary branch, two secondary branches are oppositely placed at 90° (Fig. 4B, arrow 1). Further along this primary branch, three secondary branches are closely arranged (Figs 4B, arrow 2 and circles, C, arrows, 5B, arrows), with one extending into the rock matrix (Fig. 4B, arrow 2, D, right arrow). Therefore, secondary branches are borne in an irregular helix. Tertiary branches are borne at 70–90°, and are alternately (Figs 4B, E, 6A) or suboppositely arranged (Fig. 4G). They are readily recognized by the characteristic pinnules (Figs 4B, E–H, 6A–D).
One pinnule (Figs 4B, arrow 3, E, arrows, 6B, D, arrow) or paired pinnules (Figs 4F, arrows, 6A, arrow, C) are present at the base of tertiary branches. Lateral pinnules are alternately or suboppositely arranged (Figs 4B, E–H, 6A–D). A single pinnule terminates the tertiary branches (Figs 4B, E–G, 6A). Pinnules are laminate and variously lobed. Basal pinnules are fan-shaped and uneven at the margin. Lateral pinnules are Sphenopteris-like and three- to six-lobed. Terminal pinnules are two- to four-lobed. One lateral pinnule, however, is fan-shaped (Fig. 6B). On a tertiary branch, the lobe number of lateral pinnules decreases acropetally (Fig. 4G). Pinnules at the lower part of tertiary branches may have what appears to be a ‘central axis’ (Figs 4E, 6C, D). Veins of pinnules appear parallel proximally (Figs 4J, left part, 5D, 6E, F), then dichotomize up to four times (Figs 4I, 5C) and finally terminate near the margin of leaf lobes rather than sinuses (Figs 4I, J, 5C–E, 6E, F). Some veins end in oval-like bodies, possibly representing glands or hydathodes.
Fertile branches and organs
Details of fertile branches are visible in part and counterpart of the holotype, which show five primary branches born helically at 45–90° (Fig. 7A, stars, B, stars). One primary branch (Fig. 7A, arrow 1) beneath another has been partially exposed through dégagement. Secondary branches depart at 60–90°. Three secondary branches are borne irregularly (potentially helically), with two bearing tertiary branches (Fig. 7A, arrows 2 and 3, B, arrows 1 and 2), and with one possibly being undeveloped and pendulous probably due to preservation (Fig. 7A, arrow 4, B, arrow 3). This possibly undeveloped secondary branch seems to show the base of a tertiary branch (Fig. 8B, arrow, C). Three tertiary branches are borne at 60–90°, with two being arranged alternately (Fig. 7A, arrows 2 and 3) and one singly (Fig. 7B, arrow 2). Among them, two branches are complete (Fig. 7A, arrow 2, B, arrow 2). One (Fig. 8G) or two opposite (Fig. 10A) or three alternate (Fig. 8E, F) tertiary branches occur at 45–90° on secondary branches.
One or two fan-shaped pinnules are present at the base of three secondary branches (Figs 7A, arrows 5 and 6, B, arrow 4, C, upper arrow, D, arrow, 8D), or several fragmentary pinnules along a secondary branch (Figs 7C, lower arrow, 8B, C). A pair of basal pinnules and two suboppositely arranged lateral pinnules occur on each tertiary branch (Figs 7A, arrow 3, E–G, 9). Paired basal pinnules, with one partly covered by a fertile organ (Fig. 7E, lower arrow, G, lower arrow), are fan-shaped, whereas two lateral pinnules, with one also partly covered by a fertile organ (Fig. 7E, upper arrow, G, upper arrow), are Sphenopteris-like and six-lobed. Sometimes, only one pinnule is visible at the base of a tertiary branch (Figs 8G, arrow, E, arrows, 10A, arrow).
A pendulous and complex fertile organ terminates each tertiary branch (Figs 7E–G, 8E–G, 9, 10A), and it may be sometimes detached (Fig. 10B). One fertile organ (Fig. 10A) was treated using serial dégagement to show the multiple dichotomies in three dimensions (Figs 10D–G, 11A–D). The first dichotomy results in two major branches, each of which may then dichotomize up to nine times (Fig. 11A–D, a1–9, b1–9). The width of branches within the fertile organ decreases slightly with each dichotomy. The angles between two daughter axes are 30–90°. If all axes are equally dichotomous in this single fertile organ, the number of ultimate axes would be 210=1024, and as sporangia are borne in pairs at the tips of the ultimate axes, there would be as many as 2048 elongate and distally tapered sporangia.
COMPARISONS
Table 2 presents a summary of comparisons between Shougangia and related plant groups or genera of Late Palaeozoic age. Comparisons include vegetative branching pattern, laminate leaves, planate vegetative ultimate appendages, basal aphlebiae and fertile organs (fertile ultimate appendages).
Table 2.
Comparisons of Shougangia gen. et sp. nov. with related plants
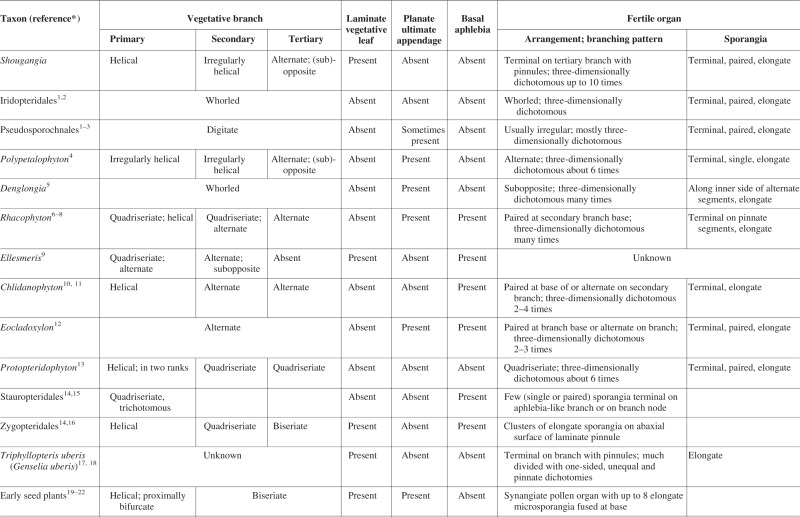 |
*References: 1, Berry and Stein (2000); 2, Meyer-Berthaud et al. (2007); 3, Leclercq and Banks (1962) ; 4, Hilton et al. (2003); 5, Xue and Hao (2008); 6, Leclercq (1951); 7, Andrews and Phillips (1968); 8, Cornet et al. (1976); 9, Hill et al. (1997); 10, Gensel (1973); 11, Hilton (1999); 12, Berry and Wang (2006); 13, Li and Hsü (1987); 14, Taylor et al. (2009); 15, Wang (2003); 16, Phillips and Galtier (2005); 17, Skog and Gensel (1980); 18, Knaus (1995); 19, Galtier (1988); 20, Stewart and Rothwell (1993); 21, Wang et al. (2014); 22, Wang and Liu (2015).
Iridopteridales, Pseudosporochnales and non-pseudosporochnaleans
Cladoxylopsida comprises Iridopteridales and Pseudosporochnales (Berry and Stein, 2000). In another scenario, this class consists of Pseudosporochnales and an informal group of non-pseudosporochnaleans with taxa of unclear affinities at the ordinal level (Meyer-Berthaud et al., 2007). Shougangia resembles iridopteridaleans and most Pseudosporochnales and non-pseudosporochnaleans in having three-dimensionally dichotomous fertile branches terminated by elongate and paired sporangia, but fertile organs of Shougangia are unusual in that they terminate short tertiary branches bearing pinnules.
Laminate leaves are absent in iridopteridaleans [morphologically known genera such as Anapaulia (Berry and Edwards, 1996), Compsocradus (Fu et al., 2011), Ibyka (Skog and Banks, 1973) and possible iridopteridalean Metacladophyton (Wang and Lin, 2007)], Pseudosporochnales [Calamophyton (Leclercq and Andrews, 1960), Lorophyton (Fairon-Demaret and Li, 1993), Pseudosporochnus (Leclercq and Banks, 1962) and Wattieza (Berry, 2000)] and non-pseudosporochnaleans [e.g. Denglongia (Xue and Hao, 2008) and Polypetalophyton (Hilton et al., 2003)].
Whorled organs (branches and ultimate appendages) typify all iridopteridaleans but are absent in Shougangia. Pseudosporochnaleans with digitate branching are also distinguished from Shougangia. Late Devonian (Frasnian) Polypetalophyton with four orders of branches (Hilton et al., 2003) is very similar to Shougangia in the vegetative branching pattern; however, each fertile organ of Polypetalophyton is simpler and bears fewer sporangia (approx. 32). Shougangia and Late Devonian (Frasnian) Denglongia (Xue and Hao, 2008) share complex fertile organs which are pendulous and dichotomize into two major axes followed by many divisions. However, the vegetative branches of Denglongia occur in whorls; two major axes of fertile organs bear pinnate segments with each dichotomizing several times.
Rhacophyton and possibly related plants
Rhacophytales (sensu Taylor et al., 2009) consists of Chlidanophyton (Gensel, 1973), Ellesmeris (Hill et al., 1997), Eocladoxylon (Berry and Wang, 2006), Protocephalopteris (Schweitzer, 1968), Protopteridophyton (Li and Hsü, 1987) and Rhacophyton (Andrews and Phillips, 1968). Chlidanophyton, Eocladoxylon and Protopteridophyton are of unknown affinity at the ordinal level because they combine the morphological and/or anatomical features of multiple plant groups (Gensel, 1973; Li and Hsü, 1987; Berry and Wang, 2006). Here we consider these three genera as fern-like plants with terminal sporangia on branches. Based on branching pattern and stelar structure, Ellesmeris is regarded either as the earliest zygopterid fern (Hill et al., 1997) or as a fern-like taxon included within Rhacophytales (Phillips and Galtier, 2005; Taylor et al., 2009; Galtier, 2010). Where known, the fertile organs of the above plants, excluding Rhacophyton, possess elongate and sometimes paired sporangia terminating three-dimensionally dichotomous axes. However, fertile organs of Shougangia terminate tertiary branches with pinnules. Furthermore, Rhacophyton and related plants have a distinctive quadriseriate branching pattern (alternate pairs of branches) and/or bear dichotomous aphlebiae at the base of branches, and, except for Ellesmeris, they lack laminate pinnules.
The Late Devonian (Famennian) Rhacophyton ceratangium (Andrews and Phillips, 1968; Cornet et al., 1976) has paired fertile organs located at the base of quadriseriate secondary branches. Each fertile organ dichotomizes several times to form many short and lateral segments terminated by dense masses of sporangia. Nevertheless, fertile organs of Shougangia are neither paired nor basal, but rather are terminal on tertiary branches. Sphenopteris-like laminate pinnules of Late Devonian (Frasnian) Ellesmeris (Hill et al., 1997) resemble those of Shougangia, but have fewer lobes and are arranged on secondary branches, as opposed to tertiary branches in Shougangia.
Stauropteridales
Stauropteridales (sensu Taylor et al., 2009) is represented by the Late Devonian Gillespiea (Erwin and Rothwell, 1989) as well as Carboniferous Multifurcatus (Wang, 2003), Rowleya (Long, 1976) and Stauropteris (Eggert, 1964). These differ from Shougangia in branching pattern, aphlebiae at the branch base, lack of laminate pinnules and simple fertile organs.
Zygopterid ferns
Shougangia and Carboniferous–Permian Zygopteridales [e.g. some species of Zygopteris (Phillips and Galtier, 2005)] share horizontal stems, upright primary branches (fronds or phyllophores or petioles in Zygopteridales) borne helically and laminate vegetative pinnules. However, fronds of many zygopterids possess quadriseriate and/or biseriate pinnae, sporangia are foliar-borne, and aphlebiae occur on stems or fronds or primary pinnae (Phillips and Galtier, 2005).
Triphyllopteris and early seed plants
Shougangia resembles Early Carboniferous Triphyllopteris uberis of uncertain affinity, in having fertile branches with laminate and lobed vegetative pinnules borne suboppositely, and a recurved and complex terminal fertile organ with sporangia of similar shape and size. Leaf veins of both plants are dichotomous and end near pinnule margins. Nevertheless, the branches of T. uberis are up to 130 mm long and 3·0–4·5 mm wide, pinnules are 10–40 mm long, and fertile organs are larger (approx. 80 mm long and 40 mm wide) and more closely resemble synangiate pollen organs (Skog and Gensel, 1980). This plant has been renamed as Genselia uberis, and the fertile organs interpreted as pollen organs of seed plants or sporangial masses of progymnosperms (Knaus, 1995). Notably, the Carboniferous spermatophyte (seed fern) Polycalyx laterale has Triphyllopteris-type foliage terminated by cupulate ovules (Vega and Archangelsky, 2001).
Early spermatophytes (e.g. Late Devonian Elkinsia) often have their fronds borne helically on the stem (Stewart and Rothwell, 1993), which conforms to the arrangement of primary branches in Shougangia. However, early seed plants are characterized by bipartite fronds with a proximally bifurcate rachis (Galtier, 1988). In contrast, the primary branches of Shougangia do not bifurcate proximally. As summarized by Wang et al. (2014), the spermatophyte pollen organs known from the Late Devonian are simple synangia often found in association with ovules. Furthermore, the microsporangia of early spermatophyte pollen organs are not borne in pairs. An individual fertile organ of Shougangia may have hundreds or even thousands of sporangia that are not basally fused, but are paired. Ovules have not been found in our large collection of over 380 specimens recovered over many years of meticulous fieldwork.
DISCUSSION
Affinity
Shougangia possesses fertile organs with three-dimensionally dichotomous axes bearing terminal elongate and paired sporangia, a feature also found in fern-like plants including iridopteridaleans, most pseudosporochnaleans, non-pseudosporochnaleans and possibly Rhacophyton-like taxa (Chlidanophyton, Eocladoxylon and Protopteridophyton). However, this newly described plant cannot be assigned to Iridopteridales with whorled organography, or to Pseudosporochnales with digitate branching. It differs from Ellesmeris, Protocephalopteris, Protopteridophyton, Rhacophyton and Stauropteridales, which typically have a quadriseriate branching pattern, and it differs significantly from zygopterid ferns characterized by foliar-borne sporangia and quadriseriate pinnae. Shougangia is then tentatively left as a fern-like genus of uncertain affinity at the order level, and is considered a derived taxon because of laminate pinnules and complex fertile organs. Amongst fern-like plants, Shougangia is more similar in branching pattern to Polypetalophyton, which has been described from the Late Devonian of China and allied with non-pseudosporochnaleans within Cladoxylopsida.
Growth habit
The stem of Shougangia may be prostrate where adventitious roots occur only on one side and at a wide angle (Figs 3, 7A, B). Other portions of the same stem lacking roots imply an upturned and erect part (Fig. 7A, B). Long stems without any roots indicate an upright habit (Fig. 4A, B). The branching angles of primary branches also provide some evidence of habit, as they depart at about 90° from horizontal portions of stem (Figs 3D, 7A, B), but at 30–90° from upright portions (Figs 4A, B, 7A, B). The primary branch (Figs 3D, 7A, B), probably originally perpendicular or attached at a wide angle to the stem, may have been compressed so that the branch and roots seem to occur from the stem in the same direction. Because of its narrow stem and multiple orders of upright branches, the roots of Shougangia may function to anchor and support the plant body. Primary and secondary branches borne helically perhaps reduce self-shading of tertiary branches with pinnules and enhance the mechanical stability of the whole shoot system.
Evolution of laminate leaves and fertile organs
The emergence of laminate leaves, with an enhanced photosynthetic capability, is an evolutionary innovation in land plants and may represent a response to a major decline of atmospheric CO2 levels during the Late Devonian (Beerling et al., 2001; Osborne et al., 2004). Early-diverging euphyllophytes usually have three-dimensionally dichotomous ultimate appendages rather than laminate leaves. Nevertheless, three lineages of euphyllophytes appear to have undergone convergent evolution, resulting in laminate leaves by the Late Devonian (Boyce and Knoll, 2002; Galtier, 2010): progymnosperms such as Archaeopteris (Taylor et al., 2009); spermatophytes such as Elkinsia and Kongshania (Serbet and Rothwell, 1992; Wang, 2000); and sphenopsids such as Xihuphyllum and Sphenophyllum (Chen, 1988; Wang et al., 2008). Shougangia clearly demonstrates both laminate pinnules and fertile organs characterizing fern-like plants. Another Late Devonian plant, Ellesmeris, also bears laminate pinnules, but unfortunately to the best of our knowledge there are no reports of fertile organs. Shougangia thus exhibits unequivocal evidence of laminate leaves for fern-like plants. This taxon and Ellesmeris indicate that the fourth lineage (fern-like plants largely representing early-diverging ferns) evolved laminate leaves similar to other early-diverging euphyllophytes. Therefore, laminate leaves appear to have independently evolved at least four times in euphyllophytes prior to the end of the Devonian.
The telome theory (Zimmermann, 1952) has long been accepted as orthodoxy for explaining megaphyllous leaf evolution. This theory holds that leaf evolution depends on essential processes of overtopping (anisotomous branching leading to determinate and three-dimensional ultimate appendages), subsequent planation, and final lamination. Molecular and cellular data recognize plausible mechanisms for overtopping and planation, but limited evidence for lamination (Beerling and Fleming, 2007). New data on fern-like plants are emerging to provide substantial support for the transformation of planation toward lamination. From the Middle (Givetian) to Late Devonian (Famennian), the planation of vegetative ultimate appendages (sometimes coupled with three-dimensional appendages) occurred to different degrees and repeatedly in fern-like plants (Pseudosporochnus nodosus, Denglongia, Polypetalophyton, Eocladoxylon and Rhacophyton), finally giving rise to fully laminate leaves (Shougangia, Ellesmeris). Additionally, during the evolutionary development of laminate leaves from homologous planate appendages, fertile organs of these plants trend toward increasing complexity. Each fertile ultimate appendage of Givetian Eocladoxylon and Pseudosporochnus nodosus divides only twice or three times (Leclercq and Banks, 1962; Berry and Wang, 2006). That of Frasnian Polypetalophyton and Denglongia divides five or six times (Hilton et al., 2003; Xue and Hao, 2008). Each fertile organ of Famennian Rhacophyton (Andrews and Phillips, 1968) and Shougangia has numerous divisions and hundreds or thousands of sporangia. The fertile organ of Shougangia terminates a branch that also bears pinnules, a highly derived character among early-diverging ferns. In light of geological age, branching pattern, planate ultimate appendages (resembling Sphenopteridium-type pinnules) and less complex fertile organs, the Frasnian Polypetalophyton may be the best candidate for the ancestor of Shougangia. These Devonian taxa suggest that early-diverging ferns present co-evolution of vegetative and fertile organs.
CONCLUSIONS
Shougangia is known from stems that are either prostrate with adventitious roots or upright and bear primary to tertiary branches, pinnules and fertile organs, and represents a derived taxon of fern-like plants. Shougangia reveals that fern-like plants, representing a major part of early-diverging ferns, had evolved laminate leaves during the Late Devonian, possibly coincident with rapidly declining levels of atmospheric CO2. In this evolutionary scenario, their laminate pinnules are homologous with planate ultimate appendages, and fertile organs show an evolutionary increase in complexity and in the number of internal divisions and terminal sporangia.
ACKNOWLEDGEMANETS
We thank Professor James Basinger and Dr Yi Zhang for important discussions, and Dun-Lun Qi, Yi Wang, Pu Huang, Li Cui and Zhen-Zhu Wan for help with fieldwork. This study was supported by the National Basic Research Program of China (grant number 2012CB821900) of the Ministry of Science and Technology of China, and the National Natural Science Foundation of China (grant numbers 41172007 and 41272001).
LITERATURE CITED
- Andrews HN, Phillips TL. 1968. Rhacophyton from the Upper Devonian of West Virginia. Botanical Journal of the Linnean Society 61: 37–64. [Google Scholar]
- Beerling DJ, Fleming AJ. 2007. Zimmermann’s telome theory of megaphyll leaf evolution: a molecular and cellular critique. Current Opinion in Plant Biology 10: 1–9. [DOI] [PubMed] [Google Scholar]
- Beerling DJ, Osborne CP, Chaloner WG. 2001. Evolution of leaf-form in land plants linked to atmospheric CO2 decline in the Late Palaeozoic era. Nature 410: 352–354. [DOI] [PubMed] [Google Scholar]
- Berry CM. 2000. A reconsideration of Wattieza Stockmans (here attributed to Cladoxylopsida) based on a new species from the Devonian of Venezuela. Review of Palaeobotany and Palynology 112: 125–146. [DOI] [PubMed] [Google Scholar]
- Berry CM, Edwards D. 1996. Anapaulia moodyi gen. et sp. nov.: a probable iridopteridalean compression fossil from the Devonian of western Venezuela. Review of Palaeobotany and Palynology 93: 127–145. [Google Scholar]
- Berry CM, Stein WE. 2000. A new iridopteridalean from the Devonian of Venezuela. International Journal of Plant Sciences 161: 807–827. [Google Scholar]
- Berry CM, Wang Y. 2006. Eocladoxylon (Protopteridium) minutum (Halle) Koidzumi from the Middle Devonian of Yunnan, China: an early Rhacophyton-like plant? International Journal of Plant Sciences 167: 551–566. [Google Scholar]
- Boyce CK, Knoll AH. 2002. Evolution of developmental potential and the multiple independent origins of leaves in Paleozoic vascular plants. Paleobiology 28: 70–100. [Google Scholar]
- Cai CY, Lu LC, Wu XY, Zhang GF. 1988. Devonian biostratigraphy of Lower Yangtze Peneplatform in Jiangsu. In: Academy of Geological Sciences, Jiangsu Bureau of Petroleum Prospecting, Nanjing Institute of Geology and Palaeontology, Academia Sinica, eds. Sinian–Triassic biostratigraphy of the Lower Yangtze Peneplatform in Jiangsu region. Nanjing: Nanjing University Press, 169–217. [Google Scholar]
- Chen QS. 1988. Fossil plants Sphenophyllales from Late Devonian Xihu Formation in Xiaoshan, Zhejiang. Acta Palaeontologica Sinica 27: 404–415. [Google Scholar]
- Chen YX, Ouyang S. 1987. A complementary study on megaspores from Devonian–Carboniferous transition in Jurong, Jiangsu. Acta Palaeontologica Sinica 26: 435–448. [Google Scholar]
- Christenhusz MJM, Chase MW. 2014. Trends and concepts in fern classification. Annals of Botany 113: 571–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet B, Phillips TL, Andrews HN. 1976. The morphology and variation in Rhacophyton ceratangium from the Upper Devonian and its bearing on frond evolution. Palaeontographica Abteilung B 158: 105–129. [Google Scholar]
- Corvez A, Barriel V, Dubuisson JY. 2012. Diversity and evolution of the megaphyll in Euphyllophytes: phylogenetic hypotheses and the problem of foliar organ definition. Comptes Rendus Palevol 11: 403–418. [Google Scholar]
- Eggert DA. 1964. The question of the phylogenetic position of the Coenopteridales. Memoirs of the Torrey Botanical Club 21: 38–57. [Google Scholar]
- Erwin DM, Rothwell GW. 1989. Gillespiea randolphensis gen. et sp. nov. (Stauropteridales), from the Upper Devonian of West Virginia. Canadian Journal of Botany 67: 3063–3077. [Google Scholar]
- Fairon-Demaret M, Li CS. 1993. Lorophyton goense gen. et sp. nov. from the Lower Givetian of Belgium and a discussion of the Middle Devonian Cladoxylopsida. Review of Palaeobotany and Palynology 77: 1–22. [Google Scholar]
- Fu Q, Wang Y, Berry CM, Xu HH. 2011. Complex branching patterns in a newly recognized species of Compsocradus Berry et Stein (Iridopteridales) from the Middle Devonian of North Xinjiang, China. International Journal of Plant Sciences 172: 707–724. [Google Scholar]
- Galtier J. 1988. Morphology and phylogenetic relationships of early ptridosperms. In: Beck CB, ed. Origin and evolution of gymnosperms . New York: Columbia University Press, 135–176. [Google Scholar]
- Galtier J. 2010. The origins and early evolution of the megaphyllous leaf. International Journal of Plant Sciences 171: 641–661. [Google Scholar]
- Gensel PG. 1973. A new plant from the Lower Mississippian of Southwestern Virginia. Palaeontographica Abteilung B 142: 137–153. [Google Scholar]
- Hao SG, Xue JZ. 2013a. The Early Devonian Posongchong flora of Yunnan — A contribution to an understanding of the evolution and early diversification of vascular plants . Beijing: Science Press. [Google Scholar]
- Hao SG, Xue JZ. 2013b. Earliest record of megaphylls and leafy structures, and their initial diversification. Chinese Science Bulletin 58: 2784–2793. [Google Scholar]
- Hao SG, Beck CB, Wang DM. 2003. Structure of the earliest leaves: adaptations to high concentrations of atmospheric CO2. International Journal of Plant Sciences 164: 71–75. [Google Scholar]
- Hill SA, Scheckler SE, Basinger JF. 1997. Ellesmeris sphenopteroides, gen. et sp. nov., a new zygopterid fern from the Upper Devonian (Frasnian) of Ellesmere, N.W.T., Arctic Canada. American Journal of Botany 84: 85–103. [Google Scholar]
- Hilton J. 1999. A Late Devonian plant assemblage from the Avon Gorge, west England: taxonomic, phylogenetic and stratigraphic implications. Botanical Journal of the Linnean Society 129: 1–54. [Google Scholar]
- Hilton J, Geng BY, Kenrick P. 2003. A novel Late Devonian (Frasnian) woody cladoxylopsid from China. International Journal of Plant Sciences 164: 793–805. [Google Scholar]
- Hou MJ, Qi DL. 2006. New views on the Devonian–Carboniferous boundary stratigraphy of the Chaohu Region, Anhui Province. Journal of Stratigraphy 30: 157–170. [Google Scholar]
- Kenrick P, Crane PR. 1997. The origin and early diversification of land plants: a cladistic study . Washington, DC: Smithsonian Institute Press. [Google Scholar]
- Knaus MJ. 1995. The species of the Early Carboniferous fossil plant genus Genselia. International Journal of Plant Sciences 156: 61–92. [Google Scholar]
- Leclercq S. 1951. Étude morphologique et anatomique d’une fougère du Dévonien Supérieur, le Rhacophyton zygopteroides nov. sp. Annales de la Société Géologique de Belgique 9: 1–62. [Google Scholar]
- Leclercq S. 1957. Etude d’une fructification de Sphenopsida à structure conservée du Devonian supérieur. Mémoires, L’Académie Royal de Belgique (Sciences) 14: 1–39. [Google Scholar]
- Leclercq S, Andrews HN. 1960. Calamophyton bicephalum, a new species from the Middle Devonian of Belgium. Annals of the Missouri Botanical Garden 47: 1–23. [Google Scholar]
- Leclercq S, Banks HP. 1962. Pseudosporochnus nodosus sp. nov., a Middle Devonian plant with Cladoxylalean affinities. Palaeontographica Abteilung B 110: 1–34. [Google Scholar]
- Li CS, Hsü J. 1987. Studies on a new Devonian plant Protopteridophyton devonicum assigned to primitive fern from South China. Palaeontographica Abteilung B 207: 111–131. [Google Scholar]
- Long AG. 1976. Rowleya trifurcata gen. et sp. nov., a simple petrified vascular plant from the Lower Coal Measures (Westphalian A) of Lancashire. Transactions of the Royal Society of Edinburgh 69: 467–481. [Google Scholar]
- Meyer-Berthaud B, Soria A, Young GC. 2007. Reconsidering differences between Cladoxylopsida and Iridopteridales: evidence from Polyxylon australe (Upper Devonian, New South Wales, Australia). International Journal of Plant Sciences 168: 1085–1097. [Google Scholar]
- Osborne CP, Beerling DJ, Lomax BH, Chaloner WG. 2004. Biophysical constraints on the origin of leaves inferred from the fossil record. Proceedings of the National Academy of Sciences, USA 101: 10360–10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S. 2000. Succession of Late Palaeozoic palynological assemblages in Jiangsu. Journal of Stratigraphy 24: 230–235. [Google Scholar]
- Phillips TL, Galtier J. 2005. Evolutionary and ecological perspectives of Late Paleozoic ferns Part I. Zygopteridales. Review of Palaeobotany and Palynology 135: 165–203. [Google Scholar]
- Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R. 2004. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. American Journal of Botany 91: 1582–1598. [DOI] [PubMed] [Google Scholar]
- Sanders H, Rothwell GW, Wyatt SE. 2009. Key morphological alternations in the evolution of leaves. International Journal of Plant Sciences 170: 860–868. [Google Scholar]
- Schweitzer HJ. 1968. Pflanzenreste aus dem Devon Nord-Westspitzbergens. Palaeontographica Abteilung B 123: 43–75. [Google Scholar]
- Serbet R, Rothwell GW. 1992. Characterizing the most primitive seed ferns. I. A reconstruction of Elkinsia polymorpha. International Journal of Plant Sciences 153: 602–621. [Google Scholar]
- Skog JE, Banks HP. 1973. Ibyka amphikoma, gen. et sp. n., a new protoarticulate precursor from the late Middle Devonian of New York State. American Journal of Botany 60: 366–380. [Google Scholar]
- Skog JE, Gensel PG. 1980. A fertile species of Triphyllopteris from the Early Carboniferous (Mississippian) of southwestern Virginia. American Journal of Botany 67: 440–451. [Google Scholar]
- Soria A, Meyer-Berthaud B. 2005. Reconstructing the Late Devonian cladoxylopsid Pietzschia schulleri from new specimens from southeastern Morocco. International Journal of Plant Sciences 166: 857–874. [Google Scholar]
- Stewart WN, Rothwell GW. 1993. Paleobotany and the evolution of plants, 2nd edn Cambridge: Cambridge University Press. [Google Scholar]
- Taylor TN, Taylor EL, Krings M. 2009. Paleobotany: the biology and evolution of fossil plants, 2nd edn Burlington: Academic Press. [Google Scholar]
- Vega JC, Archangelsky S. 2001. Austrocalyxaceae, a new pteridosperm family from Gondwana. Palaeontographica Abteilung B 257: 1–16. [Google Scholar]
- Wang DM, Guo Y. 2009. Hamatophyton from the Late Devonian of Anhui Province, South China and evolution of Sphenophyllales. Acta Geologica Sinica 83: 492–503. [Google Scholar]
- Wang DM, Lin YJ. 2007. A new species of Metacladophyton from the Late Devonian of China. International Journal of Plant Sciences 168: 1067–1084. [Google Scholar]
- Wang DM, Liu L. 2015. A new Late Devonian genus with seed plant affinities. BMC Evolutionary Biology 15: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DM, Wan ZZ, Cui L. 2008. Further study of Sphenophyllum lungtanense, with a discussion on the evolution of the Late Devonian Sphenophyllales in South China. Acta Scientiarum Naturalium Universitatis Pekinensis 44: 177–184. [Google Scholar]
- Wang DM, Liu L, Meng MC, Xue JZ, Liu T, Guo Y. 2014. Cosmosperma polyloba gen. et sp. nov., a seed plant from the Upper Devonian of South China. Naturwissenschaften 101: 615–622. [DOI] [PubMed] [Google Scholar]
- Wang Q, Geng BY, Dilcher DL. 2005. New perspective on the architecture of the Late Devonian arborescent lycopsid Leptophloeum rhombicum (Leptophloeaceae). American Journal of Botany 92: 83–91. [DOI] [PubMed] [Google Scholar]
- Wang Y. 1993. First discovery of Eviostachya hoegii Stockmans from Wutung Formation in China. Acta Palaeontologica Sinica 32: 430–441. [Google Scholar]
- Wang Y. 2000. Kongshania gen. nov. a new plant from the Wutung Formation (Upper Devonian) of Jiangning County, Jiangsu, China. Acta Palaeontologica Sinica 39: 42–56. [Google Scholar]
- Wang Y. 2003. A new plant from the earliest Carboniferous of Jiangsu, China. Alcheringa 27: 51–61. [Google Scholar]
- Xue JZ, Hao SG. 2008. Denglongia hubeiensis gen. et sp. nov., a new plant attributed to Cladoxylopsida from the Upper Devonian (Frasnian) of South China. International Journal of Plant Sciences 169: 1314–1331. [Google Scholar]
- Zhu LH, Zhang CL, Zhong JH, Zou CJ, Gao NH. 1999. The sedimentary and geochemical characteristics of the Upper Devonian Wutong Formation in lower Yangtze region. Acta Sedimentologica Sinica 17: 355–360. [Google Scholar]
- Zimmermann W. 1952. Main results of the “telome theory”. Palaeobotanist 1: 456–470. [Google Scholar]