Abstract
Background The cost–benefit model for the evolution of botanical carnivory provides a conceptual framework for interpreting a wide range of comparative and experimental studies on carnivorous plants. This model assumes that the modified leaves called traps represent a significant cost for the plant, and this cost is outweighed by the benefits from increased nutrient uptake from prey, in terms of enhancing the rate of photosynthesis per unit leaf mass or area (AN) in the microsites inhabited by carnivorous plants.
Scope This review summarizes results from the classical interpretation of the cost–benefit model for evolution of botanical carnivory and highlights the costs and benefits of active trapping mechanisms, including water pumping, electrical signalling and accumulation of jasmonates. Novel alternative sequestration strategies (utilization of leaf litter and faeces) in carnivorous plants are also discussed in the context of the cost–benefit model.
Conclusions Traps of carnivorous plants have lower AN than leaves, and the leaves have higher AN after feeding. Prey digestion, water pumping and electrical signalling represent a significant carbon cost (as an increased rate of respiration, RD) for carnivorous plants. On the other hand, jasmonate accumulation during the digestive period and reprogramming of gene expression from growth and photosynthesis to prey digestion optimizes enzyme production in comparison with constitutive secretion. This inducibility may have evolved as a cost-saving strategy beneficial for carnivorous plants. The similarities between plant defence mechanisms and botanical carnivory are highlighted.
Keywords: Action potential, botanical carnivory, carnivorous plant, cost–benefit, Dionaea, Drosera, electrical signalling, jasmonates, Nepenthes, Venus flytrap
INTRODUCTION
Carnivorous plants have long fascinated scientists, and were described by Charles Darwin in the book Insectivorous plants (Darwin, 1875). Carnivorous plants typically attract, capture and digest animal prey by modified leaves called traps. No carnivorous plant is able to capture prey by its flower. Givnish et al. (1984) proposed that a plant must fulfil two basic requirements to be considered as carnivorous. First, it must be able to absorb nutrients from dead prey, and thereby obtain some increment to fitness in terms of increased growth, pollen production or seed set. Secondly, the plant must have some adaptation or resource allocation whose primary result is the active attraction, capture and/or digestion of prey. The first is needed to differentiate carnivory from defensive adaptation that immobilizes or kills animal enemies without leading to substantial nutrient absorption and thus increased plant survival. The second is required because many plants can passively profit by absorbing some nutrients from dead animals decomposing in the soil or on leaf surfaces. A plant must have at least one adaptation (i.e. active attraction, capture and digestion) in combination with nutrient absorption to be qualified as carnivorous, because many genera of carnivorous plants lack some of these attributes. For example, Utricularia and Pinguicula probably lack resource allocation to prey attraction. Heliamphora and Darlingtonia lack digestive glands and enzymes (except probably H. tatei; Jaffé et al., 1992) and rely on symbiotic bacteria and other organisms to break down the prey (Adlassnig et al., 2011). The genus Roridula relies on a mutualistic relationship with Pameridea roridulae, a species of capsid bug, which lives on the plant and feeds on the trapped insects. The plant obtains nutrients from the droppings of this symbiotic insect (Anderson, 2005; Plachno et al., 2009). Nepenthes bicalcarata relies on the symbiotic ants Camponotus schmitzi to catch and digest its prey (Bonhomme et al., 2011a; Bazile et al., 2012; Thornham et al., 2012). These alternative methods of prey digestion, called digestive mutualisms, may represent extremely specialized adaptation to the carnivorous syndrome because they reduce the costs of having to produce digestive enzymes (Anderson and Midgley, 2003). According to this new definition, the plant Paepalanthus bromelioides is considered as a carnivorous plant (Nishii et al., 2013; Givnish et al., 2015).
Although 19 genera in 12 families and five orders of carnivorous plants are now recognized (Aldrovanda, Brocchinia, Byblis, Catopsis, Cephalotus, Darlingtonia, Drosera, Drosophyllum, Dionaea, Genlisea, Heliamphora, Nepenthes, Paepalanthus, Philcoxia, Pinguicula, Roridula, Sarracenia, Triphyophyllum and Utricularia; Givnish et al., 2015; Fig. 1), some authors argue that there are more carnivorous plant genera than previously believed and that there is a clear continuum between carnivorous and non-carnivorous plants. Recently, Chase et al. (2009) discussed as carnivorous the sticky plants Potentilla glandulosa, Geranium viscosissimum, Petunia violacea, Petunia nyctaginiflora and Solanum tuberosum, and plants that use glandular hairs to protect their flowers, including Stylidium species, Passiflora foetida and Plumbago auriculata. They also included plants that make pitchers with their leaves including Dipsacus fullonum, plants that kill birds including Puya raimondii, and more. Although that paper attracted media attention, many authors disagree with such a broad definition of carnivorous plants, because they do not fulfil the above-mentioned criteria (Brittnacher, 2011; Rice, 2011).
Fig. 1.
The carnivorous plants. (A) Cephalotus follicularis, (B) Darlingtonia californica, (C) Dionaea muscipula, (D) Nepenthes tentaculata, (E) Heliamphora nutans, (F) Sarracenia flava, (G) Drosera roraimae, (H) Pinguicula alpina, (I) Utricularia humboldtii.
A long-standing problem in evolutionary biology, i.e. an explanation for the ecological conditions under which botanical carnivory is likely to evolve repeatedly, was resolved by Givnish et al. (1984). Several comprehensive reviews of the rise of carnivorous plants have been published over the past decade, all focusing on trade-offs among physiological and morphological traits (Ellison and Gotelli, 2001, 2009; Ellison, 2006; Ellison and Adamec, 2011). Here, we review the cost–benefit model for the evolution of botanical carnivory in view of new data on the molecular biology of trap leaves. In addition to the classical ecological intepretations of that model, we highlight the importance of energetic costs of active trapping mechanisms. We also attempt to address the similarities between carnivory and plant defence mechanisms and the role of jasmonate signalling in carnivory. Finally, we extend the intepretation of the cost–benefit model to alternative nutrient sequestration strategies in carnivorous plants.
TRAPPING MECHANISMS
The traps of carnivorous plants may be active or passive, depending on whether movement aids the capture of prey. Five basic trapping mechanisms are found in carnivorous plants (Juniper et al., 1989; Król et al., 2012).
Pitfall traps (pitcher plants)
These trap prey into a modified pitcher that contains a pool of digestive enzymes or bacteria. Special adaptation such as wax layers, anisotropy of digestive glands, slippery peristome and acidic and viscoelastic digestive fluid are involved in the capture mechanism (Bohn and Federle, 2004; Gaume et al., 2004; Gorb et al., 2004; Gaume and Forterre, 2007; Bazile et al., 2015). Prey usually lose stability on slippery surfaces and fall inside the pitcher with digestive fluid. Passive pitfall traps are thought to have evolved six times independently in the families Sarraceniaceae (Sarracenia, Heliamphora, Darlingtonia), Cephalotaceae (Cephalotus), Nepenthaceae (Nepenthes) and Eriocaulaceae (Paepalanthus) and twice in the family Bromeliaceae (Brocchinia and Catopsis) (Givnish et al., 2015).
Flypaper traps
These traps involve a sticky mucilage on the leaf surface. Flypapers have evolved at least five times independently in the following genera: Drosera (family Droseraceae), Drosophyllum (family Drosophyllaceae), Triphyophyllum (family Dioncophyllaceae), Byblis (family Byblidaceae), Pinguicula (family Lentibulariaceae), Roridula (family Roridulaceae) and the recently described genus Philcoxia (family Plantaginaceae; Renner and Specht, 2011; Pereira et al., 2012; Givnish et al., 2015). Mucilage-secreting glands may be short (like those of the Pinguicula), or long and mobile (like those of Drosera). There are active and passive traps. For example, Triphyophyllum has a passive flypaper trap that secretes mucilage, but whose leaves do not move in response to prey capture. Sundew (Drosera) has active flypaper traps with tentacles and leaf bending reactions. A special category of tentacles has recently been described: fast-moving snap tentacles in Drosera glanduligera (Poppinga et al., 2012).
Snap traps
These traps utilize rapid leaf movements. There are only two closely related active snap trap genera – Dionaea and Aldrovanda (family Droseraceae). The rapid trap closure is triggered by action potentials generated by touch of sensitive hairs on the trap lobes (Volkov et al., 2008).
Bladder suction traps
These suck in prey with a bladder that generates an internal vacuum and are exclusively found in the genus Utricularia (family Lentibulariaceae). The bladders actively pump ions out of their interiors and water follows by osmosis; this generates a partial vacuum inside the bladder. Aquatic invertebrates touch the trigger hairs and deform the door by lever action, releasing the trap wall and causing water influx. The invertebrate is sucked into the bladder, where it is digested (Vincent et al., 2011a, b; Adamec, 2012).
Eel-traps
These traps force prey to move towards a digestive organ with inward-pointing hairs. These are passive traps. They are found in the genus Genlisea (family Lentibulariaceae) and in Sarracenia psittacina (family Sarraceniaceae) (Adamec, 2003).
COST–BENEFIT MODEL FOR EVOLUTION OF BOTANICAL CARNIVORY
Today >650 species of carnivorous plants have been recognized, including monocotyledons and eudicotyledons (Ellison and Adamec, 2011). They have evolved at least nine times independently and are thus an example of convergent evolution (Albert et al., 1992; Givnish et al., 2015). Recently, the first fossilized (35–47 milion years old) carnivorous plant trap of the family Roridulaceae was found (Sadowski et al., 2015). Convergent evolution is an independent evolution of similar features in species of different lineages which grow or live in a similar environment. For example, pitcher plants Sarracenia and Nepenthes are not related and pitcher traps are analogous, not homologous, structures (i.e. they have not evolved from a common ancestor). What environmental factors are the driving forces for evolution of botanical carnivory? Ecologist Thomas Givnish was the first to recognize why carnivorous plants are mainly restricted to habitats and microsites that are not only nutrient poor, but sunny and moist as well. In January 1980, Thomas Givnish visited the Gran Sabana in Venezuela, where he observed terrestrial bromeliad Brocchinia reducta. He noticed that this bromeliad has evolved some features which resembled a carnivorous lifestyle. In contrast to related species such as B. tatei, B. reducta forms bright yellow-green leaves which are held vertically enclosing its own water tank. The fluid inside the tank is highly acidic (pH 2.8–3.0) and contains abundant remains of dead insects. The tank emits a sweet odour. The leaf surface is coated with a fine waxy layer inhibiting prey escape from the central tank. After removing this layer by brushing, attempts by ants to ascend the leaf surface succeeded. Finally, the trichomes of B. reducta can absorb 3H-labelled leucine. Based on the criteria mentioned above, B. reducta fulfils all the requirements to be classified as carnivorous. Although the evidence that absorption of nutrients increases B. reducta fitness is missing, it is very likely that it occurs, because it grows in a nutrient-poor habitat that is the home of other genera of carnivorous plants (Drosera, Heliamphora, Genlisea and Utricularia), where benefit from prey capture in terms of increased growth was documented (Ellison, 2006). Givnish et al. (1984, 1997) also proposed possible mechanisms for how the carnivory in B. reducta may have evolved. The closest relative of B. reducta is B. tatei, which is a facultatively epiphytic tank species growing in shady cloud forest. Its nearly horizontal rosette-forming green leaves are well adapted for light capture (Fig. 2). The leaves of B. tatei form a spreading rosette and capture moderate amounts of precipitation and leaf litter in their bases. Under such conditions, it probably obtains some nutrients from the breakdown of such debris, like many other tank epiphytes. Invasion of sunny sterile savannah by B. reducta’s ancestors would have favoured the evolution of steeply inclined leaves with strongly reflective waxy cuticles to reduce light capture. These traits would be a pre-adaptation for the evolution of carnivory. The crucial shift to carnivory probably involved the leakage into the tank of a volatile compound stored in the leaf base of many Brocchinia species. This would attract insects into the tank and promote the evolution of other carnivorous-related functions. Thus B. reducta was the first documented case of carnivory in Bromeliaceae and even in monocotyledons. The related species B. hectioides also growing on Gran Sabana in Venezuela is considered as the second carnivorous species in the genus Brocchinia. It has evolved traits very similar to those of B. reducta. The third species is probably Catopsis berteroniana, but more research on this species is needed (Frank and O’Meara, 1984; Givnish et al., 1984; Givnish, 1989). The fact that the genus Brocchinia also possesses other specialized adaptations to nutrient capture (myrmecophytic B. accuminata, nitrogen (N) fixation in some populations of B. tatei) in nutrient-poor habitats indicates that they have evolved in the process of adaptive radiation. Givnish et al. (1997) hypothesized that the tank habit and absorptive trichomes are the two key innovations that allowed the evolution of a great diversity of specialized mechanisms of nutrient capture including carnivory.
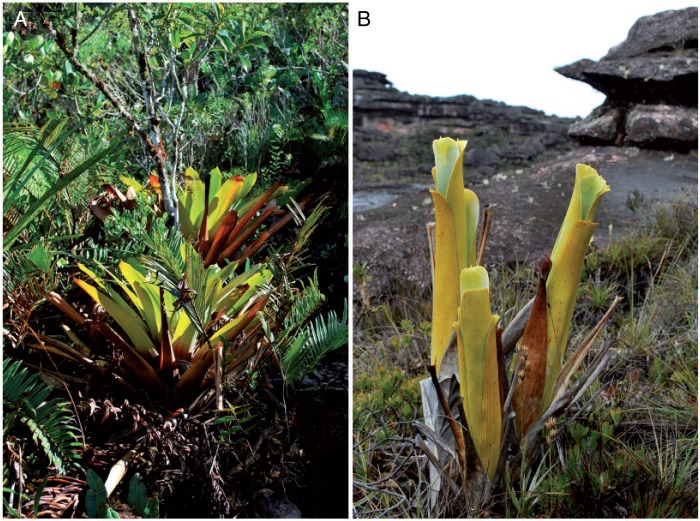
Fig. 2.
Two related species of Brocchinia growing in the Guiana Highlands, Venezuela. (A) The non-carnivorous species Brocchinia tatei often grows in cloud forest and forms nearly horizontal rosette-forming green leaves. (B) The carnivorous species Brocchinia reducta grows in open vegetation and forms bright yellow-green leaves which are held vertically.
The observations of Givnish in Gran Sabana in Venezuela led him to formulate a cost–benefit model for evolution of botanical carnivory. Cost–benefit models have been a hallmark of ecological analyses for >50 years, and ask what organismal form, physiology or behaviour would maximize energy capture in a particular environment and thus be likely to result in maximal competitive ability and fitness in that context. The amount of energy a given organism can allocate to different functions is fixed, so that there are inevitably trade-offs among allocations to those functions, with the optimal allocation almost certainly varying with environmental conditions. In other words, it is based on the assumption that organisms cannot do equally well and there must be some trade-off. Carnivorous plants are model systems for studying a wide range of ecophysiological and ecological processes, and the application of a cost–benefit model for the evolution of carnivory by plants has provided many novel insights. Carnivory should evolve if benefits from increased uptake of nutrients from animal prey exceed the cost of investment in carnivorous adaptations. In this case, the plants obtain an advantage in competing with other plants in nutrient-poor habitats. Givnish et al. (1984) considered that the costs of carnivory included the extra energy required to attract prey (e.g. production of lures), capture prey (e.g. production of wax or mucilage) and digest prey (e.g. production of digestive enzymes), as well as a decreased rate of photosynthesis per unit leaf mass or area (AN). Against these, he proposed three potential benefits from carnivory:
Carnivory may increase a plant’s AN through improved nutrient supply, particularly N status, in two ways: either by increased AN per unit leaf mass or by an increase in the total leaf mass that can be supported.
Carnivory may result in an increased seed production through improved mineral acquisition.
Carnivory may replace autotrophy partly by heterotrophy.
Givnish et al. (1984) considered the second benefit as a part of the first, as increased AN should lead to increased seed production, and the third benefit as unlikely, because experimental evidence supports the fact that carnivorous plants obtain minerals, not carbon. He concluded that the primary benefit from carnivory is increased AN through increased nutrient supply from prey. However, the AN does not increase equally well under all conditions. If the factors such as light or water are in short supply and limit photosynthesis, then AN increases more slowly with improved N content than at high light or high water supply, because of light and stomatal limitation of photosynthesis. It is known that carnivorous plants give up carnivory temporarily if they grow in nutrient-rich soil or they do not have enough water and light. For example, the genera Nepenthes and Cephalotus stop producing their pitchers, Sarracenia forms non-carnivorous phyllodia instead of traps, Dionaea decreases excitability of their trap, and Drosera and Pinguicula decrease the stickness of their leaves (Zamora et al., 1998; Ellison and Gotelli, 2002; Thorén et al., 2003; Pavlovič et al., 2010b; Escalante-Pérez et al., 2011). Thus, in summary, obviously where there is a shortage of nutrients and enough water and light, there is the greatest impact on photosynthetic gains from prey capture, and such conditions favour the evolution of plant carnivory (see fig. 3 in Pavlovič et al., 2009). In contrast, the costs to carnivory are thought to exceed the benefits in shady, nutrient-rich and dry habitats, and carnivory has no adaptive value in such an environment and does not pay off. Thus the main outcome from the cost–benefit model, that energetic benefits of carnivory are likely to exceed its costs only in sunny, moist and nutrient-poor environments, became a framework for studies in carnivorous plants (Ellison and Gotelli, 2009).
Fig. 3.
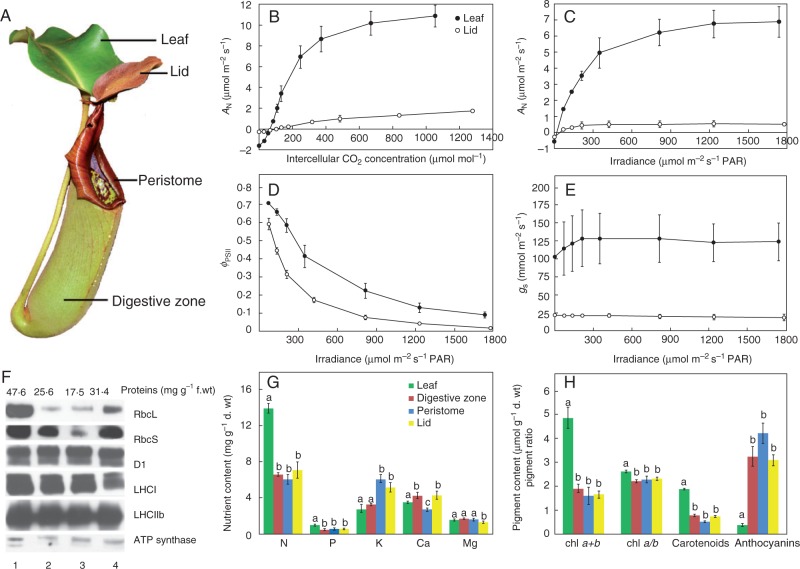
Classical interpretation of the cost–benefit model for evolution of botanical carnivory in the genus Nepenthes. Comparison of photosynthetic characteristics between the pitcher (the lid as a flat part of the pitcher) and lamina. (A) The leaf of Nepenthes truncata. (B) A/Ci response curve of photosynthesis. (C) Light response curve of photosynthesis. (D) Light response curve of effective photochemical quantum yield of photosystem II (ϕPSII). (E) Stomatal conductance (gs). (F) Protein content and protein gel blot analysis (the same amount of protein was electrophoresed), leaf (1), digestive zone (2), peristome (3), lid (4). (G) Elemental composition of the leaf. (H) Pigment content and ratio. Data are means ± s.e. (n = 5); different letters denote significant differences among plant tissues (one-way ANOVA followed by Tukey’s test). For details of the methods, see the Supplementary Data.
When Givnish proposed the cost–benefit model for the evolution of botanical carnivory 30 years ago, there was almost no compelling evidence bearing on whether his intepretation was right or wrong. Although Darwin’s son was the first who showed that the growth of the carnivorous plant Drosera rotundifolia was enhanced by insect feeding (Darwin, 1878), the final experimental proof that carnivory may enhance AN was missing for another 130 years. The first study performed by Méndez and Karlsson (1999) did not show enhanced AN in response to feeding in Pinguicula vulgaris probably due to the short feeding period. The study of Ellison and Gotelli (2002) showed that Sarracenia purpurea responded to nutrient addition by shifting from production of carnivorous pitchers to production of more photosynthetically active phyllodia. However, Wakefield et al. (2005) measured the AN after feeding in the same species with prey but he did not find any increase of AN. Later Ellison and Farnsworth (2005) showed that AN correlated with foliar N content in Darlingtonia californica from different sites. Ellison (2006) summarized data from 24 studies, and his meta-analysis showed that there is a significant positive effect of prey addition on carnivorous plant growth, but data regarding photosynthesis are still lacking. The first experimental evidence that AN increased in response to carnivory was documented at almost the same time in ten Sarracenia species (Farnsworth and Ellison, 2008), Utricularia australis (Adamec, 2008) and Nepenthes talangensis (Pavlovič et al., 2009). Later, the positive effect of feeding on AN was found in Nepenthes ampullaria (Pavlovič et al., 2011b), Nepenthes alata (He and Zain, 2012), Drosera capensis (Pavlovič et al., 2014) and Dionaea muscipula (Kruse et al., 2014). In many of these studies the increase in AN correlated well with the increased leaf N and/or phosphorus (P) concentrations and increased growth. The chlorophyll concentration also increased in response to feeding and it is the most sensitive indicator of nutrient stress in carnivorous plants (Moran and Moran, 1998; Farnsworth and Ellison, 2008; Pavlovič et al., 2009, 2011b, 2014; Bazile et al., 2012; He and Zain, 2012). Moreover, Adamec (1997, 2002) suggested that absorption of leaf mineral nutrients from prey stimulates root nutrient uptake. Nutrients taken up by the roots can enhance AN and thus increase the benefit from carnivory in Nepenthes talangensis (Pavlovič et al., 2010b), indicating a high capacity for root nutrient uptake in some carnivorous plants (Gao et al., 2015). Increased flowering frequency and seed production as a secondary benefit from carnivory is also documented in carnivorous plants (Karlsson and Pate, 1992; Thorén and Karlsson, 1998; Pavlovič et al., 2009).
All these photosynthetic studies however showed another important thing: the AN and photosynthetic nitrogen use efficiency (PNUE) in terrestrial carnivorous plants are lower than those in non-carnivorous plants, including graminoids, forbs, and deciduous and evergreen trees and shrubs. This documents the cost of carnivory in terms of a decreased rate of AN (decreased AN in traps) and in terms of the physiological consequences of slow growth and conditions of extremely low nutrient availability (decreased AN in photosynthetic lamina; Mendéz and Karlsson, 1999; Ellison and Farnsworth, 2005; Ellison, 2006; Ellison and Adamec, 2011). Although the construction costs (the amount of glucose required to synthesize 1 g of carbon skeleton/biomass) of traps are not higher than the construction costs of, for example, roots or leaves, the low AN results in long pay-back times (the time that a leaf needs to photosynthesize in order to recover the carbon investment used in its construction; Osunkoya et al., 2008; Karagatzides and Ellison, 2009). However, the construction costs do not include only construction of trap organs. Many carnivorous plants secrete sugar- (carbon) rich exudates. The mucilage of Drosera capensis comprises l-arabinose, d-xylose, d-galactose, d-mannose and d-glucuronic acid (Gowda et al., 1983). Also, the viscoelastic digestive fluid and waxy zone of the trap in the genus Nepenthes is composed of long-chain polysaccharides and aldehydes, respectively (Riedel et al., 2003; Bonhomme et al., 2011b). Similarly, rootless Utricularia plants supply easily available organic carbon (glucose, fructose and lactate) from photosynthesis to the microbial community thriving within the trap environment while benefiting from its by-products (Sirová et al., 2010, 2011). The traps of carnivorous plants are therefore probably more costly than was previously thought (Osunkoya et al., 2008).
From a morphological point of view, two types of carnivorous plants can be distinguished. Butterworts (Pinguicula), sundews (Drosera) and American pitcher plants (Heliamphora and Darlingtonia) have leaves that can both photosynthesize and capture prey. The Australian pitcher plant (Cephalotus), Venus flytrap (Dionaea), bladderworts (Utricularia), North American pitcher plants (Sarracenia), corkscrew plants (Genlisea) or Asian pitcher plants (Nepenthes) produce leaves that can photosynthesize but do not capture prey (photosynthetic laminae or phyllodes), and/or produce traps that capture prey and photosynthesize little or not at all (Ellison and Gotelli, 2001). This trait allows them to vary their investment in carnivory as a function of environmental conditions (light, water and nutrient availability sensu stricto Givnish’s cost–benefit model; Knight and Frost, 1991). These plants are suitable models for cost–benefit studies, because reworking leaf morphology and physiology to carnivory apparently reduces the efficiency of AN in traps. Pavlovič et al. (2007, 2009) compared AN in the Nepenthes alata, N. mirabilis and N. talangensis lamina/leaf and pitcher trap separately and described the traits responsible for the carnivorous syndrome. This is briefly summarized in Fig. 3, here in the case of Nepenthes truncata (Fig. 3A). The traps of Nepenthes have very low AN (close to zero), lower effective photochemical quantum yield of photosystem II (ϕPSII) and apparent quantum yield of CO2 fixation (ϕCO2, slope of the linear portion of the light response curve, 0·019 ± 0·0019 and 0·003 ± 0·0006 mol CO2 mol photons–1 for leaf and pitcher lid conditions, respectively), low stomatal density and conductance for CO2 and H2O (gs), compact mesophyll without palisade parenchyma, and lower chlorophyll and carotenoid content but higher anthocyanin content (Fig. 3B–E, H). The pitchers, in comparison with photosynthetic leaves, also have a decreased amount of N and P (Fig. 3G). The pitchers also have lower protein content and a decreased amount of total soluble proteins invested in the Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase) large and small subunits, RbcL and RbcS, respectively, but almost the same invested in the chlorophyll-binding proteins (D1, LHCI and LHCIIb; Fig. 3F). This is consistent with decreased apparent Rubisco activity (ε) in the pitcher trap expressed as the slope of the linear part of the A/Ci response curve (0·44 ± 0·04 and 0·03 ± 0·005 µmol CO2 m–2 s–1 Pa–1 for leaf and pitcher lid, respectively; Fig. 3B). In the prey-deprived plants of N. ampullaria, where the N concentration in leaves and traps was <0·5 %, the Rubisco content was almost comparable between the lamina and pitcher trap, making it tempting to assume that surplus N from prey is incorporated into Rubisco in the leaves, which may explain the higher AN in fed plants (Pavlovič et al., 2009, 2011b), an original hypothesis of Givnish et al. (1984). Rubisco is present at very high levels in photosynthesizing cells of C3 plants and may contribute up to 50 % of soluble leaf proteins and 20–30 % of total leaf N (Feller et al., 2008). Therefore, the photosynthetic capacity of leaves is related to the N content, and the proportion of total N in Rubisco increases with increasing leaf N (Evans, 1989). Recently, Galmés et al. (2014) characterized the kinetic properties of Rubisco from 28 terrestrial plant species, representing different phylogenetic lineages, environmental adaptations and photosynthetic mechanisms, and found that carnivorous plants incorporated less total soluble proteins into Rubisco than non-carnivorous plants. As a result of extremely low protein incorporation into Rubisco, the Rubisco in carnivorous plants has evolved toward a higher maximum carboxylation rate. The higher maximum carboxylation rate probably does not compensate for lower protein investment in Rubisco, which may explain the lower PNUE in carnivorous plants. It seems that oxygen evolution (He and Zain, 2012) and electron transport from water (expresssed as ϕPSII) are not reduced to such an extent as dark enzymatic reactions of photosynthesis (compare the differences between the trap and lamina in AN and ϕPSII in Fig. 3B–D, F), indicating that electrons from water are probably used in traps for competing processes other than photosynthesis. Pavlovič et al. (2007) predicted that the above-mentioned characteristics, which make photosynthesis in traps inefficient, may be responsible for the carnivorous function in nutrient-poor habitats (e.g. compact anatomy might serve for symplastic transport of nutrients gained from prey, N and carbon skeleton are allocated more into digestive fluid, enzymes and lures than to photosynthesis-related proteins).
A very low AN and chlorophyll content were also found in the bladders in comparison with leaves of aquatic Utricularia by Knight (1992) and Adamec (2006). Although nobody has studied the genus Genlisea, it is almost certain that its colourless traps also have very low AN if any. Thus, it is not surprising that such strongly modified leaves almost without photosynthetic activity are usually buried in substrate. Underground they can target a different type of prey, thus avoiding competition with sympatric terrestrial carnivorous plants. However, some other carnivorous genera have traps with not such drastically reduced AN. For example pitchers of Cephalotus, Sarracenia or Darlingtonia have AN almost comparable with the photosynthetic efficiency of assimilatory lamina (Ellison and Farnsworth, 2005; Farnsworth and Ellison, 2008; Hájek and Adamec, 2010; Pavlovič, 2011; Table 1). This indicates that some species indeed have reduced photosynthetic capacity in the trap organs (Nepenthes, Utricularia), but in others the AN in the trap is only slightly, if at all, reduced in comparison with photosynthetic lamina (e.g. Sarracenia, Cephalotus, Dionaea), but is still very low in comparison with non-carnivorous plants (Méndez and Karlsson, 1999; Ellison and Farnsworth, 2005; Table 1). Altogether, these studies unequivocally showed that there is a photosynthetic cost in producing the traps in that the carbon invested in the traps does not return as much carbon as it would if it was invested in assimilatory tissue. As a result of our analyses, there is compelling evidence that traps have lower AN than leaves (16 out of 18 cases), and that plants have higher AN after feeding (16 out of 19 cases; Table 1).
Table 1.
Rate of net photosynthesis (AN) in carnivorous plants with leaves differentiated into traps and photosynthetic lamina or in response to experimental feeding (fed plants in parentheses)
| Species (and reference) | Leaf type | AN (nmol CO2 g–1 d. wt s–1) or ANaq (mmol O2 kg–1 f. wt h–1) | AN (µmol CO2 m–2 s–1) | Lower AN in trap | Higher AN after feeding |
|---|---|---|---|---|---|
| Aldrovanda vesiculosa (aq) (Adamec, 2008) | Shoot | 34·1 ± 1·5 (21·5 ± 2·2) | N/A | – | |
| Cephalotus follicularis (Pavlovič, 2011) | Lamina | 42·8 ± 2·1 | + | N/A | |
| Trap | 27·2 ± 2·5 | ||||
| Dionaea muscipula (Hájek and Adamec, 2010) | Lamina | 90 ± 9 | 4·03 ± 0·38 | + | N/A |
| Trap | 52 ± 3 | 3·04 ± 0·20 | |||
| Dionaea muscipula (Kruse et al., 2014) | Trap | Approx. 25 (approx. 38) | N/A | + | |
| Drosera capensis (Pavlovič et al., 2014) | Trap | 2·18 ± 0·52 (4·88 ± 0·57) | N/A | + | |
| Nepenthes alata (Pavlovič et al., 2007) | Lamina | 42·3 ± 4·5 | + | N/A | |
| Trap | –2·4 ± 1·4 | ||||
| Nepenthes alata (He and Zain, 2012) | Lamina | Approx. 4 (approx. 6)* | + | + | |
| Trap | Approx. 1 (approx. 2)* | ||||
| Nepenthes ampullaria (Pavlovič et al., 2011b) | Lamina | 2·51 ± 0·55 (3·38 ± 0·37) | + | + | |
| Trap | 0·07 ± 0·01 (0·20 ± 0·01) | ||||
| Nepenthes × Coccinea (Karagatzides and Ellison, 2009) | Lamina | 29·8 ± 23·1 (s.d.) | + | N/A | |
| Trap | 2·5 | ||||
| Nepenthes mirabilis (Pavlovič et al., 2007) | Lamina | 24·4 ± 5·5 | + | N/A | |
| Trap | 0·0 ± 1·6 | ||||
| Nepenthes × Miranda (Karagatzides and Ellison, 2009) | Lamina | 36·0 | + | N/A | |
| Trap | 1·1 | ||||
| Nepenthes talangensis (Pavlovič et al., 2009) | Lamina | 19·4 ± 2·0 (37·8 ± 5·4) | 3·1 ± 0·3 (6·0 ± 0·5) | + | + |
| Trap | 2·0 ± 0·9 (3·5 ± 1·4) | 0·10 ± 0·04 (0·15 ± 0·03) | |||
| Pinguicula vulgaris (Méndez and Karlsson, 1999) | Plant | 55·6 ± 16·9 (s.d.) (52·1 ± 10·0) (s.d) | 2·2 ± 0·6 (s.d.) (2·2 ± 0·4) (s.d.) | N/A | 0 |
| Sarracenia spp. (ten species) (Farnsworth and Ellison, 2008) | Trap | Approx. 22 (38–45) | N/A | + (10×) | |
| Sarracenia flava (Karagatzides and Ellison, 2009) | Lamina | 45·5 ± 7·3. (s.d.) | 0 | N/A | |
| Trap | 43·0 ± 10·1 (s.d.) | ||||
| Sarracenia leucophylla (Karagatzides and Ellison, 2009) | Lamina | 35·8 ± 24·1 (s.d.) | 0 | N/A | |
| Trap | 36·5 ± 10·1 (s.d.) | ||||
| Sarracenia purpurea (Hájek and Adamec, 2010) | Wing | 64 ± 6 | 5·26 ± 0·46 | N/A | N/A |
| Trap | 58 ± 3 | 3·97 ± 0·22 | |||
| Sarracenia purpurea (Wakefield et al., 2005) | Trap | 3·1 ± 1·2 (s.d.) | N/A | 0 | |
| (3·1 ± 1·2) (s.d.) | |||||
| Utricularia australis (aq) (Adamec, 2008) | Leaf | 66·7 ± 1·6 (89·2 ± 3·7) | N/A | + | |
| Utricularia australis (aq) (Adamec, 2006) | Leaf | 86·5 ± 10·1 | + | N/A | |
| Trap | 9·03 ± 1·22 | ||||
| Utricularia bremii (aq) (Adamec, 2006) | Leaf | 40·0 ± 6·6 | + | N/A | |
| Trap | 5·24 ± 0·51 | ||||
| Utricularia floridana (aq) (Adamec, 2006) | Leaf | 66·4 ± 3·9 | + | N/A | |
| Trap | –5·09 ± 0·33† | ||||
| Utricularia intermedia (aq) (Adamec, 2006) | Leaf | 117 ± 13·1 | + | N/A | |
| Trap | –6·77 ± 0·57† | ||||
| Utricularia macrorhiza (aq) (Knight, 1992) | Leaf | 0·758–0·965‡ | + | N/A | |
| Trap | 0·328–0·558‡ | ||||
| Utricularia ochroleuca (aq) (Adamec, 2006) | Leaf | 111 ± 5·6 | + | N/A | |
| Trap | –5·15 ± 0·42† | ||||
| Utricularia vulgaris (aq) (Adamec, 2006) | Leaf | 96·9 ± 5·2 | + | N/A | |
| Trap | 14·7 ± 0·64 | ||||
| Summary | (+) 16 | (+) 16 | |||
| (–) 0 | (–) 1 | ||||
| (0) 2 | (0) 2 |
A positive response is indicated by ‘+’, a negative response by ‘–’, no response by ‘0’, no data by ‘N/A’.
Note the different unit for aquatic carnivorous plants (ANaq).
Means ± s.e. whenever possible (or s.d. if indicated).
*Values are in µmol O2 m–2 s–1
†The traps are achlorophyllous; a negative sign of the number indicates CO2 release (i.e. RD).
‡Values are in mg C g tissue–1 h–1; values are from a population in Grassy Lake.
Carbon uptake from prey considered as the third benefit from carnivory is negligible in terrestrial carnivorous plants, but is ecologically important for aquatic carnivorous plants (see Adamec, 1997). Although some studies have documented the uptake of carbon from prey in Drosera erythrorhiza, Nepenthes insignis or Dionaea muscipula, this absorbed carbon seems more likely to be in N-bearing amino acids than in carbohydrates (Dixon et al., 1980; Rischer et al., 2002; Kruse et al., 2014). Chandler and Anderson (1976) have shown that growth of dark-grown Drosera whittakeri was not enhanced in response to feeding, confirming the suggestion that carnivory cannot replace autotrophy by heterotrophy. This is in contrast to plants with a heterotrophic mode of nutrition (e.g. parasitic plants). Although the major source of the carbon skeleton in carnivorous plants is photosynthesis, recently the loss of genes, accelerated substitution rates and relaxation of selection in plastid genomes of Utricularia, Genlisea and Pinguicula were documented in comparison with non-carnivorous plants. Almost all genes for light and dark reactions of photosynthesis are affected and resemble the obligate photosynthetic parasitic plants such as Cuscuta, indicating that alternative paths of acquiring nutrients may promote the rapid evolution of plastid genes in Lentibulariaceae or in carnivorous plants in general (Revill et al., 2005; Wicke et al., 2014).
WATER PUMPING AND ELECTRICAL SIGNALLING
There is now a significant amount of evidence that the cost–benefit model proposed by Givnish et al. (1984) is valid for terrestrial carnivorous plants with slight modifications for aquatic carnivorous plants. Ellison and Adamec (2011) suggested that nutrient limitation is more pronounced in terrestrial carnivorous plants, which also have much lower growth rates and lower AN than aquatic carnivorous plants. Because traps of aquatic carnivorous plants are energetically very costly, it is plausible that P limitation (of, for example, ATP) might be of more consequence for aquatic than for terrestrial carnivorous plants. As demonstrated by Sydenham and Findlay (1975), ions and water pumping during the resetting of Utricularia bladders is a process requiring high amounts of metabolic energy derived from aerobic respiration. When trigger hairs situated on trap door are touched by a prey, the door opens, prey is aspirated into the trap lumen and the watertight door closes again with a speed of 5 ms. In contrast to the Venus flytrap, this process has a purely mechanical basis, as electrical signals have never been recorded in Utricularia (Vincent et al., 2011a, b; Adamec, 2012). The first attempt to include respiratory cost in the cost–benefit model was made by Knight (1992), who found at least a 10 % greater respiration rate (RD) in Utricularia bladders than in leaves. Adamec (2006) showed that RD in Utricularia after firing and during resetting the bladders is even 75–200 % greater than in the leaves (Table 2). This results in anoxia inside the bladders within 30 min after trap firing and causes captured prey to die of suffocation (Adamec, 2007, 2010b). These findings led Laakkonen et al. (2006) to modify the cost–benefit model including respiratory costs as an additional trade-off parameter. Jobson et al. (2004) documented that the rate-limiting enzyme in the cellular respiration pathway, cytochrome c oxidase subunit I (COX I), may be functionally altered in bladderworts. They sequenced an intron-containing gene for 21 Utricularia species and identified that the otherwise conserved Leu113–Ser114 motif in COX I is replaced by Cys113–Cys114 across all examined Utricularia and some Genlisea species. No other carnivorous plant families have the motif, and neither does it appear in any other of the >30 000 COX I sequences currently databased for eukaryotes (with one exception – Welwitschia mirabilis). This motif lies directly at the docking point of COX I helix 3 and cytochrome c. The authors suggested two possible implications for such a substitution. The first is that a disulphide bridge at the bladderwort C–C motif could lead to early termination of helix 3 and decrease the surface area for cytochrome c association–dissociation and upregulate COX I kinetics. The second is that early termination of helix 3 leads to uncoupling of proton pumping from electron transport. Such decoupling would permit bladderworts to optimize power output during times of needs, although with a 20 % decrease in total energy efficiency of respiration. Moreover, Ibarra-Laclette et al. (2011) have recently found that bladders overexpress genes involved in respiration in comparison with leaves. Considering a higher RD, they also found a higher rate of reactive oxygen species (ROS) production, which may be responsible for high elevated nucleotide substitution rates in the Utricularia organellar and nuclear genome and the dynamic evolution of genome size (but questioned by Wicke et al., 2014). The physiological data with some molecular support confirmed that the bladders of Utricularia are expensive structures due to their high RD/AN ratio (Ellison and Adamec, 2011).
Table 2.
Rate of dark respiration (RD) in carnivorous plants with leaves differentiated into traps and photosynthetic laminae or in response to experimental feeding (fed plants in parentheses)
| Species (and reference) | Leaf type | RD (nmol CO2 g–1 d. wt s–1) or RDaq (mmol O2 kg–1 f. wt h–1) | RD (µmol CO2 m–2 s–1) | Higher RD in traps | Higher RD after feeding |
|---|---|---|---|---|---|
| Aldrovanda vesiculosa (aq) (Adamec, 2008) | Shoot | 8·17 ± 0·81 (8·97 ± 0·72) | N/A | 0 | |
| Cephalotus follicularis (Pavlovič, 2011) | Lamina | 3·48 ± 0·46 | 0 | N/A | |
| Trap | 2·97 ± 0·66 | ||||
| Cephalotus follicularis (Adamec, 2010a) | Lamina | 4·26 ± 0·25* | – | N/A | |
| Trap | 2·22 ± 0·26* | ||||
| Dionaea muscipula (Adamec, 2010a) | Lamina | 12·3 ± 1·2* | 0 | N/A | |
| Trap | 14·2 ± 2·0* | ||||
| Dionaea muscipula (Hájek and Adamec, 2010) | Lamina | 8·2 ± 1·8 | 0 | N/A | |
| Trap | 6·8 ± 0·6 | ||||
| Dionaea muscipula (Pavlovič et al., 2010a) | Lamina | Approx. 0·3 | +† | N/A | |
| Trap | Approx. 3·3 | ||||
| Dionaea muscipula (Kruse et al., 2014) | Trap | Approx. 4·5 (approx. 8·5/4·5) (during/after feeding) | N/A | +/0 (during/after feeding) | |
| Drosera capensis (Adamec, 2010a) | Lamina | 24·9 ± 0·4* | – | N/A | |
| Trap | 20·8 ± 0·9* | ||||
| Drosera capensis (Pavlovič et al., 2014) | Trap | 0·93 ± 0·10 | N/A | 0 | |
| (0·74 ± 0·38) | |||||
| Drosera prolifera (Adamec, 2010a) | Lamina | 7·16 ± 0·70* | +† | N/A | |
| Tentacles | 52·0 ± 7·5* | ||||
| Nepenthes alata (Pavlovič et al., 2007) | Lamina | 9·7 ± 0·9 | – | N/A | |
| Trap | 6·3 ± 1·1 | ||||
| Nepenthes alata (He and Zain, 2012) | Lamina | Approx. 2‡ | – | N/A | |
| Trap | Approx. 1‡ | ||||
| Nepenthes ampullaria (Pavlovič et al., 2011b) | Lamina | 0·54 ± 0·10 (0·59 ± 0·05) | – | 0 | |
| Trap | 0·35 ± 0·06 (0·38 ± 0·06) | ||||
| Nepenthes mirabilis (Pavlovič et al., 2007) | Lamina | 8·0 ± 0·6 | 0 | N/A | |
| Trap | 11·6 ± 0·9 | ||||
| Nepenthes talangensis (Pavlovič et al., 2009) | Lamina | 5·8 ± 0·5 (7·0 ± 0·21) | 0·97 ± 0·02 (1·15 ± 0·06) | 0/− (g–1 d. wt/m–2) | 0 |
| Trap | 6·5 ± 0·4 (9·6 ± 1·7) | 0·43 ± 0·02 (0·60 ± 0·10) | |||
| Nepenthes ventricosa (Adamec, 2010a) | Lamina | 5·86 ± 0·93* | 0 | N/A | |
| Trap | 8·20 ± 1·47* | ||||
| Pinguicula vulgaris (Méndez and Karlsson, 1999) | Plant | 19·2 ± 8·1 (s.d.) (23·1 ± 8·2) (s.d.) | 0·8 ± 0·3 (s.d.) (1·0 ± 0·4) (s.d.) | N/A | 0 |
| Sarracenia minor (Adamec, 2010a) | Wing | 4·79 ± 0·27* | – | N/A | |
| Trap | 3·76 ± 0·25* | ||||
| Sarracenia psittacina (Adamec, 2010a) | Wing | 2·28 ± 0·25* | 0 | N/A | |
| Trap | 2·40 ± 0·49* | ||||
| Sarracenia purpurea (Adamec, 2010a) | Wing | 5·81 ± 0·62* | + | N/A | |
| Trap | 8·38 ± 0·32* | ||||
| Sarracenia purpurea (Hájek and Adamec, 2010) | Wing | 9·5 ± 1·1 | N/A | N/A | |
| Trap | 12·6 ± 0·9 | ||||
| Sarracenia rubra (Adamec, 2010a) | Wing | 5·57 ± 0·20* | 0 | N/A | |
| Trap | 4·98 ± 0·41* | ||||
| Utricularia australis (aq) (Adamec, 2008) | Leaf | 7·89 ± 0·68 | N/A | 0 | |
| (7·42 ± 0·93) | |||||
| Utricularia australis (aq) (Adamec, 2006) | Leaf | 4·86 ± 0·23 | +† | N/A | |
| Trap | 8·56 ± 0·35 | ||||
| Utricularia bremii (aq) (Adamec, 2006) | Leaf | 3·07 ± 0·36 | +† | N/A | |
| Trap | 7·02 ± 0·45 | ||||
| Utricularia floridana (aq) (Adamec, 2006) | Leaf | 2·65 ± 0·29 | +† | N/A | |
| Trap | 5·09 ± 0·33 | ||||
| Utricularia intermedia (aq) (Adamec, 2006) | Leaf | 3·48 ± 0·34 | +† | N/A | |
| Trap | 6·77 ± 0·57 | ||||
| Utricularia macrorhiza (aq) (Knight, 1992) | Leaf | 0·751§ | 0 | N/A | |
| Trap | 0·804§ | ||||
| Utricularia ochroleuca (aq) (Adamec, 2006) | Leaf | 1·73 ± 0·17 | + † | N/A | |
| Trap | 5·15 ± 0·42 | ||||
| Utricularia vulgaris (aq) (Adamec, 2006) | Leaf | 3·94 ± 0·36 | +† | N/A | |
| Trap | 7·49 ± 0·21 | ||||
| Summary | (+) 9† | (+) 0 | |||
| (–) 7 | (–) 0 | ||||
| (0) 9 | (0) 7 |
A positive response is indicated by ‘+’, a negative response by ‘–’, no response by ‘0’, no data by ‘N/A’.
Note the different unit for aquatic carnivorous plants (RDaq).
Means ± s.e. whenever possible (or s.d. if indicated).
*Values are in nmol O2 g–1 d. wt s–1.
†Active trap (electrical signalling or water pumping).
‡Values are in µmol O2 m–2 s–1.
§Values are in mg C g tissue–1 h–1 from a population in Grassy Lake.
As a result of our analysis, it is evident that the energetic or respiration costs of traps in comparison with leaves greatly depend on the type of the trap, whether it is active or passive (Table 2). In passive pitcher traps of Sarracenia, Cephalotus and Nepenthes, the difference in RD between traps and leaves (or pitcher walls and wings) is rather small or ambiguous. In many cases (seven out of 24 cases in Table 2), the RD in the passive traps is even lower than in the leaves, probably as a consequence of reduced AN or due to a different leaf mass area (LMA) of these two distinct organs and not to specialization for carnivory, as is documented in the case of N. talangensis (Pavlovič et al., 2007, 2009; Adamec, 2010a, Hájek and Adamec, 2010; Table 2). Because the RD in traps is comparable with that in leaves (Table 2) and traps have lower AN than leaves (Table 1), traps and carnivorous plants have a higher RD/AN ratio (Bruzzese et al., 2010; Hájek and Adamec, 2010). In the active but resting traps of Dionaea and Drosera differences in RD between the trap and petiole are also negligible (Adamec, 2010a; Hájek and Adamec, 2010; Table 2). However during their action, spatio-temporal changes in AN and RD occur. Pavlovič et al. (2010a) showed that AN and ϕPSII decreased and RD increased in response to trigger hair stimulation, simulating prey capture and retention in Dionaea muscipula. As a result of decreased photochemistry, non-photochemical quenching dissipates the absorbed light energy safely (Fig. 4A–C). Jaffe (1973) and Williams and Bennett (1982) showed that traps of Dionaea are expensive, and during trap closure about 29 % of the cellular ATP is lost; this is used for rapid transport of water, resulting in changes in turgor pressure and subsequent closure of the trap. Pavlovič et al. (2010a) showed that rapid changes of AN and RD in the Venus flytrap are due to trigger hair stimulation which generates electrical signals (action potentials, APs) and is independent of trap closure: ‘single hair irritation’ and the ‘prey struggle phase in the closed trap’ also resulted in reduction of AN and stimulation of RD (Fig. 4A–D). Volkov et al. (2007, 2008) and Pavlovič and Mancuso (2011) found that action potential signalling and changes in RD and AN in Dionaea are confined to the traps and are not propagated to the photosynthetic laminae, what may decrease the overall costs. In their subsequent studies, they found that mainly dark enzymatic reactions are targeted, with a negligible effect on light reactions of photosynthesis (Pavlovič et al., 2011a; Vredenberg and Pavlovič, 2013). The effect of electrical signals on photosynthesis and respiration in plants is now well recognized (for reviews, see Fromm and Lautner, 2007; Pavlovič, 2012b; Gallé et al., 2015). Increased RD was also found in separated tentacles of Drosera prolifera in the study of Adamec (2010a), and the author suggested that such an increase is also probably due to electrical signalling in tentacles (Williams and Pickard, 1972a, b; Table 2). Because consumption of ATP during action potentials was documented (Beilby, 2007), the decrease in the ATP/ADP ratio after APs seems to be the factor increasing RD (Pavlovič et al., 2011a). It is very likely that a similar response of APs on photosynthesis and respiration also occurs in the aquatic carnivorous plant Aldrovanda vesiculosa; however this plant has never been studied in this respect.
Fig. 4.
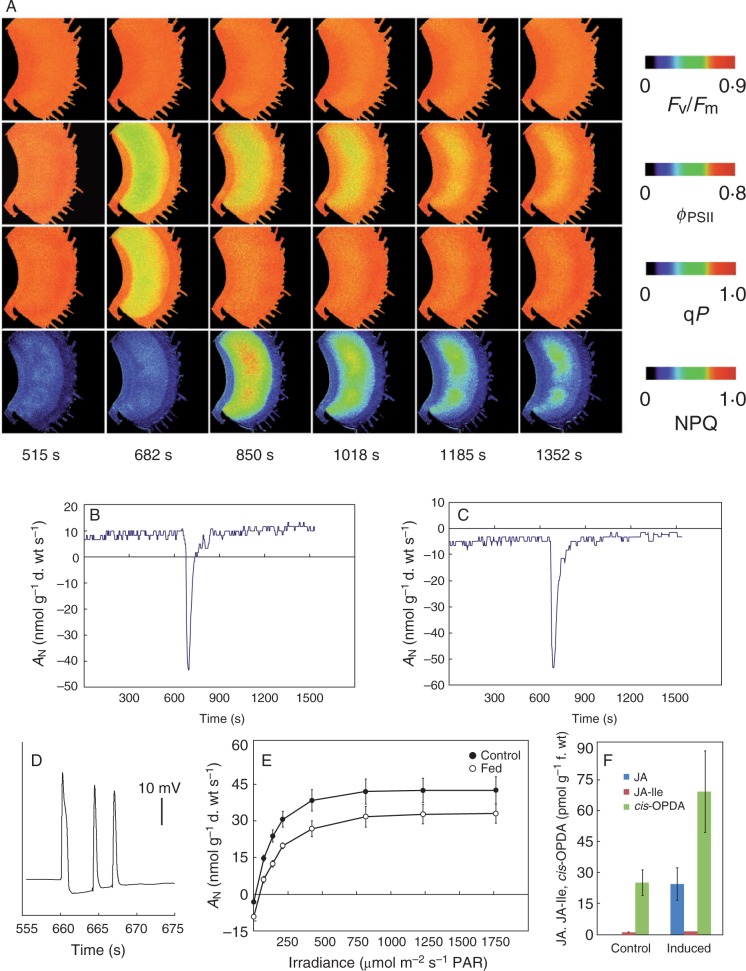
A novel insight into the cost–benefit model in the Venus flytrap (Dionaea muscipula). (A) Spatio-temporal changes of chlorophyll a fluorescence in response to mechanical touches of trigger hairs (at time 660–670 s) and generation of action potentials, maximum quantum yield of photosystem II (Fv/Fm), effective photochemical quantum yield of photosystem II (ϕPSII), photochemical quenching (qP) and non-photochemical quenching (NPQ); for detailed explanations of the kinetics of chlorophyll a fluorescence in Venus flytrap see Pavlovič et al. (2011a). (B) Simultaneous measurement of gas exchange at a light intensity of 80 µmol m–2 s–1 PAR. (C) Measurement of gas exchange in the dark indicating the rate of respiration (RD). (D) Action potentials in the trap in response to mechanical stimulation of trigger hairs. (E) Light response curve of photosynthesis in open control traps and traps in the digestive period 36 h after chemical stimulation with NH4Cl. (D) The endogenous jasmonate level (JA, jasmonic acid; JA-Ile, isoleucine conjugate of jasmonic acid; cis-OPDA, cis-12-oxophytodienoic acid) in trap tissue 36 h after induction with NH4Cl. Data are means ± s.e. (n = 5). For details of the methods, see the Supplementary Data.
The high metabolic or energetic costs of traps also occur during prey digestion. Kruse et al. (2014) found increased RD during the digestive period of the Venus flytrap, hence resulting in a significantly greater RD/AN ratio. We measured the light response curve of photosynthesis and we also found higher RD 36 h after induction of digestion (Fig. 4E, at zero irradiance). This is in accordance with the observations of Robins and Juniper (1980), who found that the mitochondria of stimulated D. muscipula glands closely resemble mitochondria of active animal tissue. Following stimulation, the mitochondria become extended, greatly increasing their surface to volume ratio accompanied by an increase in the density of cristae. Recently, Gao et al. (2015) found that fed D. muscipula traps are frequently short of organic carbon (probably spent by RD and enzyme production), so that they even attract carbon resources from the roots. The behaviour of glands in passive pitcher traps has never been investigated in this respect, so we cannot exclude the possibility that they also increase RD in response to prey capture.
Besides digestion, some chemicals in insect prey can also affect carbon metabolism in carnivorous plants. Using chlorophyll fluorescence imaging, Pavlovič (2010) found that formic acid in ants, a common prey of carnivorous plants, strongly inhibits the photosynthetic reaction in PSII during digestion in Drosera capensis. Formic acid inhibits electron transport on the acceptor side of PSII, particularly from plastoquinone A to plastoquinone B.
What is the benefit of costly electrical signalling in carnivorous plants? For a successful hunt, the fast trap closure triggered by electrical signals must be faster than the prey escape reaction. Electrical signalling is carbon costly in term of decreased AN and increased RD; however, successful insect capture and digestion provides a significant amount of nutrients which can later stimulate photosynthesis (Pavlovič et al., 2010; Kruse et al., 2014). However, the importance of cost–benefit analysis of electrical signalling in carnivorous plants goes beyond fast prey capture. After the rapid closure secures the prey, repeated mechanical stimulation of trigger hairs by struggling prey and generation of hundreds of APs result in further closure and tightening of the trap to a tightly appressed state and secretion of digestive fluid (Affolter and Olivo, 1975). Escalante-Peréz et al. (2011) found that 30 min after prey capture in the Venus flytrap, the level of the jasmonic acid (JA) precursor, 12-oxo-phytodienoic acid (OPDA), increased. Libiaková et al. (2014) also found in the same species an increased level of jasmonates in response to mechanical and chemical stimuli (Fig. 4F). Nakamura et al. (2013) and Mithöfer et al. (2014) found an increased level of JA and its bioactive isoleucine conjugate (JA-Ile) in Drosera capensis in response to prey capture. Libiaková et al. (2014) suggested that jasmonate molecules can regulate production of digestive enzymes in response to mechanical and chemical stimuli from prey. It has been found that mechanical and subsequent electrical activity is not sufficient to trigger the full enzymatic capacity in Drosera and Dionaea, and some chemical signals must be involved (Matušíková et al., 2005; Libiaková et al., 2014; Pavlovič et al., 2014). External application of JA or coronatine, a structural mimic of JA-Ile, bypasses mechanical and chemical signalling and initiates secretion of digestive fluid with high proteolytic activity of the cysteine endopeptidase Dionain, and induces transcription of type I chitinase and ammonium channels in the Venus flytrap (Escalante-Pérez et al., 2011; Scherzer et al., 2013; Paszota et al., 2014; Libiaková et al., 2014). The link between electrical and jasmonate signalling is not novel and has been documented in the systemic response in many non-carnivorous plants (e.g. Hlaváčková et al., 2006; Mousavi et al., 2013). Jasmonates are lipid-derived compounds acting as key signalling molecules in plant stress and defence responses (Wasternack and Hause, 2013), and their accumulation is probably dependent on a cytosolic Ca2+ increase (Fisahn et al., 2004; Vadassery et al., 2014). In the sites that receive the electrical signals, jasmonates mediate defence-responsive gene expression (Mousavi et al., 2013). These induced defences are generally believed to have evolved as a resource-saving strategy because JA acts as a signal to redirect the gene expression and biosynthetic capacity from photosynthesis and growth to defence, and that represent a significant allocation cost for plants, which might be offset by the fitness benefit of not incurring these costs, when defence is not needed (Heil and Baldwin, 2002; Meldau et al., 2012; Vos et al., 2013; Attaran et al., 2014). Moreover, jasmonates inhibit photosynthetic reactions and repress transcription of many photosynthesis-related genes (Herde et al., 1997; Heil and Baldwin, 2002; Hlaváčková et al., 2006; Nabity et al., 2013; Attaran et al., 2014). This cost–benefit explanation has been widely applied to evolution and maintenance of inducible defence (Baldwin, 1998; Heil and Baldwin, 2002; Vos et al., 2013). The finding that jasmonates play a role in plant carnivory is not surprising, because it is now believed that carnivory has evolved from plant defence mechanisms (Juniper et al., 1989; Hatano and Hamada, 2012). The possible hierarchy of consecutive events in trap tissue which are initiated by prey capture adopted from plant defence mechanisms is suggested in Fig. 5. Thus electrical and jasmonate signalling is beneficial for regulation of enzyme and transporter expression in carnivorous plants with active trapping mechanisms, and inducibility allows carnivorous plants to forgo the allocation costs when the prey in the trap is not present. In Fig. 4E, F, the increased accumulation of jasmonates in Venus flytraps 36 h after induction of digestion and decreased AN can be seen. Whether the reduction of AN is the result of increased RD (as discussed above) or reduced photosynthesis as a result of allocation costs is unclear. Venus flytraps strongly change their trap shape from the open to narrowed phase, which complicates interpretation of the data due to the changes of light interception. Nevertheless, an increased RD/AN ratio is an indicator of substantial cost during the digestive period in the Venus flytrap (Kruse et al., 2014). These costs are, however, offset by the benefits of not incurring these costs when digestion is not needed. It remains to be elucidated whether the jasmonates accumulate only in digestive glands or also in mesophyll tissue, and where exactly they reprogramme the gene expression.
Fig. 5.
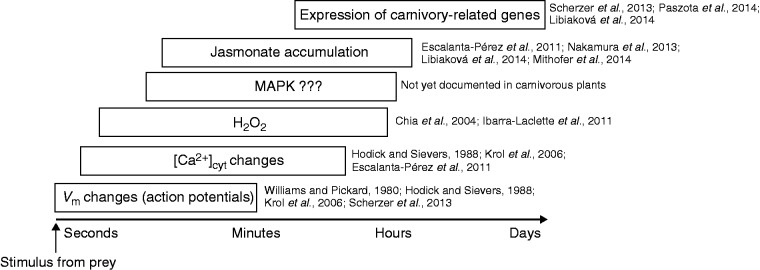
Probable timed hierarchy of consecutive events detectable in carnivorous plants with the active trapping mechanism in response to prey capture adopted from plant defence mechanisms (Maffei et al., 2007). The earliest events measurable are action potentials generated by mechanical stimuli (Williams and Pickard, 1980; Hodick and Sievers, 1988; Krol et al., 2006; Escalante-Pérez et al., 2011) or chemical stimuli from prey (Scherzer et al., 2013), which trigger a cytosolic calcium increase (Escalante-Pérez et al., 2011) and generation of H2O2 (Chia et al., 2004; Ibarra-Laclette et al., 2011). Increased cytosolic Ca2+ is probably sensed by binding to calmodulin protein (CaM) or other calcium-sensing proteins, which can interact with mitogen-activated protein kinases (MAPKs; this part of the signaling pathway has not yet been documented in carnivorous plants). MAPKs regulate biosynthesis of jasmonates which trigger the expression of carnivory-related genes (Scherzer et al., 2013; Nakamura et al., 2013; Libiaková et al., 2014; Mithöfer et al., 2014; Paszota et al., 2014).
This may seem to be in contrast to pitcher plants, which are considered to have passive traps (Juniper et al., 1989). In contrast to Venus flytraps, the digestive fluid and enzymes in carnivorous plants of the genus Nepenthes are present even in the pitcher traps without captured prey (Eilenberg et al., 2006; Hatano and Hamada 2008, 2012). Indeed, some of the digestive enzymes are constitutively expressed (type I chitinase Nkchit2b and S-like RNAase cf-I in Nepenthes khasiana and Cephalotus follicularis, respectively; Eilenberg et al., 2006; Nishimura et al., 2013). However, transcription of some carnivory-related genes in the genus Nepenthes is also regulated by the presence of prey, such as ammonium transporter NaAMT1, H+-ATPase NaPHA3, type I chitinase Nkchit1b, type III chitinase Nrchit1 and thaumatin-like protein (Schulze et al., 1999; An et al., 2001; Eilenberg et al., 2006; Rottloff et al., 2011, 2013). Hatano and Hamada (2012) identified new proteins in digestive fluid of N. alata in response to chitin addition: class III peroxidase (NaPrx1a), β-1,3-glucanase (NaBGLUC2) and class III chitinase (NaCHIT3). Expression of protease, RNase, nuclease and phosphatase is also induced by the presence of appropriate chemical signals (such as nucleic acids, protein and reduced N) in the passive trap of Sarracenia purpurea (Gallie and Chang, 1997). Although the electrical signals have never been documented in passive pitcher traps, the intracellular measurements of membrane potential in the glands of Venus flytrap showed that application of NH4+, the most effective inductor in carnivorous plants, resulted in strong depolarization of membrane potentials due to the action of the ammonium transporter DmAMT1 and even triggered APs (Scherzer et al., 2013). A similar ammonium transporter NaAMT1 was immunodetected in the glands of the carnivorous plant Nepenthes alata (Schulze et al., 1999), and NH4+ is rapidly taken up from digestive fluid (An et al., 2001; Moran et al., 2010); however, no electrophysiological measurements have been done in this species. Also what kind of molecule or phytohormone is involved in transduction of stimuli from prey to changes of gene expression is unknown. It is tempting to assume that carnivorous pitcher traps are not so passive and electrically silent as was previously believed and more investigation in this genus is needed. In contrast, very active traps of bladderworts (Utricularia sp.) probably have constitutive enzyme production and besides enzymes also rely on microbial digestion (Sirová et al., 2003, 2010, 2011; Adamec et al., 2011).
ARE ALL CARNIVOROUS PLANTS REALLY CARNIVOROUS?
Our perception of carnivorous plants as merciless killers catching anything that is moving and careless is being changed. As the first, Cresswell (1998) reported that over half of the weight of dead matter found in the pitchers of Nepenthes ampullaria in Borneo was plant derived. This observation was supported by a study of Moran et al. (2003) who proposed that N. ampullaria exhibits a leaf litter trapping syndrome. The leaf litter trapping syndrome in plants is not novel and is very common in non-carnivorous tank epiphytic bromeliads (Givnish et al., 1997, 2014; Dézerald et al., 2013). The N. ampullaria plant has adapted to this alternative nutrition by an unusual growth pattern and pitcher morphology:
the pitcher lid is reflexed away from pitcher mouth and allows debris to fall directly into the pitcher;
the pitchers sit above the soil surface in a tightly packed ‘carpet’ and the upper pitchers are only rarely produced on the climbing stem;
Nepenthes ampullaria is often found growing beneath the forest canopy, whereas most other lowland species are found predominantly in open, secondary vegetation.
The ‘rain’ of debris from the canopy and tightly packed ‘carpet’ of pitchers positioned above the soil surface may intercept this source of nutrient before it reaches roots of other non-carnivorous species and may provide competitive advantage over non-carnivorous plants co-occurring in the same habitat (Fig. 6A). On the other hand, some traits involved in prey attraction (nectar glands) and retention (waxy zone and lunate cells) are reduced or absent and some are still present (large slippery peristome, chitinase in digestive fluid) (Clarke and Moran 2001; Moran et al. 2003; Rottloff et al., 2011).
Fig. 6.
Unique nutrient sequestration strategies in three species of Nepenthes from Borneo. (A) Leaf litter utilization by N. ampullaria. (B) Pitcher plants N. lowii and N. rajah (C) have modified pitcher morphology for collecting faeces from the mountain tree shrew (Tupaia montana).
Other species, such as Nepenthes lowii, produce pitchers lacking the features normally associated with arthropod prey capture – slippery peristome, waxy zone and viscoelastic digestive fluid. This species is perhaps the most unusual in the genus, being characterized by its strongly constricted upper pitchers with a reflexed lid and numerous bristles on its lower surface (Fig. 6B). Clarke et al. (1997) found that the aerial pitcher of N. lowii contained large amounts of vertebrate faeces, but no invertebrate prey. This combination of aerial pitcher characteristics and contents indicated a possible interaction with vertebrates. Clarke et al. (2009) resolved the special adaptation of this species definitively when they filmed the tree shrew Tupaia montana defecating into the pitchers after feeding on exudates that accumulate on the pitcher lid. Stable N isotope analysis revealed that tree shrew faeces account for between 57 and 100 % of foliar N in mature N. lowii plants. This unique nutrient sequestration strategy was later also found in two other species of the genus (N. rajah and N. macrophylla; Chin et al., 2010). On the basis of unique morphological characteristics of all three species, Chin et al. (2010) concluded that extraordinary modifications to nutrient acquisition strategies in carnivorous plants may occur through simple modifications of trap geometry. The pitchers of N. lowii, N. rajah and N. macrophylla with large orifices, and lids that are concave, elongated and oriented approximately at right angles to the orifice capture faeces of the tree shrew T. montana, whose body size is optimal to defecate into the pitchers (Fig. 6C). Moreover, all three Nepenthes species were shown to produce visual signals, in which the underside of the pitcher lid stood out in high contrast to the adjacent area on the pitcher, in the blue and green wavebands visible to the tree shrews (Moran et al., 2012). Analysis of volatiles extracted from the secretions of the pitcher lids by gas chromatography coupled to mass spectrometry (GC/MS) revealed 44 volatile compounds, including hydrocarbons, alcohols, esters, ketones and sulphur-containing compounds, which are commonly present in sweet fruit and flower odours (Wells et al., 2011). The body of a drowned tree shrew is occasionally found in the pitcher of N. rajah, providing much greater benefits than droppings. Recently it has been documented that beside diurnal T. montana, the nocturnal summit rat (Rattus baluensis) also defecates into the N. rajah pitchers. Temporal segregation of pitcher visits by the two mammal species enables T. montana and R. baluensis to exploit the same resource whilst largely avoiding direct conflict (Greenwood et al., 2011; Wells et al., 2011).
The pitchers of Nepenthes rafflesiana var. elongata (recently described as the new taxon Nepenthes hemsleyana; Scharmann and Grafe, 2013) is very similar to the typical form of N. rafflesiana, but is elongated in all respects. Hardwicke’s woolly bats (Kerivoula hardwickii) roost in the upper pitchers of N. hemsleyana. The elongated pitchers provide enough space for the bats and in return the plant receives additional N input in the form of faeces. It has been estimated that the plant derives 33·8 % of its total foliar N from the bat’s droppings; however, only 20·8 % of traps are occupied (Grafe et al., 2011).
The fact that the genus Nepenthes demonstrates a remarkable variety in pitcher morphology implies that it may be a candidate model for adaptive radiation with regard to N sequestration strategies (Chin et al., 2010; Pavlovič, 2012a). If the changes in pitcher morphology are adaptive, the plant will obtain some increment in fitness. Pavlovič et al. (2011b) tested the hypothesis of whether leaf litter utilization by N. ampullaria can increase photosynthetic efficiency according to Givnish’s cost–benefit model. They found that leaf litter utilization slightly increased AN; however, the nutrient stress was not completely alleviated in comparison with experiments with insect prey (Pavlovič et al., 2009). It seems that these unique nutrient sequestration strategies make the best of a bad situation. Because the insect body contains around 9·8 % (Pavlovič et al., 2009), animal faeces 4·9 % (Chin et al., 2010) and the leaves of co-habiting plant species only 1·2 % of N (Osunkoya et al., 2007), the animal prey is still the best source of N. This might explain why the plants do not completely rely on alternative sources of nutrients and still capture some insect prey (Pavlovič et al., 2011b).
A unique sequestration strategy is not confined to Nepenthes. Roridula plants capture insects by using a sticky hydrophobic secretion on the heads of immobile tentacles. It has been hypothesized that Roridula leaves absorb N from the faeces of the obligately associated, carnivorous hemipteran bug Pameridea roridulae (Anderson, 2005; Plachno et al., 2009). Also, the non-carnivorous plants Bromelia balansae and Paepalanthus bromelioides (now considered as carnivorous) obtain a significant amount of N from spider faeces (Romero et al., 2006; Nishi et al., 2013) as does Vriesea gigantea from amphibian excrement (Inselsbacher et al., 2007). Peroutka et al. (2008) documented that algae of 45 genera were found in Utricularia bladders, which form up to 80 % of total prey. Ninety per cent of them were dead. This is the reason why the authors named their paper appropriately: ‘Utricularia – a vegetarian carnivorous plant’. The algae probably entered the traps due to an incidental and spontaneous firing (Adamec, 2011, 2012; Vincent et al., 2011a). Koller-Peroutka et al. (2015) confirmed the ecological importance of autonomous firing in Utricularia and found that the contribution of pollen grains and algae to the nutrition of Utricularia plant is comparable with the benefit gained from prey animals. Harder and Zemlin (1968) demonstrated in axenic cultures of Pinguicula lusitanica, grown on agar without N and P for 8 weeks, nutrient utilization from supplied Pinus pollen. The pollen-fed plants grew faster, contained more chlorophyll, and aged more slowly. In contrast to unfed plants, they initiated flower buds very early and flowered richly. Thus, the Pinguicula species with broad leaves (and possibly also some Drosera, e.g. Drosera schizandra growing beneath the forest canopy in Queensland) may benefit from aerial rain of pollen and probably also of spores, seeds and leaf fragments under natural conditions.
CONCLUSIONS
In 2014 the cost–benefit model for evolution of plant carnivory celebrated 30 years of existence. During that time, the cost–benefit model became a framework for interpretation of results from a wide range of experimental studies on many carnivorous plant species. The relationship between nutrients gained from prey digestion and photosynthesis has been central in cost–benefit model analyses. In the years since the cost–benefit model was initially proposed, several studies have now confirmed two of its key assumptions, showing both that traps have lower photosynthetic rates than asimilatory leaves, and that photosynthesis of carnivorous plants increases as a result of feeding (Table 1). Recent research has shown that active trapping mechanisms are costly in terms of spatial and temporal activation of respiration and inactivation of photosynthesis. Water pumping in Utricularia bladders, prey digestion, and electrical signalling in Dionaea, Drosera and probably Aldrovanda represent energetic costs for plants. Against these, jasmonate signalling has evolved as a cost-saving strategy, because instead of producing costly digestive enzymes permanently, carnivorous plants often activate their production only in response to electrical and chemical signals that implicate the presence of prey. The involvement of jasmonates and chitinases in the digestive process support the hypothesis that carnivory may have evolved from plant defence mechanisms – and it is not only the carnivory. Recent works have revealed different strategies that carnivorous plants employ in acquiring nutrients and that the cost–benefit model can also be applied here.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and give the materials and methods for the data in Figs 3 and 4.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Education, Science, Research and Sport of the Slovak Republic [VEGA 1/0304/15] and grant LO1204 from the National Program of Sustainability I. We thank Dr. Lubomír Adamec (Třeboň, Czech Republic) for elemental analyses, Eva Hirnerová and Kristýna Floková (Palacký University in Olomouc, Czech Republic) for phytohormone analyses, Professor Thomas Rost (University of California, USA) for language corrections, and two anonymous reviewers for their constructive comments, which improved our manuscript.
LITERATURE CITED
- Adamec L. 1997. Mineral nutrition of carnivorous plants – a review. Botanical Review 63: 273–299. [Google Scholar]
- Adamec L. 2002. Leaf absorption of mineral nutrients in carnivorous plants stimulates root nutrient uptake. New Phytologist 155: 89–100. [DOI] [PubMed] [Google Scholar]
- Adamec L. 2003. Zero water flow in the carnivorous genus Genlisea. Carnivorous Plant Newsletter 32: 46–48. [Google Scholar]
- Adamec L. 2006. Respiration and photosynthesis of bladders and leaves of aquatic Utricularia species. Plant Biology 8: 765–769. [DOI] [PubMed] [Google Scholar]
- Adamec L. 2007. Oxygen concentrations inside the traps of the carnivorous plants Utricularia and Genlisea (Lentibulariaceae). Annals of Botany 100: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec L. 2008. The influence of prey capture on photosynthetic rate in two aquatic carnivorous plant species. Aquatic Botany 89: 66–70. [Google Scholar]
- Adamec L. 2010a. Dark respiration of leaves and traps of terrestrial carnivorous plants: are there greater energetic costs in traps? Central European Journal of Biology 5: 121–124. [Google Scholar]
- Adamec L. 2010b. Ecophysiological look at organ respiration in carnivorous plants: a review. In: Osterhoudt G, Barhydt J, eds. Cell respiration and cell survival: processes, types and effects. New York: Nova Science Publishers, Inc., 225–235. [Google Scholar]
- Adamec L. 2011. The comparison of mechanically stimulated and spontaneous firings in traps of aquatic carnivorous Utricularia species. Aquatic Botany 94: 44–49. [Google Scholar]
- Adamec L. 2012. Firing and resetting characteristics of carnivorous Utricularia reflexa traps: physiological or only physical regulation of trap triggering? Phyton 52: 281–290. [Google Scholar]
- Adamec L, Vrba J, Sirová D. 2011. Fluorescence tagging of phosphatase and chitinase activity on different structures of Utricularia traps. Carnivorous Plant Newsletter 40: 68–73. [Google Scholar]
- Adlassnig W, Peroutka M, Lendl T. 2011. Traps of carnivorous plants as habitat: composition of the fluid, biodiversity and mutualistic activities. Annals of Botany 107: 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter JM, Olivo RF. 1975. Action potentials in Venus’s-flytraps: long term observations following the capture of prey. American Midland Naturalist 93: 443–445. [Google Scholar]
- Albert VA, Williams SE, Chase MW. 1992. Carnivorous plants: phylogeny and structural evolution. Science 257: 1491–1495. [DOI] [PubMed] [Google Scholar]
- An C-I., Fukusaki E-I, Kobayashi A. 2001. Plasma-membrane H+-ATPases are expressed in pitchers of the carnivorous plant Nepenthes alata Blanco. Planta 212: 547–555. [DOI] [PubMed] [Google Scholar]
- Anderson B. 2005. Adaptations to foliar absorption of faeces: a pathway in plant carnivory. Annals of Botany 95: 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Midgley JJ. 2003. Digestive mutualism, an alternate pathway in plant carnivory. Oikos 102: 221–224. [Google Scholar]
- Attaran E, Major IT, Cruz JA, Rosa BA, et al. 2014. Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant Physiology 165: 1302–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT. 1998. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proceedings of the National Academy of Sciences, USA 95: 8113–8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazile V, Le Moguédec G, Marshall DJ, Gaume L. 2015. Fluid physico-chemical properties influence capture and diet in Nepenthes pitcher plants. Annals of Botany 115: 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazile V, Moran JA, Moguédec GL, Marshall DJ, Gaume L. 2012. A carnivorous plant fed by its symbiont: a unique multi-faceted nutritional mutualism. PLoS One 7: e36179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilby MJ. 2007. Action potential in Charophytes. International Review of Cytology 257: 43–82. [DOI] [PubMed] [Google Scholar]
- Bohn HF, Federle W. 2004. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences, USA 101: 14138–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme V, Gounand I, Alaux C, Jousselin E, Barthélémy D, Gaume L. 2011a. The plant-ant Camponotus schmitzi helps its carnivorous host-plant Nepenthes bicalcarata to catch its prey. Journal of Tropical Ecology 27: 15–24. [Google Scholar]
- Bonhomme V, Pelloux-Prayer H, Jousselin E, Forterre Y, Labat J-J, Gaume L. 2011b. Slippery or sticky? Functional diversity in the trapping strategy of Nepenthes carnivorous plants. New Phytologist 191: 545–554. [DOI] [PubMed] [Google Scholar]
- Brittnacher J. 2011. Murderous plants. Carnivorous Plant Newsletter 40: 17–18. [Google Scholar]
- Bruzzese BM, Bowler R, Massicotte HB, Fredeen AL. 2010. Photosynthetic light response in three carnivorous plant species: Drosera rotundifolia, D. capensis and Sarracenia leucophylla. Photosynthetica 48:103–109. [Google Scholar]
- Chandler GE, Anderson JW. 1976. Studies on the nutrition and growth of Drosera species with reference to the carnivorous habit. New Phytologist 76: 129–141. [Google Scholar]
- Chase MW, Christenhusz MJM, Sanders D, Fay MF. 2009. Murderous plants: Victorian Gothic, Darwin and modern insights into vegetable carnivory. Botanical Journal of the Linnean Society 161: 329–356. [Google Scholar]
- Chia TF, Aung HH, Osipov AN, Goh NK, Chia LS. 2004. Carnivorous pitcher plant uses free radicals in the digestion of prey. Redox Report 9: 255–261. [DOI] [PubMed] [Google Scholar]
- Chin L, Moran JA, Clarke C. 2010. Trap geometry in three giant montane pitcher plant species from Borneo is a function of tree shrew body size. New Phytologist 186: 461–470. [DOI] [PubMed] [Google Scholar]
- Clarke CM. 1997. Nepenthes of Borneo. Kota Kinabalu, Malaysia: Natural History Publication. [Google Scholar]
- Clarke CM, Bauer U, Lee CC, Tuen AA, Rembold K, Moran JA. 2009. Tree shrew lavatories: a novel sequestration strategy in a tropical pitcher plant. Biology Letters 5: 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CM, Moran JA. 2001. Ecology. In: Clarke CM, ed. Nepenthes of Sumatra and Peninsular Malaysia. Kota Kinabalu, Malaysia: Natural History Publication, 29–75. [Google Scholar]
- Cresswell JE. 1998. Morphological correlates of necromass accumulation in the traps of an Eastern tropical pitcher plant, Nepenthes ampullaria Jack, and observations on the pitcher infauna and its reconstitution following experimental removal. Oecologia 113: 383–390. [DOI] [PubMed] [Google Scholar]
- Darwin C. 1875. Insectivorous plants. London: John Murray. [Google Scholar]
- Darwin F. 1878. Experiments on the nutritions of Drosera rotundifolia. Journal of Linnean Society – Botany 17: 17–32. [Google Scholar]
- Dézerald O, Leroy C, Corbara B, et al. 2013. Food-web structure in relation to environmental gradients and predator–prey ratios in tank-bromeliad ecosystems. PLoS One 8: e71735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon KW, Pate JS, Bailey WJ. 1980. Nitrogen nutrition of the tuberous sundew Drosera erythrorhiza Lindl. With special reference to catch of arthropod fauna by its glandular leaves. Australian Journal of Botany 28: 283–297. [Google Scholar]
- Eilenberg H, Pnini-Cohen S, Schuster S, Movtchan A, Zilberstein A. 2006. Isolation and characterization of chitinase genes from pitchers of the carnivorous plant Nepenthes khasiana. Journal of Experimental Botany 57: 2775–2784. [DOI] [PubMed] [Google Scholar]
- Ellison AM. 2006. Nutrient limitation and stoichiometry of carnivorous plants. Plant Biology 8: 740–747. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Adamec L. 2011. Ecophysiological traits of terrestrial and aquatic carnivorous plants: are the costs and benefits the same? Oikos 120: 1721–1731. [Google Scholar]
- Ellison AM, Farnsworth EJ. 2005. The cost of carnivory for Darlingtonia californica (Sarraceniaceae): evidence from relationships among leaf traits. American Journal of Botany 92: 1085–1093. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. 2001. Evolutionary ecology of carnivorous plants. Trends in Ecology and Evolution 16: 623–629. [Google Scholar]
- Ellison AM, Gotelli NJ. 2002. Nitrogen availability alters the expression of carnivory in the northern pitcher plant, Sarracenia purpurea. Proceedings of the National Academy of Sciences, USA 99: 4409–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. 2009. Energetics and the evolution of carnivorous plants – Darwin’s ‘most wonderful plants in the world’. Journal of Experimental Botany 60: 19–42. [DOI] [PubMed] [Google Scholar]
- Escalante-Pérez M, Krol E, Stange A, et al. 2011. A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proceedings of the National Academy of Sciences, USA 108: 15492–15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19. [DOI] [PubMed] [Google Scholar]
- Farnsworth EJ, Ellison AM. 2008. Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous plant species. Journal of Ecology 96: 213–221. [Google Scholar]
- Feller U, Anders I, Mae T. 2008. Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. Journal of Experimental Botany 59: 1615–1624. [DOI] [PubMed] [Google Scholar]
- Fisahn J, Herde O, Willmitzer L, Peňa-Cortés H. 2004. Analysis of the transient increase in cytosolic Ca2+ during the action potential of higher plants with high temporal resolution: requirement of Ca2+ transients for induction of jasmonic acid biosynthesis and PINII gene expression. Plant and Cell Physiology 45: 456–459. [DOI] [PubMed] [Google Scholar]
- Frank JH, O’Meara GF. 1984. The bromeliad Catopsis berteroniana traps terrestrial arthropods but harbors Wyeomyia larvae (Diptera: Culicidae). Florida Entomologist 67: 418–424. [Google Scholar]
- Fromm J, Lautner S. 2007. Electrical signals and their physiological significance in plants. Plant, Cell and Environment 30: 249–257. [DOI] [PubMed] [Google Scholar]
- Gallé A, Lautner S, Flexas J, Fromm J. 2015. Environmental stimuli and physiological responses: the current view on electrical signalling. Environmental and Experimental Botany 114: 15–21. [Google Scholar]
- Gallie DR, Chang S-C. 1997. Signal transduction in the carnivorous plant Sarracenia purpurea: regulation of secretory hydrolase expression during development and in response to resources. Plant Physiology 115: 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Kapralov MV, Andralojc PJ, et al. 2014. Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant, Cell and Environment 37: 1989–2001. [DOI] [PubMed] [Google Scholar]
- Gao P, Loeffler TS, Honsel A, et al. 2015. Integration of trap- and root-derived nitrogen nutrition of carnivorous Dionaea muscipula. New Phytologist 205: 1320–1329. [DOI] [PubMed] [Google Scholar]
- Gaume L, Forterre Y. 2007. A viscoelastic deadly fluid in carnivorous pitcher plants. PLoS One 2: e1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Perret P, Gorb E, Gorb S, Labat JJ, Rowe N. 2004. How do plant waxes cause flies to slide? Experimental tests of wax-based trapping mechanisms in three pitfall carnivorous plants. Arthropod Structure and Development 33: 103–111. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. 1989. Ecology and evolution of carnivorous plants. In: Abrahamson WG, ed. Plant–animal interactions. New York: McGraw-Hill, 243–290. [Google Scholar]
- Givnish TJ, 2015. New evidence on the origin of carnivorous plants. Proceedings of the National Academy of Sciences, USA 112: 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish TJ, Barfuss MHJ, Van Ee B, et al. 2014. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution 71: 55–78. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. 1984. Carnivory in the bromeliad Brocchinia reducta with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient poor habitats. American Naturalist 124: 479–497. [Google Scholar]
- Givnish TJ, Sytsma KJ, Smith JF, Hahn WJ, Benzing DH, Burkhardt EM. 1997. Molecular evolution and adaptive radiation in Brocchinia (Bromeliaceae: Pitcairnioideae) atop tepuis of the Guayana Shield. In: Givnish TJ, Sytsma KJ, eds. Molecular evolution and adaptive radiation. New York: Cambridge University Press, 259–311. [Google Scholar]
- Gorb E, Kastner V, Peressadko A, et al. 2004. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. Journal of Experimental Biology 207: 2947–2963. [DOI] [PubMed] [Google Scholar]
- Gowda DC, Reuter G, Schauer R. 1983. Structural studies of an acidic polysaccharide from the mucin secreted by Drosera capensis. Carbohydrate Research 113: 113–124. [Google Scholar]
- Grafe TU, Schöner CR, Kerth G, Junaidi A, Schöner MG. 2011. A novel resource–service mutualism between bats and pitcher plants. Biology Letters 7: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood M, Clarke C, Lee CC, Gunsalam A, Clarke RH. 2011. A unique resource mutualism between the giant Bornean pitcher plant, Nepenthes rajah, and members of a small mammal community. PLoS One 6: e21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájek T, Adamec L. 2010. Photosynthesis and dark respiration of leaves of terrestrial carnivorous plants. Biologia 65: 69–74. [Google Scholar]
- Harder R, Zemlin I. 1968. Blütenbildung von Pinguicula lusitanica in vitro durch Fütterung mit pollen. Planta 78: 72–78. [DOI] [PubMed] [Google Scholar]
- Hatano N, Hamada T. 2008. Proteome analysis of pitcher fluid of the carnivorous plant Nepenthes alata. Journal of Proteome Research 7: 809–816. [DOI] [PubMed] [Google Scholar]
- Hatano N, Hamada T. 2012. Proteomic analysis of secreted protein induced by a component of prey in pitcher fluid of the carnivorous plant Nepenthes alata. Journal of Proteomics 75: 4844–4852. [DOI] [PubMed] [Google Scholar]
- He J, Zain A. 2012. Photosynthesis and nitrogen metabolism of Nepenthes alata in response to inorganic NO3– and organic prey N in the greenhouse. International Scholarly Research Network Botany ID 263270. [Google Scholar]
- Heil M, Baldwin IT. 2002. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends in Plant Science 7: 61–67. [DOI] [PubMed] [Google Scholar]
- Herde O, Peña-Cortéz H, Willmitzer L, Fisahn J. 1997. Stomatal responses to jasmonic acid, linolenic acid and abscisic acid in wild-type and ABA-deficient tomato plants. Plant, Cell and Environment 20: 136–141. [Google Scholar]
- Hlaváčková V, Krchňák P, Nauš J, Novák O, Špundová M, Strnad M. 2006. Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta 225: 235–244. [DOI] [PubMed] [Google Scholar]
- Hodick D, Sievers A. 1988. The action potential of Dionaea muscipula Ellis. Planta 174: 8–18. [DOI] [PubMed] [Google Scholar]
- Ibarra-Laclette E, Albert VA, Pérez-Torres CA, et al. 2011. Transcriptomics and molecular evolutionary rate analysis of the bladderwort (Utricularia), a carnivorous plant with a minimal genome. BMC Plant Biology 11: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselsbacher E, Cambui CA, Richter A, Stange CF, Mercier H, Wanek W. 2007. Microbial activities and foliar uptake of nitrogen in the epiphytic bromeliad Vriesea gigantea. New Phytologist 175: 311–320. [DOI] [PubMed] [Google Scholar]
- Jaffé K, Michelangeli F, Gonzales JM, Miras B, Ruiy MC. 1992. Carnivory in pitcher plants of the genus Heliamphora (Sarraceniaceae). New Phytologist 122: 733–744. [Google Scholar]
- Jaffe MJ. 1973. The role of ATP in mechanically stimulated rapid closure of Venus’s flytrap. Plant Physiology 51: 17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobson RW, Nielsen R, Laakkonen L, Wikström M, Albert VA. 2004. Adaptive evolution of cytochrome c oxidase: infrastructure for a carnivorous plant radiation. Proceedings of the National Academy of Sciences, USA 101: 18064–18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. 1989. The carnivorous plants. London: Academic Press. [Google Scholar]
- Karagatzides JD, Ellison AM. 2009. Construction costs, payback times, and the leaf economics of carnivorous plants. American Journal of Botany 96: 1612–1619. [DOI] [PubMed] [Google Scholar]
- Karlsson PS, Pate JS. 1992. Contrasting effect of supplementary feeding of insects or mineral nutrients on the growth and nitrogen and phosphorous economy of pygmy species of Drosera. Oecologia 92: 8–13. [DOI] [PubMed] [Google Scholar]
- Knight SE. 1992. Costs of carnivory in the common bladderwort, Utricularia macrorhiza. Oecologia 89: 348–355. [DOI] [PubMed] [Google Scholar]
- Knight SE., Frost TM. 1991. Bladder control in Utricularia macrorhiza: lake specific variation in plant investment in carnivory. Ecology 72: 728–734. [Google Scholar]
- Koller-Peroutka M, Lendl T, Watzka M, Adlassnig W. 2015. Capture of algae promotes growth and propagation in aquatic Utricularia. Annals of Botany 115: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol E, Dziubinska H, Stolarz M, Trebacz K. 2006. Effects of ion channel inibitors on cold- and electrically-induced action potentials in Dionaea muscipula. Biologia Plantarum 50: 411–416. [Google Scholar]
- Król E, Plachno BJ, Adamec L, Stolarz M, Dziubinska H., Trebacsz K. 2012. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Annals of Botany 109: 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J, Gao P, Honsel A, et al. 2014. Strategy of nitrogen acquisition and utilization by carnivorous Dionaea muscipula. Oecologia 174: 839–851. [DOI] [PubMed] [Google Scholar]
- Laakkonen L, Jobson RW, Albert VA. 2006. A new model for the evolution of carnivory in the bladderwort plant (Utricularia): adaptive changes in cytochrome c oxidase (COX) provide respiratory power. Plant Biology 8: 758–764. [DOI] [PubMed] [Google Scholar]
- Libiaková M, Floková K, Novák O, Slováková Ľ, Pavlovič A. 2014. Abundance of cysteine endopeptidase Dionain in digestive fluid of Venus flytrap (Dionaea muscipula Ellis) is regulated by different stimuli from prey through jasmonates. PLoS One 9: e104424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei ME, Mithöffer A, Boland W. 2007. Before gene expression: early events in plant–insect interaction. Trends in Plant Science 12: 310–316. [DOI] [PubMed] [Google Scholar]
- Matušíková I, Salaj J, Moravčíková J, Mlynárová L, Nap JP, Libantová J. 2005. Tentacles of in vitro-grown round-leaf sundew (Drosera rotundifolia L.) show induction of chitinase activity upon mimicking the presence of prey. Planta 222: 1020–1027. [DOI] [PubMed] [Google Scholar]
- Meldau S, Ullman-Zeunert L, Govind G, Bartram S, Baldwin IT. 2012. MAPK-dependent JA and SA signalling in Nicotiana attenuata affects plant growth and fitness during competition with conspecifics. BMC Plant Biology 12: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez M, Karlsson PS. 1999. Costs and benefits of carnivory in plants: insights from the photosynthetic performance of four carnivorous plants in a subarctic environment. Oikos 86: 105–112. [Google Scholar]
- Mithöfer A, Reichelt M, Nakamura Y. 2014. Wound and insect-induced jasmonate accumulation in carnivorous Drosera capensis: two sides of the same coin. Plant Biology 5: 982–987. [DOI] [PubMed] [Google Scholar]
- Moran JA, Moran AJ. 1998. Foliar reflectance and vector analysis reveal nutrient stress in prey-deprived pitcher plants (Nepenthes rafflesiana). International Journal of Plant Sciences 159: 996–1001. [Google Scholar]
- Moran JA, Clarke CM, Hawkins BJ. 2003. From carnivore to detritivore? Isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. International Journal of Plant Sciences 164: 635–639. [Google Scholar]
- Moran JA, Clarke C, Greenwood M, Chin L. 2012. Tuning of color contrast signals to visual sensitivity maxima of tree shrews by three Bornean highland Nepenthes species. Plant Signaling and Behavior 7: 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JA, Hawkins BJ, Gowen BE, Robbins SL. 2010. Ion fluxes across the pitcher walls of three Bornean Nepenthes pitcher plant species: flux rates and gland distribution patterns reflect nitrogen sequestration strategies. Journal of Experimental Botany 61: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. 2013. Glutamate receptor-like genes mediate leaf-to-leaf wound signals. Nature 500: 422–426. [DOI] [PubMed] [Google Scholar]
- Nabity PD, Zavala JA, DeLucia EH. 2013. Herbivore induction of jasmonic acid and chemical defences reduce photosynthesis in Nicotiana attenuata. Journal of Experimental Botany 64: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Reichelt M, Mayer VE, Mithöfer A. 2013. Jasmonates trigger prey-induced formation of ‘outer stomach’ in carnivorous sundew plants. Proceedings of the Royal Society B: Biological Sciences 280: 20130228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi AH, Vasconcellos-Neto J, Romero GQ. 2013. The role of multiple partners in a digestive mutualism with a protocarnivorous plant. Annals of Botany 111: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura E, Kawahara M, Kodaira R, et al. 2013. S-like ribonuclease gene expression in carnivorous plants. Planta 238: 955–967. [DOI] [PubMed] [Google Scholar]
- Osunkoya OO, Daud SD, Di-Giusto B, Wimmer FL, Holige TM. 2007. Construction costs and physico-chemical properties of the assimilatory organs of Nepenthes species in northern Borneo. Annals of Botany 99: 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osunkoya OO, Daud SD, Wimmer FL. 2008. Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Annals of Botany 102: 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszota P, Escalante-Perez M, Thomsen LR, et al. 2014. Secreted major Venus flytrap chitinase enables digestion of Arthropod prey. Biochimica et Biophysica Acta 1844: 374–383. [DOI] [PubMed] [Google Scholar]
- Pavlovič A. 2010. Spatio-temporal changes of photosynthesis in carnivorous plants in response to prey capture, retention and digestion. Plant Signaling and Behavior 5: 1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A. 2011. Photosynthetic characterization of Australian pitcher plant Cephalotus follicularis. Photosynthetica 49: 253–258. [Google Scholar]
- Pavlovič A. 2012a. Adaptive radiation with regard to nutrient sequestration strategies in the carnivorous plants of the genus Nepenthes. Plant Signaling and Behavior 7: 295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A. 2012b. The effect of electrical signals on photosynthesis and respiration. In: Volkov A, ed. Plant electrophysiology – signaling and responses. Berlin: Springer-Verlag, 33–62. [Google Scholar]
- Pavlovič A, Mancuso S. 2011. Electrical signaling and photosynthesis. Can they co-exist together? Plant Signaling and Behavior 6: 840–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Masarovičová E, Hudák J. 2007. Carnivorous syndrome in Asian pitcher plants of the genus Nepenthes. Annals of Botany 100: 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Singerová L, Demko V, Hudák J. 2009. Feeding enhances photosynthetic efficiency in the carnivorous pitcher plant Nepenthes talangensis. Annals of Botany 104: 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Demko V, Hudák J. 2010a. Trap closure and prey retention in Venus flytrap (Dionaea muscipula Ellis.) temporarily reduces photosynthesis and stimulates respiration. Annals of Botany 105: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Singerová L, Demko V, Šantrůček J, Hudák J. 2010b. Root nutrient uptake enhances photosynthetic assimilation in prey-deprived carnivorous pitcher plant Nepenthes talangensis. Photosynthetica 48: 227–233. [Google Scholar]
- Pavlovič A, Slováková Ľ, Pandolfi C, Mancuso S. 2011a. On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis.). Journal of Experimental Botany 62: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Slováková Ľ, Šantrůček J. 2011b. Nutritional benefit from leaf litter utilization in the pitcher plants Nepenthes ampullaria. Plant, Cell and Environment 34: 1865–1873. [DOI] [PubMed] [Google Scholar]
- Pavlovič A, Krausko M, Libiaková M, Adamec L. 2014. Feeding on prey increases photosynthetic efficiency in the carnivorous sundew Drosera capensis. Annals of Botany 113: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira CG, Almenara DP, Winter CE, Fritsch CE, Lambers H, Oliviera RS. 2012. Underground leaves of Philcoxia trap and digest nematodes. Proceedings of the National Academy of Sciences, USA 109: 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroutka M, Adlassnig W, Volgger M, Lendl T, Url WG, Lichtscheidl IK. 2008. Utricularia: a vegetarian carnivorous plant? Plant Ecology 199: 153–162. [Google Scholar]
- Plachno B, Adamec L, Huet H. 2009. Mineral nutrient uptake from prey and glandular phosphatase activity as a dual test of carnivory in semi-desert plants with glandular leaves suspected of carnivory. Annals of Botany 104: 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppinga S, Hartmeyer SRH, Seidel R. 2012. Catapulting tentacles in a sticky carnivorous plant. PLoS One 7: e45735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner T, Specht CD. 2011. A sticky situation: assessing adaptations for plant carnivory in the Caryophyllales by means of stochastic character mapping. International Journal of Plant Sciences 172: 889–901. [Google Scholar]
- Revill MJW, Stanley S, Hibberd JM. 2005. Plastid genome structure and loss of photosynthetic ability in the parasitic genus Cuscuta. Journal of Experimental Botany 56: 2477–2486. [DOI] [PubMed] [Google Scholar]
- Rice BM. 2011. What exactly is a carnivorous plant? Carnivorous Plant Newsletter 40: 19–22. [Google Scholar]
- Riedel M, Eichner A, Jetter R. 2003. Slippery surfaces of carnivorous plants: composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta 218: 87–97. [DOI] [PubMed] [Google Scholar]
- Rischer H, Hamm A, Bringmann G. 2002. Nepenthes insignis uses a C2-portion of the carbon skeleton of l-alanine acquired via its carnivorous organs, to build up the allelochemical plumbagin. Phytochemistry 59: 603–609. [DOI] [PubMed] [Google Scholar]
- Robins RJ, Juniper BE. 1980. The secretory cycle of Dionaea muscipula Ellis. I. The fine structure and the effect of stimulation on the fine structure of the digestive gland cells. New Phytologist 86: 279–296. [Google Scholar]
- Romero GQ, Mazzafera P, Vasconcellos-Neto J, Trivelin PCO. 2006. Bromeliad-living spiders improve host plant nutrition and growth. Ecology 87: 803–808. [DOI] [PubMed] [Google Scholar]
- Rottloff S, Stieber R, Maischak H, Turini FG, Heubl G, Mithöfer A. 2011. Functional characterization of a class III acid endochitinase from the traps of the carnivorous pitcher plant genus, Nepenthes. Journal of Experimental Botany 62: 4639–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottloff S, Mithöfer A, Müller U, Kilper R. 2013. Isolation of viable multicellular glands from tissue of the carnivorous plant, Nepenthes. Journal of Visualized Experiments 82: e50993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski E-M, Seyfullaha LJ, Sadowski F, Fleischmann A, Behling H, Schmidt AR. 2015. Carnivorous leaves from Baltic amber. Proceedings of the National Academy of Sciences, USA 112: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmann M, Grafe TU. 2013. Reinstatement of Nepenthes hemsleyana (Nepenthaceae), an endemic pitcher plant from Borneo, with a discussion of associated Nepenthes taxa. Blumea 58: 8–12. [Google Scholar]
- Scherzer S, Krol E, Kreuzer I, et al. 2013. The Dionaea muscipula ammonium channel DmAMT1 provides NH4+ uptake associated with Venus flytrap’s prey digestion. Current Biology 23: 1649–1657. [DOI] [PubMed] [Google Scholar]
- Schulze W, Frommer WB, Ward JM. 1999. Transporters for ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant Nepenthes. The Plant Journal 17: 637–646. [DOI] [PubMed] [Google Scholar]
- Sirová D, Adamec L, Vrba J. 2003. Enzymatic activities in traps of four aquatic species of the carnivorous genus Utricularia. New Phytologist 159: 669–675. [DOI] [PubMed] [Google Scholar]
- Sirová D, Borovec J, Šantrůčková H, Šantrůček J, Vrba J, Adamec L. 2010. Utricularia carnivory revisited: plants supply photosynthetic carbon to traps. Journal of Experimental Botany 61: 99–103. [DOI] [PubMed] [Google Scholar]
- Sirová D, Borovec J, Picek T, Adamec L, Nedbalová L, Vrba J. 2011. Ecological implications of organic carbon dynamics in the traps of aquatic carnivorous Utricularia plants. Functional Plant Biology 38:583–593. [DOI] [PubMed] [Google Scholar]
- Sydenham PH, Findlay GP. 1975. Transport of solutes and water by resetting bladders of Utricularia. Australian Journal of Plant Physiology 2: 335–351. [Google Scholar]
- Thorén LM, Karlsson S. 1998. Effects of supplementary feeding on growth and reproduction of three carnivorous plant species in subarctic environment. Journal of Ecology 86: 501–510. [Google Scholar]
- Thorén LM, Tuomi J, Kämäräinen T, Laine K. 2003. Resource availability affects investment in carnivory in Drosera rotundifolia. New Phytologist 159: 507–511. [DOI] [PubMed] [Google Scholar]
- Thornham DG, Smith JM, Grafe TU, Federle W. 2012. Setting the trap: cleaning behaviour of Camponotus schmitzi ants increases long-term capture efficiency of their pitcher plant host, Nepenthes bicalcarata. Functional Ecology 26:11–19. [Google Scholar]
- Vadassery J, Reichelt M, Jimenez-Aleman GH, Boland W, Mithöfer A. 2014. Neomycin inhibition of (+)-7-iso-lasmonoyl-l-isoleucine accumulation and signaling. Journal of Chemical Ecology 40: 676–686. [DOI] [PubMed] [Google Scholar]
- Vincent O, Roditchev I, Marmottant P. 2011a. Spontaneous firings of carnivorous aquatic Utricularia traps: temporal patterns and mechanical oscillations. PLoS One 6: e20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent O, Weißkopf C, Poppinga S, et al. 2011b. Ultra-fast underwater suction traps. Proceedings of the Royal Society B: Biological Sciences 278: 2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Adesina T, Jovanov E. 2007. Closing of Venus flytrap by electrical stimulation of motor cells. Plant Signaling and Behavior 2: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Adesina T, Markin VS, Jovanov E. 2008. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiology 146: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos IA, Pieterse CMJ, van Wees SCM. 2013. Costs and benefits of hormone-regulated plant defences. Plant Pathology 62: 43–55. [Google Scholar]
- Vredenberg W, Pavlovič A. 2013. Chlorophyll a fluorescence induction (Kautsky curve) in a Venus flytrap (Dionaea muscipula) leaf after mechanical trigger hair irritation. Journal of Plant Physiology 170: 242–250. [DOI] [PubMed] [Google Scholar]
- Wakefield AE, Gotelli NJ, Wittman SE, Ellison AM. 2005. Prey addition alters nutrient stoichiometry of the carnivorous plant Sarracenia purpurea. Ecology 86: 1737–1743. [Google Scholar]
- Wasternack C, Hause B. 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany 111: 1024–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K, Lakim MB, Schulz S, Ayasse M. 2011. Pitchers of Nepenthes rajah collect faecal droppings from both diurnal and nocturnal small mammals and emit fruity odour. Journal of Tropical Ecology 27:347–353. [Google Scholar]
- Wicke S, Schäferhoff B, dePamphilis CW, Müller KF. 2014. Disproportional plastome-wide increase of substitution rates and relaxed purifying selection in genes of carnivorous Lentibulariaceae. Molecular Biology and Evolution 31: 529–545. [DOI] [PubMed] [Google Scholar]
- Williams SE, Bennett AB. 1982. Leaf closure in the Venus flytrap: an acid growth response. Science 218: 1120–1121. [DOI] [PubMed] [Google Scholar]
- Williams SE, Pickard BG. 1972a. Receptor potentials and action potentials in Drosera tentacles. Planta 103: 193–221. [DOI] [PubMed] [Google Scholar]
- Williams SE, Pickard BG. 1972b. Properties of action potentials in Drosera tentacles. Planta 103: 222–240. [DOI] [PubMed] [Google Scholar]
- Williams SE, Pickard BG. 1980. The role of action potentials in the control of capture movements of Drosera and Dionaea. In: Skoog F, ed. Plant growth substances. Berlin: Springer, 470–480. [Google Scholar]
- Zamora R, Gómez JM, Hódar JA. 1998. Fitness responses of a carnivorous plant in contrasting ecological scenarios. Ecology 79: 1630–1644. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.