Abstract
Background and Aims Contractile roots are known and studied mainly in connection with the process of shrinkage of their basal parts, which acts to pull the shoot of the plant deeper into the ground. Previous studies have shown that the specific structure of these roots results in more intensive water uptake at the base, which is in contrast to regular root types. The purpose of this study was to find out whether the basal parts of contractile roots are also more active in translocation of cadmium to the shoot.
Methods Plants of the South African ornamental species Tritonia gladiolaris were cultivated in vitro for 2 months, at which point they possessed well-developed contractile roots. They were then transferred to Petri dishes with horizontally separated compartments of agar containing 50 µmol Cd(NO3)2 in the region of the root base or the root apex. Seedlings of 4-d-old maize (Zea mays) plants, which do not possess contractile roots, were also transferred to similar Petri dishes. The concentrations of Cd in the leaves of the plants were compared after 10 d of cultivation. Anatomical analyses of Tritonia roots were performed using appropriately stained freehand cross-sections.
Key Results The process of contraction required specific anatomical adaptation of the root base in Tritonia, with less lignified and less suberized tissues in comparison with the subapical part of the root. These unusual developmental characteristics were accompanied by more intensive translocation of Cd ions from the basal part of contractile roots to the leaves than from the apical–subapical root parts. The opposite effects were seen in the non-contractile roots of maize, with higher uptake and transport by the apical parts of the root and lower uptake and transport by the basal part.
Conclusions The specific characteristics of contractile roots may have a significant impact on the uptake of ions, including toxic metals from the soil surface layers. This may be important for plant nutrition, for example in the uptake of nutrients from upper soil layers, which are richer in humus in otherwise nutrient-poor soils, and also has implications for the uptake of surface-soil pollutants.
Keywords: Apoplasmic barrier, cadmium, Cd, Casparian band, contractile roots, endodermis, heavy metal uptake, maize, root translocation, soil pollution, Tritonia gladiolaris, Zea mays
INTRODUCTION
The occurrence and function of contractile roots is usually connected to and discussed in the literature with respect to the process of axial shrinking of the basal parts of these specific roots and pulling the shoots deeper into the ground (Smith-Huerta and Jernstedt, 1990; Pütz, 1992, 1993, 1994, 2002). They are present in several families, mostly in monocotyledonous species, but also in some dicotyledonous plants (Pütz, 1994; Pütz and Sukkau, 1995) and some gymnosperms (Tomlinson et al., 2014).
Root contraction is best known in bulbs and corms, protecting these reserve organs against herbivores, drought, fire or frost. The contraction of roots and in some cases also of the hypocotyl or shoot is important also for vegetative spreading and propagation of some species and for stabilization of plants in the substrate (Pütz, 1994, Rajaei et al., 2009). The pulling force exhibited by these roots can be >1·5 N (Pütz, 1992).
A general characteristic of non-contractile root ontogeny is the presence of meristematic tissue in the apical part and the progression of cell differentiation towards the base. Consequently, the endodermal (eventually exodermal) cells develop wall modifications with gradual deposition of lignin and suberin materials, increasing from the apex to the base; contractile roots are one of the rare exceptions to this. The process of contraction is subject to dimensional changes of cells in the contracting basal part of these roots. It also requires specific anatomical adaptation of the root base and proportional changes in cells, which expand radially and contract longitudinally; occasional collapse of peripheral parts of the root cortex may also occur. The basal parts of contractile roots do not form lateral roots. Contraction of the root’s basal parts requires less lignified and suberized tissues in comparison with the subapical parts of the root body.
The abnormal course of differentiation of contractile roots in comparison with typical (non-contractile) types of roots affects the characteristics of radial water transport. It was found that the basal parts were more active in water uptake than apical–subapical parts, which is in contrast to regular root types (North and Nobel, 2000). This finding was interpreted as an evolutionary advantage for plants in arid zones, enabling fast water uptake from occasional light rains that increase moisture at the soil surface (North and Baker, 2007; North et al., 2008).
The species used for the present experiments, Tritonia gladiolaris, was selected due to its relatively small size and easy propagation from corms, making it suitable for in vitro experiments, in comparison with some other species with contractile roots, such as many bulbous plants. Maize seedlings were used for comparison. Maize is not only one of the most important economic crops, but is also a frequent experimental subject, and was used in our previous work.
Our previous work focusing on cadmium (Cd) uptake showed a relationship between uptake and translocation of this heavy metal by plants and characteristics of apoplasmic barrier development. Salix clones with high levels of Cd uptake by roots and translocation to the shoot developed endodermal tissues more distant from the root apex in comparison with clones characterized by low Cd uptake and translocation (Lux et al., 2004). A similar relationship between Cd uptake/translocation and the development of apoplasmic barriers was found in maize (Lux et al., 2011; Redjala et al., 2011). Cadmium also induced earlier development, i.e. earlier suberization and lignification of endodermal cells (Vaculík et al., 2009). This reaction is very sensitive and even local; unilateral treatment of roots with Cd induced premature suberization and lignification of endodermis from the exposed side of the root (Lux et al., 2011). The unusual structural characteristics of contractile roots with a less lignified and suberized root base and more active water transport by their basal parts led us to ask whether the basal parts are also more active in uptake and translocation of cadmium. This question was addressed in the present work using in vitro culture of T. gladiolaris, which has prominent contractile roots. The results were compared with those obtained with regular (non-contractile) primary seminal roots in maize, grown under the same experimental conditions and using the same treatment.
MATERIALS AND METHODS
Tritonia gladiolaris cultures were obtained from the Research Centre for Plant Growth and Development, University of KwaZulu-Natal Pietermaritzburg, South Africa (G. D. Ascough). This plant material, originally derived from seeds, was grown and propagated vegetatively from proliferating corms on Murashige–Skoog medium (Murashige and Skoog, 1962) supplemented with paclobutrazol (1 mgL–1), myoinositol (100 mg L–1), sucrose (3 %) and agar (0·8 %) (Ascough et al., 2010). The cultures were maintained in a growth room with cool fluorescent tubes with light intensity between 45 and 60 µmol m–2 s–1, in a 16/8 h light/dark photoperiod at 23 ± 1 °C and 60 % room air humidity.
Plants with well developed contractile roots (2 months old) were carefully removed from large test tubes and transferred to flat, square Petri dishes with Murashige–Skoog medium containing 0·8 % agar. Petri dishes were oriented vertically. The agar was divided into two parts for the experiment. In the first variant only the apical part of root system was treated with Cd [50 µmol Cd(NO3)2] and its basal part was exposed to Cd-free medium (Cd-treated apex). In the second variant only the basal part was treated with Cd [50 µmol Cd(NO3)2] and the apical part was exposed to Cd-free medium (Cd-treated base). Two agar layers were separated by a thin layer of grafting wax (Dendrosan, Fytofarm Ltd.), to prevent transfer of Cd ions between the compartments.
The same experimental design, i.e. two separated compartments of agar with or without 50 µmol Cd(NO3)2, was used also for cultivation of 4-day-old maize seedlings (Zea mays, hybrid Valentina) germinated in rolls of filter paper at 28 °C in the dark. Roots of maize after germination were reduced to the primary seminal root by removing adventitious roots. Both plant species were cultivated in a growth chamber with light intensity 125–150 µmol m–2 s–1 at 25 °C for 10 d. The preparation of media and the transfer and cultivation of plants were done in sterile conditions.
After the experiment the plants were carefully removed from the media, all agar was removed from the roots, samples were washed thoroughly in distilled water, and separated leaves were dried at 70 °C for 3 days. Cadmium content in the leaves was determined by inductively coupled plasma mass spectrometry in three plants from each treatment and for each species.
Anatomical analysis of Tritonia roots was performed using freehand cross-sections prepared and stained for visualization of Casparian bands with berberine (Brundrett et al., 1988). Suberin lamellae in endodermal or exodermal cells were stained according to Brundrett et al. (1991) with modifications according to Lux et al. (2005).
Statistical significance was assessed with Student’s t-test using Statgraphics Centurion XV v. 15.2.05 (StatPoint, Inc., Warrenton, VA, USA) and Excel (Microsoft Office 2007). A P value <0·05 or <0·01 was defined as significant. The data presented (concentration) are from three replicates, in each of which three plants were analysed.
RESULTS
Structure of T. gladiolaris roots
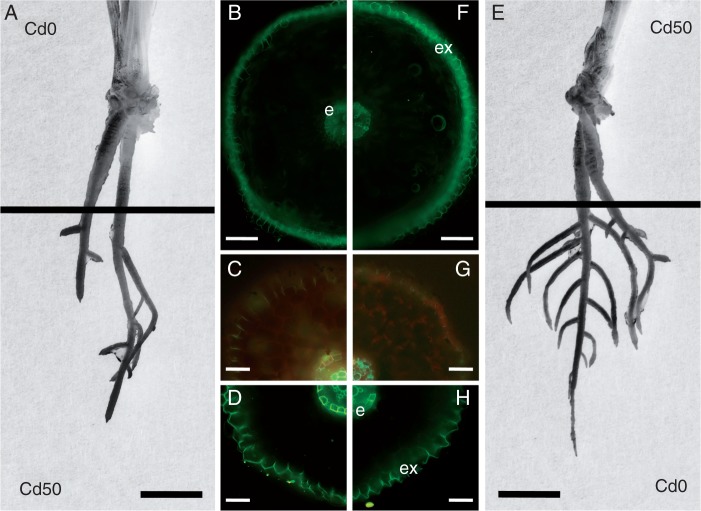
The roots were ∼5 cm long at the time of transfer to experimental treatments and the average root increment during the next cultivation was <5 mm. Basal parts of Tritonia roots represented the contractile region. This part was ∼1·5 cm long, thick and wrinkled on the surface (Figs 1 and 2A, E). No lateral roots were formed in this region. The subapical part was thinner and had branches ∼3·5 cm long. Anatomical analysis of these two parts showed prominent structural differences. In the basal, shrinking zone, only weak suberin deposition was present on staining for detection of suberin lamellae in the endodermis. These deposits were not apparently influenced by Cd treatment (Fig. 2B, F). In contrast, in the subapical parts (5–10 mm from the root apex) suberin lamellae developed in <50 % of endodermal cells and in all exodermal cells (Fig. 2G, H). In the subapical region of the Cd-treated apex, more intensive development of Casparian bands and suberin lamellae occurred in both endo- and exodermal cells (Fig. 2C, D). In the basal, longitudinally shrinking root parts, the root surface was also wrinkled perpendicularly, often with deep furrows. Mid-cortical regions exhibited radial cell extension and occasional collapse of cells. Inner cortical layers consisted of small cells.
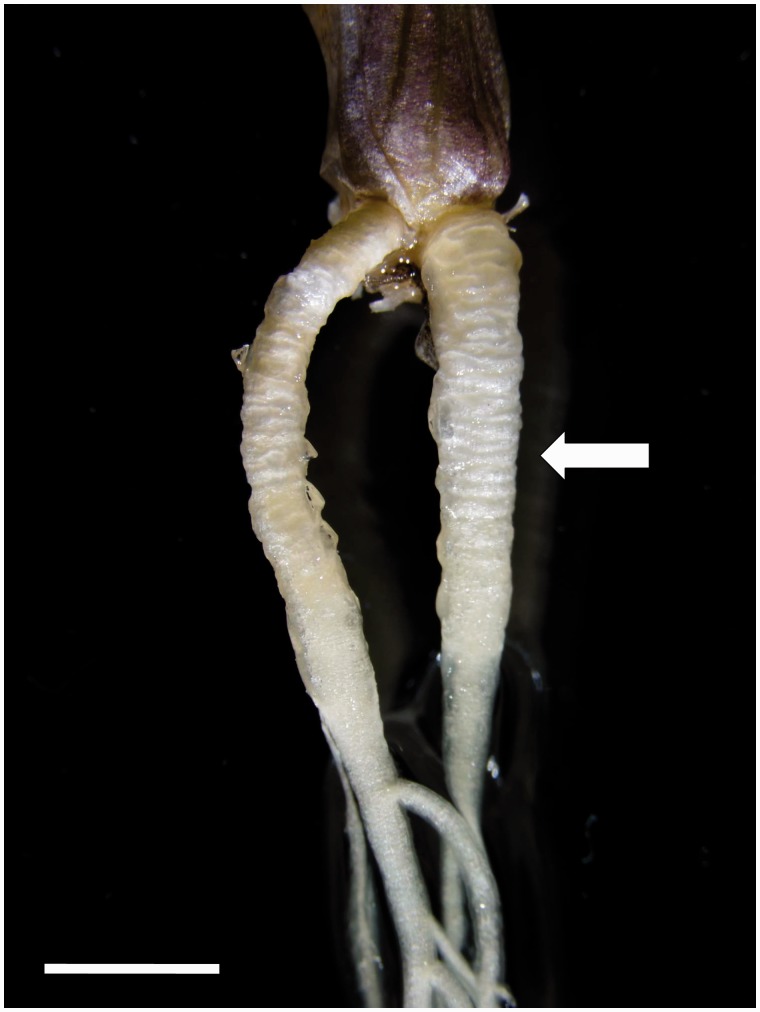
Fig. 1.
Contractile roots growing from the base of a T. gladiolaris plant cultured in vitro. The basal, contracting part of these adventitious roots was thick and wrinkled, without lateral roots (arrow). The subapical part was thinner and branching. Scale bar = 5 mm.
Fig. 2.
Roots of T. gladiolaris grown on agar medium, where either only the apical part was treated with Cd and the basal part was exposed to Cd-free medium (A) or only the basal part was treated with Cd and the apical part was exposed to Cd-free medium (E). The apical part of the root responded to Cd treatment by more prominent deposition of apoplasmic barriers such as Casparian bands (C) and suberin lamellae (D) in endodermal and exodermal cells compared with Cd-non-treated apex (G, H). Endodermal and exodermal cells of the contractile root base did not change the response to Cd treatment (F) compared with the Cd-non-treated base (B), and in both cases the suberin lamellae were less developed compared with the non-contractile apices. Staining: fluorol yellow 088 (B, D, F, H), berberine–toluidine blue (C, G). Abbreviations: e, endodermis; ex, exodermis. Scale bar (B, F) = 200 µm; (C, D, G, H) = 50 µm; (A, E) = 10 mm.
Structure of Z. mays roots
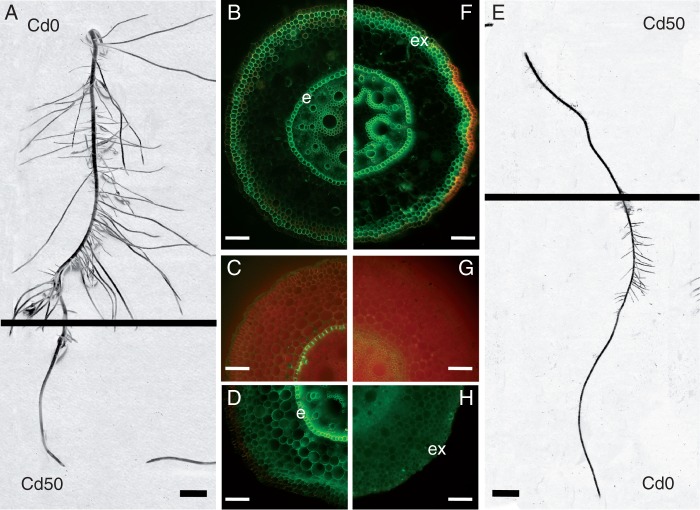
Zea mays was used to show the response of plants with non-contractile roots to the same modes of treatment (Fig. 3) as applied to T. gladiolaris. The reaction of maize roots exposed to Cd-containing medium differed between the two treatments, but in Cd-treated apical parts the reaction was opposite. Apex growth was inhibited and the lateral roots stopped growing in the Cd-treated region. However, the Cd-non-treated basal region compensated by the formation of numerous lateral roots (Fig. 3A). In Cd-treated basal parts, the apical part continued to grow and formed prominent lateral roots. On the other hand, growth of laterals in Cd-treated basal parts was inhibited (Fig. 3E). In the group in which the apex was treated with Cd, the basal part of the root developed deposits of suberin lamellae in endodermal and exodermal cells (Fig. 3 B). These deposits were even more prominent in the group in which the base was treated with Cd (Fig. 3F). In the subapical region (5–10 mm from the root apex) differences in apoplasmic barrier formation were even more evident. In Cd-treated bases the subapical part of the root developed weak Casparian bands (Fig. 3G) but no suberin lamellae in endodermal cells (Fig. 3H). In Cd-treated apexes the subapical part developed prominent Casparian bands and suberin lamellae in the endodermis (Fig. 3C, D).
Fig. 3.
Roots of Z. mays grown on agar medium where either only the apical part was treated with Cd and the basal part was exposed to Cd-free medium (A) or only the basal part was treated with Cd and the apical part was exposed to Cd-free medium (E). Apical part of the root responded to Cd treatment by more prominent deposition of apoplasmic barriers such as Casparian bands (C) and suberin lamellae (D) in endodermal cells compared with the Cd-non-treated apical part (G, H). Endodermal and exodermal cells of the root base responded to Cd treatment by developing prominent suberin lamellae (F) compared with the Cd-non-treated base (B). Staining: fluorol yellow 088 (B, D, F, H), berberine–toluidine blue (C, G). Abbreviations: e, endodermis; ex, exodermis. Scale bar (B, F) = 100 µm; (C, D, G, H) = 50 µm; (A, E) = 10 mm.
Cadmium uptake by apical and basal root parts
The concentrations of Cd in the leaves of Tritonia and Zea plants after exposure of roots to this heavy metal in the groups with Cd-treated bases or apexes differed (Figs 4 and 5). Cadmium translocation to the leaves of Tritonia was low, not exceeding 10 mg Cd kg–1 dry weight. These plants can be classified as Cd excluders. In the group with a Cd-treated base the concentration of Cd in the leaves was approximately twice as high as in the group with a Cd-treated apex (Fig. 4). This was opposite to the results obtained in maize. In this species the same experimental design with a Cd-treated base resulted in very low translocation of Cd to the leaves, the mean value being 0·5 mg Cd kg–1 dry weight. In the group with a Cd-treated apex the mean content in the leaves was almost 15 mg Cd kg–1 dry weight, indicating almost 30-fold higher translocation of Cd by the apical root part in comparison with the basal part (Fig. 5).
Fig. 4.
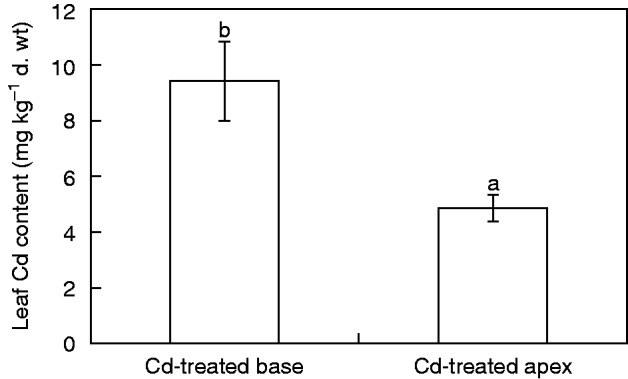
Concentration of Cd in leaves of T. gladiolaris plants grown on agar medium where either only the apical part was treated with Cd and the basal part was exposed to Cd-free medium (Cd-treated apex) or only the basal part was treated with Cd and the apical part was exposed to Cd-free medium (Cd-treated base). Values are means ± s.d. (n = 3). Different letters indicate a significant difference between treatments (P < 0·05).
Fig. 5.
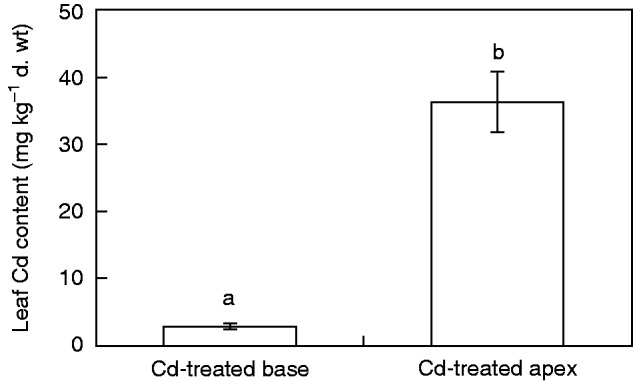
Concentration of Cd in leaves of Z. mays seedlings grown on agar medium where either only the apical part was treated with Cd and the basal part was exposed to Cd-free medium (Cd-treated apex) or only the basal part was treated with Cd and the apical part was exposed to Cd-free medium (Cd-treated base). Values are means ± s.d. (n = 3). Different letters indicate a significant difference between treatments (P < 0·01).
DISCUSSION
The process of contractile roots shrinking in their basal parts is accompanied by several cytological and anatomical peculiarities. The most unusual are dimensional changes of cortical cells and their radial expansion and longitudinal shrinking. In monocotyledonous species, collapse of cortical cells accompanies root contraction (reviewed by Pütz, 2002). G-fibres (gelatinous or tension wood fibres) may cause contraction of roots and hypocotyls in dicotyledonous seedlings (Fisher, 2008). Gelatinous fibres were also found in the secondary phloem in roots of Cycadales that undergo contraction (Tomlinson et al., 2014). These structures were not confirmed in Tritonia contractile roots in the present study. Xylem elements of wrinkled roots also appeared wrinkled, with a wavy appearance in longitudinal view (Fisher, 2008). Mechanical tissues or specialized tissues with suberized and lignified cell walls represent an obstacle to the shrinking and shortening of contractile roots. This is the reason for the reduced development of thick-walled cells in the basal, contracting parts of these roots. The unusual process of differentiation, with thicker deposits of suberin in tissues close to the root tip, in comparison with older tissues of the root base is in contrast to non-contractile root types. In maize, which was used in this experiment for comparison of Cd translocation, gradual maturation of endodermal tissues occurs under control conditions. Casparian bands develop in the endodermis close to the root tip, suberin lamellae are deposited at a distance of several centimetres from the root tip, and tertiary lignified walls develop in the basal parts of older roots (Schreiber et al., 1999; Ma and Peterson, 2003). Roots exposed to Cd treatment deposit suberin lamellae in the endodermis, both in the root base and close to the root apex. Anatomical analysis of Tritonia roots in the present study showed reduced development of endodermal tissues in the contracting basal root parts, terminating their development with Casparian bands and weak deposits of suberin lamellae. In most plant species, with the exception of some aquatic species and a few terrestrial species (Schreiber et al., 1994; Seago and Fernando, 2013), endodermis proceeds gradually to the second stage – deposition of suberin lamellae on the whole inner surface of endodermal cells. In many species, even the third stage – the development of thick tertiary walls, which become lignified – occurs in more basal root parts (von Guttenberg, 1968; Enstone et al., 2002; Ma and Peterson, 2003). In the subapical region of Tritonia roots in the present study, suberin lamellae were deposited and even lignification of endodermal cell walls occurred.
The development of apoplasmic barriers represents a checkpoint for Cd uptake by roots and translocation to the shoot. Our previous work showed a close correlation between the distance of endodermal development from the root apex and Cd uptake and translocation (Lux et al., 2004, 2011; Vaculík et al., 2009; Redjala et al., 2011). In particular, the deposition of suberin lamellae is a very sensitive parameter influencing Cd uptake and translocation. These cell wall modifications occur even after local unilateral exposure of roots to Cd for 2 d (Lux et al., 2011; Martinka et al., 2014). The importance of cell wall chemistry for the regulation of Cd uptake and translocation was recently confirmed in a rice mutant with altered cell wall properties in the conducting tissues that affected Cd translocation efficiency, largely explaining the low Cd accumulation in the mutant plants (Song et al., 2013).
The unusual development of the endodermis in shrinking root parts and prominent suberin lamellae in subapical root parts coincided with the intensity of Cd translocation, which was lower in subapical root parts and higher in the basal parts. This is in clear contrast to the regular root types, in which there was much higher Cd translocation from the apical parts of the root in comparison with the basal parts. Cadmium influx at the root tip is on average 2.9-fold higher than that in the basal region in sunflower lateral roots (Laporte et al., 2013). A similar result was obtained in our experiment with maize roots, though here the difference, in primary seminal roots, was almost 30-fold. This occurred in spite of deposition of suberin lamellae in the subapical root parts exposed to Cd treatment. Intensive uptake by young subapical root parts probably precedes the process of suberin deposition in the endodermis that occurs as a response to Cd treatment, protecting the root against the transport of toxic Cd to vascular tissues and the shoot.
Longitudinal variation in cadmium translocation, opposite in contractile roots to that in regular non-contractile roots found in the present study, confirms and extends the unusual functional characteristics of these roots. As shown by North and co-workers (North and Baker, 2007; North et al., 2008), these roots exhibit increased capacity for water absorption by their basal parts. This represents an evolutionary adaptation and an advantage in dry areas where rainfall is rare and low in amount. On the other hand, these roots sacrifice the safety of basal, older root parts, which may become more sensitive to mechanical or other abiotic and biotic stresses. This disadvantage correlates with the high sensitivity of these roots to salinity stress (Rajaei et al., 2009).
It can be concluded that specific characteristics of contractile roots may have great impact on the uptake of ions, including toxic metals, from the soil surface. These little-known characteristics may be important for plant nutrition, e.g. for the uptake of nutrients from the upper soil layers, which are relatively rich in humus in nutrient-poor soils. On the other hand, soil pollution, which is often most severe on the soil surface, may present both danger to the plant and a threat to food safety.
ACKNOWLEDGEMENTS
This contribution is a result of implementation of a project at Comenius University in Bratislava Science Park supported by the Research and Development Operational Programme funded by the ERDF grant ITMS 26240220086. The authors are thankful to T.L. Rost of the University of California, Davis, for critical reading of the manuscript.
LITERATURE CITED
- Ascough GD, Swart PA, Finnie JF, Van Staden J. 2010. Micropropagation of Romulea minutiflora, Sisyrinchium laxum and Tritonia gladiolaris – Iridaceae with ornamental potential. South African Journal of Botany 77: 216–221. [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA. 1988. A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 146: 133–142. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA. 1991. Efficient lipid staining in plant material with Sudan red 7B or Fluoral yellow 088 in polyethylene glycol-glycerol. Biotechnic & Histochemistry 66: 111–116. [DOI] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA, Ma FS. 2002. Root endodermis and exodermis: structure, function, and responses to the environment. Journal of Plant Growth Regulation 21: 335–351. [Google Scholar]
- Fisher JB. 2008. Anatomy of axis contraction in seedlings from a fire prone habitat. American Journal of Botany 95: 1337–1348. [DOI] [PubMed] [Google Scholar]
- Laporte MA, Denaix L, Pages L, et al. 2013. Longitudinal variation in cadmium influx in intact first order lateral roots of sunflower (Helianthus annuus L.). Plant and Soil 372: 581–595. [Google Scholar]
- Lux A, Šottníková A, Opatrná J, Greger M. 2004. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Plant Physiology 120: 537–545. [DOI] [PubMed] [Google Scholar]
- Lux A, Morita S, Abe J, Ito K. 2005. Improved method for clearing and staining free-hand sections and whole-mount samples. Annals of Botany 96: 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Martinka M, Vaculík M, White PJ. 2011. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany 62: 21–37. [DOI] [PubMed] [Google Scholar]
- Ma FS, Peterson CA. 2003. Current insights into the development, structure, and chemistry of the endodermis and exodermis of roots. Canadian Journal of Botany 81: 405–421. [Google Scholar]
- Martinka M, Vaculík M, Lux A. 2014. Plant cell responses to cadmium and zinc. In: Nick P, Opatrný Z. eds. Applied plant cell biology. Cellular tools and approaches for plant biotechnology . Heidelberg: Springer, 209–246. [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 472–497. [Google Scholar]
- North GB, Baker EA. 2007. Water uptake by older roots: evidence from desert succulents. Hortscience 42: 1103–1106. [Google Scholar]
- North GB, Nobel PS. 2000. Heterogeneity in water availability alters cellular development and hydraulic conductivity along roots of a desert succulent . Annals of Botany 85: 247–255. [Google Scholar]
- North GB, Brinton EK, Garrett TY. 2008. Contractile roots in succulent monocots: convergence, divergence and adaptation to limited rainfall. Plant, Cell & Environment 31: 1179–1189. [DOI] [PubMed] [Google Scholar]
- Pütz N. 1992. Measurement of the pulling force of a single contractile root. Canadian Journal of Botany 70: 1433–1439. [Google Scholar]
- Pütz N. 1993. Underground plant movement. I. The bulb of Nothoscordum inodorum (Alliaceae). Botanica Acta 106: 338–343. [Google Scholar]
- Pütz N. 1994. Vegetative spreading of Oxalis pes-caprae (Oxalidaceae). Plant Systematics and Evolution 191: 57–67. [Google Scholar]
- Pütz N. 2002. Contractile roots. In: Waisel Y, Eshel A, Kafkafi U. eds. Plant roots: the hidden half, 3rd edn New York: Marcel Dekker, 975–987. [Google Scholar]
- Pütz N, Sukkau I. 1995. Examination of the moving process in monocot and dicot seedlings using the example Lapeirousia laxa (Iridaceae) and Foeniculum vulgare (Apiaceae) . Feddes Repertorium 106: 475–481. [Google Scholar]
- Rajaei SM, Niknam V, Seyedi SM, Ebrahimzadeh H, Razavi K. 2009. Contractile roots are the most sensitive organ in Crocus sativus to salt stress. Biologia Plantarum 53: 523–529. [Google Scholar]
- Redjala T, Zelko I, Sterckeman T, Legué T, Lux A. 2011. Relationship between root structure and root cadmium uptake in maize. Environmental and Experimental Botany 71: 241–248. [Google Scholar]
- Schreiber L, Breiner HW, Riederer M, Duggelin M, Guggenheim R. 1994. The Casparian strip of Clivia miniata Reg. roots: isolation, fine-structure and chemical nature. Botanica Acta 107: 353–361. [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J. 1999. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany 50: 1267–1280. [Google Scholar]
- Seago JL, Jr, Fernando DD. 2013. Anatomical aspects of angiosperm root evolution. Annals of Botany 112: 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Huerta NL, Jernstedt JA. 1990. Orientation of cellulose microfibrils in radial longitudinal and transverse cell walls. Protoplasma 154: 161–171. [Google Scholar]
- Song X-Q, Liu L-F, Jiang Y-J, et al. 2013. Disruption of secondary wall cellulose biosynthesis alters cadmium translocation and tolerance in rice plants. Molecular Plant 6: 768–780. [DOI] [PubMed] [Google Scholar]
- Tomlinson PB, Magellan TM, Griffith MP. 2014. Root contraction in Cycas and Zamia (Cycadales) determined by gelatinous fibers. American Journal of Botany 101: 1275–1285. [DOI] [PubMed] [Google Scholar]
- Vaculík M, Lux A, Luxová M, Tanimoto E, Lichtscheidl E. 2009. Silicon mitigates cadmium inhibitory effects in young maize plants. Environmental and Experimental Botany 67: 52–58. [Google Scholar]
- Von Guttenberg H. 1968. Der primäre Bau der Angiospermenwurzel VIII/5. In: Linsbauer K. ed. Handbuch der Pflanzenanatomie. Berlin: Gebrüder Borntraegern Verlagsbuchhandlung. [Google Scholar]