Abstract
Background and Aims Strigolactones (SLs) and their derivatives are plant hormones that have recently been identified as regulating root development. This study examines whether SLs play a role in mediating production of adventious roots (ARs) in rice (Oryza sativa), and also investigates possible interactions between SLs and auxin.
Methods Wild-type (WT), SL-deficient (d10) and SL-insensitive (d3) rice mutants were used to investigate AR development in an auxin-distribution experiment that considered DR5::GUS activity, [3H] indole-3-acetic acid (IAA) transport, and associated expression of auxin transporter genes. The effects of exogenous application of GR24 (a synthetic SL analogue), NAA (α-naphthylacetic acid, exogenous auxin) and NPA (N-1-naphthylphalamic acid, a polar auxin transport inhibitor) on rice AR development in seedlings were investigated.
Key Results The rice d mutants with impaired SL biosynthesis and signalling exhibited reduced AR production compared with the WT. Application of GR24 increased the number of ARs and average AR number per tiller in d10, but not in d3. These results indicate that rice AR production is positively regulated by SLs. Higher endogenous IAA concentration, stronger expression of DR5::GUS and higher [3H] IAA activity were found in the d mutants. Exogenous GR24 application decreased the expression of DR5::GUS, probably indicating that SLs modulate AR formation by inhibiting polar auxin transport. The WT and the d10 and d3 mutants had similar expression of DR5::GUS regardless of exogenous application of NAA or NPA; however, AR number was greater in the WT than in the d mutants.
Conclusions The results suggest that AR formation is positively regulated by SLs via the D3 response pathway. The positive effect of NAA application and the opposite effect of NPA application on AR number of WT plants also suggests the importance of auxin for AR formation, but the interaction between auxin and SLs is complex.
Keywords: Adventitious root formation, auxin, IAA, strigolactone, SL, rice, Oryza sativa, root growth, plant hormone
INTRODUCTION
The root system is fundamentally important for plants to efficiently obtain nutrients and water. In contrast to the primary root system of plants, roots of monocot cereals consist almost entirely of a complex fibrous system and a mass of adventitious roots (ARs). AR formation is the process of root initiation from the stem base post-embryonically, which is tightly regulated to prevent the loss of valuable plant resources for non-essential root formation. A lack of stable and credible morphological data makes it difficult to study physiological and molecular mechanisms governing AR growth (Coudert et al., 2010; E et al., 2012). However, comprehensive understanding of AR development should have important implications for manipulating root architecture, which contributes to both improving crop yield and optimizing agricultural land use.
Several plant hormones control AR formation, in which auxin plays a pivotal role (Jiang and Feldman, 2003; E et al., 2012). Indole-3-acetic acid (IAA) is the predominant form of active auxin in plants and it induces both AR and lateral root (LR) formation (Cooper, 1936; Boerjan et al., 1995; Delarue et al., 1998; Zhao et al., 2001; Negi et al., 2010). Auxin is synthesized predominantly in the shoot apex and young leaves (Ljung et al., 2001), and is redistributed through the cooperative actions of auxin efflux carriers such as PIN-FORMED (PIN) and ABCB/PGP family proteins, and auxin influx carriers including AUX1/LAX family proteins (Friml, 2003; Blakeslee et al., 2005; Zazimalova et al., 2010; Peret et al., 2012). In rice, altered expression of PIN1b changes AR development (Xu et al., 2005). Several rice auxin-related mutants show defects in AR formation. For example, CRL1/ARL1 (an AS2/LOB transcriptional factor) acts as a positive regulator for AR formation downstream of the IAA and auxin response factor-mediated auxin signalling pathway. The crl1/arl1 mutant has been shown to exhibit few LRs (Inukai et al., 2005; Liu et al., 2005). The rice CROWN ROOTLESS4 (CRL4)/OsGNOM1 mutation affects AR initiation and development by modulating expression of PIN genes (Kitomi et al., 2008; Liu et al., 2009). Previous studies have demonstrated that AR formation is affected by auxin factors, such as transport, metabolism or the presence of free IAA (Diaz-Sala et al., 1996; Krisantini et al., 2006). Additional signalling pathways may be involved in the regulation of AR induction.
In addition to auxin, studies have shown that strigolactones (SLs) and their derivatives play a key role in modulation of root development (Kapulnik et al., 2011a; Koltai, 2011; Ruyter-Spira et al., 2011; Rasmussen et al., 2012; Sun et al., 2014). Application of GR24 (a synthetic SL analogue) under favourable growth conditions led to a MORE AXILLARY GROWTH 2 (MAX2)-dependent increase in primary root length. The inhibition of primary root length was observed in treatments with relatively higher doses of GR24, in a MAX2-independent fashion (Ruyter-Spira et al., 2011). The SL-deficient and response mutants of Arabidopsis thaliana and pea (Pisum sativum) have enhanced AR development, suggesting, conversely, that SLs suppress AR formation in both species. However, Arite et al. (2012) found that SLs positively regulate the length of ARs in rice. Lateral root densities are increased in SL-deficient and SL-insensitive arabidopsis and tomato (Solanum lycopersicum) mutants, suggesting that SLs affect LR formation (Koltai et al., 2010; Kapulnik et al., 2011b; Ruyter-Spira et al., 2011). In contrast to findings in dicots such as arabidopsis and tomato, the number of LRs on the seminal root did not differ among 14-d-old wild-type (WT) and d10 and d14 rice mutants (Arite et al., 2012). These results suggest that complex biological mechanisms underlie the regulation of root growth by SLs acting as plant hormones.
In this study, the role of SLs in regulating rice AR formation was examined in SL-deficient and SL-insensitive rice mutants. Rice is an ideal model for SL study, as it has several well-characterized SL synthetic mutants (d10, d17 and d27) and response mutants (d3, d14 and d53) (Xie et al., 2010; Waters et al., 2012; Jiang et al., 2013; Zhou et al., 2013). The d mutants exhibited reduced AR development compared with WT rice plants. Exogenous GR24 restored AR formation in the SL synthesis mutant d10, but not in the SL signalling mutant d3. In addition, endogenous IAA concentration at the shoot–root junction, SL mutants carrying the auxin reporter construct DR5::GUS, polar transport of radiolabeled [3H] IAA and exogenous application of α-naphthylacetic acid (NAA) and the auxin transport inhibitor N-1-naphthylphalamic acid (NPA) revealed that AR formation in rice is regulated by SLs and auxin status.
MATERIALS AND METHODS
Plant growth conditions
The Shiokari ecotype of rice (Oryza sativa L.) was the basis for this study. The d10 (SL synthesis) and the d3 (SL signalling) mutants were kindly provided by Shinjiro Yamaguchi of the RIKEN Plant Science Center. Rice seedlings were grown in a greenhouse under natural light at day/night temperatures of 30°C/18°C. Seven-day-old seedlings of uniform size and vigour were transplanted into holes in a lid placed over the top of pots (four holes per lid and three seedlings per hole). Nutrient solutions varying from one-quarter to one-half strength were applied for 1 week and a full-strength nutrient solution was applied thereafter. The full-strength nutrient solution of the International Rice Research Institute (IRRI) was (mm): 1·25 NH4NO3, 0·3 KH2PO4, 0·35 K2SO4, 1·0 CaCl2, 1·0 MgSO4.7H2O and 0·5 Na2SiO3, and (µm) 20·0 Fe-EDTA, 9·0 MnCl2, 0·39 (NH4)6Mo7O24, 20·0 H3BO3, 0·77 ZnSO4 and 0·32 CuSO4 (pH 5·5).
The treatments of GR24 (dissolved in acetone), NAA (dissolved in 1 m NaOH) and auxin transport inhibitor NPA (dissolved in dimethyl sulfoxide, DMSO) were applied via the plant-growth media. Seven-day-old rice seedlings were grown in hydroponic medium containing various concentrations of NAA and NPA for 3 weeks. The GR24 treatments were applied in the same IRRI nutrient solution, as described above, in hydroponic or 0·4 % agar medium. Rice seeds were germinated in trays for 3 d and then transferred into plant culture tubes and grown for 3 weeks. The control treatment for GR24 contained 0·1 % acetone (Sun et al., 2014).
Measurement of root system architecture
The average length of ARs was measured using the WinRhizo scanner-based image analysis system (Regent Instruments, Montreal, QC, Canada). The number of ARs was counted manually.
Quantification of IAA levels
The concentration of IAA in the shoot–root junction was determined as described by Song et al. (2013). Fresh weight of samples was determined, followed immediately by freezing in liquid N2. Sample measurement of free IAA by HPLC was carried out according to Song et al. (2013). A standard IAA sample was obtained from Sigma-Aldrich (St Louis, MO, USA).
Agrobacterium tumefaciens (strain EHA105) contained the construct pDR5::GUS, which was kindly provided by Professor Ping Wu’s group at Zhejiang University, Hangzhou, China. Tissues were treated with ethanol prior to observation to remove chlorophyll pigmentation. The stained tissues were photographed using an Olympus SZX2-ILLK stereomicroscope with a colour CCD camera (Olympus, Tokyo, Japan).
[3H] IAA-transport assay
To assay the effect of SLs on auxin transport, WT and the d10 mutant were pre-cultured for 3 weeks under normal nutrition with or without 1·25 µm GR24 application. Auxin transport was assayed by means of [3H] IAA, as described below.
Shoot-to-root auxin transport in intact plants was assayed according to Song et al. (2013). For this, 20 µL of [3H] IAA solution was applied to the cut surface after removal of rice shoot 4 cm above the root–shoot junction and plants were kept in darkness for 18 h. The [3H] IAA solution contained 0·5 µm [3H] (20 Ci mmol–1) in 2 % DMSO, 25 mm MES (pH 5·2) and 0·25 % agar. The root–shoot junction was dissected and weighed before being incubated in scintillation solution for more than 18 h. [3H] IAA radioactivity was detected using a multipurpose scintillation counter (LS6500, Beckman-Coulter, Fullerton, CA, USA) (Song et al., 2013).
qRT-PCR analysis
Total RNA was isolated from the roots of rice seedlings. RNA extraction, reverse transcription and quantitative reverse transcription polymerase chain reaction (qRT-PCR) were performed as described previously (Sun et al., 2014). Primer sets for PIN genes are listed in Supplementary Data Table S1.
Data analysis
Data were pooled for calculation of means and standard errors (s.e.) and assessed by one-way analysis of variance, followed by computation of least significant differences to compare means. All statistical analyses were performed using SPSS ver. 11·0 (SPSS Inc., Chicago, IL, USA). In all analyses, the targeted level of statistical significance was P < 0·05.
RESULTS
Decreased number of ARs in the d10 and d3 mutants compared with WT plants
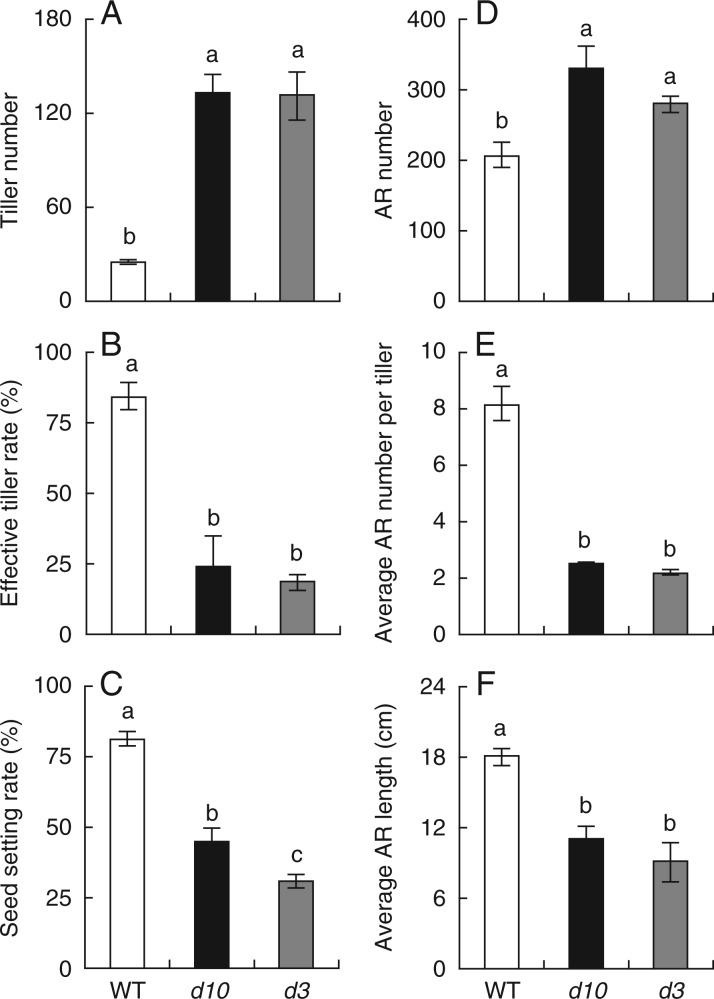
As reported by Ishikawa et al. (2005), the SL synthetic mutant d10 and signalling mutant d3 exhibited increased numbers of tillers compared with the WT (Fig. 1A). However, the effective tiller rate and seed setting rate were much lower in the d10 and d3 mutants compared with the WT (Fig. 1B, C). The reasons for the differences in effective tiller rate and seed setting were unclear. We examined AR growth among the two d mutants and the WT at maturity (Fig. 1D–F). Only two ARs per tiller were recorded in the d10 and d3 mutants, lower than for the WT. Furthermore, the average length of ARs was lower in the two d mutants compared with the WT. These results indicated that the defect in AR growth potentially reduced the effective tillering and seed setting rate in the SL mutants (Fig. 1F).
Fig. 1.
Phenotype of strigolactone-deficient (d10) and strigolactone-insensitive (d3) mutants and wild-type (WT) rice plants at maturity. Rice seedlings were grown in soil after germination. (A) Tiller number, (B) effective tiller rate, (C) seed setting rate, (D) adventitious root (AR) number, (E) average AR number per tiller and (F) average AR length of WT, d10 and d3. Data are means ± s.e. Different lower-case letters indicate significant differences (P < 0·05, ANOVA).
We examined the time course of development of tillers and ARs at 8 weeks following germination. The outgrowth of tiller buds in the d10 and d3 mutants was markedly increased and tiller numbers were greater in both mutants 3 weeks after germination compared with the WT (Fig. 2A). At an early growth stage (from 2 to 4 weeks), the AR numbers in the two mutants were lower compared with the WT (Fig. 2B, D). An increased number of tillers in the d mutants led to a greater number of ARs from 8 weeks to maturity (Fig. 2C). Although the total number of ARs in the SL mutants was higher than in the WT at later growth stages, the number of ARs per tiller was three times less than in the WT (Fig. 1E). To further investigate the formation of ARs, we used 3 weeks after germination as the experimental period in subsequent analyses.
Fig. 2.
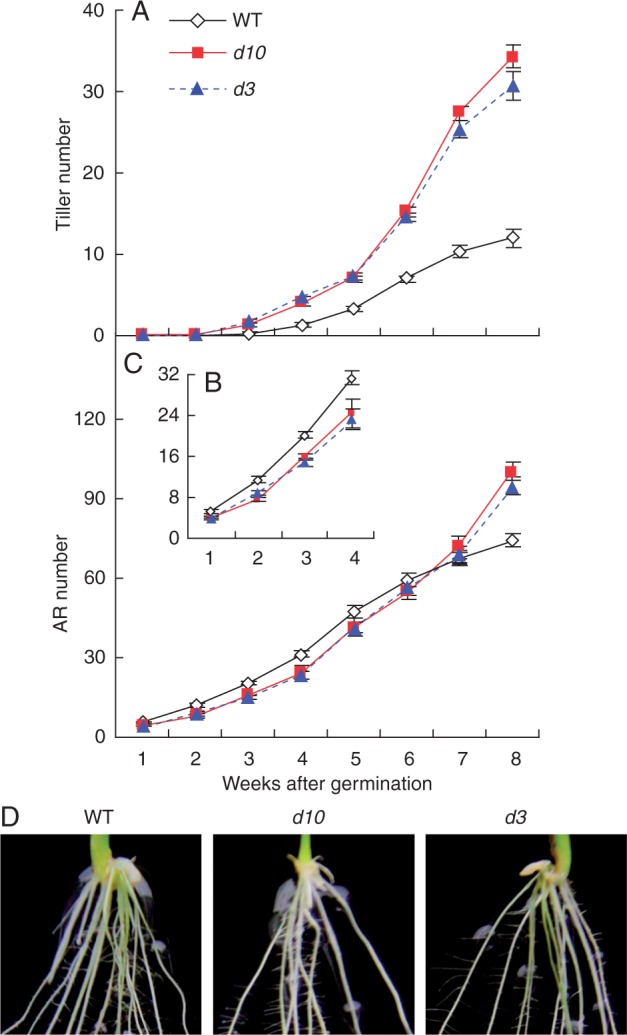
Time course of development of tillers and adventitious root (AR) in strigolactone-deficient (d10) and strigolactone-insensitive (d3) mutants and wild-type (WT) rice plants over 8 weeks. Rice seedlings were grown in hydroponic media after germination. (A) Tiller number of WT, d10 and d3. (B, C) AR number of WT, d10 and d3 over 4 weeks (B) and 8 weeks (C). (D) ARs of WT, d10 and d3 during weeks 2–3. Data are means ± s.e.
Exogenous application of GR24 increased AR numbers in the SL synthetic mutant d10 but not in d3
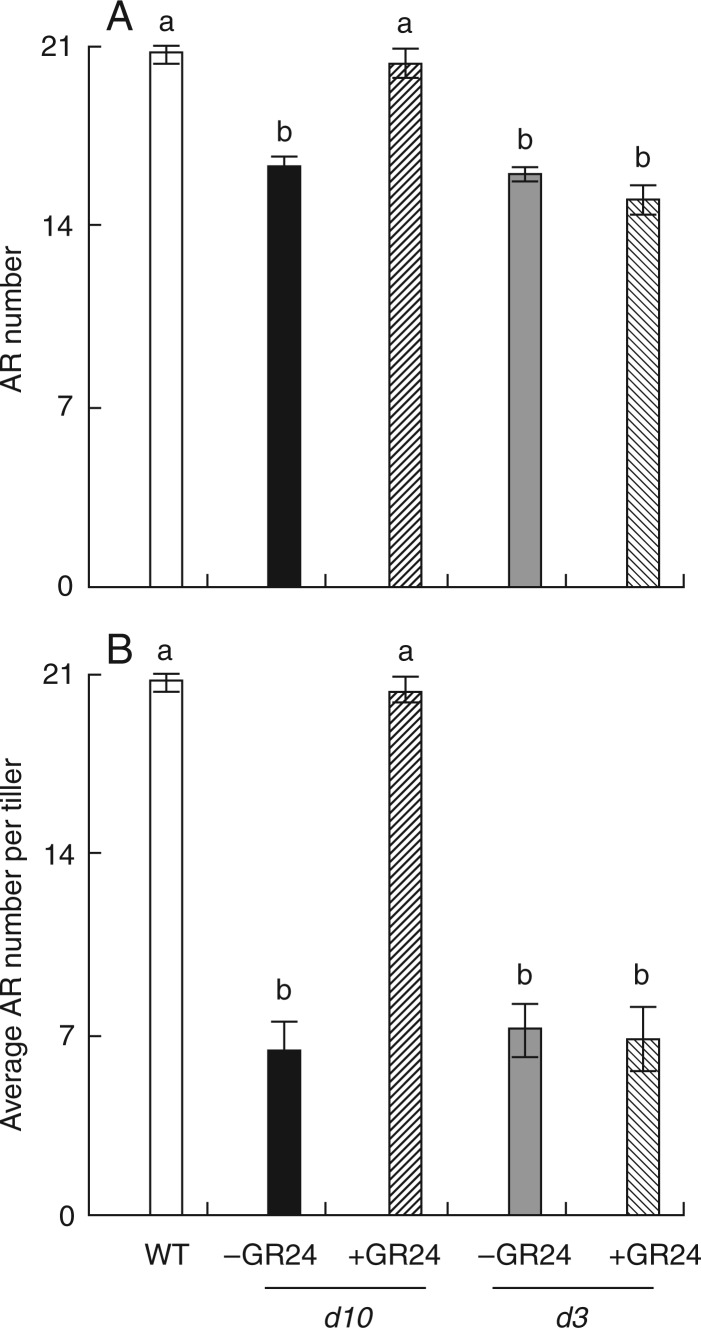
Endogenous 2′-epi-5-deoxystrigol was detected in the WT and the mutant d3 but not in the d10 mutant (Umehara et al., 2008). To determine if SLs affect the growth of ARs in rice, synthetic GR24 at 0–5 µm was applied exogenously to the WT plants and the two d mutants (Supplementary Data Fig. S1). Application of GR24 had no effect on the number of ARs in the WT, with the exception of 5 µm GR24, which caused a slight decrease in the number of ARs. Application of 1·25 µm GR24 increased AR number and average AR number per tiller in the d10 mutant to the level of the WT (Fig. 3). However, root morphology of the d3 mutant was unaffected by GR24 application, indicating involvement of the D3 gene in the SL regulatory pathway for AR formation.
Fig. 3.
Effect of the synthetic strigolactone analogue GR24 on the number of adventitious roots (AR) and average AR number per tiller in strigolactone-deficient (d10) and strigolactone-insensitive (d3) mutants and wild-type (WT) rice plants. Rice seedlings were grown for 3 weeks in agar media containing 1·25 µm GR24. Data are means ± s.e. Different lower-case letters indicate significant differences (P < 0·05, ANOVA).
Auxin levels were significantly higher in the d10 and d3 mutants relative to the WT
To assess whether auxin affected AR formation in the d10 and d3 mutants, endogenous IAA concentrations was first analysed in the shoot–root junction. IAA concentration was increased by 100 and 168 %, respectively, in the d10 and d3 mutants relative to WT (Fig. 4A). A specific reporter contains seven repeats of a highly active synthetic auxin response element, and changes in in vivo auxin levels are monitored via expression of DR5::GUS (Ulmasov et al., 1997). Expression of DR5::GUS was subsequently examined in the WT and d10 and d3 mutants. Expression of DR5::GUS in d10 and d3 plants was stronger in the first leaf, leaf sheath and junction, in comparison with the WT (Supplementary Data Fig. S2). These results indicated that d mutant plants had higher IAA concentrations in the junction relative to the WT.
Fig. 4.
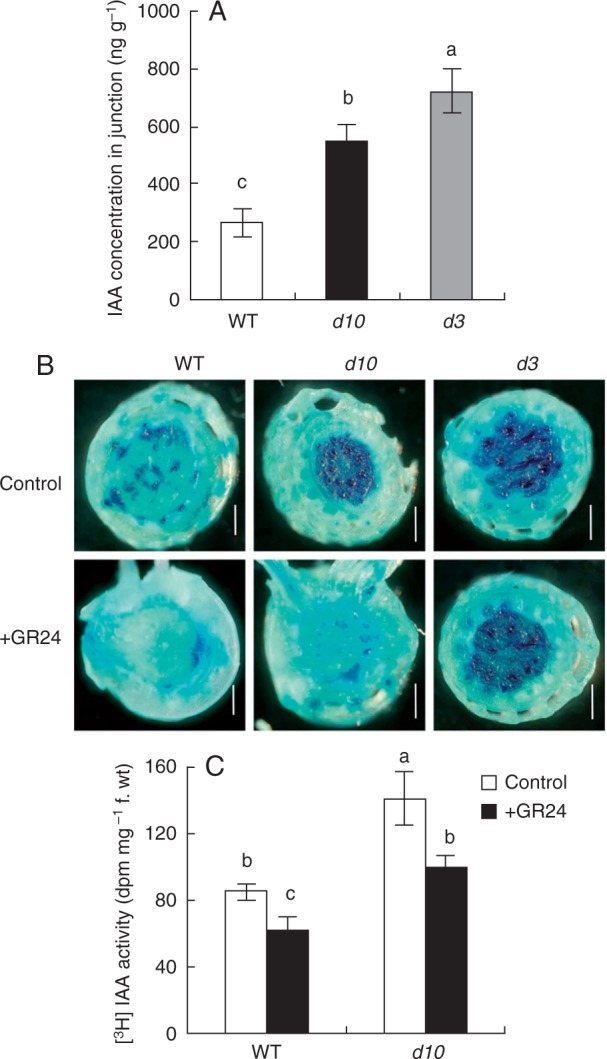
(A) Auxin concentration and (B) histochemical localization of DR5::GUS activity and (C) [3H] IAA transport in junction of wild-type (WT) and strigolactone-deficient (d10) and strigolactone-insensitive (d3) mutants. Seedlings were grown for 3 weeks in hydroponic media with or without 1·25 µm GR24. Plants were stained for GUS activity for 24 h. Scale bar = 1 mm. Data are means ± s.e. Different lower-case letters indicate significant differences (P < 0·05, ANOVA).
To determine whether SLs regulate AR formation through modulating polar auxin transport, the effect of GR24 application on the expression of DR5::GUS and [3H] IAA transport was analysed (Fig. 4B, C). GR24 application significantly decreased expression of DR5::GUS in WT and d10 but not d3 (Fig. 4B). Application of GR24 markedly reduced [3H] IAA activity in the junction of WT and d10 plants, and there were no differences in [3H] IAA activity in d10 with GR24 application relative to the WT without GR24 application (Fig. 4C). These results suggested SLs inhibited polar auxin transport in rice plants.
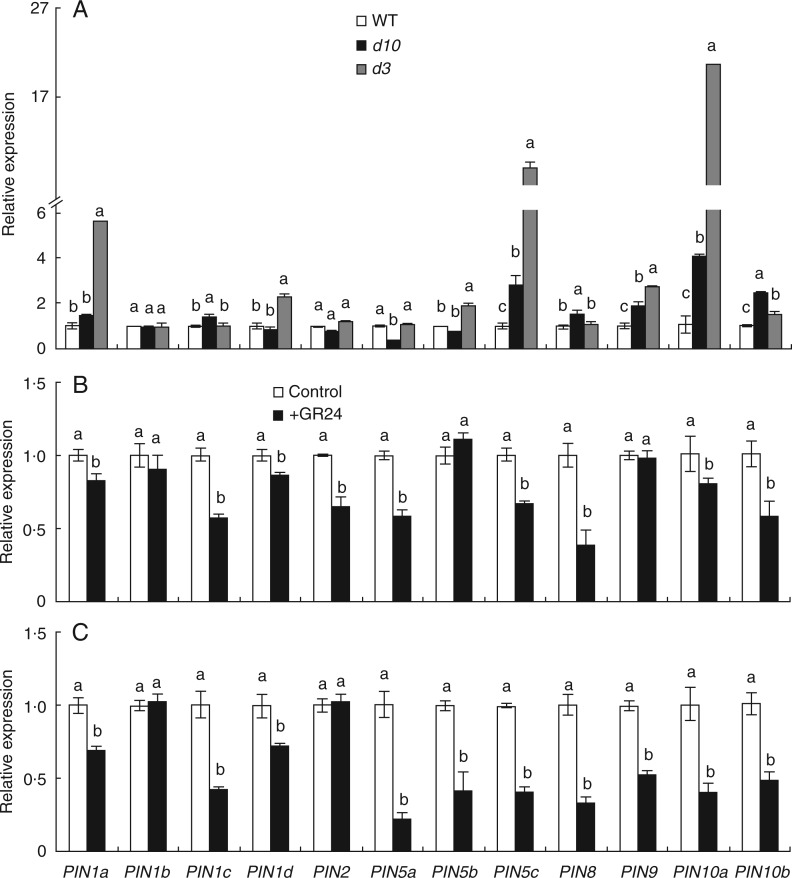
Expression of OsPIN genes in the d10 and d3 mutants
Auxin is delivered from the shoot mainly through the polar transport stream, which is facilitated by proteins of the PIN family. The expression pattern of PIN genes in the WT and the d10 and d3 mutants was examined (Fig. 5). qRT-PCR showed that the expression levels of PIN5c, PIN9, PIN10a and PIN10b increased significantly in the d10 and d3 mutants compared with the WT. The expression levels of PIN1c, PIN5c and PIN8 were increased in d10, whereas in d3 the expression levels of PIN1a, PIN1d and PIN5b were increased. The expression level of PIN5a was reduced significantly only in d10. No differences in the expression of other genes in the PIN family were observed between the WT and the two d mutants (Fig. 5A). Application of GR24 markedly downregulated the levels of most PIN family genes in WT (Fig. 5B) and d10 (Fig. 5C), with the exception of PIN1b, PIN5b and PIN9 in WT and PIN1b and PIN2 in d10. These results suggested SLs inhibited polar auxin transport by modulating transcriptional level of PIN genes.
Fig. 5.
qRT-PCR analysis of PIN family genes in the junction of wild-type (WT) and strigolactone-deficient (d10) and strigolactone-insensitive (d3) mutant rice plants. Rice seedlings were grown for 3 weeks in hydroponic media with or without 1·25 µm GR24. (A) WT, d10 and d3 mutants. (B, C) WT (B) and d10 (C) with or without GR24 application. Relative mRNA levels were normalized for individual genes relative to OsACT. Data are mean ± s.e. Different lower-case letters in the same gene indicate significant differences (P < 0·05, ANOVA).
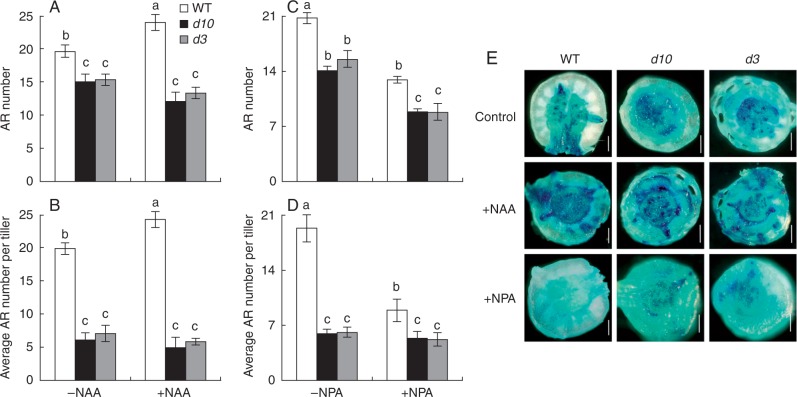
NAA and NPA application affected AR formation
The responses of AR number to application of exogenous NAA and the auxin polar transport inhibitor, NPA, were examined to assess whether increased auxin concentrations in the two SL mutants were responsible for the reduced AR formation. WT plants had higher numbers of ARs compared with the two d mutants regardless of NAA concentration (Supplementary Data Fig. S3A). Application of 10 nm NAA significantly increased AR numbers and average AR number per tiller by 22 % in the WT, but no changes in the two d mutants (Fig. 6A, B). Significant decreases were recorded in the number of ARs between the WT and the two d mutants after application of various NPA concentrations (Fig. S3B). Adventitious root numbers and average AR number per tiller were greater in the WT than d10 and d3 in response to application of 0·5 µm NPA (Fig. 6C, D). Although the WT and the d10 and d3 mutants had similar expression of DR5::GUS regardless of exogenous application of NAA or NPA (Fig. 6E), a higher AR number was always recorded in WT than in the d mutants under NAA and/or NPA applications over 5 weeks of the experiment (Fig. S4). These results suggested that the increased auxin concentrations in the two d mutants might not be responsible for reduced AR production.
Fig. 6.
Effect of synthetic NAA (A and B) and NPA (C and D) on AR number and average AR number per tiller of wild-type (WT) and strigolactone-deficient (d10) and strigolactone-insensitive (d3) mutant rice plants and DR5::GUS activity in the junction of WT and d10 and d3 (E). Rice seedlings were grown for 3 weeks in hydroponic media containing 10 nm NAA (α-naphthylacetic acid, exogenous auxin) and 0·5 µm NPA (N-1-naphthylphalamic acid, polar auxin transport inhibitor). Scale bar = 1 mm. Data are means ± s.e. Different lower-case letters indicate significant differences (P < 0·05, ANOVA).
DISCUSSION
In this study, we demonstrated that rice d mutants with impaired SL biosynthesis and signalling produced lower AR number compared with the WT. The number of ARs in d10, an SL-deficient mutant, was complemented by application of lower levels of GR24. This was not the case for d3, an SL-insensitive mutant. Based on these results, we concluded that AR formation was positively regulated by SLs in rice. A higher concentration of IAA in the root–shoot junction and [3H] IAA activity, and stronger expression of DR5::GUS were observed in the d mutants as comparing with the WT. Exogenous GR24 application decreased the expression of DR5::GUS in WT and d10, suggesting that SLs modulate AR formation by inhibiting polar auxin transport. Exogenous application of NAA and NPA induced similar expression levels of DR5::GUS in the WT and d10 and d3 mutants; however, AR production was still greater in the WT compared with the d10 and d3 mutants. These results indicated that the relationship between the SLs and auxin in regulating adventitious formation appears to be complex in rice.
In rice seedlings, the vigour of shoot growth correlated well with the total length of roots. AR growth in rice is the consequence of shoot growth (E et al., 2012). Tiller numbers of d10 and d3 increased significantly and higher tiller numbers were recorded in the d mutants compared with the WT 3 weeks after germination. However, lower effective tiller and seed setting rates were found in the two d mutants relative to the WT. Arite et al. (2012) found that d mutants exhibit a shorter AR phenotype. Spike number in rice has a close relationship with the number of elongated ARs (Zhang, 1988). Lower AR numbers were recorded in the d10 and d3 mutants during 5 weeks after germination. Because tiller numbers increased more rapidly in the d mutants than in WT plants, the more rapid AR outgrowth in the two d mutants 6 weeks after germination resulted in a 47 % increase in AR production per plant in the d mutants compared with WT plants. Therefore, the higher AR numbers in d mutants at the maturity stage observed in this study were probably the consequence of excessive shoot branching. However, the average AR number per tiller was considerably lower in the d mutants than in the WT, and these reductions may be another reason for the lower effective tiller and seed setting rates.
Several lines of evidence suggest that the SL pathway is involved in root growth, in a manner that depends on the growth conditions and plant genotypes. LR densities in tomato and Arabidopsis are increased in SL-deficient and SL-insensitive mutants, suggesting that SLs affect LR formation (Koltai et al., 2010; Kapulnik et al., 2011b; Ruyter-Spira et al., 2011). However, rice LR numbers on the seminal root did not differ among 14-d-old WT and d mutants. Application of the synthetic SL analogue GR24 decreased LR density via the signalling gene MAX2/D3, which suppressed LR outgrowth in Arabidopsis and rice (Arite et al., 2012; Sun et al., 2014). Sun et al. (2014) found that SLs affect rice LR density in response to nitrogen and phosphorus deficiencies. These results indicate that SLs function as plant hormones to regulate root growth, but the reasons remain obscure. Adventitious rooting was enhanced in SL-deficient and SL-response mutants of Arabidopsis and pea, and the CYCLIN B1 expression results suggested that SLs restrain the number of ARs by inhibiting the first formative divisions of the founder cells (Rasmussen et al., 2012). Arite et al. (2012) reported that SLs positively regulate the length of ARs by controlling cell division in the meristematic zone. In this study, rice d mutants with impaired SL biosynthesis and signalling produced lower AR number at the seedling stage and a lower number of ARs per tiller at maturity. AR number and average AR number per tiller in d10, but not in d3, were complemented by application of lower levels of GR24. These results suggest that AR formation is positively regulated by SLs via the D3 response pathway. Furthermore, the average length of ARs and the tiller number in SL synthetic mutants but not SL response mutants were complemented by application of GR24 (Arite et al., 2012; Jiang et al., 2013; Zhou et al., 2013). These results indicate that application of different concentrations of GR24 were able to compensate for both shoot and root phenotypes in rice.
The importance of auxin in promoting adventitious rooting has been well established (Blakesley et al., 1991; Coudert et al., 2010). AR emergence and development were significantly inhibited in OsPIN1b RNA interference (RNAi) transgenic plants, which was similar to the phenotype of NPA-treated WT plants. The NAA treatment rescued the mutated phenotypes in the rice RNAi plants (Xu et al., 2005). In this study, the application of NAA significantly induced AR formation in WT rice plants, while the application of NPA had the opposite effect (Fig. 6), which is consistent with reports of auxin promoting AR formation (Blakesley et al., 1991; Xu et al., 2005).
SLs have been suggested to act as modulators of auxin transport to regulate root growth (Koltai, 2011; Ruyter-Spira et al., 2011; Sun et al., 2014). Ruyter-Spira et al. (2011) suggest that SLs can modulate local auxin levels, a function that is dependent on the auxin status of Arabidopsis. The effects of SLs on root morphology in the presence of auxin have been shown to involve interference with auxin efflux carriers in tomato (Koltai et al., 2010). In this study, the higher concentration of auxin in the shoot base of SL mutants might result from up-regulation of PIN5c, PIN9 and PIN10a-b. Notably, the transcript level of PIN5c and PIN10a increased by three- to 20-fold in the junction of d mutants, which would play pivotal roles in mediating auxin acropetal flow to the junction. Conversely, application of GR24 decreased expression levels of most PIN family genes in WT and d10. These results confirmed that SLs inhibited polar auxin transport by modulating the expression of PIN genes. RT-PCR and promoter-driven GUS expression analysis showed that the high transcript levels of PIN9 and PIN10a-b, monocot-specific PINs, were observed in the shoot base of rice plants (Paponov et al., 2005; Wang et al., 2009). Furthermore, PIN9 and PIN10a are highly expressed in the primordia of ARs (Wang et al., 2009).
The interaction between the SLs and auxin signalling pathways in regulating adventitious rooting appears to be more complex. In Arabidopsis and pea, SLs can at least partially reverse the stimulation of AR formation by auxin, and auxin can further increase the numbers of ARs in more axillary branching (max) mutants that are resistant to the inhibitory effects of apical auxin. The above discussion indicates that AR formation is positively regulated by SLs via the D3 response pathway and modulation of auxin acropetal flow to the junction. Furthermore, the positive effect of NAA application and the opposite effect of NPA application on AR number of WT plants also suggested the importance of auxin for AR formation. In the two d mutants, the non-responsiveness of AR to NAA application probably resulted from higher endogenous auxin levels (Supplementary Data Figs S3 and S4). In contrast, DR5::GUS expression was similar in the WT and d10 and d3 mutants, after NAA or NPA application (Fig. 6E), while the WT had higher AR numbers than the d10 and d3 mutants. These data probably suggested that auxin may modulate AR formation depending on SL signalling, i.e. SLs play a role in AR formation downstream of auxin. In conclusion, SLs promoted AR formation in rice via the D3 component of SL signalling. The relationship between the SLs and auxin in regulating AR formation also appears to be complex.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: the primers used for qRT-PCR of PIN genes. Figure S1: number of ARs produced in response to the synthetic strigolactone analogue GR24. Figure S2: histochemical localization of GUS activity in rice plants. Figure S3: development of AR number in response to varying NAA and NPA concentrations. Figure S4: time course of AR number in response to NAA and/or NPA applications.
ACKNOWLEDGEMENTS
The SL mutants were kindly provided by Shinjiro Yamaguchi of RIKEN Plant Science Center. This work was funded by the Ministry of Science and Technology of China (No. 2011CB100302), National Nature Science Foundation of China (Nos. 31071846, 31172022 and 31471936), Innovative Research Team Development Plan of the Ministry of Education of China (No. IRT1256), the 111 Project (No. 12009), UU-COE Research Project from Utsunomiya University, PAPD in Jiangsu Province of China, China Scholarship Council (CSC), and Innovative Plan of Jiangsu Province of China (CXLX13_280 and 568).
LITERATURE CITED
- Arite T, Kameoka H, Kyozuka J. 2012. Strigolactone positively controls crown root elongation in rice. Journal of Plant Growth Regulation 31:165–172. [Google Scholar]
- Blakesley D, Weston GD, Hall JF. 1991. The role of endogenous auxin in root initiation. Plant Growth Regulation 10: 341–353. [Google Scholar]
- Blakeslee JJ, Peer WA, Murphy AS. 2005. Auxin transport . Current Opinion in Plant Biology 8: 494.–. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, et al. 1995. Super-root, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WC. 1936. Transport of root-forming hormone in woody cuttings. Plant Physiology 11: 779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert Y, Périn C, Courtois B, Khong GN, Gantet P. 2010. Genetic control of root development in rice, the model cereal. Trends in Plant Science 15: 219–226. [DOI] [PubMed] [Google Scholar]
- Delarue M, Prinsen E, Onckelen HV, Caboche M, Bellini C. 1998. Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant Journal 14: 603–611. [DOI] [PubMed] [Google Scholar]
- Diaz-Sala C, Hutchison KW, Goldfarb B, Greenwood MS. 1996. Maturation related loss in rooting competence by loblolly pine stem cuttings: the role of auxin transport, metabolism and tissue sensitivity. Physiologia Plantarum 97: 481– 490. [Google Scholar]
- E Z-G, Ge L, Wang L. 2012. Molecular mechanism of adventitious root formation in rice. Plant Growth Regulation 68: 325–331. [Google Scholar]
- Friml J. 2003. Auxin transport-shaping the plant. Current Opinion in Plant Biology 6: 7–12. [DOI] [PubMed] [Google Scholar]
- Inukai Y, Sakamoto T, Ueguchi-Tanaka M, et al. 2005. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17: 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J. 2005. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiology 46: 79–86. [DOI] [PubMed] [Google Scholar]
- Jiang K, Feldman LJ. 2003. Root meristem establishment and maintenance: the role of auxin. Journal of Plant Growth Regulation 21: 432–440. [Google Scholar]
- Jiang L, Liu X, Xiong G, et al. 2013. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y, Resnick N, Mayzlish-Gati E, et al. 2011a. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. Journal of Experimental Botany 62: 2915–2924. [DOI] [PubMed] [Google Scholar]
- Kapulnik Y, Delaux PM, Resnick N, et al. 2011b. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233: 209–216. [DOI] [PubMed] [Google Scholar]
- Kitomi Y, Ogawa A, Kitano H, Inukai Y. 2008. CRL4 regulates crown root formation through auxin transport in rice. Plant Root 2: 19–28. [Google Scholar]
- Koltai H. 2011. Strigolactones are regulators of root development. New Phytologist 190: 545–549. [DOI] [PubMed] [Google Scholar]
- Koltai H, Dor E, Hershenhorn J, et al. 2010. Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. Journal of Plant Growth Regulation 29: 129–136. [Google Scholar]
- Krisantini S, Johnston M, Williams RR, Beveridge C. 2006. Adventitious root formation in Grevillea (Proteaceae), an Australian native species. Scientia Horticulturae 107: 171–175. [Google Scholar]
- Liu H, Wang S, Yu X, Yu J, He X, Zhang S, Shou H, Wu P. 2005. ARL1, a LOB – domain protein required for adventitious root formation in rice. Plant Journal 43: 47–56. [DOI] [PubMed] [Google Scholar]
- Liu S, Wang J, Wang L, Wang X, Xue Y, Wu P, Shou H. 2009. Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Research 19: 1110–1119. [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. 2001. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant Journal 28: 465–474. [DOI] [PubMed] [Google Scholar]
- Negi S, Sukumar P, Liu X, Cohen JD, Muday GK. 2010. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant Journal 61: 3–15. [DOI] [PubMed] [Google Scholar]
- Peret B, Swarup K, Ferguson A, et al. 2012. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell 24: 2874–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paponov IA, Teale WD, Trebar M, Blilou I, Palme K. 2005. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends in Plant Science 10: 170–177. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Mason MG, De Cuyper C, et al. 2012. Strigolactones suppress adventitious rooting in Arabidopsis and Pea. Plant Physiology 158: 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T, et al. 2011. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiology 155: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Sun H, Li J, et al. 2013. Auxin distribution is differentially affected by nitrate in roots of two rice cultivars differing in responsiveness to nitrogen nutrients. Annals of Botany 112: 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Tao J, Liu S, et al. 2014. Strigolactones are involved in phosphate and nitrate deficiency-induced root development and auxin transport in rice. Journal of Experimental Botany 65: 6735–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. 1997. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, et al. 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–201. [DOI] [PubMed] [Google Scholar]
- Waters MT, Nelson DC, Scaffidi A, et al. 2012. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139: 1285–1295. [DOI] [PubMed] [Google Scholar]
- Wang J, Hu H, Wang G, Li J, Chen J, Wu P. 2009. Expression of PIN genes in rice (Oryza sativa L.): tissue specificity and regulation by hormones. Molecular Plant 4: 823–831. [DOI] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Yoneyama K. 2010. The strigolactone story. Annual Review of Phytopathology 48: 93–117. [DOI] [PubMed] [Google Scholar]
- Xu M, Zhu L, Shou HX, Wu P. 2005. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiology 46: 1674–1681. [DOI] [PubMed] [Google Scholar]
- Zazimalova E, Murphy AS, Yang H, Hoyerova K, Hosek P. 2010. Auxin transporters – why so many? Cold Spring Harbor Perspectives in Biology 2:a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, et al. 2001. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309. [DOI] [PubMed] [Google Scholar]
- Zhang Z. 1988. The relationship between spike number and the number of elongate adventitious root. Tillage and Cultivation 1: 63–64 (in Chinese). [Google Scholar]
- Zhou F, Lin Q, Zhu L, et al. 2013. D14-SCFD3-dependent degradation of D53 regulates strigolactone signaling. Nature 504: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.