Abstract
Colocalization Single Molecule Spectroscopy (CoSMoS) has proven to be a useful method for studying the composition, kinetics, and mechanisms of complex cellular machines. Key to the technique is the ability to simultaneously monitor multiple proteins and/or nucleic acids as they interact with one another. Here we describe a protocol for constructing a CoSMoS micromirror Total Internal Reflection Fluorescence Microscope (mmTIRFM). Design and construction of a scientific microscope often requires a number of custom components and a significant time commitment. In our protocol, we have streamlined this process by implementation of a commercially available microscopy platform designed to accommodate the optical components necessary for a mmTIRFM. The mmTIRF system eliminates the need for machining custom parts by the end-user and facilitates optical alignment. Depending on the experience-level of the microscope builder, these time-savings and the following protocol can enable mmTIRF construction to be completed within two months.
Keywords: TIRF, single molecule, micromirror, colocalization, fluorescence microscopy, CoSMoS
Introduction
Many essential biological processes are carried out by cellular machines which can contain multiple protein, RNA, and/or DNA components. Along with compositional complexity, many of these machines are even capable of proceeding along different reaction pathways each containing distinct intermediates.1,2,3 Single molecule techniques can provide unique insights into these complex processes. Observation of individual assemblies throughout the course of their reaction can provide detailed information about assembly order, reaction kinetics, and pathway heterogeneity that might be obscured by limitations of ensemble assays.
One common method for observing single molecules is total internal reflectance fluorescence microscopy (TIRFM). TIRFM relies on the generation of an exponentially decaying evanescent wave (typically < 100 nm in depth) by total internal reflection (TIR) to selectively excite fluorescent molecules tethered to the surface of a glass or quartz slide4 (Figure 1), thus providing the low background necessary to image individual fluorophores. Single molecule TIRFM is now routinely used for single molecule fluorescence resonance energy transfer (smFRET) experiments in which one or more molecules contain smFRET acceptor and donor fluorophores.5 Changes in the smFRET signal can be used to measure molecular distances or report on conformational dynamics. Another method is to monitor each fluorophore individually and study the colocalization of the single molecules as a means to detect biomolecular interactions. One of the earliest multi-wavelength colocalization experiments employed an alternating laser excitation scheme to observe ATP turnover by individual myosin molecules.6 Subsequently, many colocalization experiments have incorporated dual imaging optical components that split the image into short and long-wavelength images7 to allow simultaneous laser excitation of multiple fluorophores. A key advantage of colocalization microscopy is the ability to characterize complex cellular processes in which many different biomolecules participate in the overall reaction. For this reason, colocalization methods such as CoSMoS are well suited for composition, stoichiometric and kinetic analysis of essential cellular processes such as transcription, splicing and translation.8
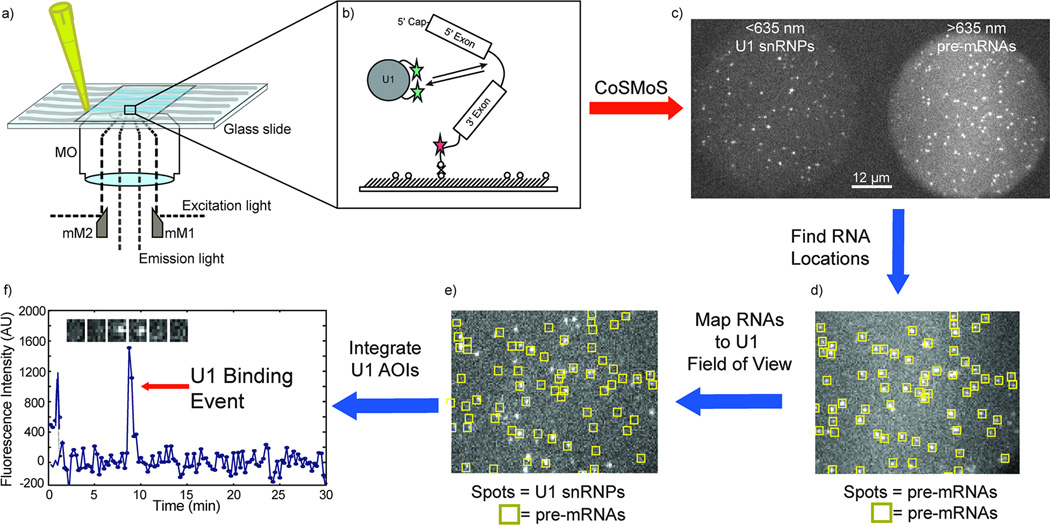
Figure 1. An example of the use of the mmTIRFM in a CoSMoS experiment to study spliceosome assembly.
a) A microfluidic flow chamber is loaded with fluorescently labeled biomolecules. The fluorescently labeled molecules at the surface are selectively excited by TIR, using the mmTIRFM constructed as described in this protocol. b) A Cy5 labeled pre-mRNA is tethered to the surface of a slide via a biotin/streptavidin/biotin linkage. Yeast whole cell extract with Dy-549 labeled U1 snRNP is subsequently flowed into the chamber where it can interact with the pre-mRNA. c) The surface tethered Cy5-pre-mRNAs are imaged using the mmTIRFM constructed here as diffraction limited spots in the >635 nm channel. The Dy549 labeled U1 snRNPs are imaged as diffraction limited spots only when the U1 snRNPs are sufficiently close to the surface of the slide and bound to the pre-mRNAs. d) Image analysis software locates the fluorescent signal from the pre-mRNA in the >635nm channel and marks them as areas of interest (AOIs). e) The AOIs are mapped onto the <635nm channel. f) The AOIs are then integrated and fluorescence intensity can be plotted over time to reveal discrete U1 snRNP-pre-mRNA binding events for individual pre-mRNAs.
Multi-wavelength TIRFM often utilizes an objective-based setup where TIR is generated by focusing the excitation lasers through a high numerical aperture (NA) (> 1.4) microscope objective (MO).9 As a consequence, both excitation and emission light pass through the objective and must be separated using additional optics. Removal of excitation light from the emission path is usually achieved on commercial microscope platforms by a combination of dichroic mirrors (DM) and emission filters (EF). The excitation light is directed into the MO through the back aperture by the DM. Consequently, the fluorescence emission must pass through both a DM and EF before being directed to the camera. One disadvantage of this approach is that as the number of lasers and distinct fluorophores increase in the experiment, separating the excitation and emission light becomes more challenging. This can be achieved with the addition of multiple DMs or more complicated DMs in the optical pathway; however, this also may result in the reduction of the collection efficiency for emitted photons.
The need for additional optics can be circumvented by spatially separating the excitation and emission light. This can be achieved using micromirror TIRF (mmTIRF). mmTIRF relies on small broadband mirrors to direct the excitation laser beams into and out of the back aperture of the MO (Figure 2).10 This approach has been used to study a number of systems by Colocalization Single Molecule Spectroscopy (CoSMoS; as depicted in Figure 1).8,11 While a mmTIRFM can achieve high photon detection efficiencies10, the additional optics below the MO adds significant spatial constraints that are often not compatible with conventional microscope platforms. Here we present a protocol for assembling a multi-wavelength mmTIRFM from commercial components for CoSMoS and other single molecule experiments. This protocol will focus exclusively on assembly of a mmTIRF microscope. General information about preparing samples for colocalization experiments have been described elsewhere.12 As with any single molecule experiment, further details will be specific to the biochemical system under investigation.
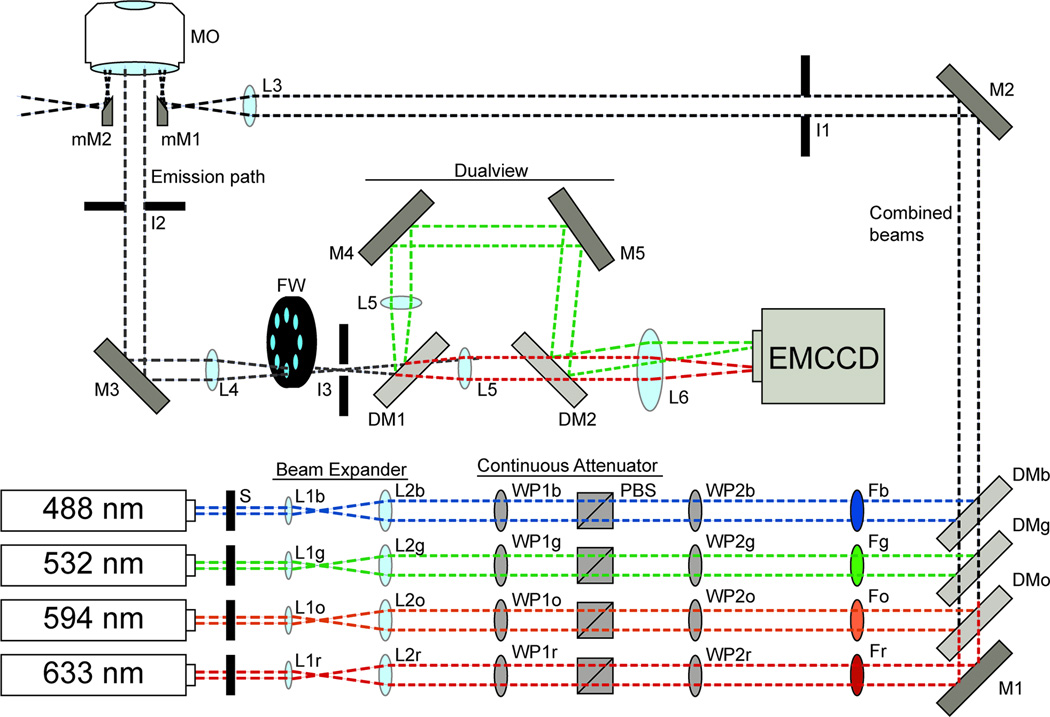
Figure 2. A general schematic of an objective-based mmTIRFM.
The individual laser beams (shown as blue, green, orange, and red dotted lines) are combined using long-pass DMs (DMb, DMg, DMo) and a broadband mirror (M1). Each DM is chosen with spectral properties specific to the wavelengths it must reflect and transmit. Laser excitation is selected using computer controlled shutters (S). Each beam is expanded and collimated using a Keplerian beam expander (lenses L1 and L2) that are selected based on the input beam diameter and desired output beam diameter for each laser. Beam intensity is controlled by a continuous attenuator composed of a λ/2 waveplate (WP1) and a polarizing beam splitting cube (PBS). Subsequently, a λ/4 waveplate (WP2) is used to circularly polarize each beam. Each beam is passed through a corresponding bandpass filter (i.e., Fb for the blue laser bandpass filter or Fg for the green laser bandpass filter). The combined beams are directed toward the input micromirror (mM1) using a broadband mirror (M2). An iris (I1) is used to reduce the beam diameter. The beams are focused near the back aperture of the microscope objective by lens L3. The beams exit the MO on the opposite side of the back aperture as from mM1 and are directed away from the emission path by an output micromirror (mM2). The emission light is directed toward an EMCCD camera by a broad band mirror (M3) in a 45° mount. Scatted light from the MO is reduced with and iris (I2). A pair of lenses collimates (L4 and L5) the emission light. A filter wheel (FW) containing filter sets specific to the experimental design remove residual laser beam light from the emission path. Iris 3 (I3) is placed at an intermediate image plane and helps define the field-of-view. The emission light is split into a >635nm and <635nm channel using a long-pass DM (DM1). A pair of broadband mirrors (M4 and M5) steer the <635nm light back toward the >635nm light. A second DM (DM2) directs the <635nm light along the same axis as the >635nm light. The two channels are focused on the detector by L6. M4 and M5 is used to steer the <635nm light to a separate area of the detector to generate separate <635nm and >635nm images.
Micrsoscope Design Rationale
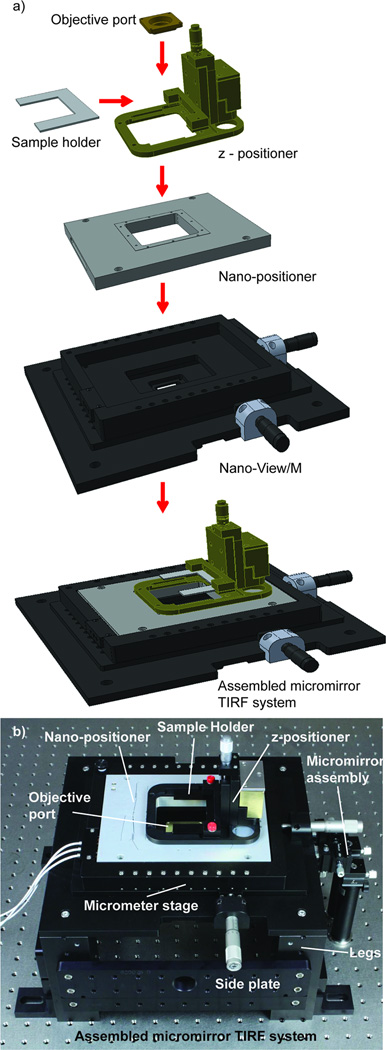
The fundamental optical design of the mmTIRFM has been previously described by Friedman et al.10 However, implementation of the published design de novo is challenging since the microscope design called for a number of custom components that are not readily available or require fabrication. To meet this challenge we developed a protocol that relies on commercially available optical components to construct a mmTIRFM11 that preserves Friedman’s optical design.10 We designed a mmTIRF stage assembly and micromirror positioning system (micromirror TIRF system, Mad City Labs, Inc., Madison, WI) to accommodate both sample positioning and optical pathway space constraints (Figure 3). The base is built around the MO to provide full and open access to both the front lens and back aperture. An objective (herein an Olympus, 60×, PlanApo, N.A. = 1.45) is statically mounted to the base plate of a manual x-y micropositioning stage, with 25mm of travel along each axis. The micropositioner itself is designed to hold an embedded 3-axis nanopositioning stage with 200 µm of travel in each dimension (Figure 3). Initial positioning of the sample in the z-dimension (up and down) is achieved with a light weight, manual z-axis micropositioner (10mm travel) mounted directly on top of the nanopositioner (Figure 3). The z-axis micropositioner contains a clamp for holding microscope slides fixed to a metal slide holder. Using this design, the sample is initially positioned and focused using the micropositioners while the nanopositioning stage is used to control sample position and maintain focus during an experiment.
Figure 3. The components of the mmTIRF system and their assembly.
a) A diagram of the mmTIRF system (Mad City Labs, Inc.) showing how each of the components interface. The system is designed around an objective holder fixed to a metal plate. A Nano-View/M 200-3 embedded-style xy-micrometer stage is then attached to this same plate. The nanopositioner itself is recessed into the micropositioner. A z-positioner with a dampening mass and a clamp to hold the U-shaped microscope slide holder is secured to the top plate of the nanopositioner. b) An image of the assembled micromirrror TRIF system showing the objective platform, stages, legs, and micromirror platform assemblies.
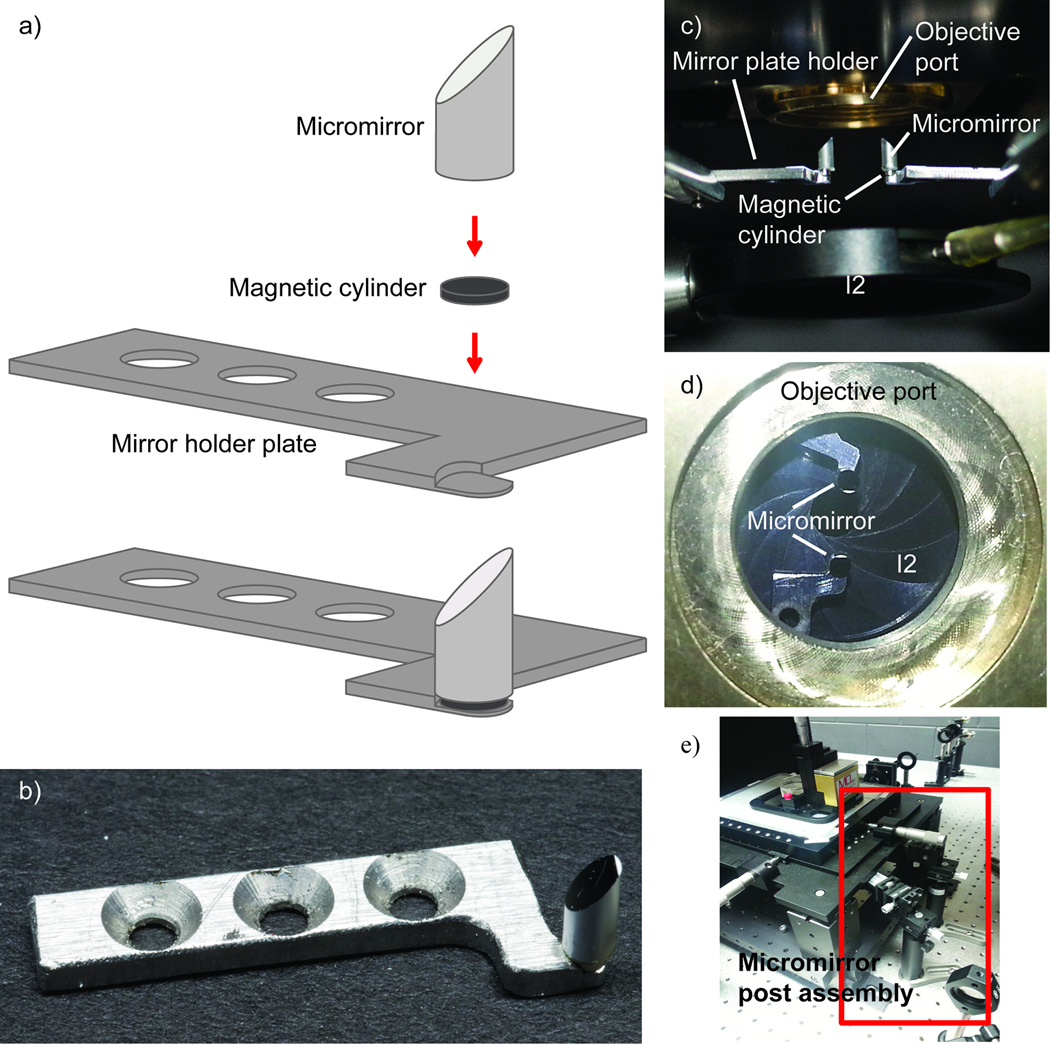
The stage assembly described above is mounted on four legs (154mm height) that are attached directly to an optical table (Figure 3), providing ample space for, and access to, the excitation and emission light pathways. To achieve and maintain TIR, the excitation beams are directed into and out of the objective lens using two cylindrical 45° micromirrors (< 3mm in diameter) that are positioned approximately 2mm below, and at opposite edges of, the back aperture of the MO (Figure 4). These mirrors are held in place by magnetic plate holders (Figure 4a and b) fixed to the ends of optical posts and post holders positioned perpendicular to the emission light pathway. The micromirror position is fine-tuned using 3-axis manual micropositioners that are themselves mounted to the table (Figure 4e). This set up allows for straightforward adjustment of the micromirrors in the x,y and z directions, as well as all three rotational axes. The mmTIRFM is mounted on a 4′×12′ isolation table with ¼′′-20 tapped holes on 1′′ centers to insulate the microscope from building vibrations. This large optical table facilitates laser beam alignment and microscope assembly but tables with smaller dimensions will also suffice.
Figure 4. Micromirror platforms and their mounting underneath the objective.
a) A diagram of the micromirrors and the micromirror plate holder. A magnetic disk is epoxied to the bottom of the cylindrical micromirror. The mirror is then magnetically secured to the mirror plate holder. The recess allows for rotation of the mirror without translation. b) An image of the micromirror and mirror plate holder. c) An image of the micromirrors mounted under the microscope objective. d) An image of the micromirrors looking down through the objective port. e) An image of the micromirror post assemblies.
The basis for the design of the mmTIRFM is to allow for observation of multiple fluorescently labeled molecules with high photon collection efficiency. Observation of up to four spectrally distinct fluorophores is achieved by combining four laser beams using long-pass DMs and focusing them on the back focal plane of the MO (Figure 2). An added benefit of this setup is the ability to independently control the TIR angle of each excitation laser using both the micromirror and dichroic mirrors to steer the beams. This feature allows adjustment and optimization of the penetration depth of the evanescent wave at each wavelength. The excitation beams are directed into the MO by the micromirror assembly described above (Figures 2 and 4). Selection of excitation wavelength is achieved using computer controlled mechanical shutters. Prior to reaching the input micromirror each laser beam is expanded and collimated with a Keplerian beam expander. Laser intensities are controlled with continuous attenuators composed of λ/2 waveplates mounted in rotary mounts and polarized beam splitters (PBS). Laser intensity is adjusted by rotating the plane of polarization with the λ/2 waveplate relative to the PBS. Beams are subsequently circularly polarized using λ/4 waveplates. Finally, band-pass filters are used to attenuate or reject undesirable laser wavelengths.
Emission light is directed toward an EMCCD camera by a Ø1′′ broadband mirror in a 45° mount. The 45° mount is itself located on a kinematic platform to enable steering of the emission light. Simultaneous imaging of two wavelength ranges (herein, < and > 635nm) is achieved by a dualview apparatus. The dualview apparatus uses a long-pass dichroic mirror to divide the emission light into a < and > 635nm path. The paths are each focused on separate regions of the EMCCD chip to generate two images. Magnification and collimation is controlled using a pair of collimating lenses placed in each channel of the dualview and focusing the emission light on them with a lens placed just before the dualview. The dualview itself splits the emission light with a long-pass DM. Emission light with a wavelength > 635nm is transmitted directly towards the EMCCD while emission light < 635 nm is reflected along a path perpendicular to the > 635nm emission path. Two Ø2′′ broadband mirrors are used to direct the <635 nm emission light back toward the original emission path, and the two paths are combined with a second long-pass DM. The <635 nm emission is steered so that the final image is placed side-by-side with the >635nm image. Finally, a focusing lens is placed in the emission path to focus the images onto the EMCCD. The DC mirrors and broadband mirrors are all placed on kinematic mounts to facilitate image steering. Two irises in the emission path are used to block stray light (I2, Figure 2) and define the edges of the field-of-view (FOV) (I3, Figure 2). With a 60× 1.45 NA objective the FOV will be approximately 50 µm in diameter. The entire emission path is contained in an enclosure made from black posterboard to block ambient light from reaching the EMCCD.
The mmTIRFM described here is suited for a variety of colocalization experiments. However, the system does have some limitations worth consideration. First, any exchange of the objective requires a significant time investment to realign the excitation and emission optics. Second, we describe here the construction of a dualview apparatus that is limited to simultaneous imaging of just two colors. However, this can easily be overcome by adaptation of an alternating laser excitation protocol13 or by expanding the emission optics to further segregate the fluorescence (e.g., construction of a tri- or quadview apparatus). The latter approach may require use of an additional camera or a camera with a larger sensor to image a reasonably sized field-of-view. Third, despite streamlining the microscope’s assembly and alignment process, construction of a mmTIRFM still represents a significant time investment, particularly when compared to purchase of commercial TIRF systems. Finally, we note that colocalization does not necessarily imply a biomolecular interaction, particularly when images are acquired at a diffraction limit much larger than the size of the individual molecules. Control experiments, for example use of mutant proteins that alter or abolish an interaction, are often required to confirm that the colocalized signals are reporting on formation of a biochemically relevant species.
Equipment
[CRITICAL] Many of the commercial components listed in the equipment section are from specific suppliers; however, similar components can be purchased from other suppliers and be used to achieve similar results.
TMC 780 series optical table with ¼′′-20 tapped holes on 1′′ centers with vibration isolating legs
Excitation Path
488 nm cyan diode laser (Spectra Physics, PC13589)
532 nm diode laser (Crystal Laser, CL532-052-L)
594 nm diode laser (Crystal Laser, CL593-025)
-
633 nm He-Ne laser (JDSU, 1144P)
[CAUTION] Class IIIb lasers can cause serious injury if the direct or specularly reflected beam enters the unprotected eye. Laser safety goggles should be worn to prevent eye injury. The area should be posted with DANGER signs to inform lab workers when the lasers are powered. Only authorized personnel who are trained on the system’s laser hazards should operate the equipment. Please check the laser safety guidelines provided by your institution for further precautions.
Shutter (Figure 2, S) [Uniblitz, LS272 (4×)]
Shutter driver (Uniblitz, VMM-D4 four channel driver)
Objective lenses for Keplerian beam expander (L1) [Newport Corporation, M-5×, M-10× (4×)]
Visible achromatic doublet lenses for Keplerian beam expander (L2) [Newport Corporation, PAC067AR.14, PAC061AR.14 (3×)]
Multiple-order quartz wave plates for continuous attenuator λ/2 retardation (WP1) (Newport Corporation, 10RP12-12 10RP12-16, 10RP12-24; Melles Griot, QWPM-594-08-2-R10)
Broadband polarizing cube beamsplitter 420–680 nm for continuous attenuator (PBS) [Newport Corporation, 10FC16PB.3 (4×)]
Multiple-order quartz wave plates for circular polarization of lasers λ/4 retardation (WP2) (Newport Corporation, 10RP14-12, 10RP14-16, 10RP14-24; Melles Griot, QWPM-594-08-4-R10)
Band-pass filters (F) (Chroma Technologies, ZET488/10×, Z532/10×, Z594/10, Z633/10×)
Broadband metallic mirrors (M) [Newport Corporation, 10D20ER.1(5×)]
Long-pass dichroic mirrors for combining excitation beams (DM) (Chroma Technologies, Z488RDC, Z532RDC, ZT594RDC)
Doublet lens for final focusing/steering lens (L3) (Newport Corporation, PAC067AR.14)
Optical support rod for mounting He-Ne laser (Newport Corporation, 70)
Rack-and-pinion rod clamp part of EMCCD camera mount (Newport Corporation, 370-RC)
Cylindrical laser mount (Newport Corporation, ULM-TILT)
Ø1/2′′ stainless steel optical posts for mounting optical components and lasers(Thor Labs, TR series posts)
Ø1/2′′ post holders for mounting optical components and lasers (Thor Labs, PH series post holders)
Post holder bases (Thor Labs BA series)
Kinematic cage mounts for mounting XY translation mounts containing doublet lenses or objectives [Thor Labs, KC1-T (9×)]
XY translation mounts for housing doublet lenses and objectives [(Thor Labs, LM1XY (9×)]
Linear stage for fine translation mounted doublet lenses [Newport, 423 (5×)]
Rotation stage for mounting wave plates. [Newport, RSP-1T (6×); Melles Griot, 1100-10 (2×)]
Kinematic platform mounts for mounting broadband polarizing cubes [Thor Labs, KM100B (4×)]
Clamping arm for mounting broadband polarizing cubes [Thor Labs, PM3 (4×)]
Ø1′′ precision kinematic mirror mounts [Newport Corporation, U100-A3K (6×)]
Kinematic prism mount for mounting dichroic mirror clamps [Thor Labs, KM100PM (3×)]
Plate holder for mounting dichroic mirror [Thor Labs, FP01 (3×)]
Post mounted iris diaphragms (I2) [Thor labs, ID25 (3×)]
Polarizing sheet plate (Thor Labs, LPVIS100)
Shearing interferometer (Thor Labs, SI100)
Objective and micromirror platforms
Olympus 60× 1.45 NA PlanApo Objective (MO)
mmTIRF system (Mad City Labs, Inc.) containing the following components: Shelf Braces (4×)(629065B); SM1 Threaded ports/side plate (2×)(629075B); RM21 legs (4×) 628415; Sample holder (made from part numbers: 629277, 629285, 629295); Z-positioner (made from part numbers: 629317, 629305, 629327, 703115); Dampening mass (made from part numbers: 629369, 629385, 629405, 629375); NanoView/M 200-3 (made from part numbers: 629217, 629227, 629237, 629258, 629245, 629397, 617045); Nano-Drive3 with USB and ISS; Mirror holder rod (2×) (629355); Mirror holder plate left and right (629336, 629346); Offset plates [629397 (2×)]; Magnetic post holder bases [PS-AZ (2×)]; Clamping Fork [PS-F (2×)]; 4′′ post holders [9608 (2×)]; 2′′ post holders [9606 (2×)]; 3-axis micropositioner [MT-XYZ (2×)]; 4′′ optical mounting posts [Sp-4 (4×)]; 2mm diameter 45° micromirror (mM1 and mM2) [54-092 Edmund Optics (2×)] [CRITICAL] The mmTIRF system from MadCity Labs is key to the construction of the microscope described here. It is possible to construct a microscope with the same functionality using other components, but significant customization and optimization may be needed.
Emission Path
Broadband metallic mirror (M3) (Newport Corporation, 10D20ER.2)
Visible achromatic doublet lenses (L4, L5, and L6) [Newport Corporation, PAC061AR.14, PAC055AR.14 (2×), PAC074AR.14]
Long-pass dichroic mirrors for dualview (DM1 and DM2) [Chroma Technologies, DCXR (2×)]
Broadband dielectric mirrors for dualview (M4 and M5) [Newport Corporation, 20Z20BD.1 (2×)]
Band-pass, long-pass and notch emission filters (e.g. Semock FF03-525/50-25 488 nm band-pass filter)
High speed filter wheel (FW) (Sutter Instruments, Lambda 10-3)
Single photon detection EMCCD camera (Andor, iXon3 897)
Alignment laser (JDS, 1508-2)
Precision optical rail for mounting lenses, dualview, filter wheel, and EMCCD camera [Newport Corporation, PRL3 (3×)]
Rail carrier (Newport Corporation, PRC series)
Ø1/2′′ stainless steel optical posts for mounting optical components (Thor Labs, TR series posts)
Ø1/2′′ post holders for mounting optical components and (Thor Labs, PH series post holders)
Kinematic cage mounts for mounting XY translation mounts containing doublet lenses [Thor Labs, KC1-T (3×)]
XY translation mounts for housing doublet lenses [Thor Labs, LM1XY (3×)]
8′′x10′′ solid aluminum bread board with ¼′′-20 tapped holes on 1′′ centers for dualview platform (Thor Labs, MB series)
Lens tubes (Thor Labs, SM30 series)
Post mounted iris diaphragms [Thor labs, ID25 (4×)]
Threaded iris diaphragms for mounting in lens tubes (Thor Labs, SM1D25)
Three axes micropositioner (Newport Corporation, MT-XYZ)
Adjustable height V-mount for mounting final focusing lens (Thor Labs, VG100)
Optical support rod for mounting EMCCD camera (Newport Corporation, 70)
Rack-and-pinion rod clamp part of EMCCD camera mount (Newport Corporation, 370-RC)
Large angle bracket for mounting camera (Thor Labs, AP90RT)
Linear translation stages for fine translation of mounted camera (Newport Corporation, 423)
Procedure
Arrangement and alignment of excitation path and installation of filters, continuous attenuators, and beam expanders Timing – 2 weeks
-
1
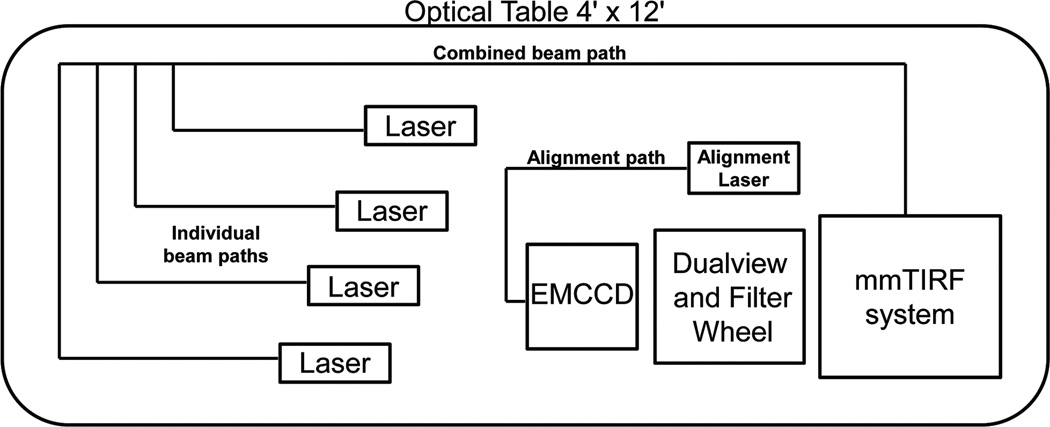
Diagram the layout of the components of the microscope on the optical table (Figure 5). Begin by assigning the location of the mmTIRF system (Mad City Labs, Inc.). The system should be located near one the corners of the optical table. There should be sufficient room for lab personal to access the system for loading samples and adjusting the micrometer stages. There should also be enough space to accommodate a computer desk to house the PC that will control the electrical components of the microscope. In addition, roughly layout the locations of the excitation path, emission path, EMCCD, and alignment laser (Figure 5).
[CRITICAL STEP] Careful consideration of the initial placement of microscope components will avoid subsequent and time-consuming rearrangement of the optical components at later stages.
-
2
Place the mmTIRF system (Mad City Labs, Inc.) and secure it to the optical table. The mmTIRF system (Mad City Labs, Inc.) should be placed first to provide a reference point for mounting the remaining microscope components. The mmTIRF system (Mad City Labs, Inc.) is designed to place the objective directly above the threaded holes on the optical table making alignment of the excitation and emission path easier. The system should be oriented so that the channel under the objective platform is running parallel with the short edge of the table. This channel provides ample room for the laser beams to access the micromirrors.
-
3
Orient the micromirrors so that the input and output beam will run parallel with the channel in the mmTIRF system (Mad City Labs, Inc.). Set the height of the micromirrors as close to the back aperture of the objective as possible (~2 mm). A focusing lens will eventually focus the excitation laser to the back focal plane of the objective. Installation of the focusing lens and locating the plane will be discussed later in the procedure (Step 6).
-
4
Set the height of the lasers to match the height of the input micromirror and align the laser beams along the rows of threaded holes in the optical table using a pair of irises set so the height of the apertures of the irises matches the height of the micromirrors. The irises are needed to ensure that the laser beams are positioned parallel to the table surface.
[CAUTION] Class IIIb lasers can cause eye injury when the direct or specularly reflected beams are viewed. Extra care needs to be taken from this point on to avoid eye injury from any spurious beams. Laser safety goggles should be used to reduce the risk of eye injury.
-
5
Using the pair of irises mount the broadband and dichroic mirrors to combine and direct the beams to the micromirrors. Broadband mirrors should be placed in kinematic mounts (Newport Corporation U100-A3K). Dichroic mirrors can be mounted on a kinematic prism mount using a plate clamp (Thor Labs, KM100PM and FP01). Direct the beam directly under the objective port using the threaded holes of the optical table and irises as a guide. This is best accomplished by first lowering the micromirrors out of the beam path.
[CAUTION] Dichroic mirrors will generate multiple beams. The beams that do not proceed along the excitation path should be blocked to limit spurious reflections that may result in poor image quality or eye injury. Beams can be blocked using a beam block (e.g., Thor Labs LB1).
-
6
Mount the final focusing lens (L3) so that the total distance of the beam path from the lens to the back aperture of the objective is very close to the focal length. L3 should be mounted in a xy-translation mount attached to a kinematic cage mount (Thor Labs, LM1XY and KCT-1). Additionally, the L3 apparatus should be placed on a linear translation stage set to translate along the direction of the beam path (Newport Corporation 423). The retro-reflection from L3 should be steered slightly off of the optical axis to avoid interference.
[CRITICAL STEP] The placement of this lens is crucial for achieving collimation within the objective. The use of a linear translation stage is critical for obtaining the correct placement.
-
7
Next, position the band-pass filters and the λ/4 waveplates. The band-pass filters are mounted in a fixed lens mount (Thors Labs, LMR1) while the λ/4 waveplates are housed in rotary mount. Again, the retro-reflections from each should be steered slightly off the optical axis (this should be done with all of the remaining optical components in the excitation path).
-
8
Assemble the remaining components of the continuous attenuators for each input laser beam. Mount the λ/2 waveplates using a rotation stage (Newport, RSP-1T; Melles Griot, 1100-10). The PBS should be mounted using a kinematic platform mount (Thor Labs, KM100B). At this point, rotation of the λ/4 waveplate should modulate the beam intensity exiting the PBS.
[CAUTION] The PBS will generate multiple beams. The reflected beams should be blocked to avoid spurious reflections that can lower image quality or cause potential eye damage.
-
9
Circular polarization of the beam is now achieved by placing the λ/4 waveplates. Correct polarization can be monitored by placing a polarizing sheet plate (Thor Labs, LPVIS100) in the beam path. Rotate the λ/4 waveplate to find the maximum and minimum power of the beam passing through the polarizing sheet plate. Calculate the mean power of the two values and adjust the λ/4 plate so that the power is equal to the mean of the maximum and minimum intensities. You can check the polarization of the beams by rotating the polarizing sheet plate and measuring the power of the transmitted beams. If the beams are circularly polarized then there should be little change in power between different orientations of the sheet polarizer. Once the attenuators are assembled, remove the polarizing sheet plate. The desired laser power can be achieved by rotation of the λ/2 waveplates and monitoring the output with a power meter.
-
10
Next, the laser beams are expanded and collimated using a Keplerian beam expander. Using the input laser beam diameter and the desired output beam diameter, select the appropriate pairs of lenses and/or objectives. Both optics should be mounted to allow for tip/tilt and×and y translation along the optical axis. This can be achieved by mounting the optics into xy-translation mount with the lens in a kinematic cage mount (Thor Labs, LM1XY and KCT-1). Additionally, the collimating lens (L2) apparatus should be placed on a linear translation stage set to translate along the direction of the beam path (Newport Corporation 423). Position these lenses on the optical table between the lasers and the λ/4 waveplates.
-
11
Collimation can be achieved using a shearing interferometer (e.g., Thor Labs SI254). Add the focal lengths of the two lenses together and mount the collimating lens approximately that distance away from the expanding lens. Use the linear stage to make fine adjustments to the distance between the two lenses. Place the interferometer into the beam path and adjust the distance until the correct orientation of the interference pattern is achieved (see manufacturer’s instructions). This will roughly collimate the beams. Final adjustments will be discussed below (Step 17).
[CRITICAL STEP] The shearing plate interferometer will only achieve a very close approximation of collimation. Final adjustments will be determined using the size of the laser beam as it exits the objective once the micromirrors have been properly aligned.
TROUBLESHOOTING
-
12
With the beam expanded, place an iris (I1) between the final focusing lens and the last mirror directing the beam to the micromirror. The distance between I3 and L3 should be one focal from L3. This iris is used to control the beam size (and the size of the field of illumination).
TROUBLESHOOTING
Figure 5. A diagram of the position of the components of the microscope on the optical table.
Alignment of the micromirrors and achievement of TIR Timing – 1 week
-
13
The micromirrors (Edmund Optics 54-094) are mounted using a mirror holder plate (Mad City Labs 629336 and 629346) attached to a mirror holder rod (Mad City Labs 629355) (Figure 4). Micromirrors are mounted to the mirror holder plate using small cylindrical magnets that are adhered to the bottom of the mirror using epoxy (Figure 4a). The position of the micromirror is controlled using a three axis stage (Newport Corporation, MT-XYZ)
-
14
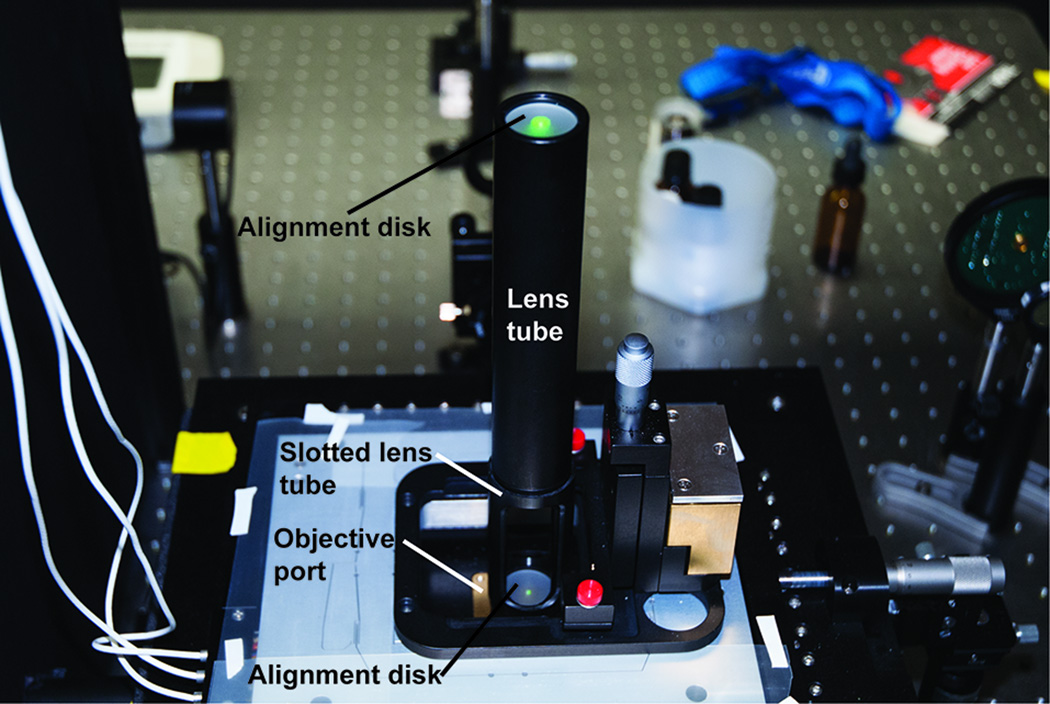
Position the input micromirror (mM1, Figure 2) underneath the objective at the center of the back aperture. Centering the input micromirror on the back aperture is best accomplished using an alignment tool (Figure 6). The alignment tool consists of a lens tube (Thor Labs SML series) with a frosted glass alignment disk (Thor Labs, DG10-1500-H1) connected to a slotted lens tube containing a second alignment disk. Screw the alignment tool into the objective holder and direct an excitation beam through so that it is centered on both the top and bottom apertures of the alignment tool. Use a forceps to position and gently rotate the micromirror to the correct position. The correct tilt of the mirror can be obtained by rotation of the mirror holder rod within its post holder. Having M2 mounted on a linear translation stage will aid in the process of centering the excitation beam on the objective aperture.
[CRITICAL STEP] Begin with alignment of a single laser (e.g., the 532 nm laser). Once this laser has been aligned, other lasers can be subsequently aligned to the first laser.
-
15
Remove the alignment tool and place a slide across the opening of the objective holder to generate a retro-reflected beam. Use this as a fine adjustment for the position of the micromirror. Adjust the mirror so that the reflected beam is directed back along the excitation path.
[CRITICAL STEP] This particular back reflection must be precisely directed back along the excitation path to ensure proper alignment of the micromirror.
-
16
Remove the slide and attach the objective (Olympus 60× 1.45NA oil immersion). If aligned correctly, the beam should be exiting straight out of the objective. Place a slide on the objective and translate the micromirror toward the edge of the back aperture. The beam should move in the opposite direction of the translating micromirror until the beam disappears.
[CAUTION] If the laser beam is not properly centered or aligned on the back of the objective, the beam will come out of the top of the objective at an unexpected angle and could potentially cause eye injury.
[CAUTION] Some objectives may contain a spring-loaded retractable nose cone; thus, it may be necessary to immobilize the spring mechanism using a compression collar around the objective to prevent the objective from hitting the micromirrors or altering the objective-mirror distance when a slide is placed on the microscope.
TROUBLESHOOTING
-
17
At this point it is possible to make the final adjustment to L2 to collimate the excitation beams. Allow the excitation laser beam to exit the objective (with a slide mounted) just out of TIR and at a steep angle. Project the beam onto a distant wall, if possible. Then, adjust the collimation for each beam independently (again using the translation stage on L2) so as to minimize the size of the projected laser spot. A collimated laser beam that is properly focused on the back focal plane of the objective will not be divergent as it exits the objective.
-
18
A beam should now appear exiting from the back aperture of the objective directly across from the input beam. Mount the second micromirror setting it to the correct height and place it directly underneath the exiting beam. Adjust it to the desired position in the same manner as the input micromirror. The quality (i.e., circular shape) and position of the exit beam can be used as an indicator for the correct position of the slide to generate TIR.
Figure 6. The alignment tool used for orientation of the excitation and emission paths through the objective.
The tool is composed of a lens tube with an alignment disk at the top and a slotted lens tube with a second alignment disk nearest the objective port. Laser alignment is achieved by guiding the beam through the small apertures in the alignment disks (shown here with the 532 nm laser beam).
Arrangement of the emission path and alignment of the dualview, lenses and filter wheel Timing – 1–2 weeks
-
19
Secure a 3′ optical rail (Newport Corporation PRL3) to the optical platform so that it is flush with the edge of the micromirror TIRF system (the rail can be extended as needed). This rail will define the emission path. The rail should be centered on the objective port and oriented so it exits from beneath the micromirror TRIF system perpendicular to the excitation path.
-
20
Place two additional 3′ rails on each side of the central rail. They should be approximately 2′′ each from the central rail.
-
21
Place a SM1 threaded iris (Thor Labs, SM1D25) in the SM1 port of the side plate of the mmTIRF system (Mad City Labs, Inc.) facing the optical rail. The center of the aperture defines the height of the emission path.
-
22
Place a second iris on the far end of the optical rail. Mount the iris to a rail carrier (Newport Corporation PRC series) so it may be translated along the rail. Set it to match the height of the iris in the side plate of the mmTIRF system (Mad City Labs, Inc.).
-
23
Secure a low-power alignment laser (e.g., JDSU 1508-2) to the optical table. With a pair of broadband mirrors mounted in kinematic mounts (Newport Corporation U100-A3K), direct the laser beam through the two irises and toward the objective port of the mmTIRF system (Mad City Labs, Inc.). This laser beam and the two irises will be used to align the emission path optics. Ideally the alignment beam will be centered on the rail and the side port so that the beam will remain centered on an iris that is translated along the rail.
-
24
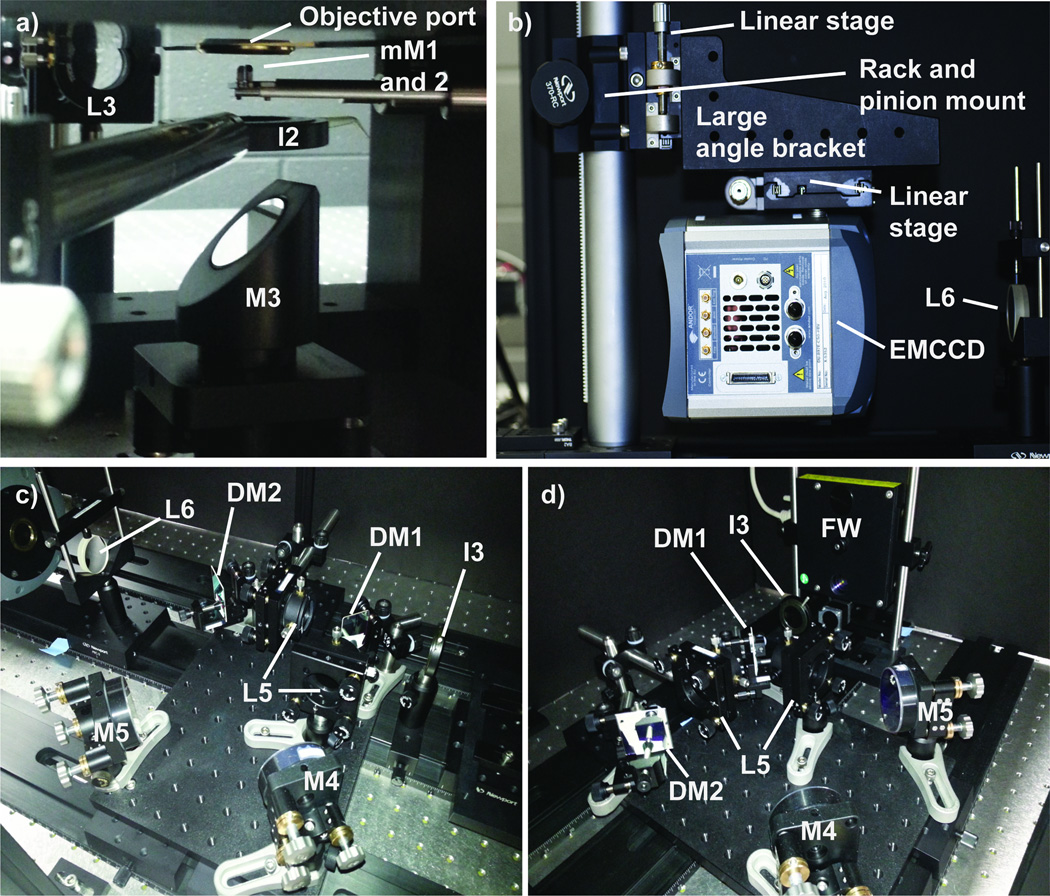
Place a broadband mirror (Newport Corporation 10D20ER.2) mounted in a 45° optic mount (Thor Labs H45) set on a kinematic platform mount (Thor Labs KM100B) directly below the objective and micromirrors (Figure 7a). Direct the alignment laser up through the center of the objective using the alignment tool as a guide (Figure 6).
-
25
Mount the final focusing lens (L6) on the optical rail using the alignment laser beam to position it in the center of the emission path. The lens is best mounted using the xy-translation and kinematic cage mounts used previously for the final focusing lens and the beam expander, but due to the size of the lens an alternative mount may be used. Mount the entire lens assembly on a rail carrier.
-
26
Translate the L6 lens so that it is next to the mmTIRF system (Mad City Labs, Inc.). If aligned correctly, the laser beam should not deviate from its set path. Turn off the alignment laser.
-
27
Attach a linear stage (Newport Corporation 423) to the top of EMCCD camera (Andor, iXon3 897) so that the camera can translate along the emission path. Secure the EMCCD/stage assembly to the long face of a large angle bracket (Thor Labs AP90RT). Attach another linear stage to the adjacent face of the large angle bracket. Orient the second stage to translate perpendicular to the emission path.
[CRITICAL STEP] It is essential that the EMCCD camera is able to translate perpendicular to the optical axis. A very robust mount is needed so the camera position does not shift over time.
-
28
Mount an optical support rod (Newport Corporation, 70) to the rail just past L6. A second rail carrier should be secured to the support rod assembly on the side furthest from the mmTIRF system (Mad City Labs, Inc.).
[CRITICAL STEP] The two rail carriers help support of the weight of the camera assembly described above.
-
29
Using a rack-and-pinion rod clamp (Newport Corporation, 370-RC) mount the camera assembly to the optical support rod. Figure 7b shows the entire assembly. Translate the camera assembly until the distance between the EMCCD camera’s chip and L6 is equal to the focal length of L6. Use the two linear translation stages to roughly center the camera in the emission path.
-
30
Prepare a slide with a surface tethered fluorescent sample that can be excited using an appropriate laser, place it on the microscope and bring it into TIR. For this step we prefer to use fluorescent beads (Life Technologies, T107171), the 532 nm laser, and a 532 long-pass filter. Place an appropriate long-pass filter in the optical path to block excitation light from entering the emission path. Use the two linear translation stages move the camera and locate the image. Translate the camera along the optical rail to find the focus.
-
31
Translate the camera and the final focusing lens away from the micromirror TIRF until the end of the rail is reached. Be sure to maintain the correct distance between the camera and L6. Mark the position of the camera on the optical rail and remove it to unblock the path for the alignment laser.
-
32
Mount a 8′′ × 10′′ aluminum optical breadboard (Thor labs MB series) between the final focusing lens and the mmTIRF system (Mad City Labs, Inc.). The plate will serve as the base for the dualview optics. The plate should span the central optical rail and one of the peripheral rails. Secure the breadboard to the rails with rail carriers so the plate can translate.
-
33
Turn on the alignment laser and use the beam as a guide to place the dualview optics. For ease of alignment it is helpful to use 2′′-sized optics (Ø2′′ for the broadband mirrors and 2′′ × 2′′ for the dichroic mirrors). Additionally, all of the optics should be mounted in kinematic mounts with xy-translation and tip/tilt capabilities. The post assemblies used to mount the dualview optics are easily positioned if the posts have a base (Thor Labs BE2) and can be secured to the breadboard using a clamping fork (Thor Labs CF series). Figure 7c and d show the dualview optics and there location on the aluminum breadboard.
-
34
Position the first dichroic mirror (DM1) (Chroma Technology DCXR 635) on the edge of the plate closest to the final focusing lens and in the beam path at a 45° angle directing the partially reflected beam along the width of the breadboard while the partially transmitted beam continues along to the objective.
[CAUTION] Dichroic mirrors will generate multiple beams. Care needs to be taken when using the alignment laser to place the mirrors. Spurious laser beams can cause serious eye injury.
-
35
Place two 2′′ broadband dielectric mirrors (Newport Corporation, 20Z20BD.1) on the breadboard so the partially reflected alignment beam is directed around the edge of the breadboard and back to the transmitted alignment beam along the optical rail. The mirrors should be mounted in kinematic platform mounts (Newport Corporation, U100-P3K) and mirror adapters for a platform mount (Newport Corporation, UPA2). Use the kinematic mounts of the first dichroic mirror and the two 2′′ mirrors to guide the partially reflected so that it remains level with transmitted alignment beam.
-
36
Place the second dichroic mirror (DM2) at the intersection of the transmitted and reflected beams at a 45° angle. Align the reflected beam with the transmitted beam using the alignment tool. A beam block may be placed in each path to independently determine the alignment of each path.
-
37
A high speed filter wheel (Sutter Instruments, Lambda 10-3) is used to quickly switch between filter sets in the emission path when imaging dyes of multiple wavelengths simultaneously. Determination of the filter required depends on dyes used in the experimental set up and will vary from setup to setup. Place the filter wheel with the appropriate filters already mounted into the beam path just before or after the dualview. Take care to leave room for the focusing lens L4 if placing the filter wheel in front of the dualview. Align the filter wheel by guiding the retro-reflected beam so that it is concentric with the alignment beam.
-
38
Place iris (I2) directly under the micromirrors. Use the alignment laser beam to center it.
-
39
Place lenses L4 and L5 into the emission paths (Figure 2). Each lens should be mounted with the xy-translation and kinematic cage mounts described previously. The first lens is placed on the optical rail before the dualview and the lenses L5 should be just after DM1. The L5 lenses need to be able to translate along their respective optical axis. Translate the two lenses until the distance between them and lens L4 is the sum of the focal lengths of the two lenses. This should effectively collimate the light in each emission path. The distance of the L5 lens in the <635 nm channel can be adjusted by sliding the entire dualview platform. The L5 lens in the >635nm channel should be mounted to the optical rail opposite of the dualview. If positioned properly, the image created by each channel of the dualview should be of equal size and magnification.
TROUBLESHOOTING
-
40
Prepare another slide with fluorescent samples that fluoresce in both the >635nm and <635nm channels. Again, we used fluorescent beads (Life Technologies, T107171), the 532 nm laser, and 532 long-pass filter. If the dualview is properly aligned there should be two separate images of the same FOV that are of equal size and magnification.
TROUBLESHOOTING
-
41
Translate the EMCCD camera to position the >635nm image,
-
42
Use the steering mirrors in the dualview to position the <635nm image so that it does not overlap with the >635m image.
-
43
Place iris (I3) at the intermediate image plane (between L4 and L5). Adjust the size of the aperture to define the size of the images.
Figure 7. The components and layout of the emission path optics.
a) Location of the micromirrors, iris, and the 45° mirror under the microscope objective. b) Mounting of the electron multiplying charge coupled device (EMCCD) camera. c) The dualview optics as seen looking towards the EMCCD camera. d) The dualview optics as viewed looking towards the microscope objective.
Timing
The time needed to assemble the microscope depends on many factors including: time dedicated to construction, experience of the person/people building the microscope, and the number of lasers included in the setup. For these reasons it is difficult to determine the timing of completing any particular microscope setup. The estimates given here assume at least 50% percent time commitment to completing the optical components of a mmTIRFM with four lasers by a graduate student with some prior experience with laser beam steering and optical alignment (e.g., from an undergraduate laboratory course in optical physics).
Placement of the mmTIRF system (Steps 1–2). 1 day.
Placement of the excitation side components and laser alignment (Steps 3–12). 13 days.
Alignment of the micromirrors (Steps 13–18). 1 week.
Placement of the emission side optical rails, 45° mirror, and alignment laser (Steps 19–23). 1 day.
Alignment of the 45° mirror and final focusing lens (Steps 24–26). 1 day.
Assembly and placement of the EMCCD camera mount (Steps 27–29). 1 day.
Acquiring initial images before inserting dualview and filter wheel (Steps 30–31). 2–3 days.
Assembly and alignment of dualview and filter wheel (Steps 32–39). 1 week.
Acquiring <635nm and >635nm images (Steps 40–43). 2 days.
Total time to complete microscope assembly. 1–2 months.
Anticipated results
The mmTIRFM microscope design described in this protocol is capable of imaging fluorescent molecules with excitation/emission spectra in the blue, green, orange, and red wavelengths. Fluorescent molecules that have been selectively tethered to a slide surface or that are interacting with a surface tethered molecule should image as diffraction limited spots. The FOV of the image and the fluorescent spot diameter should be approximately 50 µm and 300 nm respectively when using a 60× 1.45 NA PlanApo MO.
Troubleshooting
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting.
| Step(s) | Problem | Possible Reason(s) | Solution(s) |
|---|---|---|---|
| 1–12 | There is an interference pattern in the excitation beam path. | A back reflection from one of the optics is reflected directly back along the excitation path. | Check each optical component to locate the source of the interference and realign as needed. |
| 11 | The excitation beam diameter is inconsistent along the length of the excitation path | The beam is improperly collimated. | Use the shearing interferometer to check collimation. Adjust the distance between L1 and L2. |
| 13–16 | There are multiple beams exiting the back objective after placement of mM1. | mM1 is not properly aligned. (It should be noted there will always be some light scattering.) | Minimize scattered light by properly aligning mM1 and ensuring that the excitation beams are collimated. The alignment tool aids greatly in this process. Block the remaining scattered light using I2. |
| 12 | The FOV is not fully illuminated. | 1. The aperture of I1 is too small. 2. I1 is not one focal length from L3. 3. The micromirror is too small for the beam size. | 1. Increase the size of the I1 aperture. 2. Adjust the position of I1. 3. Use micromirrors of a larger diameter. |
| 39 | <635nm and >635nm images are not equal in magnification. | One or both of the two collimating lenses (L5) in the dualview are not the correct distance from L4. | While imaging, independently adjust the position of each lens until both images have equal magnification. |
| 40 | There is clipping of the <635nm and/or >635nm image. | If only one of the images is clipped, then the emission light is at the edge of one of the optics after DM1. If both are clipped then the emission light is at the edge of one of the optics before DM1. | Sequentially steer each optic starting with L6 (or DM1 if both images are clipped) to located the optic responsible. Reposition the optic as needed. |
| 40 | There is a large amount of background that extends into areas of the chip not in the FOV. | Light is entering the camera from outside the emission path. | Enclose the emission side optics in light blocking material (e.g., black posterboard). |
| 40. | There is a large amount of background only in the FOV. | Ambient light from the room is entering the front lens of the objective. | Block the light at its source if possible. Placing a light block over the objective and slide holder can also alleviate this issue. |
| 40. | The surface of the slide cannot be located. | The slide holder is not properly secured in the clamp. | Reposition the slide holder so it is securely held by the clamp. |
| 40. | There is a rapid rate of photo bleaching. | Laser intensity is too high. | Use the attenuator to reduce the laser power. |
Acknowledgements
We acknowledge support from startup funding from the University of Wisconsin – Madison, Wisconsin Alumni Research Foundation (WARF) and the Department of Biochemistry. AAH is also supported by a K99/R00 career transition award from the National Institute of Health (R00 GM086471) and is a Beckman Young Investigator of the Arnold and Mabel Beckman Foundation. JL and AAH are supported by a Hatch Act Formula Fund from the USDA (WIS 01625). LJF is supported by NIH R01 GM81648 (to Jeff Gelles, Brandeis University) and a grant from the G. Harold and Leila Y. Mathers Foundation. Funds for purchase of many of the components of the mmTIRF system described here were generously supplied by Professor Robert Landick (U. Wisconsin-Madison) and NIH administrative supplement award GM38660.
Footnotes
Author Contributions
JL constructed the microscope with assistance from AAH. MK integrated the microscope hardware with the image acquisition software. AAH, JL, EAD, WO, and JFM designed and constructed the mmTIRF system. LJF provided critical advice. AH and JL wrote the manuscript with input from the other authors.
Competing Financial Interests
The authors declare the following competing interests: MK, EAD, WOB, and JFM are employees of Mad City Labs, Inc.
References
- 1.Shcherbakova I, et al. Alternative Spliceosome Assembly Pathways Revealed by Single-Molecule Fluorescence Microscopy. Cell Reports. 2013;5:1–15. doi: 10.1016/j.celrep.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith BA, et al. Three-color single molecule imaging shows WASP detachment from Arp2/3 complex triggers actin filament branch formation. eLife. 2013;2:e01008. doi: 10.7554/eLife.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Petrov A, Tsai A, O'Leary SE, Puglisi JD. Coordinated conformational and compositional dynamics drive ribosome translocation. Nature Structural & Molecular Biology. 2013;20(6):718–727. doi: 10.1038/nsmb.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod D. Chapter 7: Total internal reflection fluorescence microscopy. Methods Cell Biol. 2008;89:169–221. doi: 10.1016/S0091-679X(08)00607-9. [DOI] [PubMed] [Google Scholar]
- 5.Roy R, Hohng S, Ha T. A Practical Guide to smFRET. Nature Methods. 2008;5(6):507–516. doi: 10.1038/nmeth.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funatsu T, et al. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature. 1995;374:555–559. doi: 10.1038/374555a0. [DOI] [PubMed] [Google Scholar]
- 7.Kinosita K, et al. Dual view microscopy with a single camera: real time imaging of molecular orientaions and calcium. The Journal of Cell Biology. 1991;115(1):67–73. doi: 10.1083/jcb.115.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larson JD, Rodgers ML, Hoskins AA. Visualizing cellular machines with colocalization single molecule microscopy. Chemical Society Reviews. 2014 doi: 10.1039/c3cs60208g. Advance Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvin PR, Ha T. Single Molecule Techniques: A Laboratory Manual. Cold Spring Harbor Laboratory Press. 2008 [Google Scholar]
- 10.Friedman LJ, Chung J, Gelles J. Viewing dynamic assembly of molecular complexes by multi-wavelength single-molecule fluorescence. Biophys J. 2006;91(3):1023–1031. doi: 10.1529/biophysj.106.084004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trang VH, et al. On Using Deubiquitinases to Catalyze Site-Specific Modification of the Ubiquitin C-Terminus. Submitted. [Google Scholar]
- 12.Anderson EG, Hoskins AA. Single Molecule Approaches for Studying Spliceosome Assembly and Catalysis. In: Hertels Klemens J., editor. Spliceosomal pre-mRNA Splicing: Methods in Molecular Biology. Vol. 1126. New York, New York, USA: Humana Press; in press. [DOI] [PubMed] [Google Scholar]
- 13.Kapanidis AN, et al. Alternating laser excitation of single molecules. Acc. Chem. Res. 2005;38:523–533. doi: 10.1021/ar0401348. [DOI] [PubMed] [Google Scholar]