Abstract
This study examined the prevalence of vitamin D deficiency in mothers and infants in Tijuana, Mexico and determined the effect of a single oral dose of 50,000 IU vitamin D3 at birth on 25-hydroxyvitamin D (25[OH]D) levels during infancy. Healthy infants were randomized to receive vitamin D3 or placebo at birth. At birth 23% of infants were vitamin D deficient and 77% had vitamin D insufficiency (mean 25[OH]D level 18.9 ng/ml); 10% of mothers were vitamin D deficient and 61% were insufficient. Infants receiving vitamin D3 had higher 25(OH)D levels at 2 months (n=29; 33.9 vs. 24.2 ng/ml) and 6 months (n=21; 36.5 vs. 27.4 ng/ml). Exclusively breastfed infants had lower 25(OH)D levels at 2 months (14.9 vs. 33.4 ng/ml). Vitamin D deficiency is common in infants and mothers in Tijuana, Mexico. A single dose of vitamin D3 at birth was safe and significantly increased 25(OH)D levels during infancy.
Keywords: Vitamin D deficiency, vitamin D receptor, vitamin D binding protein, infant nutrition, host genetics
INTRODUCTION
The vitamin D status of pregnant women has been associated with pregnancy-related complications like gestational diabetes and pre-eclampsia, infant birth weight, infant calcium and 25-hydroxyvitamin D (25[OH]D) levels, and childhood skeletal and neurocognitive development (Aghajafari et al. 2013; De-Regil et al. 2012; Javaid et al. 2006; Kovacs 2008; Mannion et al. 2006; Whitehouse et al. 2012). Beyond its traditional role in calcium homeostasis and bone mineralization, vitamin D is increasingly being recognized as an important modulator of innate and adaptive immunity (Adams et al. 2008; Campbell et al. 2012; Liu et al. 2006; Walker et al. 2011). Infants born to human immunodeficiency virus type-1 (HIV) infected women with low vitamin D levels have a significantly higher risk of acquiring HIV infection during the perinatal and postnatal period, and are more likely to die during follow-up or become stunted and underweight (Finkelstein et al. 2012; Mehta et al. 2009). Furthermore, vitamin D related host genetic variants that alter the bioavailability and function of vitamin D have been associated with an increased risk of acute lower respiratory tract infections and advanced HIV disease progression in children (Moodley et al. 2013; Roth et al. 2008).
The prevalence of vitamin D deficiency in pregnant women and infants in Mexico has not been well studied. A recent national survey in Mexico of 1025 children aged 2–12 years, reported that 24% of preschool children were vitamin D deficient (<20 ng/ml), while 30% were vitamin D insufficient (21–29 ng/ml) (Flores et al. 2013). In a study of obese children aged 6–12 years in northeastern Mexico, 62% of children were found to have vitamin D insufficiency (21–29 ng/ml) while 20% had vitamin D deficiency (<20 ng/ml) (Elizondo et al. 2010). Similarly high rates of vitamin D deficiency (26%) and insufficiency (59%) have been reported in Hispanic children in the United States, using data from the 2001–2006 National Health and Nutrition Examination Survey (NHANES) (Mansbach et al. 2009).
While the optimal vitamin D level in pregnant women and children is unknown, infant 25(OH)D levels below 20 ng/ml have been associated with bone demineralization and other adverse health outcomes (Camargo et al. 2011; Gordon et al. 2008). The American Academy of Pediatrics (AAP) currently recommends that all exclusively breastfed infants receive a daily vitamin D supplement containing at least 400 IU of vitamin D to ensure 25(OH)D levels are maintained above 20 ng/ml during infancy (Hollis 2005; Wagner et al. 2008). Despite the high prevalence of childhood vitamin D deficiency in Mexico, there are limited health policy recommendations on infant or childhood vitamin D supplementation.
The present study determined the prevalence of vitamin D deficiency in mothers and infants in Tijuana, Mexico and investigated whether a single, high dose of oral vitamin D3 administered at birth, corrected vitamin D deficiency and maintained 25(OH)D levels during early infancy. The association between seven vitamin D related host genetic variants, and maternal and infant 25(OH)D levels was also investigated.
METHODS
A single-center, double-blind, placebo controlled trial was conducted in 51 mother-infant pairs. Infants were randomized at birth to receive a single oral dose of 50,000 IU vitamin D3 (cholecalciferol) or placebo. All subjects were enrolled in a study evaluating the effect of vitamin D3 supplementation on Bacille Calmette Guerin (BCG) induced immunity in infants. Sample size was based on 90% power in showing a difference in BCG induced immunity between infants receiving vitamin D3 and placebo. Two subjects who were randomized, chose not to participate further and did not receive vitamin D or placebo and were excluded from the analyses. Thirty five infants and forty nine mothers had blood successfully obtained for vitamin D measurement at the time of enrollment.
The primary objectives were to determine the prevalence of vitamin D deficiency in mothers and infants in Tijuana, Mexico and to investigate whether high dose vitamin D3 administered at birth, corrected vitamin D deficiency and boosted 25(OH)D levels during early infancy. Secondary objectives were to investigate the association between seven vitamin D related host genetic variants and maternal and infant 25(OH)D levels. Tijuana is a coastal city in Mexico sharing a common border with San Diego County, California.
Infants were enrolled at birth and attended two follow-up visits at 2 and 6 months of age. At study visits, historical data were obtained regarding infant or maternal use of vitamin D supplements, dietary intake of the infant and predominant mode of infant feeding. At each visit 25(OH)D levels were measured.
The study was conducted between July 2011 and July 2012 at Tijuana General Hospital, Mexico and was approved by the institutional review boards of the University of California, San Diego and Tijuana General Hospital. Written informed consent was obtained from mothers through Spanish-speaking research assistants. All mothers received twenty US dollars in compensation for travel expenses related to each study visit.
Subject enrollment and randomization
Healthy infants born to women ≥18 years of age at Tijuana General Hospital, Mexico were enrolled within 24 hours after birth and prior to routine BCG vaccine administration. Infants were not eligible to participate if they were preterm (<37 weeks gestation), had low birth weight (<2,500 grams) or had received vitamin D supplementation. Infants were also excluded if their mothers had active or recent (within one year) tuberculosis disease, HIV infection, maternal fever, or maternal use of vitamin D supplements, steroids or immune-regulatory medications.
After enrollment, infant and maternal blood was obtained for DNA extraction and 25(OH)D measurement. Infants were then randomized to receive oral vitamin D3 or placebo. None of the infants vomited or regurgitated the liquid in the fifteen minutes after administration. A randomization list was generated in blocks of ten using http://www.randomizer.org.
Supplements
A clear, tasteless liquid containing 2,000 IU of vitamin D3 (cholecalciferol) per drop was used (Carlson Laboratories Inc., Arlington Heights, Illinois). The study dose of 50,000 IU was dispensed in 0.7ml of liquid vitamin D3 solution. The placebo was a tasteless, colorless liquid that contained 0.7ml of medium chain triglycerides. Vitamin D3 and placebo were administered in pre-filled, pre-coded syringes that were indistinguishable.
Vitamin D measurements and genotyping
25(OH)D levels were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS). Vitamin D deficiency was defined as 25(OH)D levels <15 ng/ml (<37.5 nmol/L), insufficiency as 15–<32 ng/ml (37.5 – 80 nmol/L), and normal as ≥32 ng/ml (≥80 nmol/L) (Hollis 2005; Misra et al. 2008; Holick 2007). The thresholds used to define severe vitamin D deficiency (<5 ng/ml) and deficiency (<15 ng/ml) were based on current AAP guidelines (Misra et al. 2008).
Seven vitamin D related single nucleotide polymorphisms (SNPs) were detected using real-time polymerase chain reaction (PCR) with melting curve analysis (Lightcycler; Roche, Indianapolis, Indiana) as described previously (Moodley et al. 2013). Two SNPs within the vitamin D receptor (VDR) gene rs1544410 (Bsm-I G/A) and rs2228570 (Fok-I C/T) were selected for their functional effects on VDR transcriptional efficiency (rs2228570) and VDR messenger RNA stability (rs1544410). Three SNPs that alter vitamin D binding protein (VDBP): rs7041, rs4588 and rs2282679 (GC A/C); and two additional SNPs that influence vitamin D synthesis: rs12785878 (DHCR7/NADSYN1 G/T) and vitamin D hydroxylation: rs10741657 (CYP2R1 G/A) were also studied (Wang et al. 2010).
Safety Monitoring
Hypervitaminosis D and hypercalcemia were not anticipated based on previous infant studies (Misra et al. 2008; Shakiba et al. 2010) using similar doses of supplemental vitamin D. Infants with severe vitamin D deficiency defined as 25(OH)D levels <5 ng/ml (<12.5nmol/L) were unblinded from the study and instructed to take an oral vitamin D supplement.
Statistical Analysis
Descriptive statistics of 25(OH)D levels were summarized for both mothers and infants at birth. Pearson’s correlation coefficient was used to estimate the correlation between maternal and infant 25(OH)D levels at birth. The Student T-test was used to compare 25(OH)D levels at birth, 2 and 6 months of age. Vitamin D levels were also compared between formula fed and breast-fed infants, adjusting for vitamin D3 supplementation at birth. Linear regression was used to test the association between 25(OH)D levels and host genetic variants, adjusting for mode of feeding and vitamin D3 supplementation at birth. All p-values are two-sided.
RESULTS
Characteristics of mothers and infants at enrollment
All infants were born by vaginal delivery and were breastfed. None had received formula or vitamin D supplementation. The mean gestational age was 39 weeks and the mean birth-weight was 3328 grams. The mean 25(OH)D level at birth was 18.9 ng/ml. Twenty-three percent of infants were vitamin D deficient while the remaining infants had vitamin D insufficiency. None of the infants had severe vitamin D deficiency (<5 ng/ml). Infant baseline characteristics and 25(OH)D levels were not significantly different between the intervention arms (Table 1).
Table 1.
Maternal and infant baseline characteristics
| Vitamin D Arm (N=27) | Placebo Arm (N=22) | All infants (N=49) | ||
|---|---|---|---|---|
| Male sex | N (%) | 13 (48%) | 12 (55%) | 25 (51%) |
| Gestational age (weeks) | Mean (range) | 39 (37–42) | 39 (37–42) | 39 (37–42) |
| Birth weight (grams) | Mean (range) | 3342 (2680–4265) | 3311 (2710–4125) | 3328 (2680–4265) |
| Infant 25(OH)D level ng/ml | N=18 | N=17 | N=35 | |
| Mean (range) | 17.7 (11.0–27.0) | 20.1 (13.0–29.0) | 18.9 (11.0–29.0) | |
| 15–<32 ng/ml | N (%) | 13 (72%) | 14 (82%) | 27 (77%) |
| Mean (range) | 19.4 (11.0–27.0) | 21 (13–29) | 20.8 (15.0–29.0) | |
| <15 ng/ml | N (%) | 5 (28%) | 3 (18%) | 8 (23%) |
| Mean (range) | 11.8 (11.0–13.0) | 13 (13) | 12.3 (11.0–13.0) | |
| Mother 25(OH)D level ng/ml | Mean (range) | 25 (10–42) | 27.5 (11.0–44.0) | 26.2 (10.0–44.0) |
| ≥32 ng/ml | N (%) | 7 (26%) | 7 (32%) | 14 (29%) |
| Mean (range) | 36.5 (33.0–42.0) | 38.7 (33.0–44.0) | 37.6 (33.0–44.0) | |
| 15–<32 ng/ml | N (%) | 17 (63%) | 13 (59%) | 30 (61%) |
| Mean (range) | 22.8 (15.0–28.0) | 23.9 (17.0–30.0) | 23.3 (15.0–30.0) | |
| <15 ng/ml | N (%) | 3 (11%) | 2 (9%) | 5 (10%) |
| Mean (range) | 11.3 (10.0–13.0) | 11.5 (11.0–12.0) | 11.4 (10.0–13.0) | |
| Plan to exclusively breast feed | % | 93% | 91% | 92% |
The women who attended Tijuana General Hospital were young and of Hispanic ethnicity with a mean age of 23.9 years. The mean 25(OH)D level in the mothers was 26.2 ng/ml. Ten percent of women were vitamin D deficient while 61% were vitamin D insufficient (Table 2). Forty-five mothers (92%) planned to exclusively breast feed their infants. Most women took prenatal vitamins that did not contain vitamin D; none had taken vitamin D supplements.
Table 2.
Correlation between maternal and infant 25(OH)D levels at birth
| Mothers | Infants | |||||
|---|---|---|---|---|---|---|
| 25(OH)D ng/ml | N | Mean | Range | N | Mean | Range |
| Mothers Vitamin D ≥ 32 | 14/49 | 37.6 | 33.0–44.0 | 10 | 24.3 | 18.0–29.0 |
| Mothers Vitamin D 15–<32 | 30/49 | 23.3 | 15.0–30.0 | 22 | 17.1 | 11.0–27.0 |
| Mothers Vitamin D <15 | 5/49 | 11.4 | 10.0–13.0 | 3 | 13.3 | 12.0–15.0 |
Pearson’s correlation of 0.68, p<0.001
Maternal and infant 25(OH)D levels were strongly correlated at birth with a Pearson’s correlation of 0.68, p<0.001 (Table 2), but not at 2 or 6 months of age.
Infant 25(OH)D levels at 2 months of age
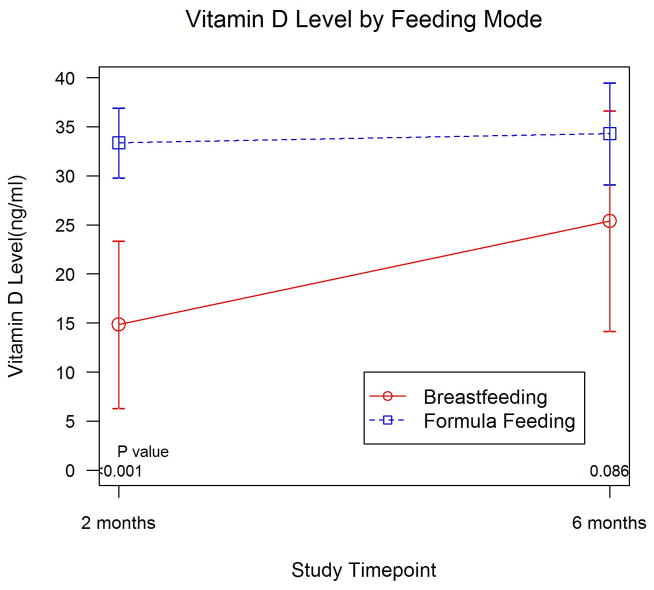
By 2 months of age, most infants (76%) had received vitamin D fortified formula in addition to breast milk. None of the infants or mothers had received oral vitamin D supplementation since hospital discharge. Exclusively breastfed infants (n=7) had significantly lower mean 25(OH)D levels at 2 months compared to formula fed infants (14.9 vs. 33.4 ng/ml, p<0.001) (Table 3, Figure 1). The effects were still significant after adjusting for vitamin D3 supplementation at birth (p<0.001). None of the exclusively breastfed infants who received vitamin D3 at birth were vitamin D deficient at 2 months of age.
Table 3.
Infant 25(OH)D levels at 2 and 6 months by predominant mode of feeding
| Feeding mode | 25(OH)D ng/ml 2 months (N=29) |
25(OH)D ng/ml 6 months (N=21) |
|---|---|---|
| Formula fed | ||
| N (%) | 22 (76%) | 16 (76%) |
| Mean (95% CI) | 33.4 (29.8–36.9) | 34.3 (29.1–39.5) |
| Breastfed | ||
| N (%) | 7(24%) | 5 (24%) |
| Mean (95% CI) | 14.9 (6.3–23.4) | 25.4 (14.2–36.6) |
| p-value | <0.001 | 0.086 |
Figure 1.
Infant 25(OH)D levels at 2 and 6 months of age by mode of feeding (n=29 at 2 months; n=21 at 6 months)
Infants who received vitamin D3 supplementation at birth had higher 25(OH)D levels at 2 months of age (n=29; 33.9 vs. 24.2 ng/ml, p=0.022) (Table 4, Figure 2). After adjusting for mode of feeding, the differences were still significant (p=0.042). At 2 months of age 5 infants (36%) in the vitamin D treated arm had vitamin D insufficiency with a mean 25(OH)D level of 24.6 ng/ml (range 17.0–31.0 ng/ml) but none were vitamin D deficient. Eight infants (53%) in the placebo arm had vitamin D insufficiency and 3 infants (20%) had vitamin D deficiency with mean 25(OH)D levels of 24.1 ng/ml (range 13.0–30.0 ng/ml) and 7 ng/ml (range 4–9 ng/ml), respectively. Only 4 infants (27%) in the placebo arm had normal 25(OH)D levels. One exclusively breastfed infant in the placebo arm of the study, who met the criteria for severe vitamin D deficiency with a 25(OH)D level of 4 ng/ml, was started on oral vitamin D supplementation. No adverse events were associated with vitamin D supplementation at birth.
Table 4.
Infant 25(OH)D levels at birth, 2 and 6 months of age by intervention arm
| Intervention arm | 25(OH)D ng/ml Birth (N=35) |
25(OH)D ng/ml 2 months (N=29) |
25(OH)D ng/ml 6 months (N=21) |
|---|---|---|---|
| Vitamin D arm | |||
| N (%) | 18 (51%) | 14 (48%) | 11 (52%) |
| Mean (95% CI) | 17.7 (15.1–20.4) | 33.9 (28.9–38.9) | 36.5 (29.5–43.6) |
| Placebo arm | |||
| N (%) | 17 (49%) | 15 (52%) | 10 (48%) |
| Mean (95% CI) | 20.1 (17.2–22.9) | 24.2 (17.7–31) | 27.4 (22–32.8) |
| p-value | 0.211 | 0.022 | 0.035 |
Figure 2.
Infant 25(OH)D levels at birth, 2 and 6 months of age by intervention arm (n=29 at 2 months; n=21 at 6 months)
Infant 25(OH)D levels at 6 months of age
By 6 months of age, most infants had been fed solid foods (86%) or received vitamin D fortified formula (76%). None of the infants or mothers had received oral vitamin D supplementation since hospital discharge. Most infants who were exclusively breastfed at 2 months of age had received solid foods by 6 months of age (n=5). Despite the introduction of solid foods however these infants still had lower mean 25(OH)D levels at 6 months compared to formula fed infants (n=16; 25.4 vs. 34.3 ng/ml, p=0.086) (Table 3, Figure 1). None of the exclusively breastfed infants who received vitamin D3 at birth were vitamin D deficient at 6 months of age.
Infants who received vitamin D3 supplementation at birth had higher 25(OH)D levels at 6 months of age (n=21; 36.5 vs. 27.4 ng/ml, p=0.035) (Table 4, Figure 2). At 6 months, 4 infants (36%) in the vitamin D3 treated arm and 6 infants (60%) in the placebo arm had vitamin D insufficiency with a mean 25(OH)D level of 25.5 ng/ml (range 20.0–31.0 ng/ml) and 22.1 ng/ml (range 16.0–27.0 ng/ml), respectively. None of the infants at 6 months were vitamin D deficient.
Association between vitamin D related SNPs and 25(OH)D levels
Large genome-wide association studies have found an association between specific host genetic variants and 25(OH)D levels (Wang et al. 2010; McGrath et al. 2010; Powe et al. 2013). Of the genetic variants evaluated, only the polymorphism in rs7041 (VDBP) was associated with maternal 25(OH)D levels. Mothers with the GG genotype, associated with higher levels of VDBP, had an estimated mean 25(OH)D level 6.4 ng/ml higher than the TT genotype (p=0.078). Although not statistically significant, the effect size of 6.40 ng/ml is large with 95% confidence interval (−0.76, 13.57). This finding is consistent with those of Powe et al who showed that black Americans compared to whites had lower levels of vitamin D and VDBP associated with the rs7041 TT genotype resulting in similar levels of bioavailable vitamin D (Powe et al. 2013). No other maternal or infant 25(OH)D levels were associated with the vitamin D related host genetic variants tested (data not shown).
DISCUSSION
The prevalence of vitamin D deficiency in pregnant women and newborn infants has not been well studied in Mexico. Despite the increased opportunity for sun exposure, a recent national survey of Mexican children reported that 24% of pre-school children had vitamin D deficiency (<20ng/ml) while 30% suffered from vitamin D insufficiency (<30 ng/ml) (Flores et al. 2013). Studies of pregnant women in North America have reported high rates of vitamin D deficiency in similar sun-rich environments (Hamilton et al. 2010). Of 559 women studied in South Carolina, 48% were found to be vitamin D deficient (<20 ng/ml) while 37% were vitamin D insufficient (<32 ng/ml). African-American and Hispanic women had the highest prevalence of either vitamin D deficiency or insufficiency at 94.3% and 78.4%, respectively. We report a similarly high prevalence (71%) of vitamin D deficiency and insufficiency in Hispanic pregnant women in Tijuana, Mexico. Infants born to these mothers were more likely to have low 25(OH)D levels at birth, emphasizing the correlation between maternal and infant 25(OH)D levels. Prenatal vitamins taken by most pregnant women in Mexico do not contain adequate concentrations of vitamin D. In North America, the Institute of Medicine (IOM) recommends that pregnant and lactating women take between 400–4000 IU of vitamin D daily. Some investigators suggest that higher doses up to 6400 IU per day may be required by lactating women (Wagner et al. 2006). High dose vitamin D supplementation during pregnancy has been reported to effectively raise maternal and cord blood 25(OH)D levels above 32 ng/ml in the majority of treated women (Roth et al. 2013). Further studies are warranted to determine the wider prevalence of vitamin D deficiency in Mexico and to evaluate the optimal dose required to prevent vitamin D deficiency and insufficiency in these women.
Healthy, term newborns in this study were found to have a high prevalence of vitamin D insufficiency (77%) and vitamin D deficiency (23%) at birth. Exclusively breastfed infants were at particularly high risk of developing vitamin D deficiency and severe vitamin D deficiency (<5 ng/ml) during early infancy. A single bolus dose of 50,000 IU of oral vitamin D3 at birth was safely tolerated and significantly increased 25(OH)D levels from 17.7 ng/ml at birth to 33.9 ng/ml by 2 months of age. While high dose vitamin D3 at birth corrected vitamin D deficiency in all infants regardless of 25(OH)D level at birth, it was not sufficient in preventing the development of vitamin D insufficiency in over a third of infants at 2 and 6 months of age however. Of note, the effect of bolus vitamin D supplementation at birth was still detectable at 6 months of age with 25(OH)D levels still elevated at 36.5 ng/ml in treated infants. The half-life of 25(OH)D is typically 2–3 weeks; however, vitamin D stored in fat may last for longer periods (Wagner et al. 2008), and the half-life may be longer in young infants. Exclusively breastfed infants who received vitamin D supplementation at birth did not develop vitamin D deficiency at 2 or 6 months of age. By 6 months of age a significant number of infants were fed formula and solid foods in varying amounts, which likely impacted vitamin D levels in both groups. However, a major limitation of this study is the small sample size and the number of subjects who were lost to follow up. Many of the women who attended Tijuana General Hospital for the delivery of their infants chose not to seek ongoing routine infant care at the same hospital. Additionally, the findings of this study may not be generalizable to the rest of the Mexican population.
Most infants need to maintain 25(OH)D levels above 20 ng/ml (50 nmol/L) to prevent the complications associated with vitamin D deficiency (Wagner et al. 2008). Infant formulas contain sufficient vitamin D to meet the 400 IU per day recommended by AAP. Preterm infants, infants born to mothers who are vitamin D deficient and dark-skinned infants who live in higher latitudes and are exclusively breastfed, may require higher doses of vitamin D (Wagner et al. 2008). As new research sheds light on the multitude of non-skeletal effects of vitamin D, the thresholds used to establish vitamin D sufficiency and insufficiency continue to shift higher. Many experts now recommend that 25(OH)D levels of 32 ng/ml may be necessary for optimal vitamin D function (Hollis 2005), and that higher doses up to 1600 IU daily of supplemental vitamin D may be required during infancy to achieve these target levels (Gallo et al. 2013). Furthermore, recent data suggests that determining an individual’s vitamin D requirements and status may be more complex than simply measuring 25(OH)D levels since bioavailable vitamin D levels are strongly influenced by vitamin D related genetic variants that may vary by race (Powe et al. 2013).
In Mexico, despite the high prevalence of childhood vitamin D deficiency, there are limited guidelines addressing vitamin D supplementation in the prevention of infant and childhood vitamin D deficiency. In this study, 50,000 IU of vitamin D3 administered at birth was well tolerated and effective in correcting vitamin D deficiency in all infants. These findings are consistent with those found in infants and children with established vitamin D deficiency (<15 ng/ml), for whom the recommended treatment is either daily vitamin D supplementation over several months or bolus high dose (100,000 to 600,000 IU) vitamin D administered orally over 1–5 days (Misra et al. 2008). Bolus dose regimens are appealing in neonates and infants as compliance with a daily regimen for several months may be challenging for caregivers. A recent study reported that bolus dose (50,000 IU) oral vitamin D3 given every 2 months with routine childhood immunizations was well tolerated, did not result in hypercalcemia and achieved 25(OH)D levels at 6 months of age of >30 ng/ml in 97% of subjects (Shakiba et al. 2010).
Several genetic variants have been associated with the function and bioavailability of vitamin D. Of the seven SNPs evaluated in this study, only the polymorphism in rs7041 (GC gene) associated with circulating VDBP levels, showed an association in postpartum women with 25(OH)D concentrations with an effect size of 6.4 ng/ml. The failure to identify other genetic associations with 25(OH)D levels likely reflects the small sample size of our study. However, our findings support the concept that genetic polymorphisms that lower levels of VDBP are likely an evolutionary compensatory mechanism to increase bioavailable levels of vitamin D in dark skinned racial and ethnic groups.
CONCLUSION
In summary, we found that a single dose of 50,000 IU of vitamin D3 at birth resulted in sustained levels of vitamin D >32 ng/ml through the first 6 months of life in most infants from Tijuana, Mexico. Single high dose oral vitamin D3 supplementation administered at birth is an attractive alternative to daily vitamin D supplementation and should be investigated further.
Acknowledgments
Source of funding: This research was supported in part by National Institutes of Health grants R21AI084573, R01NS077874 and an Early Career Award from Thrasher Research Foundation.
The authors would like to thank Rachel Bruckman, Jasmin Barcenas, Rolando Viani and Rujing Shi for their assistance with Spanish translation, venipuncture and the transportation of blood samples. The authors would also like to thank Min Qin for validating the statistical analyses and reviewing the manuscript.
Abbreviations
- 25(OH)D
25 hydroxyvitamin D
- HIV
human immunodeficiency virus
- PCR
polymerase chain reaction
- SNP
single nucleotide polymorphism
- VDBP
vitamin D binding protein
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Clinical Trial Registration: Clinicaltrials.gov (NCT01288950)
Presented in part at PAS 2013, Pediatric Academic Societies Meeting, Washington, D.C, May 4–7th, 2013.
References
- 1.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nature clinical practice Endocrinology & metabolism. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. Bmj. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 3.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127(1):e180–7. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 4.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS pathogens. 2012;8(5):e1002689. doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. The Cochrane database of systematic reviews. 2012;2:CD008873. doi: 10.1002/14651858.CD008873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elizondo-Montemayor L, Ugalde-Casas PA, Serrano-Gonzalez M, Cuello-Garcia CA, Borbolla-Escoboza JR. 25-hydroxyvitamin d concentration, life factors and obesity in Mexican children. Obesity. 2010;18(9):1805–11. doi: 10.1038/oby.2009.448. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein JL, Mehta S, Duggan C, Manji KP, Mugusi FM, Aboud S, et al. Maternal vitamin D status and child morbidity, anemia, and growth in human immunodeficiency virus-exposed children in Tanzania. The Pediatric infectious disease journal. 2012;31(2):171–5. doi: 10.1097/INF.0b013e318245636b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores M, Macias N, Lozada A, Sanchez LM, Diaz E, Barquera S. 25-hydroxyvitamin D levels among Mexican children ages 2 y to 12 y: a national survey. Nutrition. 2013;29(5):802–4. doi: 10.1016/j.nut.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA : the journal of the American Medical Association. 2013;309(17):1785–92. doi: 10.1001/jama.2013.3404. [DOI] [PubMed] [Google Scholar]
- 10.Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Archives of pediatrics & adolescent medicine. 2008;162(6):505–12. doi: 10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton SA, McNeil R, Hollis BW, Davis DJ, Winkler J, Cook C, et al. Profound Vitamin D Deficiency in a Diverse Group of Women during Pregnancy Living in a Sun-Rich Environment at Latitude 32 degrees N. International journal of endocrinology. 2010;2010:917428. doi: 10.1155/2010/917428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. The Journal of nutrition. 2005;135(2):317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 14.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. The American journal of clinical nutrition. 2008;88(2):520S–8S. doi: 10.1093/ajcn/88.2.520S. [DOI] [PubMed] [Google Scholar]
- 16.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 17.Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2006;174(9):1273–7. doi: 10.1503/cmaj.1041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansbach JM, Ginde AA, Camargo CA., Jr 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404–10. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGrath JJ, Saha S, Burne TH, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. The Journal of steroid biochemistry and molecular biology. 2010;121(1–2):471–7. doi: 10.1016/j.jsbmb.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 20.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. The Journal of infectious diseases. 2009;200(7):1022–30. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, Drug, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 22.Moodley A, Qin M, Singh KK, Spector SA. Vitamin D-related host genetic variants alter HIV disease progression in children. The Pediatric infectious disease journal. 2013;32(11):1230–6. doi: 10.1097/INF.0b013e31829e4d06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England journal of medicine. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth DE, Al Mahmud A, Raqib R, Akhtar E, Perumal N, Pezzack B, et al. Randomized placebo-controlled trial of high-dose prenatal third-trimester vitamin D3 supplementation in Bangladesh: the AViDD trial. Nutrition journal. 2013;12(1):47. doi: 10.1186/1475-2891-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. The Journal of infectious diseases. 2008;197(5):676–80. doi: 10.1086/527488. [DOI] [PubMed] [Google Scholar]
- 26.Shakiba M, Sadr S, Nefei Z, Mozaffari-Khosravi H, Lotfi MH, Bemanian MH. Combination of bolus dose vitamin D with routine vaccination in infants: a randomised trial. Singapore medical journal. 2010;51(5):440–5. [PubMed] [Google Scholar]
- 27.Wagner CL, Greer FR American Academy of Pediatrics Section on B, American Academy of Pediatrics Committee on N. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 28.Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeeding medicine : the official journal of the Academy of Breastfeeding Medicine. 2006;1(2):59–70. doi: 10.1089/bfm.2006.1.59. [DOI] [PubMed] [Google Scholar]
- 29.Walker VP, Zhang X, Rastegar I, Liu PT, Hollis BW, Adams JS, et al. Cord blood vitamin D status impacts innate immune responses. The Journal of clinical endocrinology and metabolism. 2011;96(6):1835–43. doi: 10.1210/jc.2010-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH. Maternal vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129(3):485–93. doi: 10.1542/peds.2011-2644. [DOI] [PubMed] [Google Scholar]