Abstract
IMPORTANCE
There is conflicting evidence about how different bariatric procedures impact health care use.
OBJECTIVE
To compare the impact of laparoscopic adjustable gastric banding (AGB) and laparoscopic Roux-en-Y gastric bypass (RYGB) on health care use and costs.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective interrupted time series with comparison series study using a national claims data set. The data analysis was initiated in September 2011 and completed in January 2015. We identified bariatric surgery patients aged 18 to 64 years who underwent a first AGB or RYGB between 2005 and 2011. We propensity score matched 4935 AGB to 4935 RYGB patients according to baseline age group, sex, race/ethnicity, socioeconomic variables, comorbidities, year of procedure and baseline costs, emergency department (ED) visits, and hospital days. Median postoperative follow-up time was 2.5 years.
MAIN OUTCOMES AND MEASURES
Quarterly and yearly total health care costs, ED visits, hospital days, and prescription drug costs. We used segmented regression to compare pre-to-post changes in level and trend of these measures in the AGB vs the RYGB groups and difference-in-differences analysis to estimate the magnitude of difference by year.
RESULTS
Both AGB and RYGB were associated with downward trends in costs; however, by year 3, AGB patients had total annual costs that were 16% higher than RYGB patients (P < .001; absolute change: $818; 95% CI, $278 to $1357). In postoperative years 1 and 2, AGB was associated with 27% to 29% fewer ED visits than RYGB (P < .001; absolute changes: −0.6; 95% CI, −0.9 to −0.4 and −0.4; 95% CI, −0.6 to −0.1 visits/person, respectively); however, by year 3, there were no detectable differences. Postoperative annual hospital days were not significantly different between the groups. Although both procedures lowered prescription costs, annual postoperative prescription costs were 17% to 32% higher for AGB patients than RYGB patients (P < .001).
CONCLUSIONS AND RELEVANCE
Both laparoscopic AGB and RYGB were associated with flattened total health care cost trajectories but RYGB patients experienced lower total and prescription costs by 3 years postsurgery. On the other hand, RYGB was associated with increased ED visits in the 2 years after surgery. Clinicians and policymakers should weigh such differences in use and costs when making recommendations or shaping regulatory guidance about these procedures.
The prevalence of severe obesity (body mass index [BMI, calculated as weight in kilograms divided by height in meters squared] ≥ 40) is rising faster than that of obesity (BMI ≥ 30) in the United States.1 Patients with severe obesity have greater health care use rates2 and higher levels of morbidity/mortality. Thus, it is increasingly important to evaluate the comparative effectiveness of treatments for this condition.
Bariatric surgery results in dramatic weight loss, as well as remission of many comorbidities,3 but different procedure types vary substantially in their effects and mechanisms of action.4–11 Additionally, the procedures are costly and can result in a number of short- and long-term complications.5
Laparoscopic Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric banding (AGB) represent 2 of the most common bariatric procedures in the United States.5,11 Roux-en-Y gastric bypass is more effective at producing weight loss and diabetes mellitus remission than AGB; however, it is also a more complex procedure.4,5 Despite the relative simplicity of AGB, its use has declined owing to high reoperation rates related to device failures, gastric erosions, and the inability to achieve or maintain weight loss.12–14
To our knowledge, few studies have addressed how these procedures affect total health care use or the extent to which they differentially influence postoperative use and expenditures. Most prior studies have not distinguished between surgical types15–20 or have included a substantial proportion of outdated procedures.21 For patients, health care professionals, and payers deciding between current surgical modalities, more comparative effectiveness research is needed.
Our objective was to assess the impact of laparoscopic AGB and RYGB on emergency department (ED) visits, hospital days, prescription drug costs, and total health care costs, comparing the procedures using propensity-matched groups.
Methods
Data Source
Our data source was 2000–2011 claims from a US-wide commercial insurer including enrollment and demographic information and inpatient, outpatient, and pharmacy claims for all members. For claims from 2004 onwards, the data vendor (OptumInsight) also calculated standardized costs using an algorithm to closely approximate the health plan’s amount allowed (ie, the total cost of the claim), while eliminating variability in pricing across geography and time. This study was approved by the Harvard Pilgrim Health Care institutional review board, and a waiver of informed patient consent was obtained.
Identification of Study Patients and Exposure Measure
We identified members aged 18 to 64 years who underwent AGB or RYGB between January 2005 and December 2011 using Current Procedural Terminology (CPT), and International Classification of Diseases, Ninth Revision (ICD-9) codes. We classified members with CPT code 43770 or ICD-9 procedure code 44.95 as having undergone AGB, and those with CPT codes 43644 or 43645 or with ICD-9 code 44.38 and a code for morbid obesity (278.01) as having undergone laparoscopic RYGB (eFigure in the Supplement). We used additional codes to identify other bariatric procedures, and if members had coding for more than 1 procedure type during their enrollment, we defined the earliest as their index procedure. We excluded members with CPT codes for revisional procedures (43848 and 43771–43774) occurring on the index date or within a year prior. We limited our analyses to members who were continuously enrolled for at least 1 year before and after their procedures. We excluded members with evidence of gastrointestinal malignancy within 6 months prior to surgery or perforated gastrointestinal ulcer on the date of surgery to avoid including those with surgical indications other than obesity.
The data analysis was initiated in September 2011 and completed in January 2015.
Covariates
Demographic covariates included age group (18–34, 35–49, and 50–64 years), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), and region of the United States (Pacific, Mountain, West-North Central, West-South Central, East-SouthCentral,East-NorthCentral,SouthAtlantic,Mid-Atlantic, and New England). We classified race/ethnicity according to a variable constructed using place of residence (geocoding) and surname analysis.22 Geocoding is sensitive in detecting black or white race and surname analysis provides ac-curate information on Asian and Hispanic ethnic group.23 Togenerate proxy measures of socioeconomic status, we created previously established24 categorical variables of census block group poverty and education levels derived from 2000 US Census reports.25 Variables were as follows: census block group education (level 1: <15% of people with < high school education, level 2: 15%–24.9%, level 3: 25%–39.9%, and level 4: ≥40%) and 2000 census block group poverty level (level 1: <5% of persons below poverty, level 2: 5%–9.9%, level 3: 10%–19.9%, and level 4: ≥20%). We determined baseline disease burden using claims from 12 to 3 months prior to the index procedure (9-month period), including Charlson Comorbidity Index score26 (0, 1, 2, 3, 4, and ≥5) and diagnoses of type 2 diabetes mellitus, kidney disease, liver disease, hypertension, and dyslipidemia. Finally, we included year of procedure (before or after 2008) as a covariate to minimize bias due to secular trends.
Propensity Score Matching
We used 1:1 caliper propensity score matching to create AGB and RYGB groups that were balanced with respect to baseline characteristics including age group, sex, neighborhood education and poverty levels, race/ethnicity, region of the United States, Charlson Comorbidity Index score, several comorbid conditions, and year of procedure. We also matched on standardized costs, ED visits, and hospital days for quarters −4, −3, and −2 (excluding the 3 months immediately before surgery which included a spike in use owing to preoperative workup). Propensity score matching is a well-established method that assists in generating a comparison group with similar measured characteristics when patients have not been randomly allocated into study groups.27–30 We used a caliper equal to 0.6 times the standard deviation of the propensity score, and diagnostics of the propensity score distributions revealed excellent overlap (Table 1). Our prematch pool included 5608 AGB and 6459 RYGB patients with complete covariate data, and the final sample comprised 4935 patients in each group.
Table 1.
Baseline Characteristics of AGB vs RYGB Patients Pre– and Post–Propensity Score Matching
| Variable | Pre–Propensity Score Matching, % | Post–Propensity Score Matching, % | ||||
|---|---|---|---|---|---|---|
| AGB (n = 5608a) | RYGB (n = 6459a) | P Valueb | AGB (n = 4935) | RYGB (n = 4935) | P Valueb | |
| Age group, y | ||||||
| 18–34 | 19.8 | 20.2 | .88 | 19.9 | 19.9 | .82 |
| 35–49 | 47.2 | 47.0 | 47.3 | 47.8 | ||
| 50–64 | 32.9 | 32.8 | 32.8 | 32.3 | ||
| Sex | ||||||
| Male | 21.9 | 20.0 | .02 | 21.5 | 20.9 | .47 |
| Female | 78.1 | 80.0 | 78.5 | 79.1 | ||
| Census-level education quartile | ||||||
| 1 | 53.8 | 54.4 | .07 | 53.5 | 53.4 | .98 |
| 2 | 23.8 | 25.2 | 24.4 | 24.2 | ||
| 3 | 16.7 | 15.2 | 16.5 | 16.8 | ||
| 4 | 5.6 | 5.3 | 5.5 | 5.6 | ||
| Census-level poverty quartile | ||||||
| 1 | 41.2 | 40.2 | .41 | 40.5 | 39.8 | .89 |
| 2 | 26.4 | 27.2 | 26.8 | 27.1 | ||
| 3 | 21.9 | 22.6 | 22.3 | 22.5 | ||
| 4 | 10.5 | 10.0 | 10.4 | 10.6 | ||
| Race/ethnicity | ||||||
| Non-Hispanic white | 69.8 | 72.1 | .007 | 70.4 | 70.4 | .89 |
| Non-Hispanic black | 15.2 | 13.2 | 14.8 | 14.6 | ||
| Hispanic | 10.4 | 10.1 | 10.2 | 10.4 | ||
| Other | 4.5 | 4.7 | 4.6 | 4.6 | ||
| Region | ||||||
| Pacific | 5.2 | 6.9 | <.001 | 5.7 | 5.9 | .99 |
| Mountain | 6.8 | 7.5 | 7.3 | 7.2 | ||
| West-North Central | 12.4 | 20.3 | 13.8 | 13.9 | ||
| West-South Central | 27.3 | 17.8 | 23.1 | 22.6 | ||
| East-South Central | 4.0 | 3.3 | 4.0 | 3.8 | ||
| East-North Central | 8.6 | 8.3 | 9.0 | 8.9 | ||
| South Atlantic | 24.7 | 26.8 | 26.8 | 27.2 | ||
| Mid-Atlantic | 7.7 | 5.7 | 7.0 | 6.9 | ||
| New England | 3.1 | 3.5 | 3.4 | 3.6 | ||
| Charlson Comorbidity Index score | ||||||
| 0 | 60.1 | 54.8 | <.001 | 58.0 | 57.9 | .99 |
| 1 | 28.6 | 31.7 | 30.1 | 30.1 | ||
| 2 | 8.1 | 9.2 | 8.5 | 8.5 | ||
| 3 | 1.9 | 2.7 | 2.1 | 2.2 | ||
| 4 | 0.7 | 0.9 | 0.8 | 0.8 | ||
| ≥5 | 0.6 | 0.7 | 0.5 | 0.5 | ||
| Type 2 diabetes mellitus diagnosis | 25.6 | 31.4 | <.001 | 27.3 | 27.9 | .53 |
| Cardiovascular diseasec | 5.1 | 5.3 | .68 | 5.1 | 5.2 | .89 |
| Kidney disease | 1.4 | 1.6 | .28 | 1.4 | 1.5 | .93 |
| Liver disease | 0.4 | 0.3 | .54 | 0.4 | 0.3 | .62 |
| Hypertension | 57.3 | 63.1 | <.001 | 59.7 | 60.0 | .79 |
| Hyperlipidemia | 42.5 | 47.5 | <.001 | 44.2 | 44.5 | .76 |
| Year of procedure, 2004–2007 (vs 2008–2011) | 32.4 | 42.2 | <.001 | 34.9 | 35.2 | .75 |
Abbreviations: AGB, adjustable gastric banding; RYGB, Roux-en-Y gastric bypass.
Eligible persons with complete data on all matching variables.
P value for categorical variables is for χ2 testing; t testing with Satterthwaite method for cost variable – unequal variances.
Diagnosis of myocardial infarction, congestive heart failure, or cerebrovascular or peripheral vascular disease.
Main Outcome Measures
We examined 4 measures of health care use at quarterly (91.25 days) and yearly intervals relative to a patient’s index procedure: ED visits, hospital days, standardized prescription costs, and total health care costs. We assessed person-level ED visits per quarter and year, as well as hospital days per quarter and year as count variables, with a maximum of 15 preoperative and 22 postoperative quarters, based on the distribution of follow-up time in our cohort.
We measured standardized total health care costs as a proxy for overall use. We summed prescription drug and total standardized costs at the individual level per quarter and per year. Because costs were non-normally distributed and contained some extreme outliers, we capped them at the 99th percentile. Additionally, because cost data were not available before 2004, cost outcomes relied on baseline period data from 2004 onwards, regardless of whether an individual had been enrolled earlier.
Statistical Analysis
To visualize changes in the levels and trends of outcomes, we generated time series plots of the unadjusted mean values in each quarter. We used patient-level segmented regression analysis to test for postsurgical level or trend changes using 1-part generalized estimating equations specified with the log link and Poisson variance function. We applied a first-order autoregressive working model to adjust for autocorrelation between adjacent individual quarterly measurements and estimated the variance using the empirical sandwich estimator. This approach is statistically valid even when a substantial fraction of members do not have outcomes.31 The analytic model produced population-level use or cost-rate ratios using the quarterly person-level outcomes. The primary independent variables in our analyses were time (in quarters from the start of the baseline period through postsurgical follow-up), intervention (whether a given quarter was before or after the index [surgical] date), and time after intervention (time in quarters after the index date). Thus, the exponentiated coefficient for time indicated the baseline trend of use of the referent group (the ratio of 2 adjacent quarterly use rates); the exponentiated coefficient for intervention indicated a level change (a rate ratio) in use immediately after the index date compared with the prior quarter; and the exponentiated coefficient for time after intervention indicated a trend change (a rate ratio) in use after the index date compared with the baseline trend. Models were run with each group (AGB and RYGB) serving as referent to obtain estimates for within-group changes. To calculate the differential changes in the level and trend of use between AGB and RYBG, we examined interactions between the study groups and primary independent variables listed here to estimate the ratio of rate ratios. Because differential dropout between groups over time could introduce imbalance with respect to confounders, we built multivariable models to adjust for changing population characteristics over baseline and follow-up, including all propensity-matching covariates except for outcome variables and less-common comorbidities.
Although interrupted time series designs are among the most robust for establishing causal inference, interpreting the ratio of ratios from segmented regression models can be challenging. Therefore, we performed multivariable difference-in-differences analysis on all outcomes, comparing AGB with RYGB in postoperative years 1 to 3, with a baseline consisting of the analyzed portion of the preoperative year (months −12 to −6). Estimates from these models are presented as the annual pre-to-post change for the procedure of interest (AGB) compared with the control procedure (RYGB).
Outcome analyses excluded 6 months before and 3 months after surgery, a period of higher use of medical care (eg, owing to preoperative testing) not representative of the general preoperative or postoperative course.17,32 Given that many cohort members had little more than a year of baseline data available (thus, little more than 6 months of baseline), we examined whether a longer baseline would alter our findings. We conducted a sensitivity analysis, repeating segmented regression models on a subset of patients with at least 2 years of preoperative baseline, still excluding months −6 through 3 months postoperative.
Results
Population Characteristics
Our propensity-matched cohort included 4935 AGB and 4935 RYGB patients. The groups were balanced with respect to all measured baseline characteristics (Table 1). The mean (SD) age of patients was 43.8 (10.2) years at the time of the index procedure. Seventy-nine percent were female, 70.4% were non-Hispanic white, and just over half (53.5%) resided in census tracts where 85% or more of residents had at least a high school education. Mean postoperative follow-up time was nearly 3 years (2.9 for AGB and 2.8 for RYGB; P < .001). Attrition owing to disenrollment occurred over the postoperative follow-up period, and 3763 patients (38%) (1993 AGB and 1770 RYGB) remained enrolled by the end of the third postoperative year (eTable 1 in the Supplement).
Emergency Department Visits
In time series analyses, AGB patients experienced a significantly lower level of ED visits relative to RYGB patients immediately after surgery; however, there was no significant difference in trend change between the 2 procedures (Figure 1; Table 2). In the corresponding multivariable difference-in-differences models, AGB patients had fewer annual ED visits during early follow-up than RYGB patients (Table 3). In postoperative years 1 and 2, AGB patients had 29% (95% CI, −39% to −20%) and 27% (95% CI, −41% to −13%) fewer ED visits than RYGB patients, respectively (absolute changes: −0.6; 95% CI, −0.9 to −0.4 and −0.4; 95% CI, −0.6 to −0.1 visits per member per year, respectively). There was no detectable difference between procedures by year 3 (absolute change: −0.1; 95% CI, −0.4 to 0.1 visits per member per year).
Figure 1. Mean Emergency Department Visits and Hospital Days per Quarter for Patients Undergoing Adjustable Gastric Banding vs Roux-en-Y Gastric Bypass.
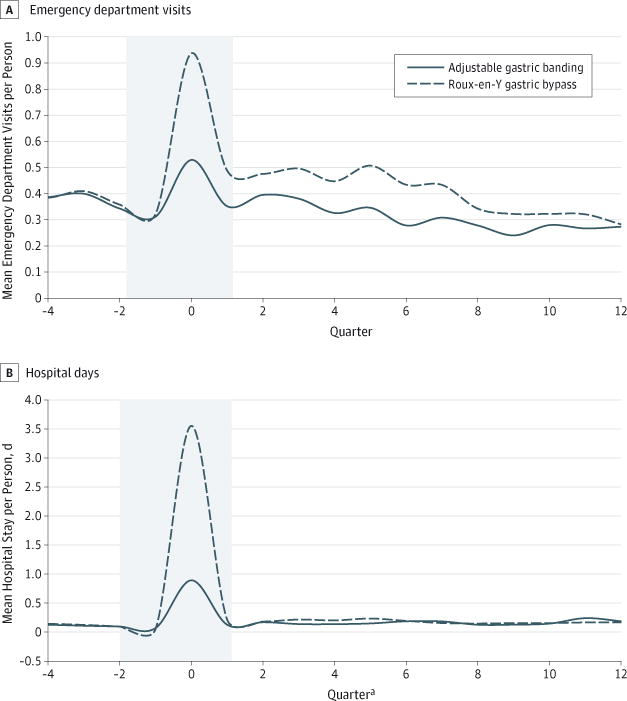
a Refers to the 91.25-day period relative to the index procedure. Note that data from quarters −2, −1, and 0 were omitted from analysis. Number (%) of patients enrolled during the presurgery and postsurgery periods are as follows: In the adjustable gastric banding group, there were 4935 (100%) in quarter −4, 4935 in quarter 0, 4935 (100%) in quarter 4, 3272 (66%) in quarter 8, and 1993 (40%) in quarter 12. In the Roux-en-Y gastric bypass group, there were 4935 (100%) in quarter −4, 4935 in quarter 0, 4935 (100%) in quarter 4, 3018 (61%) in quarter 8, and 1770 (36%) in quarter 12. Values are plotted using a smoothing function and no actual values go below zero.
Table 2.
Segmented Regression Estimates of Changes in Level and Trend for Use Presurgery to Postsurgery
| Variable | Estimate (95% CI) | |||||
|---|---|---|---|---|---|---|
| AGB | RYGB | AGB Relative to RYGB | ||||
| Level Changea | Trend Changea | Level Changea | Trend Changea | Level Changeb | Trend Changeb | |
| Quarterly emergency department visits | 0.07 (−0.10 to 0.23) | −0.06 (−0.08 to −0.03) | 0.27 (0.11 to 0.42) | −0.05 (−0.07 to −0.02) | −0.27 (−0.47 to −0.06) | −0.02 (−0.05 to 0.01) |
| Quarterly hospital days | 0.23 (−0.05 to 0.50) | −0.02 (−0.06 to 0.01) | 0.55 (0.29 to 0.82) | −0.04 (−0.09 to 0.01) | −0.40 (−0.75 to −0.04) | 0.01 (−0.04 to 0.06) |
| Standardized prescription drug costs | −0.30 (−0.34 to −0.25) | −0.10 (−0.11 to −0.09) | −0.50 (−0.55 to −0.44) | −0.08 (−0.09, −0.07) | 0.20 (0.13 to 0.27) | −0.03 (−0.04 to −0.01) |
| Standardized total health care costs | −0.06 (−0.12 to −0.01) | −0.06 (−0.07 to −0.05) | −0.07 (−0.13 to −0.01) | −0.05 (−0.06 to −0.04) | −0.01 (−0.08 to 0.07) | −0.01 (−0.03 to −0.004) |
Abbreviations: AGB, adjustable gastric banding; RYGB, Roux-en-Y gastric bypass.
Includes parameter estimates and 95% CIs for within procedure pre-post comparisons. These estimates are taken directly from the Poisson model’s parameter estimates and approximate rate ratios. A level change corresponds to the change in level of the outcome from immediately before to after surgery (akin to a change in y-intercept in a linear model), and trend change corresponds to the change in slope (trajectory of the outcome over time) from before to after surgery.
Interaction term parameter estimates and 95% CIs are reported to compare level and trend changes between procedures; parameter estimates for between-procedure comparisons are ratios of rate ratios.
Table 3.
Difference-in-Difference Estimates for Postoperative Use Measures
| Variable | AGB vs RYGB, Difference (95% CI) | |||||
|---|---|---|---|---|---|---|
| Postoperative Year 1 | Postoperative Year 2 | Postoperative Year 3 | ||||
| Absolute Differencea | Relative Difference (%)b | Absolute Difference | Relative Difference (%) | Absolute Difference | Relative Difference (%) | |
| ED visits per year | −0.6 visits/person (−0.9 to −0.4) | −29 (−39 to −20) | −0.4 visits/person (−0.6 to −0.1) | −27 (−41 to −13) | −0.1 visits/person (−0.4 to 0.1) | −15 (−35 to 6) |
| Hospital days per year | −0.1 d/person (−0.3 to 0.1) | −20 (−52 to 11) | −0.1 d/person (−0.2 to 0.1) | −10 (−40 to 19) | 0.03 d/person (−0.1 to 0.2) | 8 (−36 to 52) |
| Prescription drug costs per year, US$ | $324/person ($262 to $385) | 32 (25 to 39) | $245/person ($176 to $313) | 26 (17 to 34) | $159/person ($74 to $243) | 17 (7 to 28) |
| Total standardized health care costs per year, US$ | $233/person (−$250 to $717) | 4 (−4 to 11) | $397/person (−$87 to $880) | 7 (−2 to 15) | $818/person ($278 to $1357) | 16 (4 to 27) |
Abbreviations: AGB, adjustable gastric banding; ED, emergency department; RYGB, Roux-en-Y gastric bypass.
Refers to the estimated actual change among AGB patients minus the change among RYGB patients in each postoperative year vs baseline (months −12 to −6), accounting for all other years (eg, in postoperative year 1, AGB patients had an estimated 0.6 fewer visits per person than RYGB patients).
Refers to the estimated relative difference between AGB patients and RYGB patients in each postoperative year vs baseline (months −12 to −6), accounting for all other years (eg, in postoperative year 1, AGB patients had an estimated 29% fewer ED visits than RYGB patients).
Hospital Days
Time series analyses of quarterly hospital days revealed that AGB patients had a significantly smaller immediate increase in postoperative level of hospital days than RYGB patients but neither group experienced significant changes in trend (Figure 1; Table 2). Multivariable difference-in-differences models, which are less sensitive to immediate postoperative changes, showed no significant differences in annual hospitalization days at years 1, 2, or 3 in the AGB relative to the RYGB group (Table 3).
Standardized Prescription Costs
Adjustable gastric banding patients experienced a smaller immediate postoperative drop in prescription costs but a greater downward trend compared with RYGB patients (Figure 2; Table 2). Similarly, in difference-in-differences models, AGB patients’ prescription costs were 17% to 32% (P < .001) higher than those of RYGB patients during all 3 postoperative years (absolute differences in postoperative years 1, 2, and 3: $324, $245, and $159, respectively; P < .001; Table 3).
Figure 2. Mean Standardized Prescription Drug and Total Costs per Quarter for Patients Undergoing Adjustable Gastric Banding vs Roux-en-Y Gastric Bypass.
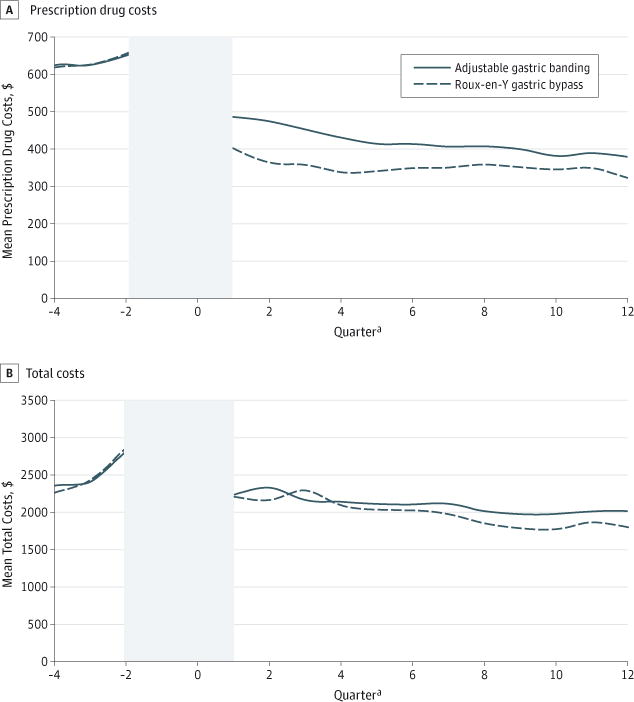
Quarters −2, −1, and 0 were omitted from the analysis and are not shown here because their extreme high values would have made visually interpreting preoperative to postoperative trend and level changes difficult.
a Refers to the 91.25-day period relative to the index procedure.
Total Health Care Costs
Mean total costs in the quarter immediately following the surgical date were $19 731 for AGB and $28 664 for RYGB patients. Total costs initially trended down slightly more after AGB relative to RYGB, but there was no significant difference in level change (Table 2; Figure 2). In difference-in-differences analyses, the cohorts had no detectable differences in total costs for the first 2 postoperative years, but by year 3, AGB patients had 16% (95% CI, 4% to 24%) higher total costs than RYGB patients (absolute increase: $818 per year; P < .01; Table 3).
Sensitivity Analysis for Subcohort With 2 Years Preoperative Baseline
In our subset of 3585 AGB and 3585 propensity score–matched RYGB patients with at least 2 years of baseline data, all observed patterns for level and trend change from preoperative to postoperative were nearly identical to the interrupted time series results from our main segmented regression analysis (eTable 2 in the Supplement).
Discussion
We found that both laparoscopic AGB and RYGB patients experienced reduced drug and total cost trends in the first 3 years after surgery, despite initial relative increases in ED visits and hospital days among RYGB patients. By year 3, AGB patients experienced total annual medical costs that were 16% higher and prescription costs 17% higher on average compared with RYGB patients. The prescription drug findings are unsurprising given RYGB’s greater impact on weight loss and chronic disease resolution.5,11 However, RYGB’s increased risk for early complications, implied by higher initial ED visits and hospital days, may have nullified any potential early use or spending reductions from remission of comorbid disease.
To our knowledge, this is the first study to compare the impact of laparoscopic AGB and RYGB on costs and use. Most previous comparative effectiveness studies have included a large proportion of patients receiving open procedures and may be less useful for health care professionals and patients deciding between current surgical modalities. Our early post operative use findings support prior clinical and claims-based studies. The increased ED visits we observed among RYGB patients are consistent with higher early complication rates vs AGB.5,11,32 Also, many prior studies have revealed short-term increases in hospitalizations after surgery, although few have differentiated by surgical type.15,17,19,21,32,33 Our findings of reduced prescription costs are not surprising, given prior studies demonstrating reductions in non-hospital–based health care use after bariatric surgery.21,33 Our findings do differ from those of a Veterans Affairs–based analysis that compared bariatric surgery patients to nonoperative control individuals and found no decreases in total costs during 3 follow-up years.17 This discrepancy may be related to the Veterans Affairs study’s focus on open rather than laparoscopic procedures34,35 and its greater inclusion of higher-morbidity male patients. Prior modeling work by Campbell et al36 suggested that bariatric procedures are most likely to be cost-effective in younger women than older men.
Our study adds several insights potentially useful to patients, clinicians, and policymakers. Our finding that both procedures were associated with flattened cost trends among commercially insured patients is novel. Presuming that the middle-aged patients comprising our sample continue on the trajectories demonstrated in the 3 years after their procedures, our analyses suggest both AGB and RYGB might be associated with long-term cost savings, although our follow-up period prevented us from definitively saying so. Therefore, payers might consider whether the higher upfront costs of RYGB are balanced by potential longer-term savings. To our knowledge, our study is also one of the few to demonstrate that ED and hospital use, while higher among RYGB patients for 2 years, is not detectably different by year 3. Therefore, clinicians and patients choosing between the procedures might weigh these early changes in ED and hospital use against expected weight loss or other factors.
Our study had several potential limitations. First, we did not attempt cost-effectiveness or return-on-investment analyses, as some prior work has done,35–37 limiting our ability to make inferences about relative changes in shorter-term costs from the societal or payer perspective. However, our longitudinal approach did permit key inferences about longer-term use trends and costs. Second, our study could be subject to confounding by indication if patients and health care professionals chose a certain procedure type based on unmeasured factors that differed between the groups and that also predicted outcomes. Related to this issue, we had no weight or BMI data. If RYGB patients were heavier than AGB patients, their higher starting BMI could have been partially responsible for greater cost/use changes independent of the procedure itself.36 Nevertheless, our propensity score–matching algorithm generated study groups with nearly identical baseline trends and measured characteristics such as rates of diabetes mellitus, hypertension, and hyperlipidemia. Controlled interrupted time series designs are among the most robust for causal inference, and accumulating evidence suggests that matching on baseline trend generates effect estimates similar to randomized clinical trials.38
Requiring at least a year of postsurgical follow-up could have biased results if sicker patients, likely to have higher costs, disenrolled early. Attrition after the first follow-up year did not differ substantially between the groups and we controlled for it using patient-level segmented regression models. Importantly, our analysis captured outcomes of surgical patients independent of whether they later had reversal of their procedure (eg, band removal). Complications, such as AGB removal, are an important component of the costs of and clinical decision-making around procedure selection. To the extent that AGB removals increased health care use or costs, we expect that these differences are captured in our study. Our analysis was also unable to explore health care provider–level factors, such as designation as a surgical Center of Excellence, that might have influenced outcomes. However, the procedures we analyzed require prior authorization from the health insurer, likely directing both groups to similar surgical facilities. Therefore, the lack of detail about this information was unlikely to impact our results.
Finally, although AGB and RYGB were the most popular procedures during much of the period we examined, surgeons are increasingly using the newer vertical sleeve gastrectomy as an alternative to both of these procedures.39 Future studies should compare this procedure relative to RYGB and AGB as well.
Conclusions
We found that laparoscopic AGB and RYGB were associated with reduced health care costs among commercially insured patients, partially driven by reductions in prescription costs. Although RYGB patients experienced higher rates of hospitalizations and ED visits than AGB patients in the first 2 postoperative years, rates were similar by year 3. For patients, clinicians, and policymakers interested in reducing overall use and prescription use, both of these laparoscopic procedures offer promise. However, future studies should assess emerging procedures, such as vertical sleeve gastrectomy, to more fully inform patients and health care professionals.
Supplementary Material
Acknowledgments
Funding/Support: This work was funded in part by a P30 grant from the National Institutes of Health (P30 HL101312; principal investigator, Dr Gillman).
Role of the Funder/Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamasurgery.com
Author Contributions: Dr Lewis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lewis, Arterburn, Ross-Degnan, Gillman, Wharam.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Lewis, Wharam.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Lewis, Zhang, Wharam.
Obtained funding: Lewis, Wharam.
Administrative, technical, or material support: Lewis, Gillman.
Study supervision: Lewis, Arterburn, Ross-Degnan, Gillman, Wharam.
Conflict of Interest Disclosures: None reported.
References
- 1.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37(6):889–891. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreyeva T, Sturm R, Ringel JS. Moderate and severe obesity have large differences in health care costs. Obes Res. 2004;12(12):1936–1943. doi: 10.1038/oby.2004.243. [DOI] [PubMed] [Google Scholar]
- 3.Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Med Clin North Am. 2011;95(5):1009–1030. doi: 10.1016/j.mcna.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–420. doi: 10.1097/SLA.0b013e31822c9dac. discussion 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neff KJ, Olbers T, le Roux CW. Bariatric surgery: the challenges with candidate selection, individualizing treatment and clinical outcomes. BMC Med. 2013;11:8. doi: 10.1186/1741-7015-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032–1040. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 7.Romy S, Donadini A, Giusti V, Suter M. Roux-en-Y gastric bypass vs gastric banding for morbid obesity: a case-matched study of 442 patients. Arch Surg. 2012;147(5):460–466. doi: 10.1001/archsurg.2011.1708. [DOI] [PubMed] [Google Scholar]
- 8.Dorman RB, Serrot FJ, Miller CJ, et al. Case-matched outcomes in bariatric surgery for treatment of type 2 diabetes in the morbidly obese patient. Ann Surg. 2012;255(2):287–293. doi: 10.1097/SLA.0b013e318232b033. [DOI] [PubMed] [Google Scholar]
- 9.Campos GM, Rabl C, Roll GR, et al. Better weight loss, resolution of diabetes, and quality of life for laparoscopic gastric bypass vs banding: results of a 2-cohort pair-matched study. Arch Surg. 2011;146(2):149–155. doi: 10.1001/archsurg.2010.316. [DOI] [PubMed] [Google Scholar]
- 10.Woodard GA, Peraza J, Bravo S, Toplosky L, Hernandez-Boussard T, Morton JM. One year improvements in cardiovascular risk factors: a comparative trial of laparoscopic Roux-en-Y gastric bypass vs. adjustable gastric banding. Obes Surg. 2010;20(5):578–582. doi: 10.1007/s11695-010-0088-0. [DOI] [PubMed] [Google Scholar]
- 11.Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? a systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121(10):885–893. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 12.Himpens J, Cadière GB, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg. 2011;146(7):802–807. doi: 10.1001/archsurg.2011.45. [DOI] [PubMed] [Google Scholar]
- 13.Lanthaler M, Aigner F, Kinzl J, Sieb M, Cakar-Beck F, Nehoda H. Long-term results and complications following adjustable gastric banding. Obes Surg. 2010;20(8):1078–1085. doi: 10.1007/s11695-010-0190-3. [DOI] [PubMed] [Google Scholar]
- 14.Arterburn D, Maggard MA. Revisiting the 2011 FDA decision on laparoscopic adjustable gastric banding. Obesity (Silver Spring) 2013;21(11):2204. doi: 10.1002/oby.20476. [DOI] [PubMed] [Google Scholar]
- 15.Bleich SN, Chang HY, Lau B, et al. Impact of bariatric surgery on health care utilization and costs among patients with diabetes. Med Care. 2012;50(1):58–65. doi: 10.1097/MLR.0b013e3182290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maciejewski ML, Livingston EH, Smith VA, Kahwati LC, Henderson WG, Arterburn DE. Health expenditures among high-risk patients after gastric bypass and matched controls. Arch Surg. 2012;147(7):633–640. doi: 10.1001/archsurg.2012.818. [DOI] [PubMed] [Google Scholar]
- 17.Maciejewski ML, Smith VA, Livingston EH, et al. Health care utilization and expenditure changes associated with bariatric surgery. Med Care. 2010;48(11):989–998. doi: 10.1097/MLR.0b013e3181ef9cf7. [DOI] [PubMed] [Google Scholar]
- 18.Makary MA, Clark JM, Shore AD, et al. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery [published correction appears in Arch Surg. 2011;146(6):659] Arch Surg. 2010;145(8):726–731. doi: 10.1001/archsurg.2010.150. [DOI] [PubMed] [Google Scholar]
- 19.Zingmond DS, McGory ML, Ko CY. Hospitalization before and after gastric bypass surgery. JAMA. 2005;294(15):1918–1924. doi: 10.1001/jama.294.15.1918. [DOI] [PubMed] [Google Scholar]
- 20.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240(3):416–423. doi: 10.1097/01.sla.0000137343.63376.19. discussion 423–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiner JP, Goodwin SM, Chang HY, et al. Impact of bariatric surgery on health care costs of obese persons: a 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA Surg. 2013;148(6):555–562. doi: 10.1001/jamasurg.2013.1504. [DOI] [PubMed] [Google Scholar]
- 22.Ethnic Technologies. http://www.ethnictechnologies.com/product_detail.php?id=9. Accessed April 30, 2015.
- 23.Fiscella K, Fremont AM. Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res. 2006;41(4, pt 1):1482–1500. doi: 10.1111/j.1475-6773.2006.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures: the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Bureau of the Census. Geographical Areas Reference Manual. Washington, DC: US Bureau of the Census; 1994. [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Coca-Perraillon M. Local and global optimal propensity score matching. 2007 http://www2.sas.com/proceedings/forum2007/85-2007.pdf. Accessed December 19, 2009.
- 28.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Cook EF, Goldman L. Performance of tests of significance based on stratification by a multivariate confounder score or by a propensity score. J Clin Epidemiol. 1989;42(4):317–324. doi: 10.1016/0895-4356(89)90036-x. [DOI] [PubMed] [Google Scholar]
- 30.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007;26(1):20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 31.Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? comparing methods of modeling Medicare expenditures. J Health Econ. 2004;23(3):525–542. doi: 10.1016/j.jhealeco.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Arterburn D, Powers JD, Toh S, et al. Comparative effectiveness of laparoscopic adjustable gastric banding vs laparoscopic gastric bypass. JAMA Surg. 2014;149(12):1279–1287. doi: 10.1001/jamasurg.2014.1674. [DOI] [PubMed] [Google Scholar]
- 33.Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. JAMA. 2012;308(11):1132–1141. doi: 10.1001/2012.jama.11792. [DOI] [PubMed] [Google Scholar]
- 34.Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg. 2008;248(1):10–15. doi: 10.1097/SLA.0b013e31816d953a. [DOI] [PubMed] [Google Scholar]
- 35.Cremieux PY, Buchwald H, Shikora SA, Ghosh A, Yang HE, Buessing M. A study on the economic impact of bariatric surgery. Am J Manag Care. 2008;14(9):589–596. [PubMed] [Google Scholar]
- 36.Campbell J, McGarry LA, Shikora SA, Hale BC, Lee JT, Weinstein MC. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care. 2010;16(7):e174–e187. [PubMed] [Google Scholar]
- 37.Finkelstein EA, Allaire BT, Burgess SM, Hale BC. Financial implications of coverage for laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2011;7(3):295–303. doi: 10.1016/j.soard.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 38.St Clair T, Cook TD, Hallberg K. Examining the internal validity and statistical precision of the comparative interrupted time series design by comparison with a randomized experiment. Am J Eval. 2014;35(3):311–327. doi: 10.1177/1098214014527337. [DOI] [Google Scholar]
- 39.Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg. 2011;21(11):1650–1656. doi: 10.1007/s11695-011-0479-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


