Abstract
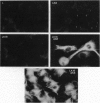
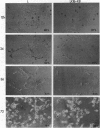
Intermediate filament proteins have been used to diagnose the origin of specific cells. Classically, vimentin is found in mesenchymal cells, and keratins are present in epithelial cells. However, recent evidence suggests that the coexpression of these phenotype-specific proteins augments tumor cell motility, and hence, metastasis. In the present study, we used the mouse L-cell model to determine if a direct correlation exists between the expression of additional keratins in these cells, which normally express only vimentin, and their migratory ability. Mouse L cells were transfected with human keratins 8, 18, and both 8 and 18. The results indicate that the cells expressing complete keratin filaments have a higher migratory and invasive ability (through extracellular matrix-coated filters) compared with the parental and control-transfected clones. Furthermore, there is an enrichment of keratin-positive cells from a heterogeneous population of L clones selected over serial migrations. This migratory activity was directly correlated with the spreading ability of the cells on Matrigel matrix, in which the keratin-positive transfectants maintain a round morphology for a longer duration, compared with the other L-cell populations. Collectively, these data suggest that keratins may play an important role(s) in migration, through a special interaction with the extracellular environment, thereby influencing cell shape.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baribault H., Oshima R. G. Polarized and functional epithelia can form after the targeted inactivation of both mouse keratin 8 alleles. J Cell Biol. 1991 Dec;115(6):1675–1684. doi: 10.1083/jcb.115.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Zöller M., Raz A. Differential expression of intermediate filament proteins in metastatic and nonmetastatic variants of the BSp73 tumor. Cancer Res. 1986 Feb;46(2):785–790. [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Letai A., Hebert A., Paller A. S., Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991 Sep 20;66(6):1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Domenjoud L., Jorcano J. L., Breuer B., Alonso A. Synthesis and fate of keratins 8 and 18 in nonepithelial cells transfected with cDNA. Exp Cell Res. 1988 Dec;179(2):352–361. doi: 10.1016/0014-4827(88)90274-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990 Dec;111(6 Pt 2):2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther A., Kinjo M., Winter H., Sonka J., Volm M. Differential expression of intermediate-filament proteins in murine sarcoma 180 ascites or solid tumor. Cancer Res. 1984 Jun;44(6):2590–2594. [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Chu Y. W., Seftor R. E., Nagle R. B., McDaniel K. M., Leong S. P., Yohem K. H., Leibovitz A. M., Meyskens F. L., Jr Coexpression of vimentin and keratins by human melanoma tumor cells: correlation with invasive and metastatic potential. J Natl Cancer Inst. 1992 Feb 5;84(3):165–174. doi: 10.1093/jnci/84.3.165. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Seftor R. E., Fidler I. J. A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett. 1987 Dec;38(1-2):137–147. doi: 10.1016/0304-3835(87)90209-6. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Seftor R. E., Misiorowski R. L., Saba P. Z., Sundareshan P., Welch D. R. Comparison of tumor cell invasion assays: human amnion versus reconstituted basement membrane barriers. Invasion Metastasis. 1989;9(5):278–297. [PubMed] [Google Scholar]
- Jones J. C., Goldman R. D. Intermediate filaments and the initiation of desmosome assembly. J Cell Biol. 1985 Aug;101(2):506–517. doi: 10.1083/jcb.101.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Bachant J. B., Domingo A. Functions of intermediate filaments. Cell Motil Cytoskeleton. 1989;14(3):309–331. doi: 10.1002/cm.970140302. [DOI] [PubMed] [Google Scholar]
- Kulesh D. A., Ceceña G., Darmon Y. M., Vasseur M., Oshima R. G. Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol. 1989 Apr;9(4):1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D. A., Oshima R. G. Cloning of the human keratin 18 gene and its expression in nonepithelial mouse cells. Mol Cell Biol. 1988 Apr;8(4):1540–1550. doi: 10.1128/mcb.8.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E. B., Hogan B. L., Kurkinen M., Garrels J. I. Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature. 1983 Jun 23;303(5919):701–704. doi: 10.1038/303701a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Markl J. Cytokeratins in mesenchymal cells: impact on functional concepts of the diversity of intermediate filament proteins. J Cell Sci. 1991 Mar;98(Pt 3):261–264. doi: 10.1242/jcs.98.3.261. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Palm S. L., Furcht L. T. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J Cell Biol. 1983 Sep;97(3):772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M., Franssila K. Immunohistochemical spectrum of malignant melanoma. The common presence of keratins. Lab Invest. 1989 Dec;61(6):623–628. [PubMed] [Google Scholar]
- Ochalek T., Nordt F. J., Tullberg K., Burger M. M. Correlation between cell deformability and metastatic potential in B16-F1 melanoma cell variants. Cancer Res. 1988 Sep 15;48(18):5124–5128. [PubMed] [Google Scholar]
- Oshima R. G. Intermediate filament molecular biology. Curr Opin Cell Biol. 1992 Feb;4(1):110–116. doi: 10.1016/0955-0674(92)90067-m. [DOI] [PubMed] [Google Scholar]
- Quaranta V., Jones J. C. The internal affairs of an integrin. Trends Cell Biol. 1991 Jul;1(1):2–4. doi: 10.1016/0962-8924(91)90046-c. [DOI] [PubMed] [Google Scholar]
- Robey H. L., Hiscott P. S., Grierson I. Cytokeratins and retinal epithelial cell behaviour. J Cell Sci. 1992 Jun;102(Pt 2):329–340. doi: 10.1242/jcs.102.2.329. [DOI] [PubMed] [Google Scholar]
- Seftor E. A., Seftor R. E., Hendrix M. J. Selection of invasive and metastatic subpopulations from a heterogeneous human melanoma cell line. Biotechniques. 1990 Sep;9(3):324–331. [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Gehlsen K. R., Stetler-Stevenson W. G., Brown P. D., Ruoslahti E., Hendrix M. J. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Goldman R. D. Recent insights into the assembly, dynamics, and function of intermediate filament networks. Cell Motil Cytoskeleton. 1991;19(2):67–79. doi: 10.1002/cm.970190202. [DOI] [PubMed] [Google Scholar]
- Soll D. R. "DMS," a computer-assisted system for quantitating motility, the dynamics of cytoplasmic flow, and pseudopod formation: its application to Dictyostelium chemotaxis. Cell Motil Cytoskeleton. 1988;10(1-2):91–106. doi: 10.1002/cm.970100114. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Thompson E. W., Paik S., Brünner N., Sommers C. L., Zugmaier G., Clarke R., Shima T. B., Torri J., Donahue S., Lippman M. E. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992 Mar;150(3):534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- Trask D. K., Band V., Zajchowski D. A., Yaswen P., Suh T., Sager R. Keratins as markers that distinguish normal and tumor-derived mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2319–2323. doi: 10.1073/pnas.87.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Southgate J., Kemshead J. T., Hodges G. M. Phenotypic analysis of cultured melanoma cells. Expression of cytokeratin-type intermediate filaments by the M5 human melanoma cell line. Exp Cell Res. 1986 Jun;164(2):388–398. doi: 10.1016/0014-4827(86)90037-6. [DOI] [PubMed] [Google Scholar]
- Tullberg K. F., Burger M. M. Selection of B16 melanoma cells with increased metastatic potential and low intercellular cohesion using Nuclepore filters. Invasion Metastasis. 1985;5(1):1–15. [PubMed] [Google Scholar]
- Vassar R., Coulombe P. A., Degenstein L., Albers K., Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991 Jan 25;64(2):365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]