Abstract
Background
COPD exacerbations requiring hospitalization increase morbidity and mortality. Although most COPD exacerbations are neutrophilic, approximately 10%–25% of exacerbations are eosinophilic.
Aim
We aimed to evaluate mortality and outcomes of eosinophilic and non-eosinophilic COPD exacerbations and identify new biomarkers that predict survival.
Methods
A retrospective observational cohort study was carried out in a tertiary teaching hospital from January 1, 2014 to November 1, 2014. All COPD patients hospitalized with exacerbations were enrolled in the study at their initial hospitalization and followed-up for 6 months after discharge. Electronic data were collected from the hospital database. Subjects’ characteristics, hemogram parameters, CRP levels, neutrophil-to-lymphocyte ratio (NLR), platelet-to-mean platelet volume ratio on admission and discharge, length of hospital stay (days), readmissions, and mortality were recorded. Patients were grouped according to peripheral blood eosinophil (PBE) levels: Group 1, >2% PBE, eosinophilic; Group 2, non-eosinophilic ≤2%. Patient survival after hospital discharge was evaluated by Kaplan–Meier survival analysis.
Results
A total of 1,704 patients hospitalized with COPD exacerbation were included. Approximately 20% were classified as eosinophilic. Six-month mortality was similar in eosinophilic and non-eosinophilic groups (14.2% and 15.2%, respectively); however, the hospital stay length and readmission rate were longer and higher in the non-eosinophilic group (P<0.001 and P<0.01, respectively). CRP and NLR were significantly higher in the non-eosinophilic group (both P<0.01). The platelet-to-mean platelet volume ratio was not different between the two groups. Cox regression analysis showed that survival was negatively influenced by elevated CRP (P<0.035) and NLR (P<0.001) in the non-eosinophilic group.
Conclusion
Non-eosinophilic patients with COPD exacerbations with high CRP and NLR values had worse outcomes than eosinophilic patients. PBE and NLR can be helpful markers to guide treatment decisions.
Keywords: chronic obstructive pulmonary disease, exacerbation, peripheral eosinophilia, mortality
Introduction
COPD is one of the major causes of morbidity and mortality in the world. It is estimated that COPD will become the third leading cause of death worldwide by 2020, up from the sixth leading cause of death in 1990.1 The disease is characterized by acute exacerbations that often require hospitalization.2 Exacerbation is the main issue affecting morbidity and mortality.3 COPD mortality rates range from 15% to 54% and are higher in patients who are hospitalized due to acute exacerbations.4 Different factors correlate with mortality associated with COPD exacerbation.2,5
COPD is characterized by chronic inflammation of the airways and lungs.6 Studies have shown that frequently neutrophilic, and to a lesser extent eosinophilic, inflammation occur in COPD exacerbations.7,8 Sputum and peripheral blood eosinophil (PBE) counts (2%–3%) can be seen in 10%–25% of COPD exacerbations;3,8–11 however, the effect of PBE on survival in patients with COPD exacerbation is not clear.
Recent studies have investigated the usefulness of neutrophil-to-lymphocyte ratio (NLR),12 mean platelet volume (MPV), and platelet-to-MPV ratio (PLT/MPV) as new inflammatory markers.13,14 Relationships between NLR and prognosis in lung cancer, colorectal cancer, and acute coronary syndrome have been shown in many studies.15–17 MPV has been identified as an inflammation marker in some chronic inflammatory diseases, and an inverse correlation between disease activity and MPV has been demonstrated.18–20 Despite these findings, there are limited data about the utility of using NLR,13 MPV, and PLT/MPV for predicting COPD exacerbation outcomes.6
Knowledge of clinically meaningful predictors of mortality and poor outcome is crucial for guiding patient management decisions.2 In the present study, we hypothesized that the presence of PBE (eosinophils > 2%) and a lower NLR could be a predictor of a better long-term outcome after hospital discharge in COPD patients with acute exacerbation. We also aimed to evaluate the effect of PLT/MPV as another inflammatory marker of COPD outcome.
Methods
A retrospective observational cohort study was conducted at Chest Diseases and Thoracic Surgery Teaching and Research Hospital, Istanbul, Turkey, between January 1, 2014 and November 1, 2014. This is the largest chest disease hospital of the country. The study protocol was approved by the Hospital’s local ethics committee and was in accordance with the Declaration of Helsinki. All data were collected retrospectively from the hospital database. Due to the retrospective nature of the study design, informed consent was not obtained.
Patients
Patients previously diagnosed with COPD by a pulmonology specialist, recorded according to the International Classification of Diseases (ICD) 10 with codes J44, J44.0, J44.1, J44.8, J44.9, and who were hospitalized were included in the study.
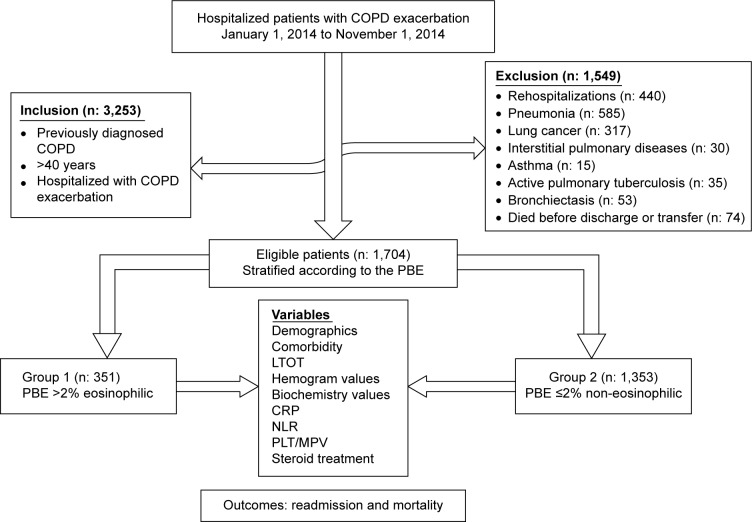
COPD patients hospitalized for specific (secondary) causes such as pneumonia, lung cancer, interstitial pulmonary disease, asthma, bronchiectasis, and active pulmonary tuberculosis were excluded. Each patient was enrolled in the study only once at their initial hospitalization. Subject inclusion is summarized in Figure 1.
Figure 1.
Flow chart of patients.
Abbreviations: PBE, peripheral blood eosinophil; LTOT, long-term oxygen therapy; NLR, neutrophil-to-lymphocyte ratio; PLT/MPV, platelet-to-mean platelet volume ratio.
Definitions
COPD
The COPD diagnosis was established by a pulmonologist who evaluated airflow obstruction on spirometry (ie, forced expiratory volume in 1 second ≤70% predicted and an forced expiratory volume in 1 second and forced vital capacity ratio ≤70%) in patients with compatible history.1
COPD exacerbation
The exacerbation of COPD was defined as acute change in a patient’s respiratory symptoms that is beyond normal variability, and that is sufficient to warrant a change in therapy.1 The most common cause of COPD exacerbation is respiratory tract infections, other causes are also defined as follows:
Infections: for a diagnosis of infectious COPD exacerbation, criteria were an increase in dyspnea, increase in sputum quantity, and purulence.1,21 In addition, the patient’s ICD code was 44.0.
Other causes: other causes of COPD exacerbations as defined by the ICD coding system are arrhythmia: (I47, I48, I49, I49.8, I49.9) heart failure: (I50, I50.0, I50.9, I11.0) pleurisy: (J91, J94.8, J94.9) pneumothorax: (J93.1, J93.8) pulmonary embolism: (I26, I26.0, I26.9).
Peripheral eosinophilia: COPD exacerbation with a PBE count higher than 2% was defined as an eosinophilic COPD exacerbation. If the eosinophil count was equal or less than 2%, it was defined as a non-eosinophilic COPD exacerbation.9,11 The cutoff value of PBEs has been determined as 2% for identifying a sputum eosinophilia of greater than 3% with a sensitivity of 90% and specificity of 60% during COPD exacerbation.9,11
NLR: a new marker of inflammation12 was determined by dividing the absolute count of neutrophils by the number of lymphocytes in the complete blood count.
MPV and PLT/MPV: MPV is an early marker of platelet activation during inflammation and thrombocytosis.14 Abnormal platelet count may be an important marker as an indication of systemic inflammatory response,22 and it is easily calculated from complete blood count data.
Readmission was defined as repeat hospitalization for the treatment of COPD exacerbation within 6 months of discharge.
Measurements
Electronic data were collected from the hospital database. Patients’ characteristics, comorbid diseases, hemogram parameters, NLR, PLT/MPV, CRP levels, biochemistry values on admission and discharge, long-term oxygen therapy (LTOT), steroid use, length of hospitalization (days), readmissions, and mortality were recorded. Spirometry values and microbiological data of patients could not be recorded because of the lack of electronically available spirometry test data.
Total leukocyte, neutrophil, eosinophil, lymphocyte, and platelet counts and MPV were determined using a Coulter LH 780 Hematology Analyzer (Beckman Coulter Inc., Brea, CA, USA). CRP was checked by the nephelometry method with a BN II System (Siemens, Munich, Germany). The normal range of CRP is 0–5 mg/L.
COPD patients have nearly similar pre-hospital and current hospital treatment as based on national and international guidelines.1 The COPD exacerbation treatment protocol of the hospital was 40 mg of intravenous prednisolone per day, nebulized bronchodilators (inhaled salbutamol and ipratropium bromide), antibiotics, and supplemental oxygen.
Comorbidities such as diabetes mellitus, hypertension, heart failure, arrhythmia, chronic ischemic heart disease, chronic renal failure, anemia, and anxiety/depression were recorded.
COPD patients were divided into two groups according to their PBE status: Group 1, eosinophilic (PBE >2%) and Group 2, non-eosinophilic (PBE ≤2%). The recorded parameters were compared between the two groups. Readmissions and mortality were recorded during a 6-month follow-up after hospital discharge.
Statistical analysis
Descriptive analysis was used to investigate patient demographics and clinical data. Groups were compared with Mann–Whitney U-tests for nonparametric continuous variables or Student’s t-tests for parametric continuous variables. Chi-square tests were employed for dichotomous variables. The median with interquartile range was employed for nonparametric continuous variables, and mean ± standard deviation was used for parametric continuous variables. Count and percentage were used when applicable. Patient survival after hospital discharge was evaluated by Kaplan–Meier survival analysis. Long-term mortality risk factors were analyzed with the Cox regression model. We included the variables that were statistically significant following univariate analyses of survival and non-survival in patients following hospital discharge. A P-value <0.05 was accepted as statistically significant.
Results
A total of 1,704 patients were assessed including 351 patients with eosinophilic COPD (Group 1, PBE >2%) and 1,353 patients with non-eosinophilic COPD (Group 2, PBE ≤2%). The eosinophilic group included 20% of all patients.
Table 1 shows the demographics, comorbidities, LTOT, hemogram, and biochemistry values and inflammatory biomarkers on admission. The majority of patients in both groups were male and older than 65 years. Comorbidities and LTOT were similar in both groups. Leukocyte count, neutrophil %, monocyte %, lymphocyte %, eosinophil %, and basophil % were significantly different in both groups. The non-eosinophilic group also had significantly higher leukocyte count, neutrophil %, blood glucose, blood urea nitrogen, and serum creatinine. CRP and NLR were significantly higher in the non-eosinophilic group on admission. PLT/MPV values were similar in both groups.
Table 1.
Patients’ characteristics and laboratory findings on admission
| Variables | Group 1 Eosinophilic (n=351) | Group 2 Non-eosinophilic (n=1,353) | P-value |
|---|---|---|---|
| Number of patients | 351 | 1,353 | |
| Male, % | 66.9 | 65.1 | |
| Age, years (median) | 70 (61–80) | 71 (63–78) | 0.70 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 45 (12.8) | 124 (9.2) | 0.60 |
| Hypertension | 44 (12.5) | 166 (12.3) | 0.89 |
| Ischemic heart disease | 16 (4.6) | 59 (4.4) | 0.87 |
| Chronic renal failure | 7 (2) | 17 (1.3) | 0.29 |
| Anemia | 3 (0.9) | 9 (0.7) | 0.70 |
| Anxiety/depression | 2 (0.9) | 16 (1.2) | 0.60 |
| Heart failure | 54 (15.4) | 223 (16.5) | 0.62 |
| Arrhythmia | 6 (1.7) | 32 (2.4) | 0.45 |
| Pleurisy | 19 (1.6) | 6 (1.2) | 0.53 |
| Emboli | 60 (5.0) | 19 (3.8) | 0.27 |
| Pneumothorax | 1 (0.1) | 1 (0.2) | 0.52 |
| LTOT, n (%) | 177 (50.4) | 696 (51.4) | 0.73 |
| Hemogram values | |||
| Leukocyte count, 109 L | 7.7 (6.2–9.5) | 9.9 (7.5–13.01) | 0.001 |
| Neutrophil, % | 68.6 (62.1–73.8) | 82.21 (74.10–88.90) | 0.001 |
| Monocyte, % | 7.19 (5.6–9.02) | 5.2 (3.1–7.2) | 0.001 |
| Lymphocyte, % | 19.3 (14.9–24.32) | 10.3 (6.3–17.1) | 0.001 |
| Eosinophil, % | 3.0 (2.4–3.99) | 0.8 (0.11–1.1) | 0.001 |
| Basophil, % | 0.5 (0.29–0.9) | 0.3 (0.1–0.6) | 0.001 |
| Erythrocyte count, 1012 L | 4.31 (3.9–4.74) | 4.36 (3.93–4.8) | 0.15 |
| Hemoglobin, g/dL | 12 (10.94–13.59) | 12.35 (11.04–13.64) | 0.11 |
| Hematocrit, % | 36.89 (33–41.1) | 37.25 (33.6–41.41) | 0.23 |
| MCV, fL | 86.99 (82–91) | 86.51 (81.9–90.25) | 0.50 |
| Platelet count, 109 L | 247 (194–311.8) | 247.6 (198–313) | 0.84 |
| Mean platelet volume, fL | 8.6 (7.9–9.2) | 8.63 (7.95–9.3) | 0.22 |
| Biochemistry values | |||
| Blood glucose, mg/dL | 110 (93–142) | 136 (103–186) | 0.001 |
| BUN, mg/dL | 22 (15–37) | 25 (18–39) | 0.002 |
| Serum creatinine, mg/dL | 0.81 (0.7–1.12) | 0.83 (0.7–1.05) | 0.90 |
| Sodium, mmol/L | 139 (137–141) | 138 (136–140) | 0.005 |
| Potassium, mmol/L | 4.2 (3.8–4.7) | 4.2 (3.8–4.7) | 0.63 |
| SGOT, U/L | 19.5 (14–26) | 19 (14–26) | 0.47 |
| SGPT, U/L | 16 (10–25) | 17 (11–27) | 0.06 |
| Protein, g/dL | 6.6 (6–7) | 6.5 (6–7.1) | 0.60 |
| Albumin, g/dL | 3.1 (2.7–3.4) | 3.1 (2.7–3.5) | 0.92 |
| Inflammatory markers | |||
| CRP, mg/dL | 19.3 (6.76–57.10) | 35.15 (11.7–95.9) | 0.001 |
| Neutrophil-to-lymphocyte ratio | 3.63 (2.57–4.95) | 7.99 (4.36–13.87) | 0.001 |
| Platelet-to-mean platelet volume ratio | 29.86 (21.37–38.29) | 28.85 (21.87–37.47) | 0.65 |
Note: Median (25%–75%).
Abbreviations: BUN, blood urea nitrogen; MCV, mean corpuscular volume; MPV, mean platelet volume; LTOT, long-term oxygen therapy; SGOT, serum glutamic-oxalacetic transaminase; SGPT, serum glutamic-pyruvic transaminase.
Table 2 shows hemogram, biochemistry values, inflammatory biomarkers measured immediately before hospital discharge, steroid use during hospitalization, and length of hospital stay. Compared to admission values, leukocyte count and neutrophil % had decreased in both groups, but the non-eosinophilic group still had significantly higher leukocyte count, neutrophil %, blood glucose, blood urea nitrogen, and serum creatinine. The length of stay was significantly shorter in the eosinophilic group. It was 6.6 (4.6–8.0) days in the eosinophilic group and 7.0 (5.5–9.0) days in the non-eosinophilic group (P<0.001). Steroid use during hospitalization was higher in the non-eosinophilic patients.
Table 2.
Laboratory findings, length of stay, and steroid use on hospital discharge
| Variables | Group 1 Eosinophilic (n=351) | Group 2 Non-eosinophilic (n=1,353) | P-value |
|---|---|---|---|
| Hemogram values | |||
| Leukocyte count, 109 L | 8.1 (6.5–10.11) | 9.69 (7.6–12.2) | 0.001 |
| Neutrophil, % | 69.71 (62.4–74.6) | 78.4 (70.6–86.0) | 0.001 |
| Monocyte, % | 7.0 (5.48–8.98) | 5.8 (4.0–7.76) | 0.19 |
| Lymphocyte, % | 18.75 (14.07–23.93) | 13.1 (8–19.4) | 0.001 |
| Eosinophil, % | 2.8 (2.2–3.84) | 0.9 (0.34–1.42) | 0.001 |
| Basophil, % | 0.46 (0.23–0.86) | 0.30 (0.10–0.60) | 0.001 |
| Erythrocyte count, 1012 L | 4.34 (3.89–4.77) | 4.37 (3.93–4.82) | 0.13 |
| Hemoglobin, g/dL | 12.02 (10.99–13.60) | 12.35 (11.04–13.70) | 0.08 |
| Hematocrit, % | 36.8 (33.29–41.31) | 37.4 (33.7–41.33) | 0.019 |
| MCV, fL | 86.95 (82–91) | 86.27 (82–90.23) | 0.64 |
| Platelet count, 109 L | 253.3 (209–314) | 258.5 (204–324) | 0.64 |
| Mean platelet volume, fL | 8.46 (7.9–9.14) | 8.47 (7.9–9.2) | 0.89 |
| Biochemistry values | |||
| Blood glucose, mg/dL | 109 (90–139) | 123 (95–166) | 0.001 |
| BUN, mg/dL | 23 (15–37) | 26 (18–41) | 0.001 |
| Serum creatinine, mg/dL | 0.73 (0.63–0.91) | 0.76 (0.64–0.92) | 0.12 |
| Sodium, mmol/L | 139 (137–140) | 138 (136–140) | 0.019 |
| Potassium, mmol/L | 4.1 (3.8–4.6) | 4.2 (3.8–4.6) | 0.23 |
| SGOT, U/L | 19 (15–27) | 18 (14–25) | 0.040 |
| SGPT, U/L | 16 (11–28) | 19 (12–30) | 0.005 |
| Protein, g/dL | 6.5 (6.0–7.0) | 6.5 (5.9–7.0) | 0.83 |
| Albumin, g/dL | 3.3 (2.9–3.6) | 3.2 (2.8–3.6) | 0.20 |
| Length of hospital stay, days, median | 6.6 (4.6–8.0) | 7.0 (5.5–9.0) | 0.001 |
| Steroid use, n (%) | 216 (61) | 1,097 (81) | 0.001 |
| Inflammatory markers | |||
| CRP, mg/dL | 12.2 (5.7–26.4) | 12.2 (4.3–31.6) | 0.91 |
| Neutrophil-to-lymphocyte ratio | 3.73 (2.6–5.15) | 6.0 (3.65–10.6) | 0.001 |
| Platelet-to-mean platelet volume ratio | 30.3 (22.87–38.31) | 29.97 (22.89–39.67) | 0.89 |
Note: Median (25%–75%).
Abbreviations: BUN, blood urea nitrogen; MCV, mean corpuscular volume; SGOT, serum glutamic-oxalacetic transaminase; SGPT, serum glutamic-pyruvic transaminase.
After discharge from the hospital, all patients were followed-up for 6 months. The readmission rate was significantly higher in the non-eosinophilic patients. Readmission within 6 months was one (0–3) for the eosinophilic and two (0–4) for the non-eosinophilic group (P<0.01). We also assessed the number of exacerbations per patient per year and this was found to be 0.61 (95% confidence interval: 0.58–0.64) in the non-eosinophilic group and 0.48 (95% confidence interval: 0.43–0.53) in the eosinophilic group.
Table 3 shows the factors affecting readmission and mortality in the 6-month follow-up period. Readmission rates were higher in the non-eosinophilic patients but the mortality rate was similar in both groups (14.2% and 15.2%, respectively). Higher CRP and NLR levels and a longer hospital stay were the factors that positively affected both readmission and mortality.
Table 3.
Hospital readmission for treatment of COPD exacerbation, and mortality within 6 months after discharge
| Variables | Readmission
|
Mortality
|
||||
|---|---|---|---|---|---|---|
| No (n: 1,200) | Yes (n: 504) | P-value | No (n: 1,448) | Yes (n: 256) | P-value | |
| Eosinophilic patients, n (%) | 266 (22.2) | 85 (16.9) | 0.014 | 301 (20.8) | 50 (19.5) | 0.64 |
| Male, % | 61.9 | 74 | 0.01 | 70 (62–77) | 76 (67–82) | 0.001 |
| Age, years (median) | 71 (63–79) | 70 (62–76) | 0.01 | 70 (62–77) | 76 (67–82) | 0.001 |
| Comorbidities, n (%) | ||||||
| Diabetes mellitus | 120 (10.0) | 49 (9.7) | 0.22 | 149 (10.3) | 20 (7.8) | 0.92 |
| Hypertension | 143 (11.9) | 67 (13.3) | 0.43 | 188 (13.0) | 22 (8.6) | 0.04 |
| Ischemic heart disease | 54 (4.5) | 21 (4.2) | 0.75 | 67 (4.6) | 8 (3.1) | 0.28 |
| Chronic renal failure | 22 (1.8) | 2 (0.4) | 0.02 | 18 (1.2) | 6 (2.3) | 0.16 |
| Anemia | 12 (1.0) | 0 (0) | 0.02 | 11 (0.8) | 1 (0.4) | 0.51 |
| Depression/anxiety | 1 (0.9) | 8 (1.6) | 0.23 | 16 (1.1) | 3 (1.2) | |
| Heart failure | 202 (16.8) | 75 (14.9) | 0.31 | 213 (14.7) | 64 (25.0) | 0.001 |
| Arrhythmia | 29 (2.4) | 9 (1.8) | 0.42 | 32 (2.2) | 6 (2.3) | 0.89 |
| Pleurisy | 19 (1.6) | 6 (1.2) | 0.53 | 19 (1.3) | 6 (2.3) | 0.20 |
| Emboli | 60 (5.0) | 19 (3.8) | 0.27 | 63 (4.4) | 16 (6.2) | 0.18 |
| Pneumothorax | 1 (0.1) | 1 (0.2) | 0.52 | 1 (0.1) | 1 (0.4) | 0.16 |
| LTOT, n (%) | 605 (50.4) | 268 (53.2) | 0.11 | 743 (51.3) | 130 (50.8) | 0.88 |
| Laboratory findings | ||||||
| Leukocyte count, 109 L | 9.2 (7.2–11.6) | 9.7 (7.6–12.0) | 0.006 | 9.3 (7.3–11.7) | 9.4 (7.2–11.8) | 0.96 |
| Hematocrit, % | 37 (33.4–41.2) | 38.2 (34.0–41.7) | 0.013 | 37.7 (33.9–41.8) | 35.5 (31.4–39.0) | 0.001 |
| Platelet count, 109 L | 257 (208–323) | 258 (202–319) | 0.79 | 257 (208–321) | 257 (192–328) | 0.40 |
| Mean platelet volume, fL | 8.5 (7.9–9.2) | 8.4 (7.8–9.1) | 0.01 | 8.4 (7.8–9.2) | 8.5 (7.9–9.2) | 0.50 |
| Blood glucose, mg/dL | 121 (94–164) | 118 (93–155) | 0.21 | 120 (94–164) | 119 (94–155) | 0.61 |
| BUN, mg/dL | 26 (17–40) | 25 (17–38) | 0.25 | 25 (17–39) | 28 (19–50) | 0.001 |
| Serum creatinine, mg/dL | 0.8 (0.6–1.0) | 0.7 (0.6–0.9) | 0.012 | 0.8 (0.6–1.0) | 0.8 (0.6–1.1) | 0.48 |
| Sodium, mmol/L | 138 (136–140) | 138 (136–141) | 0.21 | 138 (136–140) | 138 (135–141) | 0.015 |
| Potassium, mmol/L | 4.2 (3.8–4.6) | 4.2 (3.8–4.6) | 0.19 | 4.2 (3.8–4.6) | 4.1 (3.8–4.6) | 0.39 |
| Albumin, g/dL | 3.0 (2.6–3.4) | 3.1 (2.7–3.4) | 0.1 | 3.1 (2.7–3.5) | 2.8 (2.7–3.1) | 0.001 |
| Inflammatory markers | ||||||
| CRP, mg/dL | 12.9 (5.0–32.1) | 10.4 (4.0–27.7) | 0.01 | 11.2 (4.3–27.9) | 18.9 (7.2–48.7) | 0.001 |
| Neutrophil-to-lymphocyte ratio | 4.9 (3.2–8.8) | 5.8 (3.5–10.4) | 0.004 | 4.9 (3.2–8.7) | 6.7 (4.2–12.3) | 0.001 |
| Platelet-to-mean platelet volume ratio | 30.0 (22.7–39.5) | 30.2 (23.2–39.1) | 0.79 | 30.2 (23.1–39.4) | 29.6 (21.8–40.0) | 0.41 |
| Steroid use, n (%) | 844 (70.3) | 469 (93.1) | 0.001 | 1,132 (78.2) | 181 (70.7) | 0.009 |
| Length of stay, days (%) | 7.0 (5.0–8.8) | 7.0 (5.5–9.0) | 0.03 | 7.0 (5.0–8.6) | 7.5 (6.0–10.5) | 0.00 |
Note: Median (25%–75%).
Abbreviations: BUN, blood urea nitrogen; LTOT, long-term oxygen therapy; SGOT, serum glutamic-oxalacetic transaminase; SGPT, serum glutamic-pyruvic transaminase.
When the relationship between the use of steroids and readmission with mortality was evaluated, the readmission rate was found to be significantly higher, while mortality was lower, in patients receiving steroids. However, there was no difference between the eosinophilic and non-eosinophilic groups.
Kaplan–Meier analysis showed that the survival rates of eosinophilic and non-eosinophilic COPD patients after hospital discharge were not significantly different (Figure 2).
Figure 2.
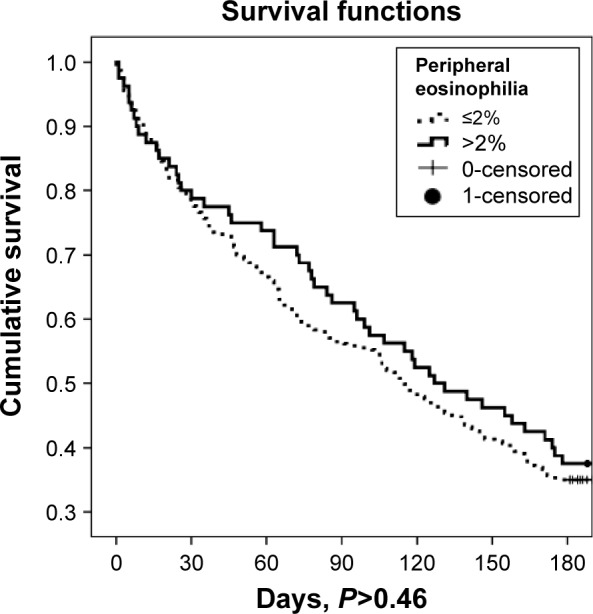
Eosinophilic and non-eosinophilic COPD patient survival after hospital discharge (days) as determined by Kaplan–Meier analysis.
Using the data shown in Table 3, we included eosinophilic or non-eosinophilic group, sex, heart failure, hypertension, steroid use, LTOT, CRP, NLR, length of hospital stay, and readmission within 6 months as parameters in the Cox regression model, and these risk factors are summarized in Table 4.
Table 4.
Cox regression analysis of survival after hospital discharge
| Variables | Odds ratio | 95% CI: lower–upper limit | P-value |
|---|---|---|---|
| Readmission | 1.93 | 1.45–2.57 | 0.001 |
| NLR equal and ≥7 at discharge | 1.79 | 1.37–2.34 | 0.001 |
| CRP >19 mg/dL at discharge | 1.32 | 1.01–1.71 | 0.035 |
| Presence of heart failure | 1.48 | 1.10–1.99 | 0.01 |
| Length of stay | 1.06 | 1.03–1.09 | 0.001 |
| Steroid use | 0.42 | 0.31–0.59 | 0.001 |
Abbreviations: CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio.
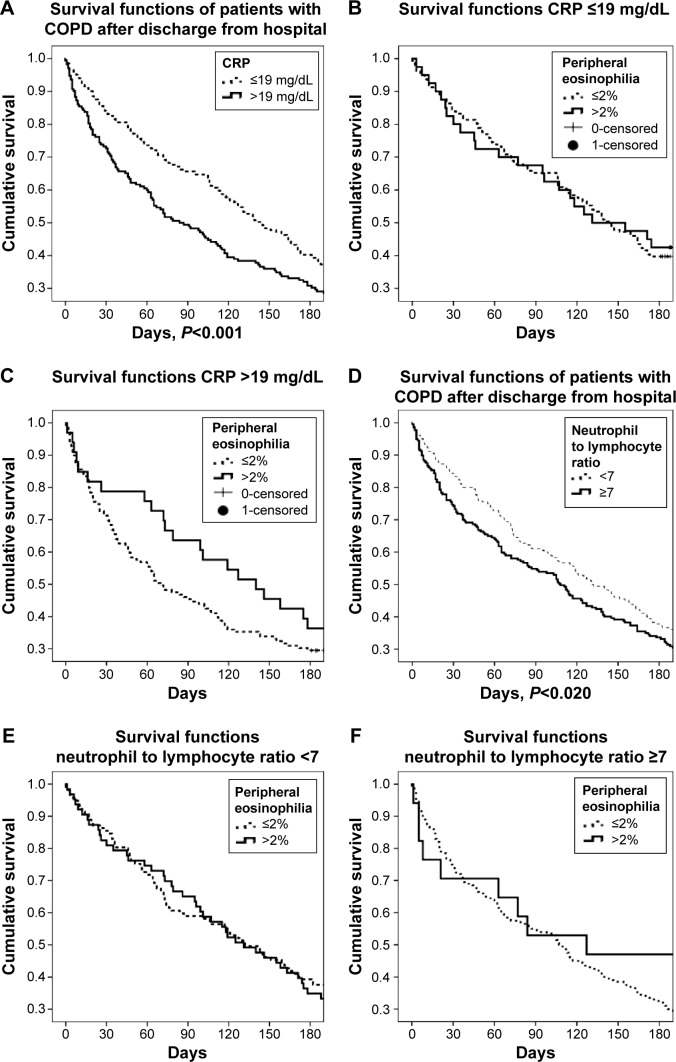
CRP and NLR values were further stratified according to the values in Table 3. Table 4 shows CRP and NLR as 19 mg/dL and 7, respectively. Kaplan–Meier survival curves show CRP and NLR categorized for 6 months survival for all patients (both the eosinophilic and non-eosinophilic groups). CRP >19 mg/dL and NLR ≥7 were negatively associated with longer survival, and both groups had similar survival curves. The association between CRP and NLR and survival is shown in Figure 3A–F.
Figure 3.
Survival functions of COPD patients according to CRP and NLR.
Notes: (A) CRP values above and below 19 mg/dL for 6-month survival after discharge. (B) CRP values >19 mg/dL and, (C) CRP ≤19 mg/dL had similar survival curves in both eosinophilic groups (eosinophils ≤2% and >2%) (P>0.36). (D) Kaplan–Meier survival curves show the neutrophil-to-lymphocyte ratio (NLR) <7 and ≥7 for all patients. (E) NLR ≥7 mg/dL and (F) NLR <7 had similar survival curves in both eosinophilic groups (eosinophils ≤2% and >2%) (P>0.98).
Discussion
Our findings provide novel information related to patient survival and identify new biomarkers that can predict the outcomes of COPD patients with eosinophilic or non-eosinophilic exacerbations after hospital discharge. In the present study, hospitalization length and readmission were found to be longer and higher in non-eosinophilic patients, respectively. The 6-month mortality in COPD patients after hospital discharge was positively associated with an NLR ≥7, CRP values >19 mg/dL, and instances of readmission. However, COPD patients in the eosinophilic and non-eosinophilic groups showed similar behavior with respect to the level of inflammatory markers.
PBE and COPD exacerbation
Although traditionally viewed as a neutrophilic inflammation, eosinophilic airway inflammation can be seen in 10% to 25% of COPD exacerbations.3,23,24 It has been shown that eosinophil numbers are increased in induced sputum and bronchial biopsies collected during exacerbations; however, PBE count is also a valid assessment. It offers an attractive biomarker for use in clinical practice as it is simple to measure, reliable, and widely available at the time of an exacerbation.7,11
The 2% threshold value is a sensitive marker of the presence of peripheral eosinophilia in COPD, as assessed by Bafadhel et al.8 Twenty percent of our patients were found to be eosinophilic. PBE was previously shown to be associated with an increase in all-cause mortality in patients with airway disease and the general population.25,26 However, we found similar mortality rates in hospitalized patients with eosinophilic or non-eosinophilic COPD exacerbations. PBE counts can be used to direct systemic corticosteroid treatment during COPD exacerbation.3,11,24 Targeting treatment to normalize sputum eosinophilia reduced the number of hospital admissions, and Saha and Brightling27 suggested that controlling eosinophilic inflammation could modify disease progression and alter mortality. Clinically relevant exacerbation phenotypes (eg, eosinophilic and bacterial) can be identified with biomarkers including PBE and CRP. Further studies are needed to validate the use of these biomarkers for optimizing therapies in COPD exacerbations.
Inflammatory biomarkers in hospitalized patients with COPD exacerbation
CRP is a well-known inflammatory biomarker that can be used to identify bacterial COPD exacerbation phenotypes and predict prognosis and adverse outcomes.3,28–31
We also found that CRP values were higher in non-eosinophilic COPD exacerbations; notably, a CRP value >19 mg/dL was associated with a 1.5-fold increase in mortality.
NLR has attracted attention as a new inflammatory marker in different diseases because it is quick and cheap; it can be easily measured in routine complete blood count analyses.6,12 However, NLR is a little-known marker for assessing inflammation in COPD patients.
Gunay et al reported that NLR was positively correlated with CRP levels in COPD patients and found higher NLR values during acute COPD exacerbation compared with both stable COPD and healthy controls, 4.2, 2.0, and 1.7, respectively.6 In this study, hospitalized patients with non-eosinophilic COPD exacerbations had two times higher NLR values than their eosinophilic counterparts. NLR was also correlated with CRP, and the Cox regression model showed that NLR (>7) was associated with a greater mortality rate in the 6 months follow-up.
MPV is another new inflammatory marker that has been investigated in different chronic diseases.18–20 Wang et al reported that COPD patients have lower MPV compared with healthy controls during exacerbation and in stable phases; moreover, they found that reduced MPV is positively related to white blood cell count and CRP levels in exacerbated COPD.14 Their findings suggest that decreased MPV values support increased systemic inflammation during COPD exacerbation.13 The present study is the first to assess MPV values in eosinophilic and non-eosinophilic exacerbations of COPD. We found that PLT/MPV was similar between non-eosinophilic and eosinophilic COPD and was not associated with mortality. However, we did find that a lower MPV value was associated with higher readmission rates.
Length of stay, readmission, and mortality of hospitalized patients with COPD exacerbation
Hospitalizations for COPD exacerbations have increased significantly over the past 10 years.32 In the USA, rates of hospital discharges from 2001 to 2012, emergency department visits from 2006 to 2011, and the 30-day readmission rate from 2009 to 2012 did not change significantly among patients with COPD. In contrast, significant reductions in length of stay were reported.33 The authors reported a decline in mean length of hospital stay from 4.5 days in 2001 to 4.0 days in 2012 among hospitalized COPD patients.33 In the current study, length of hospital stay was found to be longer in non-eosinophilic COPD exacerbations than eosinophilic ones.
In a study by Garcia-Aymerich et al, where COPD patients were followed-up for 1.1 year, 63% of patients were readmitted at least once, and 29% died.34 In another study, 54.8% of patients were readmitted at least once during the 1-year follow-up, and CRP was found to be an independent risk factor for predicting rehospitalization.35
In the present study, the readmission rate per patient per year was found to be higher in non-eosinophilic patients compared with the eosinophilic group, and instances of readmission within the 6-month follow-up also appeared to contribute to mortality.
Higher CRP, NLR, longer hospital stay, and steroid use during hospitalization were found to be factors affecting readmission. Steroid use and higher readmission rates can be explained in those patients with severe COPD. However, lower mortality rates with steroid use may suggest that steroids are still an important anti-inflammatory treatment option in COPD. Readmission was found to be significantly lower in eosinophilic patients receiving steroids where steroid use was the first choice of treatment. Further investigations are required to guide treatment management.
Coleta et al stated that the first-year mortality in a cohort of patients with very severe COPD and receiving LTOT was 15.4%.36 In a study by Gunen et al, half of all COPD patients hospitalized with acute exacerbations died within 3 years.5 Despite these values, which factors influence survival in hospitalized COPD patients with eosinophilic or non-eosinophilic exacerbations are not clear.
In our study, the 6-month mortality following hospitalized COPD exacerbation was 15% and there was no significant difference between the eosinophilic and non-eosinophilic group. Higher CRP, NLR, and instances of readmission were factors that negatively affected survival. Further studies are needed to evaluate outcomes in COPD patients with eosinophilic and non-eosinophilic exacerbations.
Limitations of this study are its retrospective nature and the fact that patients were treated at a single center. Another shortcoming is that COPD severity and spirometry results were not recorded because there were no objective diagnostic test results on file.
Nevertheless, the results from a large, disease-specific study population assessed at the largest chest disease teaching and research hospital in the region provide valuable clinical information for assessing biomarkers of mortality in hospitalized COPD patients.
Conclusion
Non-eosinophilic patients who experience COPD exacerbations have poorer outcomes than eosinophilic patients. The inflammatory markers CRP and NLR are more likely to be elevated in non-eosinophilic COPD exacerbations. CRP levels >19 mg/dL and NLR levels ≥7 are associated with increased risk of mortality, so these patients should be followed-up closely. NLR levels can be more easily obtained than CRP in clinical practice, and it is a cheaper, quicker test. PLT/MPV does not appear to be a good predictor of inflammation in COPD exacerbations. These findings support the theory that PBE with low CRP and NLR values are associated with noninfectious COPD attacks and they are useful biomarkers for guiding management of COPD exacerbations including steroid treatment. More studies on this issue are needed to clarify the utility of these biomarkers.
Acknowledgments
The authors thank Professor Ahmet Demir for his detailed statistical analysis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease [homepage on the Internet] Global strategy for diagnosis, management, and prevention of COPD [updated 2012] [Accessed October 5, 2015]. Available from: http://www.goldcopd.org.
- 2.Singanayam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. A systematic review and meta-analysis. Ann Am Thorac Soc. 2013;10(2):81–89. doi: 10.1513/AnnalsATS.201208-043OC. [DOI] [PubMed] [Google Scholar]
- 3.Brightling CE. Biomarkers that predict and guide therapy for exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(Suppl):S214–S219. doi: 10.1513/AnnalsATS.201302-023AW. [DOI] [PubMed] [Google Scholar]
- 4.Tertemiz KC, Kömüs N, Ellidokuz H, Sevinç C, Cımrın AH. Kronik obstrüktif akciğer hastalığında mortalite ve mortaliteyi etkileyen faktörler [Mortality and factors affecting mortality in chronic obstructive pulmonary disease] Tuberk Toraks. 2012;60(2):114–122. doi: 10.5578/tt.2889. Turkish. [DOI] [PubMed] [Google Scholar]
- 5.Gunen H, Hacievliyagil SS, Kosar F, et al. Factors affecting survival of hospitalized patients with COPD. Eur Respir J. 2005;26(2):234–241. doi: 10.1183/09031936.05.00024804. [DOI] [PubMed] [Google Scholar]
- 6.Gunay E, Sarınç Ulaşlı S, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation. 2014;37(2):374–830. doi: 10.1007/s10753-013-9749-1. [DOI] [PubMed] [Google Scholar]
- 7.Siva R, Gren RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29(5):906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 8.Bafadhel M, Davies L, Calverley PM, Aaron SD, Brightling CE, Pavord ID. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J. 2014;44(3):789–791. doi: 10.1183/09031936.00062614. [DOI] [PubMed] [Google Scholar]
- 9.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 10.Bafadhel M, McCormick M, Saha S, et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012;83(1):36–44. doi: 10.1159/000330667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iliaz S, Iliaz R, Ortakoylu G, Bahadir A, Bagci BA, Caglar E. Value of neutrophil/lymphocyte ratio in the differential diagnosis of sarcoidosis and tuberculosis. Ann Thorac Med. 2014;9(4):232–235. doi: 10.4103/1817-1737.140135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulasli SS, Ozyurek BA, Yilmaz EB, Ulubay G. Mean platelet volume as an inflammatory marker in acute exacerbation of chronic obstructive pulmonary disease. Pol Arch Med Wewn. 2012;122(6):284–290. doi: 10.20452/pamw.1284. [DOI] [PubMed] [Google Scholar]
- 14.Wang RT, Li JY, Cao ZG, Li Y. Mean platelet volume is decreased during an acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2013;18(8):1244–1248. doi: 10.1111/resp.12143. [DOI] [PubMed] [Google Scholar]
- 15.Cedrés S, Torrejon D, Martínez A, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14(11):864–869. doi: 10.1007/s12094-012-0872-5. [DOI] [PubMed] [Google Scholar]
- 16.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 17.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102(6):653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Sahin F, Yazar E, Yıldız P. Prominent features of platelet count, plateletcrit, mean platelet volume and platelet distribution width in pulmonary tuberculosis. Multidiscip Respir Med. 2012;7(1):38. doi: 10.1186/2049-6958-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisacik B, Tufan A, Kalyoncu U, et al. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine. 2008;75(3):291–294. doi: 10.1016/j.jbspin.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Jianan R, Han G, et al. Mean platelet volume: a controversial marker of disease activity in Crohn’s disease. Eur J Med Res. 2012;17:27. doi: 10.1186/2047-783X-17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anthonisen NR, Manfreda J, Warren CP, Hersfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Inter Med. 1987;106(6):196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 22.Mirsaeidi M, Peyrani P, Aliberti S, et al. Thrombocytopenia and thrombocytosis at time of hospitalization predict mortality in patients with community-acquired pneumonia. Chest. 2010;137(2):416–420. doi: 10.1378/chest.09-0998. [DOI] [PubMed] [Google Scholar]
- 23.Saetta M, Di Stefano A, Maestrelli P, et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 24.Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356(9240):1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 25.Hospers JJ, Schouten JP, Weiss ST, Postma DS, Rijcken B. Eosinophilia is associated with increased all-cause mortality after a follow-up of 30 years in a general population sample. Epidemiology. 2000;11(3):261–268. doi: 10.1097/00001648-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Ulric CS, Frederiksen J. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest. 1995;108(1):10–15. doi: 10.1378/chest.108.1.10. [DOI] [PubMed] [Google Scholar]
- 27.Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):39–47. doi: 10.2147/copd.2006.1.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174(8):867–874. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309(22):2353–2361. doi: 10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 30.Pinto-Plata VM, Müllerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61(1):23–28. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonescu-Turcu AL, Tomic R. C-reactive protein and copeptin: Prognostic predictors in chronic obstructive pulmonary disease exacerbations. Curr Opin Pulm Med. 2009;15(2):120–125. doi: 10.1097/MCP.0b013e3283218603. [DOI] [PubMed] [Google Scholar]
- 32.Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD. A systematic review and metaanalysis. Chest. 2008;133(3):756–766. doi: 10.1378/chest.07-1207. [DOI] [PubMed] [Google Scholar]
- 33.Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample. 2001–2012 and Nationwide Emergency Department Sample 2006–2011. Chest. 2015;147(4):989–998. doi: 10.1378/chest.14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Aymerich J, Farrero E, Felez MA, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–105. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jing Z, Chun C, Ning S, et al. Systemic inflammatory marker CRP was better predictor of readmission for AECOPD than sputum inflammatory markers. Arch Bronconeumol. 2015 May 20; doi: 10.1016/j.arbres.2015.01.011. Epub. [DOI] [PubMed] [Google Scholar]
- 36.Coleta KD, Silveira LV, Lima DF, Rampinelli EA, Godoy I, Godoy I. Predictors of first year survival in patients with advanced COPD treated using long-term oxygen therapy. Respir Med. 2008;102(4):512–518. doi: 10.1016/j.rmed.2007.12.003. [DOI] [PubMed] [Google Scholar]