Abstract
Electroretinogram (ERG) studies identified a new mouse line with a normal a-wave but lacking the b-wave component. The ERG phenotype of this new allele, nob7, matched closely that of mouse mutants for Grm6, Lrit3, Trpm1, and Nyx, which encode for proteins expressed in depolarizing bipolar cells (DBCs). To identify the underlying mutation, we first crossed nob7 mice with Grm6nob3 mutants and measured the ERGs in offspring. All the offspring lacked the b-wave, indicating that nob7 is a new allele for Grm6: Grm6nob7. Sequence analyses of Grm6nob7 cDNAs identified a 28 base pair insertion between exons 8 and 9, which would result in a frameshift mutation in the open reading frame that encodes the metabotropic glutamate receptor 6 (Grm6). Sequencing both the cDNA and genomic DNA from exon 8 and intron 8, respectively, from the Grm6nob7 mouse revealed a G to A transition at the last position in exon 8. This mutation disrupts splicing and the normal exon 8 is extended by 28 base pairs, because splicing occurs 28 base pairs downstream at a cryptic splice donor. Consistent with the impact of the resulting frameshift mutation, there is a loss of mGluR6 protein (encoded by Grm6) from the dendritic tips of DBCs in the Grm6nob7 retina. These results indicate that Grm6nob7 is a new model of the complete form of congenital stationary night blindness, a human condition that has been linked to mutations of GRM6.
Keywords: Congenital stationary night blindness, Electroretinogram, Metabotropic glutamate receptor 6
Introduction
Mutations in GRM6, which encodes the metabotropic glutamate receptor 6 (mGluR6), underlie one form of complete congenital stationary night blindness (cCSNB; Dryja et al., 2005; Zeitz et al., 2005). While rod-mediated vision is severely impaired in patients with cCSNB due to GRM6 mutations, their deficits in visual acuity or other measures of cone-mediated vision are more variable and may be normal (Dryja et al., 2005; Zeitz et al., 2005; Godara et al., 2012; Sergouniotis et al., 2012; Bijveld et al., 2013). mGluR6 is the glutamate receptor used by depolarizing bipolar cells (DBCs; Masu et al., 1995), and GRM6 mutations impair vision when DBCs can no longer modulate transient receptor potential melastatin 1 (TRPM1) channel activity in response to changes in extracellular glutamate (Morgans et al., 2010).
Our understanding of the impact of abnormal mGluR6 activity on retinal function has been expanding through the availability of mouse Grm6 mutants. Masu et al. (1995) characterized the retina of Grm6tmNak/tmNak mice, referred to here as Grm6−/−. They noted that retinal structure was intact, despite the loss of the electroretinogram (ERG) b-wave component, which reflects DBC activity (Kofuji et al., 2000). Two additional mutants were discovered when no b-wave (nob) mice were identified during screening of mice generated in a N-ethyl-N-nitrosourea mutagenesis program (Grm6nob3; Pinto et al., 2007) or mouse colonies at the Jackson Laboratory (Grm6nob4; Maddox et al., 2008). While normal retinal structure was observed in both Grm6nob3 (Pinto et al., 2007) and Grm6nob4 mice (Maddox et al., 2008), differences in receptive field abnormalities of retinal ganglion cells (RGCs) between the two mutants were noted. These studies indicate the value of mouse models of night-blinding disorders and of allelic series for further understanding human disease. Here, we report the discovery and initial characterization of a new nob mouse mutant (nob7), which involves a novel spontaneous mutation in Grm6.
Materials and methods
Mice
All procedures used in animal experiments were approved by the Institutional Animal Care & Use Committees of the institutions involved and conform to the principles regarding the care and use of animals adopted by the American Physiological Society and the Society for Neuroscience. Homozygous nob7 mice were identified in the course of ERG studies of C57BL/6J mice conducted at the National Eye Institute. After establishing that nob7 was inherited as an autosomal recessive trait, affected mice were transferred to Cleveland Clinic for further analysis including a cross to Grm6nob3 (nob3) mice (Maddox et al., 2008). C57BL/6J mice were used as controls.
Electroretinography
After overnight dark adaptation, mice were anesthetized (ketamine: 80 mg/kg; xylazine: 16 mg/kg), their pupils were dilated (1% tropicamide and 2.5% phenylephrine HCl eyedrops) and the corneal surface was anesthetized (1% proparacaine HCl). ERGs were recorded from the corneal surface using a stainless steel wire. Needle electrodes placed in the cheek and tail served as reference and ground leads, respectively. Responses to strobe light flashes were amplified (0.03–1000 Hz) and stored using an LKC (Gaithersburg, MD) UTAS E-3000 signal averaging system. ERGs were recorded under stimulus conditions that allow the response properties of rod- and cone-driven components to be defined using published protocols (Gregg et al., 2007).
Sequencing
Total RNA was isolated from retinas using TrIzol reagent as per the manufacturer’s protocol (Life Technologies, Carlsbad, CA). cDNA was synthesized from total RNA using the Superscript II reverse transcriptase as per the manufacturer’s protocol (GE Biosystems). The entire coding region of Grm6 was amplified with mGrm6-ex01F (5′-CAATGTCTTGCGCCTGTTTG-3′) and mGrm6-ex10R (5′-GTGGAGGTCTTCTTGAGGCT-3′), which spanned the open reading frame encoded by exons 2–10. The PCR fragment was cloned and then sequenced on an Applied Biosystems (Carlsbad, CA) 3130XL Sequencer (using a 50 cm array and POP7 polymer). The causative mutation was confirmed to be present in genomic DNA.
Immunohistochemistry
Dissected retinas were immersion fixed for 15 min in 4% (w/v) para-formaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB), then washed in PB, cryoprotected through a graded sucrose series, and frozen in OCT (Sakura Finetek, Torrence, CA):20% sucrose (2:1; Barthel & Raymond, 1990). 16-μm sections were cut on a cryostat, mounted onto Super-Frost glass slides, air-dried, and stored at −80°C.
Sections were brought to room temperature, washed in PB saline (PBS) for 5 min, PBX (PBS containing 0.5% (v/v) Triton X-100) for 5 min, and then incubated in a blocking solution (PBX containing 5% (v/v) normal goat serum) for 1 h. Primary antibodies were diluted in the blocking solution and incubated on retinal sections at room temperature overnight. Primary antibodies and dilutions used were: anti-mGluR6 (1:1000; Cao et al., 2011) and anti-CACNA1F (1:1000; BD Biosciences). After incubation with the primary antibody, sections were washed three times in PBS for 5 min each, and subsequently incubated with fluorescently labeled secondary antibodies (1:1000 in blocking solution) at room temperature for 1 h. Secondary antibodies were Alexa 488 goat anti-rabbit and Alexa 555 goat anti-mouse (Invitrogen, Carlsbad, CA). Slides were then washed three times in PBS and coverslipped with Immunomount (ThermoShandon, Pittsburgh, PA). Sections were imaged on an Olympus (Center Valley, PA) FV1000 confocal microscope, using a 60× oil objective (1.45 NA). Images shown are maximum projections of confocal stacks, adjusted for contrast and brightness with Fluoview software.
Results and discussion
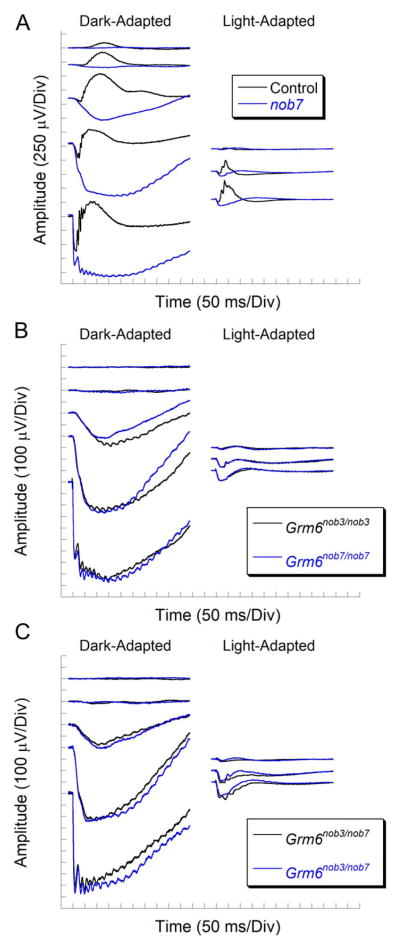
The nob7 mutant was discovered during ERG testing of mice anticipated to be wild type. Fig. 1 compares representative ERGs obtained from control and nob7 littermates. Under dark-adapted conditions (Fig. 1A, left), the control response is dominated by the cornea positive b-wave. In response to high luminance stimuli, the b-wave is preceded by the negative polarity a-wave, reflecting the light-induced closure of cation channels along rod photoreceptor outer segments (Penn & Hagins, 1969). In comparison, the b-wave component is absent from responses of nob7 mice, and the a-wave is followed by a negative polarity component, slow PIII, that is generated by Kir4.1 channel activity in Müller glial cells (Kofuji et al., 2000; Wu et al., 2004) and is normally masked by the larger amplitude b-wave (Samuels et al., 2010).
Fig. 1.
ERG characteristics of nob7 mice. (A) ERGs of nob7 mice lack the b-wave under dark-adapted (left) and light-adapted (right) conditions. (B) ERG waveforms of Grm6nob7 and Grm6nob3 mice have a comparable loss of the b-wave component. (C) Representative ERGs from two Grm6nob3/nob7 double heterozygotes.
Control and nob7 ERGs also differ under light-adapted conditions (Fig. 1A, right). Under these conditions, the ERG represents activity of the cone pathway (Peachey et al., 1993). The control cone ERG is dominated by the positive polarity b-wave and high frequency oscillatory potentials, both of which reflect activity through the DBC-pathway (Sharma et al., 2005; Shirato et al., 2008). In contrast, cone ERGs of nob7 mice are electronegative.
The nob7 ERG phenotype is inherited in an autosomal recessive fashion and is distinct from that of mouse models for proteins involved in presynaptic aspects of photoreceptor-to-bipolar synaptic transmission, where the b-wave is reduced but still present (Pardue & Peachey, 2014). Instead, the nob7 ERG phenotype closely matches that of mouse mutants for Trpm1 (Morgans et al., 2009; Koike et al., 2010; Peachey et al., 2012a), Nyx (Pardue et al., 1998), Grm6 (Masu et al., 1995; Pinto et al., 2007; Maddox et al., 2008), Gpr179 (Peachey et al., 2012b), and Lrit3 (Neuillé et al., 2014). Fig. 1B compares the responses obtained from nob7 and nob3 mice. There is an excellent agreement between the responses of the two mutants under both dark- (left) and light-adapted (right) conditions.
To identify the nob7 gene defect, we focused initially on genes expressed in DBCs. Given the close agreement between the ERG phenotypes of nob7 and nob3 mice, we crossed affected animals and examined the ERGs of compound heterozygote offspring. The ERG phenotype of nob3/nob7 compound heterozygous mice was indistinguishable from either parental line (compare Figs. 1C and 1B). This result demonstrates that the nob7 phenotype is caused by a mutation in the Grm6 gene, and the allele will be hereafter referred to as Grm6nob7.
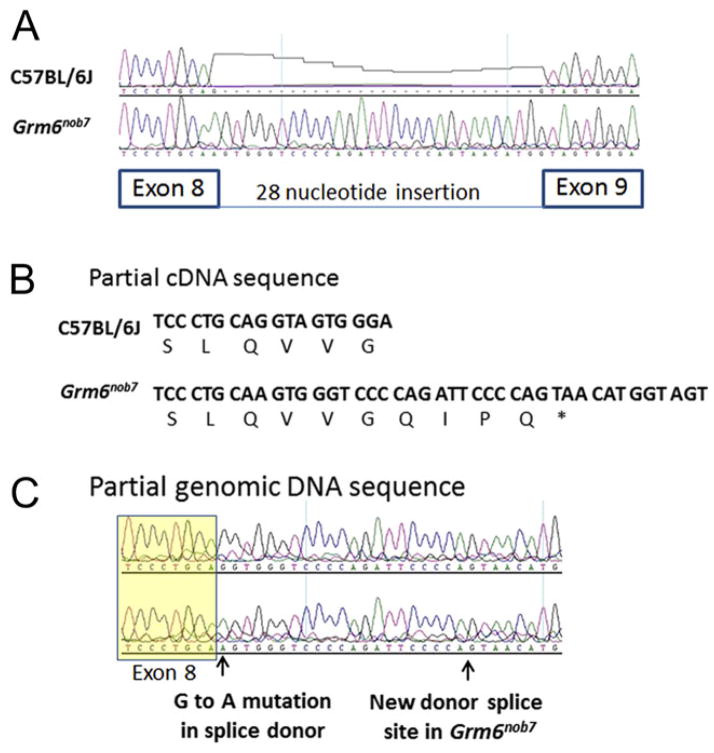
To identify the Grm6nob7 mutation, we used reverse transcription PCR to clone and sequence the cDNA fragments representing the open reading frame for mGluR6, from RNA isolated from control and Grm6nob7 retinas. Sequence analyses of the cDNA clones from the Grm6nob7 retinas (Fig. 2) identified an insertion between exons 8 and 9 of the normal transcript (ENSEMBLE Transcript ENSMUST00000000631). This insertion is predicted to cause a frameshift mutation, which will result in early termination and truncation of the mGluR6 protein. This truncation occurs at amino acid 701, which is located in predicted transmembrane domain IV (Nakajima et al., 1993). In addition, seven amino acids are added to the carboxy terminus that are encoded by the insertion and prior to a stop codon that occurs in the insertion. Given the loss of several transmembrane domains and the intracellular carboxy region, this truncation would not be functional and likely the mRNA or protein if produced would be rapidly degraded.
Fig. 2.
A splice site mutation causes the Grm6nob7 phenotype. (A) The control and Grm6nob7 cDNA and genomic sequences are shown aligned in the region of interest. The Grm6nob7 cDNA (B) and genomic DNA (C) have a G to A mutation at the last nucleotide of exon 8. This is a highly conserved base at splice sites and its mutation results in the use of a cryptic splice site 28 base pairs downstream (arrows). This frameshift truncates the normal protein at amino acid 701 and the intron insertion results in the addition of seven amino acids.
BLAST comparison (http://blast.ncbi.nlm.nih.gov/Blast.cgi) of the 28 base pair sequence inserted into the Grm6nob7 cDNA with the mouse genome database indicated that it was derived from the beginning of intron 8 of Grm6. Furthermore, the last base of exon 8, a G, was mutated to an A. This G is highly conserved at most splice donor sites because it establishes alignment with U1 snRNA during splicing. To determine if the mutation was present in the genomic DNA, we PCR-amplified fragments from genomic DNA isolated from control and Grm6nob7 mice using primers located in exon 8 and exon 9. As shown in the partial sequence of this fragment, the last base of exon 8 contains a G to A transition (Fig. 2C). Alignment of the Grm6nob7 cDNA with the genomic DNA shows that a cryptic splice 28 base pair downstream from the normal site at the end of exon 8 was used (Fig. 2C). We predict that this mutation, which creates a frameshift mutation truncating the normal protein by 170 amino acids, would result in a null allele.
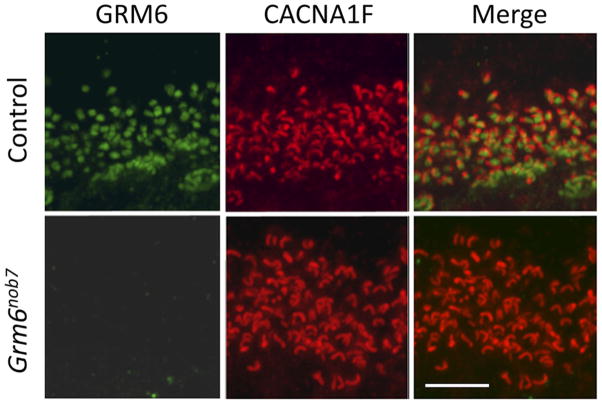
To evaluate the expression of mGluR6 on the dendritic tips of DBCs, we used immunohistochemistry and confocal microscopy. As shown in Fig. 3, mGluR6 is localized as discrete puncta in the OPL of control retinas, which are known to represent the dendritic tips of DBCs located in the outer plexiform layer (OPL). However, this staining is absent from the Grm6nob7 retina, confirming that the Grm6nob7 mutation results in a null allele. Null mutations also underlie the Grm6nob3 and Grm6nob4 models, in which more extensive anatomical studies reveal that overall retinal and synaptic structures are preserved (Pinto et al., 2007; Maddox et al., 2008). In contrast, immunohistochemical staining using an antibody to CACNA1F, a presynaptic marker (Schmitz et al., 2000), is comparable between control and Grm6nob7 retinal sections, indicative of preserved OPL structure.
Fig. 3.
Immunohistochemistry of control (top) and Grm6nob7 (bottom) retinas stained for mGluR6 (green) and CACNA1F (red). CACNA1F staining is comparable in both retinas and localizes to the outer plexiform layer where it opposes mGluR6 labeling in the control retina. In comparison, mGluR6 staining is not present in the Grm6nob7 retina. Scale bar indicates 10 μm.
The availability of animal models for human retinal diseases has facilitated the evaluation of experimental therapies for many conditions (Fletcher et al., 2011; Farrar et al., 2014), and the availability of allelic series provides resources with which to fully characterize a potential therapy. The Grm6nob7 mutant is a new model for cCSNB and will be useful in understanding this condition and evaluating experimental therapies for the GRM6 form of cCSNB as they become available.
Acknowledgments
We are grateful to Dr. Amy Lee for providing the antibody against CACNA1F. This research was supported by grants from the U.S. National Institutes of Health (R21EY021852 to N.S.P. and R.G.G.; R01EY12354 to R.G.G.), the U.S. Veterans Administration Medical Research Service, Hope for Vision, a Foundation Fighting Blindness Center Grant to the Cole Eye Institute, Cleveland Clinic, and unrestricted awards from Research to Prevent Blindness to the Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine and to the Department of Ophthalmology & Visual Sciences, University of Louisville.
References
- Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. Journal of Histochemistry & Cytochemistry. 1990;38:1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- Bijveld MC, Florijn RJ, Bergen ABB, van den Born LI, Kamermans M, Prick L, Riemslag FCC, van Schooneveld MJ, Kappers AML, van Genderen MM. Genotype and phenotype of 101 Dutch patients with congenital stationary night blindness. Ophthalmology. 2013;120:2072–2081. doi: 10.1016/j.ophtha.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Cao Y, Posokhova E, Martemyanov KA. TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. Journal of Neuroscience. 2011;31:11521–11526. doi: 10.1523/JNEUROSCI.1682-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Berson EL, Fishman GA, Sandberg MA, Alexander KR, Derlacki DJ, Rajagopalan AS. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4884–4889. doi: 10.1073/pnas.0501233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar GJ, Millington-Ward S, Chadderton N, Mansergh FC, Palfi A. Gene therapies for inherited retinal disorders. Visual Neuroscience. 2014;31:298–307. doi: 10.1017/S0952523814000133. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Jobling AI, Vessey KA, Luu C, Guymer RH, Baird PN. Animal models of retinal disease. Progress in Molecular Biology and Translational Science. 2011;100:211–286. doi: 10.1016/B978-0-12-384878-9.00006-6. [DOI] [PubMed] [Google Scholar]
- Godara P, Cooper RF, Sergouniotis PI, Diederichs MA, Streb MR, Genead MA, McAnany JJ, Webster AR, Moore AT, Dubis AM, Neitz M, Dubra A, Stone EM, Fishman GA, Han DP, Michaelides M, Carroll J. Assessing retinal structure in complete congenital stationary night blindness and Oguchi disease. American Journal of Ophthalmology. 2012;154:987–1001. doi: 10.1016/j.ajo.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Kamermans M, Klooster J, Lukasiewicz PD, Peachey NS, Vessey KA, McCall MA. Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. Journal of Neurophysiology. 2007;98:3023–3033. doi: 10.1152/jn.00608.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: Phenotypic impact in retina. Journal of Neuroscience. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox DM, Vessey KA, Yarbrough GL, Invergo BM, Cantrell DR, Inayat S, Balannik V, Hicks WL, Hawes NL, Byers S, Smith RS, Hurd R, Howell D, Gregg RG, Chang B, Naggert JK, Troy JB, Pinto LH, Nishina PM, McCall MA. Allelic variance between GRM6 mutants, Grm6nob3 and Grm6nob4 results in differences in retinal ganglion cell visual responses. Journal of Physiology. 2008;586:4409–4424. doi: 10.1113/jphysiol.2008.157289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, Takada M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Specific deficit of the ON response in visual transmission by targeted disruption of the mGIuR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Brown RL, Duvoisin RM. TRPM1: The end-point of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. BioEssays. 2010;32:609–614. doi: 10.1002/bies.200900198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. Journal of Biological Chemistry. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Neuillé M, El Shamieh S, Orhan E, Michiels C, Antonio A, Lancelot ME, Condroyer C, Bujakowska K, Poch O, Sahel JA, Audo I, Zeitz C. Lrit3 deficient mouse (nob6): A novel model of complete congenital stationary night blindness (cCSNB) PLoS One. 2014;9:e90342. doi: 10.1371/journal.pone.0090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS. A naturally-occurring mouse model of X-linked congenital stationary night blindness. Investigative Ophthalmology & Visual Science. 1998;39:2443–2449. [PubMed] [Google Scholar]
- Pardue MT, Peachey NS. Mouse b-wave mutants. Documenta Ophthalmologica. 2014;128:77–89. doi: 10.1007/s10633-013-9424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey NS, Goto Y, al-Ubaidi MR, Naash MI. Properties of the mouse cone-mediated electroretinogram during light adaptation. Neuroscience Letters. 1993;162:9–11. doi: 10.1016/0304-3940(93)90547-x. [DOI] [PubMed] [Google Scholar]
- Peachey NS, Pearring JN, Bojang P, Jr, Hirschtritt ME, Sturgill-Short G, Ray TA, Furukawa T, Koike C, Goldberg AF, Shen Y, McCall MA, Nawy S, Nishina PM, Gregg RG. Depolarizing bipolar cell dysfunction due to a Trpm1 point mutation. Journal of Neurophysiology. 2012a;108:2442–2451. doi: 10.1152/jn.00137.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey NS, Ray TA, Florijn R, Rowe LB, Sjoerdsma T, Contreras-Alcantara S, Baba K, Tosini G, Pozdeyev N, Iuvone PM, Bojang P, Jr, Pearring JN, Simonsz HJ, van Genderen M, Birch DG, Traboulsi EI, Dorfman A, Lopez I, Ren H, Goldberg AFX, Nishina PM, Lachapelle P, McCall MA, Koenekoop RK, Bergen AAB, Kamermans M, Gregg RG. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. American Journal of Human Genetics. 2012b;90:331–339. doi: 10.1016/j.ajhg.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969;223:201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Pinto LH, Vitaterna MH, Shimomura K, Siepka SM, Balannik V, McDearmon EL, Omura C, Lumayag S, Invergo BM, Glawe B, Cantrell DR, Inayat S, Olvera MA, Vessey KA, McCall MA, Maddox D, Morgans CW, Young B, Pletcher MT, Mullins RF, Troy JB, Takahashi JS. Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Visual Neuroscience. 2007;24:111–123. doi: 10.1017/S0952523807070149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Sturgill GM, Grossman GH, Rayborn ME, Hollyfield JG, Peachey NS. Light-evoked responses of the retinal pigment epithelium: Changes accompanying photoreceptor loss in the mouse. Journal of Neurophysiology. 2010;104:391–402. doi: 10.1152/jn.00088.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Königstorfer A, Südhof TC. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Sergouniotis PI, Robson AG, Li Z, Devery S, Holder GE, Moore AT, Webster AR. A phenotypic study of congenital stationary night blindness (CSNB) associated with mutations in the GRM6 gene. Acta Ophthalmologica. 2012;90:e192–e197. doi: 10.1111/j.1755-3768.2011.02267.x. [DOI] [PubMed] [Google Scholar]
- Sharma S, Ball SL, Peachey NS. Pharmacological studies of the mouse cone electroretinogram. Visual Neuroscience. 2005;22:631–636. doi: 10.1017/S0952523805225129. [DOI] [PubMed] [Google Scholar]
- Shirato S, Maeda H, Miura G, Frishman LJ. Postreceptoral contributions to the light-adapted ERG of mice lacking b-waves. Experimental Eye Research. 2008;86:914–928. doi: 10.1016/j.exer.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Marmorstein AD, Kofuji P, Peachey NS. Contribution of Kir4.1 to the mouse electroretinogram. Molecular Vision. 2004;10:650–654. [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, van Genderen M, Neidhardt J, Luhmann UF, Hoeben F, Forster U, Wycisk K, Mátyás G, Hoyng CB, Riemslag F, Meire F, Cremers FP, Berger W. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Investigative Ophthalmology & Visual Science. 2005;46:4328–4335. doi: 10.1167/iovs.05-0526. [DOI] [PubMed] [Google Scholar]