Abstract
Background
Periodontitis is a bacteria-induced inflammatory disease mainly affecting periodontal tissues, leading to periodontal inflammation, bone breakdown, and loss of the tooth. The main obstacle for treating periodontitis effectively is the difficulty in finding a target that can inhibit bone loss and inflammation simultaneously. Recent studies showed that cathepsin K (CTSK) might have functions in the immune system besides its role in osteoclasts. Thus, targeting CTSK would have a potential therapeutic effect in both the bone system and the immune system during the progression of periodontitis.
Methods
In the current study, a small molecular inhibitor (odanacatib [ODN]) is explored to inhibit the function of CTSK in a bacteria-induced periodontitis mouse model.
Results
The application of ODN decreased the number of osteoclasts, macrophages, and T cells, as well as the expression of Toll-like receptors (TLRs) in the periodontitis lesion area. Furthermore, lack of CTSK inhibited the expression of TLR4, TLR5, and TLR9 and their downstream cytokine signaling in the gingival epithelial cells in periodontitis lesions, demonstrating that the innate immune response was inhibited in periodontitis.
Conclusion
The present results show that inhibition of CTSK can prevent bone loss and the immune response during the progression of periodontitis, indicating that CTSK is a promising target for treating inflammatory diseases such as periodontitis by affecting both osteoclasts and the immune system.
Keywords: Bone resorption, cathepsin K, macrophages, osteoclasts, periodontal diseases, Toll-like receptors
Periodontitis lesions are caused by multiple bacterial infections of the gingival tissue followed by the periodontal tissue, which induce inflammation and uncontrolled immune response from the host in the oral lesion area.1,2 The pathogens of the bacteria mainly include lipopolysaccharide (LPS), flagellin, and CpG DNA, which will cause an innate immune response and the subsequent adaptive immune response.3 During this progress, the extensively activated T cells and osteoclasts (OCs) have been implicated to be involved in the production of proinflammatory cytokines and the activation of OCs.4
The current strategy in clinics to treat periodontitis is focused mainly on the local elimination of bacterial pathogens and controlling the inflammations, which still cannot target the bone loss and bacterial infection-induced periodontal inflammation and the consequential loss of alveolar bone and teeth. Cathepsin K (CTSK) is a peptidase C1 protein family member that is known to be a lysosomal cysteine protease.5,6 Furthermore, cathepsin family members are known to be crucial for the innate immune response.7,8 Studies demonstrated that CTSK is required for Toll-like receptor (TLR) 9 functions in dendritic cells, which play an essential role in innate recognition of microbial products and activation of defense responses.9 TLRs, which are an important part of the innate and adaptive immune system, have been proposed as driving the inflammation in the development of periodontitis.3,10,11 The research also led to the pivotal realization that CTSK also has significant functions in the immune system during periapical diseases.12,13
Based on recent research, there are still questions about whether CTSK has functions in activating antigen presentation cells, such as macrophages, which will lead to T-cell activation and the subsequent production of inflammatory cytokines.9,12 Therefore, the current investigation is applied with respect to the probability that CTSK has a critical role in the activation of macrophages and TLR-mediated immune responses in the pathogenesis of periodontitis lesions.
MATERIALS AND METHODS
Animals
Seven- to 8-week-old female wild-type (WT) BALB/cJ mice§ were used for introducing a periodontitis mouse model. Mice were divided into four groups: 1) a normal group with CTSK inhibitor (no bacterial infection; 3.606 mg · kg−1 · wk−1; n = 5 mice); 2) a normal group without CTSK inhibitor (no bacterial infection; n = 5 mice); 3) a bacterial infection group treated with CTSK inhibitor (3.606 mg · kg−1 · wk−1; n = 5 mice or 0.7212 mg · kg−1 · wk−1; n = 5 mice); and 4) a bacterial infection group without CTSK inhibitor (n = 5 mice). All experiments were repeated on three independent occasions, making the total 75 mice. The animals were maintained in the University of Alabama at Birmingham animal facility and were given distilled water and allowed to feed freely. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (protocol 131209236), Birmingham, Alabama.
Administration of CTSK Inhibitor
Male mice in the normal and periodontitis disease groups were treated with odanacatib (ODN) and compared to the untreated normal and disease groups. Treated mice were orally administered 3.606 or 0.7212 mg · kg−1 · wk−1 (five-fold lower dose) of pharmacologic grade ODN∥ in dimethylsulfoxide (DMSO) from 1 week before the establishment of the disease model to the end of sample harvest. The ODN dose was chosen to match the treatment of humans by converting to an equivalent dose for mice.14 The control mice were orally administered DMSO.
Infection With Bacterial Strains
The bacteria used in this study are Porphyromonas gingivalis W50 (American Type Culture Collection [ATCC] 53978), Treponema denticola (ATCC 35404), and Tannerella forsythia (ATCC 43037). These strains were grown under anaerobic conditions (80% N2, 10% H2, and 10% CO2) at 37°C in a anaerobic chamber¶ and were cultured.15,16 P. gingivalis W50 was grown for 3 days on CDC anaerobic 5% sheep-blood agar plates, and bacteria were scraped from the agar surface using sterile cotton swabs soaked and suspended in broth supplemented with hemin and vitamin K. T. forsythia was grown in trypticase soy agar II basal medium supplemented with yeast extract, phytone peptone, sheep blood (5%), and N-acetylmuramic acid (NAM) for 3 days, and bacteria were scraped from the agar surface and resuspended in NAM broth. T. denticola was grown in GM-1 broth for 48 to 72 hours, log-phase cultures were harvested by centrifugation at 9,000 × g for 15 minutes at 4°C, and the pellets were resuspended in broth. For oral mixed microbial infection, P. gingivalis W50 (3 × 1010 cells/mL), T. denticola (3 × 1010 cells/mL), and T. forsythia (3 × 1010 cells/mL) were mixed gently for 1 to 2 minutes and were allowed to interact for an additional 5 minutes for any interactions among these species. An equal volume of sterile 2% (weight/volume) carboxymethyl cellulose was added and mixed thoroughly, and 100 μL (5 × 109 cells/mL for P. gingivalis, 5 × 109 cells/mL for T. denticola and T. forsythia) was administered by oral and anal topical application eight consecutive times according to a previously described protocol with modification.17,18
Harvest and Preparation of Samples
Animals were sacrificed by CO2 inhalation at 56 days after the initial infection. For bone resorption measurements, maxillae samples from the left side were defleshed and stained with 1% methylene blue and mounted on microscope slides for bone loss measurements. Maxillae samples from the right side were fixed immediately in 4% formaldehyde and prepared for histologic analysis according to standard protocols.17 In brief, samples for paraffin sections were fixed in 4% formaldehyde for 24 hours, washed with 1× phosphate-buffered saline (PBS), decalcified in 10% EDTA in 0.1 M Tris solution (pH 7.0) for 20 days (replenished each day), washed with 1× PBS three times, and embedded in paraffin after series dehydration. Mandibular samples from the right side in every independent experiment were obtained for real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analyses, and samples from the left side were obtained for enzyme-linked immunosorbent assays (ELISAs).
Periodontal Bone Loss Measurement
The protocol for imaging was performed as described previously.17,19 Briefly, images of molar tooth roots and alveolar bone were captured using digital microscopy. The area of periodontal bone loss, including the cemento-enamel junction (CEJ), the lateral margins of the exposed tooth root, and the alveolar ridge, was determined using image analysis software.# In addition, the polygonal area was measured using the image analysis software.** Measurements were expressed in square millimeters by converting pixels to millimeters. For the hematoxylin and eosin (H&E) coronal section, the length between the CEJ to the alveolar ridge has been measured as described previously.20
Immunofluorescence Analyses
Immunofluorescence (IFC) analysis was performed as described previously,21-23 with the exception that rat polyclonal anti-cluster of differentiation 3 (CD3)†† was used as the primary antibody, and observations were performed by epifluorescence in a microscope.‡‡ Nuclei were visualized with 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI).§§ The experiments were set in triplicate on three independent occasions.
Immunohistochemistry Analyses
Immunohistochemistry (IHC) was performed using corresponding sections of mandibular tissue from the normal group (with or without inhibitor) and the disease group (with or without inhibitor), and then the slides were analyzed by IHC for expression and localization of proteins of the macrophage marker F4/80∥∥ (rat monoclonal, 1:200), TLR4¶¶ (goat polyclonal, 1:200), TLR5## (rabbit polyclonal, 1:200), and TLR9*** (rabbit monoclonal, 1:500). An avidin–biotin complex kit for anti-rat and rabbit immunoglobulin G peroxidase polymer detection systems along with a 3,3′-diaminobenzidine kit††† as a substrate were used for the peroxidase-mediated reaction. The experiments were set in triplicate on three independent occasions.
RNA Extraction and Real-Time qRT-PCR
For RNA extraction, the samples were transferred to the tube prefilled with beads‡‡‡ and homogenized using a blender.§§§ The RNA extraction was then applied to the standard procedure using a reagent.∥∥∥ The extracted RNA was reversed using a synthesis kit.¶¶¶ Real-time qPCR was performed as described previously12,13 using primers as listed in supplementary Table 1 in online Journal of Periodontology. Briefly, cDNA fragments were amplified by PCR master mix### and detected by a real-time PCR system.**** The mRNA expression level of the housekeeping gene β-actin was used as an endogenous control and enabled the calculation of specific mRNA expression levels as a ratio of β-actin. Experiments were repeated at least three times.
Protein Extraction and Cytokine for ELISA
For protein extraction, the frozen periodontitis tissue samples were transferred to a tube prefilled with beads†††† and homogenized using a blender.‡‡‡‡ The tissue fragments were dispersed in 300 μL lysis buffer. The mixture was centrifuged at 12,000 rpm for 10 minutes, and the supernatant was collected and stored at −80°C until the assay. An ELISA was used as described previously24,25 to evaluate the effect of inhibition of CTSK on the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-23 in inflammatory periodontitis tissues extracted from the normal group (with or without inhibitor) and the disease group (with or without inhibitor). Briefly, assays for cytokines in extracts used commercially available ELISA kits: TNF-α,§§§§ IL-6,∥∥∥∥ and IL-23.¶¶¶¶ All assays were conducted in accordance with the instructions of the manufacturers. Results were expressed as picograms of cytokine per milliliters of tissue.
Statistical and Data Quantification Analyses
Experimental data are reported as mean ± SD of triplicate independent samples. All experiments were performed in triplicate on three independent occasions. The figures are representative of the data. Data were analyzed with the two-tailed Student t test. Mann-Whitney U test was used for the non-parametric test. P values <0.05 or U values >1.96 were considered significant. Data quantification analyses were performed using an imaging analysis program#### as described previously.12,17,26
RESULTS
Inhibition of CTSK by a Specific Small-Molecule Inhibitor Showed Promising Bone-Protective Effects
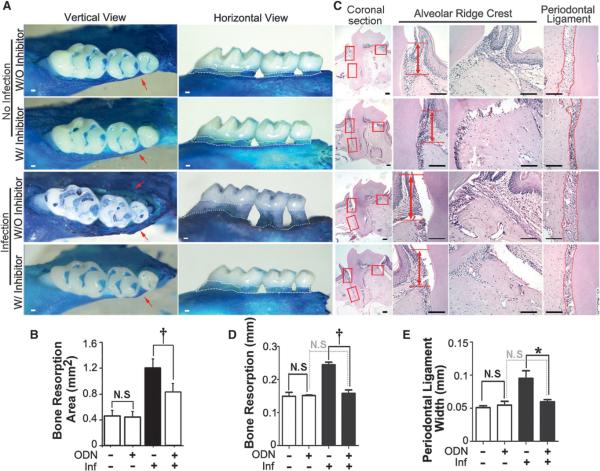
The periodontitis mouse model established by a previous study was used.17 Vertical observation of methylene blue stain showed that there was no obvious vertical bone resorption in the WT and inhibitor-treated negative control groups. Bone resorption was significant in the WT periodontitis group but not in the inhibitor-treated periodontitis group (Fig. 1A, red arrows). In the horizontal view of Figure 1A, the quantified data showed that bone resorption was obvious in the WT periodontitis group compared to the inhibitor-treated periodontitis group (Fig. 1B, white dotted area). The H&E histologic analysis showed that the alveolar bone resorption, alveolar bone loss between the CEJ and the bone crest, and the width of the periodontal ligament (PDL) revealed that each measurement was significantly higher in the WT periodontitis group than in the inhibitor-treated periodontitis group (Fig. 1C). The results showed that the inhibition of CTSK can protect the periodontitis group from bone loss (Figs. 1D and 1E). The different doses of CTSK inhibitor, 3.606 mg · kg−1 · wk−1 or five-fold lower at 0.7212 mg · kg−1 · wk−1, had a dose dependent bone-protective effect by methylene blue analysis (see supplementary Fig. 1 in online Journal of Periodontology).
Figure 1.
Inhibition of CTSK bya small-molecule inhibitor showed bone-protective effects in the periodontitis lesion area. A) Methylene blue staining of the maxilla tooth of the normal and disease groups with and without ODN (3.606 mg · kg−1 · wk−1) treatment. Red arrows indicate vertical bone resorption. White dotted areas indicate horizontal bone resorption. B) Quantification of the alveolar bone resorption area in the horizontal view of A. C) H&E staining of the periodontal tissue of different groups. The H&E stain showed increased bone breakdown in the WT disease group (red arrows). Columns 2 to 4 are enlarged images of the boxed areas in column 1 (magnification ×20). D and E) Quantification of bone resorption length and PDL width of C. W/ = with; W/O = without; Inf = infection; N.S. = not significant. * P <0.01,† P <0.001. n = 5, repeated three times. Scale bars = 100 μm.
Inhibition of CTSK Decreased OC Numbers and May Be Involved in the Immune Response in the Periodontitis Lesion Area
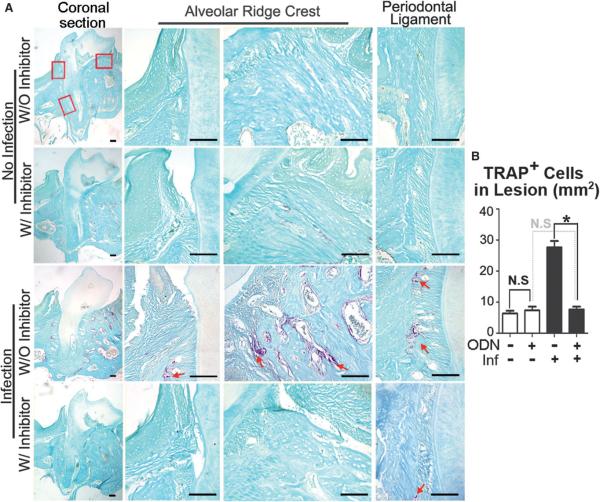
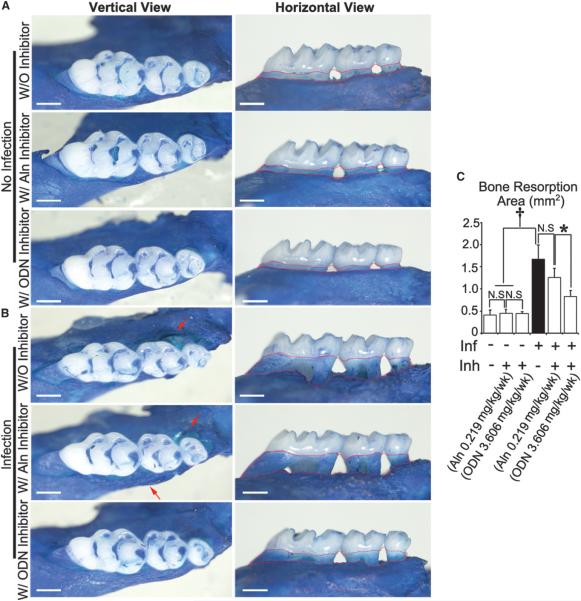
It was found that the number of OCs decreased in the disease group with inhibitor (3.606 mg · kg−1 · wk−1) compared to the disease group without inhibitor by applying tartrate-resistant acid phosphatase (TRAP) staining (Fig. 2). The distribution of OCs in the WT periodontitis group was around the tooth in alveolar bone and PDL area (red arrows). In the inhibitor-treated group, visible OCs can hardly be found in the periodontitis lesion area, which also indicates that CTSK has an important function in the immune system. To prove the hypothesis, the effect of bisphosphonate (alendronate [Aln]) and the CTSK-specific small-molecule inhibitor ODN was compared in the same periodontitis mouse model (Figs. 3A and 3B). The results showed that bone loss cannot be inhibited by Aln, but administration of ODN can significantly protect against bone loss (Fig. 3C). The TRAP stain results showed that the OC numbers in the Aln-treated disease group increased significantly compared to the ODN-treated group (see supplementary Fig. 2 in online Journal of Periodontology). This indicated strongly that CTSK has critical functions not only in OCs but also in immune cell–mediated OC activation.
Figure 2.
Inhibition of CTSK showed reduced TRAP-positive cells in the periodontitis lesion area. A) TRAP staining of the periodontal tissue area sections in different groups with and without ODN treatment. Columns 2 to 4 are enlarged images of the boxed areas in column 1 (magnification ×20). Red arrows indicate TRAP-positive cells. B) Quantification of TRAP-positive cell numbers in the lesion areas of different groups. W/ = with; W/O = without; Inf = infection; N.S. = not significant. * P <0.01. n = 5, repeated three times. Scale bars = 100 μm.
Figure 3.
Inhibition of CTSK by different inhibitors (Aln and ODN) demonstrated different bone-protective effects in the periodontitis lesion area. A) Methylene blue staining of the maxilla tooth of uninfected WT mice with or without bisphosphonate (Aln;0.219mg·kg−1·wk−1) or ODN (3.606mg·kg−1·wk−1) treatment at 8 weeks. Red dotted areas indicate horizontal bone loss. B) Methylene blue staining of the maxilla tooth from infected WT mice with or without bisphosphonate (0.219 mg · kg−1 · wk−1) or ODN (3.606 mg · kg−1 · wk−1) treatment at 8 weeks. Red arrows indicate vertical bone resorption. C) Quantification of bone resorption area in different groups. Inf = infection; Inh = inhibitor; N.S. = not significant. * P <0.05, † P <0.001. n = 5, repeated three times. Scale bars = 500 μm.
CTSK May Have Effects on Innate Immune Responses and Immune Cells at the Periodontitis Lesion Area
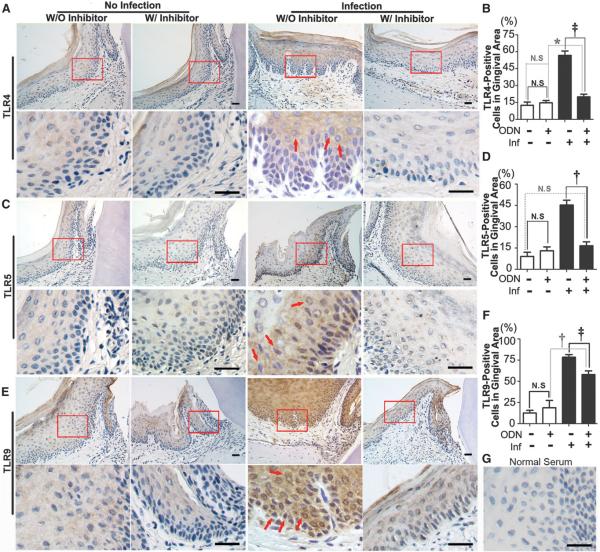
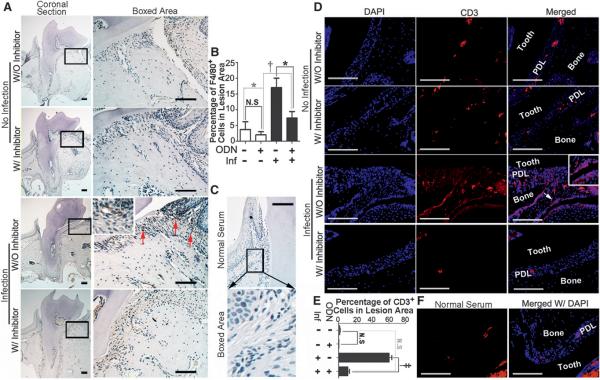
TLRs are important for the innate immune response. As a first line of defense against bacterial infections, TLRs have naturally become the most important candidate targets. In bacterial-mediated inflammation, TLR4 recognizes LPS, TLR5 recognizes flagellin, and TLR9 recognizes bacterial DNA and CpG oligodeoxynucleotide, which are critical antigens that cause an immune response.3 To explore whether these TLRs are involved in the development of periodontitis and to determine whether CTSK has an effect on the immune response during the progression of periodontitis, IHC staining was performed on the periodontitis lesion areas of different groups at 56 days (Fig. 4). A significant increase of TLR4-positive, TLR5-positive, and TLR9-positive cells was observed in the periodontitis group compared to the inhibitor-treated disease group (Figs. 4B, 4D, and 4F). More interestingly, the IHC stain of F4/80-positive macrophages decreased significantly in the inhibitor-treated periodontitis group. To further confirm the findings, CD3 IFC stain was applied to examine T-cell activation. T lymphocytes are important immune effector cells that can be divided into cytotoxic T lymphocytes, helper T lymphocytes, and suppressor T lymphocytes. The cosurface marker of lymphocytes, CD3, can be used to detect activated T lymphocytes in inflammation.27 The results showed that CD3-positive T cells decreased significantly in the lesion areas in the inhibitor-treated periodontitis group. These results indicated that inhibition of CTSK may affect the immune response that occurs in periodontitis lesion areas (Figs. 4 and 5).
Figure 4.
Inhibition of CTSK reduced TLR4-, TLR5-, and TLR9-positive cells in the periodontal gingival lesion area. A through F) IHC stains and quantification of TLR4-positive (A and B), TLR5-positive (C and D), and TLR9-positive (brown) (E and F) cells indifferent groups with or without ODN (3.606 mg · kg−1 · wk−1) treatment at 8 weeks. Red arrows indicate positive cells. Bottom rows are enlarged images of the boxed areas in the lesion area (magnification ×40). G) Normal serum served as a negative control. W/ = with; W/O = without; Inf = infection; N.S. = not significant. * P <0.05, † P <0.01, ‡ P <0.001. n = 5, repeated three times. Scale bars = 25 μm.
Figure 5.
Inhibition of CTSK showed reduced F4/80-positive and CD3-positive cells in the periodontitis lesion area. A) IHC stains of F4/80-positive (brown) macrophages in the periodontitis lesion areas of different groups treated with ODN (3.606 mg · kg−1 · wk−1) and without inhibitor at 8 weeks. Red arrows show positive cells. B) Quantification of F4/80-positive cell numbers in the PDL area of the different groups. C) Normal serum served as negative control. D) IFC staining of CD3-positive (red) T cells in the periodontitis lesion area in the different groups. White arrow and white inset box of the enlarged image show positive cells (magnification ×40). E) Quantification of CD3-positive cell analysis. F) Normal serum served as a negative control. W/ = with; W/O = without; Inf = infection; N.S. = not significant. * P <0.05, † P <0.01, ‡ P <0.001. n = 5, repeated three times. Scale bars = 100 μm.
Inhibition of CTSK Reduced the Expression of Proinflammatory Genes and TLRs and Cytokines in the Periodontitis Lesion
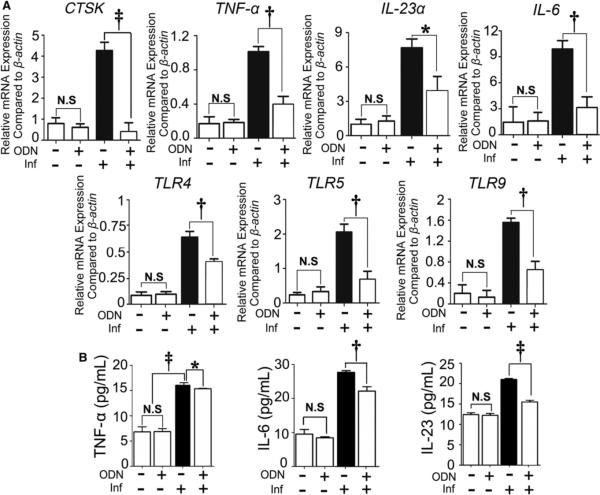
The expression of CTSK was determined to confirm whether OC function had been affected. To evaluate the effect of CTSK inhibition on the mRNA levels of TNF-α, IL-6, and IL-23α, which are related to inflammatory and OC status in periodontitis tissues, qRT-PCR was used as described previously.12 The relative mRNA expression levels showed that the expression of CTSK and proinflammatory gene expression increased in the disease group at 56 days (Fig. 6A). For the TLR gene expression, TLR4, TLR5 and TLR9 all increased significantly in the disease group (Fig. 6A), which was consistent with the IHC result (Fig. 4). The ELISA result showed that TNF-α, IL-6, and IL-23 increased significantly in the disease group without inhibitor at 56 days (Fig. 6B).
Figure 6.
Inhibition of CTSK by a specific inhibitor reduced the expression of proinflammatory genes, as well as TLR genes and cytokines in the periodontitis lesion. A) qRT-PCR of OC-specific genes (i.e., CTSK), proinflammatory genes (i.e., TNF-α, IL-23α, and IL-6), and TLR genes (i.e., TLR4, TLR5, and TLR9) in the different groups. b-Actin was used as an endogenous control. B) Expression of TNF-α, IL-6, and IL-23 in the periodontitis lesion detected by ELISA. Significance was compared between the disease group with and without inhibitor. The black bars represent the group with periodontitis disease without inhibitor treatment. Inf = infection; N.S. = not significant. * P <0.05, † P <0.01, ‡ P <0.001. n = 3, repeated three times.
DISCUSSION
In the current study, it is reported that CTSK may play a key role in mediating periodontitis by attenuating the innate TLRs’ response and the subsequent adaptive immune response. To support this, a bacterial-induced periodontitis model treated with a CTSK-specific small-molecule inhibitor (ODN) was used. It was demonstrated that inhibition of CTSK decreased the number of macrophages, OCs, and TLR4-, TLR5-, and TLR9-positive cells in the periodontitis lesion area. In light of these findings and a previous report12 showing that CTSK has important roles in periapical disease, it is plausible that CTSK is required for immune cells to mediate the immune response in periodontitis.
Periodontal diseases result from gingivitis induced by microorganism infection. The infection induces an inflammatory response first within the gingival tissue and subsequently in the PDL region after egress of bacterial pathogens, their byproducts, and/or altered periodontal tissues from the continuous infected gingival tissue. The lesion development is characterized by sustained and prolonged cellular activation in periodontitis lesion areas and often leads to tissue damage and chronic inflammation rather than repair.28 Consequently, the question faced by researchers and clinics is to control an excessive inflammatory response in the inflammatory process, while reserving corresponding alveolar bone effectively. CTSK, a lysosomal cysteine protease, is expressed extensively in OCs. It has been shown in early studies that CTSK is responsible for matrix protein (such as collagen Type I) degradation during normal bone metabolism, and its mutation may cause severe pycondysostosis, which is also proved to be upregulated by CCAAT-enhancer binding protein α in recent studies.5,23 CTSK has been shown to have a close relation with TLR9 in the immune response, which indicated that it also participates in the inflammation process.9 Beklen et al.29 showed that CTSK was expressed in the macrophage-like cells, fibroblast-like cells, vascular endothelial cells, and gingival epithelial cells in PDLs and gingival tissues. Previous studies9,30,31 showed that CTSK also has important functions in immune response. ODN is a selective inhibitor of the cysteine protease CTSK.32 ODN inhibits OC digestion function but does not reduce the number of OCs.33 However, whether inhibition of CTSK can affect not only OCs but also the immune response is still unclear. To investigate the mechanism of the effect of CTSK inhibition in vivo, TRAP staining was applied. A significant bone-protective effect and decrease in OCs in the ODN-treated periodontitis groups was found (Figs. 1 and 2). To determine the proper treatment dose for periodontal diseases, a dose experiment was performed to treat the periodontitis, which showed dose-dependent bone-protective effects (see supplementary Fig. 1 in online Journal of Periodontology). Furthermore, administration of bisphosphonate (Aln) caused severe bone breakdown and an increase of OC numbers stimulated by bacteria (Fig. 3) (see supplementary Fig. 2 in online Journal of Periodontology). Yanming et al.34 showed that administration of bisphosphonates results in more OCs and the formation of giant OC-like cells within the alveolar bone, which will cause osteonecrosis in the jaw area. Studies have indicated that the activation of OCs is not only dependent on osteoblasts but is also closely related to the immune system.35 Notably, myeloid progenitors for OCs can also differentiate to dendritic cells and macrophages.36,37 Previous studies and the current results suggest that CTSK may mediate periodontitis bone breakdown not only through its known function in OCs but also through immune cell-mediated activation of OCs.
Previous studies showed that pathogen-associated molecular patterns can directly regulate OCs via OC TLR. Activation of TLR in osteoblasts or stromal cells stimulates production of osteoclastogenic cytokines, such as receptor activator of nuclear factor-kappa B ligand and TNF-α.38 TLRs in gingival epithelial cells can increase the attachment and migration of immune cells toward antigen and also induce the production of proinflammatory cytokines.3 Importantly, the present study provides new insights into the possible connections between CTSK and the immune response in periodontitis. In the periodontitis mouse model, inhibition of CTSK resulted in significant decreases in TLR4, TLR5, and TLR9 (Fig. 4). Activated macrophages are indicated as the source of bone-resorbing cytokines in periodontal diseases,39,40 which can be activated by T lymphocytes or bacterial endotoxin. To fully demonstrate the function of CTSK in the immune response to periodontal diseases, the present study examines whether inhibition of CTSK affects the expression of macrophages by detecting the pan-marker F4/80 (Fig. 5A). The results showed that the number of macrophages increased significantly in the periodontitis group without inhibitor at 56 days but not in the inhibitor-treated group which indicated that CTSK may play an important role in both OCs and immune responses (Figs. 5A and 5B). As the effecter cells of inflammation, T cells can produce a variety of effecter molecules.41,42 Therefore, the number of T cells is an important indicator of the severity of the local inflammatory response. As a T-cell surface marker, CD3 is commonly used to detect T-cell populations, including αβ T cells and γδ T cells. The results showed that the number of CD3-positive T cells was reduced significantly in the inhibitor-treated periodontitis disease group (Figs. 5D and 5E). T cells showed aggregated distribution in the periodontitis lesion area, whereas the inhibitor-treated periodontitis group only showed minor T-cell infiltration, which indicated that the immune system–mediated inflammatory reaction is suppressed in CTSK-inhibited disease groups. T cells and macrophages decreased, which might result in a lower proinflammatory cytokine production, and subsequently the lower OC might be activated and result in less bone loss.
ELISA and qRT-PCR results confirmed the in vivo IHC stain in periodontitis lesion tissues. The expression level of proinflammatory maker genes, including TNF-α, IL-23α, and IL-6, increased significantly in the periodontal disease group at day 56 compared to that in the inhibitor-treated disease group (Fig. 6A). These results underscore the possibility that future therapies could block CTSK in periodontitis by specific small-molecule inhibitors or gene therapy. CTSK inhibition was also confirmed by qRT-PCR (Fig. 6A). Because TLRs are important in the immune response, TLR4, TLR5, and TLR9 are selected as the research objects in this experiment to detect the changes in periodontitis lesion area. In the current study, it is shown that TLR4, TLR5, and TLR9 all decreased in the inhibitor-treated disease group compared to the disease group. Beklen et al.10 showed that, except for TLR7 and TLR8, expression levels of TLR1 through TLR10 in human gingival surfaces are higher in individuals with periodontitis than in healthy individuals. The current view is that cathepsins make TLRs form a correct protein structure through proteolytic processing, thereby initiating the antigen recognition process.43,44 However, it is still unclear how cathepsins perform the corresponding processes that hydrolyze the specific TLR. The protein level expression of cytokines was also detected, including TNF-α, IL-6, and IL-23a, and all increased in the periodontitis disease group compared to the inhibitor-treated disease group (Fig. 6B). These cytokines are closely related to TLR activation and the subsequent immune response and the OC activation.45 The proinflammatory cytokines, such as TNF-α, have been reported to have a key role in regulating the inflammatory response.46 IL-6 is produced at the site of inflammation and also plays a key role in the acute-phase response, which is also shown by the qRT-PCR results (Fig. 6A).47
CONCLUSIONS
The possible mechanism of the dual effect of CTSK in targeting OCs and immune cells was explored. The inhibition of CTSK reduced bone loss by inhibition of both the OC function and inflammation, which in turn decreased OC activation and differentiation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Ms. Christie Paulson and Ms. Suzy Newton from the Department of Pathology at the University of Alabama at Birmingham for their excellent assistance with this manuscript. The authors also thank the Center for Metabolic Bone Disease (National Institutes of Health [NIH] Grant P30 AR046031), the Small Animal Phenotyping Core (NIH Grant P30 DK079626), the Metabolism Core, and the Neuroscience Molecular Detection Core Laboratory (NIH Grant P30 NS0474666), all at the University of Alabama at Birmingham. This work was supported by NIH Grant R01DE023813(YPL) and UAB Department of Pathology Start-Up funding (WC). NIH manufactured the image analysis software used in this study. LH and JC contributed equally to this manuscript.
Footnotes
The Jackson Laboratory, Bar Harbor, ME.
Odanacatib, Selleck Chem, Houston, TX.
Coy Laboratory Products, Grass Lake, MI.
Adobe Photoshop, San Jose, CA.
NIH ImageJ, National Institutes of Health, Bethesda, MD
Abcam, Cambridge, MA.
Zeiss Axioplan, Carl Zeiss Microscopy, Thornwood, NY.
Sigma-Aldrich, St. Louis, MO.
eBioscience, San Diego, CA.
eBioscience.
Santa Cruz Biotechnology, Dallas, TX.
Sigma-Aldrich.
VECTASTAIN Elite ABC kit for anti-rat and rabbit IgG peroxidase polymer detection systems with a DAB substrate kit, Vector Laboratories, Burlingame, CA.
Next Advance, Averill Park, NY.
Next Advance.
TRIzol reagent, Invitrogen, Thermo Fisher Scientific, Waltham, MA.
VILO master kit, Invitrogen, Thermo Fisher Scientific.
SYBR Green Fast Advanced Master Mix, Invitrogen, Thermo Fisher Scientific.
StepOne Plus Real-Time PCR System, Invitrogen, Thermo Fisher Scientific.
Next Advance.
Next Advance.
eBioscience.
eBioscience
Biolegend, San Diego, CA.
NIH ImageJ, National Institutes of Health.
REFERENCES
- 1.Ertugrul AS, Arslan U, Dursun R, Hakki SS. Periodontopathogen profile of healthy and oral lichen planus patients with gingivitis or periodontitis. Int J Oral Sci. 2013;5:92–97. doi: 10.1038/ijos.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Wen YM. The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci. 2011;3:66–73. doi: 10.4248/IJOS11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hans M, Hans VM. Toll-like receptors and their dual role in periodontitis: A review. J Oral Sci. 2011;53:263–271. doi: 10.2334/josnusd.53.263. [DOI] [PubMed] [Google Scholar]
- 4.Nowak M, Kramer B, Haupt M, et al. Activation of invariant NK T cells in periodontitis lesions. J Immunol. 2013;190:2282–2291. doi: 10.4049/jimmunol.1201215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Yang S, Abe Y, et al. Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum Mol Genet. 2007;16:410–423. doi: 10.1093/hmg/ddl474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodds RA, James IE, Rieman D, et al. Human osteoclast cathepsin K is processed intracellularly prior to attachment and bone resorption. J Bone Miner Res. 2001;16:478–486. doi: 10.1359/jbmr.2001.16.3.478. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson LS, Lima H, Jr, Goldberg MF, et al. Cathepsin-mediated necrosis controls the adaptive immune response by Th2 (T helper type 2)-associated adjuvants. J Biol Chem. 2013;288:7481–7491. doi: 10.1074/jbc.M112.400655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zavasnik-Bergant T, Turk B. Cysteine cathepsins in the immune response. Tissue Antigens. 2006;67:349–355. doi: 10.1111/j.1399-0039.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 9.Asagiri M, Hirai T, Kunigami T, et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science. 2008;319:624–627. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- 10.Beklen A, Hukkanen M, Richardson R, Konttinen YT. Immunohistochemical localization of Toll-like receptors 1-10 in periodontitis. Oral Microbiol Immunol. 2008;23:425–431. doi: 10.1111/j.1399-302X.2008.00448.x. [DOI] [PubMed] [Google Scholar]
- 11.Xie YF, Shu R, Jiang SY, Liu DL, Zhang XL. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int J Oral Sci. 2011;3:125–134. doi: 10.4248/IJOS11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao B, Chen W, Hao L, et al. Inhibiting periapical lesions through AAV-RNAi silencing of cathepsin K. J Dent Res. 2013;92:180–186. doi: 10.1177/0022034512468757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J, Chen W, Zhang L, et al. RNA interference-mediated silencing of Atp6i prevents both periapical bone erosion and inflammation in the mouse model of endodontic disease. Infect Immun. 2013;81:1021–1030. doi: 10.1128/IAI.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoch SA, Zajic S, Stone J, et al. Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: Two double-blind, randomized, placebo-controlled phase I studies. Clin Pharmacol Ther. 2009;86:175–182. doi: 10.1038/clpt.2009.60. [DOI] [PubMed] [Google Scholar]
- 15.Byrne SJ, Dashper SG, Darby IB, Adams GG, Hoffmann B, Reynolds EC. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol Immunol. 2009;24:469–477. doi: 10.1111/j.1399-302X.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 16.Kesavalu L, Sathishkumar S, Bakthavatchalu V, et al. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun. 2007;75:1704–1712. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Chen W, Zhu G, et al. RNAi-mediated silencing of Atp6i and Atp6i haploinsufficiency prevents both bone loss and inflammation in a mouse model of periodontal disease. PLoS One. 2013;8:e58599. doi: 10.1371/journal.pone.0058599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalla E, Lamster IB, Hofmann MA, et al. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003;23:1405–1411. doi: 10.1161/01.ATV.0000082462.26258.FE. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki H, Suzuki N, Kent R, Jr, Kawashima N, Takeda J, Stashenko P. T cell response mediated by myeloid cell-derived IL-12 is responsible for Porphyromonas gingivalis-induced periodontitis in IL-10-deficient mice. J Immunol. 2008;180:6193–6198. doi: 10.4049/jimmunol.180.9.6193. [DOI] [PubMed] [Google Scholar]
- 20.Benakanakere M, Abdolhosseini M, Hosur K, Finoti LS, Kinane DF. TLR2 promoter hypermethylation creates innate immune dysbiosis. J Dent Res. 2015;94:183–191. doi: 10.1177/0022034514557545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng S, Deng L, Chen W, Shao J, Xu G, Li YP. Atp6v1c1 is an essential component of the osteoclast proton pump and in F-actin ring formation in osteoclasts. Biochem J. 2009;417:195–203. doi: 10.1042/BJ20081073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Ma J, Zhu G, et al. Cbfb deletion in mice recapitulates cleidocranial dysplasia and reveals multiple functions of Cbfb required for skeletal development. Proc Natl Acad Sci USA. 2014;111:8482–8487. doi: 10.1073/pnas.1310617111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Zhu G, Hao L, Wu M, Ci H, Li YP. C/EBPa regulates osteoclast lineage commitment. Proc Natl Acad Sci USA. 2013;110:7294–7299. doi: 10.1073/pnas.1211383110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki H, Hou L, Belani A, et al. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol. 2000;165:3626–3630. doi: 10.4049/jimmunol.165.7.3626. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res. 2004;39:432–441. doi: 10.1111/j.1600-0765.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang S, Hao L, McConnell M, et al. Inhibition of Rgs10 expression prevents immune cell infiltration in bacteria-induced inflammatory lesions and osteoclast-mediated bone destruction. Bone Res. 2013;1:267–281. doi: 10.4248/BR201303005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owen JJT. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 28.Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–787. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- 29.Beklen A, Al-Samadi A, Konttinen Y. Expression of cathepsin K in periodontitis and in gingival fibroblasts. Oral Dis. 2015;21:163–169. doi: 10.1111/odi.12230. [DOI] [PubMed] [Google Scholar]
- 30.Conus S, Simon HU. Cathepsins and their involvement in immune responses. Swiss Med Wkly. 2010;140:w13042. doi: 10.4414/smw.2010.13042. [DOI] [PubMed] [Google Scholar]
- 31.Krieg AM, Lipford GB. Immunology. The toll of cathepsin K deficiency. Science. 2008;319:576–577. doi: 10.1126/science.1154207. [DOI] [PubMed] [Google Scholar]
- 32.Eisman JA, Bone HG, Hosking DJ, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: Three-year continued therapy and resolution of effect. J Bone Miner Res. 2011;26:242–251. doi: 10.1002/jbmr.212. [DOI] [PubMed] [Google Scholar]
- 33.Cusick T, Chen CM, Pennypacker BL, et al. Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J Bone Miner Res. 2012;27:524–537. doi: 10.1002/jbmr.1477. [DOI] [PubMed] [Google Scholar]
- 34.Bi Y, Gao Y, Ehirchiou D, et al. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol. 2010;177:280–290. doi: 10.2353/ajpath.2010.090592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li YP, Chen W. Characterization of mouse cathepsin K gene, the gene promoter, and the gene expression. J Bone Miner Res. 1999;14:487–499. doi: 10.1359/jbmr.1999.14.4.487. [DOI] [PubMed] [Google Scholar]
- 36.Charles JF, Hsu LY, Niemi EC, Weiss A, Aliprantis AO, Nakamura MC. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J Clin Invest. 2012;122:4592–4605. doi: 10.1172/JCI60920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacome-Galarza CE, Lee SK, Lorenzo JA, Aguila HL. Identification, characterization, and isolation of a common progenitor for osteoclasts, macrophages, and dendritic cells from murine bone marrow and periphery. J Bone Miner Res. 2013;28:1203–1213. doi: 10.1002/jbmr.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bar-Shavit Z. Taking a toll on the bones: Regulation of bone metabolism by innate immune regulators. Auto-immunity. 2008;41:195–203. doi: 10.1080/08916930701694469. [DOI] [PubMed] [Google Scholar]
- 39.Miyajima S, Naruse K, Kobayashi Y, et al. Periodontitis-activated monocytes/macrophages cause aortic inflammation. Sci Rep. 2014;4:5171. doi: 10.1038/srep05171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metzger Z. Macrophages in periapical lesions. Endod Dent Traumatol. 2000;16:1–8. doi: 10.1034/j.1600-9657.2000.016001001.x. [DOI] [PubMed] [Google Scholar]
- 41.Ueda H, Howson JMM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 42.Marcçal JRB, Samuel RO, Fernandes D, et al. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J Endod. 2010;36:995–999. doi: 10.1016/j.joen.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Cattaneo A, Gobert FX, Müller M, et al. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc Natl Acad Sci USA. 2012;109:9053–9058. doi: 10.1073/pnas.1115091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208:643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beutler B, Cerami A. The biology of cachectin/TNF — A primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 47.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl. 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.