Abstract
The neuropeptides oxytocin (OT) and arginine-vasopressin (AVP) have been implicated in modulating sex-specific responses to offspring in a variety of uniparental and biparental rodent species. Despite the large body of research in rodents, the effects of these hormones in biparental primates are less understood. Marmoset monkeys (Callithrix jacchus) belong to a clade of primates with a high incidence of biparental care and also synthesize a structurally distinct variant of OT (proline instead of leucine at the 8th amino acid position; Pro8-OT). We examined the roles of the OT and AVP systems in the control of responses to infant stimuli in marmoset monkeys. We administered neuropeptide receptor agonists and antagonists to male and female marmosets, and then exposed them to visual and auditory infant-related and control stimuli. Intranasal Pro8-OT decreased latencies to respond to infant stimuli in males, and intranasal AVP decreased latencies to respond to infant stimuli in females. Our study is the first to demonstrate that Pro8-OT and AVP alter responsiveness to infant stimuli in a biparental New World monkey. Across species, the effects of OT and AVP on parental behavior appear to vary by species-typical caregiving responsibilities in males and females.
Keywords: neuropeptide, paternal, maternal, biparental, primate
Despite structural and chemical similarities, oxytocin (OT) and arginine-vasopressin (AVP) differentially affect parental behavior depending on species, previous parental experience, sex, and breeding system. Evidence from uniparental species presents a clear role for OT in modulating parental behavior: increased oxytocinergic activity enhances maternal behavior (reviewed in Bosch and Neumann, 2012). Mothers with naturally high oxytocinergic activity engage in high levels of parental behavior (rat, Champagne et al., 2001; rat, Francis et al., 2000; macaque, Maestripieri et al., 2009), and females that would not ordinarily engage in parental behavior can be induced to do so with exogenous OT infusions (rat, Fahrbach et al., 1984; sheep, Kendrick et al., 1987; mouse, McCarthy et al., 1986; rat, Pedersen et al., 1982). Recent empirical and theoretical perspectives from research on humans suggest that OT exerts a multiplicity of effects on both male and female caregivers, including complex behavior such as bonding, coordination of affect, and alterations in the sensitivity of the caregiver to stimuli emanating from dependent offspring (reviewed in Feldman, 2015).
The AVP system also acts on parental care in ways that are sex- and species-specific: AVP exerts the same effects on parental behavior in males of biparental species as it does in females of uniparental species. In biparental male prairie voles and female rats alike, AVP increases and AVP V1a receptor antagonists (V1a-A) decrease parental behavior (Bosch and Neumann, 2012; Wang et al., 1994). The AVP system is also involved in modulating defensive parental aggression, and again it enhances this behavior in males of biparental rodents and females of uniparental species, though there are some strain differences (Bosch and Neumann, 2012; Nephew and Bridges, 2008; Young et al., 1997). In some species, such as the biparental California mouse, the AVP system is associated with parental care in males and females, but this association is weaker in females than it is in males, and AVP has been linked to aggression in males only (Bester-Meredith and Marler, 2012, 2003, 2001). Thus the effect of the AVP system on parental behavior, especially defensive aggression, depends not only on the sex of the individual, but also on differences in species-typical offspring care.
The OT and AVP systems are highly conserved among mammals, including primates, but New World monkeys (NWM) exhibit considerable inter-specific diversity in the OT gene and the OT and AVP receptors (OTR, V1a), while the AVP ligand remains unchanged (Lee et al., 2011; Ren et al., 2015, 2014; Vargas-Pinilla et al., 2015). Of particular interest is a clade of small-bodied monkeys (Callitrichidae). Marmosets (Callithrix) exhibit high rates of biparental care and a willingness to respond to isolated infants in distress (Lee et al., 2011; Nunes et al., 2001; Santos et al., 1997; Zahed et al., 2008), and they also produce an OT variant that substitutes leucine for proline at position eight (Lee et al., 2011). This variant (Pro8-OT) is structurally distinct from the conserved Leu8-OT, and variation in both OXTR and AVPR1a genes are associated with social behavior (Ren et al., 2015, 2014). This ligand-receptor-social system co-evolution suggests a functional role of neuropeptides in modulating social behavior, thus species in this clade can provide insight into relationships between neuropeptide systems and parental behavior. One study has shown that Leu8-OT enhances passive food sharing to offspring in adult male marmosets (Saito and Nakamura, 2011), but Pro8-OT and Leu8-OT differentially affect marmoset behavior (Cavanaugh et al., 2014), and no studies have yet investigated the roles of Pro8-OT or AVP in male and female marmosets.
Our study investigated the roles of the OT and AVP systems in modulating parental responsiveness in marmosets. If these neuropeptides modulate the brain systems that control sensitivity to infant stimuli, then marmosets exposed to intranasal Pro8-OT should exhibit increased responsiveness to and interest in infant stimuli compared to control stimuli, and marmosets exposed to OT receptor antagonists (OTA) should exhibit decreased responsiveness to and interest in infant stimuli. Also, because AVP affects parental behavior in males more so than in females in biparental rodents, we expected intranasal AVP to increase responsiveness to infant stimuli in marmoset males, but not females.
Methods
Subjects
We tested adult male (n = 8) and female (n = 8) marmosets (Callithrix jacchus). Marmosets were housed either in pairs or as the breeding pair of a family group (Table 1). One female (Arw) was pregnant during testing, three females (Kha, Lil, Nyl) were contracepted, and five males (Bat, Abu, Jax, Dac, Fab) were vasectomized (but not gonadectomized). Experimental procedures were approved by UNMC/UNO IACUC (#13-039-07-FC). Two males and two females had previous experience with infants as parents. Details of housing and husbandry protocols can be found in (Schaffner et al., 1995).
Table 1. Subject Characteristics.
| Pair (F / M) | Age (years) | Infant exposure * | Housing |
|---|---|---|---|
| Arw / Cle | 4.0 / 1.7 | Current | Pair +2 infants |
| Kha / Dex | 5.0 / 5.0 | Current | Pair +2 infants |
| Lil / Bel | 2.1 / 5.0 | No | Pair |
| Ell / Bat | 4.1 / 4.2 | No | Pair |
| Nyl / Odi | 4.4 / 4.6 | No | Pair |
| Tar / Abu | 5.4 / 4.1 | No | Pair |
| Vel / Jax | 5.5 / 4.6 | No | Pair |
| Art / Dac | 5.4 / 4.2 | No | Pair |
| Ath / Fab | 4.6 / 5.7 | No | Pair |
Note. F=Female, M=Male,
No = no exposure to infants in cage for at least 24 months
Neuropeptide manipulations
OT and AVP systems were manipulated using five treatment conditions, using a combination of oral (via preferred food item) and intranasal deliveries: Pro8-OT (150μg/kg; CYIQNCPPG-NH2) and AVP (133μg/kg; CYFQNCPRG-NH2) were each administered intranasally, and doses were based on previous research in NWMs (Cavanaugh et al., 2014; Jarcho et al., 2011; Smith et al., 2010). Intranasal delivery is a common method to administer small neuropeptide ligands to the brain non-invasively (Born et al., 2002; Neumann et al., 2013), but there are currently no pharmacological or behavioral data available for neuropeptide receptor antagonists administered intranasally. Instead, we chose ligands that are orally available and would be expected to cross the blood-brain barrier (Boccia et al., 2007; Pettibone et al., 1993; Serradeil-Le Gal et al., 1993). Doses for the OTR antagonist (OTA; 20mg/kg; L-368,899 provided by Dr. Peter Williams, Merck) and the V1a antagonist (V1a-A; 20μg/kg; SR49059) were based on previous research in NWMs and on the binding affinities of the antagonists for their respective receptors (Cavanaugh et al., 2014; Jarcho et al., 2011; Manning et al., 2012; Smith et al., 2010). Oral deliveries were administered ninety minutes prior to testing, and intranasal deliveries were administered twenty minutes prior to testing between 0800-1000h. These treatment-testing intervals allowed for maximal absorption and concentration in cerebrospinal fluid (Boccia et al., 2007; Born et al., 2002). Treatments were arranged so that the experience of each marmoset was identical during all testing sessions: Pro8-OT = oral control + intranasal Pro8-OT; AVP = oral control + intranasal AVP; OTA = oral OTA + intranasal saline; V1a-A = V1a-A+ intranasal saline; and SAL = oral control + intranasal saline. Each marmoset was tested under all five treatment conditions and received each treatment condition on two occasions. A one-week washout period occurred between the same treatments and a two-week washout between different treatments. The order of treatment was pseudorandomized and counterbalanced among subjects.
Behavioral testing
After treatment, marmosets were released into the long arm of a T-maze that had a stimulus box at the end of each arm (Figure 1). The infant stimulus box contained lifelike models of two infant marmosets on a branch, and a speaker that played distress (cry, tsik) and contact (twitter) calls recorded from two-week-old infants (Pistorio et al., 2006). The control stimulus box contained only a branch, and emitted 1s bursts of pure tone at 5500Hz, the dominant frequency of the recorded cry calls. Acoustic stimuli alternated for the duration of the test between 30s of infant calls and 30s of control tone, with a 5s period of silence between tones and calls. The placement of the stimulus boxes (left vs. right) was counterbalanced across trials. We used three measures to index the behavior of marmosets toward infant stimuli: 1) responsiveness to infant cues – latency to first “peek” into the infant stimulus box, 2) sustained interest in infant stimuli – total duration of “peeks” into the stimulus boxes, and 3) preference for one stimulus – greater duration spent within 15cm of one stimulus over the other. “Peeks” were scored when the marmoset's face was within 6cm of a viewing hole and its gaze was oriented into the box. We also recorded behavioral indicators of stress/neophobia: alarm and contact vocalizations, latency to approach and investigate control stimuli, and total activity.
Figure 1.
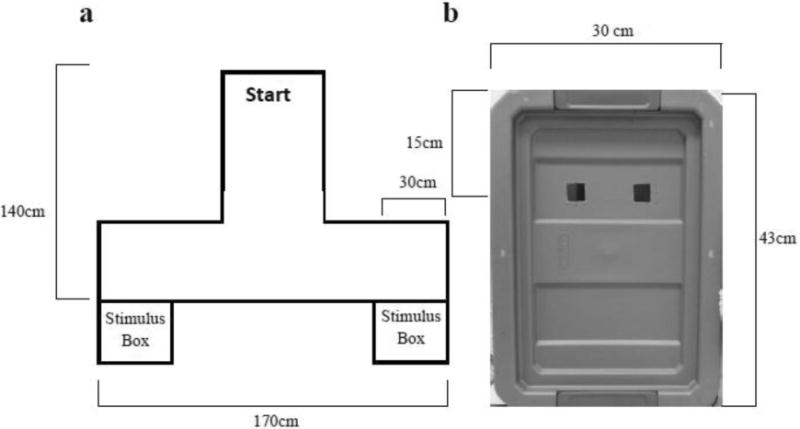
T-maze apparatus (a) and stimulus box (b). Each stimulus box contained visual and auditory stimuli, accessed through two holes in each box.
Statistical analyses
Behavioral data were averaged within treatment and analyzed using 5(treatment) × 2(stimulus type) × 2(sex) mixed factorial ANOVAs. Behavioral indicators of stress were tested for treatment effects using 5(treatment) × 2(sex) mixed factorial ANOVAs.
Results
Responsiveness to infant cues
The latencies for marmosets to perform the first peek in the stimulus boxes were affected by treatment condition, stimulus type, and sex (F(4,64) = 3.15, p = .020, η2 = .17; Figure 2). Male marmosets treated with Pro8-OT peeked in the infant stimulus box significantly faster than when treated with saline (t(8) = -3.16, p = .013, dz = -1.05), and faster than females treated with Pro8-OT (t(16) = -.247, p = .025, ds = -0.12). Additionally, females treated with AVP peeked in the infant stimulus box significantly faster than when treated with saline (t(8) = -2.50, p = .037, dz = -.83) and faster than males treated with AVP (t(16) = -3.14, p = .006, ds = -1.48). Latencies to the first peek in the control stimulus box, though, were unaffected by neuropeptide treatment condition (paired comparisons within sexes, all t(8) < ±1.19, p = n.s., dz < .40; male vs. female comparisons, all t(16) < ±1.37, p = n.s., ds < .65) demonstrating that the effects of neuropeptide treatment were specific to infant stimuli, and did not generalize to other novel auditory and visual stimuli. Oral neuropeptide antagonists had no effect on peek latencies (t(8) < ±1.18, p = n.s., dz < .39; t(16) < ±1.37, p = n.s ds < .64).
Figure 2.
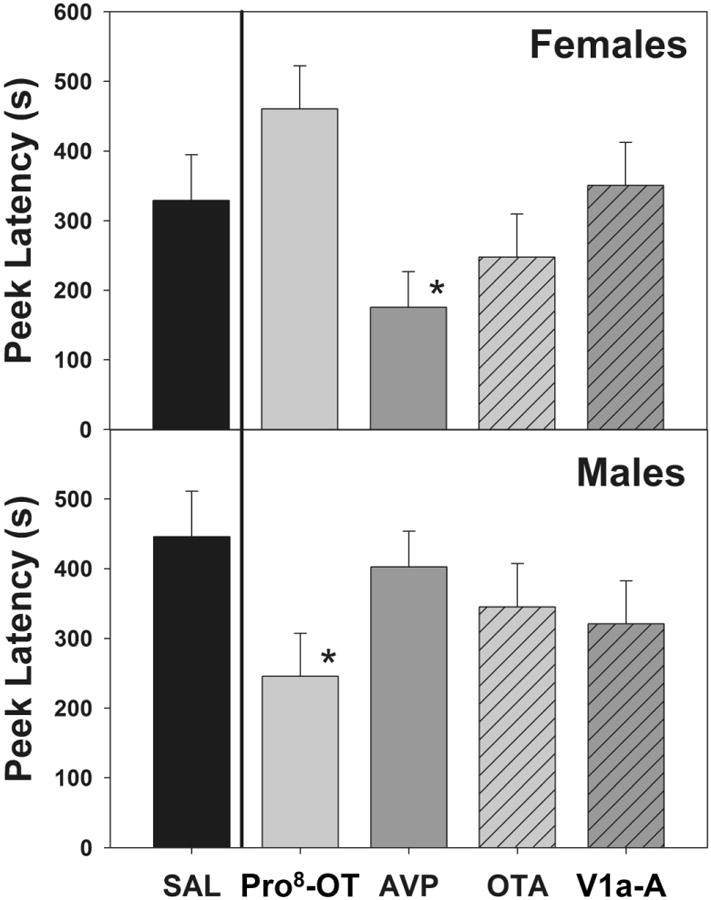
Mean(±SEM) latency (s) to the first peek in stimulus boxes in female and male marmosets. Asterisks indicate significant differences compared to saline.
Sustained interest in infant stimuli
Marmosets spent more time investigating the infant stimulus box compared to the control stimulus box (F(1,16) = 29.46, p < .001, η2 = .65; Table 2), but the duration of time that marmosets spent peeking in the stimulus boxes was not significantly affected by neuropeptide treatment, sex, or the interaction (F(4,64) < .43, p = n.s. η2 < .09).
Table 2. Sustained interest and preference for stimuli types.
| Peek Duration (s) | Proximity Duration (s) | |||
|---|---|---|---|---|
| Stimulus | Infant | Control | Infant | Control |
| Male | 4.0 (0.53) | 1.9 (0.36) | 147.7 (15.9) | 153.6 (16.5) |
| Female | 3.6 (0.53) | 2.3 (0.36) | 194.0 (15.9)* | 140.1 (16.5) |
| Combined | 3.8 (0.38)* | 2.1 (0.26) | 170.87 (11.23) | 146.84 (11.67) |
Note. Data presented are means ± SEM.
indicates a significant difference from control stimulus
Stimulus preferences
Males and females differed on time spent near each stimulus (F(1,16) = 6.53, p = .02, η2 = .29; Table 2). Female marmosets spent more time in close proximity to the infant stimulus box compared to the control stimulus box (t(8) = -3.09, p = .015 dz = -1.03), whereas males had no such preference. Neuropeptide treatment did not affect time spent near the infant box (F(4,64) < .75, p = n.s., η2 < .04).
Stress/neophobia measures
Neuropeptide treatment did not significantly affect rates of phee calling, alarm calling, latency to approach/investigate, or total locomotion (Table 3). This indicates that the effects of neuropeptide treatment on responsiveness to infant stimuli were not due to anxiety, general neophobia, or total activity.
Table 3. Neuropeptide manipulation does not affect behavioral indicators of stress.
| Behavior | AVP | Pro8-OT | AVPA | OTA | SAL | F(4,64) | p |
|---|---|---|---|---|---|---|---|
| Locomotion | 25.7(4.6) | 27.1(4.7) | 28.7(6.2) | 34.2(6.2) | 27.8(5.6) | 0.77 | .548 |
| Phee Calling | 6.9(1.8) | 7.9(2.1) | 9.0(2.1) | 8.4(2.6) | 8.6(2.0) | 0.28 | .887 |
| Alarm Calling | 3.1(1.4) | 3.5(1.7) | 3.7(1.6) | 6.4(2.7) | 1.4(0.8) | 1.62 | .182 |
| Approach control novel (s) | 161.6(51.0) | 148.6(34.4) | 161.3(41.1) | 160.8(35.5) | 127.1(31.7) | 0.174 | .951 |
| Peek in control novel (s) | 328.4(43.6) | 365.2(52.1) | 320.5(47.0) | 376.7(34.6) | 356.0(45.0) | 0.277 | .892 |
Note. Data presented under treatment columns are means (±SEM).
Discussion
This study is the first to use Pro8-OT or AVP to study parental responsiveness in a NWM, and our findings extend the growing molecular and behavioral evidence that neuropeptides modulate parental care in mammals. Neuropeptide agonists enhanced responsiveness to infant stimuli in marmosets, but the effects were sex-specific; Pro8-OT enhanced responsiveness to infant stimuli in males, and AVP enhanced responsiveness to infant stimuli in females. These results were opposite our predictions, because we had expected AVP to affect males, but not females, as in rodents. Antagonist treatments at these doses did not affect any measures, and it appears that chronic, rather than acute, oral antagonist treatment may be necessary to affect marmoset social behavior (Cavanaugh et al., 2014; Smith et al., 2010), or that the timing of the oral antagonist treatments were sub-optimal. It is also possible that when only one receptor type is blocked (either OTR or V1a), responses to infant stimuli can be rescued by activation of the other receptor by endogenous neuropeptides, as has been demonstrated in prairie voles (Bales et al., 2004). Additionally, none of the neuropeptide manipulations affected anxiety-like behavior or neophobia, indicating that Pro8-OT and AVP affected responses to infant stimuli specifically.
Previous research has shown that not only do marmoset parents respond behaviorally to infant stimuli faster than non-parents (Sánchez et al., 2014; Zahed et al., 2008), but also that the neuropeptide systems in male marmoset brains are altered by parental experience (Kozorovitskiy et al., 2006; Woller et al., 2012). Our sample included both experienced and inexperienced marmosets, and we found that neuropeptides modulated responses to infant stimuli across both categories of marmosets. It is possible though, that experience caring for offspring can alter neuropeptide-induced changes in responsiveness. Our small sample of two fathers and two mothers did not allow for the assessment of parental status as a factor in our analyses, so this will be an important avenue for future research. Only one study has manipulated OT to study actual parental behavior in marmosets; intracerebroventricular Leu8-OT increases tolerance for infant food stealing in marmoset fathers (Saito and Nakamura, 2011). The finding that Leu8-OT enhances passive food sharing in marmoset fathers (Saito and Nakamura, 2011), and our finding that Pro8-OT and AVP increase responsiveness to infant stimuli in male and female marmosets, respectively, indicate that neuropeptides are important facilitators of responsiveness to infant cues in marmosets.
The sex-specific effects of Pro8-OT and AVP were unexpected, as AVP, rather than OT, is more closely associated with male parental and social behavior in biparental rodents (Bales et al., 2004; Wang et al., 1994) This finding highlights the potential for interspecific variation in the effects of neuropeptides based on species-typical patterns of offspring care. It has been proposed that OT enhances nurturing parental behavior, while AVP enhances defensive aggression (van Anders et al., 2011), and thus the response to neuropeptide manipulation may not necessarily vary by sex, but by the specific parental care roles played by males and females in different infant-rearing systems. For instance, AVP modulates parental defense behavior in males of biparental rodents (Young et al., 1997), and affects the same behavior in females of uniparental rodents (Bosch and Neumann, 2012). Thus the influence of OT and AVP on behavior in rodents is explained better as a result of the nurturing/defending responsibilities in parental care rather than as a sex difference. Though male and female marmosets respond to infant stimuli similarly, fathers contribute more to “nuturant” parental care (i.e. infant carrying) overall during the development of offspring (French et al., 2008; Sánchez et al., 2014). Furthermore, adult female marmosets respond aggressively toward intruders, especially when they reside in family groups with infants (Schaffner and French, 1997). In this light, the effects of neuropeptides on responsiveness to infant stimuli in marmosets align with what is known from rodents. That is, AVP tends to affect the sex that exhibits more aggression and defense (which may not necessarily be the male), and OT affects the sex that exhibits more nurturant care.
In addition to their effects on social behavior, OT and AVP can have anxiolytic and anxiogenic properties, respectively. In humans, intranasal oxytocin decreases hypothalamic-pituitary-adrenal (HPA) activation during social stress and increases trust (Heinrichs et al., 2009; Van IJzendoorn and Bakermans-Kranenburg, 2012). Similarly, intranasal OT decreases HPA activation in squirrel monkeys during social isolation (Parker et al., 2005). However, we found that neither OT nor AVP treatment had any effect on behavioral indicators of stress/anxiety, including the latency to approach or investigate the novel control stimulus, suggesting that the effects of neuropeptide treatment on the latency to respond to infant stimuli were specific to parental-like behavior. A potential explanation for why OT produces anxiolysis in other primates but not in marmosets comes from work done in rodents that use localized neuropeptide manipulations to specific brain nuclei. When manipulations are diffuse across the brain, upregulation of the OT system decreases and upregulation of the AVP system increases anxiety like behavior in rats and mice (Bosch and Neumann, 2008; Gimpl and Fahrenholz, 2001). Moreover, OT agonists inhibit, and AVP agonists excite the same distinct populations of neurons in the amygdala (Huber et al., 2005). When neuropeptide manipulations are localized to the MPOA, a region implicated in the control of parental care, upregulations of the AVP and OT systems increase maternal behavior without affecting anxiety-like behavior (Bosch and Neumann, 2012, 2008). Furthermore, in another socially relevant nucleus, the PVN, upregulations of the AVP and OT systems increase maternal defense. In the central amygdala though, OT and AVP have differential effects; OT decreases and AVP increases defensive behavior (Bosch and Neumann, 2012). In rodents, OT and AVP in the amygdala are critical for fear and social recognition (Ferguson et al., 2001), but OT receptors have not been found in the amygdala of marmosets (Schorscher-Petcu et al., 2009), though V1a receptors are present (Schorscher-Petcu et al., 2009; Wang et al., 1997). Thus it is possible that intranasal neuropeptides at the doses used here activate only those circuits that control parental behavior, leaving anxiety-like behavior unchanged.
Conclusions
We showed that neuropeptides modulate responsiveness to infant stimuli in marmosets. Specifically, OT increased responsiveness to infant stimuli in males, whereas AVP increased responsiveness to infant stimuli in females. Neither OT nor AVP receptor antagonist treatment affected marmoset behavior, and none of the OT or AVP system manipulations affected anxietylike behavior. When compared to previous work in uniparental and biparental rodents, our findings are best described as a consequence of specific social/parental responsibilities; OT affects the behavior of the parent that engages in the most parental care, and AVP affects the behavior of the parent that engages in defensive behavior. The differential effects of OT and AVP on male and female behavior is especially interesting in marmosets, because recent molecular studies in New World monkeys, particularly those in the family Callitrichidae, have shown that the V1a and OTR genes are quite different from human receptors, and the OT and the OT-OTR genes have coevolved (Ren et al., 2015, 2014; Vargas-Pinilla et al., 2015). It is yet unknown whether Pro8-OT is more or less selective for the marmoset OTR compared to Leu8-OT and the OTR in most mammals, or how well Pro8-OT binds to the marmoset V1a. Experiments exploring these molecular mechanisms are needed in order to fully understand the behavioral results of neuropeptides in marmosets presented here and elsewhere. The present study, in conjunction with previous reports on marmosets and humans, supplements the body of work in uniparental and biparental rodents and primates, by highlighting the diversity and subtlety in the way in which neuropeptides affect parental behavior.
Highlights.
Male marmosets exposed to intranasal Pro8-OT responded to infant stimuli faster than when treated with intranasal saline.
Female marmosets exposed to intranasal vasopressin responded to infant stimuli faster than when treated with intranasal saline.
Neuropeptide manipulation did not affect the expression of anxiety-like behaviors, neophobia, or total activity, indicating that intranasal neuropeptides in marmosets affect parental behavior specifically, rather than via global effects on activity or fear or anxiety circuits.
We suggest that the effects of oxytocin and vasopressin depend on species-typical sex differences in nurturant and defensive parental behavior rather than on sex alone.
Acknowledgments
We would like to thank Heather Jensen, her team of volunteers, and Dr. Liz Gunkelman for their excellent care of the marmosets. We would also like to thank Jon-Ryan Cavanaugh and Aaryn Mustoe for helpful comments and discussion on previous versions of this manuscript. This research was supported by NIH (HD042882 awardee:JAF) and UNO-GRACA (awardee:JHT).
Footnotes
We declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bales KL, Kim AJ, Lewis-Reese AD, Sue Carter C. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Naturally Occurring Variation in Vasopressin Immunoreactivity Is Associated with Maternal Behavior in Female Peromyscus Mice. Brain Behav Evol. 2012;80:244–253. doi: 10.1159/000341899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav Neurosci. 2003;117:455. doi: 10.1037/0735-7044.117.3.455. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus) Horm Behav. 2001;40:51–64. doi: 10.1006/hbeh.2001.1666. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Goursaud APS, Bachevalier J, Anderson KD, Pedersen CA. Peripherally Administered Non-peptide Oxytocin Antagonist, L368,899(R), Accumulates in Limbic Brain Areas: A New Pharmacological Tool for the Study of Social Motivation in Non-Human Primates. Horm Behav. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Horm Behav, Oxytocin, Vasopressin and Social Behavior. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. Proc Natl Acad Sci. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, French JA. Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology. 2014;49:1–10. doi: 10.1016/j.psyneuen.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Oxytocin induction of short-latency maternal behavior in nulliparous, estrogen-primed female rats. Horm Behav. 1984;18:267–286. doi: 10.1016/0018-506x(84)90016-3. [DOI] [PubMed] [Google Scholar]
- Feldman R. The adaptive human parental brain: implications for children's social development. Trends Neurosci. 2015 doi: 10.1016/j.tins.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the Medial Amygdala is Essential for Social Recognition in the Mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- French JA, Fite JA, Ross CN. Parent. Brain N. Y: Elsevier Press; 2008. Family life in marmosets: causes and consequences of variation in offspring care; pp. 461–478. [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and Oxytocin Excite Distinct Neuronal Populations in the Central Amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav. 2011;10:375–383. doi: 10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology. 1987;46:56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Lee AG, Cool DR, Grunwald WC, Neal DE, Buckmaster CL, Cheng MY, Hyde SA, Lyons DM, Parker KJ. A novel form of oxytocin in New World monkeys. Biol Lett. 2011;7:584–587. doi: 10.1098/rsbl.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD. Mother–infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol Behav. 2009;96:613–619. doi: 10.1016/j.physbeh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and Vasopressin Agonists and Antagonists as Research Tools and Potential Therapeutics. J Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Bare JE, Vom Saal FS. Infanticide and parental behavior in wild female house mice: Effects of ovariectomy, adrenalectomy and administration of oxytocin and prostaglandin F2 α. Physiol Behav. 1986;36:17–23. doi: 10.1016/0031-9384(86)90066-1. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol Biochem Behav. 2008;91:77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among Paternal Behavior, Steroid Hormones, and Parental Experience in Male Marmosets (Callithrix kuhlii) Horm Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Clineschmidt BV, Guidotti MT, Lis EV, Reiss DR, Woyden CJ, Bock MG, Evans BE, Freidinger RM, Hobbs DW, Veber DF, Williams PD, Chiu SHL, Thompson KL, Schorn TW, Siegl PKS, Kaufman MJ, Cukierski MA, Haluska GJ, Cook MJ, Novy MJ. L-368,899, a potent orally active oxytocin antagonist for potential use in preterm labor. Drug Dev Res. 1993;30:129–142. doi: 10.1002/ddr.430300305. [DOI] [Google Scholar]
- Pistorio AL, Vintch B, Wang X. Acoustic analysis of vocal development in a New World primate, the common marmoset (Callithrix jacchus) a) J Acoust Soc Am. 2006;120:1655–1670. doi: 10.1121/1.2225899. [DOI] [PubMed] [Google Scholar]
- Ren D, Chin KR, French JA. Molecular Variation in AVP and AVPR1a in New World Monkeys (Primates, Platyrrhini): Evolution and Implications for Social Monogamy. PLoS ONE. 2014;9:e111638. doi: 10.1371/journal.pone.0111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Lu G, Moriyama H, Mustoe AC, Harrison EB, French JA. Genetic Diversity in Oxytocin Ligands and Receptors in New World Monkeys. PLoS ONE. 2015;10:e0125775. doi: 10.1371/journal.pone.0125775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Nakamura K. Oxytocin changes primate paternal tolerance to offspring in food transfer. J Comp Physiol A. 2011;197:329–337. doi: 10.1007/s00359-010-0617-2. [DOI] [PubMed] [Google Scholar]
- Sánchez SM, Ziegler TE, Snowdon CT. Both parents respond equally to infant cues in the cooperatively breeding common marmoset, Callithrix jacchus. Anim Behav. 2014;97:95–103. doi: 10.1016/j.anbehav.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CV, French JA, Otta E. Infant carrying behavior in callitrichid primates: Callithrix and Leontopithecus. Int J Primatol. 1997;18:889–907. [Google Scholar]
- Schaffner CM, French JA. Group size and aggression:“recruitment incentives” in a cooperatively breeding primate. Anim Behav. 1997;54:171–180. doi: 10.1006/anbe.1996.0413. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in wied's black tufted-ear marmosets (Callithrix kuhli) Am J Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupré A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, Garcia C, Lacour C, Guiraudou P, Christophe B, Villanova G, Nisato D, Maffrand JP, Le Fur G. Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. J Clin Invest. 1993;92:224. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anders SM, Goldey KL, Kuo PX. The Steroid/Peptide Theory of Social Bonds: Integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology. 2011;36:1265–1275. doi: 10.1016/j.psyneuen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37:438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Vargas-Pinilla P, Paixão-Côrtes VR, Paré P, Tovo-Rodrigues L, Vieira CM, de AG, Xavier A, Comas D, Pissinatti A, Sinigaglia M, Rigo MM, Vieira GF, Lucion AB, Salzano FM, Bortolini MC. Evolutionary pattern in the OXT-OXTR system in primates: coevolution and positive selection footprints. Proc Natl Acad Sci U S A. 2015;112:88–93. doi: 10.1073/pnas.1419399112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Toloczko D, Young LJ, Moody K, Newman JD, Insel TR. Vasopressin in the forebrain of common marmosets (Callithrix jacchus): studies with in situ hybridization, immunocytochemistry and receptor autoradiography. Brain Res. 1997;768:147–156. doi: 10.1016/s0006-8993(97)00636-7. [DOI] [PubMed] [Google Scholar]
- Wang ZX, De Vries G, Ferris CF. The role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci USA. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller MJ, Sosa ME, Chiang Y, Prudom SL, Keelty P, Moore JE, Ziegler TE. Differential Hypothalamic Secretion of Neurocrines in Male Common Marmosets: Parental Experience Effects? J Neuroendocrinol. 2012;24:413–421. doi: 10.1111/j.1365-2826.2011.02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: Behavioral consequences. Behav Neurosci. 1997;111:599. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Zahed SR, Prudom SL, Snowdon CT, Ziegler TE. Male parenting and response to infant stimuli in the common marmoset (Callithrix jacchus) Am J Primatol. 2008;70:84–92. doi: 10.1002/ajp.20460. [DOI] [PubMed] [Google Scholar]


