Abstract
Health impact assessments (HIAs) inform policy and decision making by providing information regarding future health concerns, and quantitative HIAs now are being used for local and urban-scale projects. HIA results can be expressed using a variety of metrics that differ in meaningful ways, and guidance is lacking with respect to best practices for the development and use of HIA metrics. This study reviews HIA metrics pertaining to air quality management and presents evaluative criteria for their selection and use. These are illustrated in a case study where PM2.5 concentrations are lowered from 10 to 8 µg/m3 in an urban area of 1.8 million people. Health impact functions are used to estimate the number of premature deaths, unscheduled hospitalizations and other morbidity outcomes. The most common metric in recent quantitative HIAs has been the number of cases of adverse outcomes avoided. Other metrics include time-based measures, e.g., disability-adjusted life years (DALYs), monetized impacts, functional-unit based measures, e.g., benefits per ton of emissions reduced, and other economic indicators, e.g., cost-benefit ratios. These metrics are evaluated by considering their comprehensiveness, the spatial and temporal resolution of the analysis, how equity considerations are facilitated, and the analysis and presentation of uncertainty. In the case study, the greatest number of avoided cases occurs for low severity morbidity outcomes, e.g., asthma exacerbations (n=28,000) and minor-restricted activity days (n=37,000); while DALYs and monetized impacts are driven by the severity, duration and value assigned to a relatively low number of premature deaths (n=190 to 230 per year). The selection of appropriate metrics depends on the problem context and boundaries, the severity of impacts, and community values regarding health. The number of avoided cases provides an estimate of the number of people affected, and monetized impacts facilitate additional economic analyses useful to policy analysis. DALYs are commonly used as an aggregate measure of health impacts and can be used to compare impacts across studies. Benefits per ton metrics may be appropriate when changes in emissions rates can be estimated. To address community concerns and HIA objectives, a combination of metrics is suggested.
Keywords: Health impact assessment, air quality management, health metrics, disability-adjusted life years, monetized impacts
1. Introduction
Air quality management requires the consideration of a complex array of technical, economic, legal and political factors. In the U.S., statutory obligations are placed on state and local governments to attain ambient concentrations and meet other standards set by the US Environmental Protection Agency (US EPA). Historically, compliance with standards has been achieved by emission reduction strategies that addressed a single pollutant at a time, and targeted local and culpable sources for emission reductions, at the same time incorporating effects of the broader emission reductions accomplished by national emission standards. As air quality standards continue to be strengthened and easily implemented controls become rarer, decision makers must consider a wider range of policy measures. Since interventions aimed at reducing ambient pollution levels can affect the health of those living and working in the affected area (Henschel et al., 2012), it is becoming increasingly important to assess the nature and magnitude of potential health impacts, thus avoiding both unintended health consequences and missed opportunities to improve public health (NRC, 2011).
Health impact assessments (HIAs) use a variety of techniques to evaluate and compare potential health impacts of proposed projects, policies and plans with the key objectives of understanding the direction, magnitude, severity and distribution of impacts (Bhatia et al., 2014). HIAs and similar analyses have been conducted at multiple scales and for different purposes. At the national or global scale, accountability research, burden of disease, and other studies are used to evaluate the disease burden due to pollution (Fann et al., 2012b; Lim et al., 2012), the incremental impact of alternative policies and scenarios, e.g., different levels of an ambient standards (Chanel et al., 2014; Dias et al., 2012; Heal et al., 2013), to apportion health impacts by source industry (Fann et al., 2013), and to explain the benefits of standards, e.g., the avoided 230,000 premature deaths annually by 2020 due to implementation of PM2.5 controls between 1990 and 2005 in the US (US EPA, 2011). At regional (sub-national), urban and project scales, HIAs can be conducted in a policy context, but more commonly to gauge potential impacts and benefits of specific actions. In particular, HIAs conducted by health departments, academic researchers or advocacy groups often aim to incorporate health outcomes in policy and decision making (Dannenberg and Wernham, 2013).
Due to limitations in the scope and available data, most HIAs have been qualitative rather than quantitative (Rhodus et al., 2013). While qualitative assessments can convey the direction and magnitude of impacts, quantitative methods offer more explicit information regarding impacts of potential interventions or the status of abatement policies (Bhatia and Seto, 2011). Several guides for the design and implementation of HIAs have provided recommendations for screening, scoping, and impact assessment steps of the HIA process. However, there are few recommendations for reporting and communicating results (Hebert et al., 2012). Metrics that effectively communicate impacts to stakeholders and decision-makers need to be identified.
Tools developed to facilitate the systematic quantification of impacts produce different metrics. The Environmental Benefits Mapping and Analysis Program (BenMAP) developed by the US EPA and the Air Quality Benefits Assessment Tool (AQBAT) used by Health Canada report impacts as attributable cases and monetized impacts (Judek et al., 2006; US EPA, 2015a). Air pollution accountability research tends to favor these metrics (Bell et al., 2011). The Integrated Environmental Health Impact Assessment System developed for the European Union (Briggs, 2008) uses time-based health metrics (e.g., disability adjusted life years, DALYs). Originally developed for the comparative risk assessment framework (Murray, 1994), these metrics summarize different health effects with varying degrees of severity into a single figure (de Hollander and Melse, 2006; Hofstetter and Hammitt, 2002).
Health impacts associated with air pollution vary by duration (chronic or transient), degree (severe or minor) and temporality (caused shortly after exposures or lagged by several years). Urban-scale HIAs can address projects or policies that affect the entire urban area or a specific segment of the population. Thus, the applicability of the certain health metrics may depend on the boundary of the HIA, the severity of the predicted impacts, and community values regarding health. Previous reviews have discussed differences between qualitative and quantitative HIAs (Bhatia and Seto, 2011; O’Connell and Hurley, 2009), but the types of metrics used in quantitative urban-scale HIAs have not been addressed.
The goal of this paper is to evaluate quantitative metrics used in HIAs and similar analyses that are relevant to air quality management at the urban and potentially regional scales. The metrics are evaluated using explicit criteria, and demonstrated using a case study that focuses on particulate matter less than 2.5 µm in diameter (PM2.5). The paper concludes with recommendations for those metrics that can best inform decision-makers.
2. Methods
Literature published between 2011 and 2015 was reviewed to identify HIA metrics used for both project and policy applications. Reviews and critiques of HIAs (in both the peer-reviewed and grey literature) and original peer-reviewed articles were examined, and included studies that evaluated the burden of disease attributable to ambient air pollution, the health benefits of proposed ambient air quality standards, and policies to reduce pollutant levels (e.g., active transport). The HIAs identified in the literature ranged in scale from multi-national to urban. Recent regulatory impact analyses (RIAs) by US EPA were also reviewed (US EPA, 2015b, 2014, 2012a). Selected metrics include the predicted number of cases, time-based metrics, impacts per unit emissions, and monetized impacts.
Evaluative criteria were identified from two sources. First, findings of the reviewed quantitative HIAs were used to identify key characteristics relevant to air quality metrics, e.g., metrics should account for population dynamics since pollution-related health effects can lag years behind exposures (Flachs et al., 2013). Second, review articles and commentaries from the health indicator literature were examined to identify additional criteria, e.g., the comparability of metrics across populations of different size (Walker et al., 2007).
A case study demonstrates the formulation, use, strengths and limitations of the metrics. This uses Detroit, Michigan and the surrounding county (Wayne), a mostly urban and suburban region (area of 1600 km2, population of 1.8 million) that has a mix of industrial, commercial, area and mobile emission sources. The county scale was selected due to the availability of emission and other data. A scenario is evaluated in which PM2.5 concentrations are uniformly lowered across the county from 10 to 8 µg/m3, reflecting a policy that reduces concentrations below the current national annual average standard of 12 µg/m3 (US EPA, 2013a). The analysis follows the method reported by Fann et al. (2012) with several differences. To examine potential differences between HIA methodologies, two methods (detailed below) are used to estimate mortalities attributable to changes in PM2.5 levels. In addition to the concentration-response (CR) estimates included in the BenMAP software, cause-specific mortality CR estimates developed for the recent Global Burden of Disease (GBD) study (Burnett et al., 2014; Lim et al., 2012) are used. To assess the sensitivity of results to national, county and local scale data, attributable rates for premature mortality are calculated using baseline rates for the US as a whole, Wayne County (including Detroit), and Detroit separately. To facilitate these analyses, a simple spreadsheet model is used that does not represent spatial differences in air quality, population or impacts across the study area. Uncertainty in the number of avoided cases predicted for the case study is simulated using a Monte Carlo (MC) analysis (@Risk for Excel, Palisade Corporation). For each CR estimate, the distribution around the regression coefficient is specified based on the reported standard error. The simulation uses 5000 iterations to estimate the mean number of avoided cases and to construct 95% confidence intervals around the mean. The uncertainty in the number of avoided cases is propagated to the DALY and monetized impact metrics. Other sources of uncertainty for these summary metrics, e.g., uncertainty in disability weights or monetized values, are not included.
Additional information on the case study is found in the supplemental materials.
Emissions-based metrics (e.g., benefits per ton) use sector-specific 2011 PM25 emissions information for Wayne County (US EPA, 2012b). Annual emission rates are listed in Supplemental Table S6. Following source apportionments performed for Detroit (Buzcu-Guven et al., 2007; Gildemeister et al., 2007), half (5 µg/m3) of the initial and existing PM2.5 is assumed to arise from local sources (e.g., direct PM25 emissions from industrial point sources, diesel and gasoline mobile sources, construction and road dust emissions, other non-point sources) that collectively emit approximately 7,000 tons per year; the other half arises from regional sources and the formation of secondary PM2.5. Using a “roll-back” method, a 2 µg/m3 reduction is achieved by reducing local emissions by 40%, or 2,800 tons per year. While simple, this approach attains results that reflect those from more complex methods that explicitly model sources and spatial variation (described later), and that are suitable for demonstrating the alternative health metrics. The benefits per ton metric is calculated by dividing avoided impacts (e.g., avoided cases and monetized impacts per year) estimated using a health impact function by the emission reduction.
3. Results
3.1 HIA metrics in previous air quality and other studies
HIA applications have been summarized and critiqued in several reviews published in the peer-reviewed and 'grey' literature (Bhatia and Seto, 2011; Dannenberg and Wernham, 2013; Hebert et al., 2012; O’Connell and Hurley, 2009; Rhodus et al., 2013; Schuchter et al., 2014). Many HIAs have been made publically available (Pew Charitable Trusts, 2014; UCLA HIA-CLIC, 2015). The following emphasizes HIAs involving air quality analyses.
Most urban scale HIAs have been conducted for urban planning, transportation and land use projects. In a review of 81 transportation, housing and infrastructure, land use and waste management HIAs conducted between 1999 and 2012 in the United States, 52% considered air quality impacts, in part due to the availability of models and other assessment tools, but only 28% used quantitative methods (Rhodus et al., 2013). In contrast, nearly all (37 out of 38) HIAs examined in the peer-reviewed literature used quantitative metrics, and most of these studies (71%) were conducted outside of the United States. (These studies are summarized in Supplemental Table S1.) Typically, impacts are reported as the number of (avoided) cases attributable to changes in ambient concentration. Fewer studies have reported impacts using DALYs or monetized impacts. Only eight of these HIAs used multiple metrics. Sometimes these metrics were calculated using standardized platforms, e.g., BenMAP. Other metrics in HIAs or regulatory analyses include cost-effectiveness and cost-benefit indicators. A few studies used indicators designed for life cycle assessment (LCA). These metrics are detailed below.
3.1.1 Predicted cases
As noted, the most common quantitative HIA metric is the number of morbidities or premature mortalities attributed to a change in pollutant concentration. The number of predicted cases is calculated using two similar approaches. The population attributable fraction (PAF) method, endorsed by the WHO (Prüss-Ustün et al., 2003), represents the fraction of risk for an outcome attributable to a specific exposure. It is estimated for specific exposure concentrations using concentration-dependent relative risks (RR):
| (1) |
where RR = relative risk for the outcome, e.g., eβΔx for a log-linear risk coefficient where Δx = change in ambient concentration, β = the regression coefficient, and Pe = the probability of exposure (i.e., the fraction of the population that is exposed; Steenland and Armstrong, 2006). For air pollution, the PAF is typically used to estimate the burden of disease relative to non-anthropogenic background levels. Multiplying the PAF by the baseline rate in the population (y0, cases person-1 year-1) and the number of people in the population (P) gives the number of attributable cases in the population. Recently, this approach has been used to estimate the burden of disease attributable to air pollution (Cárdaba Arranz et al., 2014; Hänninen et al., 2014), and to compare PM2.5 standards in Taiwan (Yang and Kao, 2013).
The second method uses a health impact function (HIF) to estimate changes in outcome incidence. The HIF represents a simplified PAF where the entire population is considered exposed (e.g., Pe=1). The HIF depends on the form of the CR function, e.g., a log-linear CR estimate gives:
| (2) |
where ΔY = incremental change in the number of cases, y0 = baseline incidence rate (cases person-1 year-1), β = CR estimate (log relative risk), Δx = expected or measured change in concentration (µg/m3 or ppb), and P = exposed population (US EPA, 2015a). The HIF can estimate the incidence attributable to pollution relative to 'pristine' or 'background' levels (Fann et al., 2012b), but generally is used to evaluate incremental impacts associated with a change in concentration, e.g., effects of a new standard relative to existing concentrations (Berman et al., 2012; Boldo et al., 2014; US EPA, 2012a).
Both PAF and HIF methods require information including the size of the exposed population, baseline incidence rates for diseases associated with pollutants, baseline and exposure concentrations, and CR estimates or relative risks for each pollutant-outcome pair. Prospective applications also require projections of population size and baseline rates; retrospective applications need current and historical data. CR estimates are drawn from the epidemiological literature, including large observational studies (e.g., Jerrett et al., 2009; Krewski et al., 2009), as well as smaller studies of targeted populations (e.g., Mar et al., 2004). CR estimates can be chosen from a single study or pooled across multiple studies. 'Counterfactual' concentrations (CFCs) for PM2.5 between 5.8 and 8.8 µg/m3 have been used as comparison or baseline conditions to represent non-anthropogenic 'background' levels (Burnett et al., 2014; Krewski et al., 2009; Murray et al., 2003).
3.1.2 Disability-adjusted life years
Duration metrics consider the time lived with disability or the time lost due to early death, and are derived from the number of predicted cases. Years of life lost (YLL) is the difference between the age-specific remaining life expectancy (LE) and the age of premature death. Years living with a disability (YLD) is the time spent living with a morbidity (i.e., the case duration), weighted by a disability weight (DW) that reflects the degree of impairment as assigned using trade-off methods (Prüss-Ustün et al., 2003), e.g., panel evaluation judging which hypothetical person with a randomly assigned disease is healthier (Salomon et al., 2012). YLL and YLD are calculated for each population stratum (e.g., age group, sex, race/ethnicity):
| (3) |
| (4) |
where Nj,a = number of avoided cases in stratum j and age group a, LEa = standard remaining life expectancy for age group a (in years), D = duration of the disease state (in years), and DW = disability weight for the morbidity outcome. DWs range from 0 (perfect health) to 1 (death).
The calculation of YLLs requires the use of standard life tables to determine the remaining life expectancy for each age group. Life tables can be developed for each year of life and particular age intervals using age-specific mortality rates for the population of interest (Anderson, 1999); this information is available at country and state levels (MDCH, 2015; World Health Organization, 2015).
DALY metrics sum YLL and YLD (eqs. 3 and 4) across the population, thus aggregating across different outcomes (e.g., asthma exacerbation and premature mortality). DALYs are commonly used in burden of disease studies (Flachs et al., 2013; Hänninen et al., 2014), and have been used in policy evaluations (Rojas-Rueda et al., 2013) and life cycle impact assessments (Kassomenos et al., 2013).
Quality-adjusted life years (QALYs) provide an alternative approach to DALYs. QALYs were developed to provide a comprehensive measure of health in multiple dimensions, e.g., physical health and social well-being (Gold et al., 2002) using weights that range from 1 (perfect health) to 0 (death; Sassi, 2006). Weights assigned to QALYs are not tied to a particular disease status, but rather look at an individual’s overall health state. In contrast, DALYs use disability weights that focus on a single disease and comorbidities are not considered (Gold et al., 2002). In this paper, we used DALYs as a summary measure of health given their use in previous studies (Supplemental Table S1).
3.1.3 Monetized impacts
Mortality and morbidity outcomes can be monetized to facilitate cost-benefit and cost-effectiveness analyses. For deaths, valuations often use the value of a statistical life (VSL), a monetary value assigned to a premature mortality based on willingness to pay (WTP), derived as what an individual would pay to reduce their risk of dying in the next year by a small amount, e.g., 1 in 100,000 (Hammitt, 2000). An alternative measure is the value of a statistical life year (VSLY), a value assigned to each YLL rather than to each premature death (Hammitt, 2007). For morbidity, valuations use the WTP or the average cost of an illness (COI), which incorporates medical expenses and societal costs, e.g., lost wages (Akobundu et al., 2006). Valuations can be discounted to account for the time-value of money, e.g., for an assumed 20 year lag between a concentration reduction and premature mortality, US EPA (US EPA, 2012a), suggests apportioning 30% of the mortality in the year following the concentration reduction, 50% in the 2nd through 4th years, and the remaining 20% between 6th and 20th years, and applying discount rates from 3 to 7% per year (OMB, 2003). Valuations without lags represent the “maximum impact” case since all impacts are assumed to occur immediately following the concentration change.
3.1.4 Functional unit-based metrics
Additional health metrics are used in life cycle assessments (LCA), which provide a comprehensive assessment of a product or service. Most LCAs use streamlined approaches that quantify impacts on the basis of a functional unit, e.g., per ton of PM2.5 emitted. Characterization factors relate environmental stressors evaluated in an LCA to health outcomes, e.g., the ReCiPe framework defines DALYs per kg of PM2.5 emitted (Goedkoop et al., 2009).
Regulatory analyses have used metrics expressed as outcomes per ton of emissions. Such metrics may be advantageous when changes in emissions (rather than ambient concentrations) are estimated, e.g., a rule requiring increased efficiencies for residential wood-burning heaters estimated monetized benefits of $380,000 per ton of PM2.5 emissions reduced (US EPA, 2015). This metric was derived using the expected change in emissions, dispersion modeling to estimate concentrations, HIFs to predict avoided cases, and economic valuations to monetize outcomes (US EPA, 2013).
3.1.5 Economic metrics
Economic metrics incorporate health measures along with resource constraints, typically expressed as the cost of implementing a policy or project. For example, cost-effectiveness metrics using benefit-cost ratios can compare monetized benefits, in part derived from HIAs, to expected costs (Johannesson, 1995). Such metrics are sometimes required, e.g., proposed regulation in the US undergo a regulatory impact analysis to demonstrate their cost-effectiveness (US EPA, 2010). The total cost of an air quality management strategy includes the direct expenditures made by polluters, e.g., costs of equipment, operation and maintenance, subsidies and financial incentives, and costs to air pollution control districts for planning, monitoring and enforcement; benefits include all avoided health, social and environmental impacts (Bower and Brady, 1981). It can be difficult to monetize all benefits of air quality management, particularly for secondary and tertiary impacts, e.g., climate change mitigation; the scope and uncertainty of such analyses can present challenges. In addition, cost-benefit analyses may mask equity concerns given their focus on efficiency and overall costs and benefits, rather than benefits to specific groups (de Groot, 1998). Despite their complexity and limitations, cost-benefit analyses can help select effective strategies, particularly for multi-pollutant strategies that may have high implementation costs but substantial health benefits (Chestnut et al., 2006).
The present analysis focuses on health metrics. The PM2.5 reduction in the case study might be achieved by a number of management strategies, which would likely vary in costs. Given the study's emphasis, we did not identify a specific strategy and thus did not estimate control costs or calculate economic metrics. While a full discussion of economic metrics utilizing HIAs is beyond our scope, guidelines for conducting economic analysis for environmental policy assessment have been presented elsewhere (US EPA, 2010).
3.2 Case study
The case study uses a 2 µg/m3 reduction in PM2.5 concentrations to illustrate the health impacts of the health metrics in urban scale air quality HIAs. The metrics could be used to compare control strategies directly, and they might be incorporated into broader environmental impact assessments, such as those specified by the National Environmental Policy Act (Bhatia and Wernham, 2008). As discussed previously, they also are necessary for cost-benefit and cost-effectiveness analyses, although the present paper is limited to an analysis of health impacts. The next section discusses implications for using health metrics in air quality management.
3.2.1 Predicted impacts
HIA results for the case study are summarized in Table 1. Additional details are provided in Supplemental Tables S7 and S8. Lowering PM2.5 levels from 10 to 8 µg/m3 is estimated to prevent 190 premature all-cause deaths, 230 cause-specific deaths (the sum of chronic obstructive pulmonary disease (COPD), lung, trachea and bronchus cancers, ischemic heart disease (IHD), and stroke deaths), 28,000 avoided asthma exacerbations and 37,000 minor restricted activity days per year (MRAD), i.e., days when individuals avoid typical activities and instead switch to less strenuous tasks without missing work or school. Attributable rates for avoided premature deaths are higher when based on Detroit mortality rates compared to those for all of Wayne County or the U.S. (Table 2). Similar distributions of impacts have been reported in the several studies that evaluated both mortality and morbidity outcomes (Berman et al., 2012; Chart-asa and Gibson, 2015; Fann et al., 2013, 2012b; Jakubiak-Lasocka et al., 2015; Voorhees et al., 2014). All of these studies show that less severe outcomes make up the majority of avoided cases.
Table 1.
Number of premature deaths and morbidities avoided per year in Wayne County due to a reduction in PM2.5 concentration from 10 to 8 µg/m3.
| Outcome (age group) | Avoided cases1 (cases per year) |
Percent attributable (%) |
Attributable rate (per 100,000) |
|---|---|---|---|
| All-cause premature mortality (>29 years, HIF) | 190 (130–260) | 1.16 | 10.48 |
| All-cause premature mortality (>29 years, PAF) | 190 (120–240) | 1.13 | 10.22 |
| Cause-specific mortality (>24 years, PAF method)2 | 230 | 3.55 | 12.61 |
| Infant mortality (<1 year) | 2 (0–3) | 0.77 | 0.09 |
| Minor restricted activity days (18–64 years) | 37,000 (15,000–58,000) | 0.44 | 2,040 |
| Asthma exacerbations (6–18 years)3 | 28,000 (–34,000–76,000) | 2.49 | 12,639 |
| Work loss days (18–64 years) | 21,000 (17,000–24,000) | 0.92 | 1,148 |
| Asthma emergency department visit (> 1 year)3 | 190 (49–323) | 1.11 | 86.42 |
| Non-fatal MI (≥ 18 years) | 160 (29–260) | 4.93 | 8.92 |
| CV hospitalization (≥ 20years) | 84 (56–110) | 0.30 | 4.71 |
| Pneumonia hospitalization (>64 years) | 26 (4–47) | 0.79 | 1.45 |
| COPD hospitalization (≥20 years) | 25 (15–36) | 0.40 | 1.42 |
| Asthma hospitalization (<65 years) | 19 (7–30) | 0.66 | 1.05 |
Number of avoided cases is rounded to two significant digits; 95% confidence interval in parentheses.
Sum of IHD, stroke, LC and COPD deaths estimated using the PAF method.
Among persons with asthma.
Table 2.
Rates of premature mortality, years of life lost and monetized impacts attributable to a reduction in PM2.5 concentration from 10 to 8 µg/m3 based on baseline mortality rates for the United States, Wayne County and Detroit
| Baseline Rate Source | Premature deaths (per 100,000) |
Avoided YLL (years per 100,000) |
Monetized impacts1 (1000$ per 100,000) |
|---|---|---|---|
| National | 8.6 | 124.0 | 83,000 |
| Wayne County (including Detroit) | 10.5 | 163.6 | 101,000 |
| Detroit | 11.0 | 194.6 | 105,000 |
Monetized benefits are in 2010$ projected to a 2020 income level and rounded to the nearest whole number with two significant digits
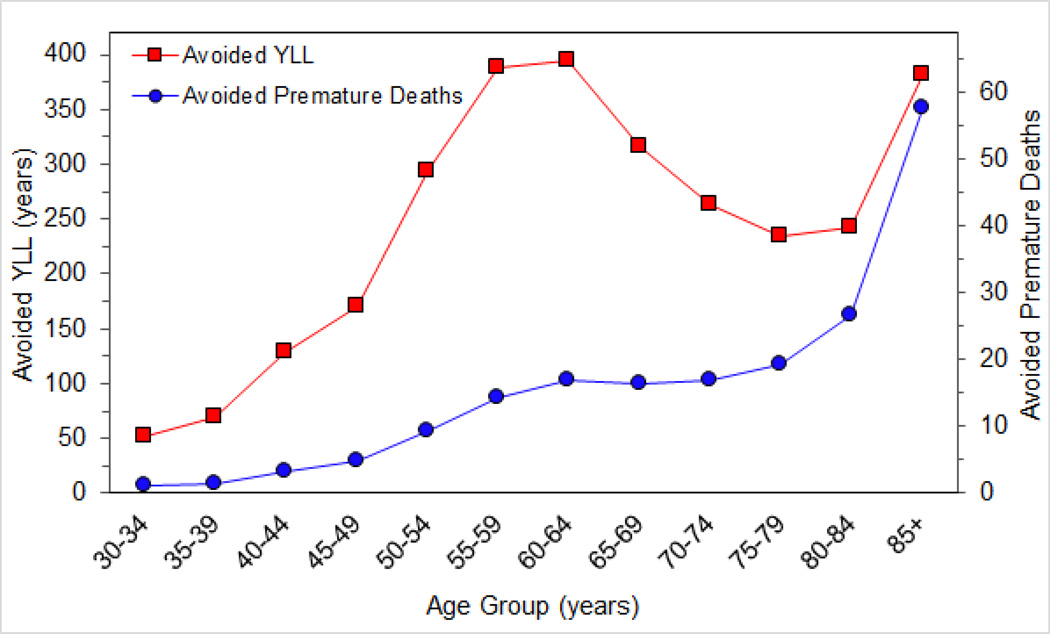
Avoided YLL, YLD and DALYs in the case study total 3052, 47 and 3099 years, respectively (Table 3). YLL is largest for the 60–64 year age group (17 premature deaths contribute 394 YLL; Figure 1). Premature deaths account for 98.5% of the total DALYs avoided in the population. Among morbidity outcomes, asthma exacerbations make the greatest contribution to population DALYs (30 YLD per year, Supplemental Table S7). Comparable contributions of YLLs and YLDs to the overall DALY have been reported elsewhere (de Hollander et al., 1999; de Hollander and Melse, 2006; Hofstetter and Hammitt, 2002).
Table 3.
DALYs and monetized impacts avoided per year for deaths, unscheduled hospitalizations and morbidity outcomes in Wayne County due to a reduction in PM2.5 concentration from 10 to 8 µg/m3.
| Outcome | DALYs1 (years)2 |
Monetized impacts3 (1000$)2 |
|---|---|---|
| Premature mortality3 | 3052 (2011, 4074) |
1,800,000 (1,200,000, 2,400,000) |
| All morbidities | 47 (−28, 108) |
36,000 (8,900, 57,000) |
| Total | 3099 (1982, 4182) |
1,900,000 (1,200,000, 25,000,000) |
| Percent attributable to mortality | 98.5 % | 94.8% |
DALYs are YLL for mortality outcomes and YLD for morbidity outcomes.
95% confidence interval in parentheses.
Monetized impacts are calculated using 2010$ projected to a 2020 income level and rounded to the nearest whole number with two significant figures.
Premature mortality is the sum of premature mortality among adults (>29 years, HIF method) and infant mortality (<1 year).
Figure 1.
Number of avoided premature deaths and years of life lost (YLL) per year by age group in Wayne County for a reduction in PM2.5 concentration from 10 to 8 µg/m3.
The total monetized health benefit of the 2 µg/m3 change in Wayne County exceeds $1.9 billion annually, most of which (95%) is due to premature mortality (Table 3, Supplemental Table S7). The most important morbidity outcomes are non-fatal myocardial infarctions (n=160, $23 million) and unscheduled hospitalizations (n= 150, $5.5 million). More common but less severe outcomes include work loss days (n=21,000, $3 million) and MRADs (n=37,000, $2.5 million). Though less frequent, hospitalization outcomes account for a large share of the monetized morbidity impacts. The large and dominant contribution of mortalities to the total monetized value parallels the PM2.5 RIA (US EPA, 2012) and a recent HIA in China (Voorhees et al., 2014).
For emissions-based metrics, the total monetized benefit (all mortality and morbidity outcomes) is $660,000 per ton of PM2.5, again, mostly due to mortality ($640,000 per ton; Table 4). Expressed using the number of cases, the more severe outcomes had the lowest benefit per ton, e.g., premature mortality were only 0.07 deaths avoided per ton, while avoided asthma exacerbations were 9.8 cases per ton and avoided MRAD were 13.1 cases per ton. The literature shows a wide range but generally lower benefits ($46,000 to $510,000 per ton PM2.5; Fann et al., 2012a). The higher estimates in the case study likely result from the simplified roll-back approach, which incompletely accounts for distance and dispersion between sources and people, e.g., reductions from elevated stacks and sources farther removed from populations are expected to have lower impacts per ton of pollutant emitted (Fann et al., 2009). Nevertheless, the estimates produced by the case study are reasonable given its limitations, e.g., uniform reduction and rollback approach.
Table 4.
Benefits per ton for a 2800 tons per year reduction of directly emitted PM2.5 in Wayne County.
| Outcome (age group) | Avoided Cases (cases per ton) |
Monetized Impacts1 ($ per ton) |
|---|---|---|
| All-cause premature mortality (>29 years, HIF method) | 0.067 | 643,845 |
| Infant mortality (<1 year) | 0.001 | 5,364 |
| Minor restricted activity days (18–64 years) | 13.058 | 888 |
| Asthma exacerbations (6–18 years)2 | 9.844 | 571 |
| Work loss days (18–64 years) | 7.346 | 1,102 |
| Asthma emergency department visit (> 1 year)2 | 0.067 | 29 |
| Non-fatal MI (≥ 18 years) | 0.057 | 8,173 |
| CV hospitalization (≥ 20years) | 0.030 | 1,247 |
| Pneumonia hospitalization (>64 years) | 0.009 | 334 |
| COPD hospitalization (≥20 years) | 0.009 | 264 |
| Asthma hospitalization (<65 years) | 0.007 | 107 |
| Total monetized benefits | 661,442 |
Monetized benefits are in 2010$ projected to a 2020 income level
Among persons with asthma
3.2.2 Case study limitations
The case study has a number of limitations. First, the same age-stratified baseline rates were applied across the population, and other sources of variability (e.g., neighborhood, gender) were omitted. Second, the population was held constant. Recent work demonstrates some sensitivity to population growth or mobility (Baccini et al., 2015; Flachs et al., 2013; Tchepel and Dias, 2011). Third, a single CR estimate was used, although other valid CR estimates are available and can be used to represent uncertainties (described later). Fourth, lags and discounting were ignored, which can overestimate premature mortality impacts and further increase the dominance of mortality impacts since YLL estimates are higher for deaths at younger ages (based on life expectancy). Fifth, the exposure scenario did not account for urban scale spatial heterogeneity (Batterman et al., 2014; Sparks et al., 2014), which should be considered to accurately predict impacts (Punger and West, 2013; Thompson et al., 2014). Sixth, impacts due to only PM2.5 were considered. Pollution control policies can affect multiple pollutants and have additional impacts and co-benefits. Lastly, both annual and daily concentrations were assumed to undergo the same change (2 µg/m3), following methods used in other HIAs (Fann et al., 2012b). However, this approach may underestimates changes in daily peak concentrations, which often arise from local sources, and it assumes that the same areas and populations are affected by annual and peak concentrations. Alternately, daily changes in PM2.5 and HIFs drawn from studies of short-term exposures could be used to determine short-term health impacts. Despite these limitations, the case study results mirror trends seen in other air pollution HIAs, and the evaluations and comparisons of the different metrics should be valid and applicable to other cities and scenarios.
3.3 Evaluation of HIA metrics
The criteria suggested for evaluating HIA metrics (Table 5) reflect several goals. First, metrics should be accurate and comprehensive with respect to the overall impacts expected on a population, otherwise impacts may be underestimated and lead to biased evaluations. Second, metrics should consider the spatial and temporal distribution of impacts, thus accounting for the variation in exposure and population susceptibility. This variation, along with equity considerations, will likely require stratification by factors related to individual or group-level susceptibilities, e.g., age, socioeconomic status, or race/ethnicity (O’Neill et al., 2008). Third, since impacts are associated with both short- and long-term exposure to pollutants, and long-term impacts may lag several years, accounting for lags is important. Fourth, metrics should be easily understood by a wide audience, particularly given limited understanding of HIA techniques, pollutant impacts, and the presence of competing interests, e.g., economic or political considerations. Fifth, predicted impacts and valuations in the metrics are inherently uncertain, and uncertainties are propagated as the number of required data inputs increases. Both quantitative uncertainty analyses and descriptive characterizations are needed to describe uncertainties and aid interpretation. The next section contrasts each metric against the evaluative criteria, drawing on case study results to highlight key points. Table 6 summarizes this evaluation.
Table 5.
Criteria used to evaluate potential metrics or urban-scale health impact assessments.
| Criterion | Description and Implications | References |
|---|---|---|
| Interpretability | Readily understood by lay audiences without need for complex technical explanations |
AbouZahr et al., 2007; Murray, 2007; |
| Comparability | Can be compared between different populations, control scenarios or policy alternatives |
Sanderson et al., 2006 Walker et al., 2007 |
| Comprehensiveness | Measures the total impact on population health by including all relevant outcomes relevant to the pollutant of interest Considers timing and severity of the outcomes Includes multiple exposure pathways or health determinants (e.g., a public transit policy may reduce air pollution exposures and promote physical activity) |
Bell et al., 2011; Rabl, 2003; Wong et al., 2003 |
| Representativeness | Data inputs reflect the baseline health status and demographics of the study population |
Bell et al., 2011; Hubbell et al., 2009 |
| Spatial Resolution | Air pollution concentration estimates and baseline health data reflect the heterogeneity in a population’s demographics, health status and exposures The boundaries of the HIA are appropriate for the proposed project or policy (i.e., city-wide policies vs. localized projects or programs) |
Batterman et al., 2014; Kheirbek et al., 2013; Thompson et al., 2014 |
| Temporal Resolution | Impacts of acute and chronic exposures assessed Accounts for anticipated changes in population (e.g., age structure, baseline rates) over time Considers lag between the timing of exposure and outcome occurrence |
Bell et al., 2011; Flachs et al., 2013 |
| Relevant environmental stressors |
Considers changes in multiple pollutants and pollutant interactions (e.g., a policy to reduce PM may also influence NOx or O3 concentrations which have additional impacts) |
Burnett et al., 2005; Dominici et al., 2010 |
| Equity | Disaggregates population subgroups by vulnerability or susceptibility (i.e., race/ethnicity, age, geographic location) |
Jerrett et al., 2004; O’Neill et al., 2008 |
| Consideration of uncertainty |
Identifies and evaluates uncertainty Uncertainties are communicated effectively |
Walker et al., 2007 |
Table 6.
Summary of strengths and weaknesses of metrics used in urban-scale health impact assessments.
| Metric | Strengths | Limitations | |
|---|---|---|---|
| 1a | Predicted number of premature deaths, disease cases or unscheduled hospitalizations e.g., 190 premature mortalities avoided in Wayne county per year |
Easy to interpret Demonstrates the magnitude of an impact on a population based on the number people potentially affected Population specific input data lead to estimates that reflect underlying health status and susceptibility to adverse outcomes Stratification based on vulnerability or susceptibility may lead to equity considerations |
Comprehensiveness is dependent on the identification and inclusion of all relevant outcomes Dependent on selection of CR and on the baseline rates for outcomes Provides no information on the duration or permanence of the impacts Not all health impacts are independent; can lead to biased estimates if this dependence is not accounted for Cannot be compared directly across populations of differing size |
| 1b | Percent attributable e.g., 1.16% of annual deaths in Wayne County would be avoided |
Explains what fraction of the population burden is attributable to air pollution Indicates which option may be more beneficial in reducing the incidence of a specific adverse outcome |
Interpretation can be limited if estimates for other exposures are not available for comparison |
| 1c | Attributable rate e.g., 10.5 avoided premature moralities per 100,000 people |
Makes metrics comparable between populations of differing size |
Rates can be harder to interpret for those unfamiliar with their use (Walker et al., 2007) |
| 2 | DALYs | Measures mortality and morbidity in one metric using time as a common unit Accounts for the severity and permanence of an outcome (e.g., duration and disability weight) |
Age-weighting and assignment of disability weights can be uncertain or controversial Diminished importance of morbidity outcomes due to weighting factors |
| 3 | Monetized impacts | Often used in regulatory analyses and HIAs; can facilitate comparisons with other types of impacts (e.g., economic) Frames health outcomes in the same manner as non-health considerations Facilitate cost-effectiveness or cost-benefit analyses |
US EPA methods for monetized premature mortality does not consider the number of years of life lost, only the total number of premature deaths May not accurately reflect the total societal costs of morbidity outcomes |
| 4 | Functional-unit based metrics (e.g., benefits per ton) |
Appropriate when changes in ambient concentrations are difficult to predict but estimated changes in emissions are available Can identify emission sources for targeted reductions (e.g., sector specific metrics) |
Need to account for the location, proximity to populations, and type of emissions source Impacts (benefits) per ton estimates can be very uncertain depending on the data inputs |
3.3.1 Predicted cases
The inclusion of MRAD, work loss days, and other transient but relatively common morbidity outcomes yields more comprehensive analyses by indicating the numbers of people potentially affected. This can increase the HIA's salience, especially for diseases like childhood asthma that represent important public health issues. The case study (like most other HIAs) included only those outcomes where the weight of evidence for an association is strong. Less evidence exists regarding associations between PM2.5 and other outcomes, including cancer and adverse birth outcomes. Following US EPA methods (US EPA, 2012a), these impacts were excluded. Such omissions may lead to systematic negative biases (O’Connell and Hurley, 2009).
The CR function is arguably the most important and most uncertain input in predicting attributable cases. The case study used a single CR function for most outcomes. Other CR estimates may be valid and available, and can be used to bound expected ranges or show uncertainties, e.g., Fann et al. (2012) noted a 2.5-fold variation in the number of premature deaths using CRs derived from two large multicity observational studies (Laden et al., 2006; RR= 1.16 per 10 µg/m3 vs. Krewski et al., 2009, RR=1.06 per 10 µg/m3). This variation may reflect differences in study population demographics, exposure patterns, and other factors. CR estimates should be drawn from well conducted epidemiological studies with sufficient statistical power. For local-scale HIAs, city-specific CR estimates, ideally from large multi-city studies, may be advantageous because they account for specific population characteristics. However, such estimates often are not available. Large multicity cohort studies or meta-analyses can have considerable statistical power, but may not be fully representative of the population for the HIA. When selecting CR estimates for local-scale HIAs, it is important to consider how the original study population differs from the one included in the HIA (Hubbell et al., 2009).
Table 2 demonstrates the sensitivity of HIA results to the baseline health data. Compared to national averages (CDC, 2014), attributable rates increase when using data specific to Wayne County (by 18%) and Detroit (by 22%). Thus, the same 2 µg/m3 reduction yields a larger impact in Detroit given the susceptible population. Using local data in urban-scale HIAs can account for population susceptibility. In addition, baseline health as well as other vulnerability or susceptibility factors are likely to be unevenly distributed across an urban region, e.g., rates of asthma hospitalizations vary 3-fold across the study region and some of the highest rates are seen in more polluted areas. Areas with higher asthma rates are expected to have more avoided asthma exacerbations than areas with lower rates, given the same PM2.5 level. The use of spatially-resolved health and exposure data should increase the accuracy of HIA results, and could allow for the development of strategies that target pollutant reductions in areas that confer the greatest benefits
Most (74%) of the cause-specific deaths are due to ischemic heart disease (IHD, Supplemental Table S8). The number of cause-specific deaths avoided (n=230) slightly exceeds the number of all-cause premature deaths (n=190; Table 1). The CR functions used for cause-specific mortality were developed specifically for the latest GBD study (Lim et al., 2012). These non-linear functions were derived from studies examining ambient air pollution, active smoking, secondhand smoke exposure, and cooking smoke exposure (Burnett et al., 2014). The shape of each cause-specific CR curve differs (see Burnett et al., 2014, Figure 1), e.g. for IHD, the slope is steeper at lower concentrations and tends to flatten at higher PM2.5 levels. At low concentrations (including the 8 to 10 µg/m3 in the present analysis) where the IHD CR function is steepest, the PAF method gives a higher number of cause-specific deaths than the all-cause deaths estimated by the HIF. Generally, predictions using non-linear CR functions depend on the baseline and scenario concentrations, e.g., lowering PM2.5 concentrations from 11 to 9 µg/m3 avoids 182 cause-specific mortalities (21% fewer deaths than the 10 to 8 µg/m3 scenario). The numbers of deaths predicted for these concentrations (679 and 498 deaths at 11 and 9 µg/m3, respectively) exceed those in the original case study (596 and 370 deaths at 10 and 8 µg/m3, respectively), but differences between the baseline and scenario deaths decreases at higher concentrations. These differences are small compared to uncertainties, as discussed below.
The case study also compared estimates of all-cause mortality predicted by the HIF and PAF methods using the same CR function from Krewski et al. (2009) (Table 1). For the specified scenario, the two methods gave the same number of avoided deaths (n=190). Differences between the HIF and PAF methods also result from differences in how health impacts are calculated, i.e., the HIF method uses the concentration difference (Δx = 2 µg/m3 in the scenario), while the PAF method compares attributable burdens across scenarios (Δx = 10 µg/m3 – CFC). Thus, lowering PM2.5 from 20 to 18 µg/m3 still gives 190 avoided all-cause premature deaths using the HIF method, but PAF predictions decrease to 176 premature deaths. While these differences are small, the influence on DALYs and monetized impacts is large given the high values assigned to premature mortalities (discussed later).
The HIF (eq. 1) in the case study can predict short-term impacts, but with greater uncertainty than for long-term impacts. This paper focused on changes in long-term (annual average) concentrations, and mortality CR functions based on studies of chronic exposures were used, although the morbidity CR estimates came from short-term exposure studies (Supplemental Table S3). To derive short term impacts, it may be preferable to use mortality CR estimates drawn from short-term exposures studies (e.g., time series studies) with estimates of short-term pollutant concentrations (e.g., daily PM2.5 concentrations). As noted earlier, short-term exposures likely will exhibit greater spatial and temporal variation than annual average concentrations, depending on proximity to the local emissions sources, meteorology, and other factors.
3.3.2 Disability-adjusted life years
YLLs, YLDs and DALYs facilitate comparison of impacts among different groups or cohorts in the population. For example, the number of avoided deaths in the 60–64 year age group is 36% lower than avoided deaths in the 80–84 year age group, but the avoided YLLs in the younger group is 62% higher (Figure 1). Given the severity of premature deaths (quantified as the YLL), the 60–64 year age group receives the greatest benefit from the PM2.5 reduction. YLL may be particularly meaningful for premature mortality since deaths are delayed, rather than avoided (Rabl, 2003).
YLDs tend to deemphasize morbidity outcomes given the short durations of these impacts (e.g., 1 to 5 days) and the small DWs assigned (Table 3, Supplemental Table S4). For example, given the duration of an asthma exacerbation (0.005 years) and its DW (0.22), the 28,000 asthma exacerbations avoided annually in the case study contributed only 30 YLDs to the total 3,100 DALYs predicted (Supplemental Table S7). For asthma, estimated YLDs due to emergency department visits or exacerbations may be underestimated since asthma exacerbations are under-reported (Reddel et al., 2009) and the time lost to avoidance behaviors (e.g., not participating in recreational activities) are excluded, potentially biasing HIA results.
This discussion highlights several issues when disparate health outcomes are combined on the basis of duration and severity. In contrast, metrics using the number of cases avoided treat each outcome equally and avoid issues related to subjective weightings (de Hollander and Melse, 2006). Others argue that consideration of duration and severity is required to make trade-off comparisons between high and low impact outcomes (Wong et al., 2003).
3.3.3 Monetized Impacts
Like DALYs, monetized benefits of air pollution depend on outcomes included, but are driven by mortality, again due to the high value assigned to a statistical life. The VSL used for mortality ($9.6 million) far exceeds values for each morbidity outcomes (Supplemental Table S5). The lack of cessation lags and discounting in the case study is not expected to substantially alter results given the low social discount rates (3 to 7%) recommended (US EPA, 2012a). The valuation method endorsed by US EPA and used in the case study does not monetize deaths based on age using the VSLY or other approaches (unlike the DALYs in the previous section that considered the timing of death in estimating YLLs) (US EPA, 2010). VSL may overstate the value of premature deaths since deaths are delayed, rather than completely avoided. However, VSLY or methods that adjust VSL based on age can raise contentious issues regarding the value of a life saved for older populations (Robinson, 2007), and US EPA found little evidence to support age adjustments in VSL estimates (US EPA, 2010).
Monetized impacts, like DALYs, deemphasize morbidity outcomes due to their low and uncertain valuations. Morbidity outcomes are difficult to monetize accurately, and the WTP may underestimate the true societal costs. For example, asthma exacerbations accounted for only 0.08% of the total monetized impacts in the case study, despite being the second-most frequently avoided morbidity outcome (Supplemental Table S7). The value of $58 assigned to each exacerbation (Rowe and Chestnut, 1986) may incompletely account for medical costs or time lost at work or school. Monetized metrics may not reflect the sentiments of affected communities in Detroit and other urban areas where asthma outcome rates greatly exceed state and national norms (Wasilevich et al., 2008). The dominance of mortality outcomes has been demonstrated in Shanghai, China where air pollution-related deaths made up 92.5% of total monetized impacts (800 deaths monetized at 1.2 billion yuan compared to 420,000 morbidities monetized at 0.09 billion yuan) (Voorhees et al., 2014). Similarly, US EPA's recent RIA for ozone (O3) showed that 98% of the total monetized benefits ($2.0 to $3.4 billion) of a 70 ppb ozone standard would be due to avoided premature mortality from both short- and long term exposures (880 to 1,020 avoided deaths) (US EPA, 2014).
Monetized metrics have been used in HIAs to facilitate comparisons among heath- and non-health outcomes. For example, greater utilization of public transport that lowers pollutant levels (due to less personal vehicle use) will increase physical activity (due to additional walking), which promotes health. In a recent assessment of a Boston area proposal to alter transit pricing, the monetized impacts of physical activity ($75 million) far exceeded air pollution’s impacts ($1.5 million) (James et al., 2014). Including the co-benefits of air quality management can provide decision makers with information about the total impact of a strategy on public health, and potentially additional impetus for recommendations. Such analyses can increase the HIA’s scope, complexity and uncertainty, but may be of particular value when the co-benefits are substantial.
3.3.4 Emissions based metrics
Emissions-based metrics are useful when it is more feasible to estimate changes in emissions rather than ambient concentrations, e.g., for a policy that requires the use of a specific control technology. Emission-based metrics also can identify specific emission sources that impose the greatest burden on the population given that contributions from specific sources to local air quality are known. The degree to which a specific source impacts the health of a population depends on a number of factors, including the proximity of the source to the population and local meteorological patterns. In order to use emissions-based metrics effectively, emissions inventory data need to be combined with dispersion modeling, population data, HIFs and the other data described previously (Fann et al., 2009). The case study assumed a 2 µg/m3 PM2.5 reduction using equal emissions reductions across multiple sectors, did not account for the distribution of sources in the population, and calculated aggregate benefits per ton. The highest benefit per ton of emissions reduced likely will occur for sources or sectors in populated areas that release pollutants near ground level, e.g., on- and off-road diesel engines in densely populated cities. Such analyses can be data intensive and potentially complex, thus data gaps and model uncertainties should be recognized and communicated to key decision makers and stakeholders. In cases, national average values for benefits per ton are available (e.g., US EPA, 2013b). However, each HIA scenario and each city may uniquely influence effects of location-specific characteristics (e.g., location, type, meteorological trends).
3.3.5 Uncertainty in health impact metrics
A simplified uncertainty analysis demonstrates the variability of HIA results due to CR estimates, identified as the single most important source of uncertainty for urban-scale HIAs (Chart-asa and Gibson, 2015). The most uncertain impacts are the low-severity outcomes, as shown by the wide confidence intervals (CIs) for the number of avoided impacts (Table 1). The CIs are symmetrical for mortality, respiratory and cardiovascular hospitalizations, emergency department visits, MRAD and WLD since the underlying CR estimates use log-linear models, while asymmetrical CIs result for non-fatal heart attacks and asthma exacerbations since these outcomes are based on logistic regression models. For the latter, the large upper "tail" of the distribution can greatly increase impacts, e.g., the MC analyses for non-fatal heart attacks and asthma exacerbations give means that are 6 and 11% higher, respectively, than the deterministic estimates that use the mean CR estimate (Table 1). Large CIs can cause additional issues, e.g., the 16% of the MC simulations for asthma exacerbations gave disbenefits (negative avoided impacts) (using the HIF estimate in Supplemental Table S3 and a CR function drawn from a single study, Mar et al., 2004). Such implausible outcomes highlight the need to use CR estimates from well-powered studies that have small standard errors, to pool CR estimates among multiple studies, or to truncate negative avoided impacts.
Uncertainty estimates due to parameter uncertainty have been estimated using MC analyses, Bayesian methods, and sensitivity analyses, and a few HIAs have considered uncertainties in model structures (e.g., Baccini et al., 2015; Chanel et al., 2014; Chang et al., 2014; Chart-asa and Gibson, 2015; Woodcock et al., 2014; Xia et al., 2015; reviewed in Mesa-Frias et al., 2013). For the number of avoided cases, uncertainty arises from CR estimate, baseline health outcome rates, and changes in exposure concentrations. These uncertainties are propagated to and potentially increase for other metrics, e.g., as mentioned, additional uncertainties in DALYs include the duration of outcomes and the subjective assignment of disability weights. Similarly, monetized metrics must contend with the subjectivity and variability of valuations. Ideally, uncertainty analyses would consider all sources of variability, including dependencies among inputs. If the total uncertainties among competing mitigation strategies are very large, then quantitative HIAs may not inform the remedy selection, and decisions may rest on economic or other criteria. However, estimates of both health impacts and uncertainties can motivate the need for mitigation, especially if decision makers are risk averse (IOM, 2013). For example, an MC analysis examining health impacts due to vehicle emissions in Chapel Hill, North Carolina (examining uncertainty in CR estimates, PM2.5 emissions, exposure concentrations and demographics) gave a substantially higher number of cases compared to deterministic results (Chart-asa and Gibson, 2015). Such uncertainties should be calculated and reported to decision makers.
Quantitative uncertainty analyses themselves have shortfalls. There are substantial data gaps regarding the variability and uncertainty of data, as well as the interactions among variables. Many analyses use a simplified bounding approach that does not indicate the likelihood or confidence intervals of possible outcomes. As mentioned, weight-of-evidence limitations may preclude consideration of potentially important outcomes. For these reasons, characterizing the limitations of the uncertainty analysis itself is important.
3.3.6 Co-benefits of air pollution management
Although excluded in the case study, HIA metrics can incorporate co-benefits of pollution control policies. For example, incentivizing active transportation to replace short car trips reduces emissions and can increase physical activity with significant health benefits (Maizlish et al., 2013). Strategies that promote active and public transportation also decrease the frequency of traffic-related car crashes (Rojas-Rueda et al., 2013; Xia et al., 2015). Increasing tree cover in cities removes pollutants from urban air sheds (Nowak et al., 2013) and can be advantageous for surface cooling and storm water management (Loughner et al., 2012; Wang et al., 2008).
Climate change mitigation and adaptation are major co-benefits of air pollution management. The transportation sector is responsible for 27% of total greenhouse gas (GHG) emissions in the US (US EPA, 2015c) and 23% of CO2 emissions globally (IEA, 2014). Transportation policies aimed at reducing primary pollutant emissions, particularly those that reduce travel demand or fuel consumption, lead to reductions in GHG emissions (McCollum and Yang, 2009). Comparisons between policy options should consider the health impacts of reduced primary pollutant emissions as well as the environmental and health benefits of reduced GHG emissions.
Co-benefits can be indirect, secondary in nature and long term, and thus difficult to assess. Still, using common metrics to link these outcomes to public health makes the health metrics more comprehensive and compelling. Again, appropriate outreach and education may be required to inform decision makers.
3.3.7. Challenges of the use of quantitative HIA methods
A number of challenges may be encountered when applying the methods in this paper to other regions. First, urban-scale HIAs are best conducted using local baseline health data that reflect the health status of the population (Hubbell et al., 2009). Sub-national health data may not be available where public health resources are limited, e.g., developing countries, especially for morbidity outcomes (Boerma and Stansfield, 2007). Second, most epidemiological studies have been conducted in the USA and Europe where concentrations tend to be lower than other parts of the world, and CR estimates derived from these studies used in other populations have limitations, e.g., while the GBD risk estimates combine several exposure sources, estimates include only mortality outcomes and respiratory infections in young children (Burnett et al., 2014). Third, suitable (e.g., long-term) air quality data for exposure assessment may not be available in many regions. While concentrations can be estimated, e.g., satellite data and global chemical transport models have been used to derive concentrations at coarse spatial resolution (10 km × 10 km, van Donkelaar et al., 2010), such methods also have uncertainties and may not capture urban-scale patterns necessary for local-scale HIAs. Fourth, regions differ with respect to pollutant sources, e.g., vehicle emissions may dominate exposures in the developed countries, while cooking and home heating emissions from biomass combustion may dominate exposures in developing countries. Such differences will shape the nature of control strategies. Due to these and possibly other reasons, site-specific, comprehensive and quantitative HIAs may not be feasible in some regions. Still, approximations using the approach with surrogate or estimated data may be valuable, and can serve to highlight data gaps.
3.4 Recommendations
Several recommendations follow from our analysis of the literature and the case study. First, if requisite data are available, HIAs should use quantitative metrics to assess health impacts and provide meaningful evidence regarding health benefits to decision makers formulating air quality management plans (Fann et al., 2011). Quantitative analyses permit explicit comparisons between options, better characterization of the magnitude of impacts for specific outcomes, estimates of the total number of people affected, and a framing of health outcomes in the same manner as other policy considerations. Quantitative metrics also enable decision makers to more readily incorporate HIA results into the policy process (Davenport et al., 2006). Because they describe concentration-dependent impacts, such metrics allow estimates of benefits for air quality improvements that go beyond standard attainment. The case study example was limited to PM2.5 reductions, but multi-pollutant frameworks should be used (Dominici et al., 2010; Johns et al., 2012; Oakes et al., 2014).
Second, since no single metric fully meets the evaluative criteria, the use of several complementary indicators is recommended. The number of cases avoided is simple and easy to interpret, but does not account for the severity of the outcome. To be comprehensive, urban-scale HIAs should report the number of avoided cases for multiple relevant health outcomes, and cases should be disaggregated into subgroups to allow consideration of equity, location, race/ethnicity, age, and other relevant factors. Consideration of multiple health outcomes in quantitative HIAs yields estimates of the total number of people affected, an important indicator itself. However, such estimates may undercount the total number of people affected since not all outcomes are captured. In addition, estimates of morbidity outcomes can have considerable uncertainty, and possible outcomes with limited evidence are excluded. Despite these limitations, inclusion of morbidity outcomes is important for evaluating strategies that may not lead to substantial numbers of avoided deaths, and for minimizing biases that would tend to underestimate the public health impacts. Estimates of morbidity outcomes and the number of people affected should be recognized as a "low end" estimate that complements all-cause mortality estimates, and that provides additional information useful for comparing among management options. DALYs incorporate the severity, duration and timing of outcomes, but are complex and require additional data inputs (including uncertain disability weights); moreover, decision makers may not readily understand this metric. Still, DALYs find widespread use in studies of population health, and can aggregate health impacts of policies and programs allowing comparisons across studies. Monetized impacts share many of the same uncertainties as DALYs, and similarly, are driven by mortality, however, these metrics are familiar to decision makers and can be used in other policy evaluations (e.g., cost-benefit analysis). No single summary measure fully captures societal impacts associated with morbidity. Still, HIAs should utilize a composite indicator like DALYs or monetized values that allow ranking of options.
Third, metrics should be tailored to the local context. Some metrics may be particularly useful and favored in certain applications. For example, emissions-based metrics can facilitate comparisons between sectors and between options within a sector, and maybe particularly useful if policy options involve control technologies or if monetized benefits per ton vary considerably by sector and source. Urban-scale HIAs are not limited to an emissions context, and can also be used to inform decision makers about the benefits of alternative exposure reduction strategies, e.g., use of vegetative buffers along highways, rerouting trucks to avoid residential neighborhoods, or use of indoor air filtration. Evidence from quantitative HIAs can encourage decision makers to implement such interventions, especially when the number of people affected is high and the intervention is viewed as cost-effective.
Fourth, community values should be considered in selecting metrics that are appropriate for urban scale HIAs. DALYs and monetized impacts place a high value on mortality. However, morbidity outcomes are far more common. DALYs or dollars might poorly capture local attitudes regarding less severe outcomes, e.g., asthma exacerbations. Engagement with stakeholders at early stages of the HIA would best serve to prioritize outcomes and metrics (Dannenberg et al., 2006).
Fifth, HIAs are strengthened by drawing on local information, including emission and dispersion data, to understand source-receptor relationships, including spatial variability, demographic and vulnerability information, and epidemiological evidence for concentration-response functions.
Sixth, environmental and health co-benefits of air quality management strategies, including climate change mitigation, should be identified. When requisite data are available, these co-benefits should be quantified using the same metrics selected for air pollution health impacts, thus increasing the comprehensiveness of the overall assessment of control strategies.
Lastly, quantitative HIAs may underestimate the total impact of a policy or program because certain environmental or health impacts cannot be reliably quantified. Qualitative methods can augment the quantitative analyses and identify potential health and environmental outcomes that do not have reliable CR estimates, e.g., cancer and adverse birth outcomes.
4. Conclusions
This study reviewed quantitative metrics in recent HIAs addressing air pollutant exposure, and developed evaluative criteria for selecting and using metrics. The metrics were illustrated in a case study for the Detroit, Michigan area. Quantitative metrics describing the direction, magnitude and severity of expected health impacts can help inform decision makers and elevate health concerns to the level of other political and economic drivers into evaluations of projects, programs and policies. Different metrics prioritize different health outcomes. For examples, the number of avoided cases emphasizes common but lower severity impacts (e.g., minor restricted activity days and asthma exacerbations), while monetized impacts and DALYs emphasize the relatively small number of premature mortalities.
A number of recommendations were developed for selecting metric appropriate for air quality applications. Metrics should be comprehensive, identify the number of people affected for each morbidity and mortality outcome, and clearly communicate both direct and indirect impacts. Further, metrics should use local data (e.g., baseline rates from the study population), incorporate outcomes of high public health importance, and represent the spatial and temporal dimensions of impacts. Uncertainties and limitations should be characterized quantitatively and qualitatively, and reported to decision makers. While appropriate metrics depend on the application, most HIAs would benefit from several metrics that capture impacts to specific population groups as well as overall health impacts.
Highlights.
HIAs have used many metrics, including avoided cases, DALYs and monetized impacts.
There is a need to identify appropriate metrics for use in urban-scale air pollution HIA.
Metrics should be comprehensive, spatially and temporally resolved, and account for vulnerability.
Metrics should evaluate and clearly present uncertainty.
The use of multiple metrics is suggested to fully characterize the impacts of a proposed policy.
Acknowledgments
Role of the funding source
Support for this research was provided by grants 5R01ES022616 and P30ES017885 from the National Institute of Environmental Health Sciences, National Institutes of Health, and by grant T42 OH008455 from the National Institute of Occupational Health and Safety. These funding sources had no involvement in the collection, analysis, interpretation, writing, or submission of this paper.
Abbreviations
- AQBAT
Air Quality Benefits Assessment Tool
- BenMAP
Environmental Benefits Mapping and Assessment Program
- CDC
Centers for Disease Control and Prevention
- CFC
Counterfactual concentration
- COI
Cost of illness
- COPD
Chronic obstructive pulmonary disease
- CR
Concentration-response
- DALYs
Disability-adjusted life years
- DW
Disability weight
- GBD
Global burden of disease
- GHG
Greenhouse gas
- HIA
Health impact assessment
- HIF
Health impact function
- IHD
Ischemic heart disease
- LC
Lung, trachea and bronchus cancer
- LCA
Life cycle analysis
- LE
Life expectancy
- MC
Monte Carlo
- MRAD
Minor restricted activity days
- NOx
Oxides of nitrogen
- O3
Ozone
- PAF
Population attributable fraction
- PM2.5
Particulate matter less than 2.5 µm in diameter
- QALY
Quality adjusted life year
- RIA
Regulatory impact analysis
- RR
Relative risk
- US EPA
United States Environmental Protection Agency
- VSL
Value of a statistical life
- VSLY
Value of a statistical life year
- WHO
World Health Organization
- WTP
Willingness to pay
- YLD
Years living with disability
- YLL
Years of life lost
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflicts to declare.
References
- AbouZahr C, Adjei S, Kanchanachitra C. From data to policy: good practices and cautionary tales. The Lancet. 2007;369:1039–1046. doi: 10.1016/S0140-6736(07)60463-2. [DOI] [PubMed] [Google Scholar]
- Akobundu E, Ju J, Blatt L, Mullins CD. Cost-of-illness studies - A review of current methods. Pharmacoeconomics. 2006;24:869–890. doi: 10.2165/00019053-200624090-00005. [DOI] [PubMed] [Google Scholar]
- Anderson RN. A method for constructing complete annual U.S. life tables. Centers for Disease Control and Prevention. 1999 [PubMed] [Google Scholar]
- Baccini M, Grisotto L, Catelan D, Consonni D, Bertazzi PA, Biggeri A. Commuting-Adjusted Short-Term Health Impact Assessment of Airborne Fine Particles with Uncertainty Quantification via Monte Carlo Simulation. Environ. Health Perspect. 2015;123:27–33. doi: 10.1289/ehp.1408218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterman S, Chambliss S, Isakov V. Spatial resolution requirements for traffic-related air pollutant exposure evaluations. Atmos. Environ. 2014;94:518–528. doi: 10.1016/j.atmosenv.2014.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Morgenstern RD, Harrington W. Quantifying the human health benefits of air pollution policies: Review of recent studies and new directions in accountability research. Environ. Sci. Policy. 2011;14:357–368. [Google Scholar]
- Berman JD, Fann N, Hollingsworth JW, Pinkerton KE, Rom WN, Szema AM, Breysse PN, White RH, Curriero FC. Health benefits from large-scale ozone reduction in the United States. Environ. Health Perspect. 2012;120:1404–1410. doi: 10.1289/ehp.1104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R, Farhang L, Heller J, Orenstein M, Richardson M, Wernham A. Minimum elements and practice standards for health impact assessments, Version 3. 2014 [Google Scholar]
- Bhatia R, Seto E. Quantitative estimation in Health Impact Assessment: Opportunities and challenges. Environ. Impact Assess. Rev. 2011;31:301–309. [Google Scholar]
- Bhatia R, Wernham A. Integrating human health into environmental impact assessment: An unrealized opportunity for environmental health and justice. Environ. Health Perspect. 2008;116:991–1000. doi: 10.1289/ehp.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma JT, Stansfield SK. Health statistics now: are we making the right investments? The Lancet. 2007;369:779–786. doi: 10.1016/S0140-6736(07)60364-X. [DOI] [PubMed] [Google Scholar]
- Boldo E, Linares C, Aragonés N, Lumbreras J, Borge R, la Paz D, de Pérez-Gómez B, Fernández-Navarro P, García-Pérez J, Pollán M, Ramis R, Moreno T, Karanasiou A, López-Abente G. Air quality modeling and mortality impact of fine particles reduction policies in Spain. Environ. Res. 2014;128:15–26. doi: 10.1016/j.envres.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Bower BT, Brady GL. Benefit-cost analysis in air quality management. Environ. Sci. Technol. 1981;15:256–261. doi: 10.1021/es00085a604. [DOI] [PubMed] [Google Scholar]
- Briggs DJ. A framework for integrated environmental health impact assessment of systemic risks. Environ. Health. 2008;7:61. doi: 10.1186/1476-069X-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett RT, Bartlett S, Jessiman B, Blagden P, Samson PR, Cakmak S, Stieb D, Raizenne M, Brook JR, Dann T. Measuring progress in the management of ambient air quality: the case for population health. J. Toxicol. Environ. Health A. 2005;68:1289–1300. doi: 10.1080/15287390590936157. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Pope CA, Ezzati M, Olives C, Lim SS, Mehta S, Shin HH, Singh G, Hubbell B, Brauer M, Anderson HR, Smith KR, Balmes JR, Bruce NG, Kan H, Laden F, Prüss-Ustün A, Turner MC, Gapstur SM, Diver WR, Cohen A. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect. 2014;122:397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzcu-Guven B, Brown SG, Frankel A, Hafner HR, Roberts PT. Analysis and apportionment of organic carbon and fine particulate matter sources at multiple sites in the Midwestern United States. J. Air Waste Manag. Assoc. 2007;57:606–619. doi: 10.3155/1047-3289.57.5.606. [DOI] [PubMed] [Google Scholar]
- Cárdaba Arranz M, Muñoz Moreno MF, Armentia Medina A, Alonso Capitán M, Carreras Vaquer F, Almaraz Gómez A. Health impact assessment of air pollution in Valladolid, Spain. BMJ Open. 2014;4:e005999. doi: 10.1136/bmjopen-2014-005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC] [accessed 12.2.14];CDC WONDER [WWW Document] 2014 URL http://wonder.cdc.gov/
- Chanel O, Henschel S, Goodman PG, Analitis A, Atkinson RW, Le Tertre A, Zeka A, Medina S Aphekom group. Economic valuation of the mortality benefits of a regulation on SO2 in 20 European cities. Eur. J. Public Health. 2014;24:631–637. doi: 10.1093/eurpub/cku018. [DOI] [PubMed] [Google Scholar]
- Chang HH, Hao H, Sarnat SE. A statistical modeling framework for projecting future ambient ozone and its health impact due to climate change. Atmos. Environ. 2014;89:290–297. doi: 10.1016/j.atmosenv.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart-asa C, Gibson JM. Health impact assessment of traffic-related air pollution at the urban project scale: Influence of variability and uncertainty. Sci. Total Environ. 2015;506–507:409–421. doi: 10.1016/j.scitotenv.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Chestnut LG, Mills DM, Cohan DS. Cost-benefit analysis in the selection of efficient multipollutant strategies. J. Air Waste Manag. Assoc. 1995. 2006;56:530–536. doi: 10.1080/10473289.2006.10464524. [DOI] [PubMed] [Google Scholar]
- Dannenberg AL, Bhatia R, Cole BL, Dora C, Fielding JE, Kraft K, McClymont-Peace D, Mindell J, Onyekere C, Roberts JA, Ross CL, Rutt CD, Scott-Samuel A, Tilson HH. Growing the Field of Health Impact Assessment in the United States: An Agenda for Research and Practice. Am. J. Public Health. 2006;96:262–270. doi: 10.2105/AJPH.2005.069880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A, Wernham A. Health impact assessment in the USA. In: Kemm J, editor. Health Impact Assessment: Past Achievement, Current Understanding, and Future Progress. Oxford, UK: Oxford University Press; 2013. [Google Scholar]
- Davenport C, Mathers J, Parry J. Use of health impact assessment in incorporating health considerations in decision making. J. Epidemiol. Community Health. 2006;60:196–201. doi: 10.1136/jech.2005.040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot WT. Environmental Management in Practice: Volume I. New York, NY: Routledge; 1998. Problem-In-Context: A framework for the analysis, explanation and solution of environmental problems. [Google Scholar]
- de Hollander AE, Melse JM, Lebret E, Kramers PG. An aggregate public health indicator to represent the impact of multiple environmental exposures. Epidemiol. Camb. Mass. 1999;10:606–617. [PubMed] [Google Scholar]
- de Hollander AEM, Melse JM. Valuing the health impact of air pollution: deaths, dalys or dollars?, in: Air Pollution and Health, Air Pollution Reviews. Imperial College Press. 2006:197–239. [Google Scholar]
- Dias D, Tchepel O, Carvalho A, Miranda AI, Borrego C. Particulate matter and health risk under a changing climate: assessment for Portugal. Scientific World Journal. 2012;2012:409546. doi: 10.1100/2012/409546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: Shifting from a single-pollutant to a multipollutant approach. Epidemiology. 2010;21:187–194. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann N, Baker KR, Fulcher CM. Characterizing the PM2·5-related health benefits of emission reductions for 17 industrial, area and mobile emission sectors across the U.S. Environ. Int. 2012a;49:141–151. doi: 10.1016/j.envint.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Fann N, Fulcher CM, Baker K. The recent and future health burden of air pollution apportioned across U.S. sectors. Environ. Sci. Technol. 2013;47:3580–3589. doi: 10.1021/es304831q. [DOI] [PubMed] [Google Scholar]
- Fann N, Fulcher CM, Hubbell BJ. The influence of location, source, and emission type in estimates of the human health benefits of reducing a ton of air pollution. Air Qual. Atmosphere Health. 2009;2:169–176. doi: 10.1007/s11869-009-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ. Estimating the National Public Health Burden Associated with Exposure to Ambient PM2.5 and Ozone. Risk Anal. 2012b;32:81–95. doi: 10.1111/j.1539-6924.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- Fann N, Roman HA, Fulcher CM, Gentile MA, Hubbell BJ, Wesson K, Levy JI. Maximizing Health Benefits and Minimizing Inequality: Incorporating Local-Scale Data in the Design and Evaluation of Air Quality Policies. Risk Anal. 2011;31:908–922. doi: 10.1111/j.1539-6924.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- Flachs EM, Sørensen J, Bønløkke J, Brønnum-Hansen H. Population dynamics and air pollution: the impact of demographics on health impact assessment of air pollution. J. Environ. Public Health. 2013;2013:760259. doi: 10.1155/2013/760259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildemeister AE, Hopke PK, Kim E. Sources of fine urban particulate matter in Detroit, MI. Chemosphere. 2007;69:1064–1074. doi: 10.1016/j.chemosphere.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Goedkoop M, Heijungs R, Huijbregts M, De Schryver A, Struijs J, van Zelm R. A life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level. 2009 [Google Scholar]
- Gold MR, Stevenson D, Fryback DG. HALYS AND QALYS AND DALYS, OH MY: Similarities and Differences in Summary Measures of Population Health. Annu. Rev. Public Health. 2002;23:115–134. doi: 10.1146/annurev.publhealth.23.100901.140513. [DOI] [PubMed] [Google Scholar]
- Hammitt JK. Valuing Changes in Mortality Risk: Lives Saved Versus Life Years Saved. Rev. Environ. Econ. Policy. 2007;1:228–240. [Google Scholar]
- Hammitt JK. Valuing Mortality Risk: Theory and Practice†. Environ. Sci. Technol. 2000;34:1396–1400. [Google Scholar]
- Hänninen O, Knol AB, Jantunen M, Lim T-A, Conrad A, Rappolder M, Carrer P, Fanetti A-C, Kim R, Buekers J, Torfs R, Iavarone I, Classen T, Hornberg C, Mekel OCL EBoDE Working Group. Environmental burden of disease in Europe: assessing nine risk factors in six countries. Environ. Health Perspect. 2014;122:439–446. doi: 10.1289/ehp.1206154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal MR, Heaviside C, Doherty RM, Vieno M, Stevenson DS, Vardoulakis S. Health burdens of surface ozone in the UK for a range of future scenarios. Environ. Int. 2013;61:36–44. doi: 10.1016/j.envint.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Hebert KA, Wendel AM, Kennedy SK, Dannenberg AL. Health impact assessment: A comparison of 45 local, national, and international guidelines. Environ. Impact Assess. Rev. 2012;34:74–82. [Google Scholar]
- Henschel S, Atkinson R, Zeka A, Tertre AL, Analitis A, Katsouyanni K, Chanel O, Pascal M, Forsberg B, Medina S, Goodman PG. Air pollution interventions and their impact on public health. Int. J. Public Health. 2012;57:757–768. doi: 10.1007/s00038-012-0369-6. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Hammitt JK. Selecting Human Health Metrics for Environmental Decision-Support Tools. Risk Anal. 2002;22:965–983. doi: 10.1111/1539-6924.00264. [DOI] [PubMed] [Google Scholar]
- Hubbell BJ, Fann N, Levy JI. Methodological considerations in developing local-scale health impact assessments: balancing national, regional, and local data. Air Qual. Atmosphere Health. 2009;2:99–110. [Google Scholar]
- Institute of Medicine [IOM] Environmental decisions in the face of uncertainty. Washington, DC: The National Acadamies Press; 2013. [PubMed] [Google Scholar]
- International Energy Agency [IEA] CO2 emissions from fuel combustion: Highlights. Paris, France: 2014. [Google Scholar]
- Jakubiak-Lasocka J, Lasocki J, Badyda AJ. The influence of particulate matter on respiratory morbidity and mortality in children and infants. Adv. Exp. Med. Biol. 2015;849:39–48. doi: 10.1007/5584_2014_93. [DOI] [PubMed] [Google Scholar]
- James P, Ito K, Buonocore JJ, Levy JI, Arcaya MC. A health impact assessment of proposed public transportation service cuts and fare increases in Boston, Massachusetts (U.S.A.) Int. J. Environ. Res. Public. Health. 2014;11:8010–8024. doi: 10.3390/ijerph110808010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett R, Brook J, Kanaroglou P, Giovis C, Finkelstein N, Hutchison B. Do socioeconomic characteristics modify the short term association between air pollution and mortality? Evidence from a zonal time series in Hamilton, Canada. J. Epidemiol. Community Health. 2004;58:31–40. doi: 10.1136/jech.58.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Pope CA, III, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-Term Ozone Exposure and Mortality. N. Engl. J. Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson M. The relationship between cost-effectiveness analysis and cost-benefit analysis. Soc. Sci. Med. 1995;41:483–489. doi: 10.1016/0277-9536(94)00353-u. [DOI] [PubMed] [Google Scholar]
- Johns DO, Stanek LW, Walker K, Benromdhane S, Hubbell B, Ross M, Devlin RB, Costa DL, Greenbaum DS. Practical advancement of multipollutant scientific and risk assessment approaches for ambient air pollution. Environ. Health Perspect. 2012;120:1238–1242. doi: 10.1289/ehp.1204939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judek S, Steib D, Jovik B. Air Quality Benefits Assessment Tool (AQBAT) release 1.0. Health Canada, Ottawa: 2006. [Google Scholar]
- Kassomenos PA, Dimitriou K, Paschalidou AK. Human health damage caused by particulate matter PM10 and ozone in urban environments: the case of Athens, Greece. Environ. Monit. Assess. 2013;185:6933–6942. doi: 10.1007/s10661-013-3076-8. [DOI] [PubMed] [Google Scholar]
- Kheirbek I, Wheeler K, Walters S, Kass D, Matte T. PM2.5 and ozone health impacts and disparities in New York City: sensitivity to spatial and temporal resolution. Air Qual. Atmosphere Health. 2013;6:473–486. doi: 10.1007/s11869-012-0185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski D, Jerrett M, Burnett RT, Ma R, Hughes E, Shi Y, Turner MC, Pope CA, III, Thurston G, Calle EE, Thun MJ, Beckerman B, DeLuca P, Finkelstein N, Ito K, Moore DK, Newbold KB, Ramsay T, Ross Z, Shin H, Tempalski B. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res. Rep. Health Eff. Inst. 2009:5–114. [PubMed] [Google Scholar]
- Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in Fine Particulate Air Pollution and Mortality. Am. J. Respir. Crit. Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughner CP, Allen DJ, Zhang D-L, Pickering KE, Dickerson RR, Landry L. Roles of Urban Tree Canopy and Buildings in Urban Heat Island Effects: Parameterization and Preliminary Results. J. Appl. Meteorol. Climatol. 2012;51:1775–1793. [Google Scholar]
- Maizlish N, Woodcock J, Co S, Ostro B, Fanai A, Fairley D. Health cobenefits and transportation-related reductions in greenhouse gas emissions in the San Francisco Bay area. Am. J. Public Health. 2013;103:703–709. doi: 10.2105/AJPH.2012.300939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar TF, Larson TV, Stier RA, Claiborn C, Koenig JQ. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane. Washington. Inhal. Toxicol. 2004;16:809–815. doi: 10.1080/08958370490506646. [DOI] [PubMed] [Google Scholar]
- McCollum D, Yang C. Achieving deep reductions in US transport greenhouse gas emissions: Scenario analysis and policy implications. Energy Policy. 2009;37:5580–5596. [Google Scholar]
- Mesa-Frias M, Chalabi Z, Vanni T, Foss AM. Uncertainty in environmental health impact assessment: quantitative methods and perspectives. Int. J. Environ. Health Res. 2013;23:16–30. doi: 10.1080/09603123.2012.678002. [DOI] [PubMed] [Google Scholar]
- Michigan Department of Community Health [MDCH] [accessed 3.15.15];Abridged Life Table, Total Michigan Residents, 2013 [WWW Document]. Mich. Mortal. Stat. 2015 URL http://www.mdch.state.mi.us/pha/osr/deaths/lifeall.asp.
- Murray CJ. Towards good practice for health statistics: lessons from the Millennium Development Goal health indicators. The Lancet. 2007;369:862–873. doi: 10.1016/S0140-6736(07)60415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull. World Health Organ. 1994;72:429–445. [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Ezzati M, Lopez AD, Rodgers A, Hoorn SV. Comparative quantification of health risks: Conceptual framework and methodological issues. Popul. Health Metr. 2003;1:1. doi: 10.1186/1478-7954-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council [NRC] Why We Need Health-Informed Policies and Decision-Making, in: Improving Health in the United States: The Role of Health Impact Assessment. Washington, DC: National Academies Press; 2011. [Google Scholar]
- Nowak DJ, Hirabayashi S, Bodine A, Hoehn R. Modeled PM2.5 removal by trees in ten US cities and associated health effects. Environ. Pollut. 2013;178:395–402. doi: 10.1016/j.envpol.2013.03.050. [DOI] [PubMed] [Google Scholar]
- Oakes M, Baxter L, Long TC. Evaluating the application of multipollutant exposure metrics in air pollution health studies. Environ. Int. 2014;69:90–99. doi: 10.1016/j.envint.2014.03.030. [DOI] [PubMed] [Google Scholar]
- O’Connell E, Hurley F. A review of the strengths and weaknesses of quantitative methods used in health impact assessment. Public Health. 2009;123:306–310. doi: 10.1016/j.puhe.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Office of Management and Budget [OMB] Circular A-4: Regulatory Analysis. Washington, DC: 2003. [Google Scholar]
- O’Neill MS, Kinney PL, Cohen AJ. Environmental equity in air quality management: local and international implications for human health and climate change. J. Toxicol. Environ. Health A. 2008;71:570–577. doi: 10.1080/15287390801997625. [DOI] [PubMed] [Google Scholar]
- Pew Charitable Trusts. [accessed 2.11.15];Health Impact Project [WWW Document] 2014 URL http://bit.ly/1sEfCXo.
- Prüss-Ustün A, Mathers C, Corvalan C, Woodward A. Environmental Burden of Disease Series: Introduction and Methods, in: Assessing the Environmental Burden of Disease at National and Local Levels. World Health Organization. 2003 [Google Scholar]
- Punger EM, West JJ. The effect of grid resolution on estimates of the burden of ozone and fine particulate matter on premature mortality in the USA. Air Qual. Atmosphere Health. 2013;6:563–573. doi: 10.1007/s11869-013-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl A. Interpretation of Air Pollution Mortality: Number of Deaths or Years of Life Lost? J. Air Waste Manag. Assoc. 2003;53:41–50. doi: 10.1080/10473289.2003.10466118. [DOI] [PubMed] [Google Scholar]
- Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, de Jongste JC, Kerstjens HAM, Lazarus SC, Levy ML, O’Byrne PM, Partridge MR, Pavord ID, Sears MR, Sterk PJ, Stoloff SW, Sullivan SD, Szefler SJ, Thomas MD, Wenzel SE. An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations. Am. J. Respir. Crit. Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- Rhodus J, Fulk F, Autrey B, O’Shea S, Roth A. A review of health impact assessments in the U.S.: Current state-of-science, best practices and area for improvement. (No. EPA/600/R-13/354) Washington, DC: U.S. Environmental Protection Agency; 2013. [Google Scholar]
- Robinson LA. How US Government Agencies Value Mortality Risk Reductions. Rev. Environ. Econ. Policy. 2007;1:283–299. [Google Scholar]
- Rojas-Rueda D, de Nazelle A, Teixidó O, Nieuwenhuijsen M. Health impact assessment of increasing public transport and cycling use in Barcelona: A morbidity and burden of disease approach. Prev. Med. 2013;57:573–579. doi: 10.1016/j.ypmed.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Rowe R, Chestnut L. Oxidants and asthmatics in Los Angeles: A benefit analysis- Executive Summary (No. EPS-230-09-86-018) Washington, DC: US EPA Office of Policy Analysis; 1986. [Google Scholar]
- Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson EG, Fudge N, Totlandsdal AI, Brunekreef B, van Bree L. Stakeholder needs for air pollution and health information. J. Toxicol. Environ. Health-Part -Curr. Issues. 2006;69:1819–1825. doi: 10.1080/15287390600631557. [DOI] [PubMed] [Google Scholar]
- Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan. 2006;21:402–408. doi: 10.1093/heapol/czl018. [DOI] [PubMed] [Google Scholar]
- Schuchter J, Bhatia R, Corburn J, Seto E. Health impact assessment in the United States: Has practice followed standards? Environ. Impact Assess. Rev. 2014;47:47–53. [Google Scholar]
- Sparks C, Reponen T, Grinshpun SA, Ryan P, Yermakov M, Simmons M, Alam M, Howard LA. Concentration Gradient Patterns of Traffic and Non-Traffic-Generated Fine and Coarse Aerosol Particles. J. Environ. Health. 2014;76:122–129. [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Armstrong B. An Overview of Methods for Calculating the Burden of Disease Due to Specific Risk Factors: Epidemiology. 2006;17:512–519. doi: 10.1097/01.ede.0000229155.05644.43. [DOI] [PubMed] [Google Scholar]
- Tchepel O, Dias D. Quantification of health benefits related with reduction of atmospheric PM10 levels: implementation of population mobility approach. Int. J. Environ. Health Res. 2011;21:189–200. doi: 10.1080/09603123.2010.520117. [DOI] [PubMed] [Google Scholar]
- Thompson TM, Saari RK, Selin NE. Air quality resolution for health impact assessment: Influence of regional characteristics. Atmospheric Chem. Phys. 2014;14:969–978. [Google Scholar]
- UCLA HIA-CLIC. [accessed 2.11.15];UCLA Health Impact Assessment Clearinghouse Learning and Information Center [WWW Document] 2015 URL http://www.hiaguide.org/
- US Environmental Protection Agency [US EPA] BenMAP User’s Manual. Research Triangle Park, NC: 2015a. [Google Scholar]
- US Environmental Protection Agency [US EPA] Regulatory Impact Analysis for Residential Wood Heaters NSPS Revision (No. EPA-452/R-15-001) Washington, DC: 2015b. [Google Scholar]
- US Environmental Protection Agency [US EPA] Inventory of U.S. greenhouse gas emissions and sinks: 1990–2013. Washington, DC: 2015c. [Google Scholar]
- US Environmental Protection Agency [US EPA] Regulatory impact analysis of the proposed revisions to the National Ambient Air Quality Standards for ground-level ozone (No. EOA-452/P-14-006) Research Triangle Park, NC: United States Environmental Protection Agency; 2014. [Google Scholar]
- US Environmental Protection Agency [US EPA] National Ambient Air Quality Standards for Particulate Matter. Fed. Regist. 2013a;78:3086–3287. [Google Scholar]
- US Environmental Protection Agency [US EPA] Technical support document: Estimating the benefit per ton of reducing PM2.5 precursors from 17 sectors. Research Triangle Park, NC: 2013b. [Google Scholar]
- US Environmental Protection Agency [US EPA] Regulatory Impact Analysis for the Final Revisions to the National Ambient Air Quality Standards for Particulate Matter. Research Triangle Park, NC: Office of Air Quality Planning and Standards; 2012a. [Google Scholar]
- US Environmental Protection Agency [US EPA] [accessed 3.10.15];National Emissions Inventory [WWW Document] 2012b URL http://www.epa.gov/ttn/chief/net/2005inventory.html#inventorydata.
- US Environmental Protection Agency [US EPA] The Benefits and Costs of the Clean Air Act from 1990 to 2020. Washington, DC: 2011. [Google Scholar]
- US Environmental Protection Agency [US EPA] Guidelines for Preparing Economic Analyses. 2010. [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Kahn R, Levy R, Verduzco C, Villeneuve PJ. Global Estimates of Ambient Fine Particulate Matter Concentrations from Satellite-Based Aerosol Optical Depth: Development and Application. Environ. Health Perspect. 2010;118:847–855. doi: 10.1289/ehp.0901623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees AS, Wang J, Wang C, Zhao B, Wang S, Kan H. Public health benefits of reducing air pollution in Shanghai: A proof-of-concept methodology with application to BenMAP. Sci. Total Environ. 2014;485–486:396–405. doi: 10.1016/j.scitotenv.2014.03.113. [DOI] [PubMed] [Google Scholar]
- Walker N, Bryce J, Black RE. Interpreting health statistics for policymaking: the story behind the headlines. The Lancet. 2007;369:956–963. doi: 10.1016/S0140-6736(07)60454-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Endreny TA, Nowak DJ. Mechanistic simulation of tree effects in an urban water balance model. J. Am. Water Resour. Assoc. 2008;44:75–85. [Google Scholar]
- Wasilevich E, Lyon-Callo S, Rafferty A, Dombkowski K. Detroit- the epicenter of asthma burden Epidemiology of Asthma in Michigan. Michigan Department of Community Health. 2008 [Google Scholar]
- Wong EY, Ponce RA, Farrow S, Bartell SM, Lee RC, Faustman EM. Comparative Risk and Policy Analysis in Environmental Health. Risk Anal. 2003;23:1337–1349. doi: 10.1111/j.0272-4332.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- Woodcock J, Tainio M, Cheshire J, O’Brien O, Goodman A. Health effects of the London bicycle sharing system: Health impact modelling study. BMJ Online. 2014:348. doi: 10.1136/bmj.g425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. [accessed 7.16.15];Life tables by country [WWW Document]. Glob. Health Obs. Data Repos. 2015 URL http://apps.who.int/gho/data/node.main.692?lang=en.
- Xia T, Nitschke M, Zhang Y, Shah P, Crabb S, Hansen A. Traffic-related air pollution and health co-benefits of alternative transport in Adelaide, South Australia. Environ. Int. 2015;74:281–290. doi: 10.1016/j.envint.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Yang C-M, Kao K. Reducing fine particulate to improve health: a health impact assessment for Taiwan. Arch. Environ. Occup. Health. 2013;68:3–12. doi: 10.1080/19338244.2011.619216. [DOI] [PubMed] [Google Scholar]