To the Editor
Skin pigmentation disorders are among the most recognizable human diseases, strongly impacting both health and quality of life. Lentigines are small, hyper-pigmented skin macules, histologically containing increased numbers of melanocytes, typically producing elevated amounts of melanin. Most lentigines are caused by UV radiation and appear with increasing age. However, lentigines can also be inherited in an autosomal dominant pattern; in these cases, lentigines typically appear in childhood (see review (Bauer and Stratakis, 2005)). We previously reported autosomal dominant inheritance of a unique lentiginous pigmentation disorder (Pacheco et al., 2002; Pacheco et al., 2004). Here, we report identification of a variant associated with this lentiginous phenotype in the SASH1 gene, with additional histological analysis of the patients’ skin. All blood samples, biopsies, photographs and information from subjects were obtained after written informed patient consent were approved by the relevant institutional review boards (including permission to publish images).
Our previous linkage analysis of this family mapped the disease locus to a 10 Mb interval between 6q24.2-q25.2 (Pacheco et al., 2002; Pacheco et al., 2004). Linkage analyses of two Chinese families also mapped a similar pigmentation disorder to the same region (Xing et al., 2003). The overlapping linkage interval of these three families is flanked by markers D6S1703 and D6S441.
In the current family, candidate genes within the linkage interval were screened for mutations, including all exons, 100 bp of introns, and 2 kb of the flanking promoter region. DNA sequence analysis of 17 affected and 18 unaffected family members identified a heterozygous missense substitution in SASH1, in exon 13, c.1556 G->A, p. S519N (Fig. 1a and b). This variant was the only one that co-segregated perfectly with disease, and was not observed in 150 ethnically matched normal controls, 20 melanoma patients with lentigines, or in the UCSC Genome, Ensembl, HapMap, NCBI dbSNP, or the Japanese SNP variant databases.
Figure 1. Identification of SASH1S519N in an inherited lentiginosis.
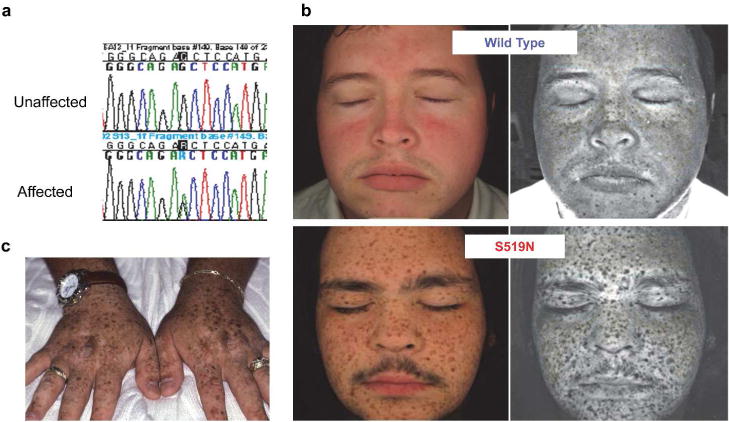
(a) Sequence chromatograms of an unaffected and an affected individual, with a heterozygous point mutation in the SASH1 gene (c. 1556G->A) in the affected individual. (b) The regular view (left panels) and melanin pigmentation view of facial images (right panels) from indicated individuals. Images were captured with a VISIA-Complexion Analysis (VISIA-CA) multi-modality facial imaging system. (c) The regular view of hands from an affected individual.
All affected family members exhibited a similar phenotype of dark brown macules of lentigines (Figure 1c and d), typically presenting in the first decade of life, most prominent in sun-exposed areas. In some cases, lentigines covered the face, trunk and extremities, and in other case, occurred principally over the face and distal extremities (Figure 1c and d).
The diagnosis of lentigines is based on the presence of increased melanin pigmentation, higher density of melanocytes, and characteristic elongation of rete ridges—the inward projections of the epidermis into the dermis (Figure 2a) (Montagna et al., 1980). Tissue sections stained with the melanocyte marker MART1 showed ~ 2 fold increase in melanocyte number per mm of skin biopsy in both the hyper-pigmented lesional and adjacent non-lesional skin of affected patients (Figure 2). However, only lesional skin has a dramatic increase of melanin (Figure 2a). Sections stained with the proliferation marker, Ki67, showed that patients had ~ 2 fold more proliferating cells in both lesional and non-lesional skin (Figure 2c and d). These findings indicate that the S519N SASH1 substitution increased the number of melanocytes and epidermal cell proliferation in skin.
Figure 2. Histological examination indicates a lentiginous phenotype in the skin from affected individuals.
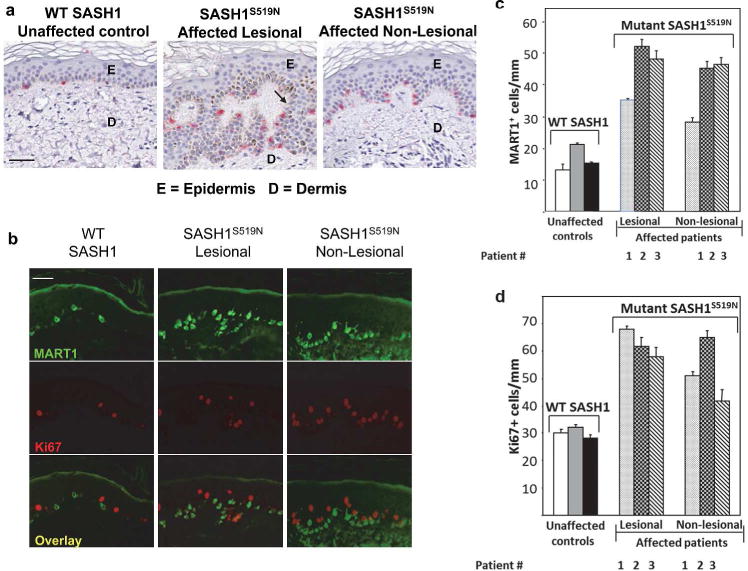
(a) The images of immuno-histochemistry staining with the melanocyte marker (MART1 in red) for the skin biopsies were collected from non-photoexposed skin of ventral forearms of an unaffected individual, and from lesional (hyper-pigmented area) and adjacent non-lesional area (1 cm or more distant normal-appearing skin) tissue from the same affected patient. Scale bar = 50 um. The upper, epidermal layer is indicated by “E”, the lower dermal layer is denoted “D”, and the arrow points to the rete ridges. (b) The same skin biopsies as in panel (a) were double-stained with antibodies to the melanocyte marker, MART1 (green), and the proliferation antigen, Ki67 (red). Scale bar = 50 um. The quantification of the MART1 (c) or Ki67 (d) staining in panel (b) was performed by counting the number of positively stained cells per millimeter of tissue across the entire length of the tissue biopsy. Three affected patients and three unaffected siblings were biopsied. Panel c shows a greater than two-fold increases in MART1 staining in the skin for all affected patients as compared to controls, and panel d shows a greater than two-fold increase in Ki67 staining in the skin for all affected patients as compared to controls. Increased proliferation was also apparent in affected patients in non-lentiginous areas.
SASH1 encodes a signal adaptor protein of 1230 amino acids that contains two nuclear localization signals, a SLY domain, a SH3 domain, and two SAM domains. The S519N substitution is located in the highly conserved SLY domain. SASH1 is expressed in many human tissues, including whole skin, keratinocytes, fibroblasts and melanocytes (NCBI Gene Expression Omnibus; http://www.ncbi.nlm.nih.gov/geo/). We also detected SASH1 expression in cultured human epidermal keratinocytes, dermal fibroblasts, and melanocytes (Supplemental Figure 1).
The function of SASH1 is unknown. Reduced SASH1 expression has been associated with tumor progression in breast and colon cancers, suggesting that it is a candidate tumor suppressor (Rimkus et al., 2006; Zeller et al., 2003). Other studies present conflicting findings. In vitro studies of various cancer cells indicate that SASH1 may inhibit cancer cell survival, proliferation, migration, or invasion (Chen et al., 2012; Lin et al., 2012; Martini et al., 2011; Meng et al., 2013; Yang et al., 2012; Zhou et al., 2013), whereas a study with a non-pigmented metastatic melanoma cell line suggests that SASH1 may increase cell migration (Zhou et al., 2013). Furthermore, in human endothelial cells SASH1 may act as a scaffold molecule in Toll-Like Receptor signaling in the innate immune response (Dauphinee et al., 2013). Thus, SASH1 may have specific but different functions in different cell types.
Recently, another missense substitution of SASH1 (c.1849G->A; p.E617K) was found to be associated with a genodermatosis in an autosomal recessive manner, which included hyper-pigmented macules on the trunk, face, and extremities, with some similarity to our patients (Courcet et al., 2015). Moreover, a non-peer-reviewed study, deposited at Nature Precedings (2011), reported three additional variants in SASH1 (E509K, L515P, and Y551D) associated with a pigmentation disorder in three Chinese families. Taken together, SASH1 thus appears to be a gene involved in regulation of human skin pigmentation and SASH1 variants may cause autosomal-dominant or -recessive genodermatosis.
Other genes associated with familial lentiginosis encode important signaling proteins such as RAF1, BRAF, SOS, SHP2, PTEN, LKB1 and PKA (see review (Bauer and Stratakis, 2005)). The identification of SASH1 as an additional gene involved in familial lentigines provides fresh insights into the development of hyper-pigmentation in human skin. Further examination of the roles of SASH1 in normal skin is needed to understand the molecular mechanisms affected. A combination of in vitro studies with human cells and in vivo studies with animal models are needed to better define SASH1’s function in skin. These investigations will determine whether SASH1 regulates or interacts with known pathways involved in hyperpigmentation disorders, and determine SASH1’s function in development, differentiation, proliferation, survival, and cell migration of skin cells.
Supplementary Material
Acknowledgments
This research was supported by funds NIH/NIAMS K23AR49214 (to TRP), R03AR064555 (to YGS), P30AR057212 (to DAN/TRP/YGS/KBA), T32AR007411 (to AB), and Academic Enrichment Funds from the University of Colorado School of Medicine. The authors thank James Fitzpatrick for dermatopathologic interpretation of skin sections, Dr. Christopher Korch at the University of Colorado DNA Sequencing Core (supported by NIH P30CA046934) for technical advice, and the University of Colorado Skin Cancer Biorepository for providing DNA samples.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Bauer AJ, Stratakis CA. The lentiginoses: cutaneous markers of systemic disease and a window to new aspects of tumourigenesis. Journal of medical genetics. 2005;42:801–10. doi: 10.1136/jmg.2003.017806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EG, Chen Y, Dong LL, et al. Effects of SASH1 on lung cancer cell proliferation, apoptosis, and invasion in vitro. Tumour Biol. 2012;33:1393–401. doi: 10.1007/s13277-012-0387-2. [DOI] [PubMed] [Google Scholar]
- Courcet JB, Elalaoui SC, Duplomb L, et al. Autosomal-recessive SASH1 variants associated with a new genodermatosis with pigmentation defects, palmoplantar keratoderma and skin carcinoma. Eur J Hum Genet. 2015;23:957–62. doi: 10.1038/ejhg.2014.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee SM, Clayton A, Hussainkhel A, et al. SASH1 is a scaffold molecule in endothelial TLR4 signaling. J Immunol. 2013;191:892–901. doi: 10.4049/jimmunol.1200583. [DOI] [PubMed] [Google Scholar]
- Lin S, Zhang J, Xu J, et al. Effects of SASH1 on melanoma cell proliferation and apoptosis in vitro. Mol Med Rep. 2012;6:1243–8. doi: 10.3892/mmr.2012.1099. [DOI] [PubMed] [Google Scholar]
- Martini M, Gnann A, Scheikl D, et al. The candidate tumor suppressor SASH1 interacts with the actin cytoskeleton and stimulates cell-matrix adhesion. The international journal of biochemistry & cell biology. 2011;43:1630–40. doi: 10.1016/j.biocel.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Meng Q, Zheng M, Liu H, et al. SASH1 regulates proliferation, apoptosis, and invasion of osteosarcoma cell. Mol Cell Biochem. 2013;373:201–10. doi: 10.1007/s11010-012-1491-8. [DOI] [PubMed] [Google Scholar]
- Montagna W, Hu F, Carlisle K. A reinvestigation of solar lentigines. Archives of dermatology. 1980;116:1151–4. [PubMed] [Google Scholar]
- Pacheco TR, Bellus GA, Oreskovich NM, et al. Exclusion of candidate genes and loci for multiple lentigines syndrome. The Journal of investigative dermatology. 2002;119:535–8. doi: 10.1046/j.1523-1747.2002.18203.x. [DOI] [PubMed] [Google Scholar]
- Pacheco TR, Oreskovich N, Fain P. Genetic heterogeneity in the multiple lentigines/LEOPARD/Noonan syndromes. American journal of medical genetics. 2004;127A:324–6. doi: 10.1002/ajmg.a.20591. [DOI] [PubMed] [Google Scholar]
- Rimkus C, Martini M, Friederichs J, et al. Prognostic significance of downregulated expression of the candidate tumour suppressor gene SASH1 in colon cancer. British journal of cancer. 2006;95:1419–23. doi: 10.1038/sj.bjc.6603452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing QH, Wang MT, Chen XD, et al. A gene locus responsible for dyschromatosis symmetrica hereditaria (DSH) maps to chromosome 6q24.2–q25.2. American journal of human genetics. 2003;73:377–82. doi: 10.1086/377007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Liu M, Gu Z, et al. Overexpression of SASH1 related to the decreased invasion ability of human glioma U251 cells. Tumour Biol. 2012;33:2255–63. doi: 10.1007/s13277-012-0487-z. [DOI] [PubMed] [Google Scholar]
- Zeller C, Hinzmann B, Seitz S, et al. SASH1: a candidate tumor suppressor gene on chromosome 6q24.3 is downregulated in breast cancer. Oncogene. 2003;22:2972–83. doi: 10.1038/sj.onc.1206474. [DOI] [PubMed] [Google Scholar]
- Zhou D, Wei Z, Deng S, et al. SASH1 regulates melanocyte transepithelial migration through a novel Galphas-SASH1-IQGAP1-E-Cadherin dependent pathway. Cellular signalling. 2013;25:1526–38. doi: 10.1016/j.cellsig.2012.12.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


