Abstract
Objective
To re-examine the relationship of hospital and surgical volume to all-cause and breast cancer-specific mortality, taking into account the potential selection bias in patients treated at high volume centers or by high volume surgeons.
Data Sources
Elderly (65+) women with early-stage, incident breast cancer surgery in 2003.
Study Design
Population-based, prospective survey study.
Methods
Two-stage, instrumental variable regression models.
Principal Findings
Women treated in high volume hospitals were significantly less likely to die of any cause by 5 years after surgery, even after adjustments for self-selection and a number of other factors. The relationship was larger and more significant for breast cancer-specific mortality. Although the general pattern of better mortality outcomes held for moderately sized hospitals, the relationships were not statistically significant. In contrast, there was no relationship of surgeon volume with all-cause or breast cancer-specific mortality.
Conclusions
Hospital volume, but not surgeon volume, is associated with better survival among women with breast cancer. The magnitude of the potential improvement was substantial and comparable to the benefit conferred by many systemic therapies. These findings highlight the importance of accounting for patient self-selection in volume-outcome analyses, and provide support for policy initiatives aimed at regionalizing breast cancer care in the U.S.
Keywords: hospital volume, breast cancer outcomes, instrumental variables, mortality
A number of studies, including a meta-analysis of 12 good quality recent studies, have now demonstrated better survival among breast cancer patients treated by higher volume providers.1 Conducted in 6 different countries, these studies have shown about 16–20% lower risk of dying over the first five years after surgery for patients treated by high volume surgeons or in high volume hospitals, compared to those treated by low volume providers. The interpretation of these findings has typically been that the better survival of patients of high volume providers is attributable to the greater experience and proficiency afforded by a larger number of cases.
This explanation is intuitive and attractive, but it is not the only explanation for a relationship between higher volume of cases and better outcomes. A plausible alternative is selection bias in the patients treated at high volume centers or by high volume surgeons, that is, that patients at lower risk of poor outcomes are more likely to be treated by high volume providers. For breast cancer, this selection bias has been demonstrated to exist. For example, breast cancer patients treated at low volume hospitals are poorer, more likely non-white, less well educated, and are more likely to have Medicaid insurance. 2 Each of these factors has a negative effect on overall survival, and could represent a confounder for evaluating the relationship between volume of cases and outcome of care. Despite this potential for selection bias, no study supporting the breast cancer volume-outcome relationship has been randomized in design.1 The available studies have been based almost entirely on observational databases without individual level information available on sociodemographic characteristics or economic status.
When evaluating observational data, one way to determine if results are robust to selection bias is to use an alternative outcome, and to determine whether the results are consistent with expectations. For example, in previous studies involving breast cancer, we used death from breast cancer as an alternative outcome to overall survival, expecting that the volume-outcome relationship would be stronger for the outcome of breast cancer deaths, compared to the outcome of overall survival. 3–4 Another method for dealing with selection bias is to employ a specific analytic technique designed for this purpose, instrumental variable analysis. This method, originally arising from the economic literature, yields results generally free of selection bias, even for unobserved confounders.5–7 Surprisingly, this method has been rarely used in volume-outcome studies to date despite the observational nature of this literature.
The purpose of this study is to evaluate the relationship of hospital volume and surgeon volume of breast cancer cases to survival, using instrumental variable (IV) techniques to address potential selection biases. If the relationship were found to persist using IV methods, this would support organizational initiatives to promote better breast cancer outcomes. One potential initiative is regionalization of breast cancer care. Despite the existing literature, little regionalization has taken place with regard to breast cancer care in the US, and case volumes for many US hospitals and surgeons remain substantially below the volumes in many European countries. 1 Another potential initiative would be determining what processes lead to better outcomes by high volume providers, and disseminating those practices more widely.
METHODS
Study Population and Data Sources
The main data source for the study was the “Improving the Care and Outcomes of Women Undergoing Breast Surgery,” a population-based, longitudinal survey of community-dwelling elderly women with incident breast cancer. The study sample consisted of elderly women in four geographic and racially diverse states--California, Florida, Illinois and New York--identified from Medicare claims as having had surgery for incident, early stage (SEER 2000 stage < 3) breast cancer between April and September of 2003.8 Potentially eligible participants were contacted by phone in early 2005 to confirm the breast cancer diagnosis and to conduct the first of four annual structured telephone interviews. (See Supplemental Digital Appendix for details on survey design and methods). Survey data were supplemented by information from Medicare Denominator and claims data, State Tumor Registry data, Census, and Area Resource File data.
The cohort for this study consisted of the 2408 women whose incident breast cancer surgery was performed at a hospital within the four study states, and for which hospital volume of breast cancer cases could be calculated from the state-wide Medicare data.
Outcome Measures
The key outcomes for this study were five-year all-cause and breast cancer-specific mortality. Because CMS only provided us with information for patients who were alive at the time of recruitment, only deaths occurring after study entry could be ascertained. For all study participants, five-year all-cause mortality was obtained from CMS Vital Statistics regardless of study completion. Cause of death was obtained from a linkage to the National Death Index (Centers for Disease Control and Prevention).
Hospital and Surgeon Volume
Hospital volume was calculated based on the annual average for the 24-month period preceding each patient’s incident surgery in 2003 for the universe of Medicare breast cancer incident cases in each study state, following the methodology used in prior work. 3–4 Hospitals were then classified based on percentile cutoffs of Medicare breast cancer patients in the four study states. As a point of departure, hospitals were categorized as high volume if they performed 40+ Medicare breast cancer surgeries annually, a threshold roughly corresponding to the upper tertile of cases, used previously to identify high volume breast cancer hospitals in the U.S. 3 We refer to this group of hospitals as “moderately high volume” hospitals. In an alternative classification, hospitals were categorized as high volume if they performed >81 Medicare breast cancer surgeries per year, corresponding to the upper decile of all incident breast cancer surgeries among Medicare beneficiaries. This cutoff corresponds more closely to the literature from the UK, Ireland, Canada, and Taiwan, as these countries exhibit a greater degree of regionalization of breast cancer care than does the U.S. 1
Surgeon volume was measured based on the universe of Medicare breast cancer incident case surgeries performed by the surgeon during the 12-month period preceding the woman’s surgery. Patients were then then classified as being treated by a “moderately high volume” surgeon if they were treated by surgeons performing 12 or more Medicare breast cancer surgeries per year (upper tertile) and, alternatively, as treated by a “high volume” surgeon if treated by surgeons performing 28 or more Medicare breast cancer surgeries per year (upper decile). Again, these cutoffs correspond to those used previously in the US literature (upper tertile), and to those more comparable to the literature outside the US (upper decile).
Other Covariates
Each subject’s address was geocoded to classify all beneficiaries into Census tracts. All hospitals in the study states were geocoded based on the American Hospital Association Survey to determine the latitude and longitude coordinates. Using these coordinates, we ascertained each woman’s distance to the closest hospital and distance to the closest moderately high volume hospital.
Information about the patient’s socio-demographic background was obtained primarily from patient surveys. Women were classified according to their reported race/ethnicity as White non-Hispanic, Black/African American non- Hispanic, Hispanic, or other race/ethnicity. Marital status categories were married/living with a partner, widowed, separated/divorced, and never married. Educational level was captured by years of formal education and categorized as less than high school, high school graduate, and some college. Respondents were also asked to estimate their annual household income in the year preceding the initial survey. Income was then classified in categories as missing income, $15,000 or less, $15,001–$30,000, $30,001–$45,000, and greater than $45,000 (reference category). Social support was assessed using the Medical Outcomes Study Social Support Scale.10 Women were coded as having low social support if they scored at the lowest quartile of the distribution for both emotional/informational and tangible support scales. Control variables were measured at study entry and, to the extent possible, reflect the patient’s circumstances at the time of the incident breast cancer surgery.
Tumor stage information (SEER 2000) was obtained from the four state tumor registries. Comorbidity was measured based on inpatient, outpatient, and Carrier Medicare data for the year preceding the incident breast cancer surgery based on the Klabunde algorithm.11 Finally, certain key structural small-area socioeconomic and health market supply characteristics, including proportion of adult population in poverty and unemployment in the patient’s county of residence, as well as density of surgeons and density of general physicians, were created based on data from the Area Resource File.
Statistical Analyses
Key to the analysis were methods to reduce the likelihood of bias based on differential patient self-selection to high volume providers. An instrumental variable analysis was employed.5–7 Following the Two-Stage Residual Inclusion estimation proposed by Terza et al.,12 in the first stage of the analysis, the probability that a breast cancer patient would choose a hospital (or surgeon) based on its case volume of breast cancer surgery, measured alternatively as “moderately high volume” (top tertile) or “high volume” (top decile) vs. all others, was estimated using a Probit specification. The IV method requires that (instrumental) variables be included in stage one that are predictive of women’s hospital choice but do not independently affect mortality. Our choice of instrumental variables was guided by the robust research indicating that geographic proximity to providers is an important determinant of the choice of high volume provider.13–14 We employed as key instruments two distance measures: distance to the nearest hospital and distance to the nearest high volume hospital. We also examined the potential of certain supply variables, e.g. density of surgeons, density of general physicians, and number of community breast cancer centers, as instruments in our models. For all analyses, we tested the validity of our instruments using Stock and Staiger’s test based on the partial R2 and Chow F-statistics on the excluded variables in the first stage regression.15
In the second stage of the instrumental variable analysis, a probit regression was employed to compute adjusted probabilities of all-cause death or death from breast cancer, using as covariates the treatment at a high volume hospital (surgeon), the residuals from the first stage estimation, as well as the demographic, economic, disease, and ecological factors included in the first stage analysis. Here again, the adequacy of the instruments was tested with respect to the extent to which they could be legitimately excluded from the mortality estimations, conditional on hospital (surgeon) volume and first stage residuals. In both stages, standard errors were adjusted to account for potential clustering (multiple patients within hospitals).
All statistical analyses were performed using STATA 12 (College Station, TX) and NLOGIT 9.0 statistical software (Econometric Software, Inc., Plainview, NY).
RESULTS
Women in this sample of 2,408 Medicare beneficiaries with incident breast cancer were predominantly Caucasian (Table 1). The racial distribution reflected the racial makeup of Medicare beneficiaries in these states as well as the predilection of breast cancer for Caucasian women. Slightly fewer than half were married. Although about half had attended at least some college, the mean household income was slightly below $30,000 for the 80% of the cohort who reported this information. About half of the sample reported low levels of social support. Most (56.7%) had stage 1 disease and no comorbid conditions. Less than 2% of the sample had advanced disease (SEER stage ≥3). Women lived an average of 5 miles from the nearest hospital, but an average of 42 miles from the nearest moderately high volume hospital and 84 miles from the nearest high volume hospital. The all-cause mortality over the average 33 months of follow-up was 7.1%, and breast cancer-specific mortality was 2.5%.
Table 1.
Sample Summary Statistics
| Characteristics | Distribution |
|---|---|
| Patient Demographic & Economic Characteristics | |
| Age (in years, mean/standard deviation) | 72.9 (5.5) |
| Race/Ethnicity (%) | |
| African American/Black | 3.6 |
| Hispanic | 2.9 |
| Other race/ethnicity (mostly Caucasian) | 93.4 |
| Marital Status: Married (%) | 48.4 |
| Education (%) | |
| Less than High School | 8.3 |
| High School Graduate | 35.3 |
| Some College | 54.0 |
| Annual Household Income (%) | |
| < $15,000 | 16.6 |
| $15,000–$29,999 | 27.5 |
| >$30,000 | 34.7 |
| Missing Information | 21.2 |
| Social Support: Low (%) | 24.4 |
| Health Status (%) | |
| No Comorbidities | 62.0 |
| 1+ Comorbid Conditions | 38.0 |
| Disease Stage (%) | |
| 0 (in situ) | 15.4 |
| 1 (localized) | 56.7 |
| 2 (regional by direct extension only) | 16.2 |
| 3 (ipsilateral regional lymph node) | 1.5 |
| 4–7 (advanced or distant disease) | 0.3 |
| Missing | 9.9 |
| Geographic Information (%) | |
| State of Residence/Treatment | |
| Florida | 32.5 |
| Illinois | 21.7 |
| California | 20.8 |
| New York | 25.0 |
| Ecological Measures of Socioeconomic Status | |
| Population in Poverty in County of Residence (%) | 13.6 |
| Unemployment Rate in County of Residence (mean/standard deviation) | 6.3 (4.0) |
| Distance to Providers | |
| Distance to Nearest Hospital (in miles, mean/standard deviation) | 4.7 (4.7) |
| Distance to Nearest Moderately High Volume Hospital (top tertile of incident Medicare cases, in miles) | 31.9 (48.0) |
| Distance to Nearest High Volume Hospital (top decile of incident Medicare cases, in miles) | 96.5 (99.7) |
| Hospital Volume where Treated (mean/standard deviation) [range] | 37.2 (31.1) [1.5–160.5] |
Notes: Sample size comprises of 2,408 Medicare women identified as having undergone incident breast cancer surgery in 2003 at a hospital within their state of residence (Florida, Illinois, New York or California).
Factors Associated with Treatment at High Volume Hospitals
Increasing age was associated with a trend toward a lower likelihood of undergoing surgery at a high volume hospital (Table 2). Compared to the poorest women, those with higher household incomes or with missing income information were considerably more likely to undergo surgery at a high volume hospital. Women residing in counties with a greater percentage of the population living in poverty had a greater chance of treatment in a high volume hospital, while women residing in a county with higher unemployment had a significantly lower chance of treatment in a high volume hospital. Disease stage, minority status, educational attainment, and marital status were not associated with treatment at a high volume hospital, when controlling for other measures of socioeconomic status.
Table 2.
Factors Associated with Breast Cancer Surgery at High Volume Hospitals
| Hospital Volume Defined as: | Moderately High Volume (Top tertile: >40 annual Medicare cases) | High Volume (Top decile: >81 annual Medicare cases) | ||
|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | |
| Patient Demographic & Economic Characteristics | ||||
| Age | − 0.01** | 0.01 | − 0.01* | 0.08 |
| Race/Ethnicity | ||||
| African American/Black | − 0.49** | 0.00 | 0.10 | 0.59 |
| Hispanic | − 0.46** | 0.03 | − 0.23 | 0.39 |
| Marital Status: Married | 0.02 | 0.72 | − 0.06 | 0.44 |
| Education: High School of Less | − 0.16** | 0.01 | − 0.10 | 0.44 |
| Household Income | ||||
| $15,000–$29,999 | 0.11 | 0.27 | 0.26** | 0.05 |
| >$30,000 | 0.19* | 0.07 | 0.38** | 0.00 |
| Missing Information | 0.22** | 0.03 | 0.35** | 0.01 |
| Social Support: Low | − 0.22** | 0.01 | − 0.10 | 0.19 |
| Extent of Disease | ||||
| Stage 0 | −0.07 | 0.41 | −0.06 | 0.57 |
| Stage 2+ | 0.02 | 0.34 | 0.05 | 0.28 |
| Unknown stage | −0.07 | 0.52 | −0.07 | 0.64 |
| Health Status | ||||
| Number of Comorbid Conditions | − 0.02 | 0.62 | − 0.04 | 0.49 |
| Geographic Information | ||||
| State of Residence/Treatment | ||||
| Florida | − 0.01 | 0.97 | 0.20 | 0.54 |
| Illinois | 0.16 | 0.57 | 0.32 | 0.29 |
| New York | 0.10 | 0.74 | 0.23 | 0.25 |
| Ecological Measures of Socioeconomic Status | ||||
| Proportion of Population in Poverty in County of Residence | 0.04* | 0.07 | 0.06** | 0.04 |
| Unemployment Rate in County of Residence | − 0.05 | 0.16 | − 0.16** | 0.00 |
| Instrumental Variables: Distance to Providers | ||||
| Distance to Nearest Hospital (÷ 10) | 0.35** | 0.00 | − 0.07 | 0.46 |
| Distance to Nearest Moderately High/High Volume Hospital (÷ 10) | − 0.31** | 0.00 | − 0.23** | 0.00 |
Note: Models estimated using a Probit specification. Statistically significantly differences at the p≤0.05 and 0.05 <p ≤0.10 levels are indicated by a double and single asterisks, respectively. Hospitals were classified as moderately high volume if they ranked at the top tertile of annual Medicare cases (hospitals performing >40 Medicare incident breast cancer cases per year) during the 24-month preceding the subjects’ incident surgery, the conventional classification of high volume hospitals in the U.S. Hospitals were classified as high volume hospitals, the subset of the moderately high hospitals that rank in the top decile of annual Medicare cases (>81 Medicare cases per year) during the 24-month preceding the subject’s incident surgery. Reference category for race/ethnicity is non-African American, non-Hispanic (primarily Caucasian) women; for marital status is unmarried; for education is some college; for household income is less than $15,000 per year; for disease stage is stage 1; for state of treatment is California.
The instrumental variables-- distance to the nearest hospital and distance to the nearest high volume hospital-- performed well as measured by the tests of adequacy of the instruments, with highly significant F-statistics of 17.3 (p<0.01) and 11.3 (p<0.01), respectively, for top decile and top tertile high volume hospitals. Greater distance to the nearest high volume hospital was associated with a much lower likelihood of treatment at such a hospital. Although the full models are not presented for treatment by a moderately high or high volume surgeon, the instrumental variables performed well in those models as well, with F-statistics at 18.5 (p<0.01) and 12.2 (p<0.01), respectively, demonstrating the validity of these factors as instrumental variables.15
Hospital Volume, Surgeon Volume, and 5-Year Mortality
In the fully instrumented model, which also controlled for demographic, socioeconomic, and extent of disease factors, women treated in high volume hospitals were significantly less likely to die of any cause by 5 years after surgery (Table 3). For breast cancer-specific mortality, this relationship was even stronger, with a larger effect size and stronger statistical significance. Although the general pattern of better mortality outcomes held when the more conventional definition of high volume (top tertile) was used, the high volume coefficient estimates were smaller in magnitude and did not achieve statistical significance. The instrumental variable analyses for surgeon volume revealed no relationship of surgeon volume with all-cause or breast cancer-specific mortality, whether volume was defined as moderately high (top tertile) or high (top decile) volume.
Table 3.
Hospital Volume, Surgeon Volume, and Five-Year Mortality Among Older Breast Cancer Patients
| All-cause 5-year Mortality | Breast Cancer-Specific Mortality | |||
|---|---|---|---|---|
| Moderately High Volume | Volume High | Moderately High Volume | High Volume | |
| (Coefficient [p-value]) | Coefficient [p-value]) | |||
| Hospital | −0.08 [0.79] | −0.98** [0.04] | −0.11 [0.76] | −1.80** [0.01] |
|
| ||||
| Surgeon | 1.31 [0.33] | 0.53 [0.63] | 0.89 [0.39] | 0.11 [0.94] |
Notes: Statistically significant coefficients at the p < 0.05 level are denoted by a double asterisks. Hospitals were classified as moderately high volume if they ranked at the top tertile of annual Medicare cases (hospitals performing >40 Medicare incident breast cancer cases per year) during the 24-month preceding the subjects’ incident surgery. Hospitals were classified as high volume hospitals, the subset of the moderately high hospitals that rank in the top decile of annual Medicare cases (>81 Medicare cases per year) during the 24-month preceding the subject’s incident surgery. For surgeon volume, the thresholds for top tertile and top decile were ≥ 12 and ≥ 28 annual incident breast cancer surgeries during the 12-month period preceding the woman’s surgery, respectively. Models also control for sociodemographic, economic, extent of disease factors, and first stage residuals. See Supplemental Digital Appendix for the full set of coefficient estimates.
To illustrate the magnitude of the effect of treatment in a high volume hospital, we used the parameter estimates from our models to estimate the adjusted excess all-cause mortality attributable to treatment in hospitals other than the high volume hospitals. We found that if women had been treated at a lower volume hospital, the predicted probability of death at 5 years was 8.9% compared to 1.1% had they been treated at a high volume hospital.
DISCUSSION
In this survey study of 2,408 women with incident, early stage breast cancer, those operated upon in high volume hospitals were significantly less likely to die from any cause at 5 years, when using IV methods to control for the substantial differences in the types of patients seen in high volume vs other hospitals. Women treated in high volume hospitals were also significantly less likely to die from breast cancer. The magnitude of the protective effect of treatment in a high volume facility was quite substantial, certainly as much as the benefit conferred by many systemic therapies, as women simulated to receive treatment in lower volume facilities had an 8-fold higher likelihood of death at 5 years than women treated at a high volume facility. There were no all-cause or breast cancer-specific survival differences when hospital volume was defined by the conventional, broader categorization of high volume (top tertile) in the U.S. literature. Similarly, surgeon case volume had no relationship to 5-year mortality when using an instrumental variable analysis to control for the differences in types of patients treated by high volume surgeons.
Our results are in partial agreement with the prior literature on case volume and outcomes for breast cancer. Much of the prior literature, as well as the meta-analysis1, finds that both surgeon and hospital case volume is positively related to overall survival among breast cancer patients, as opposed to our finding that only hospital case volume is related to survival. Our findings suggest that selection bias is a more likely explanation for the relationship between surgeon case volume and outcome.4
The findings of this study highlight the importance of addressing the issue of self-selection to higher volume providers of patients destined to do better.16 Much of the prior literature on volume-outcome relationship has been based on administrative data analyses. 3,4,17–25 Such databases may not offer the opportunity to fully control for the bias of self-selection to higher volume providers.26
There is rationale to the finding that hospital volume is associated with better mortality outcomes, while surgeon volume is not. Breast cancer surgery is not a highly morbid procedure, with in-hospital mortality rates in the range of 0.1–0.2%. 9 Therefore, purely technical expertise of the surgeon is a less likely explanation for patient differences in 5-year survival, unlike other cancer 27 or non-cancer 28 surgeries. For breast cancer, it is more likely that better processes of care account for the better observed outcomes. Hospitals with high case volumes have more incentive to invest in the development of better care processes. These processes are as yet undetermined, but might include, for example, case management (care navigators), more systematic use of multidisciplinary care, or better ancillary care.
This study has limitations, including the fact that entry into the cohort required survival to the first survey interview, roughly 27 months post-incident surgery. Although such a lag raises concerns about survival bias, evidence suggests that early breast cancer mortality is relatively low among women with incident disease. Using 2002–2004 data from the National Cancer Database (NCDB), we estimated 12- and 24-month all-cause mortality among women with incident breast cancer to be 1.7% and 4.3%, respectively. Given that (i) breast cancer mortality comprises about 30% of all-cause mortality among women with incident breast cancer,4 (ii) these NCDB mortality estimates include women with advanced stage disease who were not part of our study, and (iii) early, 30-day breast cancer mortality is relatively evenly distributed across hospitals of varying volumes,1 it is unlikely that survival bias is a significant practical concern in our study. Nonetheless, our findings must be interpreted in light of this limitation. The results are limited to 5-year, and not longer term, mortality. However, as the outcome becomes more distant in time, the likelihood of being able to ascribe outcomes to the care received around the time of the surgery becomes more and more difficult. Finally, the analyses were based on an older cohort of patients who agreed to participate in our survey, and not an unconditional all-age cohort. However, there were no significant differences between elderly women who were contacted and those who opted to participate in the study.8 2010), and previous analyses have shown a strong relationship between a provider’s volume of cases in the Medicare age group and the volume of cases overall.3
The existing literature has led at least two government entities to enact policies designed to promote regionalization of breast cancer surgery. In 2009, the New York State Department of Health set forth a policy mandating that Medicaid beneficiaries receive breast cancer surgery at centers that perform at least 30 of these procedures annually.31 In 2007, The French Ministry of Health and Solidarity issued a decree mandating that hospitals treating breast cancer be able to demonstrate performance of at least 30 of these procedures annually.32 In addition, the UK requires that breast cancer providers meet certain minimum case volumes.33
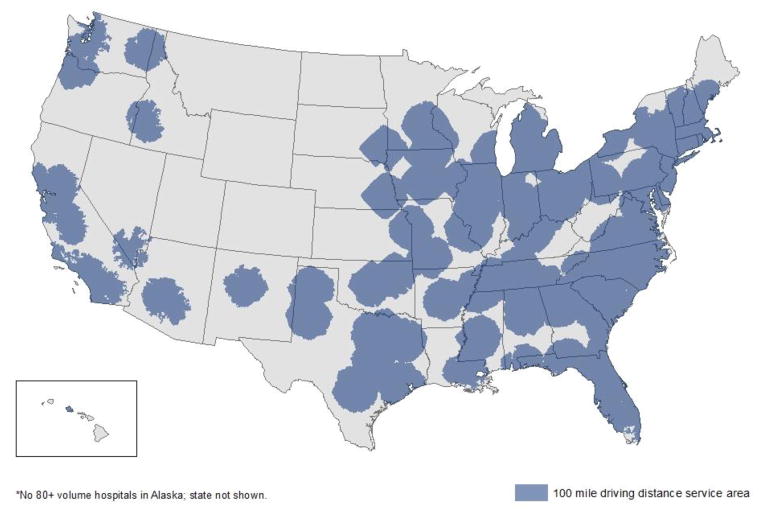
Using NCDB data of all patients with incident breast cancer in 2008, we plotted the location of high volume hospitals and their corresponding 100-mile driving distances on a map of the US in order to examine the feasibility of regionalization of breast cancer care. As evident in Figure 1, large portions of the country are not within a 100-mile driving distance of a high volume hospital, particularly in the western and plains states, a finding that raises concerns about implementing such policy initiatives in the US.
Figure 1.
High breast cancer volume hospitals and their associated 100-mile driving distances.
In summary, the 5-year all-cause and breast cancer–specific survival of women with early stage incident breast cancer could be improved if more women were treated in high volume hospitals. The magnitude of the potential improvement in survival is quite substantial, certainly as much as the benefit conferred by many systemic therapies. As women of minority race and women of lower socioeconomic status are less likely than others to live close to a high volume hospital,2 regionalization of care might also improve the rather large existing disparities in breast cancer survival for such women. Large parts of the country are not within a 100 mile driving distance of a current high volume facility, but policies to promote regionalization of care would be expected to produce a greater number of high volume facilities. Further research to determine which care processes most account for the better outcomes of the high volume hospitals could also improve outcomes in lower volume facilities.
Supplementary Material
References
- 1.Gooiker GA, van Gijn W, Post PN, et al. A systematic review and meta-analysis of the volume-outcome relationship in the surgical treatment of breast cancer. Are breast cancer patients better of with a high volume provider? Eur J Surg Oncol. 2010;36(Supplement 1):S27–S35. doi: 10.1016/j.ejso.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Kong AL, Yen TW, Pezzin LE, et al. Socioeconomic and racial differences in treatment for breast cancer at a low-volume hospital. Ann Surg Oncol. 2011;18:3220–7. 3201787. doi: 10.1245/s10434-011-2001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilligan MA, Neuner JM, Zhang X, et al. Relationship of hospital volume of breast cancer operations and five-year survival after treatment for early stage breast cancer. Am J Public Health. 2007;97:539–44. doi: 10.2105/AJPH.2005.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nattinger AB, Laud PW, Sparapani RA, et al. Exploring the surgeon volume outcome relationship among women with breast cancer. Arch Intern Med. 2007;167:1958–63. doi: 10.1001/archinte.167.18.1958. [DOI] [PubMed] [Google Scholar]
- 5.Heckman James J. Sample selection bias as a specification error. Econometrica. 1979:153–161. [Google Scholar]
- 6.Angrist Joshua D, Imbens Guido W, Rubin Donald B. Identification of causal effects using instrumental variables. J Amer Statist Assoc. 1996;91(434):444–455. [Google Scholar]
- 7.Heckman James J, Robb Richard., Jr Alternative methods for evaluating the impact of interventions: An overview. J Econom. 1985;30(1):239–267. [Google Scholar]
- 8.Nattinger AB, Pezzin LE, Sparapani RA, et al. Heightened attention to medical privacy: challenges for unbiased sample recruitment and a possible solution. Am J Epidemiol. 2010;172:637–44. doi: 10.1093/aje/kwq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. National Death Index. Accessed at http://www.cdc.gov/nchs/ndi.htm.
- 10.RAND Health. Medical Outcomes Study. Social Support Survey. Available from http://www.rand.org./health/surveys_tools/mos/mos_socialsupport.html.
- 11.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 12.Terza Joseph V, Basu Anirban, Rathouz Paul. Two-stage residual inclusion estimation: Addressing endogeneity in health econometric modeling. Health Econ. 2008;27(3):531–43. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newhouse Joseph P, McClellan Mark. Econometrics in outcomes research: the use of instrumental variables. Annual Rev Public Health. 1998;19(1):17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 14.McClellan Mark, Newhouse Joseph P. The marginal cost-effectiveness of medical technology: a panel instrumental-variables approach. J Econom. 1997;77(1):39–64. [Google Scholar]
- 15.Staiger D, Stock JH. Instrumental variables regression with weak instruments. Econometrica. 1997;65:557–86. [Google Scholar]
- 16.Luft H, Hunt S, Maerki SC. The volume-outcome relationship: practice makes perfect or selective referral patters. Health Serv Res. 1987;22(2):157–82. [PMC free article] [PubMed] [Google Scholar]
- 17.Guller U, Safford S, Pietrobon R, et al. High hospital volume is associated with better outcomes for breast cancer surgery: analysis of 233,247 patients. World J Surg. 2005;29:994–9. doi: 10.1007/s00268-005-7831-z. discussion 9–1000. [DOI] [PubMed] [Google Scholar]
- 18.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–20. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 19.Hebert-Croteau N, Brisson J, Lemaire J, et al. Investigating the correlation between hospital of primary treatment and the survival of women with breast cancer. Cancer. 2005;104:1343–8. doi: 10.1002/cncr.21336. [DOI] [PubMed] [Google Scholar]
- 20.Nomura E, Tsukuma H, Ajiki W, et al. Population-based study of the relationship between hospital surgical volume and 10-year survival of breast cancer patients in Osaka, Japan. Cancer Sci. 2006;97:618–22. doi: 10.1111/j.1349-7006.2006.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrijens F, Stordeur S, Beirens K, et al. Effect of hospital volume on processes of care and 5-year survival after breast cancer: A population-based study on 25 000 women. The Breast. 2012 Jun;21(3):261–6. doi: 10.1016/j.breast.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Peltoniemi P, Peltola M, Hakulinen T, et al. The Effect of Hospital Volume on the Outcome of Breast Cancer Surgery. Ann Surg Oncol. 2011;18:1684–90. doi: 10.1245/s10434-010-1514-1. [DOI] [PubMed] [Google Scholar]
- 23.Harcourt KF, Hicks KL. Is there a relationship between case volume and survival in breast cancer? Am J Surg. 2003;185:407–10. doi: 10.1016/s0002-9610(03)00043-6. [DOI] [PubMed] [Google Scholar]
- 24.Mikeljevic JS, Haward R, Johnston C, et al. Surgeon workload and survival from breast cancer. Br J Cancer. 2003;89:487–91. doi: 10.1038/sj.bjc.6601148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailie K, Dobie I, Kirk S, et al. Survival after breast cancer treatment: the impact of provider volume. J Eval Clin Pract. 2007;13:749–57. doi: 10.1111/j.1365-2753.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- 26.Terris Darcey D, Litaker David G, Koroukian Siran M. Health state information derived from secondary databases is affected by multiple sources of bias. J Clin Epidemiol. 2007;60(7):734–741. doi: 10.1016/j.jclinepi.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph Bellal, Morton JM, et al. Relationship between hospital volume, system clinical resources, and mortality in pancreatic resection. J Am Coll Surg. 2009;208(4):520–527. doi: 10.1016/j.jamcollsurg.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Birkmeyer JD, Finlayson VA, Birkmeyer CM. Volume standards and high risk surgical procedures: potential benefits of the Leapfrog Initiative. Surgery. 2001;130(3):415–22. doi: 10.1067/msy.2001.117139. [DOI] [PubMed] [Google Scholar]
- 29.Bound John, Jaeger David A, Baker Regina M. Problems with instrumental variables estimation when the correlation between the instruments and the endogenous explanatory variable is weak. J Amer Statist Assoc. 1995;90(430):443–450. [Google Scholar]
- 30.Andrews Donald WK, Moreira Marcelo J, Stock James H. Optimal Two-Sided Invariant Similar Tests for Instrumental Variables Regression. Econometrica. 2006;74(3):715–752. [Google Scholar]
- 31.New York State Department of Health. [Accessed April 23, 2011];Restricted breast cancer surgery facilities for Medicaid recipients. 2009 at http://www.health.ny.gov/health_care/medicaid/quality/surgery/cancer/breast.
- 32.Institut National Du Cancer. Textes Generaux. Ministere De La Sante Et Des Solidarites; 2007. [Accessed 23 April 2011]. Decrets, arrets, ciculaires. Accessed at http://www.e-cancer.fr/soins/2581-etablissements-autorises/2622-les-seuils-dactivite. [Google Scholar]
- 33.ASCO. The State of Cancer Care in America, 2014: A Report by the American Society of Clinical Oncology. J Oncol Pract. doi: 10.1200/JOP2014.001366. Published online Mar 10, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.