Abstract
Sex- and species-specific patterns of estrogen receptor (ER)-α expression are established early in development, which may contribute to sexual differentiation of behavior and determine male social organization. The current study investigated the effects of ERα and ERβ activation during the second postnatal week on subsequent alloparental behavior and ERα expression in juvenile prairie voles. Male and female pups were treated daily with 17β-estradiol (E2, ERα/ERβ agonist), PPT (selective ERα agonist), DPN (selective ERβ agonist), or the oil vehicle on postnatal days (PD) 8-14. Alloparental behavior and ERα expression were examined at PD21. PPT treatment inhibited prosocial motivation in males and increased pup-directed aggression in both sexes. E2 and DPN had no apparent effect on behavior in either sex. PPT-treated males had increased ERα expression in the medial preoptic area (MPN), medial amygdala (MEApd) and bed nucleus of the stria terminalis (BSTpr). DPN treatment also increased ERα expression in males, but only in the BSTpr. Female ERα expression was unaffected by treatment. These results support the hypothesis that ERα activation in early life is associated with less prosocial patterns of central ERα expression and alloparental behavior in males. The lack of an effect of E2 on behavior suggests that ERβ may antagonize the effects of ERα on alloparental behavior. The results in DPN-treated males suggest that ERα in the MEApd, and not the BSTpr, may be a primary determinant of alloparental behavior in males.
Keywords: estradiol, PPT, DPN, alloparental behavior, prairie vole, estrogen receptor alpha
INTRODUCTION
Prosocial behaviors consist of “positive” social interactions that benefit other individuals (Penner et al., 2005). Reproductive strategies often involve a trade-off between mating potential and prosocial behavior. Thus, highly prosocial strategies are characterized by delayed maturation, the formation of long-term social bonds and higher levels of caring for young, whereas less prosocial strategies involve rapid maturation, a focus on short-term mating opportunities and reduced care for young. Aggression and prosocial behavior, while not mutually exclusive, are typically considered to be opposite ends of social behavior, with high levels of aggression being considered to limit the expression of prosocial behavior- especially caring for young (Trivers, 1972; Wingfield et al., 1990).
Steroid hormones have been associated with both prosocial behavior and aggression (Del Giudice, 2009; Fernandez-Duque et al., 2009; Rilling and Young, 2014; Soma et al., 2008; Trainor et al., 2006; Yildirim and Derksen, 2012). However, studies on the role of estrogen in regulating these behaviors have produced mixed findings, which may reflect a number of factors including timing of treatment, sex, species, and/or the study design. Adding to this complexity, the two primary estrogen receptors (ERα and ERβ) can have opposing, synergistic or sequentially coordinated influences over behavior (Rissman, 2008). In general, ERα is associated with increased aggression, anxiety and emotionality- traits that should inhibit prosocial behavior- whereas ERβ is associated with reduced aggression and anxiety and enhanced cognition- traits that should facilitate prosocial behavior (Nomura et al., 2002; Ogawa et al., 1998; Oyola et al., 2012; Scordalakes and Rissman, 2004; Walf et al., 2009; Walf and Frye, 2005). Therefore, we hypothesized that ERα activation would reduce prosocial behavior in naïve males and females, whereas ERβ activation would enhance prosocial behavior.
Alloparental care in the prairie vole (Microtus ochrogaster) provides an excellent opportunity to study the role of estrogen receptors in regulating prosocial behavior and aggression in naïve males and females. As juveniles, both sexes are highly alloparental and rarely attack pups (Bales et al., 2004; Lonstein and De Vries, 2001). Reproductively-naïve adult males remain highly alloparental, whereas naïve adult females are more likely to show pup-directed aggression (Bales et al., 2004; Lonstein and De Vries, 1999; Lonstein and De Vries, 2000a). Thus, adolescence involves the reduction in prosocial behavior in females only, unlike most other rodent species in which both sexes show a developmental decline in alloparental behavior (Lonstein and De Vries, 2000b). The majority of adult female prairie voles will only revert to displaying high levels of alloparental behavior once they have given birth to pups (Hayes and De Vries, 2007). Estrogen and ER are thought to contribute to the reorganization of female prosocial behavior during motherhood (Olazábal et al., 2013), the mechanisms underlying its reorganization in naïve individuals during adolescence are less clear.
In part because social monogamy is distinguished by increased prosocial behavior by males, we have a greater understanding of the mechanisms regulating male prosocial behavior. While many factors contribute to male prosocial behavior, low levels of ERα expression in the medial amygdala (MEApd) and bed nucleus of the stria terminalis (BSTpm) appears to be a critical determinant (Cushing et al., 2008; Cushing and Wynne-Edwards, 2006; Lei et al., 2010). ERα expression in the MEApd and BSTpm is relatively limited during the first postnatal week and increases dramatically between the second and third postnatal weeks in both sexes, but with an attenuated rise in males that produces a significant sex difference (Yamamoto et al., 2006). Males show a further reduction in ERα expression in the MEApd and BSTpm between weaning and adulthood (Cushing et al., 2004; Kramer et al., 2006; Yamamoto et al., 2006), which renders these brain regions less sensitive to ERα activation. Several studies have shown that over-riding the reduced ERα expression in these regions with viral vectors containing ERα cDNA (Cushing et al., 2008; Lei et al., 2010) or neonatal castration (Cushing and Kramer, 2005; Lonstein et al., 2002) reduces male prosocial behavior.
Therefore, to test the hypothesis that ERα activation reduces prosocial behavior in naïve males and females, we treated voles with estradiol (E2) or ER-selective agonists during the second postnatal week and examined their alloparental behavior one week later at weaning. We predicted that selective ERα activation would increase pup-directed aggression and reduce prosocial motivation in both sexes, and increase ERα expression in the MEApd and BSTpm of males only (i.e., reorganize the brain into a less prosocial configuration). We predicted that ERβ activation would increase prosocial behavior, decrease aggression and reduce ERα expression in the MEApd and BSTpm of males; however, as control juveniles were expected to be highly prosocial, these behavioral effects might be obscured by an apparent “ceiling effect”.
MATERIALS AND METHODS
Husbandry
Prairie voles were maintained on a 14:10 hour light:dark cycle (lights on at 06:00) and provided with high fiber rabbit chow and water ad libitum. On the day of birth, animals were sexed and marked for identification with a single toe clip- a standard and approved technique for Microtines, as they lack extensive pinnae and there is no other way to reliably mark individuals for later identification across treatment and testing phases. Subjects remained with the dam, sire, and litter mates until testing at postnatal day (PD) 21, the typical age for weaning. In no case were subjects exposed to their mother's subsequent litter. Thus, the alloparental test was the first experience with pups for all subjects. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were preapproved by the University of Illinois Committee on the Use and Care of Animals.
Treatments
Animals were randomly assigned within each litter to receive one of four daily treatments between PD8-14: 5 μg of 17-β-estradiol (E2; Sigma; (Kuiper et al., 1997)), 5 μg of the ERα-selective agonist 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT; Tocris Bioscience; (Stauffer et al., 2000)), 5 μg of the ERβ-selective agonist diarylpropionitrile (DPN; Tocris Bioscience; (Meyers et al., 2001)), or sesame oil vehicle (Sigma). All injections were 25 μl in volume and given subcutaneously. Doses were based on average weight of PD8 vole (~8 g) and are within the range of doses used in other studies (Clipperton-Allen et al., 2011; Landau et al., 1978; Uban et al., 2011). The treatment period (PD8-14) was selected because it has been shown to be a sensitive period for estrogenic manipulations in voles, unlike the first postnatal week (Kramer et al., 2009; Lonstein and De Vries, 2000a; Sullivan et al., 2014), and corresponds to the developmental stage in which ERα expression begins to increase and become sexually dimorphic (Yamamoto et al., 2006). It also precedes the period during which males presumably become less sensitive to ERα activation due to their reduction in ERα expression in the MEApd and BSTpm. Additional non-treated controls were obtained from breeders that were left undisturbed outside of routine cage changes to control for potential effects of the handling procedure required for PD8-14 injections. As there were no differences between oil and non-treated controls, they were combined into a single control group.
Alloparental behavior
At PD21, subjects were removed from the home cage and allowed to acclimate to the testing apparatus for at least 45 minutes, during which time food and water were freely available. The testing apparatus consisted of two standard size mouse cages (29 cm × 19 cm × 13 cm) connected by an 8 cm long clear acrylic tube. After the acclimation period, food and water were removed and an unrelated pup (PD1-3) was introduced into the center of the unoccupied chamber. The 10-minute test began when the experimental subject placed both forepaws into the cage containing the pup and was terminated if this failed to happen within 30 minutes. The test was stopped immediately if at any time a pup was attacked and its wounds treated, or euthanized if necessary. The primary variables of interest were the percentage of attackers in each group and the total duration of pup contact, which included huddling over the pup and licking and grooming the pup. Retrieval and pup carrying were relatively rare in all groups and were not included in the measure of total pup contact. Non-attacking individuals were further divided into two alloparental categories based on their total duration of pup contact, with individuals displaying 103 seconds or more of pup contact designated “high alloparental” and those with less than 103 seconds designated “low alloparental.” The 103-second threshold was empirically derived from the lower quartile of the combined male and female controls in the present experiment (n= 71).
Immunohistochemistry and Image Analysis
Immediately after testing, experimental subjects were deeply anesthetized and their brains were removed following transcardiac perfusion with 4% paraformaldehyde and 2.5% acrolein (pH 7.4). Brains were post-fixed for 24 hours in 4% paraformaldehyde and equilibrated in 25% sucrose. 30-μm sections were cut on a freezing sliding microtome and stored in cryoprotectant at −20° C. Standard avidin:biotinylated enzyme complex (ABC) immunohistochemistry was conducted on free-floating sections using anti-ERα IgG (Santa Cruz Biotechnology, MC-20, diluted 1:7,500) generated in rabbit. Briefly, sections were treated with 1% sodium borohydride and 0.014% phenylhydrazine to quench unreacted aldehydes from the perfusion and inactivate endogenous peroxidases, respectively. Sections were incubated in the primary antibody solution for 1 hour at room temperature, and then for an additional ~60 hours at 4° C. Sections were incubated in anti-rabbit IgG (Vector Laboratories, BA-1000, diluted 1:600) for 1 hour at room temperature, followed by incubation in ABC solution (Vector Laboratories, Vectastain Elite PK-6100, prepared according to manufacturer's instructions) for 1 hour at room temperature. ERα was visualized by incubation in nickel-enhanced diaminobenzadine (Ni-DAB) solution for 15 min at room temperature. Sections were mounted on slides, air-dried, dehydrated through an ascending ethanol series, cleared with xylene and coverslipped using Enetellan rapid mounting medium. ERα-immunoreactivity(-ir) was quantified with NIH ImageJ (Schneider et al., 2012). The number of ERα-ir cells was determined in the regions of interest using a 40× objective, according to procedures described previously by our laboratory (Perry et al., 2009).
Statistics
Only data from non-attacking individuals were used in the analyses of total pup contact duration. Durations were rank transformed and aligned for sex, treatment and their interaction for nonparametric factorial analyses using ANOVA procedures (Wobbrock et al., 2011). Separate Q’ tests employing the Wilson variance were used to analyze differences in the proportion of individuals in each alloparental category (high alloparental, low alloparental and attack) due to the relatively small sample sizes and occurrence of cells with expected counts < 5 in some groups (Michael, 2007). ERα-ir was analyzed by ANOVA with sex and treatment as independent factors. Pair-wise comparisons were only made between each treatment and the control within each sex and between males and females within each treatment using Fisher's test of least significant differences and results were considered significant where p < 0.05. Eta-squared values (η2), Kramer's Phi (ϕc) or Cohen's d values are provided as indicators of effect size. ANOVA procedures were conducted in SPSS (v. 20.0), whereas the Q’ tests were performed in Excel (template downloaded from http://www.tqmp.org).
RESULTS
Effects of treatments on alloparental behavior
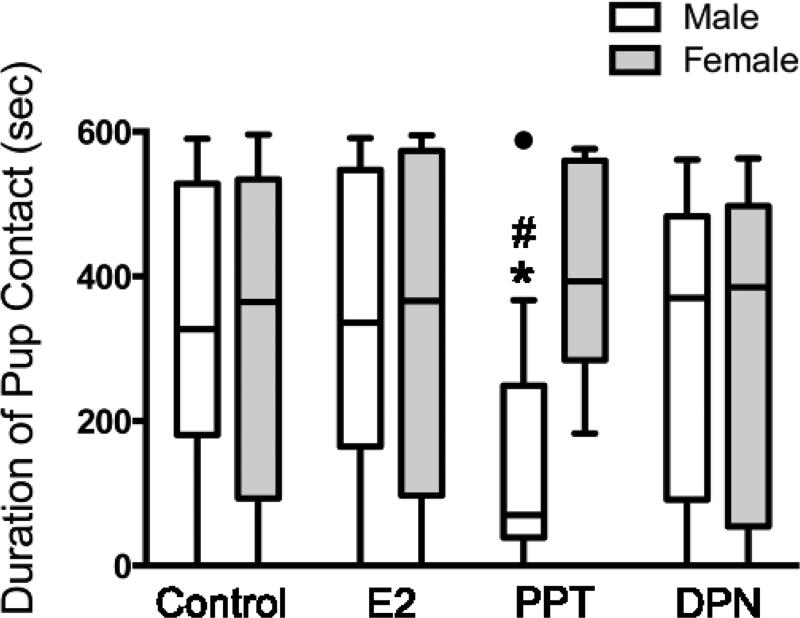
There was a significant interaction between sex and treatment on pup contact duration (Fig. 1; F3,150= 3.22, p= 0.024, η2= 0.06). PPT treatment reduced pup contact in males (p= 0.017, Cohen's d= 0.98) and tended to increase the duration of pup contact in females (p= 0.054, Cohen's d= 0.42) compared to their controls, which resulted in a significant sex difference within the PPT group (p= 0.015, Cohen's d= 1.64). In contrast, there were no apparent differences between males and females in the other groups (Control: p= 0.054, Cohen's d= 0.09; E2: p= 0.663, Cohen's d= 0.06; DPN: p= 0.473, Cohen's d= 0.03). Contact durations for the other treatments were not different from controls in either males (E2: p= 0.491, Cohen's d= 0.07; DPN: p= 0.473, Cohen's d= 0.17) or females (E2: p= 0.75, Cohen's d= 0.08; DPN: p= 0.915, Cohen's d= 0.04).
Figure 1. ERα activation reduces subsequent alloparental behavior in juvenile males.
Treatments were administered between PD8-14 and behavior was tested on PD21. Tukey box-and-whisker plot of the data for total duration of contact with the pup (e.g., huddling, licking and grooming) displaying medians and inter-quartiles ranges for each group (● = an outlier in the male PPT group). *, p< 0.05 compared to controls within the same sex and #, p< 0.05 compared to females within the same treatment. Controls (n= 35 males, 36 females), E2= 17β-estradiol (ERα and ERβ agonist, 5μg, n= 15 males, 17 females), PPT= 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (ERα agonist, 5μg, n= 11 males, 13 females) and DPN= diarylpropionitrile (ERβ agonist, 5μg, n= 18 males, 13 females).
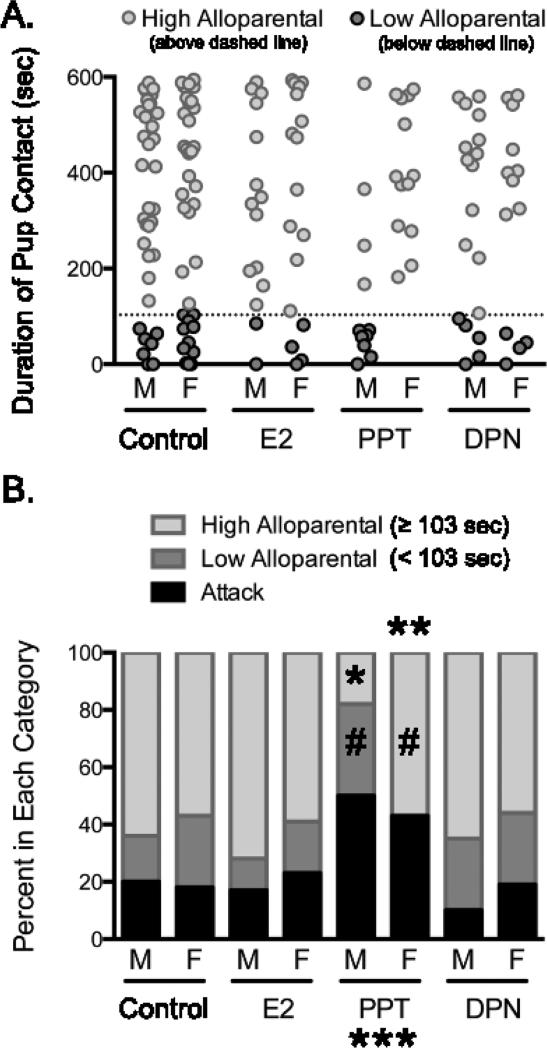
There was a significant treatment effect (Fig. 2; Q’= 8.23, d.f.= 3, p= 0.042, ϕc= 0.20) and interaction between sex and treatment in the high alloparental category (Q’ = 10.28, d.f. = 3, p = 0.016, ϕc= 0.22). The percentage of PPT-treated males in this category was significantly less than their controls (p < 0.001, Cohen's d= 0.95) and PPT-treated females (p = 0.024, Cohen's d= 0.86). The other treatments did not differ from controls in either males (E2: p= 0.917, Cohen's d= 0.17; DPN: p= 1.00, Cohen's d= 0.03) or females (E2: p= 0.998, Cohen's d= 0.04; PPT: p= 0.983, Cohen's d= 0.09; DPN: p= 0.985, Cohen's d= 0.09), and there were no other significant sex differences (Control: p= 0.997, Cohen's d= 0.05; E2: p= 0.823, Cohen's d= 0.28; DPN: p= 0.951, Cohen's d= 0.18).
Figure 2. ERα activation increases attacks and differentially affects alloparental classifications in males and females.
A. Individual variation in alloparental behavior in juvenile male (M) and female (F) prairie voles. Each point represents a single non-attacking individual. The dashed line represents the lower quartile for the combined male and female controls (103 sec), which was used to identify individuals with high (≥ 103 sec, light grey) and low (< 103 sec, dark grey) levels of alloparental behavior (refer to Figure 1 legend for numbers of non-attacking individuals). B. PPT reduced the percentage of high alloparental males (* p< 0.05) and low alloparental females (**, p< 0.05) compared to their respective controls, and increased the proportion of attackers in both sexes (***, p < 0.05). #, p< 0.05 compared to the other sex within a given treatment and category. Controls (n= 44 males, 44 females), E2= 17β-estradiol (ERα and ERβ agonist, 5μg, n= 18 males, 22 females), PPT= 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (ERα agonist, 5μg, n= 22 males, 23 females) and DPN= diarylpropionitrile (ERβ agonist, 5μg, n= 20 males, 16 females).
There was also a significant interaction between sex and treatment in the low alloparental category (Fig. 2; Q’ = 9.99, d.f. = 3, p = 0.019, ϕc= 0.22). PPT treatment reduced the percentage of females in this category compared to their controls (p = 0.036, Cohen's d= 0.59) and PPT-treated males (p = 0.017, Cohen's d= 0.98). The other treatments did not differ from controls in either males (E2: p= 0.967, Cohen's d= 0.12; PPT: p= 0.535, Cohen's d= 0.37; DPN: p= 0.865, Cohen's d= 0.22) or females (E2: p= 0.997, Cohen's d= 0.05; DPN: p= 0.985, Cohen's d= 0.10), and there were no other significant sex differences (Control: p= 0.957, Cohen's d= 0.12; E2: p= 0.937, Cohen's d= 0.20; DPN: p= 1.00, Cohen's d= 0.00).
There was a main effect of treatment (Fig. 2; Q’ = 12.07, d.f. = 3, p = 0.007, ϕc= 0.24) on the proportion of voles that attacked the pup. Voles treated with PPT were significantly more likely to attack the pup compared to controls (p = 0.012, Cohen's d= 0.60), whereas the other treatments were similar to controls (E2: p= 1.00, Cohen's d= 0.02; DPN: p= 0.90, Cohen's d= 0.13). There were no significant sex differences in any group (Control: p= 0.995, Cohen's d= 0.06; E2: p= 0.968, Cohen's d= 0.15; PPT: p= 0.973, Cohen's d= 0.13; DPN: p= 0.904, Cohen's d= 0.25).
Effects of selective estrogen receptor agonists on ERα-ir
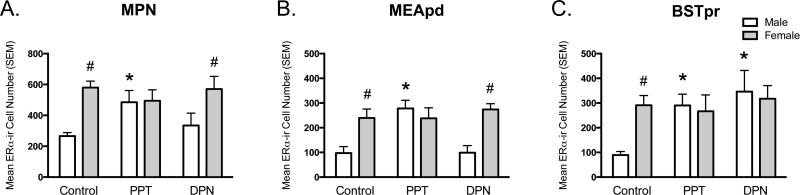
There was a significant main effect of sex on ERα-ir in the MPN (F1,36= 15.35, p< 0.001, η2= 0.22) and interaction between sex and treatment (F2,36= 3.80, p= 0.032, η2= 0.11). Females had overall greater ERα-ir in the MPN than males; however, this sex difference was only present in controls (p< 0.001, Cohen's d= 2.95) and DPN-treated voles (p= 0.013, Cohen's d= 1.45), as PPT-treated males and females were not significantly different (p= 0.916, Cohen's d= 0.07). PPT treatment increased ERα-ir in the MPN of males relative to controls (p= 0.013, Cohen's d= 2.39), whereas DPN-treated and control males were not significantly different (p= 0.377, Cohen's d= 0.64). There were no differences between the controls and either treatment in females (PPT: p= 0.247, Cohen's d= 0.60; DPN: p= 0.899, Cohen's d= 0.07).
There was a significant main effect of sex on ERα-ir in the MEApd (F1,37= 8.25, p= 0.007, η2= 0.13) and an interaction between sex and treatment (F2,37= 3.75, p= 0.033, η2= 0.12). Females had significantly more ERα-ir in the MEApd than males; however, this sex difference was only present in controls (p= 0.001, Cohen's d= 1.35) and DPN-treated voles (p= 0.007, Cohen's d= 3.34), as there was no significant difference between PPT-treated males and females (p= 0.527, Cohen's d= 0.49). PPT treatment increased ERα-ir in the MEApd of males compared to controls (p= 0.003, Cohen's d= 2.34), whereas there was no effect of DPN (p= 0.971, Cohen's d= 0.03). There were also no significant differences between the controls and either treatment in females (PPT: p= 0.986, Cohen's d= 0.01; DPN: p= 0.504, Cohen's d= 0.33).
There was a significant effect of treatment on ERα-ir in the BSTpr (F2,38= 5.00, p= 0.012, η2= 0.16) and an interaction between sex and treatment (F2,38= 4.35, p= 0.02, η2= 0.14). The controls were the only group in which there was a significant sex difference, with females having greater ERα-ir than males (p= 0.001, Cohen's d= 2.03), whereas ERα-ir was similar between the sexes in both the PPT (p= 0.775, Cohen's d= 0.19) and DPN groups (p= 0.71, Cohen's d= 0.20). In males, both PPT (p= 0.01, Cohen's d= 3.60) and DPN (p= 0.001, Cohen's d= 2.50) increased ERα-ir compared to controls, whereas these treatments were not significantly different from controls in females (PPT: p= 0.701, Cohen's d= 0.18; DPN: p= 0.68, Cohen's d= 0.21).
DISCUSSION
The results supported our hypothesis that ERα activation reduces prosocial behavior, as PPT treatment increased aggression in both sexes and reduced prosocial motivation in males. Selective activation of ERβ with DPN had no effect on alloparental behavior in males or females- consistent with our expectation of a “ceiling effect”, which is also supported by the fact that control males and females were highly prosocial and displayed low levels of aggression. Combined ERα/ERβ activation with E2 also had no apparent effect on alloparental behavior, which suggests that concurrent ERβ activation may counteract the effects of ERα on prosocial behavior and aggression. In males, PPT treatment increased ERα expression in the MPN, MEApd and BSTpr, which is generally consistent with the central pattern of ERα expression associated with low levels of prosocial behavior. Counter to our prediction, DPN treatment also increased ERα expression in males- but only in the BSTpr.
Treatment Effects on Alloparental Behavior
Our data demonstrate that ERα activation reduced alloparental behavior in both males and females, but more specifically it enhanced pup-directed aggression in both sexes and reduced prosocial motivation selectively in males. These findings demonstrate that ERα can regulate multiple dimensions of alloparental behavior, and that aggression and prosocial motivation can be independently regulated in a sex-specific fashion.
All criteria for alloparental behavior require the absence of pup-directed aggression, but the amount of prosocial motivation an individual must display in order to be considered alloparental is more varied and subjective (published studies have used minimal contact thresholds ranging from 30-180 seconds for tests lasting 10-15 minutes (Ahern and Young, 2009; Cushing et al., 2008; Lonstein and De Vries, 2000a)). Furthermore, “non-alloparental” individuals are typically lumped together irrespective of whether they attacked the pup or displayed low levels of prosocial motivation. Thus, it is presently difficult to discern whether aggression and prosocial motivation represent a single continuum or separate dimensions of alloparental behavior.
Our findings suggest that aggression and prosocial motivation represent separate dimensions of alloparental behavior, as PPT treatment differentially regulated these behaviors in males and females (i.e., increased aggression in both sexes and reduced prosocial motivation selectively in males). While ERα activation increased aggression in both sexes, it is presently unknown whether this reflects a conserved mechanism in males and females, or whether activation of ERα in sex-specific pathways converges on similar increases in aggression. Therefore, additional studies are needed to elucidate how ERα activation translates into increased pup-directed aggression in males and females.
In contrast, prosocial motivation appeared to be selectively affected by ERα activation in males. While the decrease in the number of “low alloparental” females seems to suggest that ERα activation altered their prosocial motivation, a closer inspection of the data suggests that PPT likely transformed potentially “low alloparental” females into “attackers” without affecting prosocial motivation in the “high alloparental” females (i.e., the bottom of the distribution dropped out to become attackers leaving the upper distribution unaffected). A similar phenomenon might occur in PPT-treated males, such that individuals with the lowest prosocial motivation might also be more vulnerable and rendered more likely to attack the stimulus pup. However, as prosocial motivation is also sensitive to ERα activation in males, the potentially “high alloparental” individuals would have been transformed into “low alloparental” males and ended up in the bottom of the distribution.
The majority of our findings support the hypothesis that ERα activation reduces prosocial behavior and/or increases aggression in naïve males and females. However, there is evidence that estradiol can also increase prosocial behavior in naïve individuals, as estradiol increased alloparental behavior in adult female voles (Lonstein and De Vries, 1999) and blocking estradiol production with an aromatase inhibitor during PD8-14 decreased alloparental behavior in male voles (Kramer et al., 2009). We hypothesize that these examples reflect the actions of estradiol through ERβ. While our findings from the current study do not directly support the hypothesis that ERβ activation promotes prosocial behavior, our negative findings in E2-treated voles might suggest that ERβ activation can counteract the negative effects of ERα activation on prosocial behavior and aggression- consistent with numerous other studies demonstrating an antagonistic relationship between ERα and ERβ (Mazzucco et al., 2008; SÃ et al., 2013; Song and Pan, 2012). However, future studies will need to directly address this possibility by treating subjects with combinations of PPT and DPN, or with E2 in conjunction with selective antagonists (Santollo et al., 2010). It would also be worthwhile to examine the effects of ERβ activation on alloparental behavior under conditions in which it is naturally low (e.g., adult naïve females), which would clarify whether ERβ directly increases prosocial motivation, reduces aggression and/or simply antagonizes the function of ERα.
Treatment Effects on Estrogen Receptor α Expression
The increases in ERα expression in the MPN, MEApd and BSTpm of males following PPT treatment and in the BSTpm following DPN treatment are consistent with the ability of sex steroids to modulate the expression of their receptors. Many studies have shown relatively acute effects of hormone exposure that primarily involve down-regulation of ERα expression, which is thought to reflect a negative feedback loop that can be mediated by activation of either ERα or ERβ (Kelly et al., 2013; Leite et al., 2014; Matsuda et al., 2013). However, the increases in ERα expression following PPT and DPN treatment in our study were present a full week after the final injection, suggesting a more permanent up-regulation and reorganization. Additional studies will be required to see if the increases in ERα are maintained or undergo additional modifications during adolescence and adulthood (Kramer et al., 2007; Yamamoto et al., 2006).
Future studies will also be required to determine whether the changes in ERα expression following PPT and DPN treatment are mediated directly by agonist activation of their respective receptors, or indirectly via other mechanisms and feedback loops, including those in the periphery and/or inter-connected brain regions. In this regard, examining the effects of E2 on subsequent ERα expression, both on its own and in conjunction with selective antagonists, as well as the use of site-specific infusions within discrete brain regions, would shed further light on how different patterns of ERα and ERβ activation affect the brain and prosocial behavior.
Potential Contributions of Altered ERα Expression to Alloparental Behavior
The reorganization of ERα expression following PPT treatment in males likely contributed to their increased aggression and reduced prosocial motivation. Numerous studies have implicated ERα expression in the MEApd and BSTpm as a critical factor determining male prosocial behavior (Cushing et al., 2004; 2001; Cushing and Wynne-Edwards, 2006; Roberts and Carter, 1997). Increasing ERα specifically in the MEApd or BSTpm reduces prosocial behavior in male prairie voles, with the former being particularly detrimental to alloparental care (Cushing et al., 2008; Lei et al., 2010). Thus, our results with PPT- and DPN-treated males support the hypothesis and numerous empirical studies suggesting that low levels of ERα in the MEApd, but not BSTpm, are essential for male alloparental behavior. While ERα expression was not altered in PPT-treated females, it is probable that some of the downstream effects of ERα activation persisted even after the cessation of treatment and contributed to their increased aggression. Therefore, identifying these mechanisms will be important for gaining a better understanding of the regulation of prosocial behavior in females.
It is presently unknown how high levels of ERα expression in the MEApd might translate into increased aggression and reduced prosocial motivation. However, E2 influences several aspects of MEApd structure and function in rodents, including increased soma size, regional volume, spine density, astrocytic markers and excitatory neurotransmission (Castilhos et al., 2008; Cooke et al., 2003; Gomez and Newman, 1991; Martinez et al., 2006; Morris et al., 2008; Schiess et al., 1988). Therefore, we hypothesize that increased ERα expression in the MEApd would enhance the propagation of pup-related sensory information through circuits leading to aggression (Kirkpatrick et al., 1994; Olazábal et al., 2013; Tachikawa et al., 2013). Consistent with this hypothesis, the transition from attacking pups to paternal behavior is associated with attenuated activation of neural circuits downstream of the MEApd in male mice (Tachikawa et al., 2013).
Summary/Conclusion
Alloparental behavior was disrupted by PPT treatment in both male and female prairie voles, which is consistent with our hypothesis that ERα activation reduces prosocial behavior in naïve individuals. In males, ERα activation was associated with both increased aggression and reduced prosocial motivation, whereas in females ERα activation was only associated with increased aggression. Thus, the neural substrates of prosocial behavior and/or the involvement of ERα therein might be quite different in naïve males and females. In this regard, it is noteworthy that one of the prime examples of female prosocial behavior (i.e., maternal care) is highly dependent upon ERα (Ribeiro et al., 2012). Thus, while ERα may reduce prosocial behavior and/or increase aggression in naïve females, it may take on new roles and actually promote prosocial behavior in reproductive females. Such “plasticity” in the role of ERα in female prosocial behavior might explain why only aggression, and not prosocial motivation, was sensitive to ERα activation- unlike the situation in males in which both were affected.
Highlights.
Postnatal ERα activation increased pup-directed aggression in males and females.
Postnatal ERα activation reduced pup contact duration in males.
Postnatal ERα activation reduced the number of “low alloparental” females.
Postnatal ERα activation increased ERα expression in several brain regions in males.
ERα expression was unaffected by any treatment in females.
Figure 3. PPT increases ERα-ir in the MPN (A), MEApd (B) and BSTpr (C) of male prairie voles, whereas DPN only increases ERα-ir in the BSTpr.
Significant difference from controls within the same sex (* p< 0.05). Significant difference from males within the same treatment (# p< 0.05). Controls (n= 11 males, 11-12 females per region), PPT= 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (ERα agonist, 5μg, n= 4 males, 6 females per region) and DPN= diarylpropionitrile (ERβ agonist, 5μg, n= 5 males, 5-6 females per region).
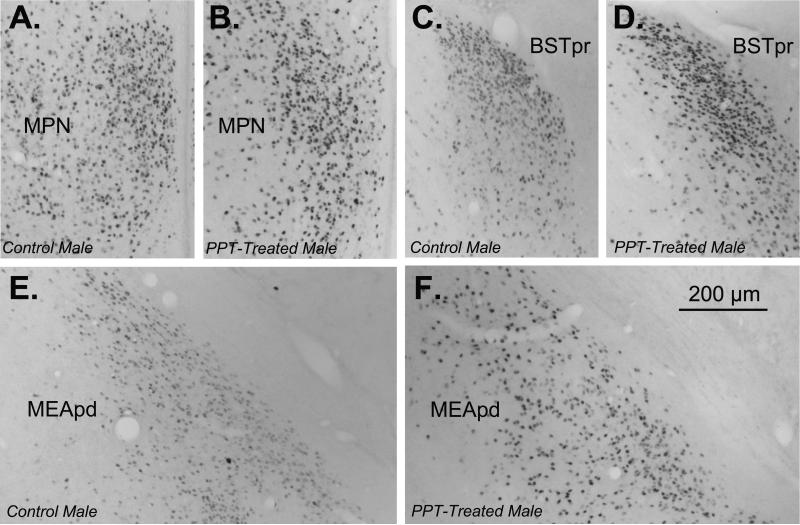
Figure 4. Representative ERα-ir in control males (A: MPN, C: BSTpr, E: MEApd) and PPT-treated males (B: MPN, D: BSTpr, F: MEApd).
Images were taken with a 10× objective and the scale bar represents 200μm.
ACKNOWLEDGEMENTS
Funding for this research was provided by the National Institutes of Drug Abuse (NIDA) to ANP (F31 DA018034). We would also like to thank Dr. Christel Westenbroek for helpful comments provided during the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster). Front. Behav. Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. doi:10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev. Psychobiol. 2004;44:123–131. doi: 10.1002/dev.10165. doi:10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- Castilhos J, de, Forti CD, Achaval M, Rasia-Filho AA. Dendritic spine density of posterodorsal medial amygdala neurons can be affected by gonadectomy and sex steroid manipulations in adult rats: A Golgi study. Brain Res. 2008;1240:73–81. doi: 10.1016/j.brainres.2008.09.002. doi:10.1016/j.brainres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Clipperton-Allen AE, Almey A, Melichercik A, Allen CP, Choleris E. Effects of an estrogen receptor alpha agonist on agonistic behaviour in intact and gonadectomized male and female mice. Psychoneuroendocrinology. 2011;36:981–995. doi: 10.1016/j.psyneuen.2010.12.010. doi:10.1016/j.psyneuen.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43:336–346. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Kramer KM. Mechanisms underlying epigenetic effects of early social experience: the role of neuropeptides and steroids. Neurosci Biobehav Rev. 2005;29:1089–1105. doi: 10.1016/j.neubiorev.2005.04.001. doi:10.1016/j.neubiorev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Martin JO, Young LJ, Carter CS. The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Horm Behav. 2001;39:48–58. doi: 10.1006/hbeh.2000.1633. doi:10.1006/hbeh.2000.1633. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Perry A, Musatov S, Ogawa S, Papademetriou E. Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. Journal of Neuroscience. 2008;28:10399–10403. doi: 10.1523/JNEUROSCI.1928-08.2008. doi:10.1523/JNEUROSCI.1928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Razzoli M, Murphy AZ, Epperson PM, Le W-W, Hoffman GE. Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Res. 2004;1016:247–254. doi: 10.1016/j.brainres.2004.05.010. doi:10.1016/j.brainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Wynne-Edwards KE. Estrogen receptor-alpha distribution in male rodents is associated with social organization. J Comp Neurol. 2006;494:595–605. doi: 10.1002/cne.20826. doi:10.1002/cne.20826. [DOI] [PubMed] [Google Scholar]
- Del Giudice M. Sex, attachment, and the development of reproductive strategies. Behav Brain Sci. 2009;32:1–21. doi: 10.1017/S0140525X09000016. doi:10.1017/S0140525X09000016. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Valeggia C, Mendoza S. The biology of paternal care in human and nonhuman primates. Annual Review of Anthropology. 2009;38:115–130. [Google Scholar]
- Gomez DM, Newman SW. Medial nucleus of the amygdala in the adult Syrian hamster: a quantitative Golgi analysis of gonadal hormonal regulation of neuronal morphology. Anat Rec. 1991;231:498–509. doi: 10.1002/ar.1092310412. doi:10.1002/ar.1092310412. [DOI] [PubMed] [Google Scholar]
- Hayes UL, De Vries GJ. Role of pregnancy and parturition in induction of maternal behavior in prairie voles (Microtus ochrogaster). Horm Behav. 2007;51:265–272. doi: 10.1016/j.yhbeh.2006.10.011. doi:10.1016/j.yhbeh.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DA, Varnum MM, Krentzel AA, Krug S, Forger NG. Differential Control of Sex Differences in Estrogen Receptor α in the Bed Nucleus of the Stria Terminalis and Anteroventral Periventricular Nucleus. Endocrinology. 2013;154:3836–3846. doi: 10.1210/en.2013-1239. doi:10.1210/en.2013-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Carter C, Newman S, Insel T. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole ( Microtus ochrogaster): Behavioral and anatomical specificity. Behav Neurosci. 1994;108:501. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- Kramer K, Yoshida S, Papademetriou E, Cushing B. The organizational effects of oxytocin on the central expression of estrogen receptor α and oxytocin in adulthood. BMC Neurosci. 2007;8:71. doi: 10.1186/1471-2202-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Carr MS, Schmidt JV, Cushing BS. Parental regulation of central patterns of estrogen receptor alpha. Neuroscience. 2006;142:165–173. doi: 10.1016/j.neuroscience.2006.05.069. doi:10.1016/j.neuroscience.2006.05.069. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Perry AN, Golbin D, Cushing BS. Sex steroids are necessary in the second postnatal week for the expression of male alloparental behavior in prairie voles (Microtus ochragaster). Behav Neurosci. 2009;123:958–963. doi: 10.1037/a0016927. doi:10.1037/a0016927. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. doi:10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Landau IT, Logue CM, Feder HH. Comparison of the effects of estradiol-17beta and the synthetic estrogen, RU-2858, on lordosis behavior in adult female rats and guinea pigs. Horm Behav. 1978;10:143–155. doi: 10.1016/0018-506x(78)90004-1. [DOI] [PubMed] [Google Scholar]
- Lei K, Cushing BS, Musatov S, Ogawa S, Kramer KM. Estrogen receptor-alpha in the bed nucleus of the stria terminalis regulates social affiliation in male prairie voles (Microtus ochrogaster). PLoS ONE. 2010;5:e8931. doi: 10.1371/journal.pone.0008931. doi:10.1371/journal.pone.0008931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite C, Madeira MD, Sá SI. Effects of sex steroids and estrogen receptor agonists on the expression of estrogen receptor alpha in the principal division of the bed nucleus of the stria terminalis of female rats. Brain Res. 2014;1582:99–106. doi: 10.1016/j.brainres.2014.07.041. doi:10.1016/j.brainres.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Lonstein J, De Vries G. Sex differences in the parental behaviour of adult virgin prairie voles: independence from gonadal hormones and vasopressin. Journal of Neuroendocrinology. 1999;11:441–450. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Influence of gonadal hormones on the development of parental behavior in adult virgin prairie voles (Microtus ochrogaster). Behav Brain Res. 2000a;114:79–87. doi: 10.1016/s0166-4328(00)00192-3. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci Biobehav Rev. 2000b;24:669–686. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster). J Comp Psychol. 2001;115:53–61. doi: 10.1037/0735-7036.115.1.53. doi:10.1037//0735-7036.115.1.53. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Rood BD, De Vries GJ. Parental responsiveness is feminized after neonatal castration in virgin male prairie voles, but is not masculinized by perinatal testosterone in virgin females. Horm Behav. 2002;41:80–87. doi: 10.1006/hbeh.2001.1740. doi:10.1006/hbeh.2001.1740. [DOI] [PubMed] [Google Scholar]
- Martinez FG, Hermel EES, Xavier LL, Viola GG, Riboldi J, Rasia-Filho AA, Achaval M. Gonadal hormone regulation of glial fibrillary acidic protein immunoreactivity in the medial amygdala subnuclei across the estrous cycle and in castrated and treated female rats. Brain Res. 2006;1108:117–126. doi: 10.1016/j.brainres.2006.06.014. doi:10.1016/j.brainres.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Matsuda KI, Yanagisawa M, Sano K, Ochiai I, Musatov S, Okoshi K, Tsukahara S, Ogawa S, Kawata M. Visualisation and characterisation of oestrogen receptor α-positive neurons expressing green fluorescent protein under the control of the oestrogen receptor α promoter. Eur J Neurosci. 2013;38:2242–2249. doi: 10.1111/ejn.12227. doi:10.1111/ejn.12227. [DOI] [PubMed] [Google Scholar]
- Mazzucco C, Walker H, Pawluski J, Lieblich S, Galea L. ER [alpha], but not ER [beta], mediates the expression of sexual behavior in the female rat. Behav Brain Res. 2008;191:111–117. doi: 10.1016/j.bbr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Michael GA. A significance test of interaction in 2 × K designs with proportions. Tutorials in Quantitative Methods for Psychology. 2007;3:1–7. [Google Scholar]
- Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res. 2008;1190:115–121. doi: 10.1016/j.brainres.2007.11.005. doi:10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Durbak L, Chan J, Smithies O, Gustafsson J-A, Korach KS, Pfaff DW, Ogawa S. Genotype/Age Interactions on Aggressive Behavior in Gonadally Intact Estrogen Receptor β Knockout (βERKO) Male Mice. Horm Behav. 2002;41:288–296. doi: 10.1006/hbeh.2002.1773. doi:10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. doi:10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, González-Mariscal G, Lévy F, Lucion AB, Morrell JI, Numan M, Uriarte N. Neuroscience and Biobehavioral Reviews. Neurosci Biobehav Rev. 2013;37:1875–1892. doi: 10.1016/j.neubiorev.2013.04.004. doi:10.1016/j.neubiorev.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Oyola MG, Portillo W, Reyna A, Foradori CD, Kudwa A, Hinds L, Handa RJ, Mani SK. Anxiolytic Effects and Neuroanatomical Targets of Estrogen Receptor-β (ERβ) Activation by a Selective ERβ Agonist in Female Mice. Endocrinology. 2012;153:837–846. doi: 10.1210/en.2011-1674. doi:10.1210/en.2011-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner LA, Dovidio JF, Piliavin JA, Schroeder DA. Prosocial Behavior: Multilevel Perspectives. Annu Rev Psychol. 2005;56:365–392. doi: 10.1146/annurev.psych.56.091103.070141. doi:10.1146/annurev.psych.56.091103.070141. [DOI] [PubMed] [Google Scholar]
- Perry AN, Paramadilok A, Cushing BS. Neonatal oxytocin alters subsequent estrogen receptor alpha protein expression and estrogen sensitivity in the female rat. Behav Brain Res. 2009;205:154–161. doi: 10.1016/j.bbr.2009.08.021. doi:10.1016/j.bbr.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Ribeiro AC, Musatov S, Shteyler A, Simanduyev S, Arrieta-Cruz I, Ogawa S, Pfaff DW. siRNA silencing of estrogen receptor-α expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proceedings of the National Academy of Sciences. 2012;109:16324–16329. doi: 10.1073/pnas.1214094109. doi:10.1073/pnas.1214094109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. doi:10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF. Roles of Oestrogen Receptors α and β in Behavioural Neuroendocrinology: Beyond Yin/Yang. Journal of Neuroendocrinology. 2008;20:873–879. doi: 10.1111/j.1365-2826.2008.01738.x. doi:10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Carter CS. Intraspecific variation and the presence of a father can influence the expression of monogamous and communal traits in prairie voles. Ann N Y Acad Sci. 1997;807:559–562. doi: 10.1111/j.1749-6632.1997.tb51968.x. [DOI] [PubMed] [Google Scholar]
- Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Hormones and Behavior. Horm Behav. 2010;58:872–877. doi: 10.1016/j.yhbeh.2010.08.012. doi:10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SÃ SI, Pereira PA, Malikov V, Madeira MD. Role of estrogen receptor α and β in the induction of progesterone receptors in hypothalamic ventromedial neurons. Neuroscience. 2013;238:159–167. doi: 10.1016/j.neuroscience.2013.02.023. doi:10.1016/j.neuroscience.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Schiess MC, Joëls M, Shinnick-Gallagher P. Estrogen priming affects active membrane properties of medial amygdala neurons. Brain Res. 1988;440:380–385. doi: 10.1016/0006-8993(88)91012-8. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. nmeth.2089. Nat Meth. 2012;9:671–675. doi: 10.1038/nmeth.2089. doi:10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Soma KK, Scotti M-AL, Newman AEM, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 2008;29:476–489. doi: 10.1016/j.yfrne.2007.12.003. doi:10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Song X, Pan Z-Z. Estrogen receptor-beta agonist diarylpropionitrile counteracts the estrogenic activity of estrogen receptor-alpha agonist propylpyrazole-triol in the mammary gland of ovariectomized Sprague Dawley rats. J Steroid Biochem Mol Biol. 2012;130:26–35. doi: 10.1016/j.jsbmb.2011.12.018. doi:10.1016/j.jsbmb.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J. Med. Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Sullivan AW, Beach EC, Stetzik LA, Perry A, D'Addezio AS, Cushing BS, Patisaul HB. A Novel Model for Neuroendocrine Toxicology: Neurobehavioral Effects of BPA Exposure in a Prosocial Species, the Prairie Vole (Microtus ochrogaster). Endocrinology. 2014;155:3867–3881. doi: 10.1210/en.2014-1379. doi:10.1210/en.2014-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa KS, Yoshihara Y, Kuroda KO. Behavioral Transition from Attack to Parenting in Male Mice: A Crucial Role of the Vomeronasal System. Journal of Neuroscience. 2013;33:5120–5126. doi: 10.1523/JNEUROSCI.2364-12.2013. doi:10.1523/JNEUROSCI.2364-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor B, Kyomen H, Marler C. Estrogenic Encounters: How Interactions Between Aromatase and the Environment Modulate Aggression. Front Neuroendocrinol. 2006;27:170–179. doi: 10.1016/j.yfrne.2005.11.001. doi:10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R. Parental investment and sexual selection. In: Campbell BG, editor. Sexual Selection and the Descent of Man (1871–1971) Aldine–Atherton; Chicago: 1972. pp. 136–179. [Google Scholar]
- Uban KA, Rummel J, Floresco SB, Galea LAM. Estradiol Modulates Effort-Based Decision Making in Female Rats. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.176. doi:10.1038/npp.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf A, Koonce C, Frye C. Adult female wildtype, but not oestrogen receptor knockout, mice have decreased depression-like behaviour during pro-oestrus and following administration of oestradiol or diarylpropionitrile. Journal of Psychopharmacology. 2009;23:442–450. doi: 10.1177/0269881108089598. doi:10.1177/0269881108089598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERβ-Selective Estrogen Receptor Modulators Produce Antianxiety Behavior when Administered Systemically to Ovariectomized Rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. doi:10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The“ challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist. 1990:829–846. [Google Scholar]
- Wobbrock JO, Findlater L, Gergle D, Higgins JJ. Presented at the Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. ACM; 2011. The aligned rank transform for nonparametric factorial analyses using only anova procedures; pp. 143–146. [Google Scholar]
- Yamamoto Y, Carter C, Cushing B. Neonatal manipulation of oxytocin affects expression of estrogen receptor alpha. Neuroscience. 2006;137:157–164. doi: 10.1016/j.neuroscience.2005.08.065. [DOI] [PubMed] [Google Scholar]
- Yildirim BO, Derksen JJL. A review on the relationship between testosterone and life-course persistent antisocial behavior. Psychiatry Research. 2012;200:984–1010. doi: 10.1016/j.psychres.2012.07.044. doi:10.1016/j.psychres.2012.07.044. [DOI] [PubMed] [Google Scholar]