Abstract
The pathogenesis of scleroderma (SSc) includes components of autoimmunity, vascular dysfunction, and accumulation of extracellular matrix. 8-isoprostane, an oxidized lipid created by oxidative stress, activates the thromboxane A2 receptor (TXAR) and ROCK pathway. In this study we determined whether the TXAR was activated by 8-isoprostane in SSc endothelial cells (ECs), and whether this pathway inhibited VEGF-induced angiogenesis. Elevated 8-isoprostane was observed in plasma and conditioned media from SSc patients. SSc conditioned media inhibited EC tube formation, while addition of vitamin E, by reducing 8-isoprostane, increased tube formation. VEGF did not induce angiogenesis in SSc ECs, but vitamin E or TXAR inhibition restored its effect. The expression of TXAR, RhoA, and ROCK1/2 were elevated in SSc ECs. ROCK activity and 8-isoprostane-induced ROCK activation were significantly higher in SSc ECs while VEGF had no effect. The hyper-activation of the TXAR leads to inhibition of VEGF-induced angiogenesis, as inhibition of the TXAR pathway results in blockade of 8-isoprostane induced ROCK activation and restoration of VEGF activity. These results suggest that the TXAR pathway plays a crucial role in angiogenesis and that 8-isoprostane is not just a by-product of oxidative stress, but also plays a significant role in the impaired angiogenesis that characterizes SSc.
Systemic sclerosis (scleroderma, SSc) is a devastating disease that involves autoimmunity, progressive fibrosis of internal organs, and vascular damage. Although the etiology of SSc is currently unknown, it is clear that vascular morphological changes appear before the onset of fibrosis. The loss of vasculature results in tissue hypoxia, which normally promotes angiogenesis through the production of pro-angiogenic factors. Among those, vascular endothelial growth factor (VEGF) is a major trigger of the angiogenic process, and exemplifies the paradox of SSc dysregulated angiogenesis. Thus, despite significant elevation of VEGF, adaptive angiogenesis is absent and the disease is shifted toward a progressive loss of capillaries. Moreover, VEGF expression is elevated in various cell types in SSC skin (Davies et al., 2006; Distler et al., 2004; Mackiewicz et al., 2002), in addition to increased circulating VEGF levels (Distler et al., 2002; Hummers et al., 2009; Kuryliszyn-Moskal et al., 2005). The cellular mechanisms of how angiogenesis becomes dysregulated in the presence of elevated VEGF in SSc are still not known.
Oxidative stress occurs when there is an imbalance between free radical production and antioxidative capacity of the biological system. During this process, lipids are oxidized by reactive oxygen species resulting in the formation of various peroxidation products. One class of oxidized lipids in abundance is the isoprostanes. They are a series of prostaglandin isomers produced independently of cyclooxygenase as products of free radical-catalyzed lipid peroxidation, and are sensitive indicators for oxidative injury (Morrow, 2000). In addition to being a biomarker, 8-isoprostane is a mediator of important biological effects (Comporti et al., 2008; Roberts and Milne, 2009). It is a potent vasoconstrictor in a variety of vascular beds, and induces endothelin release, proliferation of smooth muscle cells, and inhibition of angiogenesis through the thromboxane A2 receptor (TXAR) (Hou et al., 2004; Morrow, 2000). Inflammatory effects of 8-isoprostane have also been described. 8-isoprostane suppressed monocyte attachment to ECs via TXAR activation (Kumar et al., 2005). In addition, it increased interleukin-8/CXCL8 and macrophage inflammatory protein-1α/CCL3 expression in human macrophages through ERK1/2 and P38 pathways (Scholz et al., 2003). In SSc, elevated 8-isoprostane levels have been found in blood (Ogawa et al., 2006), urine (Avouac et al., 2010; Stein et al., 1996), bronchoalveolar lavage (Montuschi et al., 1998), and exhaled breath (Tufvesson et al., 2010) of SSc patients.
When 8-isoprostane activates the TXAR, this G-protein coupled receptor in turn activates the GTPase Ras homolog gene family-member A (RhoA), which is critical for dynamic changes in cell shape and adhesion that govern polarity and drive migration (Nobes and Hall, 1999). Activation of TXAR switches RhoA from an inactive, GDP-bound form to an active GTP-bound form that subsequently activates target molecules. The effect of RhoA is carried out by the downstream Rho-associated kinases (ROCK), which include the 2 isoforms ROCK1 and ROCK2. Activation of TXAR mediates a persistent activation of RhoA, which in turn affects actin filament assembly and contractility. Therefore RhoA and ROCK are known to play a critical role in EC motility by regulating the formation of F-actin stress fibers and focal adhesion turnover (Lamalice et al., 2007). Persistent ROCK activity may inhibit EC motility by increasing cell adhesion to the substratum or by slowing down turnover of focal adhesions (Wojciak-Stothard et al., 2007). Interestingly, VEGF transiently activates RhoA/ROCK to initiate focal adhesion turnover, which promotes angiogenesis (Lamalice et al., 2007).
Microvascular endothelial cells (ECs) are key players in SSc microangiopathy and they have been examined by us and others as useful models for studying dysregulated angiogenesis (Giusti et al., 2013; Manetti et al., 2011; Rabquer et al., 2011; Tsou et al., 2012a). In the pathogenesis of SSc, increased oxidative damage has been implicated (Bruckdorfer et al., 1995; Sambo et al., 2001; Servettaz et al., 2007; Stein et al., 1996; Svegliati et al., 2005; Tsou et al., 2014; Tsou et al., 2012b). However, the role of oxidative stress in SSc vasculopathy has not been elucidated. In this study we examined the mechanism of impaired angiogenesis in SSc ECs and focused on the interplay between 8-isoprostane/TXAR and VEGF signaling, thus providing a possible link between oxidative stress and vasculopathy in SSc.
Results
Oxidative stress in SSc
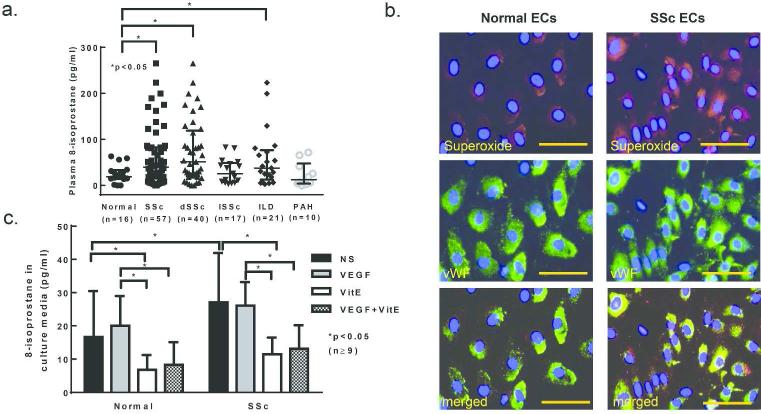
To evaluate whether oxidative stress is observed in SSc, we measured 8-isoprostane in patient's blood. Plasma 8-isoprostane levels were significantly elevated in SSc patients, specifically diffuse SSc patients as well as patients with interstitial lung disease (ILD, Figure 1A). In ECs isolated from diffuse SSc patients, increased superoxide was observed (Figure 1B). The cells were of EC origin as indicated by the positive staining of von Willebrand factor. SSc ECs released significantly more 8-isoprostane into culture media than healthy ECs, and stimulation with VEGF had no effect on 8-isoprostane production (Figure 1C). Vitamin E, an antioxidant that decreases 8-isoprostane in hypercholesterolemic patients (Davi et al., 1997), significantly reduced 8-isoprostane released from both normal and SSc ECs.
Figure 1. Elevation of 8-isoprostane in dSSc.
a. Plasma 8-isoprostane was measured using an 8-isoprostane EIA kit. Plasma 8-isoprostane was elevated in patients with diffuse SSc or interstitial lung disease but not in patients with limited SSc or pulmonary hypertension. b. Superoxide production was examined by DHE staining. The nuclei were stained using DAPI. Superoxide production was increased in SSc ECs compared to normal ECs. c. 8-isoprostane levels were measured using an 8-isoprostane EIA kit and were elevated in SSc EC culture media compared to normal EC culture media. 8-Isoprostane levels were reduced by vitamin E in both normal and SSc EC culture media. Means are shown +/− S.D. Differences were determined using the student's t test and p values less than 0.05 were significant. Scale bar=50μm. n=number of patients. dSSc: diffuse SSc; lSSc: limited SSc; ILD: interstitial lung disease; PAH: pulmonary hypertension; NS: not-stimualted; VitE: vitamin E.
Matrigel tube formation
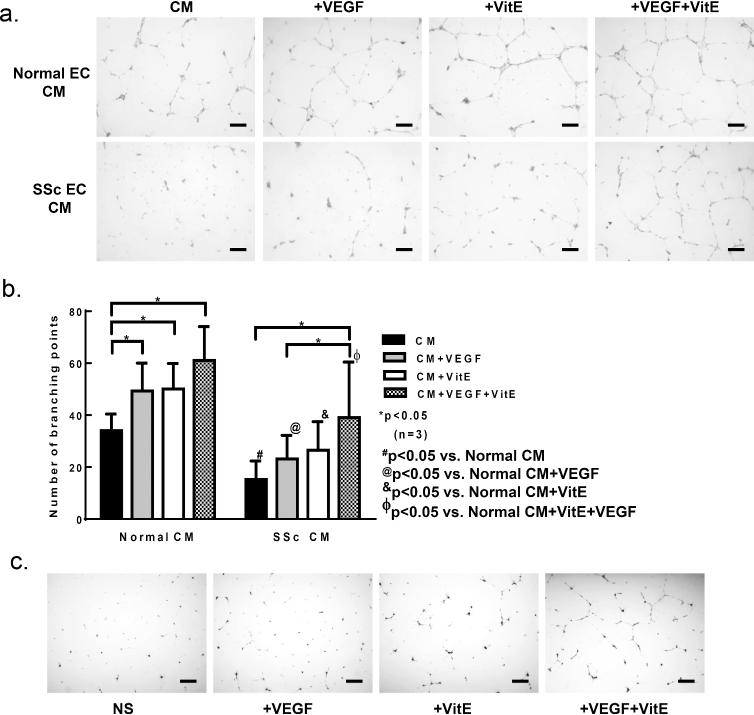
Since increased 8-isoprostane levels were observed in SSc EC conditioned media (Figure 1C), we examined whether conditioned media from SSc ECs had an inhibitory effect on EC tube formation. Conditioned media alone from healthy ECs induced healthy ECs to form tubes when they were plated on Matrigel (Figure 2A, B, and supplemental figure 1). VEGF increased the formation of tubes, and co-incubation of VEGF and vitamin E further increased tube formation. In contrast, conditioned media from SSc ECs induced fewer healthy ECs to form tubes. Addition of VEGF increased tube formation but the extent was significantly lower than that observed with healthy EC conditioned media. Addition of vitamin E with VEGF increased tube formation significantly. To test whether vitamin E has an effect on SSc ECs, we performed a Matrigel tube formation assay using SSc ECs (Figure 2C). Addition of vitamin E, alone or in combination with VEGF, increased the amount tubes formed.
Figure 2. Vitamin E reverses defection endothelial tube formation by SSc ECs.
a. To assess the effect of conditioned media from normal or SSc ECs on angiogenesis, Matrigel tube formation assay was performed. Representative Matrigel tube formation photomicrographs show that culturing normal ECs with conditioned media from normal ECs resulted in spontaneous tube formation, and that addition of VEGF (1 ng/ml) further increased tube formation. In contrast, incubating normal ECs with conditioned media from SSc ECs did not induce spontaneous tube formation, and addition of VEGF had no effect. The presence of vitamin E (50 μM) increased tube formation in both normal and SSc EC conditioned media. b. The number of branching points was quantified and graphed. c. Tube formation assay using SSc ECs. Vitamin E increased the ability of SSc ECs to form tubes, whether used alone or with VEGF. Means are shown +/− S.D. Differences were determined using two-way ANOVA and p values less than 0.05 were significant. Scale bar=200μm. n=number of patients. VitE: vitamin E; CM: conditioned media. #p<0.05 vs. normal CM; @p<0.05 vs. normal CM+VEGF; &p<0.05 vs. normal CM+VitE; ∋p<0.05 vs. normal CM+VitE+VEGF.
In vitro chemotaxis
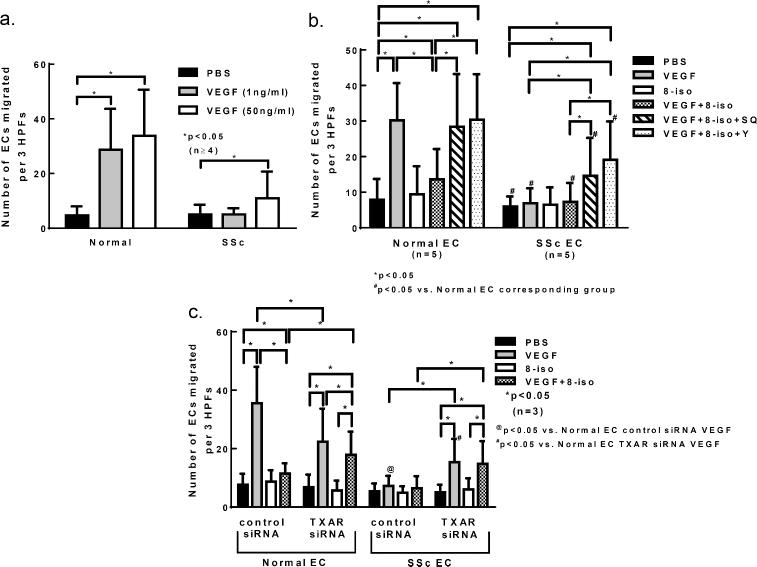
To more specifically examine the effect of 8-isoprostane on VEGF-induced cell migration, we performed chemotaxis assays. We first examined whether SSc ECs respond toward VEGF. VEGF dose-dependently induced EC migration in normal ECs, but this was markedly attenuated in SSc ECs (Figure 3A). We next examined whether 8-isoprostane had an effect on VEGF-induced cell migration. Again normal ECs migrated toward VEGF as shown in Figure 3B (VEGF 1 ng/ml vs. phosphate-buffered saline [PBS] group). 8-isoprostane significantly inhibited the ability of VEGF to induce migration of ECs. To determine whether 8-isoprostane exerted its inhibitory effect through the TXAR and its downstream ROCK pathway, a TXAR inhibitor (SQ29548) or ROCK inhibitor (Y27632) was added. The addition of the inhibitor together with VEGF and 8-isoprostane allowed significant EC migration. In contrast to normal ECs, VEGF failed to induce SSc EC migration (Figure 3B). However, this was restored partially by the addition of the inhibitors, although the extent of migration was significantly less compared to healthy ECs. These results suggest that the TXAR pathway is in part responsible for the impaired angiogenic response to VEGF in SSc.
Figure 3. Role of the TXAR in impaired angiogenesis by SSc ECs.
a. Chemotaxis assays were performed to examine whether VEGF could dose-dependently induce cell migration in normal and SSc ECs. Normal ECs migrated toward VEGF at both 1 and 50 ng/ml VEGF, while SSc ECs only responded at the higher dose. b. Chemotaxis assays were performed to determine the involvement of TXAR and its downstream pathway in 8-isoprostane- and VEGF-induced angiogenesis in both normal and SSc ECs. In normal ECs, VEGF induced significant cell migration compared to its control PBS while 8-isoprostane inhibited it. The co-incubation of either TXAR or ROCK inhibitor significantly restored VEGF-induced migration in the presence of 8-isoprostane. In SSc ECs, VEGF did not induce cell migration. The addition of the inhibitors significantly restored VEGF's ability to move cells, however the extent of migration was not as great as that seen in normal ECs. #p<0.05 vs. normal EC corresponding group. c. To further examine the role of the TXAR in VEGF-induced angiogenesis in SSc, the TXAR was knocked down and chemotaxis was performed. In both normal and SSc ECs, knocking down the TXAR restored the ability of VEGF to induce cell migration in the presence of 8-isoprostane. @p<0.05 vs. normal EC control siRNA VEGF group; #p<0.05 vs. normal EC TXAR siRNA VEGF group. Results are expressed as mean ± S.D. and p<0.05 is considered significant. HPF: high power field; n=number of experiments. 8-iso: 8-isoprostane; SQ: SQ29548 TXAR inhibitor; Y: Y27632 ROCK inhibitor.
We further explored the role of TXAR in VEGF-induced angiogenesis by knocking down TXAR expression. In normal ECs, cells treated with control siRNA migrated towards VEGF significantly compared to PBS while addition of 8-isoprostane inhibited the effect of VEGF (Figure 3C). When TXAR was knocked down, the inhibitory effect of 8-isoprostane on VEGF-induced cell migration was no longer apparent. SSc ECs, when treated with control siRNA, were unresponsive towards VEGF alone and in combination with 8-isoprostane. However when TXAR was knocked down, since 8-isoprostane could not act on TXAR, VEGF was able to induce migration of SSc ECs, whether acting alone or in combination with 8-isoprostane.
Phosphorylation of focal adhesion kinase (FAK)
Since RhoA and ROCK are key players in EC motility by regulating focal adhesion turnover (Lamalice et al., 2007), and since FAK is phosphorylated as a result of stimulation of the VEGF pathway during focal adhesion activation, we examined the extent of FAK phosphorylation in both normal and SSc ECs in relation to the expression of TXAR. In normal ECs, VEGF induced significant phosphorylation of FAK, which was inhibited by 8-isoprostane when cells were transfected with control siRNA (Figure 4A). When TXAR was knocked down, the extent of FAK phosphorylation increased significantly in the VEGF + 8-isoprostane group. In SSc ECs, VEGF failed to increase FAK phosphorylation. When TXAR was knocked down, surprisingly the basal FAK phosphorylation levels decreased significantly. However, when cells were treated with either VEGF or VEGF and 8-isoprostane together, significant phosphorylation of FAK was observed, though the extent was significantly lower compared to normal ECs.
Figure 4. Defective phosphorylation of FAK in SSc ECs.
a. Phosphorylation of FAK was examined by Western blotting. In normal ECs, VEGF induced significant FAK phosphorylation while in the presence of 8-isoprostane the phosphorylation was inhibited. When the TXAR was knocked down, the inhibitory effect of 8-isoprostane disappeared. In SSc ECs, VEGF failed to induce FAK phosphorylation in the absence or presence of 8-isoprostane. When the TXAR was knocked down, the basal levels of FAK phosphorylation decreased while VEGF and VEGF plus 8-isoprostane groups significantly increased FAK phosphorylation. The extent was still significantly lower compared to the normal ECs. #p<0.05 vs. normal EC control siRNA VEGF group; @p<0.05 vs. normal EC TXAR siRNA NS group; $p<0.05 vs. normal EC TXAR siRNA VEGF group; &p<0.05 vs. normal EC TXAR siRNA VEGF+8-iso group. b-e. The expression of the TXAR, ROCK1/2, and RhoA was examined by qPCR and Western blotting. b. Differences in the TXAR expression did not reach statistical significance at either mRNA or protein levels. c. The expression of ROCK1 was discordant between mRNA and protein levels. ROCK1 protein was significantly higher in SSc ECs. d. Similar to ROCK1, ROCK2 protein expression was significantly higher in SSc ECs. e. Both protein and mRNA levels of RhoA were elevated in SSc ECs compared to normal. Results are expressed as mean ± S.D. and p<0.05 is considered significant. n=number of subjects. 8-iso: 8-isoprostane.
Expression of the TXAR pathway
To examine whether the expression of the TXAR and its downstream signaling pathway differs between healthy and SSc ECs, we performed qPCR and Western blotting. Although there was a trend toward decreased TXAR mRNA and increased TXAR protein levels in SSc ECs, the variability rendered it not statistically significant (Figure 4B). ROCK1 mRNA expression was significantly elevated in normal ECs compared to SSc ECs (Figure 4C), however, the protein expression was significantly elevated in SSc ECs. Similar results were observed for ROCK2. ROCK2 mRNA expression in normal ECs was higher but protein expression was lower (Figure 4D). The discrepancy between the mRNA and protein levels could be due to differences in post-transcriptional/translational modifications and/or RNA and protein stability. It is possible that the lower ROCK mRNA levels in SSc ECs were due to a shorter mRNA half-life. In addition, the timing of the translation and transcription process is likely to differ. We harvested the cells after 24 hours of stimulation and it is possible that peak mRNA production occurred at an earlier time point. RhoA mRNA was significantly higher in SSc ECs when they were stimulated with 8-isoprostane or VEGF (Figure 4E). At the protein level, RhoA was significantly higher in SSc ECs under basal conditions as well as after 8-isoprostane stimulation.
ROCK activity in ECs
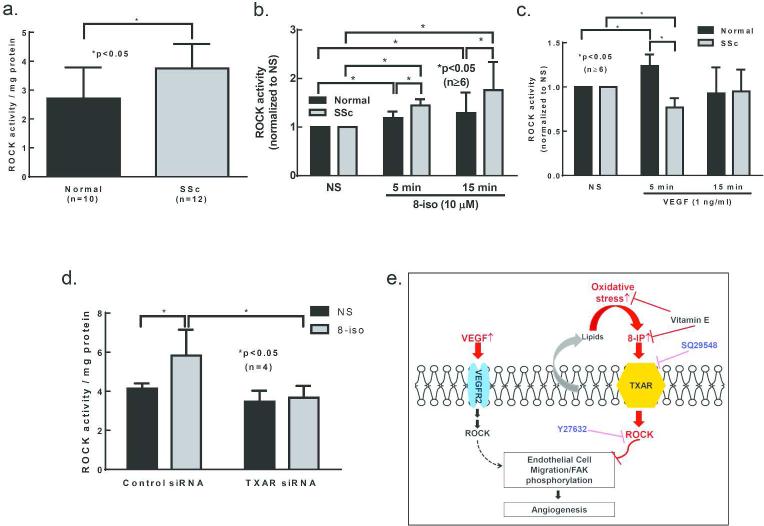
To further examine the involvement of the ROCK pathway in ECs, ROCK activity was determined after 8-isoprostane or VEGF stimulation at different time points. Under basal conditions, higher ROCK activity was observed in SSc ECs compared to healthy ECs (Figure 5A). This could be attributed to the higher expression levels of ROCK1/2 in SSc ECs as seen in Figure 4C and D. When stimulated with 8-isoprostane, both normal and SSc ECs showed significantly elevated ROCK activity at 5 and 15 min (Figure 5B). At both time points ROCK activity was significantly higher in SSc ECs compared to normal ones. On the other hand, VEGF induced a significant increase in ROCK activity in normal ECs at 5 min, and it returned to baseline at 15 min. In contrast, VEGF failed to induce ROCK activation in SSc ECs (Figure 5C). Taken together, these findings confirm that the VEGF receptor and its downstream ROCK signaling pathway function aberrantly in SSc ECs, and that 8-isoprostane stimulation results in enhanced ROCK activation, more so in SSc ECs than in normal ECs. To further examine whether 8-isoprostane signals through the TXAR and ROCK pathway, we knocked down TXAR and measured 8-isoprostane-activated ROCK levels. While 8-isoprostane increased ROCK activity in control siRNA-transfected SSc ECs, it failed to activate ROCK in TXAR knockdown cells (Figure 5D).
Figure 5. TXAR and ROCK expression and activity in SSc ECs.
a. ROCK activity was significantly higher in SSc ECs compared to normal ECs. b. When stimulated with 8-isoprostane, SSc ECs showed significant prolonged activation compared to normal ECs. c. When stimulated with VEGF, normal ECs showed significant ROCK activation while it was absent in SSc ECs. d. We confirmed that 8-isoprostane indeed activated ROCK via TXAR. Following knockdown of TXAR, 8-isoprostane failed to activate ROCK in SSc ECs. Results are expressed as mean ± S.D. and p<0.05 is considered significant. n=number of subjects. 8-iso: 8-isoprostane. e. Increased oxidative stress, as indicated by increased 8-isoprostane levels produced by SSc ECs, leads to persistent activation of TXAR and ROCK pathways, inhibition of FAK phosphorylation and EC migration, hence decreased VEGF-induced angiogenesis. Inhibition or knockdown of the TXAR pathway restores the ability of SSc ECs to migrate toward VEGF, suggesting that the TXAR plays a critical role in VEGF-induced angiogenesis in SSc. 8-IP: 8-isoprostane;  : inhibition.
: inhibition.
Discussion
In this study we determined that oxidative stress contributes to dysregulated angiogenesis in SSc skin ECs. As summarized in Figure 5E, we first established that increased 8-isoprostane levels are observed in SSc, and that vitamin E not only decreases 8-isoprostane, but also increases tube formation by both normal and SSc ECs. We then determined that 8-isoprostane affects VEGF-induced angiogenesis by applying chemical inhibitors or siRNA to block the TXAR pathway. Finally, we showed that the expression of TXAR and RhoA/ROCKs is elevated, and that the 8-isoprostane induced-ROCK pathway is hyper-activated in SSc ECs compared to normals. The direct link between 8-isoprostane, TXAR, and ROCK pathway was further supported by the effects of knocking down TXAR expression. These results provide a potential link between oxidative stress and impaired angiogenesis in SSc through the effect of 8-isoprostane on VEGF-induced angiogenesis.
The VEGF paradox in SSc vascular deficiency has been well documented. Several studies have been conducted to delineate the possible mechanism. Giusti et al. showed that the expression of pro-angiogenic kallikrein 12 was decreased in SSc patients (Giusti et al., 2005). Kallikrein 12 is involved in VEGF-mediated angiogenesis, and therefore a decrease in its expression leads to diminished VEGF response. In a recent report, an increase in a splice variant of VEGF, VEGF165b, in SSc has been observed (Manetti et al., 2011). VEGF165b, in contrast to other splice variants of VEGF, is anti-angiogenic. The authors elegantly showed that recombinant VEGF165b and SSc-EC-conditioned medium inhibited VEGF-mediated VEGF receptor phosphorylation and capillary morphogenesis in healthy ECs. In addition, anti-VEGF165b blocking antibodies abrogated the anti-angiogenic effect of SSc-EC-conditioned medium. Our data suggesting that 8-isoprostane inhibits VEGF-medicated angiogenesis adds to the mechanisms of the VEGF paradox in SSc. Although the relative contribution of each pathway is difficult to delineate, it appears that the VEGF paradox is comprised of several factors that lead to failure of angiogenesis.
We showed that elevated plasma levels of 8-isoprostane were seen only in diffuse patients but not limited patients was in agreement with the study by Tufvesson et al. (Tufvesson et al., 2010). This suggests that oxidative stress is more prominent in patients with more extensive SSc. However, in a Japanese study there was no difference between diffuse and limited patients (Ogawa et al., 2006). This might be due to racial or regional differences. In that study, the authors also showed that 8-isoprostane is associated with the severity of lung fibrosis and the extent of vascular damage. It is interesting that we observed elevated 8-isoprostane levels in patients with ILD, but not in those with PAH (Figure 1A). Since diffuse SSc patients are often afflicted with ILD and limited SSc patients develop PAH, the results seem to correspond well with the disease phenotype.
In this study, 8-isoprostane inhibited VEGF-induced angiogenesis by the TXAR activation, since the TXAR antagonist SQ29548 reversed 8-isoprostane-induced inhibition of cell migration and focal adhesion in both normal and SSc ECs. In addition, by knocking down the TXAR, the inhibitory effect of 8-isoprostane in SSc ECs was also reversed, in the context of inability to activate the ROCK pathway. These findings agree with several studies showing that TXAR is anti-angiogenic (Ashton and Ware, 2004; Benndorf et al., 2008; Gao et al., 2000). However, the role of the TXAR in angiogenesis is not quite clear, as conflicting reports exist. Thromboxane A2 (TXA2), a ligand of TXAR, can function as a critical intermediary of angiogenesis (Daniel et al., 1999; Nie et al., 2000). In addition, TXAR stimulation enhanced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression in human umbilical vascular ECs (HUVECs) (Ishizuka et al., 1998). In contrast, there are reports suggesting that TXAR stimulation inhibits EC migration and in vitro capillary formation (Ashton et al., 1999), and it induces apoptosis of ECs (Gao et al., 2000). Moreover, activation of TXAR limits angiogenesis by inhibiting VEGF signaling and antagonizing the pro-angiogenic effects of basic fibroblast growth factor (bFGF) (Ashton et al., 2004; Ashton and Ware, 2004); both pathways are inactivated in SSc. The discrepancies between these studies might be due to the size of the vasculature from which the ECs are isolated; TXAR activation tends to be pro-angiogenic using ECs isolated from larger vessels, such as HUVECs, and anti-angiogenic on ECs from microvascular structures, which were used in our study. Nonetheless, we were able to show that TXAR protein expression is increased in SSc ECs, and that inhibition of this receptor blocks the effect of 8-isoprostane and increases the effect of VEGF-induced cell migration, tube formation, and focal adhesion activation. The increased expression of the TXAR and its downstream RhoA/ROCK pathway skews the SSc ECs to an anti-angiogenic state by hyperactivating this pathway, overpowering the transiently activated VEGF-ROCK pathway, and diminishing the pro-angiogenic effect of VEGF.
The ability of both VEGF and 8-isoprostane to stimulate the ROCK pathway in ECs and result in opposite functions seems counterintuitive. We postulate the difference in the activation time course skews the cells to different pathways downstream of ROCK, governing the fate of the ECs to become pro-angiogenic or anti-angiogenic. As shown in Figure 5, VEGF transiently activated the ROCK pathway as 8-isoprostane stimulated ROCK activation for a longer period of time. Our data agrees with the study performed by Benndorf et al, where they showed brief activation of RhoA by VEGF alone and prolonged activation when 8-isoprostane was added (Benndorf et al., 2008). Although basal levels of RhoA/ROCK are essential for cell migration, its activation could also lead to inhibition of cell movement (Tkach et al., 2005). It has been shown that prolonged activation of RhoA/ROCK leads to EC motility inhibition due to increased adhesion to the substratum (Wojciak-Stothard et al., 2007). Therefore the pronounced and prolonged activation of the ROCK pathway in SSc ECs becomes the dominate pathway and gears the cells to an anti-angiogenic state. Another interesting finding is that VEGF failed to induce ROCK activation in SSc ECs (Figure 5C). The mechanism is not known however it is possible that this is a result of a dysfunctional VEGF receptor (VEGFR2) as it has been shown that although its expression is increased in SSc ECs, its ability to be phosphorylated is impaired (Manetti et al., 2011). The machinery of the VEGF pathway in SSc EC requires further investigation. It should be noted that the TXAR pathway not only affects the ROCK pathway as shown in our study, it also affects other signaling pathways induced by VEGF, including AKT, endothelial nitric oxide synthase, as well as protein kinase D1 (Ashton and Ware, 2004). Interestingly, we previously showed that in SSc ECs the AKT pathway is impaired when stimulated with various chemokines (Tsou et al., 2012a). It is possible that the 8-isoprostane/TXAR pathway affects both ROCK and AKT pathways in SSc and therefore inhibits the ability of ECs to migrate.
In summary, this study provides a better understanding of the impact of excess oxidative stress in SSc dysregulated angiogenesis. We successfully showed that, (1) the TXAR and RhoA/ROCKs are upregulated in SSc ECs; (2) the VEGF-induced ROCK pathway is impaired; (3) it adds to the mechanism of the VEGF paradox that the TXAR pathway plays an inhibitory role in VEGF-induced angiogenesis; (4) elevated 8-isoprostanes in SSc is not just a marker for oxidative stress, they in fact have biological relevance in the pathogenesis of SSc. This study provides not only a molecular mechanism to explain dysregulated angiogenesis in SSc, but also insights that can potentially be applied to other diseases that are affected by oxidative stress.
See supplemental file for more discussion.
Materials and Methods
The detailed Materials and Methods section can be found in the supplemental document.
Patients
All scleroderma (SSc) patients fulfilled the 1980 American College of Rheumatology criteria for classification of SSc (LeRoy, 1974). The details of the enrolled subject's characteristics are summarized in Table 1. For endothelial cell (EC) isolation, skin biopsies from healthy volunteers and diffuse SSc patients were obtained. Two 4mm punch biopsies were taken from the distal forearm of healthy subjects or from involved skin of SSc patients. A written informed consent was obtained from all subjects and the study was approved by the Institutional Review Board of the University of Michigan.
Table 1.
Characteristics of SSc patients and healthy volunteer subjects.
| SSc (n=61) | Diffuse SSc (n=44) | Limited SSc (n=17) | Healthy subjects (n=33) | |
|---|---|---|---|---|
| Age (years) | 55.0 ± 1.5a | 53.9 ± 1.8 | 57.6 ± 2.7 | 45.3 ± 2.5 |
| Sex | F53/M8 | F38/M6 | F15/M2 | F20/M13 |
| Disease duration (years) | 7.1 ± 1.1 | 4.5 ± 0.6 | 13.5 ± 3.0 | N.A.b |
| mRSSc | 15.0 ± 1.5 | 19.0 ± 1.8 | 4.8 ± 0.8 | N.A. |
| Raynaud's | 60 | 43 | 17 | N.A. |
| Early diseased | 36 | 30 | 6 | N.A. |
| Deceased | 5 | 3 | 2 | N.A. |
| History of Digital ulcers | 18 | 14 | 4 | N.A. |
| Teleangectasias | 34 | 24 | 10 | N.A. |
| Gastrointestinal disease | 51 | 36 | 15 | N.A. |
| ILDe | 30 | 24 | 6 | N.A. |
| PAHf | 12 | 9 | 3 | N.A. |
| Renal involvement | 2 | 2 | 0 | N.A. |
Mean ± SEM
N.A. = Not applicable
mRSS=modified Rodnan skin score (0-51)
Early disease = less than 5 years
Interstitial lung disease
Pulmonary arterial hypertension (mean pulmonary artery pressure at rest ≥ 25 mmHg; pulmonary capillary wedge pressure ≤ 15 mmHg.)
Cell culture
Dermal ECs were isolated from human skin in our laboratory. The biopsies were cut into small pieces and digested for 30 min in a solution containing 2.4 units/ml dispase (Invitrogen, Grand Island, NY), 650 units/ml type II collagenase (Sigma Aldrich, St. Louis, MO), and 10,000 Dornase units/ml DNase (Billerica, MA). After digestion, the cells were cultured in EBM-2 medium with growth factors (Lonza, Allendale, NJ). Dermal ECs were isolated using the CD31 MicroBead Kit and a MiniMACS™ Separator with an MS Column (Miltenyi Biotech, San Diego, CA). ECs, which were the positively selected cells, were maintained in EBM-2 medium with growth factors on gelatin-coated plates. If needed, the cells were re-purified when the cultures were expanded. Passages 3-6 were used for experiments and the purity of the cells are approximately 95%. Two days before experiments, ECs were grown in EBM basal media in the presence of bovine brain extract, epidermal growth factor, as well as 5% fetal bovine serum (FBS) to minimize the effect of growth factors in complete EBM-2 media. ECs were serum starved in EBM-2 basal media in the presence of 0.2% FBS overnight before stimulation experiments. To collect conditioned media, confluent ECs were washed and incubated overnight with EBM-2 basal medium with 0.2% FBS for 24 hours. The culture supernatants collected, centrifuged, and stored at −80°C until use. The ECs used in this study were isolated from 23 patients with diffuse SSc and 19 healthy subjects.
Statistical analysis
Results were expressed as mean ± S.D, except for Figure 1A, where it is presented as median ± interquartile range. To determine the differences between the groups, Student's t-test, Mann Whitney U test, or two-way ANOVA were performed. P-values of less than 0.05 with two-tailed analysis were considered statistically significant.
Supplementary Material
Acknowledgements
This work was supported by the Arthritis Foundation (to P. T.), NIH/NIAMS T32 AR007080 (to P. T.), the Scleroderma Foundation (to A.E.K.), the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, the Frederick G. L. Huetwell and William D. Robinson, MD, Professorship in Rheumatology, NIH/NIAMS K24 AR063120-02 (to D. K.), the clinical research unit at the University of Michigan, the Linda Dolce Scleroderma Research Fund, the Marvin and Betty Danto and the Jonathan and Lisa Rye Endowments for Scleroderma Research at the University of Michigan, and in part by the Tissue Procurement Core of the University of Michigan Comprehensive Cancer Center (CA46952).
Footnotes
Conflict of interest: D.K. reports grants from NIAMS (K24), during the conduct of the study; grants and personal fees from Actelion, Astra-Zeneca, Bayer, BMS, DIGNA, Genentech, Gilead, InterMune, Lycera, Merck, Takeda, Savient, and United Therapeutics, outside the submitted work.
References
- Ashton AW, Cheng Y, Helisch A, et al. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and alpha(v)beta3. Circulation research. 2004;94:735–42. doi: 10.1161/01.RES.0000122043.11286.57. [DOI] [PubMed] [Google Scholar]
- Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circulation research. 2004;95:372–9. doi: 10.1161/01.RES.0000138300.41642.15. [DOI] [PubMed] [Google Scholar]
- Ashton AW, Yokota R, John G, et al. Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2). The Journal of biological chemistry. 1999;274:35562–70. doi: 10.1074/jbc.274.50.35562. [DOI] [PubMed] [Google Scholar]
- Avouac J, Borderie D, Ekindjian OG, et al. High DNA oxidative damage in systemic sclerosis. J Rheumatol. 2010;37:2540–7. doi: 10.3899/jrheum.100398. [DOI] [PubMed] [Google Scholar]
- Benndorf RA, Schwedhelm E, Gnann A, et al. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ Res. 2008;103:1037–46. doi: 10.1161/CIRCRESAHA.108.184036. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer KR, Hillary JB, Bunce T, et al. Increased susceptibility to oxidation of low-density lipoproteins isolated from patients with systemic sclerosis. Arthritis Rheum. 1995;38:1060–7. doi: 10.1002/art.1780380807. [DOI] [PubMed] [Google Scholar]
- Comporti M, Signorini C, Arezzini B, et al. F2-isoprostanes are not just markers of oxidative stress. Free radical biology & medicine. 2008;44:247–56. doi: 10.1016/j.freeradbiomed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Liu H, Morrow JD, et al. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999;59:4574–7. [PubMed] [Google Scholar]
- Davi G, Alessandrini P, Mezzetti A, et al. In vivo formation of 8-Epi-prostaglandin F2 alpha is increased in hypercholesterolemia. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:3230–5. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- Davies CA, Jeziorska M, Freemont AJ, et al. The differential expression of VEGF, VEGFR-2, and GLUT-1 proteins in disease subtypes of systemic sclerosis. Human pathology. 2006;37:190–7. doi: 10.1016/j.humpath.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Distler O, Del Rosso A, Giacomelli R, et al. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis research. 2002;4:R11. doi: 10.1186/ar596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler O, Distler JH, Scheid A, et al. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res. 2004;95:109–16. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yokota R, Tang S, et al. Reversal of angiogenesis in vitro, induction of apoptosis, and inhibition of AKT phosphorylation in endothelial cells by thromboxane A(2). Circulation research. 2000;87:739–45. doi: 10.1161/01.res.87.9.739. [DOI] [PubMed] [Google Scholar]
- Giusti B, Margheri F, Rossi L, et al. Desmoglein-2-integrin Beta-8 interaction regulates actin assembly in endothelial cells: deregulation in systemic sclerosis. PloS one. 2013;8:e68117. doi: 10.1371/journal.pone.0068117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti B, Serrati S, Margheri F, et al. The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis. Arthritis Rheum. 2005;52:3618–28. doi: 10.1002/art.21383. [DOI] [PubMed] [Google Scholar]
- Hou X, Roberts LJ, 2nd, Gobeil F, Jr., et al. Isomer-specific contractile effects of a series of synthetic f2-isoprostanes on retinal and cerebral microvasculature. Free radical biology & medicine. 2004;36:163–72. doi: 10.1016/j.freeradbiomed.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Hummers LK, Hall A, Wigley FM, et al. Abnormalities in the regulators of angiogenesis in patients with scleroderma. J Rheumatol. 2009;36:576–82. doi: 10.3899/jrheum.080516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T, Kawakami M, Hidaka T, et al. Stimulation with thromboxane A2 (TXA2) receptor agonist enhances ICAM-1, VCAM-1 or ELAM-1 expression by human vascular endothelial cells. Clin Exp Immunol. 1998;112:464–70. doi: 10.1046/j.1365-2249.1998.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kingdon E, Norman J. The isoprostane 8-iso-PGF2alpha suppresses monocyte adhesion to human microvascular endothelial cells via two independent mechanisms. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:443–5. doi: 10.1096/fj.03-1364fje. [DOI] [PubMed] [Google Scholar]
- Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S. Soluble adhesion molecules (sVCAM- 1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: relationship to organ systemic involvement. Clin Rheumatol. 2005;24:111–6. doi: 10.1007/s10067-004-0987-3. [DOI] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–94. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- LeRoy EC. Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J Clin Invest. 1974;54:880–9. doi: 10.1172/JCI107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz Z, Sukura A, Povilenaite D, et al. Increased but imbalanced expression of VEGF and its receptors has no positive effect on angiogenesis in systemic sclerosis skin. Clin Exp Rheumatol. 2002;20:641–6. [PubMed] [Google Scholar]
- Manetti M, Guiducci S, Romano E, et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circ Res. 2011;109:e14–26. doi: 10.1161/CIRCRESAHA.111.242057. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Ciabattoni G, Paredi P, et al. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. American journal of respiratory and critical care medicine. 1998;158:1524–7. doi: 10.1164/ajrccm.158.5.9803102. [DOI] [PubMed] [Google Scholar]
- Morrow JD. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev. 2000;32:377–85. doi: 10.1081/dmr-100102340. [DOI] [PubMed] [Google Scholar]
- Nie D, Lamberti M, Zacharek A, et al. Thromboxane A(2) regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem Biophys Res Commun. 2000;267:245–51. doi: 10.1006/bbrc.1999.1840. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–44. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa F, Shimizu K, Muroi E, et al. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology (Oxford) 2006;45:815–8. doi: 10.1093/rheumatology/kel012. [DOI] [PubMed] [Google Scholar]
- Rabquer BJ, Tsou PS, Hou Y, et al. Dysregulated expression of MIG/CXCL9, IP- 10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthritis Res Ther. 2011;13:R18. doi: 10.1186/ar3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LJ, 2nd, Milne GL. Isoprostanes. J Lipid Res. 2009;50(Suppl):S219–23. doi: 10.1194/jlr.R800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambo P, Baroni SS, Luchetti M, et al. Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis Rheum. 2001;44:2653–64. doi: 10.1002/1529-0131(200111)44:11<2653::aid-art445>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Scholz H, Yndestad A, Damas JK, et al. 8-isoprostane increases expression of interleukin-8 in human macrophages through activation of mitogen-activated protein kinases. Cardiovasc Res. 2003;59:945–54. doi: 10.1016/s0008-6363(03)00538-8. [DOI] [PubMed] [Google Scholar]
- Servettaz A, Guilpain P, Goulvestre C, et al. Radical oxygen species production induced by advanced oxidation protein products predicts clinical evolution and response to treatment in systemic sclerosis. Ann Rheum Dis. 2007;66:1202–9. doi: 10.1136/ard.2006.067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CM, Tanner SB, Awad JA, et al. Evidence of free radical-mediated injury (isoprostane overproduction) in scleroderma. Arthritis Rheum. 1996;39:1146–50. doi: 10.1002/art.1780390711. [DOI] [PubMed] [Google Scholar]
- Svegliati S, Cancello R, Sambo P, et al. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J Biol Chem. 2005;280:36474–82. doi: 10.1074/jbc.M502851200. [DOI] [PubMed] [Google Scholar]
- Tkach V, Bock E, Berezin V. The role of RhoA in the regulation of cell morphology and motility. Cell motility and the cytoskeleton. 2005;61:21–33. doi: 10.1002/cm.20062. [DOI] [PubMed] [Google Scholar]
- Tsou P, Rabquer BJ, Balogh B, et al. Primary human scleroderma dermal endothelial cells exhibit defective angiogenesis. Arthritis Rheum. 2012a;64:S645. [Google Scholar]
- Tsou PS, Balogh B, Pinney AJ, et al. Lipoic acid plays a role in scleroderma: insights obtained from scleroderma dermal fibroblasts. Arthritis Res Ther. 2014;16:411. doi: 10.1186/s13075-014-0411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou PS, Talia NN, Pinney AJ, et al. Effect of oxidative stress on protein tyrosine phosphatase 1B in scleroderma dermal fibroblasts. Arthritis Rheum. 2012b;64:1978–89. doi: 10.1002/art.34336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufvesson E, Bozovic G, Hesselstrand R, et al. Increased cysteinyl-leukotrienes and 8- isoprostane in exhaled breath condensate from systemic sclerosis patients. Rheumatology (Oxford) 2010;49:2322–6. doi: 10.1093/rheumatology/keq271. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Torondel B, Tsang LY, et al. The ADMA/DDAH pathway is a critical regulator of endothelial cell motility. J Cell Sci. 2007;120:929–42. doi: 10.1242/jcs.002212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.