Abstract
Parents and children have been found to show coordination or coregulation of the hypothalamic–pituitary–adrenal (HPA) axis. This coordination may be reflected in adolescents' neural activation to parent stimuli, particularly in regions of the brain associated with social information processing.
This study reports on 22 adolescents (13 males, mean age 17 years), recruited from a longitudinal study to participate in a functional MRI (fMRI) scanning protocol. Approximately 1.5 years before the scan, these same adolescents participated in a family conflict discussion in the lab with both parents, and all three family members provided samples of salivary cortisol five times, before and after the discussion. Multilevel models found positive cross-sectional and time-lagged associations between parents' and youth cortisol. Empirical Bayes (EB) coefficients, extracted from these models to reflect the strength of the relationship between parent and adolescent cortisol, were tested in conjunction with adolescents' neural activation to video clips of their parents taken from the conflict discussion. For both mothers and fathers, youth who showed stronger cortisol coregulation with each parent (both in cross-sectional and time-lagged analyses) showed more activation to that same parent in posteromedial regions (precuneus, posterior cingulate, and retrosplenial cortex) that have been linked with social cognition, e.g. mentalizing about others' emotions. Youths' adrenocortical coregulation with their parents may be reflected in their neural processing of stimuli featuring those same parents.
Keywords: HPA axis, Cortisol, Coregulation, Adrenocortical attunement, Neural, MRI
Coordination with caregivers facilitates healthy child development, providing scaffolding to help children build self-regulatory capabilities. Parent–child synchrony helps to prepare children for complex social relationships by introducing patterned chains of interaction, shaped by social contingencies — a complex dance which provides essential input to the developing social brain (Feldman, 2007). In addition to behavioral attunement in gaze, vocalization, and movement, children may also show biological or physiological synchrony with parents. For example, autonomic arousal indices such as heart rate and temperature appear to be coordinated within parent–child dyads (Ebisch et al., 2012; Feldman et al., 2011). Another system that appears to exhibit parent–child coregulation, the hypothalamic–pituitary–adrenal (HPA) axis, releases the stress hormone cortisol and is shaped during childhood by the family and social environment (Gunnar, 1998). HPA axis coregulation has the potential to be another type of physiological synchrony that facilitates children's self-regulation.
Neuroendocrine coregulation between parents and children
In 1998, Granger found that mother–child dyads had positively correlated levels of cortisol during a conflict discussion task (Granger et al., 1998). This preliminary evidence for adrenocortical coregulation has now been replicated in multiple studies with children's ages ranging from infancy to adolescence (Hibel et al., 2009, 2014; Laurent et al., 2012; Middlemiss et al., 2012; Papp et al., 2009; Saxbe et al., 2014). Many of the recently published studies used multilevel modeling approaches to adjust for the nesting of cortisol within individuals and dyads and to control for sampling time of day, an important covariate given the diurnal slope of cortisol. Thus, these findings suggest that parents and children show correspondence in momentary cortisol levels over and above the expected diurnal change over the day and, in many cases, across several laboratory tasks and/or several days of assessment.
A number of these studies have explored moderators of within-dyad attunement, and have found maternal sensitivity to be linked with stronger mother–child cortisol coregulation, (Atkinson et al., 2013; Sethre-Hofstad et al., 2002; van Bakel and Riksen-Walraven, 2008). In the largest study of adrenocortical coregulation to date (Hibel et al., 2015), including almost 1300 dyads assessed in early infancy, late infancy, and toddlerhood, mothers' and children's cortisol levels were correlated at all three visits, and mother–child coregulation was bolstered by mothers' sensitivity and weakened by children's emotional reactivity. Similarly, Ruttle et al. (2011) found stronger cortisol correspondence between mothers and preschoolers if the dyad was more “behaviorally sensitive,” a construct including both maternal behaviors (sensitivity, structuring) and child behaviors (responsiveness, involvement) assessed during a free-play interaction. In one of the only studies focusing on adolescents (Papp et al., 2009), diurnal coordination between mothers and children was stronger if adolescents spent more time and shared more activities with their mothers and reported greater parental monitoring and supervision. Therefore, the strength of adrenocortical correspondence between mothers and adolescents appeared to be linked to the closeness of the parent–child relationship. However, importantly, this literature is mixed and a number of studies have linked coregulation with conflict and relationship distress. Indeed, within the couples' literature, coregulation has been associated with poor marital quality (Liu et al., 2013; Saxbe & Repetti, 2010), perhaps because of negative affect reciprocity processes. The implications of coregulation on relationship functioning are likely complex and may vary depending on context and on the physiological system under investigation (Timmons et al., epub before print).
Potential neural correlates of coregulation
Feldman (2007) theorized that parent–child synchrony would shape children's neural development. However, no studies have described the neural correlates of parent–child physiological coregulation or attunement. Only a small number of published studies have examined children's responses to viewing their own parents, which may represent a starting place in understanding the neural correlates of parent–child attunement. Whittle et al. (2012) found that when youth viewed their own (vs. an unfamiliar) parent showing positive affect, they showed more activation in the emotion processing, self-regulation, and mentalizing regions including the anterior and posterior cingulate, ventrolateral prefrontal cortex, and precuneus. Tottenham et al. (2012) found that the left amygdala responded to images of the mother vs. a stranger, and Saxbe et al. (2015) found that adolescents showed more medial prefrontal activation to their own parents vs. an unfamiliar peer. However, these studies all test main effects rather than specific characteristics of the parent–child relationship such as the degree of synchrony or coregulation. In a complementary literature, studies of parents have found parent–child relationship characteristics to be associated with neural activation to own-infant stimuli, but these studies have largely not included neuroendocrine measurements. In an exception to this rule, one study examined mothers' cortisol responses to their infants' distress in the laboratory, and then extracted intercept and slope coefficients from an HLM analysis in order to test whether these coefficients predicted mothers' neural activation to their infants' cry sounds (Laurent et al., 2011), finding that mothers' cortisol reactivity was associated with heightened activation in emotion-processing regions including the periaqueductal gray, insula, and orbitofrontal cortex.
If, as Feldman (2007) suggests, synchrony or coregulation prepares the social brain for the contingencies and reciprocities of social relationships, then the neural correlates of coregulation might emerge in regions that have been previously associated with social cognition. These regions could include the cortical “mentalizing network,” comprising cortical midline structures including the medial prefrontal cortex and the posteromedial cortices (PMCs; precuneus, posterior cingulate, and retrosplenial cortex), and lateral regions including the temporoparietal junctions (TPJ) and posterior superior temporal sulcus (pSTS). Activity within this network has been associated with social cognition (Van Overwalle and Baetens, 2009), empathy for others (Schnell et al., 2011) and processing of self-relevant stimuli (Northoff et al., 2006). One particular component of this network, the posteromedial cortices (PMCs) has been specifically associated with third-person perspective-taking on social interactions (Ochsner et al., 2004; Petrini et al., 2014). The ability to put oneself in another's mind may be a hallmark of the complex social cognitive abilities that are facilitated by parent–child synchrony (Feldman, 2007). As such, the PMCs may be a particularly interesting potential correlate of parent–child neuroendocrine coregulation. Additionally, subcortical regions such as the amygdala, hippocampus, and ventral striatum, known to be dense with glucocorticoid receptors, have been associated with social emotion processing (Adolphs, 2010) and processing own-parent stimuli specifically (Tottenham et al., 2012).
Limitations of current coregulation literature
Although the literature on adrenocortical coregulation between parents and children is intriguing, it is missing several important elements. Almost all of the above-cited papers focus exclusively on mother-child pairs. With few exceptions (e.g., Stenius et al., 2008), fathers have been ignored in the physiological coregulation literature and it remains unclear whether fathers also coregulate with children in the same way, and to the same degree, as mothers. Given that fathers also develop attachment relationships with children and serve a scaffolding function in child development, it is likely that they show similar coregulatory processes as mothers. In a time-lagged model of cortisol sampled before and after a family conflict interaction, Saxbe et al. (2014) found cross-sectional coregulation between fathers, mothers, and children. However, when mothers and fathers were entered together to predict youths' cortisol (with youth prior cortisol also included in the same model), only fathers' cortisol emerged as a unique predictor of youth cortisol. This suggests that fathers may not only show physiological linkage with children, but may in fact have a stronger degree of influence than mothers in some cases.
This type of time-lagged modeling (testing how one person's cortisol predicts another person's subsequent cortisol, while controlling for that person's own prior cortisol level) offers a more rigorous test of coregulation than cross-sectional analyses of coregulation, in the sense that it depicts how a person's change in cortisol over time can be driven or influenced by another person. Granger et al. (1998) found preliminary evidence for lagged effects in parent–child pairs that included school-aged children, in that mothers' pre-task cortisol predicted children's post-task cortisol and vice versa, but these analyses did not control for within-person autocorrelation. Time-lagged analyses measures what Butler (2011) calls “morphogenic covariation,” or between-partner covariation around a changing trajectory, also known as “transmission” or “contagion.” In contrast, most of the current literature measures cross-sectional intercorrelation, which Butler dubs “morphostatic covariation” and which could also be termed “concurrent synchrony.” Although morphogenic and morphostatic covariation are conceptually similar, they are statistically distinct constructs that may have different theoretical implications. For parents and children, morphostatic covariation could reflect similar reactivity to a shared context, while morphogenic covariation reflects transmission of a physiological arousal from one person to another.
Current study and hypotheses
The current study is the first to examine whether parent–child HPA axis coregulation is associated with neural activation. We do so within a longitudinal framework, testing whether parent–child cortisol coregulation coefficients extracted from a previous laboratory visit (Saxbe et al., 2014) are associated with youths' neural activation to rating the emotions of those same parents. Although the literature on the implications of physiological coregulation for relationship functioning is preliminary and mixed, several studies suggest that it may reflect parent–child closeness or at least a degree of proximity and engagement with parents. Therefore, we expected coregulation to be associated with “mentalizing network” regions, particularly the PMCs, and also with subcortical regions associated with emotion process (e.g., the amygdala, hippocampus, and ventral striatum). Given the lack of precedent in the literature for examining the neural correlates of neuroendocrine coregulation, we used whole-brain analyses as the starting point for our examination of the MRI data. We hypothesized that youth with stronger coregulation to each parent would show greater activation in social processing regions (e.g., the mentalizing network; subcortical affective processing regions) when viewing that same parent. We planned to examine coregulation with both fathers and mothers, with both cross-sectional and time-lagged coregulation coefficients as predictors. We did not have separate hypotheses for fathers vs. mothers or for time-lagged vs. cross-sectional coregulation, but rather expected that results from the four separate whole-brained analyses might provide convergent validity.
Methods
Participants
Participants were drawn from the second cohort (n = 69) of a larger longitudinal study on the impact of family aggression on youth development, conducted in Los Angeles (Margolin et al., 2010). Families were recruited from the community via advertising and word of mouth. Eligibility criteria included that the family included a child in middle school (grades 6–8), that the parents had lived together for the past 3 years, and all three family members could complete measures in English.
Approximately 1–2 years before the scan (median = 1 year 7 months; mean = 1 year 8 months; range = 1 year to 3 years 10 months; all but one participant did the scan within 2 years of the lab visit), 43 families participated in a videotaped discussion and cortisol sampling protocol including both parents and the youth. Before data collection for the next wave of the longitudinal study began, a letter was sent to these 43 families inviting youth to participate in the MRI substudy (we could not invite the entire cohort because the MRI stimuli included video from the discussion task, as described below). Eligibility criteria included that youth be right-handed, not have metal in their body or conditions that would preclude scanning, or not be taking psychoactive medications. Of the 43 families we contacted, seven youth were ineligible, five declined to participate, and seven could not be reached or had scheduling difficulties. Ultimately, 24 youth participated in the procedures and, of these, two did not sample cortisol during their laboratory visit and were not included in the current paper. Of the remaining 22 participants, two had issues with their data: one because of left-handedness, one had a brain abnormality flagged by the radiologist (specifically, a white matter hypointensity in the occipital lobe). All analyses reported in this paper were done with and without these two youth, and since the key results reported in this paper did not differ, the two youth were included. One additional participant lacked video clips of his father and cortisol from his father, but is included in analyses for the mother condition. Therefore, the final sample included 22 adolescents (13 males) averaging 17.0 years of age at the time of the scan (range 15.47–18.72). The sample was ethnically diverse: 36% (8 youth) identified as Latino, 32% (7 youth) as Caucasian, 9% (2 youth) as African–American, 14% (3 youth) as multi-racial, and 9% (2 youth) as Asian–American.
Conflict discussion and cortisol collection procedure
Families visited the lab for a 3- to 4-hour visit, scheduled after 10:30 a.m. to avoid the morning cortisol peak. Before their visit, families were informed about all aspects of the discussion task and given the option to decline saliva collection while participating in the discussion. They were instructed not to eat or drink for an hour before the appointment and not to consume tobacco, alcohol, or caffeine for 24 h prior to the appointment. Before beginning the discussion task, families participated in a relaxation induction, viewing a 10-min video with calming images and music, to establish a baseline for cortisol. The first saliva sample was collected via passive drool after the induction.
Next, the conflict portion of the task began. Each family member was given a questionnaire of 33 common family conflict topics, with the option to write in additional topics, and asked to rate the amount of conflict they typically elicited. Each family member then met with an experimenter in an individual priming interview to identify and describe conflict topics of greatest concern. Three experimenters met separately and simultaneously with one of the three family members in separate rooms. The experimenters then met briefly to identify the three greatest conflict-provoking areas of discussion for each family. Families were seated together in a room and given 15 min to discuss at least one of the three identified topics, starting with the most contentious. Families were instructed to discuss the topic as they would at home.
Six saliva samples were collected: at baseline, immediately postdiscussion (baseline + 40 min), and at four postdiscussion timepoints (baseline + 50 min, baseline + 60 min, baseline + 80 min, and baseline + 100 min). The mean baseline sample collection time was 2:10 p.m (SD + 2 h). Saliva samples were frozen immediately after the family session and later shipped in dry ice to Salimetrics, LLC, to be assayed for concentrations of free salivary cortisol, using an enzyme immunoassay with a lower limit of sensitivity of 0.003 μg/dl, and intraassay and interassay coefficients of variation of 3.5% and 5.1%, respectively. Each saliva sample was assayed twice, and analyses were repeated if any pair of results differed by N 7%.
MRI procedure
MRI stimuli
Video stimuli came from the aforementioned family discussion, which was recorded using a split-screen system. The same laboratory space, seating arrangement, and camera set-up was used to record each discussion, so that lighting, camera angles, and distance from the camera were generally consistent across families. The program Adobe Premiere Pro CS 5 was used to extract five-second clips for each family member. Any clips in which another person was visible (e.g., a hand gesturing in front of the target person) were discarded so that only the target person could be seen in each clip. We also removed sound to avoid sentence fragments or the risk of the participant hearing the voice of another family member not visible in the clip, and so that the task focused on nonverbal emotion communicated through facial expressions and gestures rather than on eliciting memories of the specific topics discussed. Thirty clips were initially produced for each family member and then culled down to 15. Before selecting clips, all clips were scored by the first author and undergraduate RAs for valence (positive/negative affect) and expression (whether the person in the clip was talking or listening), and clips were selected so as to balance both of these features, so that each participant viewed a mix of positively and negatively valenced clips in which the target person was both talking and listening. After clips were selected, at least one undergraduate research assistant, trained in observational coding of emotion, rated each family's clips in the order in which they were presented in the scanner. The intraclass coefficient coefficient (ICC) for research assistant ratings and participants' subsequent in-scanner ratings was .70, suggesting acceptable reliability.
MRI protocol
Before scanning, participants watched a minute-long clip of their own family discussion to acclimate them to seeing images of themselves and their parents. In order to prime emotional/empathetic processing, participants were told, “You may remember what you talked about in this discussion, but try not to focus on your memories. Instead, as you watch each clip, try to put yourself in that person's shoes and imagine how they are feeling.” Youth did a practice version of the task in which they rated clips on a computer outside the scanner.
In the scanner, adolescents completed three five-minute runs of the video task, which used an event-related design. Each run consisted of five 12-second trials of each condition – self, mother, father, and an unfamiliar, gender-matched peer condition (not included in the present analyses as this paper focused on parent stimuli) – and five trials of a 12-second baseline condition in which a fixation cross was shown. Condition order was optimized using a genetic algorithm (Wager and Nichols, 2003). This approach generates multiple designs and quantifies their efficiency at distinguishing among the modeled conditions in order to select a condition order that ensures optimal differential overlap among the hemodynamic responses to each condition. This eliminates the need to “jitter” the intertrial interval to create differential overlap among the hemodynamic responses to each condition. The trials contained a two-second cue screen in which the word “You,” “Mother,” “Father” or “Her/Him” (depending on peer's gender) was presented, followed by the 5-second clip, followed by a 4-second rating screen in which participants rated the person's emotional valence on a four-point scale (from Very Negative to Very Positive) using the button box, followed by a 1-second fixation cross.
Whole brain images were acquired with a Siemens 3 Tesla MAGNETON TIM Trio scanner, 12-channel matrix head coil. We used a T2* weighted Echo Planar (EPI) sequence (TR = 2 s, TE = 30 ms, flip angle = 90°); voxel resolution of 3 mm × 3 mm × 4.5 mm. Thirty-two transverse slices were continuously acquired to cover the whole brain and brain stem, with breaks between runs. Anatomical images were acquired using a magnetization prepared rapid acquisition gradient (MPRAGE) sequence (TI = 900 ms, TR = 1950 ms, TE = 2.26 ms, flip angle = 7°), isotropic voxel resolution of 1 mm.
MRI analyses
Data were preprocessed in FSL (FMRIB, Oxford, UK). We performed standard preprocessing — slice timing correction, motion correction, brain extraction, spatial smoothing (5 mm kernel), high-pass filtering, and correction for auto-correlation — prior to contrast modeling. Each of the four conditions was modeled with a separate regressor derived from a convolution of a task boxcar function and a Gamma hemodynamic response function. We modeled the whole 12-second trial including the video and video response. Six motion-correction parameters were also included in the model, as was the temporal derivative of each task regressor. FLIRT was used for registration to high resolution structural and to standard space images. After combining the three runs for each subject in a fixed-effects analysis, data were combined across subjects using FLAME mixed effects analysis with FSL's FEAT (fMRI Expert Analysis Tool), cluster corrected threshold z = 2.3, p b .05. The cluster thresholding technique used by FSL uses Gaussian Random Field Theory to estimate the probability of clusters of a given size in noise data, given the smoothness of our data. The p b .05 cluster threshold indicates that we only accept clusters which are large enough such that clusters that big occur less than 5% of the time by chance in data with comparable smoothness, after thresholding the images at Z = 2.3.
The contrasts tested for this paper were mother vs. task baseline (fixation cross) and father vs. task baseline. Associations between these contrasts and the cortisol coregulation coefficients were tested with a higher-level analysis in which the demeaned behavioral score was included as a cross-subjects regressor. For analyses using extracted signal change percentage values, we used Featquery, part of the FSL package. This program interrogates functional results using a structural mask (in this case, an anatomically defined mask of the precuneus). We converted parameter estimate values to percentage signal change values via scaling of the PE or COPE values by (100*) the peak–peak height of the regressor (or effective regressor in the case of COPEs) and then by dividing by the mean over time of the filtered functional data. Featquery generates a report with statistics derived from each image's values within the mask; we used the mean percentage signal change for each participant in our analyses.
Results
Cortisol analyses and extraction of EB coefficients
The cortisol data were analyzed using a multilevel model (HLM 6.0; Raudenbush et al., 2004a). These analyses are described in detail in Saxbe et al., 2014. Briefly, this approach adjusts for the nesting of cortisol samples within individuals and individuals within families. For the current paper, we were interested in two forms of coregulation between youth and their parents: cross-sectional, and time-lagged. Cross-sectional associations were tested with a model in which youths' cortisol level was the outcome variable and mothers' or fathers' cortisol at the same timepoint was the predictor, with sampling time of day included as a covariate. Time-lagged associations were tested with a model in which youths' cortisol was the outcome variable and mothers' or fathers' cortisol at the prior timepoint was the predictor, with sampling time and youths' own prior cortisol included as covariates. In other words, the time-lagged model would predict youths' cortisol at the second sampling timepoint by mothers' cortisol at the first timepoint, when controlling for youths' cortisol at the first timepoint. This time-lagged model therefore allows us to measure whether a parents' cortisol level influenced change in their child's subsequent cortisol level, over and above the effect of the child's own prior cortisol level.
The original paper (Saxbe et al., 2014) reported significant positive correspondence between parents' and children's cortisol during the laboratory visit, both in the aggregate and within the time-lagged models. For the current paper, our interest was in whether parents' degree of influence on youth cortisol would be linked with youths' neural responses to those same parents. In order to test this question, we first extracted the Empirical Bayes (EB) coefficients from our HLM models. These are analogous to regression coefficients but, because they are taken from a multilevel model, reflect the strength of an association across multiple timepoints nested within individuals (in this case, the five values of cortisol sampled during the laboratory visit). EB coefficients are considered to be more reliable and accurate than OLS coefficients in multilevel analyses (Raudenbush et al., 2004b).
In total, four sets of analyses were run in HLM (fathers' cortisol predicting youths' cortisol cross-sectionally; fathers' cortisol predicting youths' cortisol within the time-lagged model; mothers' cortisol predicting youths' cortisol cross-sectionally; mothers' cortisol predicting youths' within the time-lagged model), and the between-subjects (Level 2) residual file was saved each time. The Empirical Bayes (EB) coefficients were then taken from each of these residual files. The resulting four EB coefficients were generally positively associated with each other, although only there were only two statistically significant relationships out of four possible comparisons: mothers' cross-sectional cortisol coregulation coefficient correlated .32 (p = .15) with mothers' time-lagged coefficient; fathers' cross-sectional coefficient correlated .52 (p = .02) with fathers' time-lagged coefficient; mothers' and fathers' cross-sectional coefficients correlated .14 (p = .54) with each other; mothers' and fathers' time-lagged coefficients correlated .66 (p = .001). In order to establish whether the MRI sample was representative of the cortisol coregulation patterns described in Saxbe et al. (2014), we conducted t-tests comparing the EB coefficients extracted from the full sample to those extracted from the smaller MRI-only sample. These were non-significant (for fathers' time-lagged t(77,20) = − .17, p = .87; for fathers' cross-sectional t(78,21) = .90, p = .37; for mothers' time-lagged t(79, 22) = − .33, p = .74; mothers' cross-sectional t(79, 22) = .40, p = .69), suggesting that the patterns of cortisol coregulation in this sample are consistent with the patterns reported for the full sample in the original paper.
MRI results
The EB coefficients extracted from the multilevel model were used as regressors in a whole-brain analysis testing the contrast of each parent vs. baseline; the two EB coefficients for mothers' cortisol predicting youths' cortisol on the contrast of mother vs. task baseline, and the two EB coefficients for fathers' cortisol predicting youths' cortisol on the contrast of father vs. task baseline. Results for mothers are shown in Table 1 and results for fathers in Table 2.
Table 1.
Clusters in which mother–youth cortisol coregulation coefficients (mother cortisol predicting youth cortisol) associated with youths' increased signal when viewing mother vs. resting baseline; clusters extracted using FSL cluster tool.
| Area of activation | Size | Side | x | y | z | Z |
|---|---|---|---|---|---|---|
| Mother vs. rest regressed with cross-sectional EB coefficient (precuneus mask) | ||||||
| Precuneus | 159 | 2 | −62 | 10 | 3.48 | |
| Precuneus | 19 | 6 | −60 | 40 | 3.01 | |
| Mother vs. rest regressed with time-lagged EB coefficient (whole brain) | ||||||
| Lingual gyrus/precuneus | 504 | 0 | −68 | 4 | 4.53 | |
| Intracalcarine cortex/precuneus | 281 | L | −18 | −64 | 4 | 4.19 |
| Precuneus | 188 | 8 | −54 | 58 | 4.52 | |
| Cuneus | 111 | R | 24 | −72 | 28 | 3.88 |
| Lateral occipital cortex/cuneus | 27 | L | −30 | −80 | 34 | 3.48 |
| Precuneus/posterior cingulate cortex | 20 | 6 | −38 | 50 | 3.34 | |
Table 2.
Clusters in which father–youth cortisol coregulation coefficients (father cortisol predicting youth cortisol) associated with youths' increased signal when viewing father vs. resting baseline; clusters extracted using FSL cluster tool.
| Area of activation | Size | Side | x | y | z | Z |
|---|---|---|---|---|---|---|
| Father vs. rest regressed with cross-sectional EB coefficient (whole brain) | ||||||
| Occipital pole/precuneus | 393 | 14 | −98 | 22 | 3.19 | |
| Precuneus | 238 | L | −20 | −60 | 6 | 3.42 |
| Precuneus | 84 | −10 | −80 | 42 | 3.07 | |
| Father vs. rest regressed with time-lagged EB coefficient (precuneus mask) | ||||||
| Precuneus | 618 | −16 | −56 | 4 | 3.8 | |
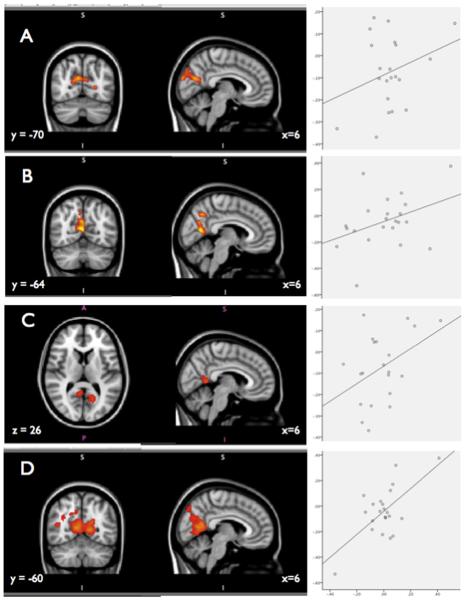
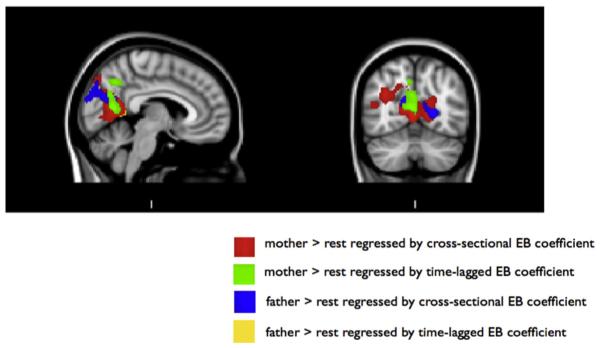
For the father cross-sectional and the mother time-lagged EB coefficients, significant activation to that parent vs. baseline appeared in the posteromedial cortices, in an area centered on the precuneus. No significant whole-brain results emerged for the mother cross-sectional or the father time-lagged EB coefficients. However, because of the findings for the father cross-sectional and mother time-lagged coefficients were centered in the precuneus, we ran these analyses using an anatomically-defined mask of the precuneus (created using the Harvard–Oxford Cortical Atlas). Significant activation appeared in both of these analyses. These results are depicted in Fig. 1. In order to illustrate the overlap between these results, Fig. 2 shows the conjunction of the four separate regression analyses.
Fig. 1.
Results for A) father vs. resting baseline contrast with regressor of cross-sectional father–youth EB coefficient; whole-brain; B) mother vs. resting baseline contrast with regressor of cross-sectional mother–youth EB coefficient; masked with anatomically defined precuneus mask; C) father vs. resting baseline contrast with regressor of time-lagged father–youth EB coefficient; masked with anatomically defined precuneus mask; D) mother vs. resting baseline contrast with regressor of time-lagged mother–youth EB coefficient; whole-brain. Corresponding scatterplots show signal change in the precuneus to that parent vs. resting baseline.
Fig. 2.
Visual depiction of the neural results for the two contrasts (mother vs. resting baseline and father vs. resting baseline) and four EB coefficients (cross-sectional mother–youth and father–youth coefficients; time lagged mother–youth and father–youth coefficients), layered on top of each other. Whole-brain results are shown for time-lagged mother–youth and cross-sectional father–youth coefficients; results for cross-sectional mother–youth and time-lagged father–youth EB coefficients are those that emerged after masking with anatomically defined precuneus mask.
In order to aid interpretation of the results, we calculated the percentage of signal change in the anatomically-defined precuneus mask from each participant. Consistent with the precuneus's role as a default mode structure that shows deactivation during goal-directed task performance (Gusnard and Raichle, 2001), the mean signal change was slightly negative for both mother and father conditions (mean signal change in precuneus for father vs. rest = − 10%, SD = 19%, range − 62% to 17%; mean signal change in precuneus for mother vs. rest = − 6%, SD = 23%, range − 73% to 38%). Therefore, our results should not necessarily be interpreted as an increase in precuneus/PMC activation linked with cortisol coregulation, but as a relatively smaller decrease or deactivation for participants with stronger coregulation. However, all four EB coefficients were positively associated with precuneus activation. Scatterplots depicting the association between signal change and cortisol coregulation coefficients are shown in Fig. 1.
Although we hypothesized that neuroendocrine coregulation would be associated with subcortical activation in emotion processing regions (amygdala, hippocampus, ventral striatum), no whole-brain results emerged above cluster-corrected statistical threshold for these regions. For viewing purposes, we thresholded the uncorrected images down to z = 2.3 (p b .01) and viewed each set of results using the EB coefficients. Bilateral activation in the hippocampus appeared and was positively associated with fathers' time-lagged coregulation with the youth. However, when we reran the cluster-corrected analysis using an anatomically defined mask of the hippocampus, this result did not survive above statistical threshold.
Correlations with button-box ratings, reaction times, and cortisol sampling times
Because youth with stronger cortisol coregulation with parents might rate those parents more positively (or negatively) and/or take more (or less) time to rate them, we tested whether youths' ratings of the clips shown in the scanner could explain any of the above-described results, and ran correlations between button-box emotion ratings and reaction times and the EB cortisol coefficients. None of these correlations reached statistical significance (range from − .18 to .27, all p values N .10), with one exception: fathers' time-lagged EB coefficient was positively correlated, at a marginal level of significance, with reaction time to fathers' video clips (r (22) = .39, p = .08), suggesting that youth with cortisol levels more closely linked with fathers rated father clips more slowly. Research assistant ratings of each family's video clips were positively correlated with participant ratings (mean correlation between participant and RA ratings across the 45 clips shown to each of the 22 participants = .53, p = .001; range .21–.72, SD = .14).
Due to evidence that coregulation within families might change across the day (Schreiber et al., 2006), we also tested correlations between EB coefficients and the start time of the cortisol sampling protocol. None of the four correlations we tested were significant (all coefficients b .17 and all p values N .20), suggesting that our results were likely not driven by time-of-day effects.
Discussion
This paper found that youths' cortisol linkage with parents during a laboratory visit predicted youths' neural activation to those same parents at a subsequent visit. We examined youths' neural activation to each parent in separate MRI contrasts, using two different types of coregulation — cross-sectional and time-lagged. Therefore, our results reflect four distinct analyses using two different neural contrasts, but our findings were remarkably convergent. The more strongly youths' cortisol was associated with parents, the more activation they showed when rating those same parents' emotions in the posteromedial cortices (PMCs; e.g., precuneus, posterior cingulate, and retrosplenial cortex). These areas have been identified as components of the default mode network, associated with self-referential and internally directed thoughts, and also the “mentalizing” network, involved in social cognition or making attributions about others. Our results did not appear to be driven by youths' ratings or reaction times to parent stimuli.
This is the first study to examine parent–child cortisol coregulation as a predictor of neural activation. We found similar results for time-lagged coefficients reflecting morphogenic coregulation (which could be conceptualized as reflecting transmission or contagion from parents to children) and for cross-sectional coefficients reflecting morphostatic coregulation (reflecting concurrent synchrony between parents and children). We also found similar results for the mother and father conditions. The degree of similarity within the neural results linked with the four EB coefficients is especially notable given that these results reflected two different neural contrasts (mother vs. rest and father vs. rest) and two different predictor coefficients within each condition, only moderately correlated. This convergence suggests that the PMCs are an important and consistent correlate of youths' adrenocortical coregulation with parents. Although we hypothesized that subcortical emotion processing regions would also be associated with parent–child coregulation, no results emerged above cluster-corrected statistical threshold.
The PMCs have been relatively understudied within the neuroscience literature until fairly recently, because they are buried deep within the brain and rarely affected by lesion or injury. These areas have among the highest resting metabolic rates within the brain, and show decreased activation during the performance of non-self-referential tasks (Cavanna and Trimble, 2006). Considered a “hub” of the default mode network (Fransson and Marrelec, 2008), the PMCs are a high-level convergence-divergence region (e.g., an associative cortex with multiple interconnections to other regions of the brain; Damasio, 2010). The PMCs appear to be recently expanded in humans relative to other animals and primates, show complex organization, and have been linked to self-consciousness and self-relevant processing (Cavanna and Trimble, 2006). Activation of the PMCs has been associated with third-person perspective on social interaction, especially when social expectations were violated (Petrini et al., 2014). The PMCs (specifically, the precuneus) also appears activated when viewing pictures with moral content (Moll et al., 2002), when experiencing complex social emotion such as admiration or compassion (Immordino-Yang et al., 2009), when making empathic and forgivability judgements (Farrow et al., 2001), and when making attributions about others' emotions (Ochsner et al., 2004). Within the parent–child literature, the PMCs have been found to respond more strongly to infant stimuli in fathers vs. non-fathers (Mascaro et al., 2014); to own-baby cry sounds in breastfeeding mothers vs. non-breastfeeding mothers (Kim et al., 2011); and to one's own vs. unfamiliar infants in mothers (Noriuchi et al., 2008; Wan et al., 2014). Therefore, our finding that PMC activation is positively associated with cortisol coregulation is consistent with these regions' apparent role in social emotions and parent–child attachment. Importantly, within this sample, the mean signal change in the precuneus decreased slightly when viewing/rating parent stimuli vs. rest. This result is not surprising and is rather consistent with the role of the PMCs within the default mode network, which tends to show deactivation during task performance. Consistent with this, an alternative interpretation of our findings is that participants who showed weaker coregulation with parents experienced increased cognitive load (accompanied by decreased self-referential processing and decreased PMC activation) when viewing those parents. Making judgments about unfamiliar people's emotions is typically more cognitively demanding than making judgments about familiar people, so youth who did coregulate as strongly with their parents might have needed to devote more processing resources to rating the emotions of that parent. This possible interpretation is belied by the lack of significant associations between EB coefficients and reaction time in the scanner; in fact, the one correlation that reached trend-level significance suggested that youth who showed stronger cortisol coregulation with fathers actually rated them more slowly. However, given that each trial lasted 12 s and participants therefore had a reasonable amount of time to generate their emotion ratings, the lack of correlations between EB coefficients and reaction times does not entirely rule out this alternative hypothesis regarding cognitive load.
This study had a number of limitations. Our sample included only 22 youth. Although the larger longitudinal study included over 100 families, we recruited from within a smaller cohort of 43 families, and not all of the youth from these families were eligible for or interested in MRI scanning. However, the small sample size is balanced by our use of cortisol and video data from the father, mother, and youth, making this a novel investigation that builds on an earlier set of cortisol coregulation findings from the full sample (Saxbe et al., 2014). The use of dynamic, self-relevant stimuli (video presentation of participants' own parents) limits the standardization of our protocol but also gives this study more ecological validity and allows for closer correspondence of the neuroendocrine predictors (which were also dynamically collected from both parents and the youth) with the MRI data. Future work can use more standardized tasks in order to build on these findings and identify the specific processes that may be most closely linked to cortisol coregulation. Additionally, both the neuroendocrine and neuroimaging literatures have focused almost exclusively on mother–child relationships (e.g., the one study to examine youths' neural responses to parents used only mother stimuli; Whittle et al., 2012), so our inclusion of fathers also advances the literature. We found that mother-to-youth and father-to-youth cortisol coregulation had a very similar neural signature when the youth was viewing either the mother or father in the scanner, providing convergent validity and suggesting that father–child coregulation does not fundamentally differ from mother–child coregulation in terms of its neural correlates. It is unfortunate that the cortisol sampling and MRI scanning session were spaced over one year apart, but also notable that consistent neural results still emerged despite this time lag. Additionally, the cortisol data were collected during a laboratory visit that included a family conflict discussion; the implications of physiological coregulation may vary depending on context (Timmons et al., epub before print) and future research can examine the neural correlates of coregulation assessed across different physiological systems (e.g. endocrine vs. sympathetic nervous system) and in different settings (e.g., conflict vs. support).
Despite its important limitations, this study is the first to explore the neural correlates of adrenocortical coregulation between parents and children. We used an ethnically diverse community sample and employed rich longitudinal data with cortisol measured at one time and neuroimaging at a subsequent time. Future studies can test whether these effects appear in younger children as well as adolescents, whether they are associated with other aspects of the parent–child relationship, whether they emerge in friendship or romantic relationship dyads as well as parent–child dyads, and what kind of impact they have on child development, social cognition, and neuroendocrine regulation. Based on the very preliminary results reported here, neuroendocrine coregulation may be an important correlate of both interpersonal closeness and of perspective-taking or emotional attribution abilities in youth.
Acknowledgments
This research was supported by NIH-NICHD NRSA Post-doctoral Fellowship F32 HD63255, awarded to Dr. Saxbe; NIH-NICHD R01 HD046807, awarded to Dr. Margolin; and an NSF Graduate Research Fellowship, awarded to Larissa Del Piero. We also thank Lauren Spies Shapiro, Aubrey Rodriguez, Michelle Ramos, and Esti Iturralde of the USC Family Studies Project; Mary Helen Immordino-Yang, Jonas Kaplan, and the participating families.
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann. N. Y. Acad. Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson L, Gonzalez A, Kashy DA, Santo Basile V, Masellis M, Pereira J, Chisholm V, Levitan R. Maternal sensitivity and infant and mother adrenocortical function across challenges. Psychoneuroendocrinology. 2013;38(12):2943–2951. doi: 10.1016/j.psyneuen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Butler EA. Temporal interpersonal emotion systems: the “TIES” that form relationships. Personal. Soc. Psychol. Rev. 2011;15(4):367–393. doi: 10.1177/1088868311411164. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Damasio A. Self Comes to Mind. Pantheon Books; New York: 2010. [Google Scholar]
- Ebisch SJ, Aureli T, Bafunno D, Cardone D, Romani GL, Merla A. Mother and child in synchrony: thermal facial imprints of autonomic contagion. Biol. Psychol. 2012;89(1):123–129. doi: 10.1016/j.biopsycho.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Zheng Y, Wilkinson ID, Spence SA, Deakin JF, Tarrier N, Griffiths PD, et al. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12(11):2433–2438. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent–infant synchrony: biological foundations and developmental outcomes. Curr. Dir. Psychol. Sci. 2007;16(6):340–345. [Google Scholar]
- Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav. Dev. 2011;34(4):569–577. doi: 10.1016/j.infbeh.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Granger DA, Serbin LA, Schwartzman AE, Lehoux P, Cooperman J, Ikeda S. Children's salivary cortisol, internalizing behaviour problems, and family environment: results from the Concordia longitudinal risk project. Int. J. Behav. Dev. 1998;22:707–728. [Google Scholar]
- Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Prev. Med. 1998;27(2):208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Cox MJ. Intimate partner violence moderates the association between mother–infant adrenocortical activity across an emotional challenge. J. Fam. Psychol. 2009;23(5):615–625. doi: 10.1037/a0016323. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Trumbell JM, Mercado E. Work/non-workday differences in mother, child, and mother–child morning cortisol in a sample of working mothers and their children. Early Hum. Dev. 2014;90(1):1–7. doi: 10.1016/j.earlhumdev.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Finegood ED. Maternal-child adrenocortical attunement in early childhood: Continuity and change. Dev. Psychobiol. 2015;57(1):83–95. doi: 10.1002/dev.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of admiration and compassion. PNAS. 2009;106(19):8021–8026. doi: 10.1073/pnas.0810363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J. Child Psychol. Psychiatry. 2011;52(8):907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Stevens A, Ablow JC. Neural correlates of hypothalamic–pituitary–adrenal regulation of mothers with their infants. Biol. Psychiatry. 2011;70(9):826–832. doi: 10.1016/j.biopsych.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, Measelle J. Taking stress response out of the box: stability, discontinuity, and temperament effects on HPA and SNS across social stressors in mother–infant dyads. Dev. Psychol. 2012;48(1):35. doi: 10.1037/a0025518. [DOI] [PubMed] [Google Scholar]
- Liu S, Rovine MJ, Cousino Klein L, Almeida DM. Synchrony of diurnal cortisol pattern in couples. J. Fam. Psychol. 2013;27(4):579. doi: 10.1037/a0033735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin G, Vickerman KA, Oliver PH, Gordis EB. Violence exposure in multiple interpersonal domains: cumulative and differential effects. J. Adolesc. Health. 2010;47(2):198–205. doi: 10.1016/j.jadohealth.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Rilling JK. Differential neural responses to child and sexual stimuli in human fathers and non-fathers and their hormonal correlates. Psychoneuroendocrinology. 2014;46:153–163. doi: 10.1016/j.psyneuen.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemiss W, Granger DA, Goldberg WA, Nathans L. Asynchrony of mother–infant hypothalamic–pituitary–adrenal axis activity following extinction of infant crying responses induced during the transition to sleep. Early Hum. Dev. 2012;88(4):227–232. doi: 10.1016/j.earlhumdev.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, Bramati IE, Mourão-Miranda J, Andreiuolo PA, Pessoa L. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. J. Neurosci. 2002;22(7):2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother's response to infant's attachment behaviors. Biol. Psychiatry. 2008;63(4):415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover GH, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004;16(10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Adam EK. Mother–adolescent physiological synchrony in naturalistic settings: within-family cortisol associations and moderators. J. Fam. Psychol. 2009;23(6):882–894. doi: 10.1037/a0017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini K, Piwek L, Crabbe F, Pollick FE, Garrod S. Look at those two!: The precuneus role in unattended third-person perspective of social interactions. Hum. Brain Mapp. 2014:00. doi: 10.1002/hbm.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [Computer software] Scientific Software International; Lincolnwood, IL: 2004a. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon R, du Toit M. HLM 6: Hierarchical linear & nonlinear modeling [computer software and manual. Scientific Software International; Lincolnwood, IL: 2004b. [Google Scholar]
- Ruttle PL, Serbin LA, Stack DM, Schwartzman AE, Shirtcliff EA. Adrenocortical attunement in mother–child dyads: importance of situational and behavioral characteristics. Biol. Psychol. 2011;88(1):104–111. doi: 10.1016/j.biopsycho.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Saxbe D, Repetti RL. For better or worse? Coregulation of couples’ cortisol levels and mood states. J. Pers. Soc. Psychol. 2010;98(1):92. doi: 10.1037/a0016959. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Margolin G, Spies Shapiro L, Ramos M, Rodriguez A, Iturralde E. Relative influences: patterns of HPA axis concordance during triadic family interaction. Health Psychol. 2014;33(3):273–281. doi: 10.1037/a0033509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Del Piero LB, Immordino-Yang MH, Kaplan J, Margolin G. Neural correlates of adolescents' viewing of parents' and peers' emotions: associations with risk-taking behavior and risky peer affiliations. Soc. Neurosci. 2015:1–13. doi: 10.1080/17470919.2015.1022216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell K, Bluschke S, Konradt B, Walter H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. NeuroImage. 2011;54:1743–1754. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff EA, Van Hulle C, Lemery-Chalfant K, Klein M, Kalin N, Essex M, Goldsmith HH. Environmental influences on family similarity in afternoon cortisol levels: twin and parent–offspring designs. Psychoneuroendocrinology. 2006;31:1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology. 2002;27(6):731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]
- Stenius F, Theorell T, Lilja G, Scheynius A, Alm J, Lindblad F. Comparisons between salivary cortisol levels in six-months-olds and their parents. Psychoneuroendocrinology. 2008;33(3):352–359. doi: 10.1016/j.psyneuen.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Timmons AC, Margolin G, Saxbe DE. Physiological linkage in couples and its implications for individual and interpersonal functioning: a literature review. J. Fam. Psychol. 2015:1–13. doi: 10.1037/fam0000115. [epub before print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Shapiro M, Telzer EH, Humphreys KL. Amygdala response to mother. Dev. Sci. 2012;15:307–319. doi: 10.1111/j.1467-7687.2011.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bakel HJA, Riksen-Walraven JM. Adrenocortical and behavioral attunement in parents with 1-year-old infants. Dev. Psychobiol. 2008;50(2):196–201. doi: 10.1002/dev.20281. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. NeuroImage. 2009;48(3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. NeuroImage. 2003;18(2):293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wan MW, Downey D, Strachan H, Elliott R, Williams SR, Abel KM. The neural basis of maternal bonding. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0088436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yücel M, Forbes EE, Davey CG, Harding IH, Sheeber L, Yap MBH, et al. Adolescents' depressive symptoms moderate neural responses to their mothers' positive behavior. Soc. Cogn. Affect. Neurosci. 2012;7(1):23–34. doi: 10.1093/scan/nsr049. [DOI] [PMC free article] [PubMed] [Google Scholar]