Abstract
Relapse of previously extinguished fear presents a significant, pervasive obstacle to the successful long-term treatment of anxiety and trauma-related disorders. Thus, identification of a novel means to enhance fear extinction to stand the passage of time and generalize across contexts is of the utmost importance. Acute bouts of exercise can be used as inexpensive, noninvasive treatment strategies to reduce anxiety, and have been shown to enhance memory for extinction when performed in close temporal proximity to the extinction session. However, it is unclear whether acute exercise can be used to prevent relapse of fear, and the neural mechanisms underlying this potential effect are unknown. The current study therefore examined whether acute exercise during extinction of auditory fear can protect against the later relapse of fear. Male, F344 rats lacking an extended history of wheel running were conditioned to fear a tone CS and subsequently extinguished within either a freely mobile running wheel, a locked wheel, or a control context lacking a wheel. Rats exposed to fear extinction within a freely mobile wheel ran during fear extinction, and demonstrated reduced fear as well as attenuated corticosterone levels during re-exposure to the extinguished CS during the relapse test in a novel context 1 week later. Examination of cfos mRNA patterns elicited by re-exposure to the extinguished CS during the relapse test revealed that acute exercise during extinction decreased activation of brain circuits classically involved in driving fear expression and interestingly, increased activity within neurons of the direct striatal pathway involved in reward signaling. These data suggest that exercise during extinction reduces relapse through a mechanism involving the direct pathway of the striatum. It is suggested that a positive affective state could become associated with the CS during exercise during extinction, thus resulting in a relapse-resistant extinction memory.
1. Introduction
Fear conditioning, during which a previously neutral conditioned stimulus (CS) comes to elicit a fear response because of its association with an aversive, unconditioned stimulus (US), contributes to anxiety and trauma-related disorders. Common behavioral therapies for such disorders, such as exposure therapy, rely on extinction phenomenon. Extinction is the decay of a fear response following repeated presentation of the fear-evoking CS in the absence of the aversive US (Pavlov, 1927). However, fear memories are particularly pervasive and patients are susceptible to relapse of fear and anxiety even following successful extinction. Fear renewal, the return of fear in contexts different from the extinction context (Bouton, 1988; Bouton, Westbrook, Corcoran, and Maren, 2006), and spontaneous recovery, the return of fear after the passage of time (Pavlov, 1927), are two relapse phenomenon that contribute to the return of extinguished fear and the poor long-term efficacy of extinction-based treatments (Boschen, Neumann, and Waters, 2009; Craske, Kircanski, Zelikowsky, Mystkowski, Chowdhury, and Baker, 2008; Neumann and Kitlertsirivatana, 2010). Identification of novel means to reduce the relapse of fear following extinction would be of vast clinical significance for the treatment of anxiety and trauma-related disorders.
Exercise represents a potentially inexpensive and noninvasive strategy to enhance conventional therapeutic options for the treatment of anxiety and trauma-related disorders (Powers, Medina, Burns, Kauffman, Monfils, Asmundson, Diamond, McIntyre, and Smits, 2015). Indeed, exercise can be successfully used on its own (For reviews, see (Asmundson, Fetzner, Deboer, Powers, Otto, and Smits, 2013)(Herring, O’Connor, and Dishman, 2010) (Stonerock, Hoffman, Smith, and Blumenthal, 2015)) or as an adjunct to conventional therapies (Broocks, Bandelow, Pekrun, George, Meyer, Bartmann, Hillmer-Vogel, and Ruther, 1998; Merom, Phongsavan, Wagner, Chey, Marnane, Steel, Silove, and Bauman, 2008) to reduce anxiety. However, clinical studies typically prescribe exercise programs lasting several weeks, and adherence to exercise programs presents a challenge for patients. Investigating whether acute exercise in previously sedentary subjects can improve mental health could therefore lead to clinically important discoveries. Siette et al. reported that previously sedentary rats allowed access to running wheels immediately before or after extinction of contextual fear conditioning demonstrated enhanced extinction memory the following day (Siette, Reichelt, and Westbrook, 2014). However, whether acute exercise can be used to prevent the relapse of fear remains unknown, along with the neurochemical mechanisms underlying these potential effects.
The dopaminergic (DA) system is sensitive to exercise (Bailey, Davis, and Ahlborn, 1993; Hattori, Naoi, and Nishino, 1994; Meeusen, Smolders, Sarre, de Meirleir, Keizer, Serneels, Ebinger, and Michotte, 1997) (Clark, Ghasem, Mika, Day, Herrera, Greenwood, and Fleshner, 2014; Foley and Fleshner, 2008; Greenwood, Foley, Le, Strong, Loughridge, Day, and Fleshner, 2011) and has been implicated in fear extinction (Abraham, Neve, and Lattal, 2014; de la Mora, Gallegos-Cari, Arizmendi-Garcia, Marcellino, and Fuxe, 2010; Singewald et al., 2015). Blockade of DA receptor D2 (Drd2) in the nucleus accumbens can impair extinction learning (Holtzman-Assif, Laurent, and Westbrook, 2010), while enhancing DA by systemic DA reuptake blockade can enhance extinction memory consolidation (Abraham, Cunningham, and Lattal, 2012). Importantly, fear extinction memory facilitated by DA may be resistant to relapse. Systemic administration of L-DOPA immediately after extinction can reduce later fear renewal and reinstatement in both mice and humans (Haaker, Gaburro, Sah, Gartmann, Lonsdorf, Meier, Singewald, Pape, Morellini, and Kalisch, 2013). It is therefore possible that exercise during fear extinction could reduce later relapse of fear through a mechanism involving DA.
Motor activity per se and/or the rewarding effects of exercise could recruit DA systems during acute exercise during fear extinction. DA release in the striatum due to movement or reward could result in signaling at the DA 1 receptor subtype (Drd1) located on medium spiny neurons of the direct pathway in the dorsal striatum and nucleus accumbens (Bertran-Gonzalez, Bosch, Maroteaux, Matamales, Herve, Valjent, and Girault, 2008; Bertran-Gonzalez, Herve, Girault, and Valjent, 2010). Indeed, Drd1 is thought to be preferentially activated following reward- or movement- related burst firing of DA neurons (Dreyer, Herrik, Berg, and Hounsgaard, 2010). Emerging data suggest that Drd1 signaling on direct pathway neurons in both the dorsal and ventral striatum can contribute to reward and reinforcement (Ilango, Kesner, Keller, Stuber, Bonci, and Ikemoto, 2014; Kravitz and Kreitzer, 2012; Kravitz, Tye, and Kreitzer, 2012). These data allow the possibility that the positive affective state elicited by potential activation of the direct pathway of the striatum during exercise during fear extinction could support fear extinction learning through a transfer of positive affect to the CS; perhaps through second-order conditioning. This idea is consistent with an emerging theory of fear extinction supporting the involvement of affective processes (Abraham et al., 2014; Tronson, Corcoran, Jovasevic, and Radulovic, 2012).
We first examined if exercise during extinction would reduce later relapse of conditioned fear, and subsequently explored the potential neural mechanisms involved in this effect. If a transfer of positive affective state contributes to the mechanisms by which exercise during fear extinction reduces later relapse of fear, then one would expect to be able to observe evidence of this second order conditioning during re-exposure to the extinguished CS. To explore this possibility, we used cfos mRNA mapping to examine activation patterns in the dorsal and ventral striatum, prefrontal cortex, and amygdala, elicited by re-exposure to the extinguished auditory CS in rats extinguished in the presence of locked or mobile running wheels. Double fluorescent in situ hybridization (FISH) was also used to quantify cfos mRNA co-localization with neurons of the direct (dynorphin-positive) and indirect (enkephalin-positive) striatal pathways. We hypothesized that if exercise during fear extinction reduced later fear relapse by facilitating the association of a positive emotional state to the CS during extinction, then the extinguished CS should come to elicit conditioned activation of the striatal direct pathway during re-exposure to the extinguished CS. To our knowledge, this is the first study to examine the impact of acute exercise during fear extinction on later relapse of conditioned fear and underlying neurochemical mechanisms.
2. Materials and Methods
2.1 Animals
Young adult (~P63), male F344 rats (n=48) were housed in a temperature (22 °C) and humidity controlled environment and maintained on a 12:12-hr light:dark cycle at the University of Colorado Boulder. All rats were pair housed in Nalgene Plexiglas cages (45 x 25.2 x 14.7 cm) during the inactive cycle and placed into their respective locked or mobile running wheels during the active cycle. Food and water were available ad libitum in the home cages and in the wheels for all groups. All rats were weighed weekly and acclimatized to the housing conditions for 1 week prior to experimental manipulations. Care was taken to minimize discomfort during all procedures, and all experimental protocols were approved by the University of Colorado Boulder Animal Care and Use Committee.
2.2 Voluntary exercise
Rats were randomly assigned to either sedentary or voluntary wheel running conditions by cage. At the onset of the active cycle, all rats were transferred into either locked (Locked group) or unlocked (Run group) running wheels (1.1 m circumference; Lafayette Instruments, Lafayette, IN, USA). Rats were placed in their respective locked or unlocked wheels nightly throughout the entire experiment for 10 consecutive days, beginning 3 days prior to conditioning. Conditioning, extinction training and relapse testing took place on days 4, 5 and 11, respectively. Despite a report that rats will engage in acute wheel running activity without a history of wheel access (Siette et al., 2014), in our experience this running is minimal and rats require a few days of access to running wheels in order to demonstrate robust wheel running behavior. Nightly wheel running continued following fear extinction (from days 5 – 10) in order to avoid potential stress of forced withdrawal from wheel running, which could influence freezing during the relapse test (Greenwood, Loughridge, Sadaoui, Christianson, and Fleshner, 2012). Rats in the Locked condition were transferred nightly into locked wheels in order to control for any effects of handling. All rats were returned to their home cages at the end of the active cycle. Wheel running behavior during the fear extinction sessions was recorded by Activity Wheel Monitor software (Lafayette Instruments; Lafayette, IN, USA).
2.3 Fear conditioning protocols
A timeline of the experiment appears in Figure 1. All experimental phases were completed during the inactive cycle, from 0800–1100. Freezing behavior, defined by the absence of all movement except that required for respiration, was scored by multiple experimenters blind to treatment condition of the animals using a random sampling procedure in which rats were scored every 10 s as either freezing or not freezing. Different experimenters’ scores were highly correlated (p<0.0001) and inter-rater reliability was calculated at 90%. Each behavioral chamber/context was rinsed with water between each rat.
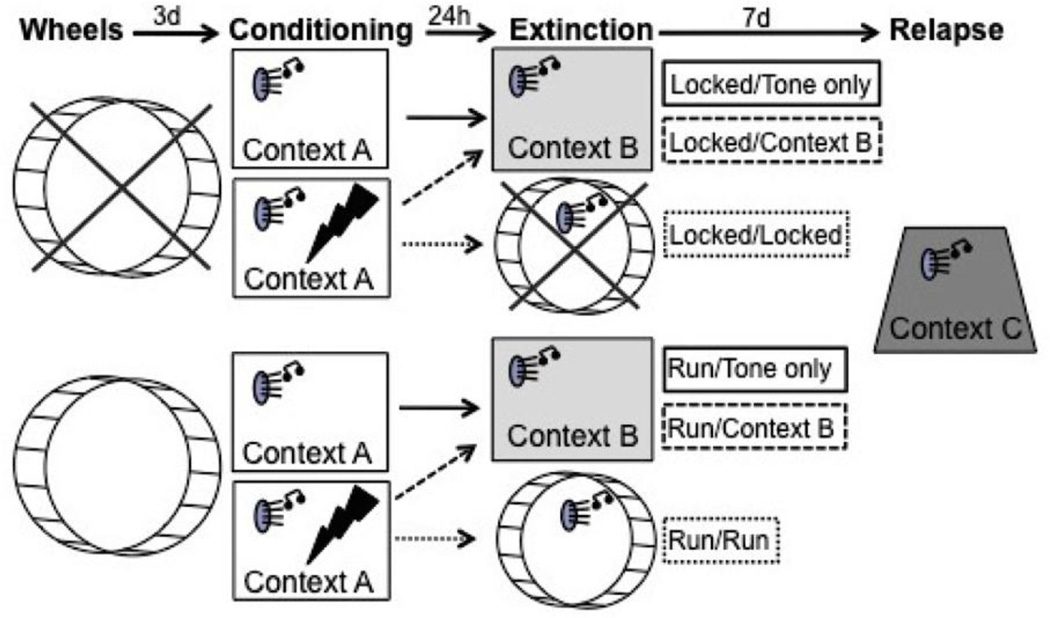
Fig. 1.
Experimental timeline. Rats were assigned to either sedentary (locked wheels) or voluntary running (mobile wheels) conditions three days prior to conditioning in Context A (Tone only rats were exposed to the auditory conditioned stimulus (CS) in each context but did not receive a foot shock on the day of conditioning). Twenty four hours later, all rats were exposed to unreinforced presentations of the auditory CS in either Context B or their familiar locked or mobile wheels. Relapse testing took place 7 days later in a novel Context C.
Conditioning
On the 4th day of the experiment, rats were placed into conditioning boxes (Context A; 25.4 x 25.4 x 30.48 cm; Coulbourn Instruments, Allentown, PA) contained in sound-attenuating chambers. Chambers were fan ventilated and illuminated by white overhead lights. Room lights remained off during conditioning. Freezing behavior was scored for 5 min prior to exposure to a single CS-US pairing. The CS consisted of a 30s, 1 KHz tone CS emitted from speakers mounted within the sound attenuating chambers (Coulbourn Instruments) that co-terminated with a 2s, 0.8 mA foot shock US transmitted through the grid floor. Freezing behavior was scored for 30s after shock termination. Tone only rats were treated identically but did not receive the foot shock US. All rats were immediately returned to their home cages following conditioning or control treatment.
Extinction
24 h following conditioning, rats were assigned to undergo extinction in either a novel environment (Context B) or their familiar locked or unlocked running wheels.
Extinction in the novel Context B was performed to evaluate how a brief history of nightly wheel running may impact fear extinction and relapse. Here, rats in Locked and Run conditions were placed in Context B (Locked/Context B and Run/Context B, respectively), consisting of vanilla-scented opaque mouse cages (19 x 29 x 13 cm) placed in fan ventilated, sound-attenuating chambers. Vanilla scent was provided to alter the olfactory cues present during conditioning and fear extinction. Room lights remained on. The conditioned CS tones (30 s, 1.0 KHz) were presented 15 times (variable 60–90s inter-trial interval; ITI) from the same speakers used during conditioning, mounted within the sound attenuating chambers. Freezing was scored for 3 min prior to the first tone presentation, during the tones, and between each tone. Locked and Run/ Tone only rats were placed into the novel Context B for approximately 35 min and exposed to the same pattern of tone presentations.
Extinction in familiar locked or mobile running wheels served to evaluate how exercise during extinction may impact later fear relapse. Here, rats in Locked and Run conditions were placed in their familiar locked (Locked/Locked) or their familiar unlocked (Run/Run) running wheels with the room lights on. Vanilla scent was provided. Rats were presented with 15 tone CS presentations (30 s, 1.0 KHz, variable 60–90 s ITIs) through speakers placed approximately 1 inch in front of or behind the running wheels (counterbalanced). Freezing behavior in the familiar locked or mobile running wheels was scored during the 3 min prior to the first tone presentation, during the tones, and during the ITIs. Scoring of freezing behavior during fear extinction in the familiar locked or mobile running wheels could not, by the nature of the experiment, be performed blind to treatment condition. Wheel revolutions were recorded by Activity Wheel Monitor software. All rats were immediately returned to their home cages immediately following the 15th tone CS presentation.
Relapse
All rats were exposed to Context C one week after fear extinction for assessment of fear relapse. A novel context was used to maximize fear renewal, and a one week duration was used to maximize spontaneous recovery. Context C consisted of a peppermint-scented transparent Plexiglas chamber (29 x 22 x 18 cm) with wire mesh flooring housed in a sound-attenuating chamber illuminated by red overhead lights and ventilated by fans. A plastic insert was placed in one corner of the cage to make one wall rounded. This context was therefore novel in shape, flooring, lighting and olfactory cues. Following a 3 min exploration period, the tone CS (30 s, 1.0 KHz) was presented 3 times with 4 min ITIs. Freezing behavior was scored prior to the first tone, during the tone presentations, during the ITIs, and for an additional 16 min following the third tone. Tone only rats were placed into the test chambers for an equivalent period of time (approximately 25 min) and exposed to the same pattern of tone presentations. All rats were sacrificed via rapid decapitation immediately after the relapse test phase, and trunk blood and brains were collected for later analyses.
2.4 Corticosterone ELISA
Plasma corticosterone was measured using an enzyme-linked immunosorbent assay (ELISA) manufactured by Arbor Assays (catalog number K014-H5) according to manufacturer’s instruction with a heat-extraction step used in place of Steroid Displacement Reagent. Briefly, plasma was first diluted 1:200 in distilled water in polypropylene tubes, and placed in a 65°C water bath for 1 h in order to degrade corticosterone-binding protein. Samples were allowed to cool at room temperature for 20 min before continuing according to the manufacturer’s instructions. The optical density was measured using SpectraMax plate reader at 450nm, and analyzed using Softmax Pro software.
2.5 Single label radioactive in situ hybridization
Brains were frozen rapidly in isopentane and dry ice (−40 to −50°C) and stored at −80°C until sliced (Leica CM 1850) and thaw mounted onto FisherBrand Colorfrost Plus slides (Fisher Scientific). Slices were stored at −80 °C until in situ hybridization. In situ hybridization was conducted following previously published protocols (Day and Akil, 1996). Briefly, 10 µm frozen brain tissue slices through the prefrontal cortex, striatum, and amygdala were fixed in 4% paraformaldehyde for 1 h, washed 3 times in 2X saline-sodium citrate buffer, acetylated with 0.25% acetic anhydride containing 0.1 M triethanolamine for 10 min, and dehydrated with graded ethanol. cRNA ribroprobes complementary to cfos (680 mer) created from cDNA subclones in transcription vectors were labeled with 35 S-UTP using standard transcription methods. Riboprobes were diluted in 50% hybridization buffer containing 50% formamide, 10% dextran sulfate, 2X saline sodium citrate, 50 mM PBS (pH = 7.4), 1X Denhardt’s solution, & 0.1 mg/ml yeast tRNA. Brain sections and probe were hybridized in humidified chambers overnight (55°C). The next morning, slices were washed in 2X saline sodium citrate and incubated in RNase A (200 µg/ml) at 37 °C for 1 h. Slices were washed to a final stringency of 0.1X saline sodium citrate at 65°C for 1 h, then dehydrated through a series of alcohols and exposed to X-ray film (Kodak, Biomax-MR) for up to 1 week.
2.6 Semi-quantitative mRNA analysis
Levels of cfos mRNA were analyzed by computer-assisted optical densitometry following previously published protocols (Greenwood, Foley, Day, Burhans, Brooks, Campeau, and Fleshner, 2005; Greenwood, Strong, Loughridge, Day, Clark, Mika, Hellwinkel, Spence, and Fleshner, 2012). Brain slice images from in situ hybridization experiments were digitally captured (CCD camera, model XC-77, Sony, Tokyo, Japan), and analyzed using Scion Image version 4.0.3 for Windows (Scion Corporation). A macro written by Dr. Serge Campeau allowed for the signal above background to be determined automatically. Thus, for each slice, background was sampled over a region of white matter, and the signal was calculated as mean gray value of background +3.5 standard deviations. Only pixels with gray values adhering to these criteria were included in the analysis. The number of pixels above background was multiplied by the signal above background to determine the integrated density value. For each subject, an average integrated density value was calculated using 2–4 slices (using both left and right hemispheres). Slices chosen for analysis were between the following coordinates: the IL and prelimbic (PL) subregions of the prefrontal cortex from +3.2 to +1.7 mm anterior to bregma; the dorsolateral (DLS) and dorsomedial striatum (DMS) as well as the AbcS and nucleus accumbens core (AcbC) of the ventral striatum from +1.60 to +0.20 mm anterior to bregma; the basal (BA), lateral (LA) and CeA nuclei of the amygdala from −2.3 to −3.3 mm posterior to bregma based on the atlas by Paxinos and Watson (Paxinos, 1998).
2.7 Double-label fluorescent in situ hybridization (FISH)
Striatal slices were thaw-mounted onto FisherBrand Colorfrost Plus slides and stored at −80°C until double-labeled fluorescence in situ hybridization (FISH) as previously described (Babb, Masini, Day, and Campeau, 2013; Clark et al., 2014). Briefly, cRNA ribroprobes complementary to dynorphin (744 mer), enkephalin (693 mer) or cfos created from cDNA subclones in transcription vectors were labeled with fluorescein-12-UTP (for dynorphin and enkephalin; Roche) and digoxigenin-11-UTP (for cfos; Roche) using customary transcription methods. Riboprobes were diluted in 50% hybridization buffer (50% formamide, 10% dextran sulfate, 2X saline sodium citrate, 50 mM PBS (pH = 7.4), 1X Denhardt’s solution, and 0.1 mg/ml yeast tRNA). Brain slices spanning the rostral to caudal extent of the striatum were hybridized with the probes in humidified chambers overnight (55°C). The next morning, slices were washed in 2X saline sodium citrate, treated with RNase A (200 mg/ml) for 1 h at 37°C, and washed to a final stringency of 0.1X SSC at 65°C for 1 h, then placed into 0.05 M PBS overnight at 4°C. The next morning, endogenous peroxidases were quenched (2% H2O2 in PBS for 30 min, RT, with agitation). Next, slices were washed with 1X TBS (consisting of 0.05% Tween-20 pH 7.5; TBS-T), incubated in blocking buffer (30 min, RT; FP1012; Perkin Elmer), then incubated with anti-fluorescein-HRP (1:100 in blocking buffer, 80 µl/slide; NEF710, Perkin Elmer) for 2 h in humidified chambers. Next, slides were washed in TBS-T, and a tyramide signal amplification kit was used to detect the fluorescein-UTP-probe complex, using fluorescein as the fluorophore (1h at RT in humidified chambers; 1:100, 80 µl/slide; TSA-Plus Kit, Perkin Elmer). Treated slices were then washed in TBS-T, rinsed in 1X TBS and transferred to PBS. Subsequently, slices were washed with TBS-T and incubated with anti-digoxigenin–peroxidase (1:750 in blocking buffer, 80 µl/slide; Roche) for 30 min at RT in humidified chambers. The digoxigenin–UTP–cfos complex was detected as stated above with cyanine-3 as the fluorophore. Slides were then cover-slipped with Vectashield hard-set mounting medium (containing DAPI; H-1500, Vector Labs). Control slides of the same experimental tissue without the probe or without amplification were also included.
2.8 FISH image capture and analysis
A fluorescence microscope (AxioImager Z1; Zeiss Microscopy, Thornwood, NY, USA) was used to identify cells expressing fluorescence markers for cfos, dynorphin, and enkephalin in the DMS and DLS, AcbC and AcbS. Images from within each region were captured at 20X, then uploaded into ZEN vision software (Zeiss) for quantification. Single dynorphin+, enkephalin+ and cfos+ labeled cells, as well as double-labeled cfos+/dynorphin+ and cfos+/enkephalin+ cells were counted within each region by blind experimenters using the ‘measure events’ tool in Zen vision to prevent multiple counts of the same cell. 2–4 images were quantified per brain region (including both left and right hemispheres) for each subject.
2.9 Statistical Analysis
For conditioning, pre-shock freezing scores were averaged into 5, 1 min blocks, and post-shock freezing scores were averaged into 1, 30s post-shock score. Repeated-measures ANOVA was used to make group comparisons of freezing behavior across the entire conditioning session and pre-shock separately, and ANOVA was used for group comparisons of post-shock averages. For extinction, pre-tone freezing scores were averaged into 1 pre-tone score and compared with ANOVA. Freezing scores during ITIs were collapsed into 15, 60–90s blocks and were analyzed with repeated measures ANOVA. Extinction scores were also averaged for the entire session and these collapsed scores were analyzed with ANOVA. Freezing observed during the tones was collapsed into 15, 30s tone scores and analyzed with repeated measures ANOVA. For freezing during the relapse phase, pre-tone freezing scores were again averaged into 1 pre-tone score and analyzed with ANOVA. Freezing during ITIs was collapsed into 25, 1 min blocks and analyzed with repeated measures ANOVA. Freezing during ITIs were also averaged across all 25 min to yield 1 average freezing score, which was analyzed with ANOVA. Finally, freezing during the tones was collapsed into 3, 30s tone scores and analyzed with repeated measures ANOVA. The relationship between distance run during extinction and level of fear during relapse was explored using a simple regression analysis. Group differences in plasma corticosterone and cfos mRNA expression in the DMS, DLS, AcbC, AcbS, IL, and PL sub regions of the prefrontal cortex, as well as the LA, BA, and CeA were analyzed with ANOVA, as were group differences in total number of dynorphin neurons and % of dynorphin expressing cfos mRNA and total number of enkephalin neurons and % of enkephalin expressing cfos mRNA in the DMS, DLS, AcbC, and AcbS. Fisher’s least significant difference post-hocs were performed when appropriate. Significant results were detected at p ≤ 0.05.
3. Results
3.1 Conditioning
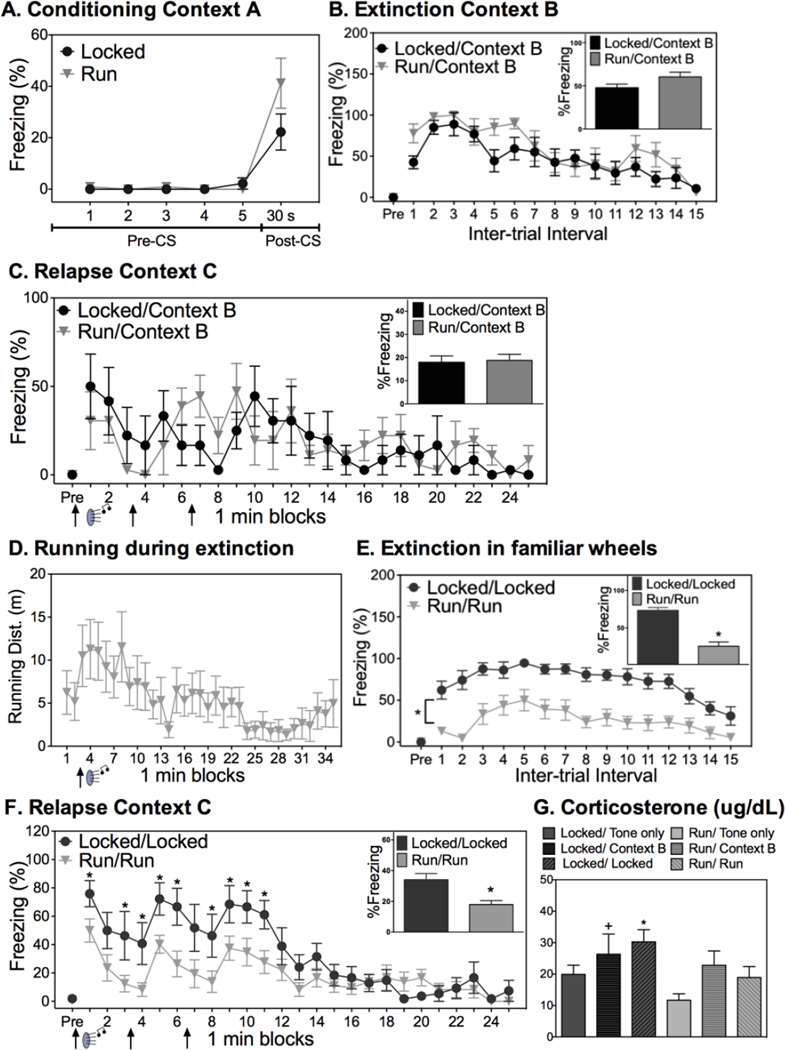
Freezing prior to and immediately following conditioning is shown in Figure 2A. Both Locked and Run rats exhibited minimal levels of freezing prior to conditioning (pre-CS). Similarly, both groups displayed comparable levels of freezing during the 30s following the CS-US pairing (post-CS; p>0.05).
Fig. 2.
Acute exercise during fear extinction reduces fear relapse. (A) All rats conditioned similarly. (B) Prior exercise had no effect on average freezing behavior during extinction or (C) relapse testing. (D) Distance run during extinction for Run/Run rats. (E) Acute exercise during fear extinction reduced freezing levels during extinction. (F) Acute exercise during extinction reduced relapse of fear. (G). Corticosterone levels during renewal testing paralleled levels of freezing during the relapse test; The Locked/Locked group exhibited greater corticosterone than Run/Tone only and Run/Run (*); The Locked/Context B group displayed greater corticosterone than Run/Tone only (+). Data are represented as mean ± SEM; +/* p<0.05.
3.2 Short-term daily exercise does not impact extinction or relapse of auditory conditioned fear
Extinction
To assess the effects of short term, nocturnal wheel running on fear extinction and relapse, half of the rats in the Locked and Run groups were randomly assigned to undergo extinction in Context B. Freezing behavior following each auditory CS during the extinction session is shown in Figure 2B. Rats displayed minimal freezing behavior during the 3 min exposure to Context B prior to the first tone CS presentation, indicating that little fear transferred between contexts A and B. Freezing behavior increased following the first CS presentation, and then dissipated across time (ITIs: F(14, 140)=8.872; p<0.0001; tones: F(14, 140)=3.162; p=0.0002). A history of prior exercise had no impact on levels of fear during extinction.
Relapse test
Figure 2C shows freezing behavior during the relapse testing phase across time and averaged over the entire 25 min session (Figure 2C inset). Again, minimal freeing behavior was present prior to the first CS. Rats demonstrated within-session extinction (F(24, 240)=3.089; p<0.0001) that did not differ between exercise groups (p>0.05). Similarly, no group differences in freezing were observed during presentations of the tone CS (p>0.05; data not shown).
3.3 Acute exercise during fear extinction reduces the relapse of fear
Extinction
To assess the effects of acute exercise during fear extinction on the relapse of conditioned fear, rats in the Locked and Run groups were exposed to extinction in their familiar locked (Locked condition) or unlocked (Run condition) running wheels. Run rats ran minimally upon placement into wheels prior to the first tone CS exposure, as is typical of running behavior during the light cycle (Greenwood et al., 2011). However, an increase in running behavior coincided with presentation of the first CS (Figure 2D), and running bouts were observed to coincide with subsequent tones. Running during fear extinction decreased with repeated tone CS presentations (F(34, 374)=2.015; p=0.0009). Percent freezing throughout the extinction session is shown in Figure 2E. Freezing was minimal prior to the first CS presentation. Freezing during the ITIs (F(14, 266)=7.423, p<0.0001) as well as tones (F(14, 266)=6.142; p<0.0001; data not shown) dissipated for both Locked/Locked and Run/Run groups across the extinction session, although the Locked/Locked group froze significantly more than the Run/Run group throughout the entirety of the extinction session (ITIs: F(1, 19)=42.898; p<0.0001; tones: (F(1, 19)=28.369, p<0.0001). This pattern of freezing is not surprising given that rats in the Run/Run group were running in their wheels during extinction.
Relapse test
Minimal fear expression upon placement in Context C 7 days after extinction indicated that little fear transferred from the conditioning or extinction contexts to the novel Context C. Re-exposure to the CS resulted in significant relapse of fear in both groups, as indicated by higher freezing levels during the ITI following the first CS presentation during the relapse test phase relative to the last ITI during the extinction phase (paired sample t-test t(8)=2.572; p=0.03 for Locked/Locked; one sample t-test t(11)=5.245; p=0.0003 for Run/Run). However, rats that ran during fear extinction displayed significantly less fear during the relapse test compared to rats in the Locked/Locked group. Repeated measures ANOVA revealed main effects of both exercise (F(1, 19)=12.021; p=0.002), time (F(24, 456)=10.182; p<0.001), and a significant interaction between exercise and time (F(24, 456)=2.031; p=0.003). The reduction in freezing in the Run/Run relative to the Locked/Locked group was observable immediately following the first of 4 CS presentations, and continued for the remainder of CS presentations (see Figure 2F for results of post hoc tests). A similar trend in freezing was observed during the 3 brief tone CS presentations (exercise x time interaction: F(2,38)=2.715; p=0.07; data not shown). The relationship between distance run during extinction and freezing during the relapse test did not reach statistical significance (R2= 0.26; p=0.09). Non-conditioned (tone only) rats did not display any freezing behavior in either the extinction or relapse tests, thus these data are omitted.
Corticosterone
Levels of freezing during the fear relapse test were largely paralleled by levels of CORT (Figure 2G). ANOVA revealed group differences in plasma CORT levels (F(5, 39)=2.398; p<0.05). Locked/Locked rats displayed elevated CORT during the relapse test session relative to Run/Run rats and Run/Tone only rats. The increase in CORT levels observed in the Locked/Locked group relative to the Locked/Tone only group did not reach significance (p=0.08).
3.4 Running during fear extinction reduces cfos mRNA induction in fear-related brain regions elicited by re-exposure to the extinguished CS during relapse testing
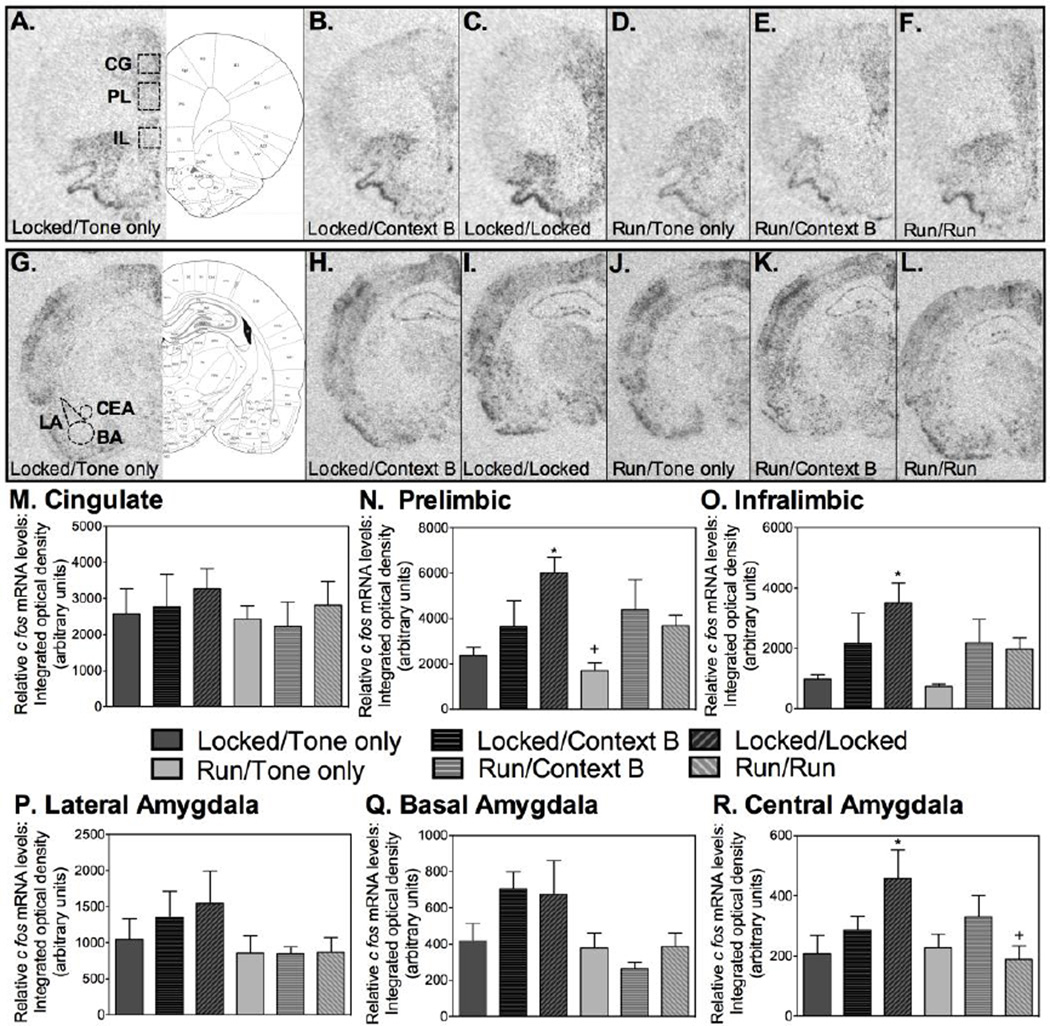
To investigate possible mechanisms underlying the reduction in fear relapse observed in rats that ran during extinction, levels of cfos mRNA were examined within subregions of the prefrontal cortex and amygdala, brain regions classically involved in fear conditioning and extinction (Knapska et al., 2012; Sierra-Mercado et al., 2011) and known to display differences in immediate early gene expression during relapse testing depending on the context in which testing occurs (Knapska and Maren, 2009). Brain atlas sections from Paxinos and Watson (Paxinos, 1998) showing the areas within the prefrontal cortex and amygdala where sampling occurred are shown in Figures 3A and 3G. Representative autoradiographs of cfos mRNA labeling in the PFC of each experimental condition are shown in Figures 3A – 3F. No differences between experimental conditions were found in the CG. However, significant group differences were observed in the PL (F (5, 32) = 4.8; p=0.002). Although levels of cfos mRNA in the PL was similar between tone-only groups and Locked and Run groups extinguished in Context B, exposure to the extinguished CS elicited an increase in cfos mRNA levels in the Locked/Locked group (p<0.05) but not the Run/Run group (see Figure 3N for post-hoc comparisons). Moreover, levels of cfos mRNA in the PL were greater in the Locked/Locked group relative to Run/Run. A similar pattern was observed in the IL (F(5, 32)=2.969; p=0.02), where levels of cfos mRNA induced by re-exposure to the extinguished CS was highest in the Locked/Locked group (Figure 3O). However, unlike the PL, levels of cfos mRNA in the IL of Locked/Locked rats did not differ from the Run/Run group (p>0.05).
Fig. 3.
Running during fear extinction reduces cfos mRNA induction in fear-related brain regions elicited by re-exposure to the extinguished CS during relapse testing. (A) - (F) depict representative autoradiographs of cfos mRNA labeling of each experimental condition in the prefrontal cortex and (G) – (L) in the amygdala. (M) No significant group differences were detected within the cingulate gyrus. (N) Within the prelimbic, the Locked/Locked group exhibited greater cfos mRNA than Locked/Tone only, Locked/Context B, Run/Tone only, and Run/Run (*), and the Run/Tone only group exhibited less cfos mRNA than Run/Context B and Run/Run (+). (O) Within the infralimbic, the Locked/Locked group exhibited greater cfos mRNA than Locked/Tone only, Run/Tone only and Run/Run (*). (P) No significant group differences were detected within the lateral amygdala or the (Q) basal amygdala. (R) Within the central amygdala, the Locked/Locked group exhibited greater cfos mRNA than Locked/Tone only, Run/Tone only, and Run/Run (*). Data are represented as mean ± SEM; +/*p<0.05.
Representative autoradiographs of cfos mRNA labeling in the amygdala of each experimental condition are shown in Figures 3G – 3L. In general, relatively low levels of cfos mRNA were detected throughout the amygdala following re-exposure to the extinguished CS. No significant group differences were found in the LA (Figure 3P) or the BA (Figure 3Q). Levels of cfos mRNA in the CeA, however, differed between groups (F(5, 35)=2.605; p=0.04). Post-hoc comparisons revealed that cfos mRNA levels were higher in the Locked/Locked group relative to the Run/Run group (Figure 3R; p<0.05).
3.5 Running during fear extinction increases cfos mRNA induction in direct pathway neurons within the striatum elicited by re-exposure to the extinguished CS during relapse testing
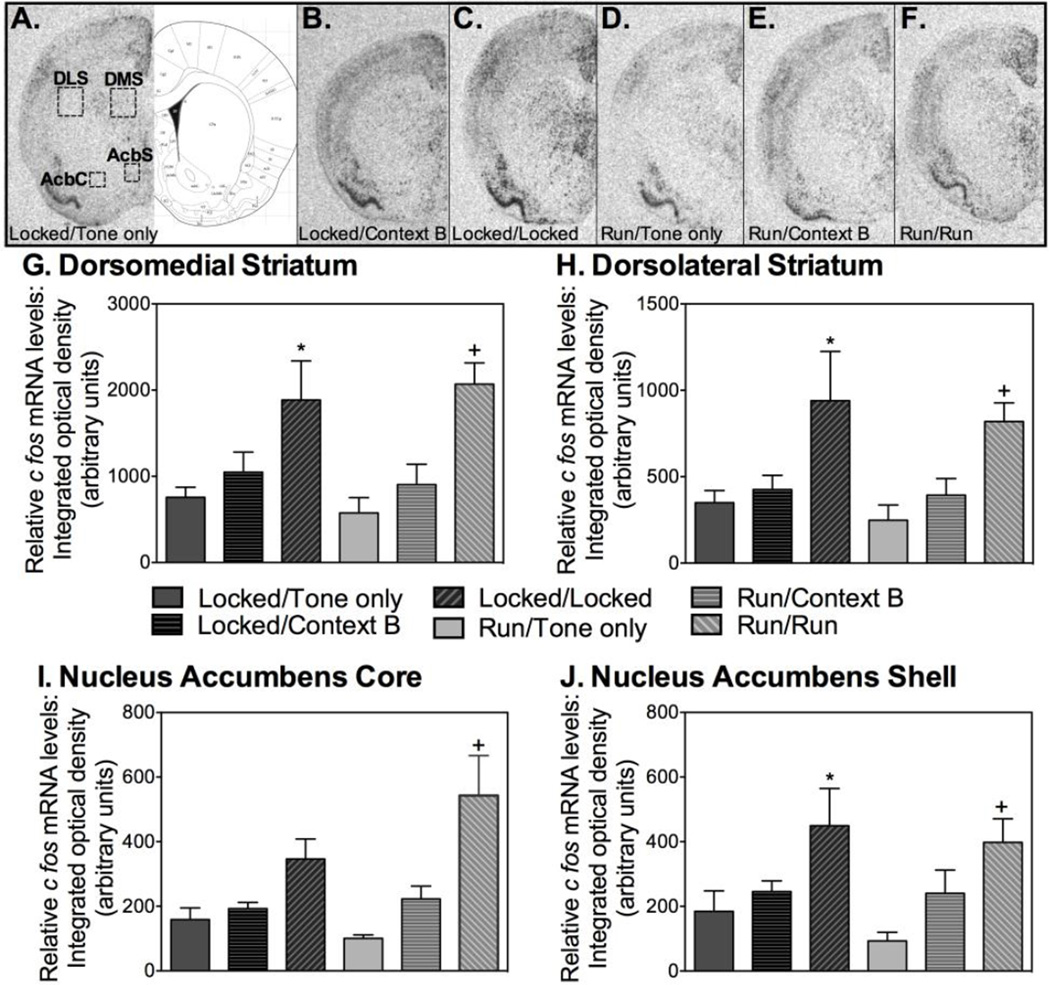
Levels of cfos mRNA within both the dorsal (DMS, DLS) and the ventral (AcbS, AcbC) aspects of the striatum were examined using single label radioactive in situ hybridization. Figure 4A shows a figure from Paxinos and Watson (Paxinos, 1998) depicting the striatal regions chosen for analyses. Representative autoradiographs showing cfos mRNA in the striatum of the various experimental conditions are shown in Figures 4A – 4F. Patterns of cfos mRNA levels within all striatal subregions differed between experimental conditions (DMS: F(5, 38)=4.977; DLS: F(5, 38)=3.336; AcbS: F(5, 37)= 2.805; AcbC: F(5, 36)=3.629; p<0.05). Consistent with levels of freezing, cfos mRNA levels following re-exposure to the extinguished CS were higher in rats that were placed in their familiar wheels during extinction (both Locked/Locked and Run/Run rats) than other groups. See graphs for detailed post hoc comparisons.
Fig. 4.
Extinction in familiar wheels increases cfos mRNA induction in striatal regions associated with affect and movement during re-exposure to the extinguished CS during relapse testing. (A) – (F) depict representative autoradiographs of cfos mRNA labeling of each experimental condition in the striatum. (G) Within the dorsomedial striatum, the Locked/Locked group exhibited greater cfos mRNA than Locked/Tone only, Run/Tone only, and Run/Context B (*); The Run/Run group exhibited greater cfos mRNA than Locked/Tone only, Locked/Context B, Run/Tone only, and Run/Context B (+). (H) Within the dorsolateral striatum, the Locked/Locked group exhibited greater cfos mRNA than Locked/Tone only, Locked/Context B, Run/Tone only, and Run/Context B (*); The Run/Run group differed from Locked/Tone only, Run/Tone only, and Run/Context B (+). (I) Within the nucleus accumbens core, the Run/Run group exhibited greater cfos mRNA than Locked/Tone only, Locked/Context B, Run/Tone only, and Run/Context B (+). (J) Within the nucleus accumbens shell, the Locked/Locked group exhibited greater cfos mRNA than Locked/Tone only and Run/Tone only (*); The Run/Run group exhibited less cfos mRNA than Run/Tone only and Locked/Tone only (+). Data are represented as mean ± SEM; +/*p<0.05.
To more closely examine cfos expression within neurochemically identified striatal pathways, double FISH was used to quantify cfos mRNA within dynorphin and enkephalin mRNA-containing medium spiny neurons of the striatum. Dynorphin is almost exclusively contained within direct pathway neurons of the dorsal striatum and nucleus accumbens, whereas enkephalin is expressed almost exclusively in indirect pathway neurons in the same regions (Bertran-Gonzalez et al., 2008; Bertran-Gonzalez et al., 2010). Dynorphin and enkephalin can therefore be used as indirect markers of the striatal direct and indirect pathways.
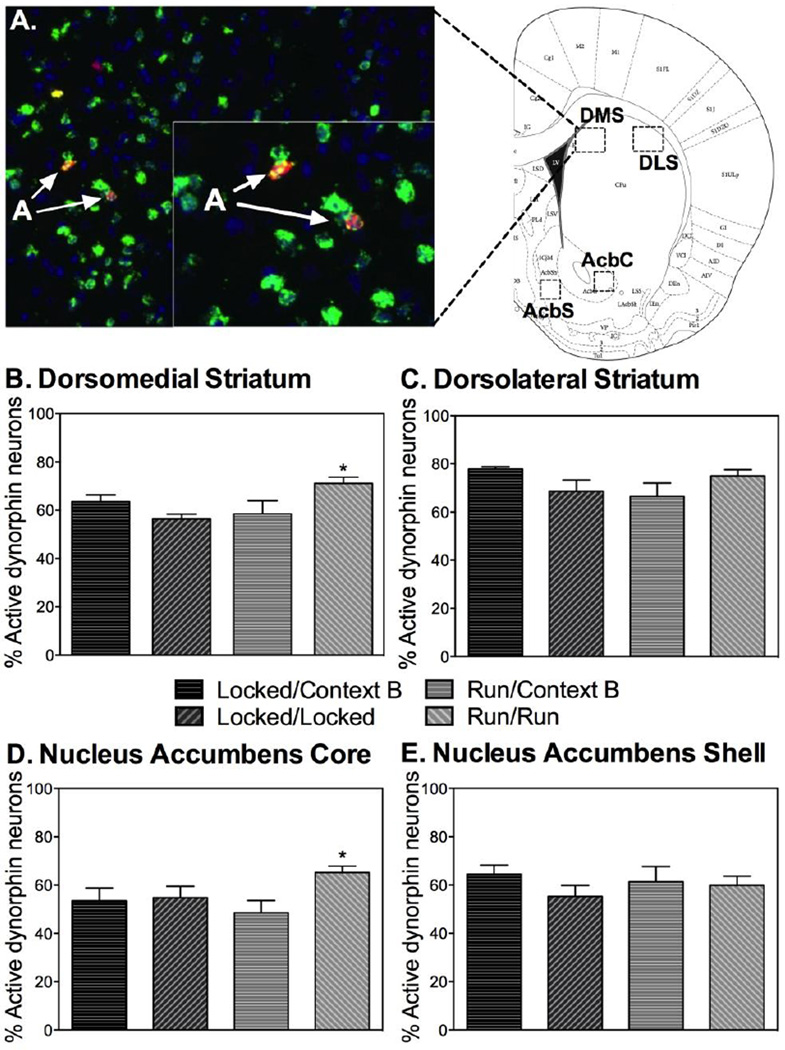
A representative photomicrograph showing double cfos+/dynorphin+ FISH in the DS, as well as a Paxinos and Watson figure (Paxinos, 1998) showing regions chosen for analyses, are depicted in Figure 5A. ANOVA revealed significant group differences in the percent of double cfos+/dynorphin+ neurons within the DMS (F(3,28)=5.850; p<0.01; Figure 5B) and the AcbC (F(3, 28)=3.049; p<0.05; Figure 5D), but not the DLS (Figure 5C) or AcbS (Figure 5E). Interestingly, the highest percentage of double cfos+/dynorphin+ -labeled neurons in the DMS and AcbC were observed in rats allowed to run in wheels during extinction.
Fig. 5.
Running during fear extinction increases cfos mRNA induction in direct pathway neurons within the striatum elicited by re-exposure to the extinguished CS during relapse testing (A) depicts a representative photomicrograph showing double cfos+/dynorphin+ FISH in the dorsal striatum, as well as regions chosen for analyses. (B) Within the dorsomedial striatum, the Run/Run group displayed greater cfos+/dynorphin+ expression than Locked/Locked and Run/Context B. (C) No significant group differences were found within the dorsolateral striatum. (D) Within the nucleus accumbens core, the Run/Run displayed greater cfos+/dynorphin+ expression than Locked/Locked and Run/Context B and Locked/Context B. (E) No significant group differences were found within the nucleus accumbens shell. Data are represented as mean ± SEM; +/*p<0.05.
No significant group differences in the percent of double cfos+/enkephalin+ neurons, single enkephalin, or single cfos mRNA-positive cells were found in any of the striatal subregions (data not shown). Because of the relatively low levels of cfos mRNA in the striatum regions of tone-only rats (Figure 4), rats exposed to tone only were not included in the double FISH.
4. Discussion
The current results are the first to demonstrate that acute exercise during extinction of auditory fear conditioning can reduce the subsequent relapse of conditioned fear upon re-exposure to the extinguished CS. The reduction in fear relapse exhibited by rats allowed to run during extinction was accompanied by an attenuation of the corticosterone and cfos mRNA responses in brain areas that drive conditioned fear during re-exposure to auditory CS, such as the PL and CeA. Together, these data indicate that exercise during fear extinction can reduce the return of fear-related responses and activation of the fear circuit that typically occurs during fear reinstatement and renewal. Moreover, following re-exposure to the extinguished CS, rats that ran during extinction demonstrated enhanced cfos mRNA in movement- and reward-related direct pathway neurons within the DMS and the AcbC; downstream targets of nigrostriatal and mesolimbic dopaminergic pathways. These data point toward a role for striatum direct pathway neurons in the mechanism by which exercise during fear extinction reduces fear relapse. Collectively, these results suggest that acute exercise during fear extinction can reduce subsequent fear responses elicited by the extinguished CS, perhaps by facilitating the association of a positive emotional state to the CS during extinction through a mechanism involving DA and the direct striatal pathway. Given that fear relapse considerably limits the effectiveness of extinction based exposure therapy (Bouton, Garcia-Gutierrez, Zilski, and Moody, 2006; Neumann, Lipp, and Cory, 2007), these data may lead to novel therapies for the treatment of anxiety and trauma-related disorders in humans.
Importantly, nightly exercise did not impact relapse of fear. Rats allowed nightly access to running wheels but extinguished in a novel context without a running wheel (Run/ Context B) displayed levels of fear, corticosterone, and cfos mRNA that were indistinguishable from those displayed by rats placed nightly into locked wheels (Locked/ Context B). These data are consistent with our prior observations that 6 weeks of nightly wheel running does not impact later within-session extinction of shock-elicited fear (Greenwood et al., 2005) or within or between session extinction of contextual fear conditioning (Greenwood, Strong, Foley, and Fleshner, 2009). In these prior studies and the current study (Context B), the preceding running bout occurred more than 2 hours prior to extinction, and the proceeding running bout occurred 8 hours later. These data demonstrate that a history of nightly wheel running has no impact on fear extinction or relapse. Interestingly, Siette et al (2014) observed that when acute wheel running occurred immediately prior to or following extinction of contextual fear conditioning, exercise enhanced extinction retention. However, when exercise occurred 6 hours after extinction no such benefit was observed (Siette et al., 2014). Together, these data along with data from the current study suggest that an acute bout of exercise in close temporal proximity to the learning phase of fear extinction may augment fear extinction, regardless of history of physical activity.
The current results extend the findings of Siette et al. by demonstrating that, in addition to enhancing consolidation of fear extinction memory (Siette et al., 2014), an acute bout of exercise during extinction can suppress the subsequent relapse of fear. In the current study, however, it is unclear whether acute exercise during extinction of auditory fear conditioning is suppressing the subsequent relapse of fear by enhancing memory for extinction. The current experiments were not designed to assess extinction memory retention; doing so would require testing rats in the same context in which they were extinguished. Nevertheless, given that acute wheel running temporally proximal to fear extinction can enhance retention of extinction (Siette et al., 2014), it is possible that acute wheel running during fear extinction is reducing the relapse of fear by enhancing retention of fear extinction memory. Our observations are similar to an exciting recent pilot study in humans reporting that acute bouts of exercise immediately prior to exposure therapy can reduce symptoms in patients with post-traumatic stress disorder (Powers et al., 2015).
In order to investigate the neural mechanisms by which exercise during extinction can attenuate relapse phenomena, levels of cfos mRNA in brain circuits classically involved in fear expression were assessed. Critical regions involved in modulating fear within this circuitry are the LA, BLA, CeA of the amygdala and the IL and PL of the medial prefrontal cortex. The PL and CeA are thought to drive the fear response during exposure to fear-eliciting cues (Burgos-Robles, Vidal-Gonzalez, and Quirk, 2009; Vidal-Gonzalez, Vidal-Gonzalez, Rauch, and Quirk, 2006), while the IL can inhibit the expression of fear by inhibiting output of the CeA through its neural projections to intercalated inhibitory neurons (Quirk, Likhtik, Pelletier, and Pare, 2003). Examination of cfos mRNA patterns within nuclei of the amygdala revealed that rats exposed to fear extinction in their locked wheels displayed enhanced cfos mRNA expression within the CeA following re-exposure to the CS relative to tone-only controls. This pattern of activation is consistent with previous reports demonstrating that high levels of cfos in the CeA is indicative of enhanced fear expression (Knapska and Maren, 2009), and, indeed, is consistent with the increased levels of fear relapse exhibited by this group. In contrast, rats that ran in wheels during fear extinction demonstrated attenuated cfos mRNA in the CeA elicited by re-exposure to the CS. A similar pattern of data was observed in the PL, whereby exercise during extinction attenuated cfos expression in the PL induced by re-exposure to the CS during the relapse test. This pattern of cfos mRNA expression in the CeA and PL parallels the reduced levels of fear exhibited by the Run/Run group. Together, these data suggest that exercise during extinction attenuates activity of brain regions thought to drive the conditioned fear response during re-exposure to the extinguished CS.
Levels of cfos mRNA were also examined within the IL, a critical region for fear extinction. Fear extinction produces synaptic plasticity within IL neurons, and disrupting the molecular events that lead to this plasticity (Izquierdo, Wellman, and Holmes, 2006) or temporarily inactivating the IL (Sierra-Mercado et al., 2011) interferes with the learning and/or recall of fear extinction memory. Moreover, several manipulations that enhance fear extinction memory are thought to act through the IL (Bukalo et al., 2014). Given these data, if exercise during extinction reduces relapse by enhancing extinction memory as suggested above, a potentiation of the cfos mRNA response in the IL of the Run/Run group relative to the Locked/Locked group would be expected (Knapska and Maren, 2009). This pattern of data, however, was not observed. Exercise during extinction had no significant impact on levels of cfos mRNA in the IL elicited by re-exposure to the CS during the relapse test (Figure 3O). These data are interesting because they are not consistent with exercise during extinction reducing later relapse of fear through a mechanism involving enhanced fear extinction memory in the IL. Exercise during extinction could therefore be reducing relapse of fear through a mechanism independent of IL-mediated inhibition of CeA output.
One possibility is that exercise during fear extinction enhances extinction memory and subsequent relapse by facilitating the association between a positive emotional state and the CS, perhaps through a transfer of the positive emotional state elicited by acute exercise to the CS. The current results are consistent with this possibility. Recent data implicate direct- and indirect-pathway neurons of the striatum in reward and aversion, respectively. Indeed, Kravits et al. reported that optogenetic activation of direct pathway neurons in either the dorsal and ventral striatum elicits approach behavior and reward, whereas activation of indirect pathway neurons elicits avoidance and aversion (Kravitz et al., 2012). In the current study, double FISH revealed that, although no group differences in % double cfos/enkephalin-positive, indirect-pathway neurons were observed, preferential cfos mRNA expression in dynorphin-positive, direct-pathway neurons was observed within the DMS and the AcbC in rats that were allowed to run during fear extinction (Figure 5). Since dynorphin is localized almost exclusively to Drd1-expressing, direct-pathway neurons in the DS and AcbC, these data suggest that the reduction of fear relapse observed in rats allowed to run during fear extinction is associated with activation of dorsal and ventral striatal direct-pathway neurons. The observed direct-pathway activity could be related to a positive emotional value associated with the CS in rats allowed to run during extinction. Indeed, striatal direct-pathway neurons are thought to be preferentially activated following burst firing of midbrain DA neurons (Dreyer, Herrik, Berg, and Hounsgaard, 2010), and DA has been suggested to contribute to an affective component of fear extinction (Abraham et al. 2014). The involvement of DA in the present results requires further exploration.
In addition to their involvement in reward, direct pathway neurons of the striatum are also involved in promoting movement (Kravitz, Freeze, Parker, Kay, Thwin, Deisseroth, and Kreitzer, 2010; Kravitz and Kreitzer, 2012). However, it is unlikely that the greater % of double cfos/dynorphin-positive neurons in the Run/Run group relative to the Locked/Locked group was due to increased locomotor activity during the relapse test. Analyses of single cfos mRNA in these same striatal regions indicated that higher levels were observed in the rats that expressed fear during the relapse test (the conditioned rats, Figure 4), rather than the rats that were simply exploring the context instead of freezing (the tone-only rats). Thus, the locomotor activity associated with moving about in the relapse context may not be enough to elicit appreciable cfos mRNA responses in striatal neurons.
The circuitry and mechanisms through which activation of striatal direct pathway neurons could be inhibiting the fear response during re-exposure to the extinguished CS remains unknown. Activation of the striatal direct pathway leads to disinhibition of the cortex, suggesting that direct pathway activation during re-exposure to the extinguished CS could reduce fear expression by increasing activation of the IL. The cfos mRNA data, however, revealed no evidence for this mechanism. Indeed, cfos mRNA responses in the IL of Run/Run rats were no higher than those observed in the IL of Locked/Locked rats during the relapse test (Figure 3O). Alternatively, the prefrontal cortex can be modulated by the amygdala during fear expression (Senn, Wolff, Herry, Grenier, Ehrlich, Grundemann, Fadok, Muller, Letzkus, and Luthi, 2014). Thus, it is possible that potential communication between striatal direct pathway neurons and the amygdala could be contributing to the effects of exercise during extinction on prefrontal cortical activity and fear relapse observed in the current study.
In summary, the data presented here indicate that exercise during extinction can reduce the relapse of fear following extinction, even in subjects exposed to minimal prior exercise. The clinical utility of using a non-invasive method such as acute exercise to prevent relapse of fear cannot be over-emphasized; exercise is a feasible, inexpensive, and non-invasive manipulation that is easily implemented in a clinical setting. A recent paper detailing the results of a promising pilot study demonstrates this utility, and shows that repeated, acute bouts of exercise just prior to sessions of exposure therapy may successfully augment treatment outcomes for patients suffering from PTSD (Powers et al., 2015). Commonly utilized behavioral therapies for anxiety and trauma-related disorders, including cognitive behavioral therapy as well as exposure-based behavioral approaches have low adherence, take an extended period of time, and still suffer from poor long-term efficacy (Choy, Fyer, and Lipsitz, 2007; Singewald et al., 2015). Thus, investigating feasible means by which to augment such therapies, and the mechanisms by which these manipulations work, is of great clinical importance. Future research should further elucidate the mechanisms and clinical utility of exercise as an augmentation strategy for exposure therapy for the treatment of anxiety and trauma-related disorders. Finally, the current data are consistent with a role for striatal direct pathway neurons in reducing the relapse of fear following extinction.
Highlights.
Given the opportunity, rats will run in wheels during fear extinction
Running during fear extinction reduces relapse of fear
Running during extinction alters brain activity patterns during the relapse test
Acute exercise represents a potential means to augment exposure therapy
Acknowledgements
This work was funded by NIH R01-MH068283-06A1 and NIH R03-MH086665-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AD, Cunningham CL, Lattal KM. Methylphenidate enhances extinction of contextual fear. Learn Mem. 2012;19:67–72. doi: 10.1101/lm.024752.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiol Learn Mem. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, Fetzner MG, Deboer LB, Powers MB, Otto MW, Smits JA. Let’s get physical: a contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Depress Anxiety. 2013;30:362–373. doi: 10.1002/da.22043. [DOI] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience. 2013;234:40–52. doi: 10.1016/j.neuroscience.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J Neurosci. 2012;32:15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SP, Davis JM, Ahlborn EN. Neuroendocrine and substrate responses to altered brain 5-HT activity during prolonged exercise to fatigue. J Appl Physiol (1985) 1993;74:3006–3012. doi: 10.1152/jappl.1993.74.6.3006. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the Degree of Segregation between Striatonigral and Striatopallidal Projections? Front Neuroanat, 4. 2010 doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC. Species-specific defense reactions and avoidance learning. Psychological Review. 1970;77:32–48. [Google Scholar]
- Boschen MJ, Neumann DL, Waters AM. Relapse of successfully treated anxiety and fear: theoretical issues and recommendations for clinical practice. Aust N Z J Psychiatry. 2009;43:89–100. doi: 10.1080/00048670802607154. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and ambiguity in the extinction of emotional learning: implications for exposure therapy. Behav Res Ther. 1988;26:137–149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Garcia-Gutierrez A, Zilski J, Moody EW. Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behav Res Ther. 2006;44:983–994. doi: 10.1016/j.brat.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, Hillmer-Vogel U, Ruther E. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. Am J Psychiatry. 1998;155:603–609. doi: 10.1176/ajp.155.5.603. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Pinard CR, Holmes A. Mechanisms to medicines: elucidating neural and molecular substrates of fear extinction to identify novel treatments for anxiety disorders. Br J Pharmacol. 2014;171:4690–4718. doi: 10.1111/bph.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy Y, Fyer AJ, Lipsitz JD. Treatment of specific phobia in adults. Clin Psychol Rev. 2007;27:266–286. doi: 10.1016/j.cpr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Ghasem PR, Mika A, Day HE, Herrera JJ, Greenwood BN, Fleshner M. Wheel running alters patterns of uncontrollable stress-induced cfos mRNA expression in rat dorsal striatum direct and indirect pathways: A possible role for plasticity in adenosine receptors. Behav Brain Res. 2014;272:252–263. doi: 10.1016/j.bbr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Day HE, Akil H. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology. 1996;63:207–218. doi: 10.1159/000126959. [DOI] [PubMed] [Google Scholar]
- de la Mora MP, Gallegos-Cari A, Arizmendi-Garcia Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Prog Neurobiol. 2010;90:198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Kincheski GC, Pavesi E, Sordi R, Assreuy J, Carobrez AP. Role of beta-adrenergic receptors in the ventromedial prefrontal cortex during contextual fear extinction in rats. Neurobiol Learn Mem. 2010;94:318–328. doi: 10.1016/j.nlm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Seemann JR, Maren S. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull. 2014;105:46–60. doi: 10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10:67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Mechanisms Underlying the Relationship Between Physical Activity and Anxiety: Animal data. New York, NY: Routledge; 2013. [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b–adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Loughridge AB, Sadaoui N, Christianson JP, Fleshner M. The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behav Brain Res. 2012;233:314–321. doi: 10.1016/j.bbr.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Loughridge AB, Day HE, Clark PJ, Mika A, Hellwinkel JE, Spence KG, Fleshner M. 5-HT2C receptors in the basolateral amygdala and dorsal striatum are a novel target for the anxiolytic and antidepressant effects of exercise. PLoS One. 2012;7:e46118. doi: 10.1371/journal.pone.0046118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Gaburro S, Sah A, Gartmann N, Lonsdorf TB, Meier K, Singewald N, Pape HC, Morellini F, Kalisch R. Single dose of L-dopa makes extinction memories context-independent and prevents the return of fear. Proc Natl Acad Sci U S A. 2013;110:E2428–E2436. doi: 10.1073/pnas.1303061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain Res Bull. 1994;35:41–49. doi: 10.1016/0361-9230(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch Intern Med. 2010;170:321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- Holtzman-Assif O, Laurent V, Westbrook RF. Blockade of dopamine activity in the nucleus accumbens impairs learning extinction of conditioned fear. Learn Mem. 2010;17:71–75. doi: 10.1101/lm.1668310. [DOI] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J Neurosci. 2014;34:817–822. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, Pieprzyk M, Cymerman IA, Werka T, Sheng M, Maren S, Jaworski J, Kaczmarek L. Functional anatomy of neural circuits regulating fear and extinction. Proc Natl Acad Sci U S A. 2012;109:17093–17098. doi: 10.1073/pnas.1202087109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology (Bethesda) 2012;27:167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- Meeusen R, Smolders I, Sarre S, de Meirleir K, Keizer H, Serneels M, Ebinger G, Michotte Y. Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta Physiol Scand. 1997;159:335–341. doi: 10.1046/j.1365-201X.1997.00118.x. [DOI] [PubMed] [Google Scholar]
- Merom D, Phongsavan P, Wagner R, Chey T, Marnane C, Steel Z, Silove D, Bauman A. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22:959–968. doi: 10.1016/j.janxdis.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Neumann DL, Kitlertsirivatana E. Exposure to a novel context after extinction causes a renewal of extinguished conditioned responses: implications for the treatment of fear. Behav Res Ther. 2010;48:565–570. doi: 10.1016/j.brat.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Neumann DL, Lipp OV, Cory SE. Conducting extinction in multiple contexts does not necessarily attenuate the renewal of shock expectancy in a fear-conditioning procedure with humans. Behav Res Ther. 2007;45:385–394. doi: 10.1016/j.brat.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev. 2012;36:1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflex: An investigation of the physiological activity of the cerebral cortex. Oxford, England: Oxford University Press; 1927. [Google Scholar]
- Paxinos GWC. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Powers MB, Medina JL, Burns S, Kauffman BY, Monfils M, Asmundson GJ, Diamond A, McIntyre C, Smits JA. Exercise Augmentation of Exposure Therapy for PTSD: Rationale and Pilot Efficacy Data. Cogn Behav Ther. 2015:1–14. doi: 10.1080/16506073.2015.1012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci U S A. 2012;109:8764–8769. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, Fadok JP, Muller C, Letzkus JJ, Luthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siette J, Reichelt AC, Westbrook RF. A bout of voluntary running enhances context conditioned fear, its extinction, and its reconsolidation. Learn Mem. 2014;21:73–81. doi: 10.1101/lm.032557.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonerock GL, Hoffman BM, Smith PJ, Blumenthal JA. Exercise as Treatment for Anxiety: Systematic Review and Analysis. Ann Behav Med. 2015 doi: 10.1007/s12160-014-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Corcoran KA, Jovasevic V, Radulovic J. Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends Neurosci. 2012;35:145–155. doi: 10.1016/j.tins.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hast TA, Bennett RZ, Merjanian M, Nocera NA, Ponnusamy R, Fanselow MS. Cholinergic blockade frees fear extinction from its contextual dependency. Biol Psychiatry. 2013;73:345–352. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]