Abstract
We measured diurnal rhythms of food intake, as well as body weight and composition, while varying three major classes of sex-biasing factors: activational and organizational effects of gonadal hormones, and sex chromosome complement (SCC). Four Core Genotypes (FCG) mice, comprising XX and XY gonadal males and XX and XY gonadal females, were either gonad-intact or gonadectomized (GDX) as adults (2.5 months); food intake was measured second-by-second for 7 days starting 5 weeks later, and body weight and composition were measured for 22 weeks thereafter. Gonadal males weighed more than females. GDX increased body weight/fat of gonadal females, but increased body fat and reduced body weight of males. After GDX, XX mice had greater body weight and more fat than XY mice. In gonad-intact mice, males had greater total food intake and more meals than females during the dark phase, but females had more food intake and meals and larger meals than males during the light phase. GDX reduced overall food intake irrespective of gonad type or SCC, and eliminated differences in feeding between groups with different gonads. Diurnal phase of feeding was influenced by all three sex-biasing variables. Gonad-intact females had earlier onset and acrophase (peak) of feeding relative to males. GDX caused a phase-advance of feeding, especially in XX mice, leading to an earlier onset of feeding in GDX XX vs. XY mice, but earlier acrophase in GDX males relative to females. Gonadal hormones and SCC interact in the control of diurnal rhythms of food intake.
Keywords: Food intake, sex differences, testosterone, estradiol, sex chromosomes, four core genotypes, body composition, circadian rhythm, night eating, obesity, adiposity
Introduction
Males and females have distinct patterns of metabolic regulation, including the control of food intake, which contributes to sex differences in the development and progression of metabolic disease. Until recently, sex differences in feeding in mammals were attributed exclusively to the effects of gonadal hormones, especially estrogens and androgens, which regulate food intake and energy metabolism by acting on the brain and diverse peripheral tissues (Asarian and Geary, 2006, 2013; Karastergiou et al., 2012; Petersen, 1978; Witte et al., 2010). We recently reported that the complement of sex chromosomes also causes sex differences in body weight, adiposity, and susceptibility to metabolic dysregulation caused by a high fat diet. Using Four Core Genotypes (FCG) mice in which sex chromosome complement (SCC, XX vs. XY) is varied in mice with testes (gonadal males) or ovaries (gonadal females), we found that the presence of two X chromosomes (vs. one) is associated with greater body weight and adiposity, in both gonadal males and gonadal females that had been gonadectomized (GDX) as adults (Chen et al., 2012). The increased body weight and adiposity of XX male and female mice was related to increased food intake specifically during the inactive period of the diurnal cycle, without alterations in activity or energy expenditure relative to XY mice.
Emerging evidence in humans and rodents suggests that disruption of the diurnal feeding pattern, especially increased caloric intake during the inactive phase, is associated with obesity and metabolic syndrome (Colles et al., 2007; Ma et al., 2003; Sierra-Johnson et al., 2008). For example, night-time eating in humans is associated with enhanced weight gain and obesity compared to similar caloric intake during normal meal times (Gallant et al., 2012). Mice that consume a greater proportion of their daily calories than normal during the inactive light phase have increased body weight, adiposity, hepatic steatosis, and hyperinsulinemia (Arble et al., 2009; Chaix et al., 2014; Hatori, et al., 2012). Conversely, restricting feeding in mice to the night prevents obesity and metabolic disease (Chaix et al., 2014; Hatori et al., 2012). Mice with mutations of the Clock gene, a key component of the molecular circadian clock, have altered diurnal feeding rhythms, light phase hyperphagia, hyperleptinemia, hyperlipidemia, hepatic steatosis, hyperglycemia, and hypoinsulinemia (Turek et al., 2005).
Our previous finding of greater food intake during the light phase in gonadectomized (GDX) XX mice, relative to XY, raises the question whether the greater adiposity in XX mice results from a diurnal shift in feeding caused by the presence of a second X chromosome. Here, we examine diurnal rhythms of food intake of FCG mice in greater detail, in groups of mice that differ in three major classes of sex-biasing factors: SCC (XX vs. XY), reversible activational effects of gonadal hormones (gonad-intact vs. GDX mice), and permanent organizational effects of gonadal hormones (differences between gonadal males vs females that persist after GDX). Thus, the design allows assessment of the relative impact of each type of sex-biasing factor, and the potential interaction of the three factors.
Materials and Methods
Mice
We used Four Core Genotypes (FCG) mice on a C57BL/6J (B6) background (B6.Cg-Tg(Sry)2Ei Srydl1Rlb/ArnoJ, Jackson Laboratories stock 10905; backcross generation greater than 23), bred at UCLA (Arnold and Chen, 2009; De Vries et al., 2002). In FCG mice, the testis-determining gene Sry is deleted from the Y chromosome and inserted as a transgene on chromosome 3 (Itoh et al., 2015), so that gonadal sex is no longer determined by SCC. Here, “male” refers to a mouse with Sry, born with testes, and “female” refers to a mouse without Sry, born with ovaries. FCG mice include XX males and females, and XY males and females (called XXM, XXF, XYM, XYF, respectively). The model is a 2 × 2 comparison in which gonadal sex and SCC (XX vs. XY) are varied independently, so that the independent and interacting effects of these variables can be assessed. The Y chromosome of FCG mice derives from strain 129. FCG B6 mice were studied with gonads intact or GDX at 75 days of age, as indicated.
The experimental design is intended to assess the roles of three major sources of sex-biasing factors: activational and organizational effects of gonadal hormones, and SCC. We compared the four genotypes with their gonads and after removal of the gonads, to test the role of testicular and ovarian secretions in adulthood (“activational effects”). Also compared were GDX mice that lack gonadal hormones as adults but that had either ovaries or testes prior to GDX, which allows detection of long-lasting effects of testicular (in mice with Sry) or ovarian secretions (in mice without Sry) up to several months after GDX. These long-lasting “organizational effects” in this model are confounded with direct effects of Sry outside the gonad in this model. Finally, the design tests if either activational or organizational effects of hormones affect XX and XY mice differently (sex chromosome effects).
Two cohorts of mice were studied, one for longitudinal measurement of body weight and composition (Fig. 1), and the second for detailed studies of patterns of feeding (Figs. 2–5). Males and females were housed as single-sex groups (except when in BioDAQ cages, see below) and maintained at 23°C with 12:12 LD (6am/6pm). The standard chow diet Purina 5001 contains approximately 5% fat (PMI Nutrition International, St. Louis, MO and Lab Diet 5001, www.labdiet.com). For studies in BioDAQ chambers, rodent diet AIN-93M was used (Research Diets, Inc., New Brunswick, NJ) which contains 4.1% fat. AIN-93M is a solid balanced rodent diet that causes minimum spillage, required for accurate measurement of food intake in BioDAQ chambers. Animal studies were performed under approval of the UCLA and Veterans Affairs Institutional Animal Care and Use Committees.
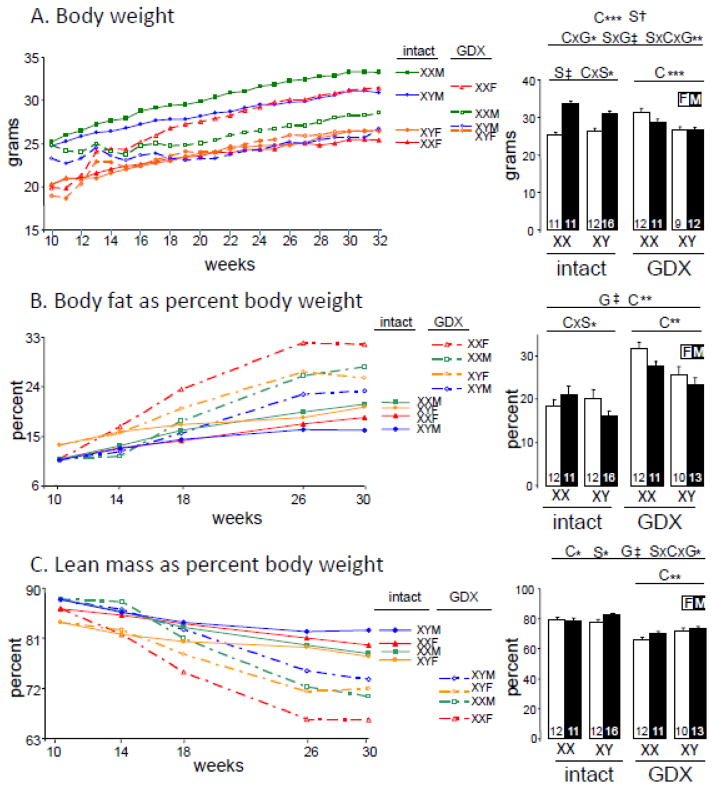
Fig. 1.
Body weight and composition in gonad-intact and GDX mice. Mice were GDX or not at 75 days of age (10 weeks) and then measured until 30–32 weeks of age. A. Body weight. B. Body fat expressed as percentage of body weight. C. Lean mass expressed as percent of body weight. F, gonadal female. M, gonadal male. On right side are final measurements at 32 weeks of age for body weight, or body fat and lean mass at 30 weeks of age. Group size is shown at the bottom of histogram bars. Results of the 3-way ANOVA for all groups (horizontal line above spanning all groups), and of 2-way ANOVAs for gonad-intact alone or GDX alone (horizontal lines above spanning only each condition), indicate significant effects (when present) of G (gonadal status, intact vs. GDX), S (gonadal sex, female vs. male), or C (sex chromosome complement, XX vs. XY). Significant interactions are shown as GxS, GxC, SxC, or GxSxC, * p<0.05, ** p<0.01, *** p<0.001, † p<0.0001, ‡ p<0.00001.
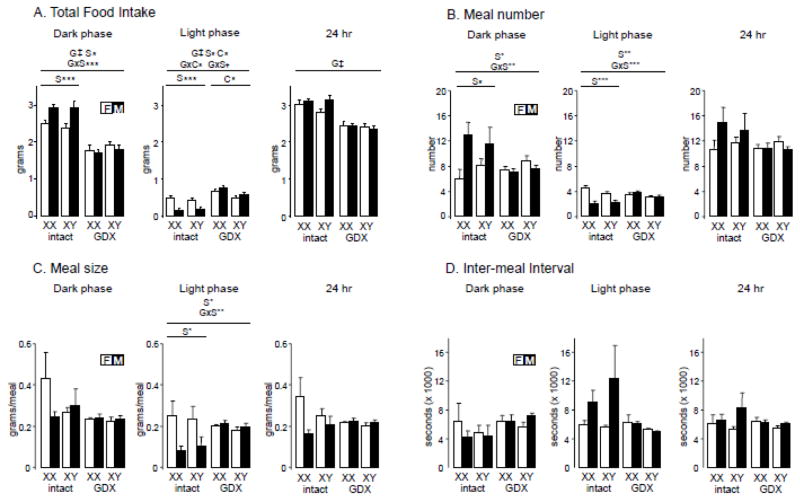
Fig. 2.
Food intake measured in BioDAQ cages. A. Total food intake measured over the dark phase, light phase, or summer over 24 hours. B. Meal number. C. Meal size. D. Inter-meal interval. Results of the 3-way ANOVA for all groups (horizontal line above spanning all groups), and of 2-way ANOVAs for gonad-intact alone or GDX alone (horizontal lines above spanning only each condition), indicate significant effects (when present) of G (gonadal status, intact vs. GDX), S (gonadal sex, female vs. male), or C (sex chromosome complement, XX vs. XY). Significant interactions are shown as GxS, GxC, SxC, or GxSxC, * p<0.05, ** p<0.01, *** p<0.001, † p<0.0001, ‡ p<0.00001.
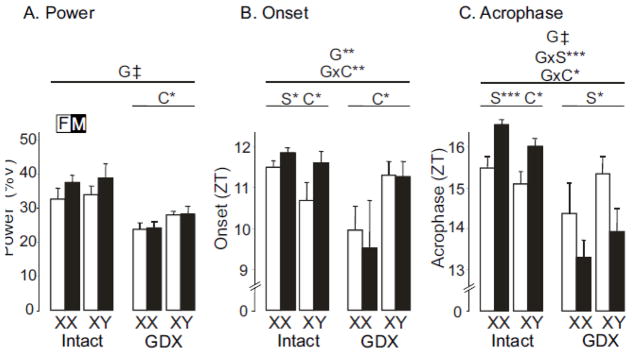
Fig. 5.
Diurnal parameters of food intake. A. Power, a measure of the strength of the rhythm. B. Onset of food intake. C. Acrophase or peak of feeding rhythm. Zeitgeber Time (ZT) of 0 represents the time of lights on (6AM) and ZT 12 represents lights off (6PM) during a 24-hour day. Results of the 3-way ANOVA for all groups (horizontal line above spanning all groups), and of 2-way ANOVAs for gonad-intact alone or GDX alone (horizontal lines above spanning only each conditions), indicate significant effects (when present) of G (gonadal status, intact vs. GDX), S (gonadal sex, female vs. male), or C (sex chromosome complement, XX vs. XY). Significant interactions are shown as GxS, GxC, SxC, or GxSxC, * p<0.05, ** p<0.01, *** p<0.001, † p<0.0001, ‡ p<0.00001
Genotyping
DNA was extracted from tails using Chelex resin (Bio-Rad, Hercules, CA). The genotype of mice was determined by PCR to amplify Sry (which determines gonadal sex) and the Y-chromosome repetitive sequence Ymt. Gonadal sex was confirmed at the time of gonadectomy or at the end of the experiment. Myogenin was used as an amplification control. The primers were myogenin-F: TTACGTCCATCGTGGACAGCAT, myogenin-R: TGGGCTGGGTGTTAGTCTTAT; Ymt-F: CTGGAGCTCTACAGTGATGA, Ymt-R CAGTTACCAATCAACACATCAC; Sry-F: AGCCCTACAGCCACATGATA, Sry-R: GTCTTGCCTGTATGTGATGG.
Measurement of body weight and body composition
Body composition was determined at 30 weeks of age with a Mouse Minispec apparatus (Bruker Woodlands, TX) with Echo Medical Systems (Houston, TX) software (Taicher et al., 2003). This apparatus uses NMR spectroscopy for fat and lean mass measurements with coefficients of variation of <3%. Correlation between NMR and gravimetric measurements is better than 0.99.
BioDAQ food intake monitoring system
Food intake behavior and meal patterns were continuously monitored, with minimal human interference, using by the BioDAQ Biological Data Acquisition episodic food intake monitor for mice (Research Diets, Inc., New Brunswick, NJ). Three weeks after gonadectomy at age 75 days, mice were habituated for one week to the AIN-93N diet, and then were housed as individuals in cages containing the BioDAQ monitors (n=4 mice of each genotype). While housed in BioDAQ cages, mice received AIN-93M diet through a feeding hopper, which weighed the food every second at 0.01g resolution. Water was provided ad libitum from regular water bottles. Gonad-intact FCG mice (n=4 per group) were tested at the same age.
Mice were deemed not to be feeding if the hopper weight was stable, and feeding if the hopper weight changed by more than 0.01g. Episodic feeding bouts, the smallest measured unit of feeding, were recorded automatically when the change in food hopper weight was greater than 0.01g. Bouts were separated by an inter-bout interval, the time period between bouts during which there was no feeding activity. Meals were defined as comprising a series of bouts within 300 seconds of each other when the sum of food intake over all bouts was equal to or greater than 0.02 g. Meal beginning in one period (e.g., the light phase) was recorded in that period even if it extended into another period (e.g., dark phase). Measurements of meal patterns included total food eaten, meal size (food eaten per meal), meal number, and inter-meal interval (the time period between meals during which there was no feeding activity) as previously described (Stengel et al., 2010). The data were analyzed with BioDAQ Monitoring software 2.2.02 and spreadsheet software.
Baseline food intake for GDX and gonad-intact mice
It usually takes 3–5 days for mice to settle into a stable pattern of food intake in the novel BioDAQ cage environment. We report on feeding patterns for 7 days beginning on the 6th day after introduction into the BioDAQ cages. Thus, feeding is reported here during the period about 5–6 weeks after GDX. Patterns were measured for mice that were placed in BioDAQ cages at 4 weeks after GDX at 75 days of age, and for gonad-intact mice that were also approximately 103 days old.
Determination of the onset, acrophase, duration, and power of feeding rhythms
Food consumption data were separated into 15 minute bins under 12:12 LD. Average waveforms of food consumption data were produced using Clocklab software (Actimetrics, Wilmette, IL). Onset was determined by the start of feeding that crossed the threshold of the mean using the 7 day average waveform. Feeding duration (alpha, α) was determined by the duration of food consumption over the threshold of the mean using the average waveform. The acrophase of each day of feeding was determined using Clocklab and an average of the 7 days is reported. The same 7 days of feeding data were analyzed by χ2 periodogram (Sokolove and Bushell, 1978) and the power of the rhythm (% variance, %V) was determined by multiplying the amplitude, Qp, by 100/n, where n = number of data points examined using the El Temps program (A. Diez-Noguera, Barcelona, Spain). Onset and acrophase times are reported in Zeitgeber Time (ZT), where ZT 0 represents the time of lights on (6AM) and ZT 12 represents lights off (6PM) during a 24-hour day. Values are reported as mean ± standard error of the mean (SEM).
Statistical analyses
Groups were compared using a three-way ANOVA (3WA) (NCSS 2001; Number Cruncher Statistical Systems, Kaysville, UT, USA) with main factors of sex (gonadal male (Sry present) vs. gonadal female (Sry absent)), SCC (XX vs. XY), and gonadal status (gonad-intact vs. GDX). Some of our previous studies focused exclusively on GDX mice (Chen et al., 2012), and therefore we began the present experiments with specific hypotheses concerning the effects of sex and sex chromosome complement in GDX mice only, as well as in goand-intact mice. Accordingly, independent of the outcome of the 3WA, two two-way ANOVAs (2WAs) were used to test the effects of sex and SCC separately in gonad-intact and GDX mice. We report selected group comparisons using Tukey-Kramer (TK) post-hoc tests, to help explain significant interactions of factors when needed. Effect size was calculated as partial eta squared ηp2, which varies between 0 and 1 and is a measure of the amount of variability accounted for by main effects or interactions of factors (Lakens, 2013).
Results
Longitudinal study of the acute effects gonadal secretions on body weight and composition
To assess the role of gonadal secretions during adulthood on body weight and body composition, we made measurements under ad libitum feeding conditions in group-housed FCG mice that were either gonad-intact or GDX at 10 weeks of age. When the experiment was concluded at 32 weeks of age, gonad-intact males were heavier than females (2WA, F(1,46)=86.9, p<0.000001, ηp2= 0.64)(Fig. 1A). This effect of sex depended on SCC (2WA, interaction of sex and SCC, F(1,46)=6.4, p=0.015, ηp2= 0.05), because the mean sex difference was larger in XX than XY mice (Fig. 1A, both sex differences TK p<0.05). Removing the gonads showed that GDX XX mice have higher body weight than XY mice (2WA, F(1,40)=13.1, p<0.0009, ηp2= 0.22), a result that was consistent with our previous study (Chen et al., 2012). GDX also led to reduced body weight in males, but increased weight in females (3WA, interaction of sex x gonadal status, F(1,86)=45.8, p<0.000001, ηp2= 0.25, Fig. 1A). The acute effects of ovarian but not testicular secretions depended on SCC (3WA, interaction of sex x SCC x gonadal status, F(1,86)=8.5, p<0.005, ηp2= 0.05), because of the four genotypes, the only genotype not showing a significant effect of GDX was XYF (TK p<0.05 for all GDX effects in XXF, XYM, and XYF).
We determined body composition by NMR spectroscopy at 30 weeks of age. Relative fat mass among gonad-intact mice showed weak interacting effects of sex and sex chromosome complement (2WA, interaction of SCC and sex, F(1,47)=4.1, p<0.05, ηp2= 0.08, Fig. 1B, SCC effect in each sex TK p>0.05). Relative lean mass did not differ among groups of gonad-intact mice at the end of the experiment (Fig. 1C, 2WA). GDX robustly increased relative fat mass (3WA, GDX effect, F(1,89)=49.8, p<0.000001, ηp2= 0.30, Fig. 1B, intact vs. GDX TK p<0.05 for XXF and XYM) and led to correspondingly lower relative lean mass (3WA, GDX effect, F(1,89)=66.3, p<0.000001, ηp2= 0.37, Fig. 1C, GDX effect TK p<0.05 for all genotypes except XYF). The effect of GDX on relative lean mass was weakly dependent on the interaction of sex and SCC (3WA, interaction of sex x SCC x gonadal status, F(1,89)=4.0, p<0.05, ηp2= 0.02, Fig. 1C, because for GDX effect TK<0.05 in all genotypes except XYF). After GDX, relative fat was higher in XX than XY (2WA, SCC F(1,42)=10.6, p<0.003, ηp2= 0.18), and relative lean mass was correspondingly lower in XX than XY (2WA, SCC F(1,42)=8,4, p<0.007, ηp2= 0.15).
The diurnal feeding pattern is affected by gonadal status and sex chromosome complement
We assessed feeding behavior (amount of food consumed, meal number and size, and inter-meal interval) continually over 24 hours for 7 days using BioDAQ cages, which monitor food intake every second at a sensitivity of 0.01 gram. Gonadally intact mice ate substantially more than GDX mice during the dark phase (3WA, gonadal status effect F(1,24)=129.0, p<0.000001, ηp2= 0.73), and over the 24-hour diurnal cycle (3WA, GDX effect, F(1,24)=93.3, p<0.00001; ηp2= 0.74, Fig. 2A). During the dark phase, gonad-intact males ate more than females (2WA, sex F(1,12)=19.46, p<0.0009, ηp2= 0.60), a difference that disappeared after GDX (3 WA, interaction of sex x gonadal status, F(1,24)=14.2, p<0.001, ηp2= 0.08, sex difference TK p<0.05 in intact but p>0.05 in GDX). By contrast, during the light phase, gonad-intact females ate more than males (2WA, F(1,12)=32.4, p=0.0001, ηp2= 0.71). After GDX, the male-female sex difference in food intake disappeared (3WA, interaction of sex and GDX, F(1,24)=21.7, p<0.0001, ηp2= 0.19, sex difference TK p<0.05 in intact but p>0.05 in GDX), and an SCC effect emerged (3WA, interaction of sex chromosome complement and GDX F(1,24)=4.3, p<0.05, ηp2= 0.04, GDX effect TK p>0.05 for XX but p>0.05 for XY), with XX mice consuming more than XY mice during the light phase (2WA, F(1,12)=8.7, p<0.02, ηp2= 0.39, Fig. 2A).
We also assessed the effects of gonadal hormones and sex chromosomes on meal number and size, and inter-meal interval. The number of meals in a 24-hour period showed no difference across groups (Fig. 2B). In gonad-intact mice, males ate more meals than females during the dark phase (2WA, F(1,12)=7.2, p<0.03, ηp2= 0.36, Fig. 2B), leading to greater food intake (Fig. 2A), a difference that was eliminated by GDX (3WA, interaction of GDX and sex, F(1,24)=8.68, p=0.007, ηp2= 0.20, sex difference TK p<0.05 for intact but p<0.05 for GDX). In the light phase, intact females ate more meals than males (2WA, F(1,12)=26.9, p<0.0003, ηp2= 0.63), leading to greater food intake in females (Fig. 2A), a sex difference that was also eliminated by GDX (3WA, interaction of GDX and sex, F(1,24)=18.4, p<0.0003, ηp2= 0.29, comparison of sexes TK p<0.05 for gonad-intact but p>0.05 for GDX).
There were no significant group differences in meal size measured over 24 hours or during the dark phase (Fig. 2C). In the light phase, gonad-intact females ate larger meals than males (2WA, F(1,12)=8.3, p<0.02, ηp2= 0.41, Fig. 2C), a sex difference that was abolished by gonadectomy (3WA, interaction of sex and gonadal status, F(1,24)=8,41, p<0.008, ηp2= 0.21, sex difference TK p<0.05 for intact but p<0.05 for GDX). Inter-meal interval did not differ among groups over the 24-hour period or in the dark or light periods.
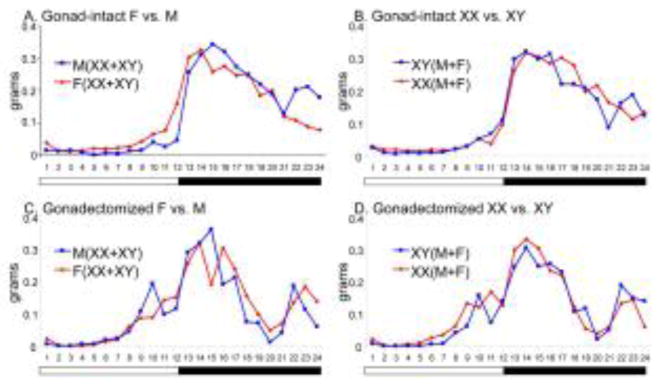
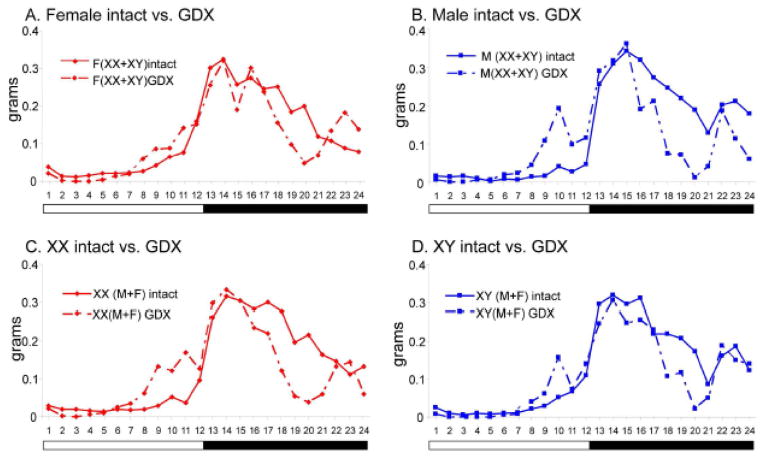
An examination of food intake at hourly intervals throughout the 24-hour period suggested sex and SCC effects on the diurnal feeding rhythm (Figs. 3 and 4, see Fig. 5 for statistical analysis of rhythms). In gonad-intact mice, females began eating in the late afternoon about two hours before lights off, whereas males began their main phase of eating later, closer to the time of lights off (Fig. 3A). Gonad-intact XX and XY mice had similar hourly patterns of food intake (Fig. 3B). After GDX, all groups showed a phase-advance of feeding, so that more food was consumed during the late afternoon (light phase) and less during the dark phase (Figs. 3CD and 4ABCD), and the oscillation in feeding between the two phases was weakened. The phase-advance in GDX mice was similar in M and F (Figs. 3C and 4AB), but slightly more pronounced in XX mice than in XY mice (Fig. 3D, 4CD), leading to the sex chromosome effect on food intake during the light phase that is shown in Fig. 2A.
Fig. 3.
Effects of gonadal sex and sex chromosome complement on diurnal rhythms of food intake. Food intake was averaged for each hour for 7 days for n=8 mice per condition. A. Gonad-intact females vs. males. B. Gonad-intact XX vs. XY. C. Gondectomized females vs. males. D. Gonadectomized XX vs. XY.
Fig. 4.
Effects of gonadectomy on diurnal rhythms of food intake of mice differing in gonadal sex or sex chromosome complement. Food intake was averaged for each hour for 7 days for n=8 mice per condition. These curves are the same as in Fig. 3, but are re-graphed for direct assessment of the effects of GDX, A. Female intact vs. GDX. B. Male intact vs. GDX. C. XX intact vs. GDX. D. XY intact vs. GDX.
To analyze rhythms of feeding further and determine which FCG group differences illustrated in Figs. 3 and 4 were statistically significant, we applied several analytical algorithms from the field of circadian biology (Fig. 5). We assessed the power of feeding rhythms using the χ2 periodogram test. The power (strength of the diurnal oscillation) of the feeding rhythms was decreased by GDX across groups (3WA, F(1,24)=39.2, p<0.00001, ηp2= 0.54, Fig. 5A), which is also seen in the pattern of the rhythm in Figs. 3 and 4. Although the 3WA did not detect an interaction between the effects of GDX and SCC on rhythm strength, the 2WA on GDX mice found a significant impact of SCC (F(1,12)=7.3, p<0.02, ηp2= 0.38), because GDX XY mice had greater rhythm strength than GDX XX mice (Fig. 5A).
To assess further the temporal pattern of food consumption in FCG mice, we generated average waveforms over 7 days of food consumption. The onset of food intake was determined as the time at which food consumption reached half the maximum of the waveform, and acrophase denoted the time at which the peak of the feeding rhythm occurred. The onset of food intake was generally phase-advanced in most groups (i.e., occurred earlier in the diurnal cycle) by GDX (3WA, F(1,24)=7.6), p=0.011, ηp2= 0.16, Fig. 5B). SCC altered the onset of feeding differently depending on the gonadal status of the mice (3WA, interaction of SCC and GDX, F(1,24)=, p=0.004 ηp2= 0.22, Fig. 5B, SCC effect TK p<0.05 for GDX but p>0.05 for intact); intact XX mice began feeding later than intact XY mice (2WA, F(1,12)=4.7, p=0.05, ηp2= 0.19), but GDX XX mice began feeding earlier than GDX XY mice (2WA, F(1,12)=6.5, p<0.03. ηp2= 0.35). In addition, in gonad-intact mice, females began feeding earlier than males (2WA, F(1,12)= 6.8,, p<0.03. ηp2= 0.27), an effect not found in GDX mice despite the absence of a significant interaction of sex and gonadal status in the 3WA.
GDX also shifted feeding earlier in most genotypes (confirming the results from Figs. 4 and 5B) as measured by the peak of the feeding rhythm (acrophase, Fig. 5C)(3WA, effect of GDX, F(1,24)=36.7, p<0.00001 ηp2= 0.42). The effect of GDX differed according to sex (3WA, interaction of GDX and sex, F(1,24)=19.1, p<0.001, ηp2= 0.22,) with a greater effect of GDX in males (TK p<0.05) than in females (TK p>0.05). The effect of GDX also differed according to SCC (3WA, interaction of GDX and SCC, F(1,24)= 6.0, p<0.03, ηp2= 0.07) because the effect of GDX was greater in XX (TK p<0.001) than XY mice (Tukey, p=0.02). In gonad-intact mice, acrophase was earlier in females than males (2WA, effect of sex, F(1,12)=24.8. p<0.001 ηp2= 0.59), and was earlier in XY than XX mice (2WA, F(1,12)=5.4, p<0.04, ηp2= 0.13). GDX induced a reversal of the sex effect found in gonad-intact mice, with GDX males having earlier acrophase than females (2WA, F(1,12)=7.0, p<0.03, ηp2= 0.32, Fig. 5C).
Although the onset and acrophase of food intake were phase-advanced by gonadectomy, the duration through which mice consumed food was not dependent on gonadal state. Three-way ANOVA did not detect any significant effect of any of the three factors on duration of feeding (data not shown).
Discussion
Here we report complex interactions of the effects of gonadal hormones and SCC on diurnal feeding rhythms, body weight, and body composition in adult C57BL/6 FCG mice. In gonad-intact mice in the dark phase, males ate more meals and more total food than females, whereas in the light phase females ate more and larger meals, and more food overall, than males. Removing the gonads of adult mice caused a phase-advance of the diurnal rhythm of feeding, reducing food intake in the dark phase but increasing it in the light phase, especially in XX mice and males. After GDX, XX mice began to feed in the late afternoon, earlier than XY mice, but gonadal males had earlier acrophase (peak) of the feeding rhythm relative to gonadal females. GDX resulted in an overall reduction in total food intake over the diurnal cycle because of the large reduction during the dark phase, probably leading to weight loss in gonadal males, but paradoxically associated with weight gain in gonadal females, especially XX females. Removal of the gonads abolished most of the differences between gonadal males and females that occur in gonad-intact mice, indicating that testicular and ovarian hormones act in a reversible fashion in adulthood to cause sex differences in feeding. However, these effects depended both on the diurnal phase and SCC, illustrating the complex interaction of sex-biasing factors controlling food intake.
Gonadal sex differences and activational effects of gonadal hormones on diurnal rhythms of feeding
To our knowledge, this is the first report that gonad-intact female mice have earlier onset and acrophase of feeding relative to males (Fig. 5BC), resulting in greater food intake of females during the light phase and greater food intake of males during the dark phase (Fig. 2A). These sex differences in feeding are attributable to a greater number of dark-phase meals in males than females, and more and larger light-phase meals in females than males (Fig. 2BC). These gonadal differences were abolished by GDX of adults, indicating that they were caused by acute (activational) effects of gonadal hormones. Although ovarian and testicular hormones both contributed to the changes caused by GDX, it appears that testicular hormones played a larger role in causing the sex differences in diurnal phase of feeding. The greater influence of testicular hormones was evident in several measurements: (a) GDX of males caused greater change in the amount of daytime feeding and number and size of daytime meals than was caused by GDX of females (Figs. 2ABC, significant interactions of effects of GDX and sex). (b) GDX of males had a larger effect on dark-phase feeding and meal number than did GDX of females (Figs. 2AB, detected as significant interactions of GDX and sex). (c) GDX of males caused greater phase-advance of acrophase of feeding relative to GDX of females (Fig. 5C, significant interaction of GDX and sex), leading to a sex difference in acrophase in GDX mice (males phase-advanced relative to females, Fig. 5C).
In the present study, GDX caused robust increases in body fat irrespective of SCC, suggesting that gonadal secretions were having grossly similar effects in XX and XY mice. Ovariectomy of XY females, however, had less effect on body weight, and on onset and acrophase of food intact, than in XX females. This is not likely due to differences in levels of gonadal hormones in the XX vs. XY females, as several studies report similar levels of testosterone and of estradiol in FCG mice of the same sex (Gatewood et al., 2006; Sasidhar et al., 2012, Corre et al., 2014; Holaskova et al., 2015). Moreover, anogenital distance does not differ in postnatal XX vs. XY mice of the same sex, suggesting that SCC does not after prenatal levels of androgens in either sex, which affect anogenital distance (Itoh et al., 2015). Nevertheless, XX females cycle normally and are fertile, but XY females are infertile in this strain, suggesting that XX and XY females may differ in their estrous cycles and levels of ovarian hormones.
Previous studies support the conclusion that ovariectomy increases body weight and increases food intake via an increase in meal size in female rats, because of the appetite-suppressing effects of estradiol (reviewed by Asarian and Geary, 2006, 2013; Eckel, 2011). However, studies of mice have not uniformly confirmed this conclusion (Witte et al., 2010). For example, although most studies report an increase in body weight and adiposity after ovariectomy of adult female mice (but in one study not at all ages after ovariectomy (Vieira Potter et al., 2012), some studies report a concomitant increase in food intake relative to ovary-intact controls, especially at short intervals after ovariectomy (Blaustein et al., 1976, but other studies found no increase in food intake (Gomori et al., 2007; Witte et al., 2010; Zengin et al, 2013) or a decrease in food intake at some but not all weeks after ovariectomy (Vieira Potter et al., 2012). The increase in meal size in female mice at proestrous (Peterson, 1976) might suggest that females have overall greater meal size than males; although the data of Fig. 2C trend in that direction, the sex difference was not significant. Differences among previous studies and the present study are potentially attributable to strain differences or differences in the interval between ovariectomy and time of measurement of food intake. In the present study, GDX deceased daily food intake in both sexes, relative to gonad-intact controls, measured at 5–6 weeks after GDX, but not because of an overall reduction in meal size or number measured across the entire 24-hour cycle. Rather, the effects of GDX were found to be dependent on diurnal phase, which has not been extensively studied previously in mice.
Effects of Sex Chromosome Complement on diurnal phase of feeding
SCC had a significant effect on diurnal phase of feeding in gonad-intact mice, as seen in the earlier onset and acrophase of food intake of XY mice relative to XX mice (Figs. 5BC). The effects of SCC were more evident after GDX. The power or strength of the feeding rhythm was greater in GDX XY than GDX XX mice, suggesting that a stronger rhythm contributes to reduced body weight and adiposity of GDX XY mice relative to GDX XX (Fig. 1AB). GDX caused a greater phase-advance in onset and acrophase of feeding in XX than XY mice (Figs. 5BC, interaction of GDX and SCC). That change resulted in earlier onset of feeding in GDX XX than XY (Fig. 5B) and greater total food intake in the light phase in GDX XX mice, relative to XY (Fig. 2A). There were no significant sex chromosome effects on measures of meal number, size, or intermeal interval (Fig. 2). Thus, the slow and progressive gain in body weight and adiposity that occurs after GDX, which is more pronounced in XX than XY mice (Fig. 1AB), is potentially attributable, at least in part, to a shift in diurnal phase of feeding rather than an increase in total feeding.
Effects of long-term gonadectomy on feeding and body weight/composition
The largest effects in the present study were caused by GDX of either sex. Long-term gonadectomy caused opposite changes in body weight in the two sexes (increased in females, decreased in males, Fig. 1B). In contrast, relatively similar effects across groups were seen in the effect of GDX on relative adiposity (increased, Fig. 1B) and relative lean mass (decreased, Fig. 1C), although effects of GDX were more pronounced in some groups. The GDX-induced differences in body weight are not explained solely by the effects of GDX on food intake. Total food intake was reduced dramatically in both sexes measured over the 24-hour day and during the dark phase when the majority of food intake occurs (Fig. 2A), although increased modestly in males during the light phase. These similar changes in both sexes were associated with decreased body weight in males but increased body weight in females. The power or strength of the diurnal rhythm was reduced by GDX (Fig. 5A), and GDX caused a strong phase-advance of onset and acrophase feeding (Fig. 5BC). The GDX-induced phase-advance in onset and acrophase was greater in XX than XY mice, and the phase-advance in acrophase was greater in males than females.
Although the opposite effect of GDX on body weight in the two sexes is not explained by the effect of GDX on feeding, is it possible that the sex differences in effect of GDX on body weight are explained by the diurnal shift in feeding rather than the total amount eaten? That explanation is not likely. The robust GDX-induced phase-advance in onset of feeding was about the same in the two sexes, although males showed a significantly greater phase-advance of acrophase (Fig. 5C, interaction of GDX and gonadal sex). If a phase-advance of feeding leads to weight gain and greater adiposity, then the diurnal patterns would predict either a similar GDX-induced gain in body weight and adiposity in the two sexes, or greater weight gain and adiposity in males than females. Neither pattern was observed. Instead, GDX led to loss of weight in males and increase in relative adiposity. The metabolic effects of GDX are likely to be diverse and to change with time after GDX. For example, ovariectomy of female mice reduces activity, metabolic rate, oxygen consumption and energy expenditure in the first 3 weeks after GDX (Witte et al., 2010; Zengin et al., 2013), but some these effects subside by 7 weeks after GDX (Zengin et al., 2013).
Interaction of hormones and sex chromosomes
We have previously suggested that the effects of sex chromosomes on body weight are reduced when gonads are present, based on a comparison of the large effect of SCC on body weight 10 months after GDX, relative to the more modest effect of SCC in 10 week old young mice just before GDX (Chen et al., 2012; Arnold et al., 2013;). The present study confirms this conclusion based on comparison of gonad-intact vs. GDX mice of the same age (20–22 weeks after GDX at 10 weeks of age, or at comparable age without GDX). In gonad-intact mice, the effect of SCC was either not significant at this age (for relative fat and lean mass), or for body weight was greater in males than females (Fig. 1A, interaction of sex and SCC).
The present study sheds some light on the question of which major sex-biasing factors cause sex differences in food intake and body weight and adiposity, because the three factors of our ANOVAs measured the three major classes of sex-biasing factors. The comparison of gonad-intact and GDX mice reflects activational hormone effects, sex differences in GDX mice reflects organizational hormone effects, and comparisons of XX and XY reflects sex chromosome effects. The effects of GDX were generally greatest, and interacted with sex and SCC. Sex differences that persisted after GDX (organizational effects) were observed only in acrophase of food intake (Fig. 5C). Moreover, because sex effects after GDX are confounded in the FCG model with effects of Sry acting outside of the gonad, it is not clear if this “organizational” effect can be attributed to long-lasting effects of gonadal hormones. Sex chromosome effects were smaller than effects of GDX, and observed predominantly in GDX mice. Other experimental approaches are needed to help detect each of these effects, for example by manipulating levels of individual gonadal hormones in adults or in neonates that differ in SCC.
Importance of diverse sex-biasing factors, and of time of day
A major conclusion of the present study is that circulating gonadal hormones, both testicular and ovarian, interact with sex-biasing factors encoded by the sex chromosomes, to change the diurnal phase of food intake in a manner than can contribute to sex differences in adiposity, body weight, and body composition. Ingestion of food has an important impact on metabolism and body composition, not only because of the total amount eaten, but also when in the diurnal cycle food intake occurs. Thus, sex biasing factors may act predominantly at specific diurnal phases, or on the control of rhythms themselves, to alter metabolism and adiposity.
Highlights.
Gonad-intact male and female mice differed in the diurnal phase of eating
Gonadectomy reduced food intake in males and females.
Gonad-intact females had earlier onset and peak of feeding than males.
Gonadectomy caused a phase-advance of food intake, especially in XX mice, leading to earlier onset of feeding in XX than XY mice irrespective of their gonad type.
Gonadectomy increased percent body content of fat, and led to greater adiposity in XX than XY mice.
Acknowledgments
Supported by NIH grants DK083561 (APA, XC, KR), NS043196 (APA), DK041301 (YT, Animal Models Core), VA Career Scientist Award (YT), and the O’Keefe Foundation (CSC). Thanks to Rebecca McClusky, Maureen Ruiz-Sundstrom, and Shawn Aarde for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity(Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X, Link JC, Itoh Y, Reue K. Cell-autonomous sex determination outside of the gonad. Dev Dyn. 2013 doi: 10.1002/dvdy.23936. epub Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Gentry RT, Roy EJ, Wade GN. Effects of ovariectomy and estradiol on body weight and food intake in gold thioglucose-treated mice. Physiol Behav. 1976;17:1027–1030. doi: 10.1016/0031-9384(76)90028-7. [DOI] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles SL, Dixon JB, O’Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 2007;31:1722–1730. doi: 10.1038/sj.ijo.0803664. [DOI] [PubMed] [Google Scholar]
- Corre C, Friedel M, Vousden DA, Metcalf A, Spring S, Qiu LR, Lerch JP, Palmert MR. Separate effects of sex hormones and sex chromosomes on brain structure and function revealed by high-resolution magnetic resonance imaging and spatial navigation assessment of the Four Core Genotype mouse model. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0952-0. epub Dec 2. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104:517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant AR, Lundgren J, Drapeau V. The night-eating syndrome and obesity. Obes Rev. 2012;13:528–536. doi: 10.1111/j.1467-789X.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomori A, Ishihara A, Ito M, Matsushita H, Ito M, Mashiko S, Iwaasa H, Matsuda M, Bednarek MA, Qian S, MacNeil DJ, Kanatani A. Blockade of MCH1 receptor signalling ameliorates obesity and related hepatic steatosis in ovariectomized mice. Br J Pharmacol. 2007;151:900–908. doi: 10.1038/sj.bjp.0707292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaskova I, Franko J, Goodman RL, Arnold AP, Schafer R. The XX Sex Chromosome Complement is Required in Male and Female Mice for Enhancement of Immunity Induced by Exposure to 3,4-Dichloropropionanilide. Am J Reprod Immunol. 2015;74:136–147. doi: 10.1111/aji.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Mackie R, Kampf K, Domadia S, Brown JD, O’Neill R, Arnold AP. Four Core Genotypes mouse model: Localization of the Sry transgene and bioassay for testicular hormone levels. BMC Research Notes. 2015;8:69. doi: 10.1186/s13104-015-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Bertone ER, Stanek EJ, III, Reed GW, Hebert JR, Cohen NL, Merriam PA, Ockene IS. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158:85–92. doi: 10.1093/aje/kwg117. [DOI] [PubMed] [Google Scholar]
- Petersen S. The temporal pattern of feeding over the oestrous cycle of the mouse. Anim Behav. 1976;24:939–955. doi: 10.1016/s0003-3472(76)80023-1. [DOI] [PubMed] [Google Scholar]
- Petersen S. Effects of testosterone upon feeding in male mice. Anim Behav. 1978;26:945–952. doi: 10.1016/0003-3472(78)90158-6. [DOI] [PubMed] [Google Scholar]
- Sasidhar MV, Itoh N, Gold SM, Lawson GW, Voskuhl RR. The XX sex chromosome complement in mice is associated with increased spontaneous lupus compared with XY. Ann Rheum Dis. 2012;71:1418–1422. doi: 10.1136/annrheumdis-2011-201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Johnson J, Unden AL, Linestrand M, Rosell M, Sjogren P, Kolak M, De FU, Fisher RM, Hellenius ML. Eating meals irregularly: a novel environmental risk factor for the metabolic syndrome. Obesity (Silver Spring) 2008;16:1302–1307. doi: 10.1038/oby.2008.203. [DOI] [PubMed] [Google Scholar]
- Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, Tache Y. Activation of brain somatostatin 2 receptors stimulates feeding in mice: analysis of food intake microstructure. Physiol Behav. 2010;101:614–622. doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, DeFuria J, Obin MS, Greenberg AS. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinol. 2012;153:4266–4277. doi: 10.1210/en.2011-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol. 2010;166:520–528. doi: 10.1016/j.ygcen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin A, Nguyen AD, Wong IP, Zhang L, Enriquez RF, Eisman JA, Herzog H, Baldock PA, Sainsbury A. Neuropeptide Y mediates the short-term hypometabolic effect of estrogen deficiency in mice. Int J Obes (Lond) 2013;37:390–398. doi: 10.1038/ijo.2012.71. [DOI] [PubMed] [Google Scholar]