Abstract
Up to now, the ability of target cells to activate protein kinase C (PKC) and protein kinase D (PKD) (which is often a downstream target of PKC) has not been examined in natural killer (NK) lymphocytes. Here we examined whether exposure of human NK cells to lysis sensitive tumor cells activated PKC and PKD. The results of these studies show for the first time that activation of PKC and PKD occurs in response to target cell binding to NK cells. Exposure of NK cells to K562 tumor cells for 10 and 30 minutes increased phosphorylation/activation of both PKC and PKD by roughly 2 fold. Butyltins (tributyltin (TBT); dibutyltin (DBT)) and brominated compounds (tetrabromobisphenol A (TBBPA)) are environmental contaminants that are found in human blood. Exposures of NK cells to TBT, DBT or TBBPA decrease NK cell lytic function in part by activating the mitogen activated protein kinases (MAPKs) that are part of the NK lytic pathway. We established that PKC and PKD are part of the lytic pathway upstream of MAPKs and thus we investigated whether DBT, TBT, and TBBPA exposures activated PKC and PKD. TBT activated PKC by 2–3 fold at 10 min at concentrations ranging from 50–300 nM while DBT caused a 1.3 fold activation at 2.5 μM at 10 min. Both TBT and DBT caused an approximately 2 fold increase in phosphorylation/activation of PKC. Exposures to TBBPA caused no statistically significant changes in either PKC or PKD activation.
Keywords: NK Cells, PKC, PKD, butyltins, brominated flame retardants
INTRODUCTION
Natural killer (NK) cells are a part of the innate immune system and lyse tumor, virally infected, and antibody-coated cells without prior sensitization (Moretta et al., 2002). NK cells are found in the bone marrow, spleen, liver, and peripheral blood and account for approximately 10 – 20% of lymphocytes in peripheral blood (Cooper et al 2001; Wu and Lanier, 2003; Lanier, 2005; Moretta et al., 2002; Vivier et al., 2004;). NK cells can stop the spread of both metastases and primary tumors (Hanna, 1980; Kiessling and Haller, 1978) and individuals who lack NK cells have an increased risk of developing viral infections and tumors (Biron et al, 1989; Fleisher et al, 1982; Purdy and Campbell, 2009).
The Protein kinase C (PKC) family of serine/threonine kinases plays an important role in signal transduction and the regulation of various cellular functions (Nishizuka 1995; Rosse et al., 2010). The binding of tumor cells to NK cells stimulates protein-tyrosine kinase(s) (PTK) which activate phospholipase Cγ (PLCγ) leading to production of diacylglycerol (DAG) and inositol trisphosphate (IP3) (Steele and Brahmi, 1988a; Ting et al., 1992; Whalen et al., 1993) known activators of PKC (Nishizuka 1992). PKC inhibitor and activator studies indicated that protein kinase C was part of the lytic signaling pathway (Graves et al., 1986; Procopio et al., 1989; Steele and Brahmi, 1988b). Activated PKC stimulates Ras which in turn activates Raf-1 the mitogen activated protein kinase (MAPK) kinase kinase (MAP3K) component of the lytic pathway (Pearson et al., 2001; Trotta et al., 1998). Additionally, PKC may also directly activate Raf-1, leading to MAPK activation (Ueffing et al., 1997; Xuan et al., 2005).
Protein kinase Ds (PKD)s are another family of serine/threonine kinases that regulate a variety of cellular processes, including trafficking, survival, migration, differentiation, and proliferation (Kunkel et al., 2007; Johannessen et al., 2007; Newton 1995; Rey et al., 2001; Rozengurt et al, 2005). PKD activation occurs by phosphorylation of Serine residues 744 and 748 found on its catalytic domain by PKC (Rozengurt et al., 2005). PKD activation can also be independent of PKC (Lemonnier et al, 2004). Activated PKD can activate MAPKs such as p44/42 (Hauser et al., 2001; Wong and Jin 2005) and cAMP-response element binding protein (CREB) (Johannessen et al., 2007).
Butyltins (BTs) are found in household, industrial, and agricultural products (Yamada et al., 1993). Tributyltin (TBT) has been used in wood preservation and marine antifouling paints (Kotake, 2012; Roper, 1992; Yamada et al., 1993). It is found in shower curtains, silicon baking parchments (Yamada et al., 1993), fish (Kannan et al., 1995) and drinking water (Sidiki et al., 1996) leading to human blood levels (as high as 260 nM) (Kannan et al., 1999; Loganathan et al., 2000; Whalen et al., 1999). TBT-exposed mammals show increased incidences of tumors (Wester et al., 1990) and decreased NK cell function (Ghoneum et al., 1990). Dibutyltin (DBT) has been used as a stabilizer in plastic products (Omae, 1989; Roper 1992) and in polyvinyl chloride (PVC) products used to store drinking water and other drinks (Forsyth et al., 1992a,b; Sidiki et al., 1996) as well as a deworming agent in poultry (Epstein et al., 1991). DBT has been detected in human blood (as high as 0.3 μM) (Kannan et al., 1999; Whalen et al., 1999). Thymus atrophy and pancreatitis are consequences of DBT exposure in rats (Merkord et al., 1997; Pieters et al., 1994).
Tetrabromobisphenol A (TBBPA), a flame retardant used in plastics, fabrics, and epoxy resin circuit boards (De Wit, 2002; HSDB 2001; IPCS/WHO 1995), has been found in seafood (IPCS/WHO, 1995) and drinking water (Peterman et al., 2000). A Japanese study showed serum levels of TBBPA at an average of 4.5 ng/g lipid (approximately 33.8 pM) (Nagayama et al., 2001), while a Norwegian study found serum levels of 0.24 – 0.71 ng/g lipid (approximately 1.8 – 5.3 pM) (Thomsen et al., 2002). TBBPA interferes with thyroid hormone (T4) binding to the human thyroid hormone transport protein transthyretin in vitro (Meerts et al., 2000; Hamers et al., 2006). Studies of Wistar rats have shown that TBBPA decreases the levels of the circulating thyroid hormone thyroxine (T4) while increasing triiodothyronine (T3) levels (Van der Ven et al., 2008) and TBBPA given to newborn rats caused polycystic kidney lesions (Fukuda et al., 2004).
Previously we have shown that exposures to TBT, DBT, and TBBPA decrease the lytic function of NK cells (Dudimah et al., 2007a, b; Kibakaya et al., 2009) with an accompanying activation of MAPKs (Aluoch and Whalen., 2005; Aluoch, et al., 2006; Cato et al., 2014; Odman-Ghazi et al. 2010). If MAPKs in NK cells are activated by exogenous compounds such as TBT, DBT, or TBBPA, then they could not be subsequently activated by the appropriate target, since the binding site on these enzymes would not be available to be activated. PKC is needed for the TBT-induced activation of MAPKs in that PKC inhibitors block phosphorylation of p44/42 in response to TBT in human NK cells (Abraha et al., 2010).
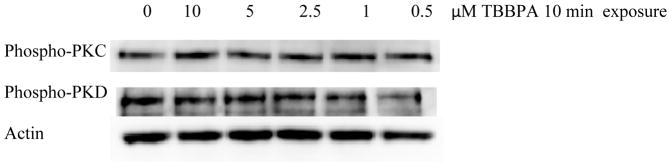
Direct activation of PKC by the physiological stimulus of target cells has not previously been demonstrated and there are to our knowledge no studies looking at target cell stimulation of PKD. The aim of the current study was to examine whether lysis-sensitive target cells activate PKC and PKD in NK cells and to determine if these kinases are activated by exposure of NK cells to the environmental contaminants, TBT, DBT, and TBBPA. These compounds were chosen as they represent different chemical classes, which have been demonstrated to activate components of the lytic signaling pathway and decrease NK lytic function (Dudimah et al., 2007a, b; Kibakaya et al., 2009; Aluoch and Whalen, 2005; Aluoch, et al., 2006; Cato et al., 2014; Odman-Ghazi et al. 2010). Neither PKC nor PKD were activated by TBBPA (Figure 1), however TBT and DBT caused activation of both kinases.
Figure 1.
Effects of exposures to TBBPA on the phosphorylation/activation state of PKC and PKD in NK cells: Representative experiment for 10 min exposures to TBBPA. Experiment was repeated using cells from seven different donors.
MATERIALS AND METHODS
Preparation of Human NK Cells
Peripheral blood (buffy coat) used in this study was from healthy adult volunteer donors (Red Cross, Portland, OR and Key Biologic LLC, Memphis, TN). Highly purified NK cells were obtained using a rosetting procedure. NK cells were purified by mixing 30–40 mL of buffy coat with 0.6 mL of RosetteSep human NK cell enrichment antibody cocktail (Stem Cell Technologies, Vancouver, BC, Canada). The mixture was mixed and incubated for 20 min at room temperature (25 °C). 7 – 9 mL of the mixture was layered onto 4 mL of Lymphosep cell separation media (1.077 g/mL; MP-Biomedicals, Santa Ana, CA) and centrifuged at 1200g for 30–50 min. The cell layer was removed and washed twice with phosphate buffered saline (PBS, pH 7.2) and stored in complete media (RPMI-1640 supplemented with 10% heat inactivated bovine calf serum (BCS), 2 mM L-glutamine and 50 U penicillin G with 50 mg streptomycin/mL) at 1 million cells/mL.
Chemical Preparation
TBT chloride was purchased from Aldrich Chemical Co. (Milwaukee, WI, USA) as a neat solution. TBT was suspended in double de-ionized water to give a 1 mM solution. This TBT solution was diluted in cell culture media to achieve the final concentrations. The concentration of TBT used in treating the cells ranged from 25 to 300 nM, based on previous studies (Whalen et al., 1999, 2002). The concentration of TBT used in the experiments is not far greater than the highest concentration that was detected in human blood (as high as 260 nM; Kannan et al., 1999; Whalen et al., 1999).
DBT dichloride was purchased from Aldrich Chemical Co. (Milwaukee, WI, USA) and is a solid at room temperature. DBT was dissolved in dimethylsulfoxide (DMSO). This DBT solution was diluted in cell culture media to achieve the final concentrations that ranged from 0.5 μM to 10 μM, based on previous studies (Whalen et al., 1999).
TBBPA was purchased from Fisher Scientific. TBBPA was dissolved in DMSO (Sigma-Aldrich, St. Louis, MO, USA) to yield a 100 mM solution. This TBBPA solution was diluted in cell culture media to achieve the concentrations used in treating the cells ranged from 0.5 μM to 10 μM. The final concentration of DMSO in any of the TBBPA exposures did not exceed 0.01%.
Cell Viability
Cell viability was determined by trypan blue exclusion before and after each exposure period. Cell numbers and their viability did not vary among experimental conditions. Cell viability was greater than 90% for control, TBT, DBT, and TBBPA treated cells.
Cell Lysates
Cell lysates for western blot were prepared from NK cells treated in the following ways: 1) NK cells (2 million per condition) were exposed to K562 (human chronic myelogenous leukemia) targets at a 12:1 ratio for 10 min, 30 min, and 1 h. Controls for the presence of target cells were done by adding the same number of targets after lysis of the NK cells to NK cells that had been incubated with media only; 2) NK cells exposed to 25–300 nM TBT or control for 10 min, 1 h, and 6 h; 3) NK cells were exposed to 0.5–10 μM DBT or control for 10 min, 1 h, and 6 h; 4) NK cells were exposed to 0.5–10 μM TBBPA or control for 10 min, 1 h, and 6 h. Following these treatments, the cells were centrifuged and washed with 1 mL of PBS. The cell pellets were then lysed using 500 μL of lysis buffer per 10 million cells. The cell lysates were stored frozen at −80° C up to the point when they were run on sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Each experiment was repeated a minimum of three times using cells from different donors.
Western Blot
Cell lysates were run on 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. The PVDF was immunoblotted with anti-phospho-PKD, anti-phospho-PKC, and anti-β-Actin antibodies (Cell Signaling Technologies, Beverly, MA, USA). Antibodies were visualized using a chemiluminescent detection system (Fisher, Atlanta GA, USA) and a Kodak Image Station or an UVP Imager (Kodak, Rochester, NY, USA; UVP, Upland CA, USA). The density of each protein band was determined by densitometric analysis using the Kodak Image Station or UVP Imager analysis software. A given experimental setup (as described in cell lysates section) always had its own internal control. Thus, differences and changes in protein expression were determined relative to the internal control. This provides relative quantitation by evaluating whether a given treatment changed expression of phospho-PKD, PKD, or phospho-PKC compared to the untreated cells. β-Actin levels were determined for each condition to verify that equal amounts of protein were loaded. The density of each protein band was normalized to β-Actin to correct for small differences in protein loading among the lanes.
Statistical Analysis
Analysis of variance (ANOVA) followed by pair-wise comparison of data was done for all western blot analyses. A minimum of three separate determinations were carried out for each western blot experiment (n≥3) and statistical significance was noted at P < 0.05.
RESULTS
Activation of PKC and PKD in NK cells stimulated with K562 target cells
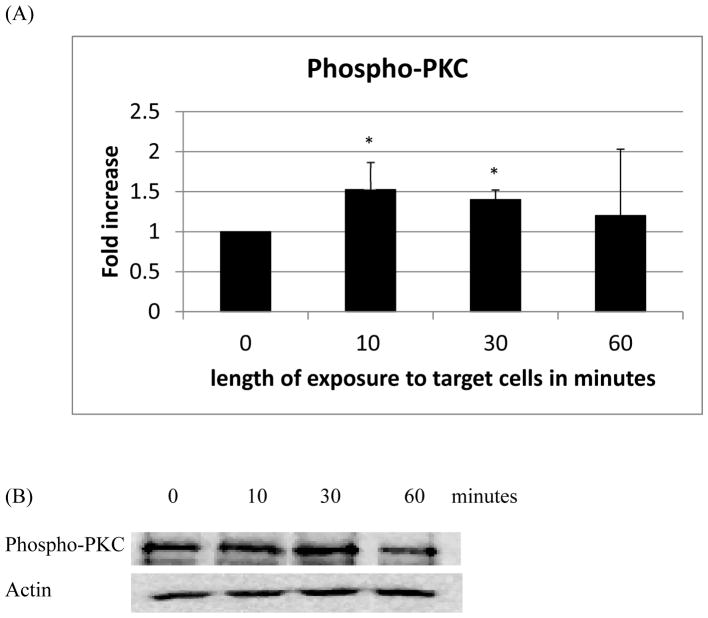
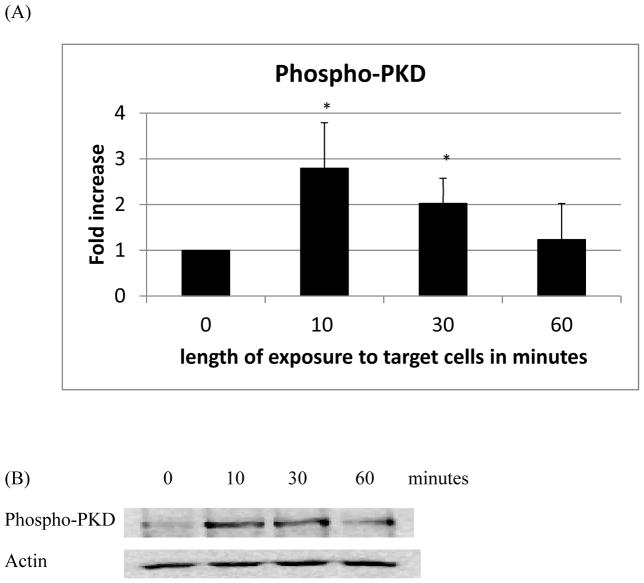
NK cells exposed to K562 tumor cells for 10 min showed increased phosphorylation of both PKC (1.5 fold, p<0.05) (Figure 2A) and PKD (2.8 fold, p<0.05) (Figure 3A) as compared to control. Exposure to K562 cells for 30 min also significantly increased phosphorylation of both PKC and PKD (Figures 2A and 3A). However, after 1 h of exposure there was no longer any significant increase in the activation of either kinase. Representative western blots are shown in Figures 2B and 3B.
Figure 2.
Effects of incubations with K562 target cells on phosphorylation/activation state of PKC in NK cells: A) levels of phospho-PKC normalized to the control. B) Representative Western blot. Experiments were repeated using cells from five different donors. Asterisks indicate a significant difference as compared with control, p<0.05.
Figure 3.
Effects of incubations with K562 target cells on phosphorylation/activation state of PKD in NK cells: A) levels of phospho-PKD normalized to the control. B) Representative Western blot. Experiments were repeated using cells from five different donors. Asterisks indicate a significant difference as compared with control, p<0.05.
TBT exposures and activation of PKC and PKD in NK Cells
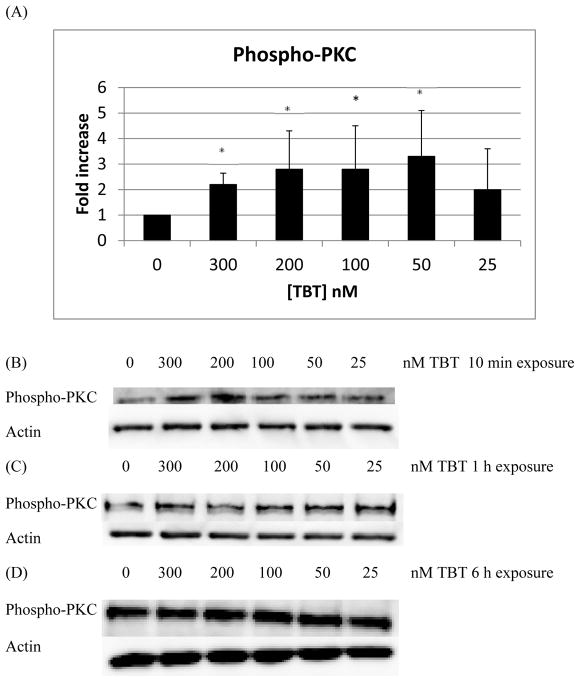
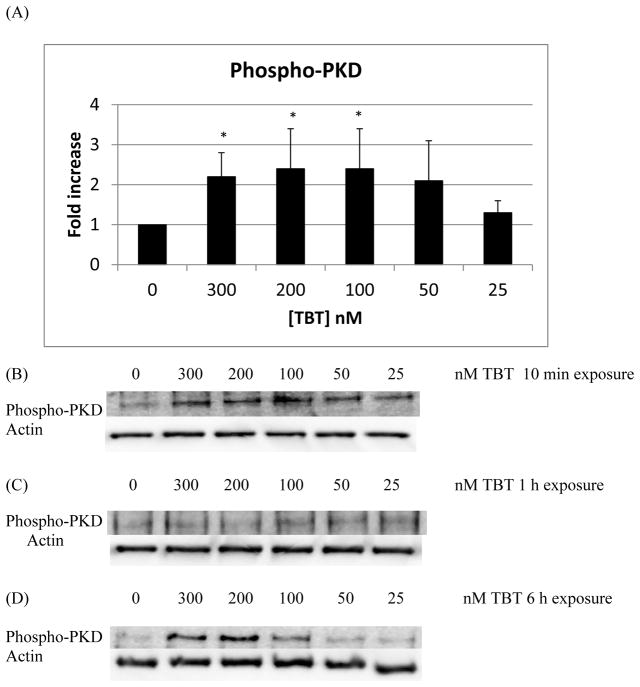
There were statistically significant increases in the phosphorylation/activation of PKC of approximately 2 to 3 fold, with respect to control, when NK cells were exposed to 300, 200, 100, and 50 nM TBT for 10 min (Figure 4A; p < 0.05). A representative western blot of the 10 min exposures to TBT is shown in Figure 4B. Exposure of NK cells to TBT for 1 h or 6 h did not result in any statistically significantly increase in the phosphorylation of PKC among the treatment conditions (representative blots Figures 4C and 4D). PKD phosphorylation/activation increased by about two fold when cells were exposed to 300, 200, and 100 nM TBT for 10 min (Figure 5A and 5B; p < 0.05), relative to control. Exposures of NK cells to TBT for 1 h (Figure 5C) or 6 h (Figure 5D) did not cause statistically significant increases in the phosphorylation of PKD. While a 6 h exposure of NK cells to TBT caused no statistically significant change in the phosphorylation of PKD, there were increases seen in PKD activation/phosphorylation seen in all donors tested. The specific concentration(s) at which an increase occurred varied from donor to donor. Thus, when experiments from all donors were combined there was no statistically significant increase seen in the activation of PKD (Figure 5D).
Figure 4.
Effects of exposures to TBT on the phosphorylation/activation state of PKC in NK cells: A) levels of phospho-PKC normalized to the control for 10 min exposures to TBT. B) Representative experiment for 10 min exposures to TBT. C) Representative experiment for 1 h exposures to TBT. D) Representative experiment for 6 h exposures to TBT. Experiments were repeated using cells from five different donors. Asterisks indicate a significant difference as compared with control, p<0.05.
Figure 5.
Effects of exposures to TBT on the phosphorylation/activation state of PKD in NK cells: A) levels of phospho-PKD normalized to the control for 10 min exposures to TBT. B) Representative experiment for 10 min exposures to TBT. C) Representative experiment for 1 h exposures to TBT. D) Representative experiment for 6 h exposures to TBT. Experiments were repeated using cells from five different donors. Asterisks indicate a significant difference as compared with control, p<0.05.
DBT exposures and activation of PKC and PKD in NK Cells
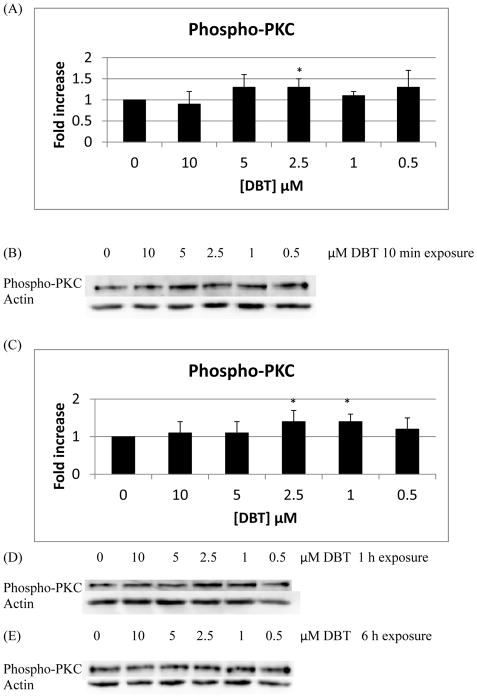
Phosphorylation of PKC was increased 1.3 fold compared to control in NK cells exposed to 2.5 μM DBT for 10 min (Figure 6A and 6B; p<0.05). 1 h exposures to 2.5 and 1 μM DBT increased phosphorylation of PKC 1.4 fold with respect to control (Figure 6C and 6D; p < 0.05). while 6 h exposures caused no significant increases in phospho-PKC (Figure 6E). Phosphorylation of PKD was increased approximately 1.4 fold (Figure 7A and 7B; P<0.05) with DBT exposure to 5 μM DBT for 10 min. Exposure of NK cells to DBT for 1 h or 6 h caused no statistically significant increase in the phospho-PKD (Figures 7C and 7D).
Figure 6.
Effects of exposures to DBT on the phosphorylation/activation state of PKC in NK cells: A) levels of phospho-PKC normalized to the control for 10 min exposures to DBT B) Representative experiment for 10 min exposures to DBT. C) levels of phospho-PKC normalized to the control for the 1 h exposures to DBT. D) Representative experiment for 1 h exposures to DBT. E) Representative experiment for 6 h exposures to DBT. Experiments were repeated using cells from six different donors for 10 min and 1h and form three different donors for 6 h. Asterisks indicate a significant difference as compared with control, p<0.05.
Figure 7.
Effects of exposures to DBT on the phosphorylation/activation state of PKD in NK cells: A) levels of phospho-PKD normalized to the control for 10 min exposures to DBT. B) Representative experiment for 10 min exposures to DBT. C) Representative experiment for 1 h exposures to DBT. D) Representative experiment for 6 h exposures to DBT. Experiments were repeated using cells from five different donors (10 min), six different donors (1 h) and three different donors (6 h). Asterisks indicate a significant difference as compared with control, p<0.05.
DISCUSSION
The current study investigated whether exposure of NK cells to the physiological stimulus of tumor target cells resulted in activation of PKC and PKD. Additionally it addressed whether these two important kinases were activated when NK cells were exposed to the environmental toxicants, TBT, DBT, and TBBPA. We found that PKC is activated within 10 minutes of exposing NK cells to target cells, such an activation had not been previously demonstrated. Additionally, PKD, a known substrate for certain PKCs, was also activated by NK interaction with target cells. These data suggest that activation of PKD, most likely due to activation of PKC, is a component of the lytic pathway activated in NK cells by interaction with their targets. To our knowledge, activation of NK cell PKD by targets has not previously been shown.
Previous studies have shown that butyltin (TBT and DBT) and brominated flame retardant (TBBPA) compounds, known to contaminate human tissues (Whalen et al., 1999; Kannan et al., 1999; Nagayama et al., 2001; Thomsen et al., 2002) are able to interfere with the lytic function of human NK cells (Dudimah et al., 2007a, b; Kibakaya et al., 2009). Further, the loss of lytic function appears to be at least in part due to the ability of these compounds to activate MAPK activity in the NK cell (Aluoch and Whalen 2005; Aluoch et al., 2006; Odman-Ghazi et al., 2010; Cato et al., 2014). The activation of MAPKs by the compounds leaves the NK cell unable to activate MAPKs in response to a subsequent encounter with an appropriate target cell.
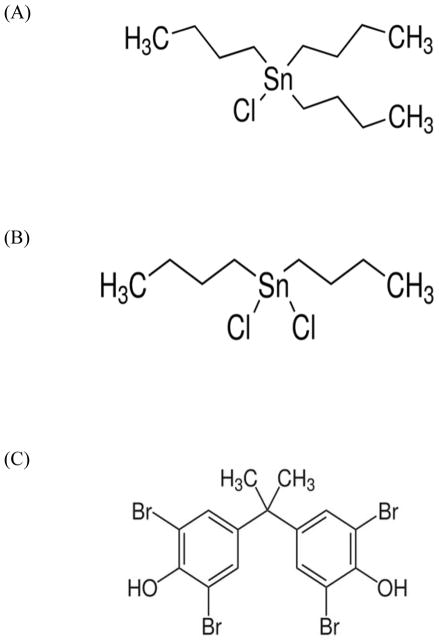
Here we show that both PKC and PKD are activated by the butyltin compounds (BTs) but not by the brominated flame retardant, TBBPA. Structurally, BTs and TBBPA differ considerably (Figure 8A,B,C). All of these compounds are quite hydrophobic. Octanol-water partition coefficients (Kow) for TBT range from 5000–7000 (Laughlin et al., 1986) while the Kow of DBT is about 10–1300 (Harper, 2005). The differences in hydrophobicity between TBT and DBT are quite significant and are due to the loss of the third butyl group. TBBPA has a wide range of measured Kow (1584–2,511,866) at neutral pH (ECB, 2006). TBBPA has some structural similarity to thyroid hormones and has been shown to compete with thyroid hormone binding (T4) to transthyretin (Meerts et al., 2000; Hamers et al., 2006). TBBPA may also increase serum estrogen levels due to its ability to compete with estrogen for the glucuoronyltransferases and sulfotransferases needed for estrogen elimination (Lai et al., 2015). BTs have no such structural similarity to T4 (Kotake, 2012). However, TBT binds to the retinoid X receptor with high affinity and acts as agonist for this nuclear receptor/transcription regulator (le Maire et al., 2009). TBT also increases the activity of aromatase (estrogen synthetase) (Nakanishi et al., 2002), while DBT has been shown to decrease ligand binding to the glucocorticoid receptor (Gumy et al., 2008). Additionally, TBT increases the phosphorylation and total of levels of the c-Jun component of the AP-1 transcription regulator (Person and Whalen, 2010). Thus, each of these compounds affects the status of transcriptional regulators. Activation of PKC (and possibly PKD) by TBT as demonstrated here may lead to the observed activation of MAPKs (Aluoch et al. 2006) resulting in alteration of AP-1 regulated transcription. A number of functions of NK cells can be regulated by MAPK controlled transcription regulators such as AP-1 including synthesis of the cytolytic protein, granzyme (Hanson et al., 1993), and the cytokine, interferon gamma (Samten et al., 2008).
Figure 8.
Structures of A) Tributytin chloride, B) Dibutytin dichloride, and C) Tetrabromobisphenol A.
The data in the current study indicate that TBT exposure can cause activation of both PKC and PKD in human NK cells. It appears that TBT may be activating the MAPK pathway(s) by activating PKC and PKD. There is data in other cell types indicating that PKD is part of the upstream activation pathway for p44/42 MAPK (Johannessen et al., 2007). The most upstream component of the MAPK activation/NK lytic pathway that has previously been shown to be activated by TBT is MAP3K (Raf-1) (Celada and Whalen, 2014). The data from this study indicate that TBT activation of further upstream components of the pathway, PKC and PKD, is occurring with exposure to TBT. As mentioned earlier, the activation of MAPKs (especially p44/42) by TBT could lead to loss of lytic function (Dudimah et al. 2007a), due to the fact that they would no longer be available for activation by a subsequent encounter with target cells. As with TBT, exposures to DBT lead to activation of both PKC and PKD, even if to a lower extent. Due to its lower hydrophobicity with respect to TBT, DBT is probably less capable of penetrating cell membranes, especially because of the hydrophobic barrier of the lipid bilayer. It is also possible that DBT interacts with a larger array of intracellular targets some of which may have effects that counteract its ability to activate PKC and PKD.
TBBPA has also been shown to decrease the lytic function of human NK cells (Kibakaya et al., 2009) with accompanying activation of MAPKs (Cato et al., 2014). However, in this study we found that the potential upstream activator of the MAPK pathway(s), PKC, was not activated when NK cell were exposed to TBBPA. Additionally, PKD was not activated when NK cells were exposed to TBBPA. This is in contrast to TBT and DBT, which both activate MAPKs and also activated PKC. It is also distinct from the activating effect that TBBPA has on PKC in the immune cells (hemocytes) of marine mussels (Canesi et al., 2005). TBBPA is structurally very distinct from the two butyltins (Figure 8), and clearly interacts with a different set of target molecules (Meerts et al., 2000; Hamers et al., 2006; Lai et al., 2015) than the BTs (le Maire et al., 2009; Nakanishi et al., 2002; Gumy et al., 2008). Thus it is not surprising that it appears to utilizes a mechanism for activating the NK lytic pathway that does not include PKC and PKD activation.
In summary, the results from this study indicate: 1) Exposure of human NK cells to lysis sensitive tumor target cells increases phosphorylation/activation of both PKC and PKD within 10 minutes of exposure and this activation maintains for 30 minutes; 2) NK cell exposure to the butyltin compounds, TBT and DBT, increase phosphorylation/activation of both PKC and PKD; 3) There was no increased phosphorylation/activation of PKC and PKD in NK cells exposed to the brominated flame retardant, TBBPA, for 10 min, 1 h, and 6 h. The spurious activation by TBT and DBT of PKC and PKC, upstream components of the lytic signaling pathway in NKs, is consistent with the described MAPK pathway activation (Aluoch et al. 2006; Odman-Ghazi et al., 2010) and loss of lytic function (Dudimah et al., 2007a b) caused by BT exposures. However, the demonstrated activation of MAPKs and decreases in lytic function caused by TBBPA (Kibakaya et al., 2009; Cato et al., 2014) cannot be attributed to the activation of these upstream components of the lytic signaling pathway.
Acknowledgments
This research was supported by 5U54CA16066 from the National Institutes of Health.
Footnotes
Declaration of Interest
The Authors report no conflicts of interest. The Authors are alone responsible for the content and writing of the paper.
References
- Abraha A, Rana K, Whalen MM. Role of protein kinase C in the TBT-induced inhibition of lytic function and MAPK activation in human natural killer cells. Arch Environ Cont Toxicol. 2010;59:661–669. doi: 10.1007/s00244-010-9520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluoch AO, Whalen MM. Tributyltin-induced effects on MAP kinases p38 and p44/42 in human natural killer cell. Toxicology. 2005;209:263–277. doi: 10.1016/j.tox.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Alteration of an essential NK cell signaling pathway by low doses of tributyltin in human natural killer cells. Toxicology. 2006;224:229–237. doi: 10.1016/j.tox.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpes virus in an adolescent without natural killer cells. New Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Canesi L, Lorusso LC, Ciacci C, Betti M, Gallo G. Effects of the brominated flame retardant tetrabromobisphenol-A (TBBPA) on cell signaling and function of Mytilus hemocytes: Involvement of MAP kinases and protein kinase C. Aquatic Toxicol. 2005;75:277–287. doi: 10.1016/j.aquatox.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Cato A, Celada L, Kibakaya EC, Simmons N, Whalen MM. Brominated Flame Retardants, Tetrabromobisphenol A and Hexabromocyclododecane activate mitogen-activated protein kinases (MAPKs) in human natrual killer cells. Cell Biology and Toxicology. 2014;30:345–360. doi: 10.1007/s10565-014-9289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada LJ, Whalen MM. Effects of Butyltins (BTs) on Mitogen-Activated-Protein Kinase Kinase Kinase (MAP3K) and Ras Activity in Human Natural Killer Cells. Journal of Appl Toxicol. 2014;34:1002–1011. doi: 10.1002/jat.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell susets. Trends in Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- De Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Odman-Ghazi SO, Hatcher F, Whalen MM. Effect of tributyltin (TBT) on ATP levels in human natural killer (NK) cells. J Appl Toxicol. 2007a;27:86–94. doi: 10.1002/jat.1202. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Odman-Ghazi SO, Gibson C, Whalen MM. Effect of dibutyltin (DBT) on ATP levels in human natural killer (NK) cells. Environ Toxicol. 2007b;22:117–123. doi: 10.1002/tox.20252. [DOI] [PubMed] [Google Scholar]
- ECB. Institute for Health and Consumer Protection, European Chemicals Bureau, European Commission Joint Research Centre., 4th Priority List. Luxembourg: Office for Official Publications of the European Communities; 2006. European Union Risk Assessment Resport-2,2′,6,6′-tetrabromo-4, 4′-isoproylidenediphenol (tetrabromobisphenol-A or TBBP-A) (CAS :79-94-7) Part II-human health. [Google Scholar]
- Epstein RL, Phillippo ET, Harr R, Koscinski W, Vosco G. Organotin residue determination in poultry and turkey sample survey in the United States. J Agric Food Chem. 1991;39:917–921. [Google Scholar]
- Fleisher G, Koven N, Kamiya H, Henle W. A non-X-linked syndrome with susceptibility to severe Epstein-Bar virus infections. J Pediatr. 1982;100:727–730. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- Forsyth DS, Weber D, Barlow L. The determination of organotin compounds in fruit juices using gas chromatography-atomic absorption spectrometry. Appl Organomet Chem. 1992a;6:579–585. [Google Scholar]
- Forsyth DS, Weber D, Cldroux C. Determination of butyltin, cyclohexyltin and phenyltin compounds in beers and wines. Food Addit Comtam. 1992b;9:161–169. doi: 10.1080/02652039209374058. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Ito Y, Yamaguchi M, Mitumori K, Koizumi M, Hasegawa R, Kamata E, Ema M. Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicol Lett. 2004;150:145–155. doi: 10.1016/j.toxlet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Ghoneum M, Hussein AE, Gill G, Alfred LJ. Suppression of murine natural killer cell activity by tributyltin: In vivo and in vitro assessment. Environ Res. 1990;52:178–186. doi: 10.1016/s0013-9351(05)80252-x. [DOI] [PubMed] [Google Scholar]
- Graves SS, Bramhall J, Bonavida B. Studies on the lethal hit stage of natural killer cell- mediated cytotoxicity. I Both phorbol ester and ionophore are required for release of natural killer cytotoxicity factor (NKCF), suggesting a role for protein kinase C activity. J Immunol. 1986;137:1977–1984. [PubMed] [Google Scholar]
- Gumy C, Chandsawangbhuwana C, Dzyakanchuk AA, Kratschmar DV, Baker ME, Odermatt A. Dibutyltin disrupts glucocorticoid receptor function and impairs glucocorticoid-induced suppression of cytokine production. PloS ONE. 2008;3:e3545. doi: 10.1371/journal.pone.0003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Hanna N. Expression of metastatic potential of tumor cells in young nude mice is correlated with low levels of natural-killer cell mediated cytotoxicity. Int J Cancer. 1980;26:675–690. doi: 10.1002/ijc.2910260521. [DOI] [PubMed] [Google Scholar]
- Hanson RD, Grisolano JL, Lay TJ. Consensus AP-1 and CRE motifs upstream from the human cytotoxic serine protease B (CSP-B/CGL-1) gene synergizes to activate transcription. Blood. 1993;82:2749–2757. [PubMed] [Google Scholar]
- Harper C. United States Agency for Toxic Substances and Disease Registry. US EPA; 2005. Toxicological profile for tin and tin compounds. [PubMed] [Google Scholar]
- Hauser A, Storz P, Hubner S, Braendlin I, Martinez-Moya M. Protein kinase C mu selectively activates the mitogen-activated protein kinase (MAPK) p42 pathway. FEBS Lett. 2001;492:39–44. doi: 10.1016/s0014-5793(01)02219-0. [DOI] [PubMed] [Google Scholar]
- HSDB (Hazardous Substances Data Bank) 2, 2′, 6, 6′-Tetrabromobisphenol A. Bethesda, MD: National Library of Medicine; 2001. http://www.toxnet.nlm.nih.gov/cgi-bin/sis/search. [Google Scholar]
- IPCS/WHO (International Program on chemical Safety/World Health Organization) Environmental Health Criteria 172: Tetrabromobisphenol A and Derivatives. Geneva: World Health Organization; 1995. [Google Scholar]
- Johannessen M, Delghandi MP, Rykx A, Dragset M, Vandenheede JR, Van Lint J, Moens U. Protein Kinase D Induces Transcription through Direct Phosphorylation of the cAMP-response Element-binding Protein. J Biol Chem. 2007;282:14777–14787. doi: 10.1074/jbc.M610669200. [DOI] [PubMed] [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R. Occurrence of butyltin residues in certain foodstuffs. Bull Environ Contam Toxicol. 1995;55:510–516. doi: 10.1007/BF00196029. [DOI] [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP. Occurrence of butyltin compounds in human blood. Environ Sci Technol. 1999;33:1776–779. [Google Scholar]
- Kibakaya EC, Stephen K, Whalen MM. Tetrabromobisphenol A has immunosuppressive effects on human natural killer cells. J Immunotoxicol. 2009;6:285–292. doi: 10.3109/15476910903258260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R, Haller O. Natural killer cells in the mouse, an alternative surveillance mechanism? Contemp Top Immunobiol. 1978;8:171–200. doi: 10.1007/978-1-4684-0922-2_6. [DOI] [PubMed] [Google Scholar]
- Kotake Y. Molecular mechanisms of environmental organotin toxicity in mammals. Biol Pharm Bull. 2012;35:1876–1880. doi: 10.1248/bpb.b212017. [DOI] [PubMed] [Google Scholar]
- Kunkel MT, Toker A, Tsien RY, Newton AC. Calcium-dependent Regulation of Protein Kinase D Revealed by a Genetically Encoded Kinase Activity Reporter. J Biol Chem. 2007;282:6733–6742. doi: 10.1074/jbc.M608086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai DY, Kacew S, Dekant W. Tetrabromobisphenol A (TBBPA): Possible modes of action of toxicity and carcinogenicity in rodents. Food and Chem Toxicol. 2015;801:206–214. doi: 10.1016/j.fct.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK Cell Recognition. Annu Rev Immunol. 2005;23:224–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Laughlin RB, Guard HE, Coleman WM., III Tributyltin in seawater: Speciation and octanol-water coefficient. Environ Sci Technol. 1986;20:201–204. doi: 10.1021/es00144a016. [DOI] [PubMed] [Google Scholar]
- Le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, Bourguet W. Activation of RXR-PPAR heterodimes by organotin environmental endocrine disruptors. EMBO Rep. 2009;10:367–373. doi: 10.1038/embor.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonnier J, Ghayor C, Guicheuz J, Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J Biol Chem. 2004;279:259–264. doi: 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- Loganathan BG, Kannan K, Owen DA, Sajwan KS. Butyltin compounds in freshwater ecosystems. In: Lipnick RL, Hermens J, Jones K, Muir D, editors. In Persistent, Bioaccumulative and Toxic Chemicals. I Fate and Exposure. American Chemical Society/Oxford University Press; London: 2000. [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brower A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Merkord J, Jonas L, Weber H, Kroning G, Nizze H. Acute interstitial pancreatitis in rats induced by dibutyltin dichloride (DBDC) pathogenesis and natural cause of lesions. Pancreas. 1997;15:392–401. doi: 10.1097/00006676-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Natural killer cells: a mystery no more. Scandinavian Journal of Immunology. 2002;55:229–232. doi: 10.1046/j.1365-3083.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- Nagayama J, Takasuga T, Tsuji H. Human Levels and Trends. 4. 2001. Contamination levels of brominated flame retardants, dioxins, and organochlorine compounds in the blood of Japanese adults; pp. 218–221. [Google Scholar]
- Nakanishi T, Kohroki J, Suzuki S, Ishizaki J, Hiromori Y, Takasuga S, Itoh N, Watanabe Y, Utoguchi N, Tanaka K. Trialkyltin compounds enhance human CG secretion and aromatase activity in human placental choriocarcinoma cells. J Clin Endocrinol Metab. 2002;87:2830–2837. doi: 10.1210/jcem.87.6.8540. [DOI] [PubMed] [Google Scholar]
- Newton A. Protein Kinase C: Stucture, Function, and Regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis pf phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Odman-Ghazi SO, Abraha A, Isom ET, Whalen MM. Dibutyltin activates MAP kinases in human natural killer cells, in vitro. Cell Biology and Toxicology. 2010;26:469–479. doi: 10.1007/s10565-010-9157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omae I. J Organomettalic Chemistry Library. Vol. 21. Organotin Chemistry Elsevier; Amsterdam, Netherlands: 1989. Application of Organotin Compounds; p. 355. [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu B-E, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocrine Rev. 2001;22:153183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Person RJ, Whalen MM. Effects of butyltin exposures on MAP kinase-dependent transcription regulators in human natural killer cells. Toxicol Mech Meth. 2010;20:227–233. doi: 10.3109/15376511003746034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman PH, Orazio CE, Gale RW. Detection of tetrabromobisphenol A and formation of Brominated [13C]=bisphenol A’s in commercial drinking water stored in reusable polycarbonate containers. ACS Div Environ Chem Extended Abstr. 2000;40:431–433. [Google Scholar]
- Pieters RH, Bol M, Seinen W, Penninks AH. Cellular and molecular aspects of organotin-induced thymus atrophy. Hum Exp Toxicol. 1994;12:876–879. doi: 10.1177/096032719401301210. [DOI] [PubMed] [Google Scholar]
- Procopio AD, Paolini R, Gismondi A, Piccoli M, Adamo S, Cavallo G, Frati L, Santoni A. Effects of protein kinase C (PK-C) activators and inhibitors on human large granular lymphocytes (LGL): role of PK-C on natural killer (NK) activity. Cell Immunol. 1989;118:470–81. doi: 10.1016/0008-8749(89)90394-8. [DOI] [PubMed] [Google Scholar]
- Purdy AK, Campbell KS. Natural killer cells and cancer: regulation by the killer cell Ig-like receptors (KIR) Cancer Biol Ther. 2009;8:13–22. doi: 10.4161/cbt.8.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey O, Young SH, Cantrell D, Rozengurt E. Rapid protein kinase D translocation in response to G protein-coupled receptor activation. Dependence on protein kinase C. J Biol Chem. 2001;276:32616–32626. doi: 10.1074/jbc.M101649200. [DOI] [PubMed] [Google Scholar]
- Roper WL. Toxicological profile for tin. US Department of Health and Human Services, Agency for Toxic substances and Disease Registry; 1992. [Google Scholar]
- Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–12. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- Rozengurt E, Rey O, Waldron RT. Protein Kinase D Signaling. J Biol Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- Samten B, Townsend JC, Weis SE, Bhoumik A, Klucar P, Shams H, Barnes PF. CREB, ATF, and AP-1 transcription factors regulate IFN-γ secretion by human T cells in response to mycobacterial antigen. The Journal of Immunology. 2008;181(3):2056–2064. doi: 10.4049/jimmunol.181.3.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiki A-I, Williams DT, Carrier R, Thomas B. Pilot Study on the contamination of drinking water by organotin compounds from PVC materials. Chemoshpere. 1996;32:2389–23998. doi: 10.1016/0045-6535(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Steele TA, Brahmi Z. Phosphatidylinositol metabolism accompanies early activation events in tumor target cell-stimulated human natural killer cells. Cell Immunol. 1988a;112:402–413. doi: 10.1016/0008-8749(88)90309-7. [DOI] [PubMed] [Google Scholar]
- Steele TA, Brahmi Z. Inhibition of human natural killer cell activity by the protein kinase C inhibitor 1-(5-isoquinolinesulfonyl)-2-methylpiperazine is an early but post-binding event. J Immunol. 1988b;141:3164–3169. [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: A study on temporal trends and the role of age. Environ Sci Technol. 2002;36:1414–1418. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- Ting AT, Karnitz LM, Schoon RA, Abraham R, Leibson PJ. Fc gamma receptor activation induces the tyrosine phosphorylation of both phospholipase C (PLC)-gamma 1 and PLC-gamma 2 in natural killer cells. J Exp Med. 1992;176:1751–1755. doi: 10.1084/jem.176.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta R, Puorro KA, Paroli M, Azzoni L, Abebe B, Eisenlohr LC, Perussia B. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-regulated kinases. J Immunol. 1998;161:6648–6656. [PubMed] [Google Scholar]
- Trotta R, Puorro KA, Paroli M, Azzoni L, Abebe B, Eisenlohr LC, Perussia B. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-related kinases. J Immunol. 1998;161:6648–6656. [PubMed] [Google Scholar]
- Ueffing M, Lovrić J, Philipp A, Mischak H, Kolch W. Protein kinase C-epsilon associates with the Raf-1 kinase and induces the production of growth factors that stimulate Raf-1 activity. Oncogene. 1997;15:2921–2927. doi: 10.1038/sj.onc.1201477. [DOI] [PubMed] [Google Scholar]
- Van der Ven L, Ven de Kuil T, Verhoef A, Verwer CM, Lilienthal H, Leonards PE, Schauer UM, Canton RF, Litens S, De Jong FH, Visser TJ, Dekant W, Stern N, Hakansson H, Slob W, Van den Berg M, Vos JG, Piersma AH. Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproductive study and a subacute toxicity study. Toxicology. 2008;245(1–2):76–89. doi: 10.1016/j.tox.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Wester PW, Krajnc EI, van Leeuwen FX, Loeber JG, van der Heijden CA, Vaessen HA, Helleman PW. Chronic toxicity and carcinogenicity of bis(tri-n-butyltin)oxide (TBTO) in the rat. Food Chem Toxicol. 1990;28:179–196. doi: 10.1016/0278-6915(90)90006-9. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Doshi RN, Homma Y, Bankhurst AD. Phospholipase C activation in the cytotoxic response of human natural killer cells requires protein tyrosine kinase activity. Immunology. 1993;79:542–547. [PMC free article] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Kannan K. Immunotoxicity of environmentally relevant concentrations of butyltins on human natural killer cells in vitro. Environ Res. 1999;81:108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- Wong C, Jin ZG. Protein Kinase C-dependent Protein Kinase D Activation Modulated ERK Signal Pathway and Endothelial Cell Proliferation by Vascular Endothelial Growth Factor. J Biol Chem. 2005;280:33262–33269. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, Bolli R. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112 :1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Fuji Y, Mikami E, Kawamura N, Hayakawa J. Small-scale survey of organotin compounds in household commodities. J AOAC Int. 1993;76:436–441. [Google Scholar]