Abstract
The hippocampus is heavily involved in the learning and memory processes necessary to successfully encode environmental stimuli and representations over time. Impairment of hippocampal function is associated with numerous neuropsychiatric diseases and can lead to detriments in the quality of life. In order to take full advantage of preclinical models of these disorders, there is a need for the development of more refined measures of clinically relevant hippocampal behaviors. While arena-based navigation tasks have provided fundamental information regarding the role of the hippocampus in spatial memory, the development of automated operant variants have had mixed results. Recently, an automated touch-screen paradigm has been shown to be highly sensitive to hippocampal function in the rat and eliminated mediating strategies that arose in previous tasks. Here we show that mice with lesions encompassing the entire ventral portion of the dorsal hippocampus are impaired on pattern separation behavior using a delayed nonmatching-to-location (TUNL) adapted for mice. Lesioned mice readily acquired the task at control rates when separations were maximal and delay periods were short while decreasing separations significantly impaired lesion mice. However, in contrast to previously reported results in the rat, consistently increasing delays did not significantly impair performance in the lesion group. Presentation of a variable delay within a session significantly impaired performance in lesion mice across delay periods. The current results demonstrate the utility of a touch-screen paradigm for measuring hippocampal-dependent pattern separation in the mouse and establish the paradigm as an important platform for future studies in disease models.
Keywords: pattern separation, hippocampus, spatial and temporal memory, lesion
1. Introduction
There is a wealth of data demonstrating the involvement of the hippocampus in learning and memory processes (Deadwyler et al., 1996, Bannerman et al., 1999, Burgess et al., 2002). The ability to successfully encode and discriminate distinct environmental stimuli is essential for survival and the hippocampus has been shown to be essential for these processes across species (Scoville and Milner, 1957, Dunnett et al., 1990, Gilbert et al., 2001, Sloan et al., 2006a). Loss of hippocampal function has been associated with neuropsychiatric disease such as Alzheimer’s disease, schizophrenia and neurodevelopmental insults and can have major impacts on quality of life (Braak et al., 1993, Daenen et al., 2002, Brady et al., 2012). The development of more refined behavioral endpoints in order to increase the translational potential of data generated in animal models has become an increasing focus in biomedical research. Traditionally, studies examining insults and disease models targeting the hippocampus have relied primarily on arena-based tasks such as the radial arm and Morris water mazes. These tasks have provided important information regarding the role of the hippocampus in spatial location memory, but also have disadvantages (Xavier et al., 1999). While automated tracking and analysis can decrease experimenter demands and potential bias in these tasks, they still require a high level of motor response in the animals. Further, tasks utilizing escape from aversive environments can cause undue stress in subjects which may lead to confounded results. With the increasing use of techniques to examine and control neuronal activity, such as in vivo electrophysiology and optogenetic stimulation/inhibition, there is also an increasing need for assays that allow for easy integration with these systems.
In an attempt to address these issues, operant tasks have been developed that require both spatial and delay dependent memory. These paradigms, categorized as matching and nonmatching-to-position tasks, require an animal to respond (typically via lever press) in either a novel or familiar spatial location for reward while ignoring a non-rewarded lever. Studies examining the effects of hippocampal lesion on delay nonmatching-to-position (DNMTP) paradigms have had mixed results. While several studies have reliably shown that loss of hippocampal function impairs DNMTP (Dunnett et al., 1990, Aggleton et al., 1992, Hampson et al., 1999), others suggest that these behaviors may not require intact hippocampal functioning (Sloan et al., 2006b). It has been suggested that these conflicting results may stem from the nature of the tasks. Due to the limited number of response locations, animals can develop mediating behaviors (positioning themselves on the side where the correct choice will appear) that allow the animals to subvert the need to recall the information after a delay (Herremans et al., 1996, Chudasama and Muir, 1997, Talpos et al., 2010).
Recently, Talpos and colleagues developed an operant task designed to exclude mediating behaviors and increase dependency on hippocampal function (Talpos et al., 2010). This task, trial unique nonmatching-to-location (TUNL), is similar to the DNMTP task, but with several crucial differences. First, it utilizes a touch-screen in order to provide an array of possible locations for visual stimuli, causing a wide variety of patterns. This circumvents an animal’s ability to use mediating behaviors to “cheat” during a delay period and allows for the ability to test spatial memory rigorously in a pattern specific manner by varying the distance between the two stimuli causing gradients of impairment. Secondly, by using visual stimuli and direct response (touch) to the stimuli, it more closely models clinically relevant measures of memory currently used, such as the Cambridge Neuropsychological Test Automated Battery (CANTAB). Importantly, it was demonstrated that loss of hippocampal function via targeted lesion impaired TUNL performance in a separation and delay specific manner in rats (Talpos et al., 2010).
Given the increasing reliance on the mouse as a preclinical model of multiple neuropsychiatric and developmental disorders that can alter hippocampal function, we wished to examine the effects of hippocampal loss on pattern separation behavior using the touch-screen TUNL paradigm in mice. We found that mice with loss of hippocampal function can learn the TUNL task when task demands were sufficiently low. Additionally, we show that excitotoxic lesions of the hippocampus impair the ability to perform the TUNL task when decreased separation or variable delay conditions make the cognitive demands high. Together, these results demonstrate the involvement of the mouse hippocampus in delay-dependent memory and pattern-separation learning, as well as the utility of the touch-screen TUNL task for screening these behaviors.
2. Materials and Methods
2.1 Subjects
Male C57BL/6J mice (n=18 at beginning of pre-training) were used in this study (Jackson Labs). Mice were housed in groupings of 2 per cage in a temperature and humidity-controlled vivarium under a reverse 12 h light/dark cycle (lights off 0800 h) and tested during the dark phase. Mice were aged 6 weeks at the onset of behavioral testing. All experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee.
2.2 Operant Apparatus
All operant behavior was conducted in a chamber measuring 21.6 × 17.8 × 12.7 cm (model # ENV-307W, Med Associates, St. Albans, VT, USA) housed within a sound- and light-attenuating box (Med Associates) as previously described (Marquardt et al., 2014a, Marquardt et al., 2014b). The standard grid floor of the chamber was covered with a solid acrylic plate to facilitate ambulation. A pellet dispenser delivering 14 mg dustless pellets (#F05684, BioServ, Frenchtown, NJ, USA) into a magazine, a house-light, tone generator and an ultra-sensitive lever was located at one end of the chamber. At the opposite end of the chamber there was a touch-sensitive screen (Conclusive Solutions, Sawbridgeworth, UK) covered by a black acrylic aperture plate allowing 2 rows of 5 touch areas measuring 2.5 × 2.5 cm separated by 0.6 cm and located at a height of 1.6 cm from the floor of the chamber. Stimulus presentation in the response windows and touches were controlled and recorded by the K-Limbic Software Package (Conclusive Solutions, Sawbridgeworth, UK).
2.3 Pre-training
Mice were first slowly reduced and then maintained at 85% free-feeding body weight. Prior to testing, mice were acclimated to the 14 mg dustless pellet food reward (Bioserv, Flemington, NJ) by provision of ~10 pellets/mouse in the home cage for 3-5 days. After becoming acclimated to the reward pellets mice were then habituated to the operant chamber and eating out of the pellet magazine by being placed in the chamber for 30 min with 10 pellets available. Mice retrieving 10 pellets within 30 min were moved to a pre-training regimen. First, mice were able to obtain reward by pressing a lever within the chamber. Mice pressing and collecting 30 rewards in under 30 min were moved to touch training. In touch training, a lever press led to the presentation of a white square stimulus in 1 of the 10 response windows (spatially pseudorandomized). The stimulus remained on the screen until a response was made. Touches in the blank response window had no response. Criterion for touch training was touching, retrieving and eating 30 pellets within 30 min.
2.4 Excitotoxic Lesions of the Hippocampus
After 1 week of feeding to facilitate recovery, mice were assigned to lesion or sham groups via matched-pair random assignment. Mice were anesthetized with isoflurane and fixed in a stereotaxic apparatus (1900 Stereotaxic Alignment System, David Kopf Instruments, Tujunga, CA) as previously described (Brigman et al., 2013). A 33-gauge infusion cannula (Plastics One, Roanoke, VA) attached with polyurethane tubing to a Hamilton syringe (Hamilton, Reno NV) was directed at 6 sites bilaterally targeting the hippocampus (−1.50, −1.80 and −2.25 mm AP, ±1.00, ±1.40 and ±1.75 mm ML, −2.00, −2.00 and −2.25 mm DV to Bregma). 0.2 μL N-methyl-D-aspartate (12.5 mg/mL, Sigma-Aldrich, St. Louis, MO) or saline vehicle was infused over 5 min using a pump (GenieTouch, Kent Scientific, Torrington, CT), with the cannula left in place for an additional 2.5 min to allow full diffusion. On completion of the last infusion, mice were sutured, given .05 mL Diazepam (.5 mg/mL), with an additional .025 ml as needed, to control seizures and returned to their home cages. Mice were given 1 week of recovery before being returned to food restriction. Mice began TUNL testing approximately 2 weeks after completion of surgery.
2.5 Trial-Unique Nonmatching-to-Location
Following recovery mice were given pre-training reminder sessions to ensure retention of touch behavior and then tested on the TUNL paradigm adapted from that previously validated in the rat (Talpos et al., 2010). Briefly, mice lever pressed to initiate the onset of a trial. In the sample phase, 1 of the 10 response locations illuminated with a white square. The mouse was required to nose poke the illuminated square in order to complete the sample phase. Thirty-three percent of sample phase responses were rewarded to ensure motivation. After an inter-phase interval delay period (varied across experiments), mice were required to lever press a second time to initiate the choice phase. In the choice phase, the stimulus from the sample phase and a stimulus in a novel location were illuminated concurrently. A touch at the novel stimulus resulted in delivery of a food reward and comitant onset of the magazine light and a tone. A touch at the previously presented stimulus resulted in a 15 sec house light-on timeout period before a new trial could be initiated. To aid learning and extinguish position bias, incorrect responses were followed by correction trials in which the same stimulus configurations were repeated until a correct response sequence was made. Correct trials were followed by a 5 sec inter-trial interval before the next trial could be initiated by lever press. Mice were tested for 1 session daily for a maximum of 60 first presentation trials or 2 hours, whichever came first. Throughout testing all 10 response locations were utilized and touch at non-illuminated windows during any phase had no response.
2.6 Experimental Design
Immediately following successful retention, lesion and sham control mice were tested on a series of 4 TUNL problems that differed in delay between trial phases and separation between stimuli: 1) Mice were first tested on acquisition of a version of the task that minimized difficulty by utilizing a large separation and a minimum delay (Fig. 1A) for 10 consecutive daily sessions. The inter-phase interval was set at 1 sec and the separation between stimuli during the choice phase was at a maximum separation (4-5 spaces apart) throughout testing. 2) To test the effects of increasing the delay between the sample and the choice phase, mice were next tested for 10 consecutive sessions with a maximum separation and an inter-phase interval of 12 sec. 3) To test the effects of decreasing the separation on performance mice were next tested for 10 consecutive sessions on a 1 sec delay but with a decreased separation (3 spaces apart; Fig. 1A). 4) Finally, based on results in the consistent separation variant, sham and lesion mice were tested for 10 sessions on a variable delay problem in which the inter-phase interval varied pseudorandomly between 1, 12, 20 or 30 sec at maximum separation.
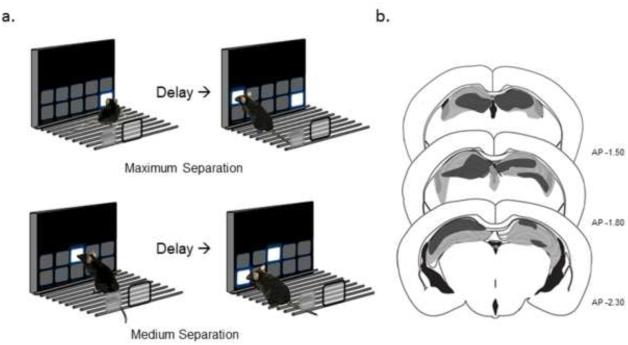
Fig. 1. TUNL behavioral paradigm and lesion reconstruction.
(a) Representation of the touchscreen operant TUNL task for mice. Separation between stimuli and delay period can be readily varied between maximum (≥4 spaces apart, top) versus medium (3 spaces apart, bottom) separations. (b) Coronal sections representing the maximum (gray) and minimum (black) extent of excitotoxic hippocampal lesions.
2.7 Histology
At the completion of testing mice were heavily anesthetized with ~400mg/kg Ketamine and transcardially perfused with 20 ml of 4% paraformaldehyde. Brains were removed and placed in 4% paraformaldehyde for 48 hours to increase fixation. Fifty μm coronal sections were cut with a vibratome (Classic 1000 model, Vibratome, Bannockburn, IL) and stained with cresyl violet. Lesion extent was quantified using a reference to a mouse brain atlas (Paxinos and Franklin, 2001) and the aid of a microscope. The maximum and minimum extent of lesions are reconstructed in Fig. 1B. Lesions primarily encompassed the ventral portion (dentate gyrus (DG) and CA3 regions) while also encompassing the dorsal (CA2 and CA1) regions of maximal lesion animals. One lesion animal was excluded from analysis due to a complete lack of lesion in one hemisphere and one sham control was excluded due to evidence of extensive tissue damage during sham surgery leaving 8 mice per treatment in the analysis.
2.8 Statistical Analysis
The following dependent measures were taken during each phase of TUNL testing: Accuracy (correct responses/first presentation trials attempted), Correction Trials, Sample Phase Response Latency (time from trial initiation to touch-screen response), Choice Phase Response Latency (time from initiation of choice phase to touch-screen response), and Reward Latency (time from correct choice phase touch to reward retrieval). Individual sessions were organized in blocks (2 sessions per block) main effects of session, treatment (lesion vs. sham), delay (when applicable) and interactions were compared for all measures using analysis of variance (ANOVA).
3. Results
3.1 Initial Acquisition of TUNL Task at maximal separation
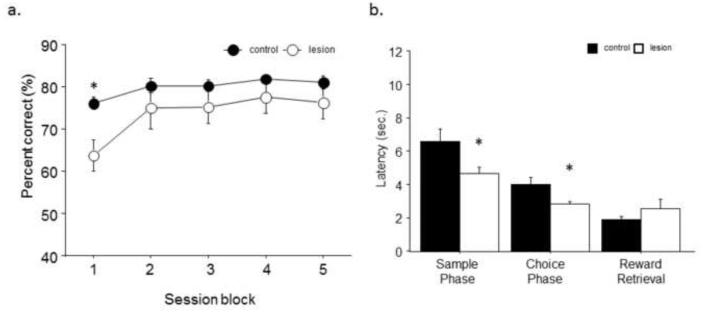
All mice were able to re-attain chance criterion within 2 sessions with no significant differences between lesion (1.78±.14) and sham (1.56 ±.18) groups. Similarly, no significant difference was seen in session completion as measured by average trials omitted for lesion (3.8±1.1) and sham (4.08±1.7) animals. Analysis of initial TUNL performance at maximum separation and 1 sec delay revealed no significant main effect of treatment (F1,1=2.77; p=.12). However there was a significant main effect of session block (F1,4=15.17; p<.01) and a significant interaction (F1,14=2.84; p<.05). Post hoc tests revealed that lesion mice had significantly reduced accuracy in session bin 1 but were equivalent to sham control thereafter (Fig. 2A). Analysis of secondary response measures revealed that lesion mice had significantly faster response latencies during both the sample (t(14)=2.23, p<.05) and choice phase (t(14)=2.60, p<.05) but no significant differences in their latency to retrieve reward after a correct response (t(14)=−1.03, p=.32; Fig. 2B).
Fig. 2. Lesion mice reach control performance with training on the maximum separation/short delay variant of TUNL.
(a) Lesion mice initially performed significantly worse than control on the initial acquisition of the TUNL with maximum separation (≥4 spaces apart) and a 1 sec delay but readily attained similar performance levels by block 2. (b) Lesion animals had significantly faster reaction times during the sample and choice phases across the problem, but no significant differences in latency to retrieve reward. n = 8/treatment. *p<.05.
3.2 Consistent Separation and Delay Alterations
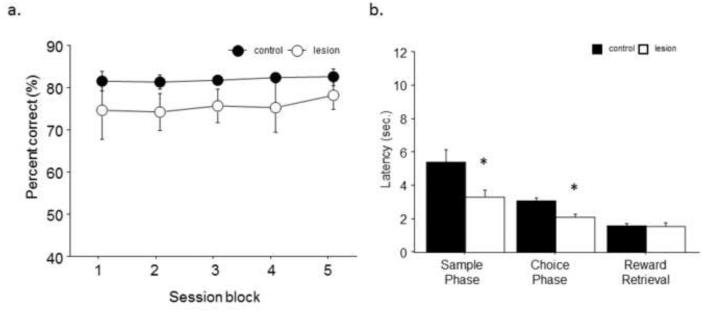
When the delay between the sample phase and the ability to initiate the choice phase was consistently increased from 1 to 12 sec, there was no significant main effect of treatment (F1,1=1.65; p>.05), session block (F1,4=.71; p=.59) and no interaction (F1,14=.24; p=.91; Fig. 3A). However, lesion mice had significantly faster response latencies during the sample (t(14)=2.50, p<.05) and choice phase (t(14)=3.67, p<.01), but no significant difference to retrieve reward after a correct response (t(14)=0.63, p=.87; Fig. 3B). Next, the separation was decreased from the maximum to medium separation with a delay of 1 sec. Consistently decreased separation significantly impaired lesion mice as shown by a significantly main effect of treatment (F1,1=15.02; p<.001). No significant main effect of session block (F1,4=2.16; p=.08) or interaction (F1,14=1.16; p=.33) was seen (Fig. 4A). Analysis of secondary response measures revealed that lesion mice had similar response latencies during the sample phase (t(14)=1.18, p=.26), but significantly faster responses during the choice phase (t(14)=2.92, p<.05). No significant difference was seen in latency to retrieve reward after a correct response (t(14)=−0.23, p=.82; Fig. 4B). In order to investigate the use of mediating behaviors we examined trials where they could be successfully applied versus those that precluded their use. Lesion mice showed impaired performance versus controls both on middle presentation sample trials (t(14)=6.18, p<.01) where positioning could not predict correct response in the choice phase and left and right sample presentation trials (t(14)=2.40, p>.05) where positioning could be effectively used. Analysis of trial type showed that mice of both treatments performed significantly better on middle presentation versus left or right presentation trials (F1,2=49.83; p<.01).
Fig. 3. Consistent increased delay between sample and choice phases did not significantly decrease accuracy of lesion or sham mice.
(a) No significant difference in accuracy seen when delay between stages was increased to 12 sec, with a constant maximum separation (≥4 spaces apart). (b) Lesion animals had significantly faster reactions times during the sample and choice phases across the problem, but no significant difference in latency to retrieve reward. n = 8/treatment. *p<.05.
Fig. 4. Decreased separation significantly impaired performance after hippocampal lesion.
(a) A consistent decreased separation (medium, 3 spaces apart, 1 sec delay) decreased accuracy of all mice and revealed a significant impairment in hippocampal lesion animals after the first 2 session blocks. (b) Lesion animals had significantly faster reaction times during the choice phase, but no significant differences in reaction times during the sample phase or latency to retrieve reward. n = 8/treatment. *p<.05.
3.3 Effects of Variable Delays
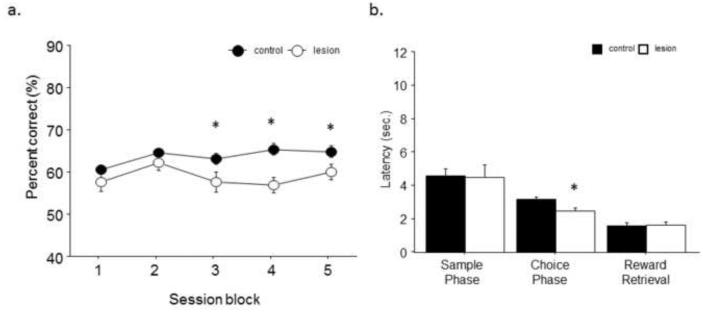
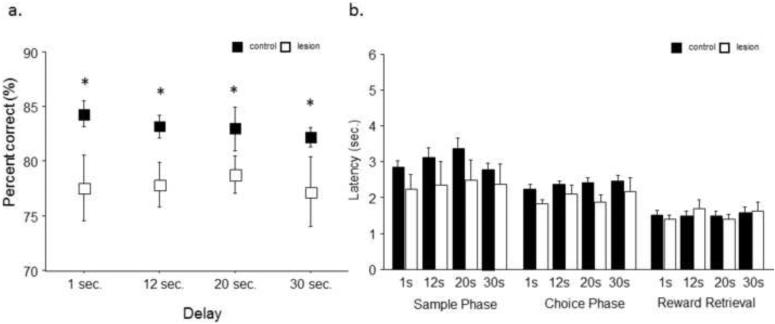
Analysis of a variable delay version of the maximum separation variant revealed that lesion mice were significantly impaired with a main effect of lesion condition (F1,4=5.82; p<.05) and no main effect of delay length (F1,4=0.28; p=.84) and no significant interaction (F1,4=.25; p=.86; Fig. 5A). Analysis revealed no main effect of treatment on response latency during the sample phase (F1,3=1.54; p=.24), choice phase (F1,1=2.51; p=.14) or their latency to retrieve reward (F1,3=.01; p=.97) after a correct response during any of the variable durations (Fig. 5B).
Fig. 5. Various delays significantly impair the accuracy of hippocampal lesion mice across delay durations.
(a) When animals were tested on an unpredictable variable delay (maximum separation of ≥4 spaces apart), hippocampal lesion mice had significantly worse accuracy across all delay periods. (b) No significant difference between lesion and control were seen on latency during sample phase, choice phase or reward retrieval on any of the 4 delay durations. n = 8/treatment. *p<.05.
4. Discussion
In the current study we show that mice with extensive hippocampal lesions were able to successfully perform the task under training conditions where cognitive load was low. When the separation distance between the stimuli was decreased in the choice phase, all mice had reduced accuracy, but lesioned animals performed significantly worse compared to control. Interestingly, this effect was not seen when the delay between phases was increased to 12 sec. Rather, hippocampal lesion mice only showed delay-dependent deficits at large separations when the delay was variable and unpredictable. Taken together, these results further support the role of the mouse hippocampus in pattern separation and suggest that mice may be insensitive to delay conditions in this paradigm compared to other species.
Numerous aspects of learning and memory have been posited to be mediated by the hippocampal formation including spatial memory, delay-dependent memory and the ability to remember and utilize information about distinct locations and experiential episodes (Morris et al., 1982, Rondi-Reig et al., 2001, Leutgeb and Leutgeb, 2007, Place et al., 2012). While our fundamental understanding of the role of the hippocampus has been constructed from results in a wide variety of spatial tasks, these paradigms have drawbacks including large footprints and corresponding motor demands, as well as different levels of stress and motivational factors. Automated operant tasks such as DNMTP, spatial discrimination and paired associates learning tasks have been developed to address these problems specifically. While helping to eliminate experimental bias and increase throughput, these tasks allow animals to use spatial mediating behavior to overcome loss of hippocampal function. As previously mentioned, due to the limited number of response options, animals can use head or body position as an egocentric cue to “cheat” across delay periods. These mediating behaviors therefore reduce the cognitive demands of the task and may obscure the involvement of the hippocampus in the task as designed.
Several aspects of the TUNL paradigm were specifically designed to moderate or eliminate these behaviors and allow for a clear picture of hippocampal involvement. First, the requirement that animals initiate the choice phase of the task in a consistent location at the rear of the chamber means that animals cannot use body position throughout the delay as a cue to the familiar location of the stimuli presented in the sample phase. More importantly, the trial-unique aspect of the task means that the novel stimuli during the choice phase may appear on either the left or right side of the familiar stimuli, greatly reducing the effectiveness of mediating behaviors across trials (Fig. 1A). In the current study we found that mice had acquisition rates analogous or faster than those seen in the rat TUNL variant (Talpos et al., 2010). Several differences between the rat and our mouse paradigm may influence the acquisition rates reported here. In our study, mice were initially trained on the easiest variant (short delay, maximum separation) in order to facilitate acquisition of task behaviors. While this method might allow for the development of mediating behaviors as seen in rats, analysis of later performance showed that these were not effectively utilized on later problems. Next, rather than initiating trial via head entry, the mouse TUNL task used in these studies required initiation via lever-press to disambiguate reward-seeking from stimulus-seeking behaviors (Marquardt et al., 2014a, Marquardt et al., 2014b). Finally, our mouse TUNL paradigm did not use cues (magazine light or lever presentation) to signal when trial phases were available to be initiated. These alterations may have increased acquisition rates reported in the mice in two ways. First, utilizing large separations consistently during the first TUNL problem likely improved accuracy in initial learning as seen in maze-based pattern separation measures. Secondly, while alterations in initiation and phase-cues may potentially have increase task difficulty, they may have increased focus during inter-phase and inter-trial intervals, leading to better acquisition overall.
Consistent with data previously reported in the rat, hippocampal lesions in the mouse significantly impaired performance when separation distance was decreased. Lesion mice showed a deficit in acquisition of the TUNL task even during initial testing under the least demanding conditions (maximal separation, 1 sec delay). However, after several training session lesion mice were able to attain performance levels similar to those of sham controls. When the separation between the familiar and novel stimulus was reduced from maximum (4-5 spaces apart) to medium (3 spaces apart), there was a corresponding reduction in the performance in both groups, as well as a significant impairment in the hippocampal lesion versus sham surgery mice. While previous studies tested a minimum separation (adjacent space), pilot experiments revealed that similar to results reported in early rat experiments (Talpos et al., 2010), further reduction (2 space apart) in separations reduced performance in intact C57BL/6J mice to no better than chance (data not shown). Importantly, analysis of trials where the sample position (middle) precluded positioning behaviors versus those that were open to mediating behavior (far left or right) revealed that hippocampal lesion mice were impaired on both. Interestingly, both lesion and control groups showed significantly higher performance on middle position sample trials, suggesting that mice may not utilize mediating behaviors effectively, as has been reported in the rat.
The involvement of the hippocampus in the ability to separate similar but distinct spatial locations has been found across numerous studies and, as in the current study, increasing the overlap between spatial representations by decreasing the separation between locations impairs an animal’s ability to distinguish the correct option (Gilbert et al., 1998, Hampson et al., 1999, White, 2004, McTighe et al., 2009). Studies using targeted loss of function or targeting of neurogenic processes have implicated the dentate gyrus (DG), the major input from entorhinal cortex into the hippocampal formation, and its outputs in the CA3 sub-region of the hippocampus in pattern separation behavior (Kesner et al., 2004, Gilbert and Kesner, 2006, Leutgeb et al., 2007, Bakker et al., 2008, Clelland et al., 2009). Studies using extracellular recording during or after spatial exploration and re-exposure have also implicated dentate gyrus granule cells and CA3 outputs in the ability to form stable and specific representation of spatial representations, and therefore successful pattern separation performance (Leutgeb et al., 2004, Leutgeb et al., 2005, Leutgeb et al., 2007, Nakashiba et al., 2012, Neunuebel and Knierim, 2014). The current study utilized a hippocampal lesion encompassing all aspects DG and CA3 and found results consistent with previous studies showing significantly reduced performance when at decreased, and therefore more similar, separations.
In contrast to results in the rat variant of the TUNL paradigm, we found that hippocampal compromised mice were insensitive to a consistent change in delay in this paradigm. While rats with a similar hippocampal lesion extent were significantly impaired by increasing the delay to only 6 sec on the TUNL task, increasing the delay to 12 sec revealed no significant differences between groups. Importantly, this was not due to alterations in sham controls that maintained performance levels of approximately 80% across delay periods. Previous studies have consistently found that the CA1 is involved in the timing required to remember spatial representations and integrate stimuli over time both in rats and mice (Brigman et al., 2010, MacDonald et al., 2011, MacDonald et al., 2013). The lack of significant effects seen in the current study may be due to sparing of anterior CA1 regions in a subset of lesion mice (Fig. 1B). However, the lack of any performance decrease in the sham controls as delay increased is in contrast to previous DNMTP studies in the mouse that have shown that even small increases in delay can lead to significant reduction in performance (Estape and Steckler, 2001). This suggests that the lack of delay-dependent effects in the current study may be due to differences in motivational factors leading to insensitivity to delay magnitude or necessary changes to TUNL protocols required to accommodate mice. The adaptation of the TUNL task for mice required a reduction in the number of response windows from 14 in the rat (7 × 2 array) to 10 in the mouse (5 × 2 array). This was necessary in order to adapt to a smaller testing chamber while not reducing stimulus salience and ensure accessibility to all locations for the smaller species. However, it may have also reduced the cognitive demands required to remember the maximum separation location across the longer delay period. In addition, increased delay was added in the current study only after mice had been previously trained for 10 sessions on an easier variant of the task. While this was done to maximize the utility of experimental subjects, the previous training may have led to decreased sensitivity to delay as previous studies have shown that training on easy variants can improve later performance when task demands increase (Gluck and Myers, 1993). Interestingly, across all consistent variants of the task, lesion mice responded to stimuli significantly more quickly in choice stages, although they were consistently less accurate overall. Given previous results showing that hippocampal loss can lead to impulsive choice in a variety of tasks, these results suggest that an inability to properly sample stimuli prior to choice may contribute to the deficits in lesion mice (Gray and McNaughton, 1983, Rawlins et al., 1985, Chudasama et al., 2012). Further studies specifically examining attention and behavioral disinhibition could help resolve these questions.
While consistently increasing the delay failed to significantly alter performance in lesion mice, altering the delay period was found to be sensitive to hippocampal loss when the delay was variable within a session. When mice were unable to predict the required delay between task phases, sham mice showed no loss of performance, maintaining >80% correct even at a considerable delays (20 and 30 sec). In contrast, hippocampal compromised animals showed significantly impaired performance across all delay periods, even those previously shown to be insensitive (1 and 12 sec). These results suggest that, even with a substantial loss of the dorsal hippocampus, mice can still use other strategies to hold representations in memory when the time required can be estimated in this paradigm. Increasing cognitive load by pseudorandomly presenting variable delays can reveal impairments in hippocampal function that may otherwise not be apparent. Future studies are needed in order to investigate whether increased delays past 30 sec will impair performance in intact mice and reveal impairments after loss of hippocampal function.
In conclusion, we found that loss of hippocampal function spared performance of pattern separation when separations were sufficiently distinct and delays were sufficiently low. However, even a moderate decrease in separation revealed significant impairments in hippocampal compromised mice. While consistent increases in delay did not significantly reduce performance, the unpredictable nature of variable delays revealed significant impairments in lesion mice. These results support the role of the mouse hippocampus in mediating pattern separation behavior and demonstrate the utility of touch-screen paradigms for measuring hippocampal-mediated memory in the mouse. Importantly, the current results suggest that mice may be insensitive to delay periods previously shown to disrupt performance in the rat. In conclusion, the development of high-throughput hippocampal-sensitive measures that utilize a small space requirement and automatize testing procedures will greatly facilitate screening of preclinical models of hippocampal insults and also greatly ease the integration of increasingly available techniques, including in vivo electrophysiological recording, optogenetic stimulation and in vivo microscopy.
Highlights.
Validation of a high-throughput trial-unique delayed nonmatching-to-location (TUNL) task in the mouse
Decreased pattern separation distance decreases accuracy in shams and specifically impairs hippocampal lesioned mice
Variable, but not consistent, increases in delay significantly impaired performance after lesion
Demonstrate the utility of touch-screen paradigms for measuring hippocampal-dependent learning and memory across species
Acknowledgements
We are very grateful to John Talpos’ for comments on this manuscript and Shannon M. Thompson for assistance with data analysis. Supported by National Institutes of Health grant P50-AA022534-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Keith AB, Rawlins JN, Hunt PR, Sahgal A. Removal of the hippocampus and transection of the fornix produce comparable deficits on delayed non-matching to position by rats. Behavioural brain research. 1992;52:61–71. doi: 10.1016/s0166-4328(05)80325-0. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behavioral neuroscience. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. European neurology. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM, Caldwell KK. A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcohol Clin Exp Res. 2012;36:457–466. doi: 10.1111/j.1530-0277.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang ZH, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci. 2013;16:1101–U1176. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Doobay VM, Liu Y. Hippocampal-prefrontal cortical circuit mediates inhibitory response control in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:10915–10924. doi: 10.1523/JNEUROSCI.1463-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology. 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. Amygdala or ventral hippocampal lesions at two early stages of life differentially affect open field behaviour later in life; an animal model of neurodevelopmental psychopathological disorders. Behavioural brain research. 2002;131:67–78. doi: 10.1016/s0166-4328(01)00350-3. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed- nonmatch-to-sample performance in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:354–372. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Wareham AT, Torres EM. Cholinergic blockade in prefrontal cortex and hippocampus disrupts short-term memory in rats. Neuroreport. 1990;1:61–64. doi: 10.1097/00001756-199009000-00017. [DOI] [PubMed] [Google Scholar]
- Estape N, Steckler T. Effects of cholinergic manipulation on operant delayed non-matching to position performance in two inbred strains of mice. Behavioural brain research. 2001;121:39–55. doi: 10.1016/s0166-4328(00)00379-x. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behavioural brain research. 2006;169:142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Myers CE. Hippocampal mediation of stimulus representation: a computational theory. Hippocampus. 1993;3:491–516. doi: 10.1002/hipo.450030410. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neuroscience and biobehavioral reviews. 1983;7:119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Jarrard LE, Deadwyler SA. Effects of ibotenate hippocampal and extrahippocampal destruction on delayed-match and -nonmatch-to-sample behavior in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1492–1507. doi: 10.1523/JNEUROSCI.19-04-01492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans AH, Hijzen TH, Welborn PF, Olivier B, Slangen JL. Effects of infusion of cholinergic drugs into the prefrontal cortex area on delayed matching to position performance in the rat. Brain research. 1996;711:102–111. doi: 10.1016/0006-8993(95)01404-7. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Reviews in the neurosciences. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learning & memory. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Carrow S, Place R, Eichenbaum H. Distinct hippocampal time cell sequences represent odor memories in immobilized rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:14607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal "time cells" bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Saha M, Mishina M, Young JW, Brigman JL. Loss of GluN2A-containing NMDA receptors impairs extra-dimensional set-shifting. Genes, brain, and behavior. 2014a;13:611–617. doi: 10.1111/gbb.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Sigdel R, Caldwell K, Brigman JL. Prenatal ethanol exposure impairs executive function in mice into adulthood. Alcohol Clin Exp Res. 2014b;38:2962–2968. doi: 10.1111/acer.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81:416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Place R, Lykken C, Beer Z, Suh J, McHugh TJ, Tonegawa S, Eichenbaum H, Sauvage MM. NMDA signaling in CA1 mediates selectively the spatial component of episodic memory. Learning & memory. 2012;19:164–169. doi: 10.1101/lm.025254.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins JN, Feldon J, Ursin H, Gray JA. Resistance to extinction after schedules of partial delay or partial reinforcement in rats with hippocampal lesions. Experimental brain research. 1985;59:273–281. doi: 10.1007/BF00230907. [DOI] [PubMed] [Google Scholar]
- Rondi-Reig L, Libbey M, Eichenbaum H, Tonegawa S. CA1-specific N-methyl-D-aspartate receptor knockout mice are deficient in solving a nonspatial transverse patterning task. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3543–3548. doi: 10.1073/pnas.041620798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Dobrossy M, Dunnett SB. Hippocampal lesions impair performance on a conditional delayed matching and non-matching to position task in the rat. Behavioural brain research. 2006a;171:240–250. doi: 10.1016/j.bbr.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behavioural brain research. 2006b;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. Trial-unique, delayed nonmatching-to- location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem. 2010;94:341–352. doi: 10.1016/j.nlm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. The role of stimulus ambiguity and movement in spatial navigation: a multiple memory systems analysis of location discrimination. Neurobiol Learn Mem. 2004;82:216–229. doi: 10.1016/j.nlm.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Xavier GF, Oliveira-Filho FJ, Santos AM. Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: difficulties in "place strategy" because of a lack of flexibility in the use of environmental cues? Hippocampus. 1999;9:668–681. doi: 10.1002/(SICI)1098-1063(1999)9:6<668::AID-HIPO8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]