Abstract
Purpose
The purpose of this paper is to review exposure assessment issues that need to be addressed in designing and interpreting epidemiology studies of phthalates, a class of chemicals commonly used in consumer and personal care products. Specific issues include population trends in exposure, temporal reliability of a urinary metabolite measurement, and how well a single urine sample may represent longer-term exposure. The focus of this review is on seven specific phthalates: diethyl phthalate (DEP); di-n-butyl phthalate (DBP); diisobutyl phthalate (DiBP); butyl benzyl phthalate (BBzP); di(2-ethylhexyl) phthalate (DEHP); diisononyl phthalate (DiNP); and diisodecyl phthalate (DiDP).
Methods
Comprehensive literature search using multiple search strategies
Results
Since 2001, declines in population exposure to DEP, BBzP, DBP, and DEHP have been reported in the United States and Germany, but DEHP exposure has increased in China. Although the half-lives of various phthalate metabolites are relatively short (3 to 18 hours), the intraclass correlation coefficients (ICCs) for phthalate metabolites, based on spot and first morning urine samples collected over a week to several months, range from weak to moderate, with a tendency toward higher ICCs (greater temporal stability) for metabolites of the shorter-chained (DEP, DBP, DiBP and BBzP, ICCs generally 0.3 to 0.6) compared with those of the longer-chained (DEHP, DiNP, DiDP, ICCs generally 0.1 to 0.3) phthalates. Additional research on optimal approaches to addressing the issue of urine dilution in studies of associations between biomarkers and different type of health effects is needed.
Conclusions
In conclusion, the measurement of urinary metabolite concentrations in urine could serve as a valuable approach to estimating exposure to phthalates in environmental epidemiology studies. Careful consideration of the strengths and limitations of this approach when interpreting study results is required.
Keywords: Phthalates, Exposure Assessment, Environmental Epidemiology, Biomarkers, Metabolites, Trends, Temporal Reliability, Biomonitoring
1.1 INTRODUCTION
Epidemiology studies can and should play a key role in the evaluation of health effects of chemical exposures. Well-designed epidemiology studies, particularly when combined with toxicology research demonstrating biological plausibility, can be highly informative in drawing conclusions regarding risks to human health. Most human studies of environmental exposure and health outcome must rely on observational study designs, and population-based observational study designs have inherent limitations as well as inherent strengths. A systematic method for assessing and categorizing relative quality of environmental epidemiology studies that may be used in the risk assessment process is needed (LaKind et al. 2014).
One important aspect that needs to be carefully considered when critiquing and comparing environmental epidemiology studies is the exposure assessment approach used in each study. Exposure assessment has historically been considered the weakest component in most environmental epidemiology studies (Nieuwenhuijsenm 2003) due to a number of challenges that result in gaps between the exposure measure that is available for use in an epidemiology study and true biologically effective dose. These gaps are associated with varying degrees of exposure measurement error, which can be either systematic or random, and differential or non-differential with respect to the outcome of interest. Systematic error can result in biased effect estimates in either direction, whereas random error will nearly always bias effect estimates toward the null hypothesis (Armstrong 2003). Systematic measurement error can typically be traced back to deficiencies in study design, participant recruitment, sample collection, and/or laboratory analysis. In contrast, all studies are certain to have at least some degree of random measurement error due to the lack of precise measures of the biologically effective dose at the target tissue for the entire duration of the relevant exposure. Characterization of the degree and direction of measurement error (e.g., via validity and reliability studies of exposures) is necessary to account for these errors, when possible, in statistical analyses and for the proper interpretation of environmental epidemiology studies.
Depending on the study and the exposure/disease relationship of interest, an exposure assessment may involve self-report, surrogate measures, exposure modeling, and/or quantitative measurements of pollutant sources, environmental media, or biological fluids. Measurements based on exposure biomarkers that lie closer to biologically effective dose on the environmental source-to-disease continuum are in many cases thought to be associated with less measurement error, especially for chemicals like phthalates that have many sources and multiple exposure pathways and routes. However, numerous factors can impact the utility of an exposure biomarker in environmental epidemiology and these factors should be carefully considered when using epidemiology data for risk assessment purposes.
The purpose of this paper is to review exposure assessment issues within the context of epidemiological studies of phthalates. Phthalates, the diesters of 1,2-benzenedicarboxylic acid (phthalic acid), are a class of synthetic chemicals that are used widely in industrial applications. Human exposure is widespread due to the common use of phthalates in consumer and personal care products (CDC (Centers for Disease Control and Prevention) 2010). Ingestion, inhalation and dermal contact are considered important routes of exposure for the general population but exposure source, pathway and route vary by phthalate due to differences in how they are used and chemical or physical properties (Meeker et al. 2009b). Phthalates have short biologic half-lives, and are quickly excreted from the body. Most studies involving human health effects from phthalates include only a single exposure measure that may or may not reflect an individual’s long-term exposure level. Because outcomes of interest may be influenced by the timing and duration of exposure, information on the temporal variability of phthalate exposure markers is needed to interpret the results from studies using only one measurement. Temporal variability in exposure can result from changes in exposure sources, such as diet and product use, as well as from variation in xenobiotic metabolism. Therefore, an individual’s exposure level may depend on several factors, and levels may vary considerably over short time periods, such as days. Another consideration, however, is that consistent individual time-activity patterns from day to day and month to month coupled with stable microenvironmental phthalate concentrations (or stable concentrations in food) may lead to “pseudo-steady state” phthalate exposure levels over long periods of time (NRC (National Research Council) 2006).
Recent developments in analytical chemistry and epidemiology have led to an increase in the number of human studies of phthalates in the last 10–15 years. Since urinary biomarkers have been the most common method for assessing exposure in environmental epidemiology studies of phthalates to date, our aim was to review the current state of the science on the following issues relevant to determining study quality and aiding in interpretation of study results: 1) human data on phthalate metabolism patterns, known metabolites that are formed, and what portion of total exposure to the parent compound each of the commonly measured metabolites represent; 2) data on population trends in exposure over the past 10–15 years in the United States and in other countries; 3) human data on the agreement between urinary phthalate metabolite concentrations and phthalate levels measured in blood or other biologic matrices that may more closely represent target tissues of interest; 4) available data on the temporal reliability of a urinary metabolite measurement and how well a single urine sample may represent longer-term (months to a couple of years) exposure in non-occupational populations; and 5) correlations among metabolites of various phthalates, and correlations with bis-phenol A or other environmental contaminants. We also addressed additional issues related to the use of urinary biomarkers for assessing exposure to phthalates in epidemiology studies.
The focus of this review is on seven specific phthalates: 1) diethyl phthalate (DEP); 2) din-butyl phthalate (DBP); 3) diisobutyl phthalate (DiBP); 4) butyl benzyl phthalate (BBzP); 5) di(2-ethylhexyl) phthalate (DEHP); 6) diisononyl phthalate (DiNP); and 7) diisodecyl phthalate (DiDP). The nomenclature and common abbreviations for these parent compounds and metabolites commonly measured in epidemiology studies are listed in Table 1.
Table 1.
Nomenclature of Phthalates and Their Metabolites
| Parent Compound, Abbreviation(s)a |
Major Metabolite(s), Abbreviation(s)a |
||
|---|---|---|---|
| Diethyl phthalate | DEP | Mono-ethyl phthalate | MEP |
|
Dibutyl phthalate Di-n-butyl phthalate Butyl phthalate n-Butyl phthalate Bis(n-butyl) phthalate |
DBP DnBP, DnBuP |
Monobutyl phthalate Mono-n-butyl phthalate |
MBPb MnBP, MnBuP |
| Di-isobutyl phthalate | DiBP | Mono-isobutyl phthalate | MiBP, MiBuP |
|
Butyl benzyl phthalate Benzyl butyl phthalate n-butyl benzyl phthalate |
BBzP, BBP |
Mono-benzyl phthalate | MBzP, MBeP |
|
Di(2-ethylhexyl) phthalate Di-octyl phthalate Bis(2-ethylhexyl) phthalate |
DEHP, DOP |
Mono(2-ethylhexyl) phthalate Mono(2-ethyl-5-hydroxyhexyl) phthalate Mono(2-ethyl-5-oxohexyl) phthalate Mono(2-ethyl-5-carboxypentyl) phthalate |
MEHP MEHHP, 5-OH-MEHP MEOHP, 5oxo-MEHP MECPP, 5cx-MEPP |
| Di-isononyl phthalate | DiNP | Mono-iso-nonylphthalate Mono(hydroxyl-isononyl) phthalate Mono(oxoisononyl) phthalate Mono(carboxy-isooctyl) phthalate |
MiNP MHiNP, OH-MiNP, 7OH-MMeOP MOiNP, oxo-MiNP, 7oxo-MMeOP MCiOP, MCOP, cx-MiNP, 7cx-MMeHP |
| Diisodecyl phthalate | DiDP | Monocarboxyisononyl phthalate | MCNP, MCINP |
Additional synonyms shown in italics
In the CDC Biomonitoring Report, monobutyl phthalate (MBP) refers to the measurement of total DBP metabolites (i.e., MiBP + MnBP=MBP) (http://www.cdc.gov/biomonitoring/DBP_BiomonitoringSummary.html)
There are both commonalities and differences among phthalates in their uses and thus sources of exposures. All or most of these 7 phthalates are used, or have been used, as plasticizers in a variety of common industrial products and in tapes or adhesives. Many personal care products and cosmetics, such as fragrances, skin lotions, nail polish, and eye shadows, may contain some types of phthalates (e.g., DEP, DBP, DiBP) as a solvent, fixative or alcohol denaturant (ATSDR (Agency for Toxic Substances and Disease Registry) 1995; CPSC (United States Consumer Product Safety Commission) 2010c; European Chemicals Bureau 2004; Koniecki et al. 2011). DEP, DiBP and DEHP have been used as a component of food and pharmaceutical packaging (ATSDR (Agency for Toxic Substances and Disease Registry) 1995; ATSDR (Agency for Toxic Substances and Disease Registry) 2002; CPSC (United States Consumer Product Safety Commission) 2010c; ECHA (European Chemicals Agency) 2010; Kelley et al. 2012; NICNAS (National Industrial Chemicals Notification and Assessment Scheme) 2010). Other uses of phthalates have included vinyl flooring (BBzP, DiNP, DiDP) and medical tubing and devices (DEHP). Importantly, over the last decade, alternative chemicals have been increasingly substituted for polyvinyl chloride and/or certain phthalates (e.g., DEHP and DBP) in some consumer products (e.g., cosmetics and children’s toys) and medical devices (e.g., blood storage bags) (Chiellini et al. 2013; CPSC (United States Consumer Product Safety Commission) 2010b). Thus, the above descriptions may not be entirely representative of the current phthalate content of these products.
1.2 METHODS
We searched PubMed in September, 2014 for any publications between 1950 and September, 2014 using multiple search strategies designed to capture papers relevant to the objectives described previously (Supplemental Table 1). Each search strategy included the phthalates of interest (including synonyms identified from ChemIDPlus) as well as additional keywords used to narrow the scope to the topics of interest. All keyword searches combined resulted in 908 unique citations; the title and abstracts (and when necessary, full text) were reviewed to determine relevancy to any of the topics. These searches were supplemented with auxiliary strategies including review of references in the identified papers and daily PubMed alerts to identify papers published subsequent to the initial search. Each study with data relevant to any of these topics was entered into a data file, with specific data extracted pertaining to the question (e.g., sample size, population, metabolites, intraclass correlation coefficient, method of urinary dilution adjustment). These studies are discussed in the relevant subsections in Section 1.3.
1.3 RESULTS
1.3.1 Metabolism Patterns and Metabolites
Following exposure and uptake, phthalates are rapidly metabolized and excreted in urine and feces. Phthalates typically undergo phase I hydrolysis followed by phase II conjugation, but metabolism patterns can differ by phthalate (Frederiksen et al. 2007). In phase I the phthalate diester is hydrolyzed into the potentially more bioactive monoester metabolite by lipases and esterases in the intestinal epithelium, liver, blood and other tissues, and systemically distributed (Calafat et al. 2006a; Calafat et al. 2006b). The monoester metabolites then: 1) undergo phase II biotransformation, catalyzed by UGTs (uridine 5’-diphosphate glucuronosyltransferases), to form glucuronide-conjugated monoesters that are excreted in the urine (Koch et al. 2005; Silva et al. 2003); 2) go through phase I biotransformation reactions (e.g., oxidation) to form more hydrophilic (and likely less bioactive) secondary oxidized metabolites prior to glucuronidation (Albro and Moore 1974; Barr et al. 2003); and/or 3) a portion of the unconjugated (free) monoester and/or secondary metabolites may also be directly excreted in urine.
Results from human studies of phthalate excretion patterns are presented in Supplemental Table 2. The extent to which hydrolytic monoesters are further oxidized to secondary metabolites depends on the alkyl chain length of the parent compound. For the shorter chained phthalates (DEP, DBP, DiBP, BBzP), approximately 70–80% of an oral dose is excreted as the simple monoester metabolite in urine compared to less than 10% and 2% of long-chained phthalates DEHP and DiNP, respectively (Anderson et al. 2001; CPSC (United States Consumer Product Safety Commission) 2014; Koch and Angerer 2007; Koch et al. 2004; Koch et al. 2005; Koch and Calafat 2009; Koch et al. 2012). The hydrolytic monoesters of long-chained phthalates (DEHP, DiNP, or DiDP) are further metabolized to oxidative side chain products such as alcohols, ketones, and carboxylic acids. Most hydrolytic monoesters are specific to a single parent diester with the exception of, for example, MCPP, a nonspecific metabolite of several long-chained phthalates, and MBP, a major metabolite of DBP and minor metabolite of BBzP. Notably, concerning the latter, since only a small proportion of total BBzP is excreted as MBP (6%, in high exposure conditions), exposures to DBP and BBzP can be assessed independently in population studies without major confounding effects (Anderson et al. 2001). However, using hydrolytic monoesters as sole biomarkers of exposure to the longer-chained phthalates in environmental epidemiology studies will likely result in some degree of exposure misclassification (Hauser and Calafat 2005). This is because hydrolytic monoesters of longer-chained phthalates, especially phthalate isomeric mixtures such as DiNP and DiDP, represent only a minor fraction of the total urinary metabolites excreted (Hauser and Calafat 2005). For longer-chained phthalates, oxidized secondary metabolites are the primary metabolites excreted in human urine (Anderson et al. 2001; CPSC (United States Consumer Product Safety Commission) 2014; Koch et al. 2005). The biological half-lives of the various phthalate metabolites have been estimated to be between approximately 3 to 18 hours.
1.3.2 Trends in Phthalate Exposures
In response to increasing scientific research and subsequent public concern about the toxicity of phthalates, various regulatory actions directed at restricting the use of certain phthalates in consumer products, particularly those concerning infants and children, have been recommended and/or enacted in the United States and abroad (Kamrin 2009). Within the last decade, the European Commission has prohibited certain phthalates (e.g., DEHP, DBP, and BBzP) for use in cosmetics, childcare articles and toys, and food-contact applications (EU (European Union) 2004; EU (European Union) 2005; EU (European Union) 2007; Kamrin 2009). Under the Consumer Product Safety Improvement Act (CPSIA) of 2008, the United States government banned the use of DEHP, DBP, and BBzP in amounts greater than 0.1% in children’s toys and childcare articles, and placed a temporary restriction on DiNP and DiDP in toys that can be put in children’s mouths (CPSC (United States Consumer Product Safety Commission) 2010a; Kamrin 2009; NICNAS (National Industrial Chemicals Notification and Assessment Scheme) 2012). Recent changes in the composition of phthalate-containing products as a result of governmental legislation in addition to pressure from consumer advocate groups such as the Campaign for Safe Cosmetics, have ultimately reshaped the plasticizer market (Campaign for Safe Cosmetics 2015; ECHA (European Chemicals Agency) 2013; Zota et al. 2014). The varying degrees to which domestic and foreign governments have taken steps to limit use of certain phthalates in consumer products, coupled with the reformulation of products over time based on other factors, have important considerations for exposure assessment and population risk.
Time trends in various phthalate exposures have been reported using biomonitoring data from the United States (spot urine samples from a representative sample of the national population; (Zota et al. 2014)) and Germany (24-hour samples from young adults collected as part of the German Environmental Specimen Bank; (Goen et al. 2011; Wittassek et al. 2007)) (Supplemental Table 3). In the United States, the primary DEP metabolite, MEP, declined 42% from 2001 to 2010; the greater decline in adults and adolescents compared with children may reflect differences in personal care product use. BBzP metabolite concentrations also decreased in both countries. Smaller declines were seen with the DBP metabolite but, in contrast, an increasing trend in population exposure to DiBP has been seen. These trends may reflect the use of DiBP as a substitute for DBP in some products (CPSC (United States Consumer Product Safety Commission) 2010c; NICNAS (National Industrial Chemicals Notification and Assessment Scheme) 2008). DEHP metabolites concentrations declined in 2009–2010 in the United States; the decline in Germany was seen beginning in the mid-1990s. Helm (2007) found a nearly perfect correlation between daily intake of DEHP as estimated by Wittassek and colleagues (2007) and the annual industrial production of DEHP in Germany between 1988 and 2003 (Spearman’s rho, r=0.94, p<0.001). An increase in DiNP metabolites was seen in the studies from the United States and Germany, likely a result of increased use in place of the more heavily regulated DEHP (Wittassek et al. 2007; Zota et al. 2014). Jensen and colleagues (2012) observed a similar decreasing trend in DEHP metabolites and increasing trend in DiNP metabolites measured in second-trimester amniotic fluid samples collected from Danish mothers between 1980 and 1996. These patterns may not apply to other countries, however. Chen et al. (2012) used environmental monitoring data (e.g., air, water, soil samples) to examine temporal and geographic variation in phthalate exposures in China. DEHP concentrations (the major component of the total phthalate measure) in air increased from 2000 to 2010, and were higher in the area that was the center of the developing plastics producing industry.
1.3.3 Biomarkers of Phthalate Exposure
Exposure biomarkers can have the advantage of being a measure of internal dose that accounts for all routes of exposure. This can be desirable in epidemiology studies of chemicals with multiple routes and pathways of exposure, but a disadvantage is that the biomarker is typically unable to singly discern how one was exposed to the chemical and whether the exposure was to the parent compound or the metabolite itself. High cost can be another disadvantage to using exposure biomarkers in environmental epidemiology studies, particularly for chemicals like phthalates that require highly sensitive and specific methods and instrumentation.
Many different types of biological specimens have been used for assessing environmental exposure to chemicals. Phthalates or their metabolites have been measured in urine and blood (serum or plasma), as well as saliva, sweat, semen, breast milk, amniotic fluid, and umbilical cord blood. However, measured concentration and detection rates in matrices other than urine are often much lower compared to urine. The measurement of phthalate metabolites in urine is the most common exposure biomarker used in epidemiology studies, and offers many advantages over measuring the diesters or their metabolites in blood. These advantages include: ease of sample collection, larger sample volumes of urine compared with other matrices, higher concentrations of the metabolites, and reduced potential for contamination by the parent diester and subsequent formation of metabolites by enzymes present in blood (Koch and Calafat 2009). In addition, for several phthalates, including DEHP, the monoester metabolite is thought to be more biologically active than the parent diester (CDC (Centers for Disease Control and Prevention) 2010).
Moderate to strong correlations between urine and serum concentrations (i.e., correlation coefficients between 0.45 and 0.90, with most between 0.55 and 0.65) have been reported for the secondary metabolites of DEHP and for the primary metabolite of DEP (Frederiksen et al. 2010; Hines et al. 2009; Hogberg et al. 2008; Kato et al. 2004), with somewhat lower correlations (r = 0.37 and 0.39) between these matrices for metabolites of DiBP and DiNP (Frederiksen et al. 2010) (Table 2). These studies were conducted in adult women and men (general population) and in postpartum women. Oral dosing studies in humans have shown that DEHP metabolite concentrations peak in serum within several hours of exposure (after approximately 2 hours) and then rapidly decline (Koch et al. 2005; Koch et al. 2006). Reflexively, urinary metabolite concentrations rise, with almost all of the orally administered dose excreted by 24 hours after exposure (Koch et al. 2005; Koch et al. 2006). At the individual-level, significant correlations between phthalate metabolite concentrations in serum and urine collected at the same time may not be expected, but at the population-level among individuals continuously exposed to these ubiquitous chemicals, moderate to strong correlations between concentrations in these two matrices are plausible.
Table 2.
Correlations between phthalate metabolite concentrations in urine and serum
| Reference | Parent | Metabolite | Concentration (ng/mL)a | Serum |
|---|---|---|---|---|
|
Frederiksen et al. 2010b Denmark Men N=60 |
DEP | MEP | Urine median 54.5 Serum mean = 4.15, median < LOD |
0.92 |
|
DEHP |
MEHP |
Urine median 4.85 Serum median 7.88 |
0.06 |
|
| MECCP | Urine median 17.04 Serum median 0.52 |
0.56 | ||
| DINP | MCIOP | Urine median 4.26 Serum mean = 0.67, median < LOD |
0.37 | |
| DiBP | MiBP | Urine median 54.4 Serum mean = 4.15, median < LOD |
0.39 | |
|
Kato et al., 2004 United States Adults (gender not specified) N=127 |
DEHP | MEHHP | Urine median 17.4 Serum median < LOD |
0.80 |
| MEOHP | Urine median 15.6 Serum median < LOD |
0.62 | ||
|
Hines et al., 2009c United States Women, postpartum N=33 Visit 1: 2–7 weeks postpartum Visit 2: 3–4 months postpartum |
DEHP | MECCP Visit 1 |
Urine median 27.3 Serum mean 2.0 |
0.45 |
| Visit 2 | Urine median 24.8 Serum mean 2.3 |
0.62 | ||
|
Hogberg et al., 2008 Sweden Women, postpartum N=36–38 |
DEP | MEP | Urine median 35 Serum median 0.50 (only 7 samples > LOD) |
0.48 |
Results are unadjusted for creatinine or did not report if the analysis was adjusted for urine dilution
Correlations were calculated for metabolites that were detectable in ≥33% of the serum samples. MnBP and MBzP were detected in 13.3% and 10% of the serum samples, respectively.
Hines et al., 2009 also presented creatinine-adjusted concentrations; correlations were higher at Visit 1 (0.74) and lower at Visit 2 (0.56) using creatinine-adjusted compared with unadjusted results.
Additionally, one study reported a strong correlation between the primary DEP metabolite in urine and semen samples (r=0.75) (Frederiksen et al. 2010), but the concentration of metabolites of other phthalates in the semen samples were too low to support this type of analysis. Lin et al. (2011) examined metabolites measured in maternal urine samples collected during the third trimester, cord blood, and breast milk in 30 women. Weak to moderate correlations (ranging from 0.1 to 0.5) between maternal urine and cord blood metabolites of DEHP and DiNP were seen, but negative correlations were seen between urine and cord blood metabolites of the other phthalates and between urine and breast milk measures for all of the metabolites (Table 3). Although limited in number (of studies, and in most cases in sample size), these studies suggests that exposure biomarkers measured in urine samples may be reasonable indicators of certain phthalate levels in other (and potentially more biologically relevant) compartments, but that cord blood and breast milk samples may not reflect exposures during gestation.
Table 3.
Correlations between phthalate metabolite concentrations in maternal urine collected during pregnancy and concentrations in cord blood or breast milk (µg/L converted to ng/mL)
| Reference | Parent | Metabolite | Median Concentration (ng/mL) | Cord blood (r) |
Breast milk (r) |
Amniotic Fluid (r) | |
|---|---|---|---|---|---|---|---|
|
Lin et al., 2011a Taiwan Women, during pregnancy, at delivery and postpartum N=30 [Urine collected in third trimester] |
DEHP | MEHP | Urine Cord blood Breast milk |
10.46 3.02 3.60 |
0.11 | −0.10 | |
| MECPP | Urine Cord blood Breast milk |
27.91 1.28 < 0.25 |
0.53 | −0.43 | |||
| MEOHP | Urine Breast milk |
20.8 < 0.25 |
−0.43 | ||||
| Sum of 5 metabolites |
0.22 | −0.05 | |||||
| DiNP | MCIOP | Urine Cord blood |
<LOD < LOD |
0.15 | |||
| MHINP | Urine Cord blood |
<LOD <LOD |
0.30 | ||||
| Summed of 3 metabolites |
Urine | <LOD | 0.35 | ||||
| DiBP | MiBP | Urine Cord blood Breast milk |
10.32 3.44 0.50 |
−0.11 | < LOD | ||
| DBP | MnBP | Urine Cord blood Breast milk |
52.39 23.90 4.05 |
−0.01 | −0.08 | ||
| BBP | MBzP | Urine Cord blood Breast milk |
1.23 < LOD <0.25 |
−0.09 | −0.27 | ||
|
Wittassek et al., 2009a,b Germany Women at delivery (Cesarean section) N=11 |
DEHP | MEHP | Urine Amniotic fluid |
55.6 1.6 |
0.91 | ||
| DiBP | MiBP | Urine Amniotic fluid |
33.6 4.2 |
0.93 | |||
Urinary phthalate metabolite concentrations unadjusted for urinary dilution
Contamination of samples possible because urine was collected via bladder catheter into urine bag
1.3.4 Temporal Variability in Urinary Phthalate Metabolites
Because phthalates are metabolized and excreted rapidly, concentrations in a single urine sample reflect exposure to the parent compound or the metabolite itself in the preceding hours or days depending on the phthalate. Thus, there is concern when interpreting environmental epidemiology studies, specifically among non-occupational populations, as to how representative a single sample is for longer-term exposure (e.g., months to a couple of years) relevant to the health outcome of interest. In addition, most studies rely on spot urine samples due to logistical issues surrounding more time-integrated samples.
Variability in phthalate metabolite concentrations by time of day has been examined in several studies ranging in size from 8 to 267 participants (Supplemental Table 4); MEP and DEHP metabolites have been examined most often, with more limited data available for other phthalates. Preau and colleagues (2010) reported that for both MEP and MEHHP for all spot urine samples collected, the within-day variance was higher than the between-day variance contribution (to the total variance) for each adult participant (Preau et al. 2010). Results from studies investigating the effect of time of day of sample collection showed that concentrations of the DEHP metabolites tended to increase between morning and evening, while there was less consistency in the results for MEP among these studies (Cantonwine et al. 2014; Fisher et al. 2014; Meeker et al. 2012; Preau et al. 2010). These patterns likely reflect differences in the primary source of exposure to these chemicals (i.e., dietary intake and personal care products, respectively for DEHP and DEP). Fasting time may also be important to consider for DEHP (Aylward et al. 2011). In a controlled 48-hr fasting study, DEHP, DINP, and DiDP metabolite concentrations in urine declined and remained low during the fasting period, while MEP, MBzP, MBP, and MiBP concentrations followed a more cyclical pattern (Koch et al. 2013).
These issues with respect to temporal variability of phthalate metabolite concentrations over the course of a day (or in relation to time since specific types of exposures), raise questions about the optimal choice of exposure measures or sampling time in epidemiology studies. Preau et al. (2010) reported that the collection of first-morning and 24-hour urine samples did not reduce the within-individual variability in MEP or MEHHP over the course of one week compared to spot urine samples, and concluded that larger studies should devote resources to collecting multiple spot samples for each individual rather than collecting 24-hour voids. A two-stage approach to modeling phthalates in relation to health effects has been proposed, where phthalate concentrations are first standardized for time of day and other sources of variability prior to regression analysis with a health outcome (Mortamais et al. 2012). However, even though phthalate metabolite concentrations are associated with time of day at the population level, it is not likely that these concentrations would follow the same pattern or time course for every individual. Thus, whether or not this approach reduces or adds random error has not been determined.
If a spot urine sample is used, how reliable is it as a measure of the relative exposure level of an individual? A number of studies have collected repeated spot or first morning urine samples from the same individuals to assess the degree of temporal variability in those measures. The most common measure of temporal reliability is the intraclass correlation coefficient (ICC), which is the amount of between-individual variance divided by the total (between individual plus within-individual) variance. An ICC near zero represents very poor temporal reliability (i.e., poor reproducibility) in a person’s phthalate metabolite concentration over time due to high within-individual variability, whereas an ICC of 1.0 would reflect perfect temporal reliability.
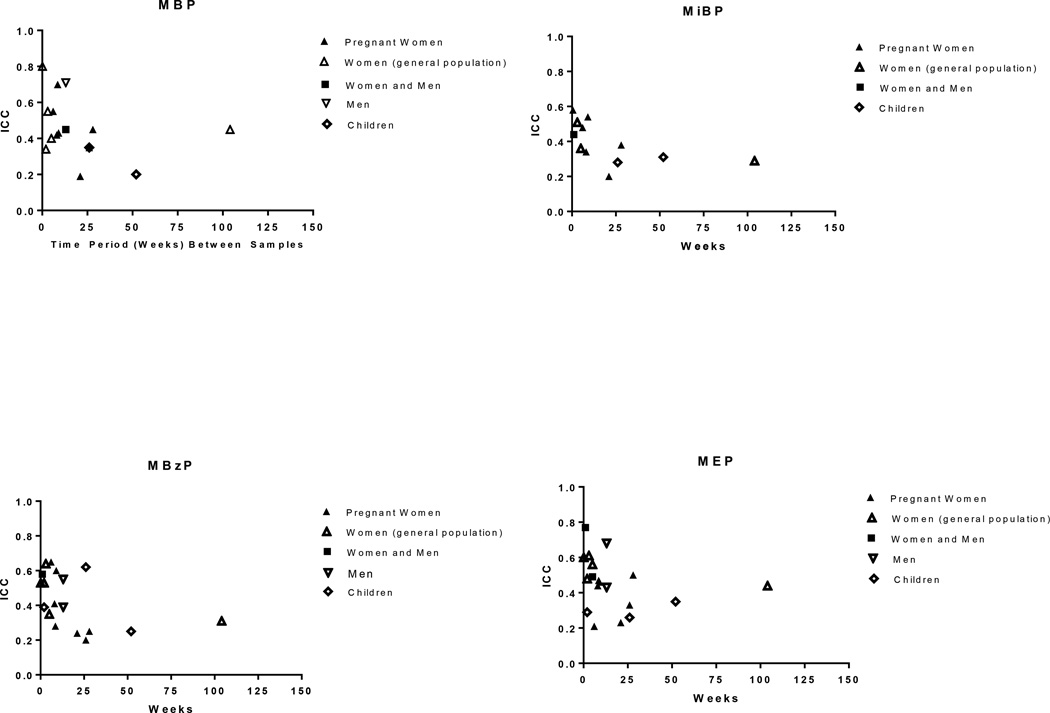
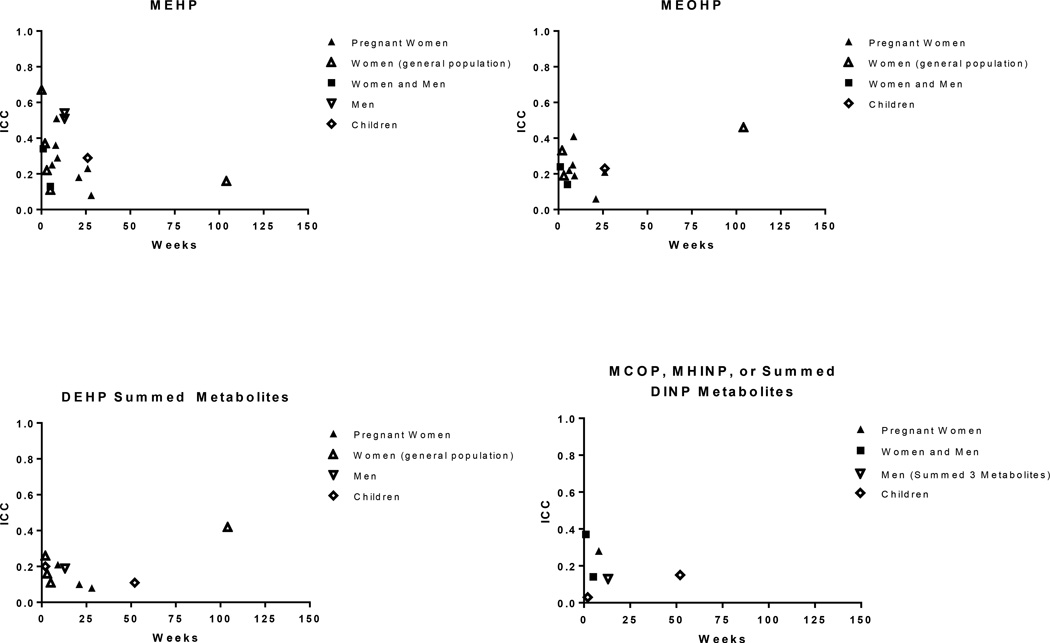
The ICCs for phthalate metabolites, based on spot and first morning urine samples collected across a week to several months, range from weak (<0.4) to moderate (0.4–0.75) (Supplemental Table 5), with a tendency toward higher ICCs (greater temporal stability) for metabolites of the shorter-chained (DEP, DBP, DiBP and BBzP, ICCs generally 0.3 to 0.6, Figure 1) compared with those of the longer-chained (DEHP, DiNP, DiDP, ICCs generally 0.1 to 0.3, Figure 2) phthalates. This pattern may reflect differences in exposure sources for these two groups, where exposure to the shorter-chained phthalates stems from sources that may be more consistent day-to-day for an individual such as use of personal care products (e.g., DEP, DBP, DiBP) and/or time spent in environments with phthalates present in building materials such as flooring (e.g., BBzP), compared to the longer-chained phthalates for which exposure is primarily through the diet. MCPP, an oxidized metabolite of di(n-octyl) phthalate (DnOP), DBP, and several long-chained phthalates (Calafat et al. 2006a) had ICCs ranging from 0.13 to 0.59.
Figure 1.
Intraclass correlation coefficients (ICC) based on repeated spot or first morning urine samples by sampling time for phthalate metabolites of DBP (MBP), DiBP (MiBP), BBzP (MBzP), and DEP (MEP).
Figure 2.
Intraclass correlation coefficients (ICC) based on repeated spot or first morning urine samples by sampling time for phthalate metabolites of DEHP (MEHP, MEOHP, and summed metabolites) and DINP (MCOP, MINP, and summed metabolites)
Only two of the studies examined ICCs over a period of time longer than 6 months (Townsend et al. 2013; Watkins et al. 2014), with most studies spanning < 3 months. There is a tendency toward higher ICCs in the studies with the shorter time interval (e.g., ≤ 4 weeks), but it is difficult to draw conclusions on long-term stability given the limited data available. The baseline collection period in the study by Townsend et al. (2013) was 2000–2001 and 1996–1999 respectively, for Nurses Health Study and Nurses Health Study II participants), with follow-up 1–3 years later; this may have been a period of relative stability in terms of phthalate-content of products, compared to the changes seen more recently. There were no clear patterns in temporal reliability when comparing results among select subgroups, such as children or pregnant women, though ICCs among children for MEP were on the low end of the range for that metabolite. A lower ICC in children could be related to differences in personal care product use, as children may have less frequent and consistent contact with DEP-containing product than adults; this may be supported by the finding that children have been shown to have lower urinary MEP concentrations compared to adults in the United States National Health and Nutrition Examination Survey (NHANES) study (CDC (Centers for Disease Control and Prevention) 2010).
Since many epidemiology studies analyze exposure as a categorical, rather than a continuous variable, several reports have also assessed the sensitivity and specificity of using one or more samples to properly categorize or rank longer-term exposure. In a study in adult men attending an infertility clinic, the sensitivity for a single sample to correctly classify the highest exposure group (top tertile) based on 3-month average metabolite concentrations ranged from 0.56 (MEHP) to 0.67 (MEP and MBP) (Hauser et al. 2004). In pregnant women living in New York City, the sensitivity for a single sample correctly predicting someone above the median based on repeated samples collected over the pregnancy was 0.50 for MiBP, and ranged from 0.60 to 0.74 for MCPP, MEP, MBP, MBzP, and metabolites of DEHP (Adibi et al. 2008). A more recent study among pregnant Canadian women reported similar sensitivities, though they were somewhat higher for several metabolites (Fisher et al. 2014). The ability for a phthalate metabolite concentration measured in a single sample to reliably rank individuals according to longer-term exposures have also been demonstrated using a surrogate categories analysis approach in women (Peck et al. 2010), children (Teitelbaum et al. 2008), and men (Hauser et al. 2004). Finally, a Korean study of elderly participants that collected up to 5 urine samples over the course of three years reported moderate to strong correlations (ranging from 0.45 to 0.79) between single MBP or summed DEHP metabolite concentrations from a single sample and the five-sample average concentration (Kim et al. 2013). While results from these sensitivity and surrogate category calculations suggest that a single sample may adequately represent one’s exposure category over several months or possibly even up to a couple of years, the measurement of phthalate metabolites in multiple urine samples from the same individual would serve to further reduce exposure misclassification.
1.3.5 Correlations Among Phthalates and Correlations with Co-Exposures
Multiple phthalates may be correlated with one another if they are used in the same applications and thus share exposure sources. This could create issues with potential confounding and in the interpretation of results for individual phthalates if exposures to the other phthalates that are correlated with the phthalate in question were not taken into consideration. We examined this question using five cycles (2003–2012) of the publically available NHANES data, a representative sample of the United States population. We also examined similar analyses in studies focusing on two specific populations (pregnant women and children) with 150 or more participants, and which explicitly delineated measures of MiBP and MBP (Table 4). Very strong correlations (i.e., r > 0.9) were seen among secondary oxidative metabolites of DEHP. In general (i.e., except for the study from China by (Wang et al. 2013), moderate correlations (e.g., r = 0.5 to 0.7) were seen between MBP and MiBP. Relatively weak correlations (e.g., r < 0.3) were seen between MEP and the DEHP metabolites, with somewhat stronger correlations (r=0.3 to 0.4) seen between MEP and MnBP, MiBP, and MBzP. The correlation between MBzP and other metabolites (MnBP, MiBP) generally ranged from 0.5 to 0.7, with lower correlations seen between MBzP and the metabolites of DEHP (general range from 0.2 to 0.5); lower correlations were seen in the study by (Ferguson et al. 2014b). Because of geographic and temporal differences in the use of products and in the phthalate content of specific products, generalization of patterns from one setting or time to others may require additional data.
Table 4.
Correlations Among Urinary Concentrations of Phthalate Metabolites
| MEP | MnBP | MiBP | MBzP | MEHP | MEOHP | MEHHP | MCOP | |
|---|---|---|---|---|---|---|---|---|
|
Adults, General Population, NHANES, United States, 2003–2012, n=8,006 men and non-pregnant women, ages 20–80 years, Spearman rho [2005–2012, n=6,661 for MCOP]a | ||||||||
| MEP | 1.0 | 0.44 | 0.34 | 0.35 | 0.27 | 0.35 | 0.35 | 0.11 |
| MnBP | 1.0 | 0.69 | 0.70 | 0.49 | 0.63 | 0.62 | 0.27 | |
| MiBP | 1.00 | 0.56 | 0.45 | 0.51 | 0.51 | 0.40 | ||
| MBzP | 1.0 | 0.43 | 0.56 | 0.56 | 0.28 | |||
| MEHP | 1.0 | 0.77 | 0.78 | 0.29 | ||||
| MEOHP | 1.0 | 0.98 | 0.36 | |||||
| MEHHP | 1.0 | 0.34 | ||||||
| MCOP | 1.0 | |||||||
| Pregnant women, Kobrosly et al., 2014; United States (4 sites), 1999–2005, n=153, ln-transformed Pearson rb | ||||||||
| MEP | 1.0 | 0.38 | 0.17 | 0.31 | 0.07 | 0.23 | 0.22 | |
| MnBP | 1.0 | 0.51 | 0.55 | 0.22 | 0.39 | 0.41 | ||
| MiBP | 1.0 | 0.46 | 0.26 | 0.30 | 0.29 | |||
| MBzP | 1.0 | 0.20 | 0.31 | 0.32 | ||||
| MEHP | 1.0 | 0.76 | 0.72 | |||||
| MEOHP | 1.0 | 0.98 | ||||||
| MEHHP | 1.0 | |||||||
| MCOP | Not measured | |||||||
| Pregnant women, Ferguson et al., 2014b; Boston, 2006–2008, n=482, Spearman rhoc | ||||||||
| MEP | 1.0 | 0.36 | 0.32 | 0.24 | 0.05 | 0.05 | 0.01 | |
| MnBP | 1.0 | 0.62 | 0.18 | 0.15 | 0.20 | 0.19 | ||
| MiBP | 1.0 | 0.46 | 0.16 | 0.21 | 0.14 | |||
| MBzP | 1.0 | 0.09 | 0.11 | 0.11 | ||||
| MEHP | 1.0 | 0.78 | 0.68 | |||||
| MEOHP | 1.0 | 0.91 | ||||||
| MEHHP | 1.0 | |||||||
| MCOP | Not measured | |||||||
|
Adolescents, General Population, NHANES, United States, 2003–2012, n=2,282 boys and girls, ages 13–19 years, Spearman rho [2005–2012, n=1,655 for MCOP]a | ||||||||
| MEP | 1.0 | 0.48 | 0.33 | 0.40 | 0.30 | 0.38 | 0.37 | 0.09 |
| MnBP | 1.0 | 0.70 | 0.74 | 0.48 | 0.62 | 0.60 | 0.31 | |
| MiBP | 1.0 | 0.58 | 0.42 | 0.46 | 0.46 | 0.41 | ||
| MBzP | 1.0 | 0.41 | 0.56 | 0.54 | 0.32 | |||
| MEHP | 1.0 | 0.79 | 0.79 | 0.36 | ||||
| MEOHP | 1.0 | 0.99 | 0.36 | |||||
| MEHHP | 1.0 | 0.34 | ||||||
| MCOP | 1.0 | |||||||
|
Children, General Population, NHANES, United States, 2003–2012, n= 2,221 boys and girls, ages 6–12 years, Spearman rho [2005–2012, n=1,794 for MCOP]a | ||||||||
| MEP | 1.0 | 0.54 | 0.44 | 0.44 | 0.39 | 0.46 | 0.46 | 0.24 |
| MnBP | 1.0 | 0.73 | 0.74 | 0.54 | 0.69 | 0.67 | 0.36 | |
| MiBP | 1.0 | 0.57 | 0.47 | 0.55 | 0.55 | 0.44 | ||
| MBzP | 1.0 | 0.45 | 0.61 | 0.59 | 0.34 | |||
| MEHP | 1.0 | 0.80 | 0.80 | 0.39 | ||||
| MEOHP | 1.0 | 0.99 | 0.42 | |||||
| MEHHP | 1.0 | 0.41 | ||||||
| MCOP | 1.0 | |||||||
| Children, Teitelbaum et al., 2012; United States (New York), 2004–2007, n=299 girls, ages 6–8 years, Spearman rhob | ||||||||
| MEP | 1.0 | 0.23 | 0.24 | 0.12 | 0.05 | 0.05 | 0.04 | |
| MnBP | 1.0 | 0.63 | 0.61 | 0.23 | 0.34 | 0.31 | ||
| MiBP | 1.0 | 0.51 | 0.16 | 0.27 | 0.25 | |||
| MBzP | 1.0 | 0.19 | 0.28 | 0.25 | ||||
| MEHP | 1.0 | 0.84 | 0.85 | |||||
| MEOHP | 1.0 | 0.99 | ||||||
| MEHHP | 1.0 | |||||||
| MCOP | Not measured | |||||||
| Children, Bertelsen et al., 2013; Norway, 2001–2004, n=623 boys and girls, age 10 years, Spearman rhoa | ||||||||
| MEP | 1.0 | 0.45 | 0.35 | 0.39 | 0.19 | 0.34 | 0.32 | 0.27 |
| MnBP | 1.0 | 0.64 | 0.55 | 0.34 | 0.51 | 0.48 | 0.36 | |
| MiBP | 1.0 | 0.50 | 0.26 | 0.55 | 0.51 | 0.39 | ||
| MBzP | 1.0 | 0.30 | 0.48 | 0.46 | 0.41 | |||
| MEHP | 1.0 | 0.62 | 0.62 | 0.27 | ||||
| MEOHP | 1.0 | 0.98 | 0.51 | |||||
| MEHHP | 1.0 | 0.50 | ||||||
| MCOP | 1.0 | |||||||
| Children, Wang et al., 2013; China, 2011, n=259 boys and girls, ages 8–15 years, ln-transformed Pearson ra | ||||||||
| MEP | 1.0 | 0.34 | 0.33 | 0.33 | 0.34 | 0.29 | ||
| MnBP | 1.0 | 0.28 | 0.43 | 0.56 | 0.46 | |||
| MiBP | 1.0 | 0.42 | 0.52 | 0.42 | ||||
| MBzP | Not measured | |||||||
| MEHP | 1.0 | 0.62 | 0.53 | |||||
| MEOHP | 1.0 | 0.93 | ||||||
| MEHHP | 1.0 | |||||||
| MCOP | Not measured | |||||||
Unadjusted for urinary dilution
Creatinine-adjusted
Specific gravity-adjusted
There is also some concern for the potential of confounding between phthalates and health outcomes if the phthalate of interest is strongly correlated to a different chemical that may be independently associated with the same outcome. One example is that of DEP, for which exposure is thought to primarily come from the use of personal care products, but which may also contain other biologically active chemicals. A limited number of studies have reported correlations coefficients ranging from zero to 0.5 between phthalates and parabens or environmental phenols in children and adult women, with the strongest correlations for MEP (Frederiksen et al. 2013; Tefre de Renzy-Martin et al. 2014). Patel et al. (2012) examined correlations among 188 blood and urine biomarkers of environmental exposures using 4 NHANES cycles. The 26 classes of compounds included phthalates, heavy metals, hydrocarbons, volatile compounds, and polychlorinated biphenyls (PCBs). The mean interclass correlation (Pearson r, creatinine-adjusted) between the phthalate metabolite and hydrocarbon measures was 0.2, with lower correlations seen between phthalates and the other classes.
1.3.6 Other Considerations for Urinary Biomarkers: Dilution Adjustment
There are a number of additional factors that need to be considered when using urinary phthalate metabolites as biomarkers of exposure. One major limitation to measuring biomarkers in spot urine samples compared to blood is that the concentration of an analyte in urine is dependent on the degree of urine dilution; samples collected from well-hydrated individuals will have a lower concentration of a given analyte per unit volume than a dehydrated individual. Several approaches to account for urinary dilution have been incorporated into exposure and epidemiology studies. Historically the most common approach to account for urine dilution is to measure the concentration of creatinine in the urine sample, and correct the mass of the analyte by the mass of creatinine from the same sample. The concentration of creatinine, a breakdown product of muscle metabolism, in urine is thought to be a relatively stable indicator of renal elimination rate (Boeniger et al. 1993). However, creatinine levels vary by gender, age, muscle mass, race, diet, activity, season, and time of day. Furthermore, dilution adjustment with creatinine is not appropriate for compounds that undergo active tubular secretion (Boeniger et al. 1993). Previous research has shown that terephthalic acid – one of three phthalic acid isomers, another being ortho-phthalic acid which is commonly referred to as “phthalates” in the literature as well as the present discussion – is both actively secreted and actively reabsorbed by the kidney (Tremaine and Quebbemann 1985). Since organic compounds such as phthalates can be conjugated by the liver in the form of glucuronides or sulfates that are subsequently actively excreted by the renal tubules, creatinine adjustment may not be appropriate for these compounds (Boeniger et al. 1993). Urinary specific gravity, in contrast, is the ratio of densities between a urine sample and pure water. Specific gravity is typically correlated with creatinine concentration, and adjusting urine metabolite concentrations to specific gravity may be susceptible to some of the same factors as creatinine, especially size, diet and sweating. However, specific gravity is not as influenced by individual factors compared to creatinine (Pearson et al. 2009; Sauve et al. 2015; Suwazono et al. 2005). The use of specific gravity over creatinine may also decrease some variability associated with urine flow (Boeniger et al. 1993; Elkins et al. 1974). Recent studies have concluded that specific gravity is superior to creatinine, demonstrated in studies using urinary biomarkers in children (Pearson et al. 2009) and in occupational cohorts (Sauve et al. 2015), based on the sources of variability in creatinine listed above, in addition to the fact that specific gravity is currently simpler and less expensive to measure than creatinine. Specific gravity is an indicator of urinary osmolality; osmolality is considered the gold-standard measurement of soluble particle measurements in urine but urine osmolality has not been widely used in epidemiology studies of phthalates. However, a recent NHANES study comparing osmolality to creatinine found a strong correlation between the two (Pearson r = 0.75) but reported that osmolality was less influenced than creatinine by socio-demographic or medical conditions (Yeh et al. 2015).
An alternative but less used method to account for urinary dilution is to collect 24-hour void samples on all participants to allow for assessment of phthalate levels on both a per-volume basis and as an excretion rate. However, this approach is often not feasible in large-scale epidemiology studies and, as described earlier, may not offer a large advantage when assessing exposure to phthalates (Preau et al. 2010). A less invasive approach to estimating flow rate is to collect a single urine sample with detailed information on total void volume and duration since previous void. Although this latter method may also be associated with feasibility and recall issues in a large-scale study, it has been incorporated into NHANES laboratory protocols since the 2009–2010 study cycle.
Creatinine-corrected (e.g., nanogram of phthalate metabolite per gram of creatinine) or specific gravity-corrected phthalate metabolite concentrations may be useful for comparing biomarker levels between studies and possibly for calculating ICC or predictors of exposure. This approach may introduce bias, however, when assessing the relationship between exposure biomarker and a health outcome since creatinine, and to a lesser extent specific-gravity, may be associated with other variables commonly associated with health outcomes of interest (e.g., age, sex, race/ethnicity, BMI). For this reason it has been recommended that studies using multiple regression analysis to estimate relationships between urinary biomarkers and a health outcome should model the urinary exposure analyte concentration (e.g., phthalate) as an independent variable in the model with urinary creatinine added as a separate independent variable, thus allowing for the exposure analyte to be appropriately adjusted for creatinine independent of other variables associated with urinary creatinine concentration (Barr et al. 2005). The same advice should also hold for other dilution correction variables such as specific gravity or osmolality.
Two recent reports have additionally called attention to the potential issue of correcting or adjusting for urinary biomarker concentrations by creatinine in studies of outcome measures that are also associated with creatinine or urinary flow rate, such as BMI, waist circumference, or obesity (Christensen et al. 2014; Hays et al. 2015). While there is still no consensus on this issue, results from studies of urinary phthalate metabolite concentrations in relation to these outcomes should be interpreted with caution. Additional research on optimal approaches to addressing the issue of urine dilution in studies of associations between biomarkers and different type of health effects are needed.
1.3.7 Free vs. Bound Metabolites
Most, if not all, epidemiologic studies to date that have utilized urinary biomarkers measured the concentration of the free plus glucuronidated species of phthalate metabolites (i.e., total concentration). However, because the metabolites are more bioactive than the parent diester, and the free form of the metabolite may be more bioactive than the glucuronidated form, it has been hypothesized that measurement of the free metabolite concentration may be a better metric of biologically effective dose (Silva et al. 2003). To our knowledge, only two studies have measured free urinary phthalate metabolites in a human population previously (Meeker et al. 2012; Silva et al. 2003). In the study by Meeker (2012) the median percentage of the total metabolite concentration present in the free form (%free) was highest for MEP (77%), followed by MCPP (59%), MCNP (58%), MCOP (47%), and MECPP (41%). The high %free for MEP was not unexpected since the more hydrophilic metabolites like MEP are more likely to be rapidly excreted in urine before undergoing phase II metabolism compared to more nonpolar and lipophilic phthalate metabolites (e.g., MEHP) (Silva et al. 2003). Free fractions of MnBP, MBzP, and MEHP were rarely detected. Thus, it was concluded in this study that unless free metabolite concentrations are demonstrated to be a superior estimate of biologically effective dose in future studies, measurement of free in addition to total metabolite concentrations in large-scale studies may not be worth the doubling of effort and expense required to obtain this information.
1.3.8 Metabolite Summary Measures
Epidemiology studies of phthalates often examine a large number of metabolites due to the multi-analyte nature of the most widely used urinary phthalate metabolite assays. Various approaches have been taken or have been proposed to reduce the number of exposure variables in these analyses. These approaches include summing metabolites of the same parent compound (Boas et al. 2010; Meeker et al. 2009a; Sathyanarayana et al. 2014; Wang et al. 2013), summing metabolites of “low molecular weight” and “high molecular weight” phthalates separately (Teitelbaum et al. 2012; Wang et al. 2013; Wolff et al. 2008), summing all measured metabolites regardless of parent chemical (Dirtu et al. 2013), or constructing global summary phthalate scores (Boas et al. 2010; Swan et al. 2005). Additionally, the National Research Council has recommended devising a summary measure for all phthalates based on observed potency each phthalate has on a specific endpoint in experimental studies (e.g., binding to a receptor) similar to the TEQ system used for dioxins (NRC (National Research Council) 2008). While each of these approaches would successfully reduce the number of statistical comparisons tested, they each have important disadvantages. All of the simple summed measures assume similar biological activities and targets for the chemicals or metabolites being summed. However, this may not be a reasonable assumption, even when summing metabolites from the same parent phthalate if the monoester itself is the most biologically active form. Likewise, even when a more nuanced approach is taken to weight the toxicities of the individual metabolites being combined based on empirical data (e.g., a TEQ method), there is an assumption that the same basis being used for the weightings represents the most sensitive endpoint for each phthalate, in addition to assumptions for additivity of effects and dose-response shapes that are identical. Another disadvantage to all of these approaches is that if associations with health endpoints are only presented with respect to a group of chemicals, the results are difficult to use in the evaluation of individual chemicals included in the summary measure. Future studies should consider modern statistical techniques to account for false positives rather than using the approaches described above, with an eye toward current methods that are not overly conservative due to assumptions of independence between all comparisons and that appropriately balance false positives and false negatives. In general, having access to results on individual chemicals and metabolites is a benefit to those individuals or groups charged with making decisions and describing the weight of evidence. The availability of such data allows the evaluation of the patterns of associations between a chemical and a health endpoint across studies, taking into account the differing research study designs, populations, and methods.
For phthalates with more complex metabolic pathways, examination of metabolites from a shared parent compound may have utility for other purposes as well. It has been proposed that the ratio of MECPP to MEHHP could be an indicator of duration since time of DEHP exposure based on the differences in half-lives between the metabolites (Lorber et al. 2011). Further, because MEHP is bioactive and subsequent phase II biotransformation produces the more hydrophilic oxidized metabolites (Barr et al. 2003; Silva et al. 2003), it has also been proposed that the ratio of MEHP to all DEHP metabolites (referred to as MEHP%) could serve as a phenotypic marker of individual susceptibility to DEHP exposure since it may represent a person’s relative efficiency to form the more hydrophilic and potentially less biologically active secondary metabolites (Hauser 2008; Meeker et al. 2012). MEHP% tends to have a higher ICC than the individual DEHP metabolites (Adibi et al. 2008; Fisher et al. 2014; Meeker et al. 2012), supporting the notion that it may be associated with individual metabolism, and there is evidence that pregnant women may have higher MEHP% compared to other populations (Cantonwine et al. 2014).
1.3.9 Additional Sources of Variability
There are a number of other potential sources of variability or error that are common to all studies that utilize biomarkers. These include a range of issues involving proper selection of sample collection, processing, storage, and analysis protocols to ensure the biomarker of choice is relevant to the question being asked, to ensure method sensitivity and selectivity, and steps to prevent and detect sample contamination. Methods for handling sample concentrations below the limit of detection in statistical analyses also need to be well thought-out; however, detection rates for most phthalates using the most common analytical method (liquid chromatography-isotope dilution-tandem mass spectrometry, LC-ID-MS/MS) are typically close to 100%, thus minimizing this issue.
1.4 DISCUSSION AND RECOMMENDATIONS
The number of epidemiology studies of phthalates which can inform and help update health risk assessments has grown rapidly in recent years. However, it is important that individual studies be interpreted in the context of their strengths and weaknesses, including how a study estimated phthalate exposure. Many of the exposure measurement issues discussed here for phthalates correspond to issues addressed in the “Biomonitoring, Environmental Epidemiology, and Short lived Chemicals” (BEES-C) instrument for studies of commonly encountered non-persistent chemicals described by (LaKind et al. 2014).
The measurement of phthalate metabolites in urine is currently the most accepted method for assessing exposure in epidemiology studies. Strengths of this approach are the wide availability of sensitive analytical methods, the specificity for most phthalate metabolites to represent a specific parent chemical, longer biological half-lives of monoester metabolites compared to the respective phthalate diester typically measured in serum and reduced likelihood of contamination or enzymatic activity compared to measuring the parent chemical or metabolites in other matrices, the availability of information on temporal variability, and evidence for correlations with phthalate concentrations in blood. Limitations of this approach include considerations of half-lives on the order of hours, the need to account for urinary dilution (as well as uncertainty as to the most appropriate method to use for urinary dilution), metabolite concentrations measured in urine which represent only an approximation for the dose at the target site, and the inability for a biomarker measure alone to provide information on route of exposure needed for risk management. Studies assessing the sensitivity and specificity suggest that a single measure may be able to reasonably predict longer-term exposure categories (i.e., up to several months, with limited data out to a couple of years) for certain phthalates. However, the collection of additional samples would improve sensitivity, and investigators should strive to conduct studies that collect multiple samples for measurement of phthalate metabolites throughout the exposure window of most relevance to the outcome of interest. Notably, the variability in phthalate exposure associated with individual behavior changes with age, coupled with the reformulation of phthalate-containing products over time, most likely preclude the ability of a single biomarker measure to approximate an individual’s exposure over the course of several decades or even a lifetime.
Since the time of day that urine samples are collected is also associated with metabolite concentrations of certain phthalates, investigators may consider collecting urine samples within carefully selected time windows during the day, if possible. In addition, since there is currently no consensus on the most appropriate method to adjust for urinary dilution, investigators should record information on duration since participant’s previous urine void and total void volume for spot samples where feasible. Since this may not be feasible in many large-scale studies, investigators should present results from sensitivity analyses that use different methods for urinary dilution adjustment with the data available.
In conclusion, the measurement of urinary metabolite concentrations in urine could serve as a valuable approach to estimating exposure to phthalates in environmental epidemiology studies. However, careful consideration of the strengths and limitations of this approach when interpreting study results is needed.
Supplementary Material
HIGHLIGHTS.
Comprehensive review of exposure assessment topics pertinent to seven phthalates.
Trends in population exposures vary by country and phthalate.
Spot urine samples may be predictive of phthalate exposure throughout one day.
Greater temporal reliability has been reported for shorter-chain phthalates.
Additional research on optimal methods for urinary dilution correction is needed.
ACKNOWLEDGEMENTS
The authors thank Dr. Krista Christensen for her review of an earlier version of this manuscript. This work is supported by grants P20ES018171, P42ES017198, R01ES018872, P30ES017885, P01ES022844, and T32ES007062, from the National Institute of Environmental Health Sciences (NIEHS) and RD834800 and RD835436 from the US Environmental Protection Agency (USEPA). The views expressed are those of the authors and do not necessarily reflect the policies of the US Environmental Protection Agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lauren E. Johns, Email: laujohns@umich.edu.
Glinda S. Cooper, Email: Cooper.Glinda@epa.gov.
Audrey Galizia, Email: Galizia.Audrey@epa.gov.
John D. Meeker, Email: meekerj@umich.edu.
REFERENCES
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ, Hauser R. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116:467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro PW, Moore B. Identification of the metabolites of simple phthalate diesters in rat urine. J Chromatogr. 1974;94:209–218. doi: 10.1016/s0021-9673(01)92368-4. [DOI] [PubMed] [Google Scholar]
- Anderson WA, Castle L, Hird S, Jeffery J, Scotter MJ. A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional excretion of primary and secondary urinary metabolites of di-2-ethylhexylphthalate and di-iso-nonylphthalate. Food Chem Toxicol. 2011;49:2022–2029. doi: 10.1016/j.fct.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Anderson WA, Castle L, Scotter MJ, Massey RC, Springall C. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit Contam. 2001;18:1068–1074. doi: 10.1080/02652030110050113. [DOI] [PubMed] [Google Scholar]
- Armstrong B. Exposure measurement error: consequences and design issues. In: Nieuwenhuijsen MJ, editor. Exposure assessment in occupational and environmental epidemiology. New York, NY: Oxford University Press; 2003. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological profile for diethyl phthalate. Atlanta, Georgia: U.S. Department of Health and Human Services Public Health Service; 1995. [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological profile for di(2-ethylhexyl) phthalate (DEHP) Atlanta, Georgia: U.S. Department of Health and Human Services Public Health Service; 2002. [PubMed] [Google Scholar]
- Aylward LL, Lorber M, Hays SM. Urinary DEHP metabolites and fasting time in NHANES. J Expo Sci Environ Epidemiol. 2011;21:615–624. doi: 10.1038/jes.2011.28. [DOI] [PubMed] [Google Scholar]
- Baird DD, Saldana TM, Nepomnaschy PA, Hoppin JA, Longnecker MP, Weinberg CR, Wilcox AJ. Within-person variability in urinary phthalate metabolite concentrations: measurements from specimens after long-term frozen storage. J Expo Sci Environ Epidemiol. 2010;20:169–175. doi: 10.1038/jes.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, Sadowski M, Needham LL, Calafat AM. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1148–1151. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen RJ, Carlsen KC, Calafat AM, Hoppin JA, Haland G, Mowinckel P, Carlsen KH, Lovik M. Urinary biomarkers for phthalates associated with asthma in Norwegian children. Environ Health Perspect. 2013;121:251–256. doi: 10.1289/ehp.1205256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedus L, Hilsted L, Juul A, Main KM. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118:1458–1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120:739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Silva MJ, Reidy JA, Earl Gray L, Samandar E, Preau JL, Herbert AR, Needham LL. Mono-(3-carboxypropyl) phthalate, a metabolite of di-n-octyl phthalate. J Toxicol Environ Health A. 2006a;69:215–227. doi: 10.1080/15287390500227381. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Silva MJ, Kuklenyik Z, Needham LL. Human exposure assessment to environmental chemicals using biomonitoring. Int J Androl. 2006b;29:166–171. doi: 10.1111/j.1365-2605.2005.00570.x. discussion 181–165. [DOI] [PubMed] [Google Scholar]
- Campaign for Safe Cosmetics. Campaign Victories and History. 2015. [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, Calafat AM, Crespo N, Jimenez-Velez B, Padilla IY, Alshawabkeh AN, Meeker JD. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int. 2014;62:1–11. doi: 10.1016/j.envint.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Fourth National Report on Human Exposure to Environmental Chemicals. Washington, D.C.: 2010. [Google Scholar]
- Chen L, Zhao Y, Li L, Chen B, Zhang Y. Exposure assessment of phthalates in non-occupational populations in China. Sci Total Environ. 2012;427–428:60–69. doi: 10.1016/j.scitotenv.2012.03.090. [DOI] [PubMed] [Google Scholar]
- Chiellini F, Ferri M, Morelli A, Dipaola L, Latini G. Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Progress in Polymer Science. 2013;38:1067–1088. [Google Scholar]
- Christensen K, Sobus J, Phillips M, Blessinger T, Lorber M, Tan YM. Changes in epidemiologic associations with different exposure metrics: a case study of phthalate exposure associations with body mass index and waist circumference. Environ Int. 2014;73:66–76. doi: 10.1016/j.envint.2014.07.010. [DOI] [PubMed] [Google Scholar]
- CPSC (United States Consumer Product Safety Commission) Overview of phthalates toxicity. Bethesda, Maryland: 2010a. [Google Scholar]
- CPSC (United States Consumer Product Safety Commission) Review of Exposure and Toxicity Data for Phthalate Substitutes. Bethesda, Maryland: 2010b. [Google Scholar]
- CPSC (United States Consumer Product Safety Commission) Toxicity Review of Diisobutyl Phthalate (DiBP) Bethesda, Maryland: 2010c. [Google Scholar]
- Sciences DfH., editor. CPSC (United States Consumer Product Safety Commission) Chronic hazard advisory panel on phthalates and phthalates alternatives. Bethesda, MD: 2014. [Google Scholar]
- Dirtu AC, Geens T, Dirinck E, Malarvannan G, Neels H, Van Gaal L, Jorens PG, Covaci A. Phthalate metabolites in obese individuals undergoing weight loss: Urinary levels and estimation of the phthalates daily intake. Environ Int. 2013;59:344–353. doi: 10.1016/j.envint.2013.06.023. [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency) Background document for diisobutyl phthalate (DiBP) 2010 [Google Scholar]
- ECHA (European Chemicals Agency) Evaluation of new scientific evidence concerning DINP and DIDP in relation to entry 52 of Annex XVII to REACH Regulation (EC) No 1907/2006. 2013 [Google Scholar]
- Elkins HB, Pagnotto LD, Smith HL. Concentration adjustments in urinalysis. Am Ind Hyg Assoc J. 1974;35:559–565. doi: 10.1080/0002889748507072. [DOI] [PubMed] [Google Scholar]
- EU (European Union) Commission Directive 2004/93/EC of 21 September 2004 Amending Council Directive 76/768/EEC for the Purpose of Adapting Its Annexes II and III to Technical Progress. Official Journal of the European Union. 2004;300:13–14. [Google Scholar]
- EU (European Union) Directive 2005/84/EC of the European Parliament and of the Council 14 December 2005 Amending for the 22nd time Council Directive 76/769/EEC on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Phthalates in Toys and Childcare Articles) Official Journal of the European Union. 2005;344:40–43. [Google Scholar]
- EU (European Union) Commission Directive 2007/19/EC of 30 March 2007 Amending Directive 2002/72/EC Relating to Plastic Materials and Articles Intended to Come into Contact with Food and Council Directive 85/572/EEC Laying Down the List of Simulants to Be Used for Testing Migration of Constituents of Plastic Materials and Articles Intended to Come into Contact with Foodstuffs. Official Journal of the European Union. 2007;91:17–36. [Google Scholar]
- European Chemicals Bureau. Dibutyl phthalate: European Union risk assessment report. 2004 [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014a;70:118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014b;168:61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Mallick R, LeBlanc A, Hauser R, Feeley M, Koniecki D, Ramsay T, Provencher G, Berube R, Walker M. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. J Expo Sci Environ Epidemiol. 2014 doi: 10.1038/jes.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Jorgensen N, Andersson AM. Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J Anal Toxicol. 2010;34:400–410. doi: 10.1093/jat/34.7.400. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Kranich SK, Jorgensen N, Taboureau O, Petersen JH, Andersson AM. Temporal variability in urinary phthalate metabolite excretion based on spot, morning, and 24-h urine samples: considerations for epidemiological studies. Environ Sci Technol. 2013;47:958–967. doi: 10.1021/es303640b. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007;51:899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- Fromme H, Bolte G, Koch HM, Angerer J, Boehmer S, Drexler H, Mayer R, Liebl B. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hyg Environ Health. 2007;210:21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Goen T, Dobler L, Koschorreck J, Muller J, Wiesmuller GA, Drexler H, Kolossa-Gehring M. Trends of the internal phthalate exposure of young adults in Germany--follow-up of a retrospective human biomonitoring study. Int J Hyg Environ Health. 2011;215:36–45. doi: 10.1016/j.ijheh.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Hauser R. Urinary phthalate metabolites and semen quality: a review of a potential biomarker of susceptibility. Int J Androl. 2008;31:112–117. doi: 10.1111/j.1365-2605.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SM, Aylward LL, Blount BC. Variation in Urinary Flow Rates According to Demographic Characteristics and Body Mass Index in NHANES: Potential Confounding of Associations between Health Outcomes and Urinary Biomarker Concentrations. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm D. Correlation between production amounts of DEHP and daily intake. Sci Total Environ. 2007;388:389–391. doi: 10.1016/j.scitotenv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE. Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ Health Perspect. 2009;117:86–92. doi: 10.1289/ehp.11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Hakansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect. 2008;116:334–339. doi: 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Norgaard-Pedersen B, Toft G, Hougaard DM, Bonde JP, Cohen A, Thulstrup AM, Ivell R, Anand-Ivell R, Lindh CH, Jonsson BA. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ Health Perspect. 2012;120:897–903. doi: 10.1289/ehp.1104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamrin MA. Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev. 2009;12:157–174. doi: 10.1080/10937400902729226. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Reidy JA, Hurtz D, 3rd, Malek NA, Needham LL, Nakazawa H, Barr DB, Calafat AM. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect. 2004;112:327–330. doi: 10.1289/ehp.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KE, Hernandez-Diaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ Health Perspect. 2012;120:379–384. doi: 10.1289/ehp.1103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park HY, Bae S, Lim YH, Hong YC. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: a panel study. PLoS One. 2013;8:e71392. doi: 10.1371/journal.pone.0071392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, Swan SH. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ Health Perspect. 2014;122:521–528. doi: 10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Angerer J. Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int J Hyg Environ Health. 2007;210:9–19. doi: 10.1016/j.ijheh.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Koch HM, Bolt HM, Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol. 2004;78:123–130. doi: 10.1007/s00204-003-0522-3. [DOI] [PubMed] [Google Scholar]
- Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79:367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci. 2009;364:2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Christensen KL, Harth V, Lorber M, Bruning T. Di-n-butyl phthalate (DnBP) and diisobutyl phthalate (DiBP) metabolism in a human volunteer after single oral doses. Arch Toxicol. 2012;86:1829–1839. doi: 10.1007/s00204-012-0908-1. [DOI] [PubMed] [Google Scholar]
- Koch HM, Lorber M, Christensen KL, Palmke C, Koslitz S, Bruning T. Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health. 2013;216:672–681. doi: 10.1016/j.ijheh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure-- an update and latest results. Int J Androl. 2006;29:155–165. doi: 10.1111/j.1365-2605.2005.00607.x. discussion 181–155. [DOI] [PubMed] [Google Scholar]
- Koniecki D, Wang R, Moody RP, Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res. 2011;111:329–336. doi: 10.1016/j.envres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Sobus JR, Goodman M, Barr DB, Furst P, Albertini RJ, Arbuckle TE, Schoeters G, Tan YM, Teeguarden J, Tornero-Velez R, Weisel CP. A proposal for assessing study quality: Biomonitoring, Environmental Epidemiology, and Shortlived Chemicals (BEES-C) instrument. Environ Int. 2014;73:195–207. doi: 10.1016/j.envint.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Ku HY, Su PH, Chen JW, Huang PC, Angerer J, Wang SL. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere. 2011;82:947–955. doi: 10.1016/j.chemosphere.2010.10.073. [DOI] [PubMed] [Google Scholar]
- Lorber M, Koch HM, Angerer J. A critical evaluation of the creatinine correction approach: can it underestimate intakes of phthalates? A case study with di-2-ethylhexyl phthalate. J Expo Sci Environ Epidemiol. 2011;21:576–586. doi: 10.1038/jes.2010.43. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women. J Expo Sci Environ Epidemiol. 2012;22:376–385. doi: 10.1038/jes.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Tellez-Rojo MM. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 2009a;117:1587–1592. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci. 2009b;364:2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Chevrier C, Philippat C, Petit C, Calafat AM, Ye X, Silva MJ, Brambilla C, Eijkemans MJ, Charles MA, Cordier S, Slama R. Correcting for the influence of sampling conditions on biomarkers of exposure to phenols and phthalates: a 2-step standardization method based on regression residuals. Environ Health. 2012;11:29. doi: 10.1186/1476-069X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen A, Frederiksen H, Sorensen K, Aksglaede L, Hagen C, Skakkebaek NE, Main KM, Andersson AM, Juul A. Urinary phthalates from 168 girls and boys measured twice a year during a 5-year period: associations with adrenal androgen levels and puberty. J Clin Endocrinol Metab. 2013;98:3755–3764. doi: 10.1210/jc.2013-1284. [DOI] [PubMed] [Google Scholar]
- NICNAS (National Industrial Chemicals Notification and Assessment Scheme) Existing chemical hazard assessment report: Diisobutyl phthalate. Sydney, Australia: Australian Department of Health and Ageing; 2008. [Google Scholar]
- NICNAS (National Industrial Chemicals Notification and Assessment Scheme) Existing chemical hazard assessment report: Diethylhexyl phthalate. Sydney. Australia: Australian Department of Health and Ageing; 2010. [Google Scholar]
- NICNAS (National Industrial Chemicals Notification and Assessment Scheme) Existing chemical hazard assessment report No 35: Diisononyl phthalate. Sydney, Australia: Australian Department of Health and Ageing; 2012. [Google Scholar]
- Nieuwenhuijsenm MJ, editor. Exposure Assessment in Occupational and Environmental Epidemiology. New York, NY: Oxford University Press; 2003. [Google Scholar]
- NRC (National Research Council) Human biomonitoring for environmental chemicals. Washington, D.C.: National Academies Press; 2006. [Google Scholar]
- NRC (National Research Council) Phthalates and Cumulative Risk Assessment The Task Ahead. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- Patel CJ, Cullen MR, Ioannidis JP, Butte AJ. Systematic evaluation of environmental factors: persistent pollutants and nutrients correlated with serum lipid levels. Int J Epidemiol. 2012;41:828–843. doi: 10.1093/ije/dys003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MA, Lu C, Schmotzer BJ, Waller LA, Riederer AM. Evaluation of physiological measures for correcting variation in urinary output: Implications for assessing environmental chemical exposure in children. J Expo Sci Environ Epidemiol. 2009;19:336–342. doi: 10.1038/jes.2008.48. [DOI] [PubMed] [Google Scholar]
- Peck JD, Sweeney AM, Symanski E, Gardiner J, Silva MJ, Calafat AM, Schantz SL. Intra- and inter-individual variability of urinary phthalate metabolite concentrations in Hmong women of reproductive age. J Expo Sci Environ Epidemiol. 2010;20:90–100. doi: 10.1038/jes.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preau JL, Jr, Wong LY, Silva MJ, Needham LL, Calafat AM. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ Health Perspect. 2010;118:1748–1754. doi: 10.1289/ehp.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction. 2014;147:401–409. doi: 10.1530/REP-13-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve JF, Levesque M, Huard M, Drolet D, Lavoue J, Tardif R, Truchon G. Creatinine and specific gravity normalization in biological monitoring of occupational exposures. J Occup Environ Hyg. 2015;12:123–129. doi: 10.1080/15459624.2014.955179. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Kato K, Malek NA, Hodge CC, Hurtz D, 3rd, Calafat AM, Needham LL, Brock JW. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch Toxicol. 2003;77:561–567. doi: 10.1007/s00204-003-0486-3. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Akesson A, Alfven T, Jarup L, Vahter M. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005;10:117–126. doi: 10.1080/13547500500159001. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Niwa M, Yoshinaga J, Watanabe C, Mizumoto Y, Serizawa S, Shiraishi H. Exposure assessment of phthalate esters in Japanese pregnant women by using urinary metabolite analysis. Environ Health Prev Med. 2009;14:180–187. doi: 10.1007/s12199-009-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL Study for Future Families Research, T. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefre de Renzy-Martin K, Frederiksen H, Christensen JS, Boye Kyhl H, Andersson AM, Husby S, Barington T, Main KM, Jensen TK. Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction. 2014;147:443–453. doi: 10.1530/REP-13-0461. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Mervish N, Moshier EL, Vangeepuram N, Galvez MP, Calafat AM, Silva MJ, Brenner BL, Wolff MS. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environ Res. 2012;112:186–193. doi: 10.1016/j.envres.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MK, Franke AA, Li X, Hu FB, Eliassen AH. Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environ Health. 2013;12:80. doi: 10.1186/1476-069X-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaine LM, Quebbemann AJ. The renal handling of terephthalic acid. Toxicol Appl Pharmacol. 1985;77:165–174. doi: 10.1016/0041-008x(85)90277-7. [DOI] [PubMed] [Google Scholar]
- Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, Vrijheid M. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int J Hyg Environ Health. 2015;218:220–231. doi: 10.1016/j.ijheh.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou Y, Tang C, He Y, Wu J, Chen Y, Jiang Q. Urinary phthalate metabolites are associated with body mass index and waist circumference in Chinese school children. PLoS One. 2013;8:e56800. doi: 10.1371/journal.pone.0056800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, Braun JM. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol. 2014;48:8881–8890. doi: 10.1021/es501744v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Angerer J, Kolossa-Gehring M, Schafer SD, Klockenbusch W, Dobler L, Gunsel AK, Muller A, Wiesmuller GA. Fetal exposure to phthalates--a pilot study. Int J Hyg Environ Health. 2009;212:492–498. doi: 10.1016/j.ijheh.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Wiesmuller GA, Koch HM, Eckard R, Dobler L, Muller J, Angerer J, Schluter C. Internal phthalate exposure over the last two decades--a retrospective human biomonitoring study. Int J Hyg Environ Health. 2007;210:319–333. doi: 10.1016/j.ijheh.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HC, Lin YS, Kuo CC, Weidemann D, Weaver V, Fadrowski J, Neu A, Navas-Acien A. Urine osmolality in the US population: implications for environmental biomonitoring. Environ Res. 2015;136:482–490. doi: 10.1016/j.envres.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findingsfrom the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect. 2014;122:235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.