Abstract
Generalized pustular psoriasis is a severe skin disease characterized by epidermal hyperplasia, neutrophil rich abscesses within the epidermis and a mixed inflammatory infiltrate in the dermis. The disease may be caused by missense mutations in the interleukin-36 receptor antagonist, IL-36Ra. Curiously, the related IL-1Ra has therapeutic effects in some of these latter patients. Here, using an experimental mouse model of psoriasiform skin inflammation, we demonstrate in vivo connections between IL-36 and IL-1 expression. After disease initiation IL-36α deficient mice exhibited dramatically diminished skin pathology, including absence of epidermal neutrophils, reduced keratinocyte acanthosis, and less dermal edema. In contrast, IL-36β and IL-36γ knockout mice developed disease indistinguishable from that of wild type mice. The endogenous IL-36α was not processed through proteolysis. While IL-36α expression was strongly induced in an IL-1 signaling-dependent manner during disease, expression of IL-1α was also dependent upon IL-36α. Hence, after being up-regulated by IL-1α, IL-36α acts through a feedback mechanism to boost IL-1α levels. Analyses of double knockout mice further revealed that IL-36α and IL-1α co-operate to promote psoriasis-like disease. In conclusion, IL-1α and IL-36α form a self-amplifying inflammatory loop in vivo that in patients with insufficient counter regulatory mechanisms may become hyper-engaged and/or chronic.
Introduction
Psoriasis encompasses a number of non-infectious inflammatory conditions of the skin (Raychaudhuri et al., 2014). Generalized pustular psoriasis (GPP) is the most severe form involving not only skin inflammation, but also systemic symptoms such as fever and malaise. GPP skin inflammation is characterized by reddening of the skin and formation of epidermal pustules filled with neutrophils. When large areas of the skin are affected by GPP the condition can be life threatening. Palmoplantar pustulosis affects specifically the hands and feet, and like GPP involves formation of neutrophil-containing pustules in the epidermis. The most common form of psoriasis is plaque psoriasis. This disease is characterized by red plaques of inflamed skin, which are often scaly due to dysregulated differentiation of the epidermis. While pustules do not form in plaque psoriasis per se, increased neutrophil recruitment into the epidermis is still observed. Typically, these neutrophils cluster together within the stratum corneum of the epidermis and are known as Munro’s microabscesses.
The etiology of the different types of psoriasis is poorly understood; however, a seminal advancement of our knowledge was made in 2011, when missense mutations within the gene, IL36RN, encoding the interleukin-36 receptor antagonist (IL-36Ra) were identified in patients with GPP (Marrakchi et al., 2011; Onoufriadis et al., 2011). IL-36Ra is a natural inhibitor of three related cytokines: IL-36α, IL-36β and IL-36γ (formerly known as IL-1F6, IL-1F8 and IL-1F9, respectively). The physiological function of the IL-36 cytokines remains unknown; however, they were linked to psoriasis pathology even before the discovery of the IL36RN mutations. Early studies using transgenic mice over-expressing IL-36α in keratinocytes revealed skin inflammation with some resemblance to psoriasis (Blumberg et al., 2007). Furthermore, several studies have observed increased IL-36α and IL-36γ mRNA expression in plaque psoriatic skin (reviewed in (Jensen, 2010)).
Imiquimod is a ligand for toll-like receptor 7 (Colak et al., 2014) and adenosine receptors (Kan et al., 2012; Schon et al., 2006), and has therapeutic effects in humans against basal cell carcinomas, actinic keratoses and warts caused by human papillomaviruses. A known side effect of the drug is psoriasiform skin inflammation (Fanti et al., 2006; Gilliet et al., 2004; Patel et al., 2011; Rajan and Langtry, 2006; van der Fits et al., 2009; Wu et al., 2004). This has been exploited to develop a mouse model of psoriasis, which has rapidly become very popular due to its strong resemblance to the human condition (reviewed in (Flutter and Nestle, 2014)). Using the imiquimod-induced psoriasiform skin inflammation model, we previously demonstrated that IL-1R1 signaling, via the chemokines CXCL1 and CXCL2, plays an essential role in recruiting neutrophils to the epidermis (Uribe-Herranz et al., 2013). Furthermore, IL-1, here referring to both IL-1α and IL-1β, promoted psoriasis-like epidermal hyperplasia via IL-1α as the dominant form of the two cytokines expressed in the model (Uribe-Herranz et al., 2013). Given the recently identified genetic link between IL-36 and excessive epidermal recruitment of neutrophils (Marrakchi et al., 2011; Onoufriadis et al., 2011) in addition to the over-expression of IL-36 in plaque psoriasis (Jensen, 2010), we here examined the role of the IL-36 cytokines and their interplay with the IL-1 axis in the imiquimod induced psoriasis mouse model.
Results
IL-36α, but not IL-36β or IL-36γ, is essential for the development of psoriasiform skin disease
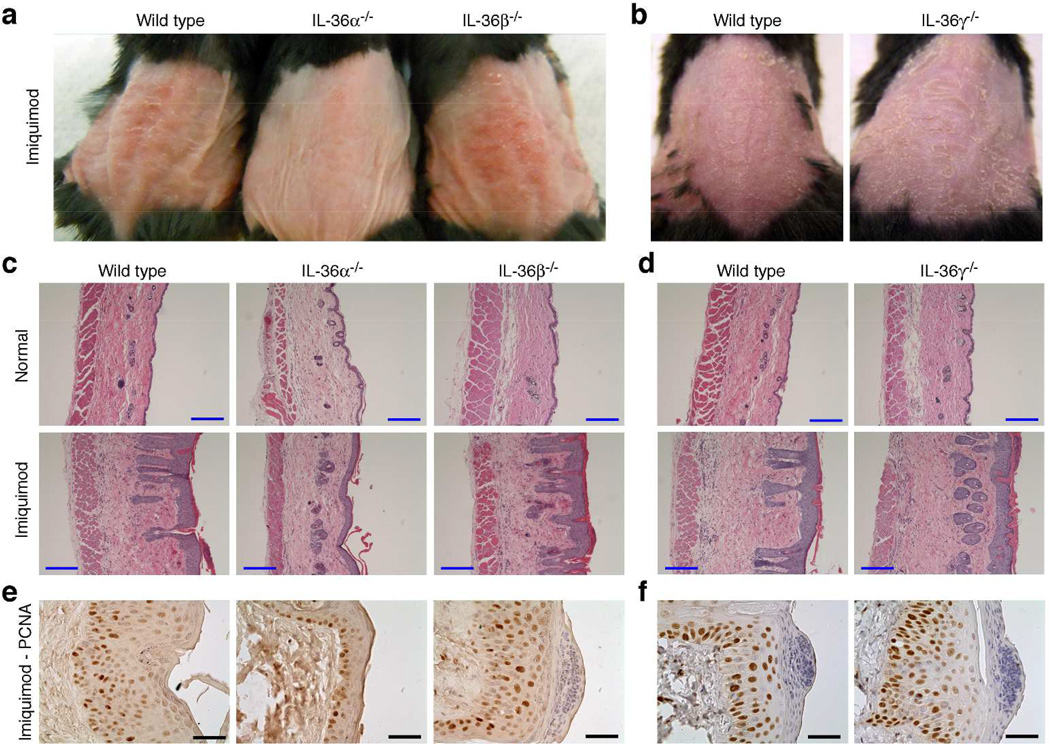
Although the IL-36 cytokines have been linked to inflammatory responses, their physiological functions remain unknown. Curiously, there are three agonist IL-36 cytokines, but only one receptor, IL-36R. With the long-term goal of elucidating the role of this apparent redundancy, we assembled a portfolio of knockout (KO) mice representing each individual IL-36 cytokine (Table S1 and Figs. S1–2). The strains have no apparent defects or obvious phenotypes. This is in agreement with studies involving IL-36R−/− mice (Blumberg et al., 2010; Blumberg et al., 2007; Lamacchia et al., 2013; Tortola et al., 2012; Vigne et al., 2012). Since IL-36 signaling has recently been linked to GPP (Marrakchi et al., 2011; Onoufriadis et al., 2011), we examined the role of each individual IL-36 cytokine in the imiquimod-induced psoriasis model (Fig. 1 and Fig. S3). Somewhat surprisingly, we found that while ablation of neither IL-36β nor IL-36γ affected the imiquimod-induced phenotype, IL-36α KO mice exhibited a dramatically reduced phenotype compared to wild type mice (Fig. 1 and Fig. S3). Externally, the IL-36α KO skin appeared thinner, less red and less scaly (Fig. 1a). In agreement with these observations, histological and immunohistochemical analyses revealed reduced epidermal acanthosis (Fig. 1c, e and Fig. S3a) and dermal edema (Fig. 1c and Fig. S3c) in IL-36α KO mice compared to wild type. These results demonstrate that IL-36α, but not IL-36β or IL-36γ, plays a significant role in driving the imiquimod associated psoriasiform skin pathology.
Figure 1. IL-36α plays a significant role in psoriasiform skin disease induced by imiquimod.
Wild type, IL-36α−/−, IL-36β−/− and IL-36γ−/− mice were treated with imiquimod for 4 days. The day after the last application anesthetized (a) or euthanized (b) mice were photographed and skin collected after euthanasia. Control mice were denuded and left untreated until skin collection. Skin was examined by H&E staining (c–d) and immunohistochemistry for PCNA (e–f). Representative images from 5 (IL-36α−/− and IL-36β−/−) and 3 (IL-36γ−/−) independent imiquimod experiments are shown. Blue scale bars = 200 µm. Black scale bars = 50 µm.
Formation of neutrophil rich microabscesses in the epidermis is IL-36α dependent
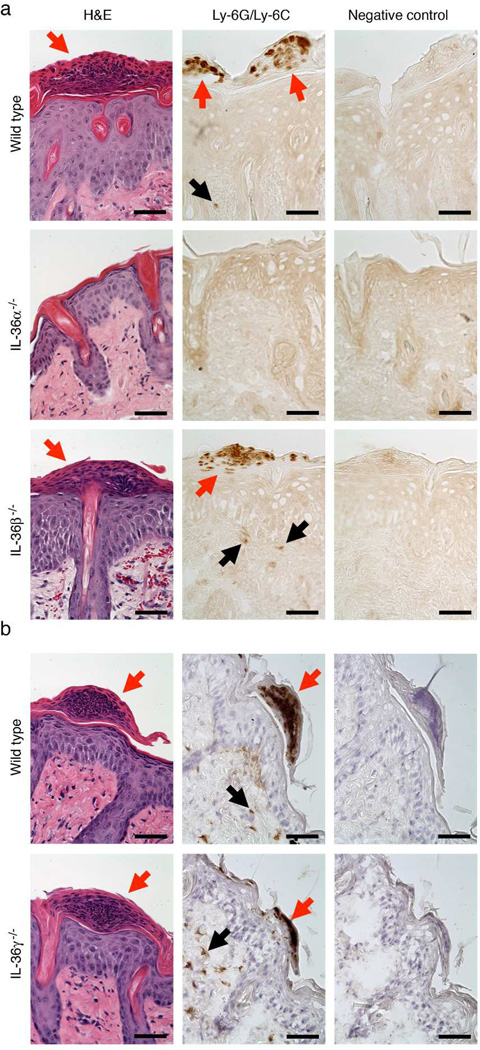
A characteristic feature of psoriasis is diffuse neutrophilic dermal inflammation and especially formation of epidermal abscesses containing neutrophils (Raychaudhuri et al., 2014). Our previous studies of IL-1 signaling in the imiquimod model demonstrated that IL-1 plays a crucial role in the formation of such abscesses (Uribe-Herranz et al., 2013). Similar analyses here revealed that IL-36α KO mice had diminished dermal infiltration by neutrophils and fewer epidermal microabscesses than wild type mice (Fig. 2 and Fig. S4). No differences between wild type and either IL-36β or IL-36γ deficient mice could be detected (Fig. 2 and Fig. S4). This, in agreement with the above-described IL-36α-dependent phenotypes, demonstrates that of the three IL-36 cytokines, IL-36α is the primary driver of neutrophil recruitment to the epidermis in the utilized skin inflammation model.
Figure 2. IL-36α, but not IL-36β or IL-36γ, is required for neutrophil recruitment.
Wild type (a and b), IL-36α−/− (a), IL-36β−/− (a) and IL-36γ−/− (b) mice were treated with imiquimod for 4 days as described in Fig. 1. Skin was collected the day after the last imiquimod application and examined by H&E staining or immunohistochemistry for neutrophils (Ly-6G/Ly-6C). Red arrows indicate Munro’s microabscesses (clusters of neutrophils) at the top of the epidermis. Black arrows indicate individual neutrophils within the dermis. Representative images from the experiments also analyzed in Fig. 1 and Fig. S3 are shown. Scale bars = 50 µm.
Neutrophil recruitment to IL-36α deficient epidermis can be rescued through CXCL1
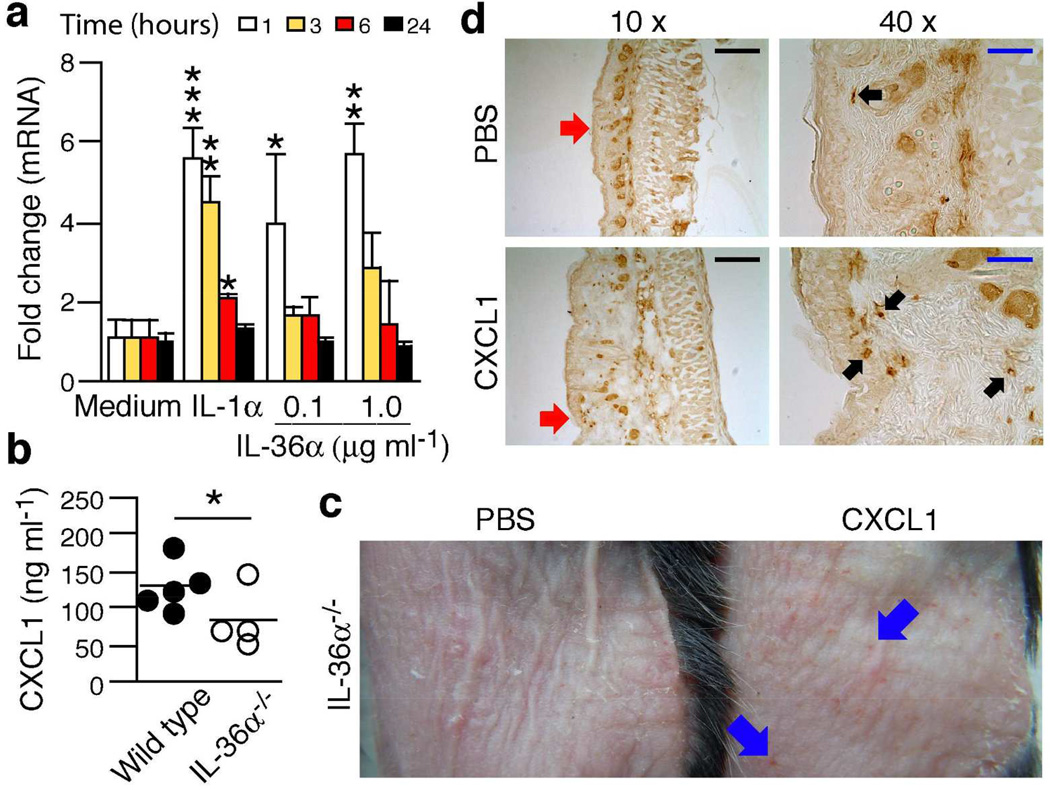
The neutrophil chemotactic CXCL1 is expressed at elevated levels in psoriasis (see (Uribe-Herranz et al., 2013) for refs.) and human IL-36α stimulates CXCL1 production by human keratinocytes (Foster et al., 2014). Using mouse keratinocytes, we observed that mouse IL-36α induced CXCL1 mRNA (Fig. 3a) and protein (data not shown) in a transient and concentrationdependent manner. Furthermore, in vivo levels of CXCL1 secreted from inflamed skin were lower in IL-36α KO mice compared to wild type (Fig. 3b). In the imiquimod model, we have shown that neutrophils are recruited to the epidermis in an IL-1-dependent manner via, for example, CXCL1 (Uribe-Herranz et al., 2013). In the IL-36α KO mice, topical application of physiologically relevant levels of CXCL1 (Fig. 3b) caused formation of inflammatory foci in the skin (Fig. 3c) and recruitment of neutrophils to the epidermis and dermis (Fig. 3d). The latter demonstrates that the migratory capacity of the neutrophils is intact in the IL-36α KO mice. Overall, these observations suggest that IL-36α promotes neutrophil recruitment, at least in part, through CXCL1.
Figure 3. IL-36α may promote neutrophil recruitment through CXCL1.
(a) Mouse primary keratinocytes were treated with medium only, IL-1α (10ng ml−1) or IL-36α (as indicated). CXCL1 mRNA levels were determined using real-time PCR (means + SD). (b) Wild type (black circles, n = 5) and IL-36α−/− (white circles, n = 4) mice were treated with imiquimod four times and 4 mm skin biopsies explanted the day after the last drug application. Production of CXCL1 was examined by ELISA. (c–d), IL-36α−/− mice were treated with imiquimod + PBS or imiquimod + 25 ng CXCL1 per mouse twice. External skin appearance was photodocumented (c) and neutrophil recruitment examined using immunohistochemistry for Ly-6G/Ly-6C (d). Blue arrows, examples of focal inflammation (red spots). Black arrows, examples of neutrophils (Ly-6G/Ly-6C positive cells). Red arrows, regions enlarged in 40×images. Black bars = 200 µm. Blue bars = 50 µm. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IL-36α is produced and secreted at high levels in imiquimod treated skin
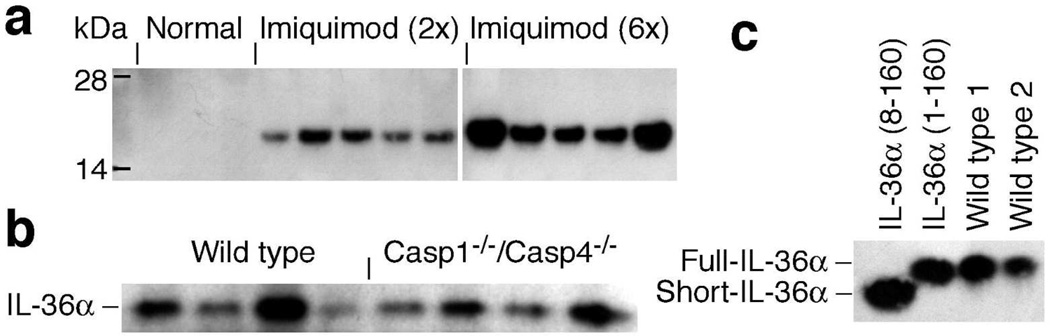
To explore the specific involvement of IL-36α, but not IL-36β or IL-36γ, in regulating imiquimod induced skin inflammation, we examined expression of these. All three IL-36 mRNAs were significantly up-regulated in response to topical imiquimod treatment (Fig. S5a–c), with IL-36α being the most dramatically induced (≅ 60-fold). IL-36α protein could be detected readily in culture medium from explanted skin after only two imiquimod applications (Figs. 4a and S5d). Similar IL-36β and IL-36γ secretion was not detected (data not shown). Hence, the specific involvement of IL-36α, but not IL-36β nor IL-36γ, in the examined skin inflammation model (Fig. 1) likely reflects the dramatic induction of IL-36α gene expression (Fig. S5a) and the subsequent correspondingly high protein levels achieved (Figs. 4a and S5d).
Figure 4. Topical imiquimod treatment leads to dramatic induction and secretion of unprocessed IL-36α in an inflammatory caspases-independent manner.
(a) Wild type mice (n = 3–5 per time point) were treated with imiquimod as indicated. Skin was collected and used for 4 mm explant cultures. Levels of secreted IL-36α were examined by Western blotting. (b) Wild type and Casp1−/−/Casp4−/− mice (n = 4 per group) were treated with imiquimod 4 times and IL-36α secretion examined. (c) Two independent samples of secreted proteins (Wild type 1 and Wild type 2) were analyzed by Western blotting next to full-length recombinant IL-36α (IL-36α (1–160)) and predicted processed short recombinant IL-36α (IL-36α (8–160)) in EpiLife medium.
We previously reported that imiquimod induces expression of IL-1α and IL-1β both in vitro and in vivo (Uribe-Herranz et al., 2013). In comparison, we here found that the IL-36α, but not IL-18, mRNA is induced at higher concentrations (Fig. S6) than those activating IL-1α and IL-1β gene expression (Uribe-Herranz et al., 2013). Despite the dramatic induction of the IL-36α mRNA (Fig. S6), we were not able to detect IL-36α protein production (data not shown). This is in agreement with our reported observations that these drug levels are cytotoxic to cultured keratinocytes (Uribe-Herranz et al., 2013). Given the topical application of imiquimod in the here employed mouse model, it is difficult to access the drug concentrations experienced by the epidermal keratinocytes; however, it is possible that imiquimod plays a significant role as a direct activator of IL-36α gene expression in this model.
IL-36α is released in an inflammatory caspases-independent manner
A curious characteristic of IL-1 and IL-36 cytokines is that they lack signal peptides for conventional protein secretion (reviewed in (Jensen, 2010)). The release mechanism for IL-1β, which is the best characterized, often involves the inflammasomes and the inflammatory caspases caspase-1 and caspase-4 (Shin and Brodsky, 2015). In macrophages imiquimod induces IL-1β secretion through NLRP3 inflammasome mediated activation of caspase-1 (Kanneganti et al., 2006). We previously established that release of IL-36γ from cells treated with double stranded RNA is dependent upon the inflammatory caspases (Lian et al., 2012), whereas IL-1α release during herpes simplex virus-1 infection is caspase-1/-4 independent (Milora et al., 2014). Hence, we examined whether IL-36α release in the here utilized imiquimod model was dependent or independent of the inflammatory caspases. Interestingly, our data revealed that IL-36α release is independent of caspase-1 and -4 (Fig. 4b). This suggests an IL-36α release mechanism distinct from that controlling IL-1β.
IL-36α does not undergo processing in vivo
Based on size, the IL-36 cytokines were predicted upon discovery to be synthesized as mature proteins unlike the related IL-1β, which requires proteolytic cleavage for activation and extracellular release (Shin and Brodsky, 2015). Full length recombinant IL-36 proteins, expressed in bacteria, are active, albeit only at high concentrations (see (Jensen, 2010) for refs.). Through genetic engineering it has been demonstrated that truncation of 17 amino acids from the N-terminus of IL-36γ led to enhanced activity (Towne et al., 2011). Curiously, removal of fewer, or more, amino acids did not have similar effects. Consequently, it was proposed that the IL-36 cytokines require proteolytic processing, at very specific sites, to be activated (Towne et al., 2011). By sequence comparison, processing of mouse IL-36α was predicted to involve 7 amino acids ((Towne et al., 2011), shown in blue in Fig. S1b). To determine if IL-36α is processed in vivo, we examined the molecular weight of secreted endogenous IL-36α compared to full length and truncated (predicted) recombinant proteins. Interestingly, we found that endogenously secreted IL-36α migrated identically to full-length recombinant IL-36α (Fig. 4c). Consequently, we conclude that IL-36α is released as an unprocessed protein.
IL-36α expression is induced by IL-1 in vitro and in vivo
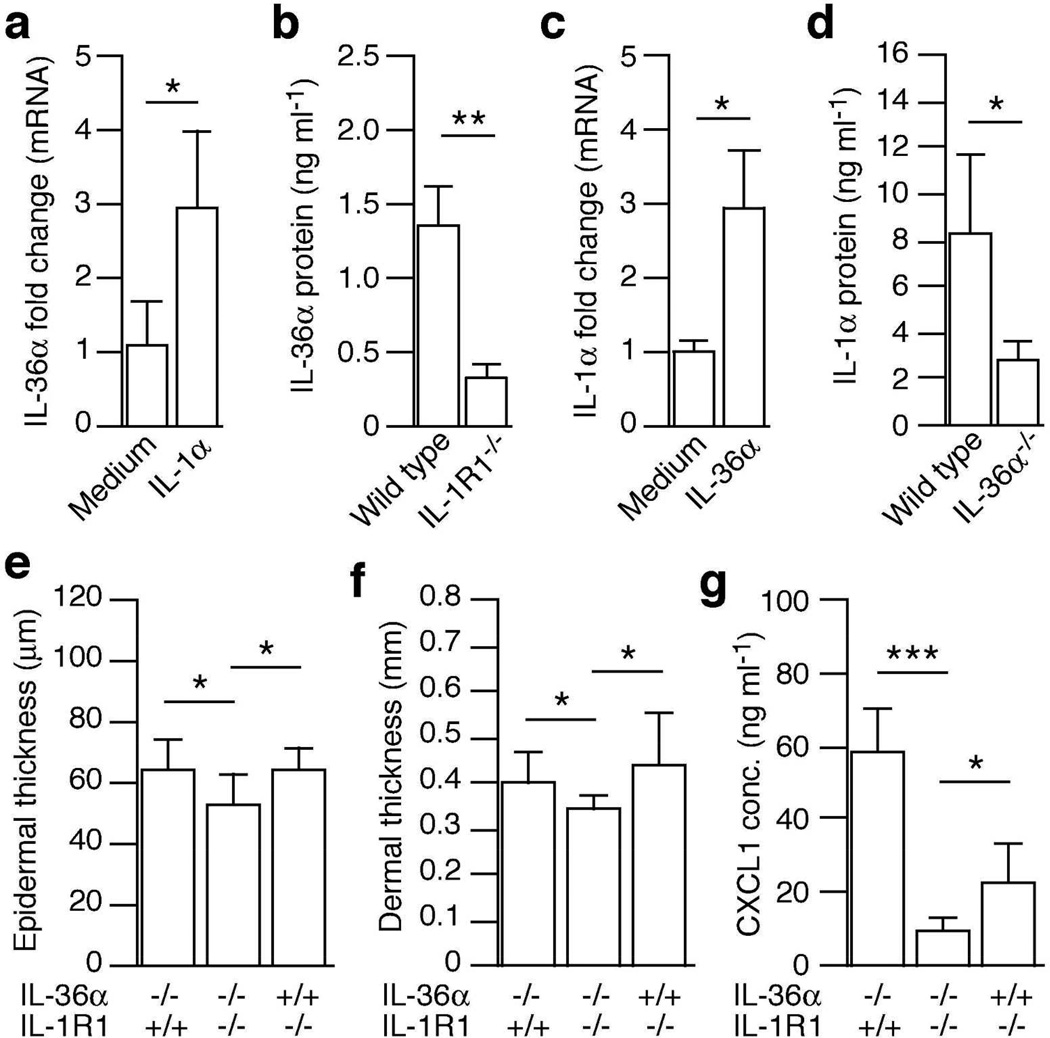
In vitro studies have established that IL-1β can stimulate expression of the human IL-36α mRNA in bronchial epithelial cells (Chustz et al., 2011). In a similar manner, we found that mouse IL-1α induced IL-36α gene expression in mouse keratinocytes (Fig. 5a). We previously demonstrated that IL-1 signaling, via IL-1R1 and primarily IL-1α, plays an essential role in initiating skin disease in the imiquimod model (Uribe-Herranz et al., 2013). Hence, we hypothesized that IL-1α signaling could be involved in driving the inducible expression of IL-36α found in vivo (Figs. 4 and S5). Consistent with this hypothesis, we observed significantly lower levels of IL-36α secretion from inflamed IL-1R1 deficient skin than wild type (Fig. 5b). Hence, IL-1α is an important regulator of IL-36α expression in vivo.
Figure 5. IL-36α and IL-1 co-operate to drive psoriasiform skin inflammation through mutual regulation.
(a and c) Mouse primary keratinocytes were treated with medium only, IL-1α (10 ng ml−1) or IL-36α (1 µg ml−1) for 1 hour. IL-36α (a) and IL-1α (c) mRNA levels were evaluated using real-time PCR. (b) Wild type (n = 4) and IL-1R1 KO (n = 3) mice were treated with imiquimod two times. Skin was explanted and maintained for another 24 hours. Levels of IL-36α secreted into the medium were determined by ELISA. (d) Wild type (n = 4) and IL-36α−/− (n = 4) mice were treated with imiquimod 4 times. Treated skin was explanted and levels of IL-1α secreted into the medium determined by ELISA. (e–g) IL-36α (n = 6), IL-1R1 (n = 7) and IL-36α/IL-1R1 double KO (n = 7) mice were treated with imiquimod 4 times. Epidermal (e) and dermal (f) thickness was examined using H&E staining and ImageJ. (g) Levels of CXCL1 secretion were determined by ELISA. Data points are shown as mean + SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IL-36α regulates IL-1α in a feedback-loop
Ex vivo studies of peripheral blood mononucleated cells from a single patient with GPP and a healthy control revealed potentiated activation of IL-1α gene expression in GPP cells in response to IL-36α (Onoufriadis et al., 2011). Using primary mouse keratinocytes we observed rapid induction of the IL-1α mRNA in response to IL-36α (Fig. 5c). In our employed mouse model of psoriasis we observed progressive up-regulation of IL-1α mRNA during disease development (Fig. S7). This is in agreement with our previously reported data demonstrating upregulation of IL-1α protein (Uribe-Herranz et al., 2013). The induction of IL-1α correlated with increased IL-36α mRNA and protein expression (Figs. 4 and S5), and we therefore examined whether the inducible IL-1α expression was dependent, at least in part, upon IL-36α. Indeed we found that levels of IL-1α released from imiquimod treated skin were significantly lower in the absence of IL-36α than in the presence of IL-36α (Fig. 5d). Levels of IL-18 (Fig. S8), but not IL-1β and IL-17A (not shown), was also found to be IL-36α dependent. Hence, we conclude that once expression of IL-36α is initiated, via IL-1R1 signaling (Fig. 5a–b), IL-36α feeds back to induce IL-1α expression (Fig. 5c–d).
IL-1α and IL-36α co-operate to promote epidermal hyperplasia, dermal edema and chemokine production
Previously, we showed that IL-1R1 signaling plays a significant role in initiating epidermal hyperplasia and neutrophil recruitment through CXCL1 in response to imiquimod (Uribe-Herranz et al., 2013). Since IL-36α expression is induced via IL-1R1 (Fig. 5a–b), we wondered whether IL-36α acts down-stream of IL-1 in a sequential linear pathway or whether the two cytokines act together when present at the same time. To address this, we generated IL-36α and IL-1R1 double KO mice and compared epidermal hyperplasia, dermal edema and CXCL1 expression to that induced in single KO mice (Figs. 5e–g). In IL-36α−/−/IL-1R1−/− mice the thickness of both the epidermis (Fig. 5e) and the dermis (Fig. 5f) was significantly thinner than that in single KO mice. Additionally, production of the neutrophil chemotactic CXCL1 was reduced in the IL-36α−/−/IL-1R1−/− mice compared to single KO mice (Fig. 5g). This demonstrates that once expression of IL-36α is induced, IL-1α and IL-36α act together in vivo to promote psoriasiform skin disease.
Discussion
The discovery of missense mutations in IL36RN causing generalized GPP (Marrakchi et al., 2011; Onoufriadis et al., 2011), implicates overzealous IL-36 signaling in the disease pathology. Here we show that in the imiquimod-induced psoriasiform skin disease model, IL-36α is the primary form of IL-36 driving skin pathology, including epidermal hyperplasia, dermal inflammation and neutrophil recruitment to the epidermis (Figs. 1–2 and S4). The active form of IL-36α is, against expectations, not proteolytically processed in connection with its release from cells (Fig. 4c). Our study further reveals that elevated expression of both IL-36α and IL-1α are intricately linked during disease, as each cytokine is involved in regulating expression of the other (Fig. 5a–d). Through this mutual induction, IL-36α and IL-1α act together to boost the pathogenic function of the other cytokine’s signaling pathway (Fig. 5e–g).
Anakinra, recombinant human IL-1Ra, has been successfully used off label to treat individual cases of GPP associated with IL-36Ra missense mutations (Huffmeier et al., 2014; Rossi-Semerano et al., 2013). Analyses of cytokine production by peripheral-blood mononuclear cells from a patient with GPP revealed elevated IL-1α production in response to IL-36α compared to a healthy control sample (Onoufriadis et al., 2011). In our employed in vivo model IL-1α is indeed a down-stream target of IL-36α signaling (Fig. 5d). Interestingly, we also find that IL-36α expression is dependent upon IL-1 signaling (Fig. 5b), suggesting feedback mechanisms between the IL-1 family cytokines (Fig. S9). Due to this inter-regulation, IL-1α and IL-36α form a self-amplifying cycle (Fig. S9), which may run out of control in the absence of proper regulatory mechanisms such as the IL-1Ra and IL-36Ra. This cycle may not only explain the often sudden and dramatic disease flares seen in patients with inflammatory diseases, for example GPP, but may also pinpoint the chronic nature of these conditions. As such, this cycle (Fig. S9) may represent an ideal therapeutic target to restore homeostatic balance.
In addition to a direct feedback loop between IL-1α and IL-36α (Figs. 5a–d and S8), additional factors may contribute to further amplification of the pathogenic process. The IL-1 and IL-36 cytokines regulate their own expression ((Carrier et al., 2011; Uribe-Herranz et al., 2013) and refs. therein); hence, they further fuel the process (Fig. S9). A second connected cycle of amplification may involve other cytokines (Fig. S9) as IL-36 expression is directly induced in keratinocytes by IL-17A, IL-22 and TNF-α (Carrier et al., 2011) and expansion of IL-17 producing γδ T cells is IL-36 dependent (Tortola et al., 2012). Interestingly, IL-36R KO mice exhibit a milder imiquimod induced phenotype than mice deficient of factors from the well-known IL-23/IL-17/IL-22 pathogenic axis, suggesting that the IL-36 system has an additional distinct activity (Tortola et al., 2012). The here identified IL-1α/IL-36α loop may be this activity (Fig. S9). While Torlola et al. did not observe an effect of IL-1R1 ablation upon epidermal acanthosis, our previous (Uribe-Herranz et al., 2013) and the here present study did find such an effect. This apparent discrepancy may be due to the different application sites. Tortola et al. applied imiquimod to the ears, whereas we apply it to the back as in the original model report (van der Fits et al., 2009). The acanthosis observed in the ears is significantly milder (Tortola et al., 2012) than that observed in the back skin (van der Fits et al., 2009) (Figs. 1–2 and S3). Hence, the IL-1R1-dependent phenotype may be missed in the ear-version of the model. Consequently, caution should be taken when comparing studies utilizing the imiquimod mouse model of skin inflammation.
Keratinocytes appear to be the source of IL-36 in at least GPP (Marrakchi et al., 2011). While we readily detected IL-1-dependent IL-36α expression in the imiquimod model (Fig. 5b), we have not been able to detect protein production in keratinocyte cultures. This could suggest that signals, additional to IL-1, are required to achieve the in vivo observed levels. Such additional factors are also suggested by our in vivo data demonstrating only a partial reduction in IL-36α expression in IL-1R1 KO mice (Fig. 5b) compared to the dramatic full induction (Figs. 4a and S5). IL-17A may be one such factor as it activates IL-36 mRNA expression in vitro (Carrier et al., 2011). Although we did not observe a difference in IL-17A levels at the here examined time-point associated with the skin phenotype, IL-17A is known to be transiently induced in the model (van der Fits et al., 2009); hence, IL-17A could contribute to the IL-36α induction at an earlier time point. Imiquimod cream may also directly induce IL-36α in a manner distinct, or similar to, that previously described for IL-1α (Uribe-Herranz et al., 2013; Walter et al., 2013). The latter is a deviation from human psoriasis pathology where the disease triggers are poorly understood. Identification of initiator factors in both the imiquimod-model and human pathology may represent new therapeutic and preventive targets.
In response to inflammatory signals IL-1β is synthesized as a pro-protein. This pro-IL-1β is cleaved by caspase-1, an independent signaling event, to gain functional activity (reviewed in (Jensen, 2010)). Based on genetic engineering, it has been suggested that the IL-36 cytokines require similar proteolytic cleavage to be activated (Towne et al., 2011). Our studies here demonstrate that IL-36α is released from skin cells in an unprocessed form (Fig. 4c). This unprocessed IL-36α appears to be active, as dramatically reduced skin pathology is observed in IL-36α deficient mice (Figs. 1–2 and S4). While it was previously shown that IL-36α gained activity upon removal of 7 or 5 amino acids from the N-terminus (mouse (Fig. S1B, blue highlight) versus human), the full-length IL-36α, unlike IL-1β, still has signaling capacity (reviewed in (Jensen, 2010)). The observed in vivo activity of unprocessed IL-36α reported here, may be due to high local production of the cytokine leading to a microenvironment conducive for the relatively low activity of full length IL-36α. However, it is a possibility that under conditions different from the here studied skin inflammation, IL-36α can undergo processing to enhance its activity, and that the protease involved is not activated in the here utilized model.
Interestingly, in vitro processing of IL-36γ appears to be associated with keratinocyte differentiation, as it is induced by high calcium concentrations (Li et al., 2014). Since epidermal differentiation is dysregulated in psoriasis and associated with aberrant calcium levels (Menon and Elias, 1991), it is possible that the IL-36 processing protease is not activated in psoriatic skin. If IL-36γ activity is strictly dependent upon processing, then lack of cleavage could explain the absence of a phenotypic effect in our IL-36γ−/− mice (Fig. 1). IL-36γ is well known to be up-regulated in psoriatic skin (reviewed in (Jensen, 2010)), and it has been proposed as a biomarker to distinguish psoriasis from other inflammatory skin conditions (D'Erme et al., 2015). If IL-36γ is to be considered a therapeutic target and not just a biomarker, it will be critical to determine if IL-36γ is, aside from up-regulated, responsible to disease pathology and/or progression. Such activity may be dependent upon site-specific processing and consequently, it would be very interesting to determine whether IL-36γ is processed in healthy and diseased human skin.
In summary, we have linked IL-1α and IL-36α in an in vivo feedback loop (Fig. S9). Such a loop could explain both sudden and dramatic disease onset as seen in, for example GPP, and the chronic nature of psoriatic diseases. As such, this self-amplifying cycle (Fig. S9) represents a promising therapeutic target; however, as discussed above, many questions remain unresolved. Through further studies, an improved insight into the mechanisms whereby IL-36 and keratinocytes initiate and maintain psoriatic disease may reveal novel therapeutic strategies and targets.
Materials and Methods
Mice
IL-1R1 and Casp1/Casp4 deficient mice were obtained from the Jackson Laboratory. Il1f6+/− mice were generously donated by GlaxoSmithKline and backcrossed onto the C57BL/6 background. Il1f9+/− and Il1f8+/− mice were procured from the Mutant Mouse Regional Resource Center (University of North Carolina) and Knockout Mouse Project University of California Davis (project ID: VG13041), respectively. Mice were interbred to generate KO (single or double) and wild type mice (all on the C57BL/6 background). Homozygous founders were used for further breeding. All experimental mice were bred in house in a specific pathogen free animal facility. All housing, breeding and experimental procedures involving mice were approved by the Temple University Institutional Animal Care and Use Committee and in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals. Experimental mice (male and female) were used at age 8–10 weeks and matched for sex in each independent experiment.
Genotyping
Mice were genotyped using a previously described protocol (Jensen et al., 2006). PCR was performed using primers listed in Table S2.
Flow analyses
Blood was collected by cardiac puncture and erythrocytes lysed with RBC lysis buffer (eBioscience). Neutrophils, monocytes and lymphocytes were identified based on forward and side scatter using a FACSCanto Cell Analyzer (BD Biosciences).
Induction of psoriasiform skin inflammation
Mice were denuded by shaving and subsequent treatment with depilating cream. Imiquimod cream (5%, 62.5 mg, Medicis) was applied daily to a 6 cm2 area as indicated for each experiment. Skin was collected the day after the last application unless indicated otherwise.
Quantification of epidermal and dermal thickness
PBS buffered formaldehyde (4%) fixed skin was examined by standard H&E staining. For each skin specimen 3 independent images were acquired. Epidermal and dermal thickness was measured in 3 independent locations using ImageJ. An average thickness of the epidermis and dermis was calculated for each tissue. In most experiments, including those involving double KOs, the measurements were performed blinded to the strain genotype.
Immunohistochemistry
Neutrophils were detected using rat anti-mouse Ly-6G/Ly-6C antibody (BD Biosciences) as previously described (Uribe-Herranz et al., 2013). PCNA was visualized using the PCNA staining kit (Life Technologies) according to the manufacturer’s instructions.
Real-time RT-PCR
RNA was isolated using the RNeasy Plus Universal system (Qiagen) according to the manufacturer’s instructions. RNA was reverse transcribed and cDNA analyzed as previously described (Sanmiguel et al., 2009). Additional primers are listed in Table S2. Actin was used as the housekeeping gene for ΔΔCt analyses.
Protein preparations
For analyses of secreted protein levels, standardized 4 mm circular skin biopsies (Miltex) were placed in 200 µl EpiLife medium supplemented with EDGF (Life Technologies) for 24 hours. Explant medium was collected for ELISA analyses. Total protein was extracted from skin in previously described lysis buffer (Jensen and Whitehead, 2003) using a Bio-Gen PRO200 homogenizer equipped with interchangeable Multi-Gen 7XL probes (PRO Scientific). Protein levels were determined using Bio-Rad Protein Assay Dye Reagent (Bio-Rad).
Cell cultures
Human and mouse primary keratinocytes were obtained and maintained as previously described (Uribe-Herranz et al., 2013). Mouse cells were treated with mouse cytokines (IL-1α (Peprotech) and IL-36α (aa 8–160) (R&D Systems) as indicated. Water-soluble imiquimod was from InvivoGen.
ELISA
Sandwich ELISAs (Peprotech (CXCL1, IL-1α) and R&D Systems (IL-17A and IL-18)) were performed according to the manufacturer’s instructions. Direct ELISAs for mouse IL-36 were performed as previously described (Lian et al., 2012). Individual IL-36 cytokines were detected by sequential incubation with: 1) goat anti-IL-36α (AF2297, R&D Systems), or goat anti-IL-36β (AF2298, R&D Systems) and 2) appropriate species-specific horseradish peroxidase conjugated secondary antibodies. The detection limit for the mouse IL-36β ELISA was 400 pg/ml (IL-36α levels were detected in the 500–10,000 pg/ml range).
Western blotting
Proteins were separated by PAGE, transferred to PVDF membranes and detected by ECL using anti-IL-36α (AF2297), anti-IL-36γ (custom synthesized at Genscript) or anti-GAPDH (FL-335, Santa Cruz) and appropriate secondary antibodies.
Statistical analyses
All experiments were performed at least 3 times unless indicated otherwise. Data shown are arithmetic means standard deviations (SD) unless indicated otherwise. Statistical significance was calculated using ANOVA or students t test.
Supplementary Material
Acknowledgements
We wish to express our gratitude to numerous investigators at GlaxoSmithKline, many of who remain unknown to us, who made the transfer of founders for the unpublished IL-36α KO mouse strain possible. We thank Samantha L. Miller for help with skin thickness measurements. This study was supported in part by an award (AR053672) from the National Institute of Arthritis, Musculoskeletal and Skin Diseases to L. E. Jensen.
Footnotes
Conflicts of interests
None
References
- Blumberg H, Dinh H, Dean C, Jr, et al. IL-1RL2 and its ligands contribute to the cytokine network in psoriasis. J Immunol. 2010;185:4354–4362. doi: 10.4049/jimmunol.1000313. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Dinh H, Trueblood ES, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier Y, Ma H-L, Ramon HE, et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J Invest Dermatol. 2011;131:2428–2437. doi: 10.1038/jid.2011.234. [DOI] [PubMed] [Google Scholar]
- Chustz RT, Nagarkar DR, Poposki JA, et al. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2011;45:145–153. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak E, Leslie A, Zausmer K, et al. RNA and imidazoquinolines are sensed by distinct TLR7/8 ectodomain sites resulting in functionally disparate signaling events. J Immunol. 2014;192:5963–5973. doi: 10.4049/jimmunol.1303058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Erme AM, Wilsmann-Theis D, Wagenpfeil J, et al. IL-36γ (IL-1F9) is a biomarker for psoriasis skin lesions. J Invest Dermatol. 2015;135:1025–1032. doi: 10.1038/jid.2014.532. [DOI] [PubMed] [Google Scholar]
- Fanti PA, Dika E, Vaccari S, et al. Generalized psoriasis induced by topical treatment of actinic keratosis with imiquimod. Int J Dermatol. 2006;45:1464–1465. doi: 10.1111/j.1365-4632.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- Flutter B, Nestle FO. TLRs to cytokines: Mechanistic insights from the imiquimod mouse model of psoriasis. Eur J Immunol. 2014;43:3138–3146. doi: 10.1002/eji.201343801. [DOI] [PubMed] [Google Scholar]
- Foster AM, Baliwag J, Chen CS, et al. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J Immunol. 2014;192:6053–6061. doi: 10.4049/jimmunol.1301481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Conrad C, Geiges M, et al. Psoriasis triggered by Toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490–1495. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- Huffmeier U, Wätzold M, Mohr J, et al. Successful therapy with anakinra in a patient with generalized pustular psoriasis carrying IL36RN mutations. Br J Dermatol. 2014;170:202–204. doi: 10.1111/bjd.12548. [DOI] [PubMed] [Google Scholar]
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11:1211–1220. [PMC free article] [PubMed] [Google Scholar]
- Jensen LE, Etheredge AJ, Brown KS, et al. Maternal genotype for the monocyte chemoattractant protein 1 A(−2518)G promoter polymorphism is associated with the risk of spina bifida in offspring. Am J Med Genet A. 2006;140A:1114–1118. doi: 10.1002/ajmg.a.31212. [DOI] [PubMed] [Google Scholar]
- Jensen LE, Whitehead AS. Ubiquitin activated tumor necrosis factor receptor associated factor-6 (TRAF6) is recycled via deubiquitination. FEBS Lett. 2003;553:190–194. doi: 10.1016/s0014-5793(03)00998-0. [DOI] [PubMed] [Google Scholar]
- Kan Y, Okabayashi T, Yokota S-i, et al. Imiquimod suppresses propagation of herpes simplex virus 1 by upregulation of cystatin A via the adenosine receptor A1 pathway. J Virology. 2012;86:10338–10346. doi: 10.1128/JVI.01196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti T-D, Ozoren N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- Lamacchia C, Palmer G, Rodriguez E, et al. The severity of experimental arthritis is independent of IL-36 receptor signaling. Arthritis Res Ther. 2013;15:R38. doi: 10.1186/ar4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Yamasaki K, Saito R, et al. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36 induction in human epidermal keratinocytes. J Immunol. 2014;193:5140–5148. doi: 10.4049/jimmunol.1302574. [DOI] [PubMed] [Google Scholar]
- Lian L-H, Milora KA, Manupipatpong KK, et al. The double-stranded RNA analogue polyinosinic-polycytidylic acid induces keratinocyte pyroptosis and release of interleukin-36γ. J Invest Dermatol. 2012;132:1346–1353. doi: 10.1038/jid.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. New Engl J Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- Menon GK, Elias PM. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol. 1991;127:57–63. [PubMed] [Google Scholar]
- Milora KA, Miller SL, Sanmiguel JC, et al. Interleukin-1α released from HSV-1 infected keratinocytes acts as a functional alarmin in the skin. Nat Commun. 2014;5:5230. doi: 10.1038/ncomms6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Gen. 2011;89:432–437. doi: 10.1016/j.ajhg.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel U, Mark NM, Machler BC, et al. Imiquimod 5% cream induced psoriasis: a case report, summary of the literature and mechanism. Br J Dermatol. 2011;164:670–672. doi: 10.1111/j.1365-2133.2010.10124.x. [DOI] [PubMed] [Google Scholar]
- Rajan N, Langtry JAA. Generalized exacerbation of psoriasis associated with imiquimod cream treatment of superficial basal cell carcinomas. Clin Exp Dermatol. 2006;31:140–141. doi: 10.1111/j.1365-2230.2005.01938.x. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev. 2014;13:490–495. doi: 10.1016/j.autrev.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Rossi-Semerano L, Piram M, Chiaverini C, et al. First clinical description of an infant with interleukin-36-receptor antagonist deficiency successfully treated with Anakinra. Pediatrics. 2013;132:e1043–e1047. doi: 10.1542/peds.2012-3935. [DOI] [PubMed] [Google Scholar]
- Sanmiguel JC, Olaru F, Li J, et al. Interleukin-1 regulates keratinocyte expression of T cell targeting chemokines through interleukin-1 receptor associated kinase-1 (IRAK1) dependent and independent pathways. Cell Signal. 2009;21:685–694. doi: 10.1016/j.cellsig.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön MP, Schön M, Klotz K-N. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- Shin S, Brodsky IE. The inflammasome: Learning from bacterial evasion strategies. Semin Immunol. 2015;27:102–110. doi: 10.1016/j.smim.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Tortola L, Rosenwald E, Abel B, et al. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest. 2012;122:3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne JE, Renshaw BR, Douangpanya J, et al. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity. J Biol Chem. 2011;286:42594–42602. doi: 10.1074/jbc.M111.267922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Herranz M, Lian L-H, Hooper KM, et al. IL-1R1 signaling facilitates Munro’s microabscess formation in psoriasiform imiquimod-induced skin inflammation. J Invest Dermatol. 2013;133:1541–1549. doi: 10.1038/jid.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JSA, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Martin P, et al. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood. 2012;120:3478–3487. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- Walter A, Schäfer M, Cecconi V, et al. Aldara activates TLR7-independent immune defence. Nat Commun. 2013;4:1560. doi: 10.1038/ncomms2566. [DOI] [PubMed] [Google Scholar]
- Wu JK, Siller G, Strutton G. Psoriasis induced by topical imiquimod. Australas J Dermatol. 2004;45:47–50. doi: 10.1111/j.1440-0960.2004.00030.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.