Abstract
Introduction
Predicting which patients will be free from atrial fibrillation (AF) after pulmonary vein isolation (PVI) remains challenging. Clinical risk prediction scores show modest ability to identify patients at risk for AF recurrence after PVI. B-type natriuretic peptide (BNP) is associated with risk for incident and recurrent AF, but is not currently included in existing AF risk scores. We sought to evaluate the incremental benefit of adding pre-operative BNP to existing risk scores for predicting AF recurrence during the 6 months after PVI.
Methods and Results
161 patients with paroxysmal or persistent AF underwent an index PVI procedure between 2010 and 2013; 77 patients (48%) had late AF recurrence after PVI (>3 months post-PVI) over the 6-month follow-up period. A BNP ≥100 pg/dL (p = 0.01) and AF recurrence within 3-months post-PVI (p<0.001) were associated with late AF recurrence in multivariate analyses. Addition of BNP to existing clinical risk scores significantly improved the areas under the curve (AUC) for each score, with an integrated discrimination improvement (IDI) of 0.08 (p=0.001); and a net reclassification improvement (NRI) of 60% (p=0.001) for all risk scores.
Conclusions
Circulating BNP levels are independently associated with late AF recurrence after PVI. Inclusion of BNP significantly improves the discriminative ability of CHADS2, CHA2DS2-VASc, R2CHADS2, and the HATCH score in predicting clinically significant, late AF recurrence after PVI and should be incorporated in decision making algorithms for management of AF. B-R2CHADS2 is the best score model for prediction of late AF recurrence.
Keywords: Cryoablation, Radiofrequency ablation, CHADS2, CHA2DS2-VASc, R2CHADS2, HATCH
Introduction
Pulmonary vein isolation (PVI) reduces atrial fibrillation (AF) burden and increases quality of life in symptomatic patients with AF. Nevertheless, reported rates of AF recurrence after PVI vary widely in the published literature (between 10%-60%) 1, and few predictors are available to identify individuals at high-risk for recurrence of AF. Since increasing numbers of patients are undergoing AF ablation, more sophisticated risk assessment tools are needed to help predict response and therefore guide physicians, patients, and families with respect to the appropriate treatment pathway.
Since many of the clinical factors included in the most common stroke risk prediction instruments 2, 3, CHADS2, and CHA2DS2-VASc, are also associated with development of AF 4, 5, several studies have examined the predictive capacities of these same instruments for recurrent AF after PVI. Unfortunately, although CHADS2 and CHA2DS2-VASc show excellent performance for stroke prediction, they demonstrate only modest discriminative ability with respect to identifying individuals at risk for AF recurrence after PVI 6-9. Recently, another clinical risk score, the HATCH score (Hypertension, age, prior transient ischemic event, chronic obstructive pulmonary disease, and heart failure), has been proposed to predict progression from paroxysmal to persistent AF in patients receiving pharmacological therapy 10. This instrument has also been examined as a predictor of recurrent AF. Unfortunately, the HATCH instrument shows only limited ability to predict AF recurrence after catheter ablation 7, 11.
Brain type natriuretic peptide (BNP) and NT-pro-BNP levels are associated with risk for incident and recurrent AF, thromboembolic events, and overall cardiovascular mortality 12-16. Likely due to the fact that BNP is not routinely measured in clinical practice outside of the context of heart failure, the incremental benefit of adding BNP to existing clinical risk scores has not been examined. Conducted within the context of the University of Massachusetts Medical Center's AF Treatment Program, we systematically measured pre-ablation BNP. We hypothesized that not only would serum BNP levels be associated with AF, but also enhance clinical AF risk prediction scores. The objectives of this investigation were to evaluate the incremental benefit of adding pre-procedural BNP values to known clinical AF risk scores for predicting AF recurrence after an index PVI.
Methods
Study Population
Between February of 2010 and December 2013, 229 consecutive patients with AF underwent a clinically indicated catheter ablation and were enrolled in the UMass Memorial Center (UMMC) AF Treatment Registry. As part of his or her standard workup, each AF Registry participant had a pre-procedural BNP level measured (normal lab range <100 pg/dl). Forty-six participants were excluded from the study since they had a history of prior AF ablation. Twenty-two participants were lost to follow up, leaving a total of 161 in the present analysis.
Demographic, clinical, and laboratory characteristics of participants were abstracted from the UMMC AF Treatment Registry and/or hospital electronic records by trained study staff. 12-lead ECGs were reviewed to validate the diagnosis of AF. Baseline venous blood samples for BNP were obtained within 1 week prior to catheter ablation for measurement as part of a standardized protocol. This study was approved by the University of Massachusetts Medical School Institutional Review Board (IRB #H00003865).
BNP and Composite Scores
A BNP level of ≥ 100 pg/dl was chosen as a cut-point based on results of prior analyses, which suggest that a level > 100 pg/dL is helpful in identifying AF patients at risk for progression from paroxysmal to persistent AF 17-19. Prediction scores often assign points for clinical and/or laboratory variables and translate point totals to estimated patient-level risks, typically assigning point values for baseline characteristics based on relative risks derived from multivariate logistic regression models 20. In keeping with this approach, and to remain consistent with previously proposed risk scores such as CHADS2, we sought to develop a new composite score that incorporated serum BNP into existing risk scores. In the proposed novel composite scores including BNP, previously proposed point values for other baseline clinical variables were retained, and we assigned points for a baseline BNP≥100 pg/dL based on the relative risk of BNP≥100 pg/dL for prediction of AF recurrence. We also compared the impact of addition of 1 point for BNP≥100 pg/dL as compared to the points derived through relative risk, for calculating the novel composite scores, on the risk of clinically significant late AF recurrence. Since the aim of the study was to evaluate the incremental predictive value of including pre-procedural BNP to the current risk prediction scores, we did not measure 3 month post-procedural BNP levels.
Pulmonary vein isolation procedure and post-procedure treatment
Patients enrolled in the UMMC AF Treatment Registry underwent PVI using either radiofrequency ablation (RFA) 21 or cryoablation (CRA) 22 by 1 of 5 cardiac electrophysiologists. The decision to use cryoablation or radiofrequency ablation, as well as which lesion set to employ, was determined by the attending electrophysiologist. However, the standardized approach outlined below was agreed upon and was performed in the vast majority of cases. In all patients undergoing RFA, wide area circumferential ablation was performed using an open-irrigated RFA catheter or 8 mm RFA catheter. In patients undergoing CRA, an Arctic Front cryoablation balloon catheter (28 mm or 23 mm, Medtronic, Inc.) was utilized to perform PVI 22. CRA in each vein was performed twice for 150-240 s. For both RFA and CRA patients, assessment of entrance and/or exit blockage was conducted using a circular mapping catheter. In patients with persistent AF, additional linear lesions were added at the LA roof, the basal posterior wall, and the LA isthmus at the discretion of the performing electrophysiologist.
After PVI, proton pump inhibitors were prescribed for 4 weeks and anti-arrhythmic therapies were generally discontinued at a routine 3-month follow-up visit if no symptomatic AF recurrences were reported or seen on routine post-procedure electrocardiographic examinations. Oral anticoagulation was prescribed for 3-6 months after ablation and long-term based on stroke risk as predicted by CHADS2 or CHA2DS2-VASc scores, all at the discretion of the treating physician.
AF Recurrence
Recurrence of AF during follow-up was ascertained from multiple sources, including clinical notes, telemetry data, and 12-lead ECGs from the participant's index hospitalization and outpatient follow up visits. All patients enrolled in the UMMC AF Treatment Registry were followed for at least 6 months after the index ablation to evaluate procedural complications and pre-specified clinical endpoints, including freedom from AF recurrence. All participants had a 12-lead ECG obtained 1, 3 and 6 months after their PVI and underwent a 7-day cardiac event monitor 1-month post-procedure. Additional ECGs and Holter recordings were obtained when patients’ symptoms were suggestive of AF. AF recurrence was defined on the basis of presence of AF on a 12-lead ECG or any AF lasting ≥ 20 seconds on a cardiac event monitor. If electric or pharmacological cardioversion or repeat procedure for AF were needed, this was also considered as an AF recurrence.
In order to focus on clinically important recurrences of AF, we divided AF recurrences into early and late. Early recurrences were defined as presence of AF during the traditional blanking period within 3 months after PVI 1. Late, or clinically significant, AF recurrences were defined AF on any tracing 3 or more months after ablation. Follow-up data was available for 6-months after ablation in all study participants. For the purpose of our study, risk of AF recurrence was defined as low (<40%), moderate (40-60%), and high (>60%) based on a priori assumptions about recurrence rates after PVI based on clinical trial and registry data 1.
Statistical analysis
Baseline statistics are presented as mean ± standard deviation (continuous variables) or as proportions (binary and categorical variables). Differences in proportions were tested using the chi-square test and differences in means by t-tests. Multivariate logistic regression was used to examine the relations of baseline predictors with AF recurrence. Variables to be included in multivariable models were selected a priori based on clinical relevance. Only factors associated with AF recurrences with a p value of <0.1 in univariate analyses were entered into the final model. Multivariate analyses examining the relations of baseline factors with clinically significant AF recurrence was performed separately for each composite score (Model 1 B-CHADS2, Model 2 B-CHA2DS2-VASc, Model 3 B-R2CHADS2, Model 4 B-HATCH) adjusting for adjusting for body mass index, gender, AF type (persistent vs. paroxysmal), chronic kidney disease, echocardiographic left atrial size, and ablation time. A two-sided p value of <0.05 was considered significant. We used Akaike's information criterion (AIC) and Bayesian information criterion (BIC) for determining the best composite score model 23. AIC and BIC are both penalized-likelihood criteria, and are used for choosing best predictor subsets in regression. Both criteria are based on various assumptions and asymptotic approximations; however a lower AIC and BIC means that the model is closer to the truth.
Receiver operating characteristic curves were generated to show score performance in predicting long-term, clinically significant AF recurrence. The C index was used to quantify the predictive value for a score. Reclassification tables were plotted to re-stratify the risk category of AF recurrence. In order to assess the discriminative ability and incremental yield of each composite risk score including BNP, net reclassification improvement (NRI) and Integrated Discrimination Improvement (IDI) were utilized 24. Statistical analysis was performed using Stata (Version 12, StataCorp, College Station, Texas).
Results
Baseline characteristics are shown in Table 1. Similar to other studies describing the characteristics of AF patients electing to undergo PVI 6-8, the mean age of study participants was 59 years (range 30-78 years), 30% were women, and participants had a moderate to severe burden of comorbid cardiovascular disease. The majority of participants had paroxysmal AF (60%) and 3 out of 4 were treated with an anti-arrhythmic drug at the time of ablation, reflecting the fact that the vast majority of participants had a consensus Class IA indication for catheter ablation 1. Despite the fact that only seven participants had heart failure at baseline, 40% of the overall sample had a BNP ≥100 pg/dL and the median BNP level was 80 pg/dl. A total of 77 participants (48%) experienced a clinically significant late AF recurrence.
Table 1.
Baseline characteristics of 161 study participants by atrial fibrillation recurrence status.
| Variable | AF recurrence* (N=77) | No AF recurrence (N=84) | p-value |
|---|---|---|---|
| Age (years) | 61.6 ± 8.8 | 56.9 ± 10.1 | 0.002 |
| Female sex | 23 (30) | 25 (30) | 0.99 |
| Body Mass Index (kg/m2) | 32.2 ± 6.2 | 32 ± 5.9 | 0.84 |
| Clinical characteristics | |||
| Persistent atrial fibrillation | 34 (44) | 30 (36) | 0.27 |
| Congestive heart failure | 3 (4) | 4 (5) | 0.79 |
| Hypertension | 57 (74) | 57 (68) | 0.39 |
| Diabetes mellitus | 22 (29) | 15 (18) | 0.11 |
| Stroke | 10 (13) | 4 (5) | 0.2 |
| Coronary artery disease | 22 (29) | 18 (21) | 0.29 |
| Obstructive sleep apnea | 25 (32) | 36 (43) | 0.17 |
| Chronic kidney disease | 10 (13) | 4 (5) | 0.2 |
| Chronic obstructive pulmonary disease | 6 (8) | 4 (5) | 0.43 |
| Laboratory characteristics | |||
| B-natriuretic peptide (pg/dl) | 187.2 ± 169.5 | 104.4 ± 120.4 | 0.002 |
| C-reactive protein (mg/dl) | 4.9 ± 7.2 | 4.7 ± 9.2 | 0.89 |
| Echocardiographic characteristics | |||
| Ejection fraction (%) | 57.0 ± 11 | 59.5 ± 7.8 | 0.1 |
| Left atrial diameter (mm) | 42.0 ± 7.3 | 40.4 ± 6.3 | 0.2 |
| Medications | |||
| Class I or III antiarrhythmic drug | 52 (68) | 63 (75) | 0.29 |
| Beta blocker | 42 (55) | 42 (50) | 0.56 |
| Calcium channel blocker | 22 (29) | 15 (18) | 0.11 |
| Oral anticoagulant | 59 (77) | 57 (68) | 0.22 |
| Risk scores | |||
| CHADS2 | 1.3 ± 0.9 | 1.0 ± 1.0 | 0.05 |
| CHA2DS2-VASC | 1.6 ± 1.2 | 1.2 ± 1.1 | 0.04 |
| R2CHADS2 | 1.4 ± 1.1 | 1.0 ± 1.0 | 0.02 |
| HATCH | 1.1 ± 0.9 | 0.9 ± 1.0 | 0.19 |
Data expressed as mean ± SD or N (%).
AF recurrence here defined as a clinically significant AF recurrence occurring between 3 and 6 months after ablation.
Predictors of clinically significant, late AF recurrence after PVI
Factors associated with clinically significant, late AF recurrence after PVI are presented in Table 1. Patients with late AF recurrence were on average older, were more likely to have experienced an early AF recurrence, and had higher CHADS2, CHA2DS2-VASC, and R2CHADS2 scores as compared to those who did not have an AF recurrence (p for all < 0.05). There was no statistically significant difference in HATCH scores between the two groups. Baseline serum BNP levels (pg/dl) were, on average, 80 pg/dL higher among participants with AF recurrence as compared to those who did not experience a recurrence (p=0.002). A BNP level of ≥100 pg/dl was seen in 59% of the participants with AF recurrence as compared to 29% with no AF recurrence (p<0.001). Compared to other baseline risk score components, BNP≥100 was found to increase the risk of recurrent atrial fibrillation with a relative risk of 3 in the current study, and hence a value of 3 points was assigned for BNP≥100 pg/dl for the calculation of novel composite scores.
Logistic regression was used to analyze relations of clinical and laboratory factors and late AF recurrence (Tables 2 and 3). In age and sex-adjusted analyses, BNP (p=0.01) and early AF recurrence (p<0.001) were associated with late AF recurrence. In multivariate analyses, CHADS2 (p=0.31), CHA2DS2-VASC (p=0.27), R2CHADS2 (p=0.25), and HATCH (p=0.5) scores were not associated with late AF recurrence. In contrast, B-CHADS2 (p=0.004), B-CHA2DS2-VASc (p=0.005), B-R2CHADS2 (p=0.003), and B-HATCH (p=0.003) remained associated with clinically significant late AF recurrence after adjustment (Table 3).
Table 2.
Age- and sex-adjusted odds of recurrent AF recurrence at 6 months for clinical, echocardiographic, and laboratory factors.
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Age* | 1.05 (1.02-1.09) | 0.003 |
| Female gender* | 1.01 (0.51-1.98) | 0.99 |
| Body Mass Index | 1.02 (0.97-1.08) | 0.43 |
| Persistent AF | 1.38 (0.71-2.68) | 0.34 |
| Congestive heart failure | 0.88 (0.17-4.50) | 0.88 |
| Hypertension | 1.11 (0.54-2.26) | 0.78 |
| Diabetes mellitus | 1.69 (0.79-3.63) | 0.18 |
| Stroke | 1.57 (0.67-3.71) | 0.3 |
| Coronary artery disease | 1.02 (0.47-2.23) | 0.96 |
| Obstructive sleep apnea | 0.70 (0.36-1.37) | 0.3 |
| Chronic kidney disease | 1.43 (0.60-3.38) | 0.42 |
| Chronic obstructive pulmonary disease | 1.63 (0.43-6.16) | 0.47 |
| Laboratory variables | ||
| C-reactive protein | 1.00 (0.96-1.05) | 0.92 |
| B-natriuretic peptide^ | 1.5 (1.11-2.02) | 0.008 |
| Echocardiographic variables | ||
| Ejection Fraction | 0.97 (0.93-1.00) | 0.09 |
| Left atrial diameter | 1.03 (0.97-1.09) | 0.32 |
| Medications | ||
| Class I or III antiarrhythmic drug | 0.62 (0.30-1.28) | 0.2 |
| Beta blocker | 1.14 (0.60-2.17) | 0.69 |
| Calcium channel blocker | 2.03 (0.94-4.40) | 0.07 |
| Anticoagulant | 1.18 (0.56-2.48) | 0.66 |
Not adjusted for age and sex
Odds ratio for every 100 point increase in BNP
Table 3.
Multivariate model for 6-month AF recurrence.
| MV Model 1 | MV Model 2 | MV Model 3 | MV Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | OR | p-value | OR | p-value | OR | p-value | OR | p-value |
| Female gender | 0.93 | 0.9 | 0.89 | 0.85 | 0.92 | 0.89 | ||
| Body Mass Index | 1.0 | 0.92 | 1.00 | 0.91 | 1.00 | 0.95 | 1.01 | 0.75 |
| Persistent AF | 0.85 | 0.78 | 0.92 | 0.89 | 0.78 | 0.66 | 0.82 | 0.74 |
| Ablation time | 1.01 | 0.28 | 1.01 | 0.24 | 1.01 | 0.31 | 1.01 | 0.26 |
| 3-month AF recurrence | ||||||||
| Diabetes mellitus | 0.82 | 0.77 | ||||||
| Left atrial diameter | 1.0 | 0.93 | 1.01 | 0.84 | 1.01 | 0.86 | 1.01 | 0.86 |
| Chronic kidney disease | 2.19 | 0.24 | 2.03 | 0.28 | 2.01 | 0.31 | ||
| BNP ≥100^ | 3.4 | 3.39 | 3.36 | 3.44 | ||||
| B-CHADS2 | 1.47 | 0.004 | ||||||
| B-CHA2DS2-VASC | 1.43 | 0.005 | ||||||
| B-R2CHADS2 | 1.46 | 0.003 | ||||||
| B-HATCH | 1.49 | 0.003 | ||||||
Odds ratios derived from logistic regression models using each baseline risk score and BNP>100 as predictors of AF recurrence at 6 months
Reclassification of AF risk by adding BNP to CHADS2 and CHA2DS2-VASc Risk Scores
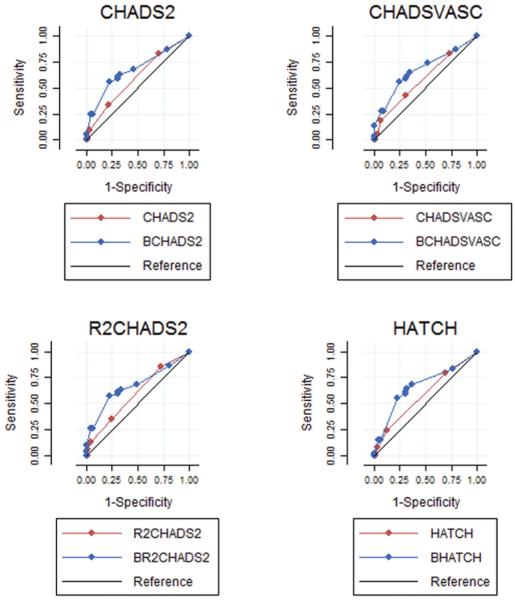
We compared the performance of CHADS2 and CHA2DS2-VASc to that of a composite risk score comprised of each score plus BNP for prediction of clinically significant late AF recurrence in relation to the original outcome in the present study. We derived a “B-CHADS2” and a “B-CHA2DS2-VASc” score by adding 3 points if a participant had a BNP level ≥100 pg/dl before their index ablation. The BCHADS2 score (C statistic 0.67 vs. 0.59, p=0.05; NRI 60%; IDI 0.08) and the B-CHA2DS2-VASc score (C statistic 0.67 vs. 0.59, p=0.04; NRI 60%; IDI 0.08) showed superior discriminatory ability and risk reclassification compared to CHADS2 and CHA2DS2-VASc, respectively (Table 4, Figure 1).
Table 4.
Comparison of each clinical AF risk score to a composite score adding 3 points for BNP>100 pg/dL with respect to prediction of clinically significant AF recurrence.
| Clinical AF Risk Score | AF Risk score AUC (95% CI) | Composite Score AUC (95% CI) | Comparison of composite score vs risk score model | % of individuals correctly classified by risk score ≥4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| p-value (ROC curve equality) | NRI (%) | p-value (NRI) | IDI | p-value (IDI) | Original score | Composite score | |||
| CHADS2 | 0.59 (0.51-0.67) | 0.67 (0.57-0.77) | 0.05 | 60 | 0.001 | 0.08 | 0.001 | 52.17 | 64.59 |
| CHA2DS2-VASC | 0.59 (0.50-0.67) | 0.67 (0.57-0.77) | 0.04 | 60 | 0.001 | 0.08 | 0.001 | 53.41 | 63.35 |
| R2CHADS2 | 0.57 (0.50-0.65) | 0.66 (0.56-0.76) | 0.03 | 60 | 0.001 | 0.08 | 0.001 | 52.17 | 64.59 |
| HATCH | 0.6 (0.51-0.68) | 0.67 (0.57-0.77) | 0.05 | 60 | 0.001 | 0.08 | 0.001 | 54.03 | 65.21 |
AUC- Area under the curve; NRI - Net reclassification improvement; IDI – Integrated discrimination improvement
Figure 1.
Receiver-Operating curve (ROC) comparisons of clinical risk scores compared to composite risk scores including BNP data.
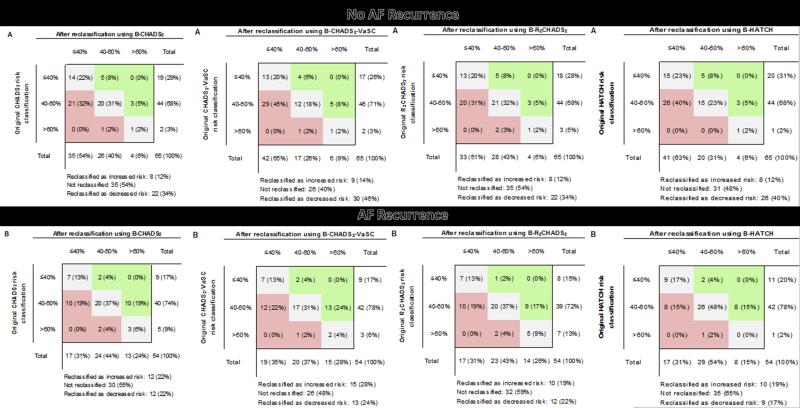
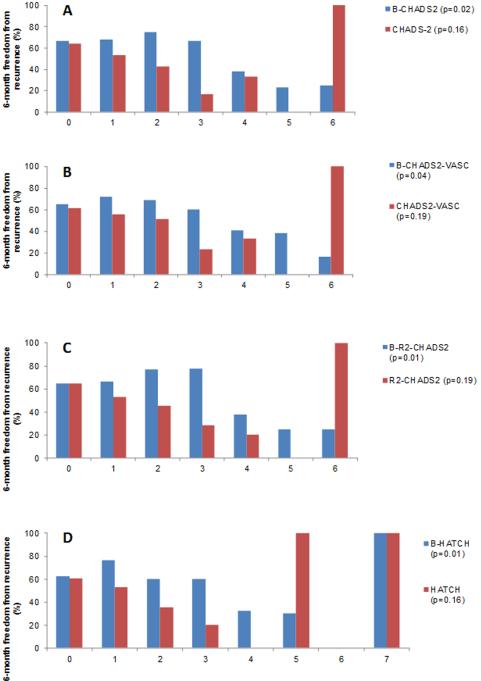
The BNP-informed B-CHADS2 score reclassified 34% of participants without an AF recurrence as being at lower risk as compared to CHADS2 score alone; and reclassified 22% of participants with AF recurrence as being at higher risk (Figure 2). The BNP-informed B-CHA2DS2-VASc score reclassified 46% of participants without an AF recurrence as being at lower risk; and reclassified 28% of participants with AF recurrence as being at higher risk in comparison to CHA2DS2-VASc alone. Risk of late AF recurrence for a B-CHADS2 score of ≥4 is 67.4% as compared to 46% for a score of <4, and for a B-CHA2DS2-VASc score of ≥4 is 63% as compared to 47.9% for a score of <4 (Figure 3). B-CHADS2 and B-CHA2DS2-VASc had an AIC score of 155.4 and 155.5; and a BIC score of 161 and 161.1 respectively.
Figure 2.
Reclassification of risk for 6-month AF recurrence using composite risk scores, compared to original risk classification; A) among subjects with no AF recurrence at 6 months, and B) among subjects with AF recurrence at 6 months.
Figure 3.
Percentage of participants free from AF at 6-months by type and number of clinical or composite risk score points. Panel A: CHADS2 vs. B-CHADS2; Panel B: CHA2DS2-VASc vs. BCHA2DS2-VASc; Panel C: R2CHADS2 vs. B-R2CHADS2; Panel D: HATCH vs. B-HATCH
Reclassification of AF risk by adding BNP to R2CHADS2 and HATCH Risk Scores
We compared the performance of R2CHADS2 and HATCH to that of a composite risk score comprised of each score plus BNP for prediction of clinically significant late AF recurrence in relation to the original outcome in the present study. We derived a “B-R2CHADS2” and a “B-HATCH” score by adding 3 points if a participant had a BNP level ≥100 pg/dl. The B-R2CHADS2 score (C statistic 0.66 vs. 0.57, p=0.03; NRI 60%; IDI 0.08) and the B-HATCH score (C statistic 0.67 vs. 0.6, p=0.05; NRI 60%; IDI 0.08) showed superior discriminatory ability and risk reclassification compared to R2CHADS2 and HATCH, respectively (Table 4, Figure 1).
The BNP-informed B-R2CHADS2 score reclassified 34% of participants without an AF recurrence as being at lower risk as compared to R2CHADS2 score alone; and reclassified 19% of participants with AF recurrence as being at higher risk (Figure 2). The BNP-informed B-HATCH score correctly reclassified 40% of participants without an AF recurrence as being at lower risk; and reclassified 17% of participants with AF recurrence as being at higher risk in comparison to HATCH alone. Risk of late AF recurrence for a B-R2CHADS2 score of ≥4 is 66.7% as compared to 44% for a score of <4; and for a B- HATCH score of ≥4 is 68.9% as compared to 46% for a score of <4 (Figure 3). B-HATCH had an AIC score of 156.5 and a BIC score of 162. B-R2CHADS2 had the lowest AIC score of 155.2, and a BIC score of 160.7 as compared to the other composite score models thus indicating it to be the best score model for prediction of late AF recurrence. Even if adding 1 point instead of 3 points for a BNP level ≥100, the p values for the composite scores would still be associated with AF recurrence, with a p value <0.03 with a NRI of 60% and an IDI of 0.08.
Discussion
In this moderately-sized sample of participants undergoing an index PVI for symptomatic AF, we found that higher BNP levels were associated with clinically significant AF recurrence and that inclusion of BNP into CHADS2, CHA2DS2-VASc, R2CHADS2 and HATCH scores significantly improved the ability of these scores to identify individuals at risk for clinically significant, late AF recurrence. B-R2CHADS2 was found to be the best score in prediction of late AF recurrence.
BNP and AF Recurrence
Likely due to the associations between subtle left ventricular dysfunction, atrial structural remodeling, and AF, higher BNP levels are frequently observed in participants with AF even if they do not have clinically overt heart failure 25-29. Several studies have shown that pre-ablation BNP levels relate to AF recurrence after catheter ablation for AF 30-33. For example, in a study of 68 participants with AF, baseline BNP levels were over 2-fold higher in those with AF recurrence at 3 months than among those who remained free from AF 30. Another retrospective study of 726 participants with lone AF showed that higher BNP levels pre-ablation were also associated with increased risk of AF recurrence during follow-up 32. In addition, several studies have built on these findings by showing that reduction in BNP levels after AF ablation also predicts of freedom from AF 34-37. However, in the only recent study to distinguish between early and late, clinically significant AF ablation, the authors found that serum BNP levels rose in the context of AF, but pre-ablation BNP levels did not relate to AF ablation success 38. In light of these conflicting findings, there is currently lack of consensus with respect to the usefulness of BNP for predicting incident or recurrent AF.
In one of the largest published studies to date to examine the relations between pre-ablation BNP levels with clinically significant AF recurrence, we observed that BNP levels were significantly higher in participants with a clinically significant AF recurrence as compared to those who remained free from AF. Moreover, we found that a BNP level of ≥100 pg/dl was strongly related to AF recurrence. Associations between BNP and AF recurrence persisted after multivariate adjustment for factors known to be associated with higher AF risk, including age, sex, chronic kidney disease and echocardiographic left atrial size.
Composite scores as predictors of AF recurrence
Several investigations involving fewer participants than the present study have shown that a CHADS2 score ≥2 is associated with higher rates of late AF recurrence with modest AUCs 8, 39-41. However, a recent study from the Leipzig Heart Center AF Ablation Registry analyzing data from 2069 patients undergoing AF ablation found a CHADS2 score was not associated with early or late AF recurrence 6. Our findings are similar to those of the Leipzig study, namely that CHADS2 was not associated with clinically significant, late AF recurrence after adjustment for other clinically significant factors.
Prior studies have shown an incremental predictive benefit of adding BNP to CHADS2 with respect to important AF-related outcomes, including thromboembolism, heart failure and coronary artery disease 42. We found that the B-CHADS2 score was superior to CHADS2 alone with respect to identifying participants with clinically significant AF recurrence and that a composite risk score including BNP showed reasonable discriminatory capabilities. B-CHADS2 was also an independent predictor of late AF recurrence.
Several investigators have shown that a CHA2DS2-VASc score ≥2 is associated with AF recurrence (AUC 0.627) 8. The Leipzig Heart Center AF study suggested, however, that the CHA2DS2-VASc score did not identify individuals at risk for AF recurrence after PVI (AUC=0.57) 6. Consistent with this prior study, we found that the CHA2DS2-VASc score was not associated with AF recurrence. The B-CHA2DS2-VASc score was superior to CHA2DS2-VASc score in identifying participants with late AF recurrence. A composite risk score including BNP showed reasonable discriminatory capabilities.
Chronic kidney disease is associated with AF and poor rhythm outcomes after catheter ablation 9, 43, 44. Kornej et al., showed that R2CHADS2 score, a score informed by eGFR, was associated with AF recurrence (HR 1.14), despite the fact that eGFR <60 ml/min/1.73m2 was not associated with AF recurrence in multivariate analyses 6. We did not observe that chronic kidney disease was predictive of AF recurrence. This could, however, be explained by the small proportion (n=14, 9%) of participants with chronic kidney disease in our sample. We did, however, observe that the B-R2CHADS2 score was superior to R2CHADS2 score alone in identifying participants with late AF recurrence and was an independent predictor of late AF recurrence. A B-R2CHADS2 score cut-off of ≥4 was a significant predictor of late AF recurrence. B-R2CHADS2 had the lowest AIC and BIC indicating it to be a better model as compared to the other composite risk scores in prediction of late AF recurrence.
Chronic obstructive pulmonary disease (COPD) has been associated with increased risk of AF 45. The HATCH score, an AF risk score that incorporates information about severity of pulmonary disease, was recently proposed to identify individuals at risk for progression from paroxysmal to persistent AF 10. We did not find COPD as an independent risk factor for AF recurrence. However, only 6% of the total sample had COPD. Schmidt et al. showed that patients with a HATCH score of >3 had a higher risk of AF recurrence after catheter ablation 7. However, another study demonstrated that the HATCH score was not related to AF recurrence. However, only 10% of study participants had a score ≥2 46. We did not find that the HATCH score was associated with late AF recurrence. However, the B-HATCH was superior to HATCH score alone in identifying participants with late AF recurrence and was associated with late AF recurrence.
Strengths and Limitations
This study of participants with AF undergoing an index ablation procedure is strengthened by its use of a registry with comprehensive contemporary clinical, electrophysiological, and laboratory data and standardized longitudinal monitoring for AF recurrence. However, several limitations of our study warrant consideration. Although participants did undergo per protocol ECG and 7-day cardiac event monitoring as well as symptom-triggered assessments after ablation, it is possible that we missed asymptomatic episodes of AF. However, this would lead to an underestimation of AF recurrence and therefore is unlikely to explain our primary study findings. We included only patients undergoing index catheter ablation, thus we are not able to explore the usefulness of AF recurrence risk scores after redo AF ablation. The observational nature of the present investigation precludes assumptions of causality and it is possible that unmeasured factors introduced confounding. Treating physicians were not blinded to BNP status and thus it is possible that this influenced frequency of screening or lowered their threshold to order more intensive AF monitoring. The study participants were predominantly white and male, and therefore results may not be generalizable to other racial or ethnic groups.
Conclusions
In a moderate-sized, contemporary cohort of patients undergoing an index ablation procedure for symptomatic AF, pre-ablation BNP levels were associated with clinically significant AF recurrence. Clinical risk scores showed modest ability to discriminate between participants with an AF recurrence during follow-up and those who remained free from AF. However, an AF-recurrence score comprised of clinical risk elements plus 3 points for BNP ≥100 pg/dL significantly improved the discriminative ability of all clinical risk scores. Our findings suggest that knowledge of baseline BNP may augment clinical risk prediction and better inform therapeutic decision making around whether or not to recommend PVI for AF. Further work is needed to validate our findings in other cohorts of patients undergoing PVI and monitored long-term for AF recurrence.
Acknowledgments
Funding: This study was funded by the Cardiology Division, University of Massachusetts Medical School, by the National Heart, Lung and Blood Institute (1U01HL105268-01) and by KL2RR031981 (DDM).
Abbreviations
- AF
Atrial fibrillation
- PVI
Pulmonary vein isolation
- BNP
B-type natriuretic peptide
Footnotes
Author Contributions: AYS – Study design and concept, Data collection, Statistical Analysis, Manuscript writing; NE – Data collection, Manuscript writing; WMD – Data collection, Statistical Analysis, Manuscript writing; MK – Data collection, Manuscript writing; IN – Data collection; KCF – Manuscript writing; CB – Manuscript writing; CE – Manuscript writing; JKD – Manuscript writing; LSR – Manuscript writing; DDM – Study design and concept, Data collection, Statistical Analysis, Manuscript writing.
Financial disclosures:
AYS – None; NE – None; WMD – None; MK – None; IN – None; KCF – None; CB – None; CE – None; JKD – None; LSR – Moderate funding: Biotronik, Inc.; DDM – Grant support: University of Massachusetts Center for Clinical and Translational Science Award, National Heart Lung and Blood Institute, Sanofi Aventis, Medtronic, Biotronik, and Philips Healthcare.
References
- 1.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696. e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 3.Kornej J, Hindricks G, Kosiuk J, Arya A, Sommer P, Husser D, Rolf S, et al. Renal dysfunction, stroke risk scores (CHADS2, CHA2DS2-VASc, and R2CHADS2), and the risk of thromboembolic events after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2013;6:868–74. doi: 10.1161/CIRCEP.113.000869. [DOI] [PubMed] [Google Scholar]
- 4.Guo Y, Tian Y, Wang H, Si Q, Wang Y, Lip GY. Prevalence, incidence and lifetime risk of atrial fibrillation in China: New insights into the global burden of atrial fibrillation. Chest. 2014 doi: 10.1378/chest.14-0321. [DOI] [PubMed] [Google Scholar]
- 5.Hung CY, Hsieh YC, Huang JL, Lin CH, Wu TJ. Statin Therapy for Primary Prevention of Atrial Fibrillation: Guided by CHADS2/CHA2DS2VASc Score. Korean Circ J. 2014;44:205–9. doi: 10.4070/kcj.2014.44.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornej J, Hindricks G, Kosiuk J, Arya A, Sommer P, Husser D, Rolf S, et al. Comparison of CHADS2, R2CHADS2, and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2014;7:281–7. doi: 10.1161/CIRCEP.113.001182. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt EU, Schneider R, Lauschke J, Wendig I, Bansch D. The HATCH and CHA2DS 2-VASc scores. Prognostic value in pulmonary vein isolation. Herz. 2014;39:343–8. doi: 10.1007/s00059-013-3835-x. [DOI] [PubMed] [Google Scholar]
- 8.Letsas KP, Efremidis M, Giannopoulos G, Deftereos S, Lioni L, Korantzopoulos P, Vlachos K, et al. CHADS2 and CHA2DS2-VASc scores as predictors of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace. 2014;16:202–7. doi: 10.1093/europace/eut210. [DOI] [PubMed] [Google Scholar]
- 9.Chao TF, Tsao HM, Ambrose K, Lin YJ, Lin WS, Chang SL, Lo LW, et al. Renal dysfunction and the risk of thromboembolic events in patients with atrial fibrillation after catheter ablation--the potential role beyond the CHA(2)DS(2)-VASc score. Heart Rhythm. 2012;9:1755–60. doi: 10.1016/j.hrthm.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 10.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–31. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Jongnarangsin K, Suwanagool A, Chugh A, Crawford T, Good E, Pelosi F, Jr., Bogun F, et al. Effect of catheter ablation on progression of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:9–14. doi: 10.1111/j.1540-8167.2011.02137.x. [DOI] [PubMed] [Google Scholar]
- 12.Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34:1475–80. doi: 10.1093/eurheartj/eht024. [DOI] [PubMed] [Google Scholar]
- 13.Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–74. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck-da-Silva L, de Bold A, Fraser M, Williams K, Haddad H. Brain natriuretic peptide predicts successful cardioversion in patients with atrial fibrillation and maintenance of sinus rhythm. Can J Cardiol. 2004;20:1245–8. [PubMed] [Google Scholar]
- 15.Freynhofer MK, Jarai R, Hochtl T, Bruno V, Vogel B, Aydinkoc K, Nurnberg M, et al. Predictive value of plasma Nt-proBNP and body mass index for recurrence of atrial fibrillation after cardioversion. Int J Cardiol. 2011;149:257–9. doi: 10.1016/j.ijcard.2011.02.043. [DOI] [PubMed] [Google Scholar]
- 16.Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, et al. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF Consortium of community-based cohort studies. Europace. 2014;16:1426–33. doi: 10.1093/europace/euu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maisel A. B-type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next? Circulation. 2002;105:2328–31. doi: 10.1161/01.cir.0000019121.91548.c2. [DOI] [PubMed] [Google Scholar]
- 18.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–6. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knudsen CW, Omland T, Clopton P, Westheim A, Wu AH, Duc P, McCord J, et al. Impact of atrial fibrillation on the diagnostic performance of B-type natriuretic peptide concentration in dyspneic patients: an analysis from the breathing not properly multinational study. J Am Coll Cardiol. 2005;46:838–44. doi: 10.1016/j.jacc.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 20.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. Jama. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 21.Eitel C, Hindricks G, Sommer P, Gaspar T, Kircher S, Wetzel U, Dagres N, et al. Circumferential pulmonary vein isolation and linear left atrial ablation as a single-catheter technique to achieve bidirectional conduction block: the pace-and-ablate approach. Heart Rhythm. 2010;7:157–64. doi: 10.1016/j.hrthm.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 23.Jones RH. Bayesian information criterion for longitudinal and clustered data. Stat Med. 2011;30:3050–6. doi: 10.1002/sim.4323. [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Wang L. B-type natriuretic peptide levels in patients with paroxysmal lone atrial fibrillation. Heart Vessels. 2006;21:137–40. doi: 10.1007/s00380-005-0884-y. [DOI] [PubMed] [Google Scholar]
- 26.Yamada T, Murakami Y, Okada T, Okamoto M, Shimizu T, Toyama J, Yoshida Y, et al. Plasma atrial natriuretic Peptide and brain natriuretic Peptide levels after radiofrequency catheter ablation of atrial fibrillation. Am J Cardiol. 2006;97:1741–4. doi: 10.1016/j.amjcard.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 27.Kim BJ, Hwang SJ, Sung KC, Kim BS, Kang JH, Lee MH, Park JR. Assessment of factors affecting plasma BNP levels in patients with chronic atrial fibrillation and preserved left ventricular systolic function. Int J Cardiol. 2007;118:145–50. doi: 10.1016/j.ijcard.2006.03.088. [DOI] [PubMed] [Google Scholar]
- 28.Morello A, Lloyd-Jones DM, Chae CU, van Kimmenade RR, Chen AC, Baggish AL, O'Donoghue M, et al. Association of atrial fibrillation and amino-terminal pro-brain natriuretic peptide concentrations in dyspneic subjects with and without acute heart failure: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Am Heart J. 2007;153:90–7. doi: 10.1016/j.ahj.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Richards M, Di Somma S, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, et al. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: results from the BACH Study (Biomarkers in ACute Heart Failure). JACC Heart Fail. 2013;1:192–9. doi: 10.1016/j.jchf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Shin DI, Deneke T, Gorr E, Anders H, Buenz K, Paesler M, Horlitz M. Predicting successful pulmonary vein isolation in patients with atrial fibrillation by brain natriuretic Peptide plasma levels. Indian Pacing Electrophysiol J. 2009;9:241–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang HJ, Son JW, Nam BH, Joung B, Lee B, Kim JB, Lee MH, et al. Incremental predictive value of pre-procedural N-terminal pro-B-type natriuretic peptide for short-term recurrence in atrial fibrillation ablation. Clin Res Cardiol. 2009;98:213–8. doi: 10.1007/s00392-009-0744-3. [DOI] [PubMed] [Google Scholar]
- 32.Hussein AA, Saliba WI, Martin DO, Shadman M, Kanj M, Bhargava M, Dresing T, et al. Plasma B-type natriuretic peptide levels and recurrent arrhythmia after successful ablation of lone atrial fibrillation. Circulation. 2011;123:2077–82. doi: 10.1161/CIRCULATIONAHA.110.007252. [DOI] [PubMed] [Google Scholar]
- 33.Mohanty S, Mohanty P, Di Biase L, Rong B, Burkhardt D, Gallinghouse JG, Horton R, et al. Baseline B-type natriuretic peptide: a gender-specific predictor of procedure-outcome in atrial fibrillation patients undergoing catheter ablation. J Cardiovasc Electrophysiol. 2011;22:858–65. doi: 10.1111/j.1540-8167.2011.02036.x. [DOI] [PubMed] [Google Scholar]
- 34.Date T, Yamane T, Inada K, Matsuo S, Miyanaga S, Sugimoto K, Shibayama K, et al. Plasma brain natriuretic peptide concentrations in patients undergoing pulmonary vein isolation. Heart. 2006;92:1623–7. doi: 10.1136/hrt.2005.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada T, Murakami Y, Okada T, Yoshida N, Toyama J, Yoshida Y, Tsuboi N, et al. Plasma brain natriuretic peptide level after radiofrequency catheter ablation of paroxysmal, persistent, and permanent atrial fibrillation. Europace. 2007;9:770–4. doi: 10.1093/europace/eum157. [DOI] [PubMed] [Google Scholar]
- 36.Kurosaki K, Tada H, Hashimoto T, Ito S, Miyaji K, Naito S, Oshima S, et al. Plasma natriuretic peptide concentrations as a predictor for successful catheter ablation in patients with drug-refractory atrial fibrillation. Circ J. 2007;71:313–20. doi: 10.1253/circj.71.313. [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Murakami Y, Okada T, Yoshida N, Toyama J, Yoshida Y, Tsuboi N, et al. Plasma brain natriuretic peptide level after hybrid therapy with pulmonary vein isolation and antiarrhythmic drugs for atrial fibrillation. Int Heart J. 2008;49:143–51. doi: 10.1536/ihj.49.143. [DOI] [PubMed] [Google Scholar]
- 38.Pillarisetti J, Reddy N, Biria M, Ryschon K, Nagarajan D, Murray C, Atkins D, et al. Elevated brain natriuretic peptide level in patients undergoing atrial fibrillation ablation: is it a predictor of failed ablation or a mere function of atrial rhythm and rate at a point in time? J Interv Card Electrophysiol. 2014;40:161–8. doi: 10.1007/s10840-014-9898-7. [DOI] [PubMed] [Google Scholar]
- 39.Saad EB, d'Avila A, Costa IP, Aryana A, Slater C, Costa RE, Inacio LA, Jr., et al. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score </=3: a long-term outcome study. Circ Arrhythm Electrophysiol. 2011;4:615–21. doi: 10.1161/CIRCEP.111.963231. [DOI] [PubMed] [Google Scholar]
- 40.Chao TF, Tsao HM, Lin YJ, Tsai CF, Lin WS, Chang SL, Lo LW, et al. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012;5:514–20. doi: 10.1161/CIRCEP.111.968032. [DOI] [PubMed] [Google Scholar]
- 41.D'Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, di Biase L, Natale A, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. Int J Cardiol. 2013;167:1984–9. doi: 10.1016/j.ijcard.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura M, Koeda Y, Tanaka F, Onoda T, Itai K, Ohsawa M, Tanno K, et al. Plasma B-type natriuretic peptide as a predictor of cardiovascular events in subjects with atrial fibrillation: a community-based study. PLoS One. 2013;8:e81243. doi: 10.1371/journal.pone.0081243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkowitsch A, Kuniss M, Greiss H, Wojcik M, Zaltsberg S, Lehinant S, Erkapic D, et al. Impact of impaired renal function and metabolic syndrome on the recurrence of atrial fibrillation after catheter ablation: a long term follow-up. Pacing Clin Electrophysiol. 2012;35:532–43. doi: 10.1111/j.1540-8159.2012.03350.x. [DOI] [PubMed] [Google Scholar]
- 44.Chao TF, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, et al. Associations between renal function, atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Circ J. 2011;75:2326–32. doi: 10.1253/circj.cj-11-0178. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Agarwal SK, Alonso A, Blecker S, Chamberlain AM, London SJ, Loehr LR, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129:971–80. doi: 10.1161/CIRCULATIONAHA.113.004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang RB, Dong JZ, Long DY, Yu RH, Ning M, Jiang CX, Sang CH, et al. Efficacy of catheter ablation of atrial fibrillation beyond HATCH score. Chin Med J (Engl) 2012;125:3425–9. [PubMed] [Google Scholar]