Abstract
Inactivation of the rodent medial prefrontal cortex (mPFC) and hippocampus or disconnection of the hippocampus from the mPFC produces deficits in spatial working memory tasks. Previous studies have shown that delay length determines the extent to which mPFC and hippocampus functionally interact, with both structures being necessary for tasks with longer delays and either structure being sufficient for tasks with shorter delays. In addition, inactivation of the nucleus reuniens (Re) / rhomboid nucleus (Rh) of the thalamus, which has bidirectional connections with the mPFC and hippocampus, also produces deficits in these tasks. However, it is unknown how delay duration relates to the function of Re/Rh. If Re/Rh are critical in modulating mPFC-hippocampus interactions, inactivation of the RE/Rh should produce a delay-dependent impairment in spatial working memory performance. To investigate this question, groups of rats were trained on one of three different spatial working memory tasks: continuous alternation (CA), delayed alternation with a five-second delay (DA5), or with a thirty-second delay (DA30). The Re/Rh were inactivated with muscimol infusions prior to testing. The results demonstrate that inactivation of RE/Rh produces a deficit only on the two DA tasks, supporting the notion that the Re/Rh is a critical orchestrator of mPFC-HC interactions.
1. Introduction
Working memory is a specific type of memory requiring the active maintenance of information that is pertinent to ongoing behavior (Baddeley, 2003). One brain region involved in working memory is the medial prefrontal cortex (mPFC) (Euston et al., 2012). Lesions of the mPFC produce deficits in tasks that are thought to require the maintenance of relevant action-guiding information (Eichenbaum et al. 1983; Kolb et al.1994; Floresco et al. 1997; Urban et al. 2014). The hippocampus is another important structure for working memory in addition to its role in declarative memory (Squire, 1992; Squire, 2004) and spatial cognition (O'Keefe and Nadel, 1978; Morris et al., 1982; for review see Hartley et al., 2014). Specifically, lesions of hippocampus produce deficits in delayed alternation tasks (Ainge et al., 2007; Czerniawski et al., 2009; Hallock et al., 2013), which depend on a working memory strategy for successful performance.
Electrophysiological studies in rodents have shown that neurons in both the mPFC and hippocampus demonstrate firing patterns that correlate with task relevant information (Jung et al., 1998; Ainge et al., 2007; Pastalkova et al., 2008). Moreover, the hippocampus and mPFC functionally synchronize during working memory tasks (Jones and Wilson 2005, Gordon, 2011, O'Neill et al., 2013), indicating that these regions form a neural circuit that is important for working memory performance. The hippocampus sends efferent projections from its ventral/intermediate subregion directly to all subregions of the mPFC (Ferino et al., 1987; Jay and Witter, 1991; Floresco & Grace, 2003; Ishikawa & Nakamura, 2003). Although there are no return projections from the mPFC to the hippocampus, a prominent indirect bidirectional pathway between these two regions includes the reuniens and rhomboid nuclei of the ventral midline thalamus (Re/Rh), which are reciprocally connected to both hippocampus and mPFC (Vertes, 2006; Vertes et al., 2007). In line with the neuroanatomical data, inactivation of Re/Rh produces deficits in the performance of spatial working memory tasks, which are similar to deficits seen after hippocampus and mPFC lesions or inactivation (Hembrook & Mair, 2011; Cholvin et al., 2013; Hembrook et al., 2012; Hallock et al., 2013).
Results from behavioral studies suggest that the mPFC and hippocampus interact in working memory tasks with longer inter-trial delays and act in parallel during tasks with shorter delays. These findings suggests that delay length is a factor that determines the reliance of mPFC-hippocampus interactions on task performance (Floresco et al., 1997; Lee & Kesner, 2003). To explicitly test this notion, Churchwell and Kesner (2011) disconnected the mPFC and intermediate CA1 of the hippocampus using a crossed unilateral muscimol inactivation approach while rats performed a spatial delayed non-match to sample working memory task with a ten second delay or a 5 minute delay. They found that pharmacological disconnection of intermediate CA1 from mPFC impairs task performance for the longer delay but not for the shorter delay.
The extent to which Re/Rh nuclei are necessary for communication between hippocampus and mPFC over a range of delays is not known. Therefore, the current study investigated the role of the Re/Rh in working memory by inactivating Re/Rh prior to the performance of one of three spatial alternation tasks: continuous alternation (CA), delayed alternation with a five-second delay (DA5), or with a thirty-second delay (DA30). The results showed that inactivation of Re/Rh did not disrupt CA task performance. However, DA30 task performance was impaired across all muscimol doses and DA5 task performance was impaired only for the highest muscimol dose. These results add evidence to the growing body of literature implicating Re/Rh activity in coordinated information transfer between PFC and hippocampus, setting the stage for future studies to determine the functional role of the Re/Rh in cross-regional synchronization and working memory.
2. Methods
2.1 Subjects
Adult male Long-Evans hooded rats (Harlan, Indianapolis) were individually housed in a temperature and humidity-controlled colony room on a 12 h light/dark cycle. Rats were food restricted in order to maintain an 80-90% ad libitum body weight and given ad libitum access to water. All animal procedures were carried out in accordance with the University of Delaware Institutional Animal Care and Use Committee.
2.2 Apparatus
Tasks were performed on a wooden T-maze, which consisted of a central stem (116 × 10 cm), two return arms (112 × 10 cm each), and two goal arms (56.5 × 10 cm each) and surrounded by 6 cm high wooden walls. Plastic bottle caps were located at the end of each goal arm containing the chocolate sprinkle reward. For the delayed alternation (DA) tasks, rats were confined to a start box located at the base of the maze between trials using a large, removable wooden barrier.
2.3 Handling, Pretraining, and Behavioral Training
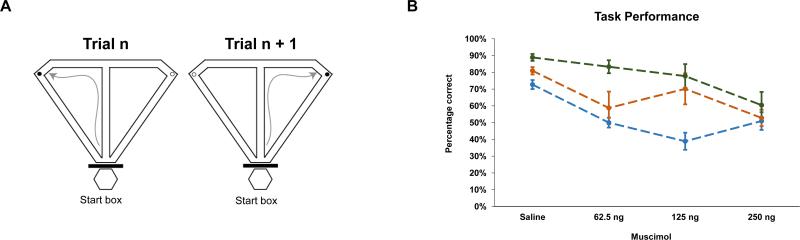
The handling and pre-training procedures were performed as previously described (Hallock et al., 2013). After completing pretraining, rats were assigned to one of three tasks: CA, DA with a five-second delay (DA5), or a thirty-second delay (DA30) (see Figure 1a). In the CA task, rats were required to run in a continuous “figure 8” pattern alternating visits to the left and right goal arms to obtain the chocolate sprinkle reward. Similarly, rats were required to alternate between the left and the right goal arms in the DA tasks. However, rats were confined to the start box for five (DA5) or thirty (DA30) seconds between trials. Each CA and DA session began with a free choice trial, during which both goal arms were baited, followed by 24 alternation trials.
Fig 1.
(A) Task schematic; all tasks were performed on the same T-maze. For each task, rats were required to run up the central stem, choose a goal arm, and consume the chocolate sprinkle reward (the black dots). Rats were rewarded for alternating between the left and right goal arms on successive trials. For the CA task, the start box was blocked off from the rest of the maze, and the rat was required to run in a figure eight pattern. For both of the DA tasks, rats waited in the start box between trials (5 seconds for DA5, 30 seconds for DA30). (B) Percentage correct for CA (green), DA5 (orange), and DA30 (blue) across the saline and 3 muscimol sessions. Error bars represent ±SEM.
2.4 Surgical Procedures
Rats were anesthetized with isoflurane (1.5 - 3% in oxygen) and placed in a stereotaxic frame. A scalp incision was made and bone screws were secured to the skull with dental acrylic (Lang Dental, Wheeling, Illinois.) A hole was then drilled 1.8 mm posterior to bregma and 2.0 mm lateral to the midline. An 8.0 mm guide cannula (PlasticsOne, Roanoke, Virginia) was lowered 6.5 mm ventral to the surface of the brain at a 15-degree angle. The cannula was cemented to the skull and the bone screws with dental acrylic, and a dummy cannula with a 1.0 mm projection was inserted. Banamine (2.5 mg/kg) was injected subcutaneously thirty minutes prior to the end of surgery for pain relief. Post-surgery, children's Ibuprofen (20 mg/ml) was mixed into each rat's drinking water for 2 days. Rats recovered for 5 days post-surgery.
2.5 Behavioral Testing
Following the post-surgery recovery period, rats were retrained on their designated tasks until they reached a performance criterion of >80% correct choices for three consecutive sessions. The following day after meeting criterion, 0.5 μl of vehicle (phosphate-buffered saline (PBS)) was infused, and choice accuracy was measured. Immediately prior to the next session, one of three concentrations of the GABAA receptor agonist muscimol (in PBS) was infused and choice accuracy measured. Muscimol was given in three concentrations: 0.125 (62.5 ng), 0.25 (125 ng), and 0.5 ug/ul (250 ng). The order of infusions was counterbalanced and rats were given infusion-free sessions between muscimol infusions until they reached at least 80% choice accuracy.
2.6 Infusion Protocol
The dummy cannula was removed, and an internal cannula made to fit the guide cannula with a one-millimeter projection was inserted. The internal cannula was attached to a plastic tube that contained either phosphate-buffered saline (PBS) or muscimol. A polyethylene tube was attached to a microinfusion syringe (Hamilton), and placed into an automated infusion pump (World Precision Instruments) with a controlled infusion rate and volume of (0.25 μl/minute and 0.5 μl, respectively). The position of the infusate was monitored, marking an air bubble that separated the infusate from distilled water. Internal cannulae remained in brain for two minutes post-infusion to avoid capillary diffusion. Rats were placed back in their home cages for twenty minutes prior to behavioral testing.
2.7 Histology
After behavioral testing, rats were given an infusion (0.5 μl volume) of fluorophore-conjugated BODIPY TMR-X muscimol (Life Technologies, Carlsbad, CA) to visualize the spread of muscimol (Allen et al. 2008). The fluorescent muscimol was diluted to a concentration of 0.25 μg/μl in solution with equal parts PBS and vehicle DMSO. Twenty minutes after infusion of the fluorescent muscimol, rats were transcardially perfused with 0.9% saline followed by 10% buffered formalin. Brains were removed and allowed to sit in 10% buffered formalin for two days, and were then transferred to a 30% sucrose solution (30 mg sucrose/100 ml PBS). After sinking, the brains were sectioned (40 μm) with a cryostat and mounted on slides. Half the slides were stained with cresyl violet (0.5%) and photographed using a camera mounted microscope. The other half of the slides were stained with ProLong Gold with DAPI (Life Technologies, Carlsbad, CA), and visualized with a confocal microscope. Cannula placement and spread of the fluorescent muscimol was characterized by placing digital plates from the Paxinos and Watson (2005) rat brain atlas over pictures of the cresyl-stained and DAPI-stained brain slices using Adobe Illustrator.
2.8 Data Analysis
A mixed-factor 3 (delay) × 4 (muscimol) ANOVA was used to compare choice accuracy on saline and muscimol sessions between the CA, DA5 and DA30 groups, followed by posthoc one-way repeated-measures ANOVAs on each group. Post-hoc Bonferroni multiple comparison tests were then used to compare choice accuracy between each of the muscimol sessions and the saline session.
3. Results
3.1 Cannula Placements
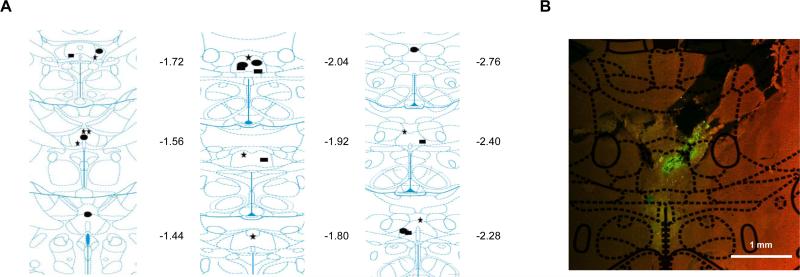
Twenty two rats were confirmed to have placements in the Re/Rh, with nine rats in the DA30 group, seven rats in the DA5 group, and ten rats in the CA group (see Figure 2A). The majority of cannula placements were in the Re/Rh. Two rats, one from DA5 and one from DA30 group had cannula placements localized to ventral Re. Two rats from the CA group had cannula terminations partially in the paraxiphoid nucleus, though the spread of the muscimol from the cannula terminations was largely confined to Re/Rh nuclei (see Fig 2B).
Fig 2.
(A) Coronal plates showing cannula terminations for all three groups (CA –rectangle, DA5 –circle, and DA30 – star). Numbers next to each plate indicate distance from bregma in mm. (B) Representative coronal slice detailing the spread of the fluorophore-conjugated muscimol (green). Plate shows the regions of the thalamic nuclei, and that the spread of the fluorescent muscimol is largely confined to RE/Rh. All plates are re-printed with permission from The Rat Brain in Sterotaxic Coordinates: 5th ed., pp. 87–100, by G. Paxinos and C. Watson, 2005, Burlington, MA, Elsevier Academic Press. Copyright 2005 by Elsevier
3.2 Inactivation of Re significantly impairs DA30 and DA5
A 3 × 4 mixed-factor ANOVA on the percentage of correct choices revealed a significant main effect of delay (F(2,24) = 7.602, p = .003), a significant main effect of muscimol (F(3,72) = 17.689, p < .001), and a significant delay × muscimol interaction (F(6,73) = 3.435, p = .005) (see Figure 1B). Posthoc repeated measures one-way ANOVAs followed by Bonferroni multiple comparisons tests for each task revealed that for the CA task, choice accuracy on the saline-infusion session did not significantly differ from any of the muscimol-infusion sessions (p>.1 in all cases). For the DA5 task, choice accuracy on the saline-infusion session did not differ from the choice accuracy on the 62.5 ng session (p = .314) or the 125 ng session (p = 1.000), but was significantly greater than choice accuracy on the 250 ng session (p = .013). For the DA30 task, choice accuracy on saline-infusion sessions was significantly better than choice accuracy on each of the muscimol-infusion sessions (62.5 ng (p = .007), 125 ng (p = .011), 250 ng (p = .013)). Choice accuracy on the muscimol-infusion sessions did not differ from one another on any of the tasks.
4. Discussion
The hypothesis of the current study was that the extent of Re/Rh involvement in working memory increases with the delay interval over which information needs to be held, which would implicate the Re/Rh in mediating hippocampal-mPFC interactions. We tested this hypothesis by comparing the effects of Re/Rh inactivation on choice accuracy on three versions of a spatial alternation task: CA, which has no delay between trials, and two variants of a DA task, one with a short intertrial delay (DA5) and one with a long intertrial delay (DA30). Consistent with our predictions, inactivation of Re/Rh impaired choice accuracy only on DA5 and DA30 , leaving CA performance unaffected. Moreover, only the highest dose of muscimol produced a performance deficit on DA5, while all three doses of muscimol produced deficits on DA30. This difference in dose-response curves between the DA5 and DA30 groups suggests a gradient of sensitivity to Re/Rh inactivation that parallels the increase in delay length. These results are in agreement with other studies demonstrating that Re/Rh are necessary for spatial working memory tasks (Hembrook et al., 2011; Hallock et al., 2013). Our results extend these previous results showing that the DA task not only requires Re/Rh for successful performance, but also that its dependence upon Re/Rh increases with longer delays.
One of the likely functions of Re/Rh, based on its anatomical connections, is its modulation of communication between hippocampus and mPFC, two regions believed to be crucial for spatial working memory (Colgin, 2011; Gordon, 2011). Re/Rh neurons make direct synaptic connections onto mPFC and hippocampus neurons and mPFC fibers synapse onto hippocampal projecting neurons, suggesting that Re/Rh is positioned to directly affect mPFC and hippocampus activity (Vertes et al. 2007). It has been demonstrated that DA is dependent on the hippocampus (Ainge et al. 2007; Czerniawski, Yoon, and Otto, 2009; Hallock et al. 2013) and is also dependent on the mPFC (Kolb et al. 1994). Previous studies have demonstrated that the duration of the delay determines whether hippocampus and mPFC work together or independently during task performance, with either structure being sufficient for tasks with shorter delays, but hippocampus-PFC communication being necessary for tasks with longer delays (Lee & Kesner, 2003; Churchwell & Kesner, 2011). How Re/Rh affects mPFC-hippocampus communication is not known, as there has yet to be a study directly investigating the role of Re/Rh in mPFC-hippocampus interactions. Previous findings suggest that Re/Rh activity is important only if the task requires both mPFC and hippocampus, but not hippocampus or mPFC individually (Cholvin et al., 2013; Hembrook et al., 2012).
Although the Re/Rh have been implicated in non-cognitive behavioral functions, including reproduction (Iwasaki et al. 2010), nociception (Gholami et al., 2006; Bullitt, 1990; Dostrovsky and Guilbaud, 1990), and feeding (Wilmot et al. 1988; Chait et al., 1995), attention has largely shifted to its role in higher-level cognition, with Re/Rh activity being linked to attention, executive functions (i.e. impulse control, behavioral flexibility), and spatial memory consolidation (for review see Cassel et al. 2013). The Re/Rh has also been shown to be one of many loci of head direction cells (Jankowski et al. 2014). Another hypothesis of Re/Rh function based on its involvement in spindle generation and cortical arousal is that the Re/Rh is part of a hippocampo-cortico-thalamic network underlying consolidation of declarative memories (Pereira de Vasconcelos & Cassel, 2015). Recent work shows that Re/Rh is necessary for trajectory-specific firing of hippocampal CA1 neurons during a spatial alternation task, suggesting that Re/Rh serves as a crucial component of a hippocampal-mPFC circuit responsible for goal-directed behavior (Ito et al. 2015). This notion is consistent with an earlier study showing that Re is part of a circuit involving the mPFC and hippocampus that determines the extent of fear memory generalization (Xu and Südhof, 2013). Further investigation into the function of Re/Rh and its role in the hippocampal-mPFC circuit will further our understanding of the mechanisms responsible for spatial working memory, and more generally, help us to understand how thalamic nuclei modulate synchrony across cortical and subcortical structures.
Highlights.
We show that inactivation of the ventral midline thalamus impairs working memory.
Task performance was disrupted in a delay-dependent manner.
The findings suggest that this region regulates prefrontal-hippocampal synchrony.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant 1P20GM103653 – 01A1 (A.L.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainge JA, van der Meer MAA, Langston RF, Wood ER. Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus. 2007;17(10):988–1002. doi: 10.1002/hipo.20301. [DOI] [PubMed] [Google Scholar]
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods. 2008;171(1):30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-Fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. Journal of Comparative Neurology. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature Reviews Neuroscience. 2003;4:829–837. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Cassel JC, Pereira VA, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP. The reuniens and rhomboid nuclei: Neuroanatomy, electrophysiological characteristics and behavioral implications. Progress in Neurobiology. 2013;111:34–52. doi: 10.1016/j.pneurobio.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait A, Suaudeau C, De BR. Extensive brain mapping of calcitonin-induced anorexia. Brain Research Bulletin. 1995;36(5):467–472. doi: 10.1016/0361-9230(94)00223-n. [DOI] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De SND, Raingard H, Cassel JC. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. Journal of Neuroscience. 2013;33(20):8772–83. doi: 10.1523/JNEUROSCI.0771-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: Interactions and independent parallel processing. Behavioural Brain Research. 2011;225:389–395. doi: 10.1016/j.bbr.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurobiology. 2011;21:467–474. doi: 10.1016/j.conb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czemiawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19(1):20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Guilbaud G. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain. 1990;40:93–104. doi: 10.1016/0304-3959(90)91056-O. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Clegg RA, Feeley A. Reexamination of functional subdivisions of the rodent prefrontal cortex. Experimental Neurology. 1983;79(2):434–51. doi: 10.1016/0014-4886(83)90224-8. [DOI] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from ammon's horn to the medial prefrontal cortex in the rat. Experimental Brain Research. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective Roles for Hippocampal, Prefrontal Cortical, and Ventral Striatal Circuits in Radial-Arm Maze Tasks With or Without a Delay. Journal of Neuroscience. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. Journal of Neuroscience. 2003;23:3930–43. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami S, Lambertz D, Hoheisel U, Mense S. Effects on c-Fos expression in the PAG and thalamus by selective input via tetrodotoxin-resistant afferent fibres from muscle and skin. Neuroscience Research. 2006;56:270–278. doi: 10.1016/j.neures.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurobiology. 2011;21:486–491. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Arreola AC, Shaw CL, Griffin AL. Dissociable roles of the dorsal striatum and dorsal hippocampus in conditional discrimination and spatial alternation T-maze tasks. Neurobiology of Learning and Memory. 2013;100:108–116. doi: 10.1016/j.nlm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL. Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behavioral Neuroscience. 2013;127:860–866. doi: 10.1037/a0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Phil. Trans. R. Soc. B. 2014;369:20120510. doi: 10.1098/rstb.2012.0510. http://dx.doi.org/10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Mair RG. Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus. 2011;21(8):815–26. doi: 10.1002/hipo.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Onos KD, Mair RG. Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus. 2012;22(4):853–860. doi: 10.1002/hipo.20945. [DOI] [PubMed] [Google Scholar]
- Hölscher C, Jacob W, Mallot HA. Learned association of allocentric and egocentric information in the hippocampus. Experimental Brain Research. 2004;158(2):233–240. doi: 10.1007/s00221-004-1896-z. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. Journal of Neuroscience. 2003;23:9987–95. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, Moser EI, Moser MB. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522(7554):50–55. doi: 10.1038/nature14396. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Jodo E, Kayama Y, Kawauchi A, Miki T, Koyama Y. Role of the lateral preoptic area and the bed nucleus of stria terminalis in the regulation of penile erection. Brain Research. 2010;1357:70–78. doi: 10.1016/j.brainres.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Jankowski MM, Islam MN, Wright NF, Vann SD, Erichsen JT, Aggleton JP, O'Mara SM. Nucleus reuniens of the thalamus contains head direction cells. Elife. 2014;3 doi: 10.7554/eLife.03075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. Journal of Comparative Neurology. 1991;313(4):574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta Rhythms Coordinate Hippocampal–Prefrontal Interactions in a Spatial Memory Task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. doi:10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cerebral Cortex. 1998;8:437–50. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, McDonald R, Sutherland RJ. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cerebral Cortex. 1994;4:664–80. doi: 10.1093/cercor/4.6.664. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. Journal of Neuroscience. 2003;23:1517–23. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behavioral and Neural Biology. 1994;61(3):260–70. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- O'Neill P-K, Gordon JA, Sigurdsson T. Theta Oscillations in the Medial Prefrontal Cortex Are Modulated by Spatial Working Memory and Synchronize with the Hippocampus through Its Ventral Subregion. The Journal of Neuroscience. 2013;33:14211–14224. doi: 10.1523/JNEUROSCI.2378-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annual Review of Neuroscience. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–7. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. Elsevier Academic Press; San Diego: 2005. [Google Scholar]
- Pereira de Vasconcelos A, Cassel J. The nonspecific thalamus: A place in a wedding bed for making memories last? Neuroscience and Biobehavioral Reviews. 2015;54:175–196. doi: 10.1016/j.neubiorev.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory. 2004;82(3):171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Urban KR, Layfield DM, Griffin AL. Transient inactivation of the medial prefrontal cortex impairs performance on a working memory-dependent conditional discrimination task. Behavioral Neuroscience. 2014;128(6):639–43. doi: 10.1037/bne0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do VAC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. Journal of Comparative Neurology. 2006;499(5):768–96. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: Link between the medial prefrontal cortex and the hippocampus. Brain Research Bulletin. 2007;71(6):601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot CA, Sullivan AC, Levin BE. Effects of diet and obesity on brain alpha1- and alpha2-noradrenergic receptors in the rat. Brain Research. 1988;453:157–166. doi: 10.1016/0006-8993(88)90154-0. [DOI] [PubMed] [Google Scholar]
- Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–5. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]