Abstract
Ultraviolet (UV) radiation-induced systemic immune suppression is a major risk factor for skin cancer induction. The migration of dermal mast cells from the skin to the draining lymph nodes plays a prominent role in activating systemic immune suppression. UV-induced keratinocyte-derived platelet-activating factor (PAF) activates mast cell migration, in part by up regulating the expression of CXCR4 on the surface of mast cells. Others have indicated that epigenetic mechanisms regulate CXCR4 expression, so we asked whether PAF activates epigenetic mechanisms in mast cells. Human mast cells were treated with PAF and the effect on DNA methylation and/or acetylation was measured. PAF suppressed the expression of DNA methyltransferase (DNMT) 1 and 3b. On the other hand, PAF increased p300 histone acetyltransferase expression, and the acetylation of histone H3, which coincided with a decreased expression of the histone deacetylase HDAC2. Chromatin immunoprecipitation assays indicated that PAF-treatment activated the acetylation of the CXCR4 promoter. Finally, inhibiting histone acetylation blocked p300 up-regulation and suppressed PAF-induced surface expression of CXCR4. Our findings suggest a novel molecular mechanism for PAF, activation of epigenetic modifications. We suggest that PAF may serve as an endogenous molecular mediator that links the environment (UV radiation) with the epigenome.
INTRODUCTION
Platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine), is a naturally occurring, phospholipid mediator of inflammation (Prescott et al., 2000). PAF is produced by epidermal keratinocytes in response to UVB-irradiation (Marathe et al., 2005; Travers et al., 2010) and is linked to UVB-induced systemic immune suppression (Walterscheid et al., 2002), a major risk factor for skin cancer induction (Yoshikawa et al., 1990). Because most UVB radiation is absorbed within the upper layers of the skin, indirect mechanisms must be involved in transmitting the suppressive signal from the skin to the immune system. Migration of mast cells from the irradiated skin to the draining lymph nodes plays a critical role (Byrne et al., 2008). Mast cell-deficient mice are resistant to the immunosuppressive effects of UV radiation (Hart et al., 1998), and inhibiting mast cell migration in UVB-irradiated mice treated with a CXCR4 antagonist blocks the induction of immune suppression (Byrne et al., 2008). PAF activates the up-regulation of the chemokine CXCR4 on the mast cell surface and promotes the migration of mast cells from the skin to the lymph nodes (Chacón-Salinas et al., 2014). PAF also up-regulates the expression of the CXCR4 ligand (CXCL12), on lymph node cells (Byrne et al., 2008), thus setting the chemokine gradient for directing mast cell migration from the skin to the draining lymph node, where they secrete IL-10 and suppress the immune response (Chacón-Salinas et al., 2011). In addition to skin cancer (Sahu et al., 2012; Sreevidya et al., 2008), PAF is involved other types of cancer (Aponte et al., 2008; Bussolati et al., 2000; Denizot et al., 2006; Denizot et al., 2005), and likely plays an important role in inflammation-related carcinogenesis. Hence, a better understanding of the mechanism(s) by which inflammatory mediators such as PAF contribute to inflammation, carcinogenesis and immune suppression is needed.
Evidence suggests that epigenetic mechanisms may be involved in modulating the expression of pro-inflammatory signals (Adcock et al., 2007; Medzhitov and Horng, 2009; Shuto et al., 2006; Sullivan et al., 2007). Epigenetic alterations have also been linked to tumor development, including skin cancer (Counts and Goodman, 1995; Jones, 2002; Jones and Baylin, 2007). The up-regulation of CXCR4 expression on melanoma cells treated with DNA demethylating agents suggests that epigenetic mechanisms regulate the expression of CXCR4 (Mori et al., 2005). Sato and colleagues (Sato et al., 2005) reported hyperacetylation of the CXCR4 promoter in pancreatic cancer. Similarly, epigenetic regulation of CXCR4 in cutaneous and uveal melanoma has been reported (Mori et al., 2005; Li et al., 2013). Furthermore, the absence of methylation on the CXCR4 promoter is associated with poorer overall survival in breast cancer patients (Ramos et al., 2011). Inhibition of histone deacetylase by valproic acid increased CXCR4 expression in hematopoietic stem/progenitor cells (Gul et al., 2009). These studies support the concept that CXCR4 may be regulated at the transcriptional level by both DNA methylation and chromatin remodeling.
It is now recognized that environmental stimuli can modify the epigenetic profile of a gene, and some suggest that epigenetic mechanisms connect the genome with the external environment (Suarez-Alvarez et al., 2013). Chromatin structure is un-raveled by the acetylation of lysine tails of the nucleosomal core histones H3 and H4 activating gene expression, whereas histone deacetylation is associated with gene silencing (Cheung et al., 2000; Pazin and Kadonaga, 1997). Gene silencing may also be controlled by DNA methylation in which the addition of cytosines to the promoter region of target genes is mediated by DNA methyltransferase enzymes (Rhee et al., 2002). Because PAF is an important inducer of CXCR4 on mast cells, we asked if PAF activates epigenetic mechanisms that affect the expression of CXCR4.
RESULTS
PAF up-regulates CXCR4 expression on human mast cells
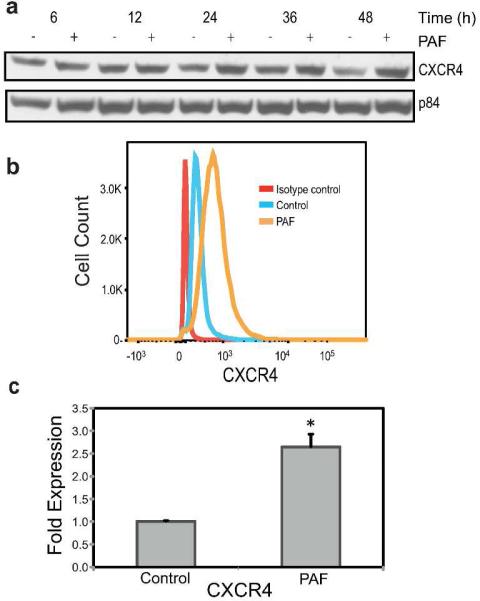
Because all of our previous data demonstrating that PAF up-regulates CXCR4 expression was obtained with murine mast cells, we asked if PAF similarly affects human mast cells. We used the human mast cell line HMC-1 (Butterfield et al., 1988; Juremalm et al., 2000; Ma et al., 2013). Based on previous studies, we used concentrations of PAF that activated significant physiological changes in vitro (Feuerherm et al., 2013; Chacón-Salinas et al., 2014; Dy et al., 1999; Pei et al., 1998; Walterscheid et al., 2002), without affecting cell viability (Puebla-Osorio et al., 2015). The metabolically stable PAF analog, carbamyl-PAF (cPAF) was used. Immunoblotting demonstrated up-regulation of CXCR4 in HMC-1 cells 24, 36 and 48 hours after cPAF treatment (Figure 1a). Using flow cytometry we confirmed that cPAF induced the expression of CXCR4 on the surface of HMC-1 cells (Figure 1b), and RT-qPCR was used to document that cPAF induced close to a three-fold increase in CXCR4 mRNA expression (Figure 1c). These results demonstrate that cPAF up-regulates human mast cell CXCR4 expression.
FIGURE 1.
CXCR4 expression in PAF-treated HMC-1 cells. (a) Immunoblotting analysis of CXCR4 protein expression 6 to 24 hours after cPAF (10 μM) treatment. p84 was the loading control. (b) Flow cytometry was used to measure CXCR4 surface expression 24 hours post cPAF treatment. (c) CXCR4 mRNA levels were increased 24 hours post cPAF treatment. Data represent the mean ± SEM (N=3), *p < 0.05 vs. control (Student's t-test).
PAF depresses DNA methyltrasferase expression in HMC-1 cells
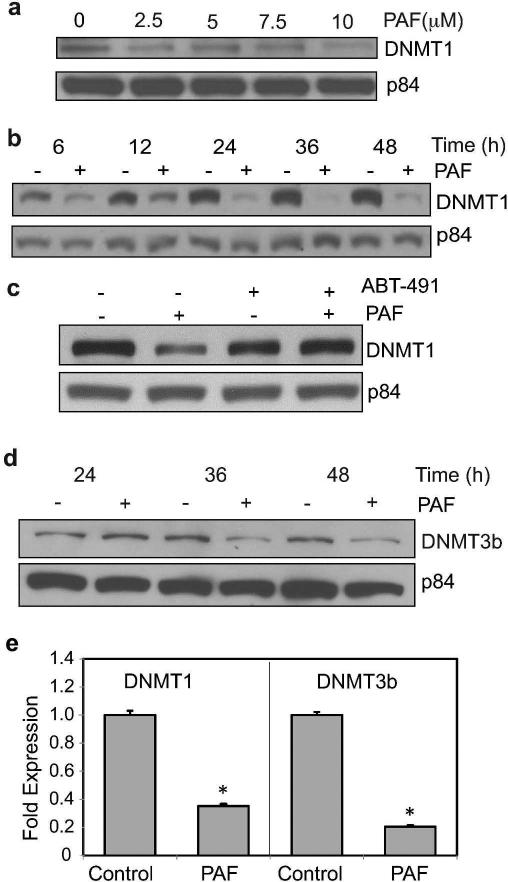
To determine whether PAF affected methylation patterns in mast cells, we first addressed its effect on common DNA methyltransferases. cPAF induced a concentration- and time-dependent reduction of DNMT1 in HMC-1 cells (Figure 2a). cPAF induced a reduction in DNMT1 as early as 6 hours, and a dramatic decrease in DNMT1 expression was observed 24 hours after cPAF treatment (Figure 2b). The effect was sustained for up to 48 hours post exposure. To confirm the effect on DNMT1 expression was attributed to cPAF binding to its receptor on mast cells, we used a PAF receptor antagonist ABT-491 (Albert et al., 1997). Failure to see a down regulation of DNMT1 expression in cells treated with the PAF-receptor antagonist indicates that the cPAF-induced reduction in DNMT1 is via cPAF binding to its receptor (Figure 2c). Next, we assessed the effect of cPAF on DNMT3b expression and observed a similar effect. cPAF induced a decrease in DNMT3b protein expression 36 and 48 hours post treatment (Figure 2d). These results were confirmed by RT-qPCR, which indicated that cPAF induced a significant reduction in mRNA levels, including a five-fold decrease in DNMT3b and a three-fold decrease in DNMT1 (Figure 2e). However, when we measured the methylation pattern of CpG islands on the promoter region of CXCR4, by bisulfite modification coupled with pyrosequencing, we found no statistical differences between the control group and the cPAF-treated group (Supplemental Figure 1). This observation led us to focus on the effects of cPAF on histone acetylation.
FIGURE 2.
PAF decreases the expression of DNMT 1 and 3b. (a) DNMT1 Protein expression in cPAF-treated HMC-1 cells was analyzed by immunoblotting; p84 is the loading control. (b) Time course of cPAF (10 μM)-induced DNMT1 depression. (c) Treating the cells with a PAF-receptor antagonist (AMT-491) blocks the cPAF-induced depression of DNMT1. (d) Time-course of cPAF-induced depression of DNMT3b expression. Effect of cPAF on DNMT1 and 3b mRNA expression as measured with RT-qPCR. Data represent the mean ± SEM (N=3), *p < 0.05 vs. control (Student's t-test).
PAF effects acetylation of the CXCR4 promoter
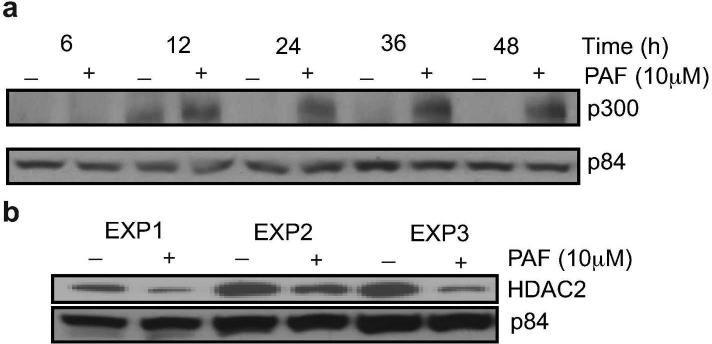
First we asked whether cPAF affected the expression of known acetylating and deacetylating enzymes, which could be indicators of active gene expression (Gong and Miller, 2013). cPAF induced a time-dependent increase in the expression of p300, which has an intrinsic histone acetyltransferase activity (Counts and Goodman, 1995). Increased protein expression of p300 was observed starting at 12 hours and high expression continued for the duration of the experiment (Figure 3a). This coincided with decreased expression of the deacetylating enzyme, HDAC2 (Figure 3b).
FIGURE 3.
PAF affects the expression of p300 and HDAC2 in HMC-1 cells. (a) Time-course expression of p300 after incubation with 10 μM cPAF. p84 is the loading control. (b) HDAC2 expression was depressed 24 hours post cPAF (10 μM) treatment. Data from three independent experiments are shown.
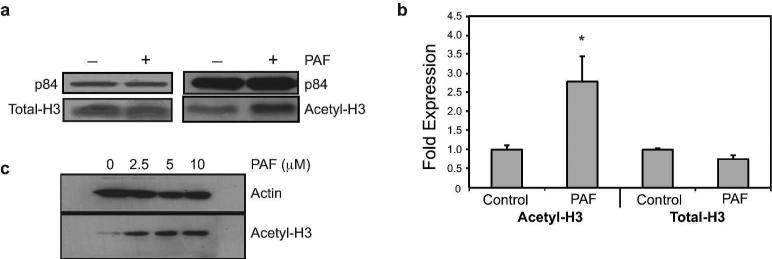
Next we determined whether cPAF affected the protein expression of acetylated H3 (H3K9/14/18/23/27) and H4 (H4K5/8/12/16) (Figure 4). As shown in Figure 4a the protein expression of acetyl-H3 was increased by cPAF. On the other hand, we were unable to reproducibly document acetylation of H4 (data not shown).
FIGURE 4.
Effect of PAF on histone acetylation. (a) Protein levels of total-H3 and acetylated-H3 in HMC-1 cells 24 hours post cPAF (10 μM) treatment. p84 is the loading control. (b) Acetylation of CXCR4 promoter by ChIP analysis. HMC-1 cells were treated with 10 μM cPAF and harvested 24 hours later. Total-H3 was used as positive control and the data were normalized against input DNA. Data represent the mean ± SEM (N=4), *p < 0.05 vs. control (Mann-Whitney U-test,). (c) PAF up-regulates the expression of acetyl-H3 mRNA in normal mast cells. Buffy coat-derived mast cells were treated with increasing concentrations of cPAF and immunoblotting was used to determine the expression of acetyl-H3(K9/14/18/23/27). Actin is the loading control.
To confirm that an increase in acetyl-H3 expression affected the acetylation status of the CXCR4 promoter, we performed chromatin immunoprecipitation assay (ChIP). Our results showed that cPAF induced an almost three-fold increase in the acetylation status of H3 (H3K9/14/18/23/27) on the promoter region of the CXCR4 gene (Figure 4b).
Next, we tested whether cPAF had a similar effect on non-transformed cells. We isolated buffy coat mast cells, as described previously (Puebla-Osorio et al., 2015), and treated them with different concentrations of cPAF. Our results indicate that cPAF up-regulates the expression of Acetyl-H3 (H3K9/14/18/23/27) in non-transformed mast cells, in a concentration dependent manner (Figure 4c), similar to that found in HMC-1 cells.
Inhibiting acetylation blocks cPAF-induced up-regulation of CXCR4
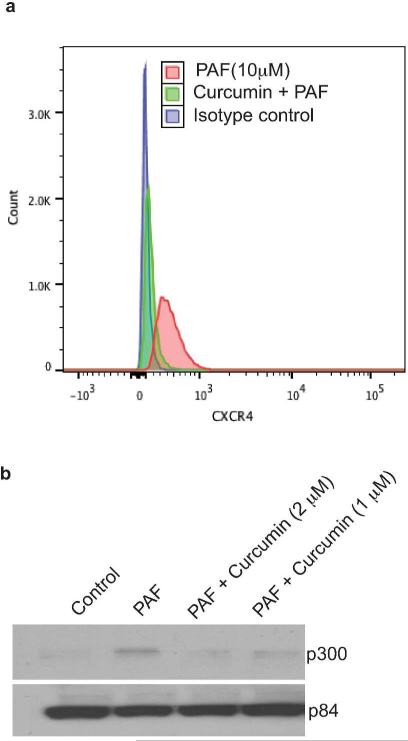
Because our findings to this point indicate that cPAF up-regulates mast cell surface expression of CXCR4 and histone acetylation of the CXCR4 promoter, we wanted to determine if the cPAF-induced up-regulation of mast cell CXCR4 surface expression was linked to increased acetylation. We used curcumin, a specific inhibitor of histone acetylation (Balasubramanyam et al., 2004), to block acetylation in cPAF-treated cells. The data presented in Figure 5a indicate that curcumin effectively reduced mast cell expression of CXCR4. Furthermore, curcumin depressed cPAF-induced up-regulation of p300 (Figure 5b). These data support the hypothesis that cPAF up-regulates CXCR4 expression on mast cells by activating epigenetic mechanisms.
FIGURE 5.
Blocking acetylation inhibits PAF-induced cell surface expression of CXCR4. (a) Mast cells treated with cPAF (red), or cPAF in the presence of curcumin (green) were analyzed by FACS 24 hours post cPAF treatment. The isotype control is shown in blue. (b) cPAF-induced up-regulation of p300 is inhibited by curcumin. An aliquot of the same cells analyzed by FACS was removed and lysed and p300 expression determined by immunoblotting. p84 is the loading control.
DISCUSSION
Here we show that CXCR4 expression is up regulated in human mast cells by cPAF and that this is associated with changes in epigenetic marks. As mentioned above, the dose of cPAF used here was chosen based on studies in the literature in which cPAF was used to activate cells in vitro (Feuerherm et al., 2013; Puebla-Osorio et al., 2015). Generally, tissue concentrations of PAF are reported to be in the picomolar range (Marathe et al., 2005; Travers et al., 2010), however in certain conditions, such as inflammation and cancer, serum levels as high as 10-7 molar, which approach the concentrations used here, have been reported (Lehr et al., 1997; Pitton et al., 1989). One must also keep in mind that PAF in the serum has a limited half-life (minutes) due to the action of PAF-acetylhydrolase (Stafforini et al., 1996). Furthermore, platelets and endothelial cells are known to produce PAF but not secrete it, rather the cell- associated form of PAF is active (Lorant et al., 1991). This suggests that the local concentration of PAF may be very high in inflamed tissues.
The down-regulation of DNA methyltransferase DNMT1 and DNMT3b is suggestive of reduced promoter methylation, which is associated with gene reactivation. These results are in line with the findings of Przybylski et al. (Przybylski et al., 2010) who showed that the reduction of both DNMT1 and DNMT3b results in an increased demethylation of the CXCR4 promoter, leading to an increased expression of CXCR4. Although our results on the methylation status, as assessed by bisulphite sequencing, showed no significant difference between cPAF-treated and the control samples, we showed that DNMTs are down regulated upon PAF exposure. Because cPAF promotes rather than depresses the expression of CXCR4, rather than pursuing methylation, we focused our attention on acetylation. We observed a significant up-regulation of the acetylation of H3 (H3K9/14/18/23/27) on the promoter region of CXCR4 in cPAF-treated mast cells. Further, we observed increased expression of p300 acetyltransferase, which promotes chromatin relaxation on transcriptionally active genes (Liu et al., 2008), and a decrease in HDAC2 histone deacetylase, a key player in deacetylation of lysine residues on core histones. These changes are generally associated with gene activation (Hildmann et al., 2007). Moreover, when we inhibited acetylation in cPAF-treated cells with curcumin, we were able to decrease both the expression of p300 and the cPAF-induced expression of CXCR4 on the surface of mast cells. These findings demonstrate that cPAF induced expression of CXCR4 is associated with increased acetylation of the promoter region of CXCR4, which presumably activates its transcription. cPAF also up-regulated histone acetylation in normal mast cells suggesting that its effect was not exclusive to transformed cells. Interestingly, these findings are supported by the work of Conte et al. who recently reported that HDAC2 reduces gene expression by repressing areas of chromatin that do not allow p300 binding and consequent acetylation, whereas silencing of HDAC2 activates p300 recruitment and H3K9-14 acetylation (Conte et al., 2014). Others have suggested that there is synergy between de-methylation and histone deacetylase inhibition in re-expression of genes (Cameron et al., 1999; Gray and Teh, 2001). In this respect, cPAF appears to share similarities in its action with both HDAC inhibitors and methylation inhibitors used in the development of drugs that target epigenetic regulators, such as Trichostatin A and 5-Aza 2-deoxycytidine (Li et al., 2013; Mori et al., 2005; Przybylski et al., 2010; Ramos et al., 2011).
Our findings provide evidence for a novel molecular mechanism for PAF, in that it may affect physiological and pathological processes via epigenetic modifications in human mast cells, and likely other cell types. Our findings allow us to speculate that a possible mechanism by which PAF mediates photo-immune suppression is through epigenetic modulation of the CXCR4 gene. Therefore, PAF may serve as a critical molecular mediator that links the environment (UVB radiation) with the epigenome.
MATERIALS AND METHODS
Antibodies and reagents
Carbamyl-PAF (cPAF), a non-hydrolyzable bioactive analog of PAF was obtained from Sigma-Aldrich (St Louis, MO). A 10 mM stock solution was prepared in water, aliquoted, and stored at −20 °C until use. The PAF receptor antagonist, ABT-491, was purchased from Sigma-Aldrich and prepared as a 24 mM stock solution in water. Antibodies specific for DNMT1 (sc-135886) and HDAC2 (sc-9959) were purchased from Santa Cruz (Dallas, TX). Antibodies specific for DNMT3b (ab13604), CXCR4 (ab2074), Acetyl-H3 (ab47915), and Total-H3 (ab1791) were purchased from Abcam (Cambridge, MA). Anti-p84 (GTX70220) was purchased from Genetex (Kennesaw, GA). Anti-mouse (7076S), and anti-rabbit horseradish peroxidase (7074S) and anti-p300 (K1499) were obtained from Cell Signaling Technology (Danvers, MA). Anti-rabbit Alexa488 was purchased from Molecular Probes (Eugene, OR). All other chemicals were purchased from Sigma-Aldrich.
Cell Cultures
The human mast cell line, HMC-1 (kindly provided by Dr. J.H. Butterfield, May Clinic, Rochester, MN) (Butterfield et al., 1988) was cultured in complete RPMI-1640 medium containing 10% heat inactivated fetal calf serum (FCS) under standard culture conditions (37°C, 5% CO2, humidified atmosphere). The cells were passaged every 3-4 days. Normal mast cells were isolated from a buffy coat obtained from an undisclosed healthy donor from the Gulf Coast Regional Blood Center (MDACC IRB LAB-03-0390), as described previously (Puebla-Osorio et al., 2015). Briefly, CD34+ cells were cultured in complete medium with human IL-6, IL-3, and recombinant human Stem Cell factor. After 4-6 weeks in culture the all the viable cells stained positive for CD117 (cKit), tryptase and toluidine blue.
Treatment with cPAF
HMC-1 cells were seeded at a density of 5 × 105 cells/ml in 60 × 15 mm petri dishes and treated with 10 μM cPAF for various time points (6-48 hours). When the PAF receptor antagonist was used, the cells were pre-treated with 100 μM ABT-491, 1 h prior to treatment with cPAF.
Western Blot Analysis
Cells were harvested, centrifuged, washed with cold PBS, and the cell pellet was then lysed with 200 μl RIPA buffer. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). The proteins were separated on 6%, 8% or 15% SDS-PAGE gels. Proteins were transferred onto polyvinylidene difluoride membranes overnight at 4°C. Non-specific binding sites were blocked in 5% non-fat dry milk/Superblock blocking buffer in PBS (Thermo Scientific) for 1 hour at room temperature, followed by 2 hour incubation at room temperature with primary antibodies, appropriately diluted, against DNMT1 (1:300), DNMT3b (1:300), CXCR4 (1:300), HDAC2 (1:250), p300 (1:250), Acetyl-H3 (1:800), Total-H3 (1:2000), p84 (1:1000). After washing in PBS/0.1% Tween 20, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (mouse, 1:3000; rabbit 1:1000) for 1 hour at room temperature. Band detection was carried out using an enhanced chemiluminescent substrate (Supersignal West Dura; Thermo Scientific) and captured on X-ray films. p84 was used as loading control because it is contained in the nucleus where most of the proteins that were assessed in this study reside, and it is not affected by cPAF treatment.
Quantitative real-time PCR
Cells were harvested, washed with cold PBS and 500 μl of Trizol reagent (Invitrogen) was added to the resulting pellet. Total RNA was extracted according to the manufacturer's instructions for Trizol reagent, and stored at −80°C. The concentration and purity of RNA obtained was determined by UV spectrophotometry and 0.2 μg from each sample was then reverse-transcribed using iScript™ Reverse Transcription Supermix (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. All cDNA samples were diluted five times and 3 μl of each dilution was used as template in RT-qPCR reactions (total volume 15 μl). Manufacturer-supplied standardized primer pairs from Bio-Rad were used to measure DNMT1 and DNMT3b while the following primers pair for CXCR4 amplification was used: 5’-GGAAGCTGTTGGCTGAAAAG-3’ (forward) and 5’-CTCACTGACGTTGGCAAAGA-3’ (reverse). As an internal reference, beta-2-microglobulin was used to normalize the amount of mRNA in each qPCR reaction, using the following primers: 5’-CATTCCTGAAGCTGACAGCATTC-3’ (forward), 5’-TGCTGGATGACGTGAGTAAACC-3’ (reverse). The reactions were run on a CFX96 Real-Time PCR Detection System (Bio-Rad) under the following PCR conditions: 95°C for 2 minutes, 40 cycles at 95°C for 5 seconds, and 60°C for 30 seconds. The results obtained were analyzed using the CFX Manager Software (Bio-Rad). The fold changes in relative mRNA expression levels were normalized to the expression level of the beta-2-microglobulin mRNA in each sample using the cycle threshold (Ct) method and using the 2-ΔΔCt formula according to Arya et al. (Arya et al., 2005).
Flow Cytometry analysis
Cells were harvested, washed twice with PBS and maintained in 1 ml Superblock blocking buffer in PBS (Thermo Scientific) for 20 minutes. Cells were then incubated with anti-human CXCR4-specific antibody, diluted 1:200 in PBS/0.2% FBS for 45 min at room temperature. Cells were washed twice with PBS and incubated with anti-rabbit Alexa488 antibody diluted 1:750 in PBS/0.2% FBS for 45 min at room temperature. Cells were then washed twice with PBS, fixed in 2% PFA for 10 minutes at room temperature in the dark, washed twice with PBS, re-suspended in 0.2 ml PBS and fluorescence measured using an LSRII (Becton Dickinson, San Jose, CA). The data were analyzed using FlowJo software (Ashland, OR).
Chromatin Immunoprecipitation
ChIP was conducted according to the manufacturer's protocol (EMD Millipore Co; Billerica, MA). After a 24-hour incubation with 10 μM cPAF, the treated mast cells were exposed to 1% formaldehyde for 10 minutes at room temperature to cross-linked chromatin. Cells were harvested and washed twice with ice-cold PBS. The cell pellet was re-suspended in SDS lysis buffer on ice for 10 minutes and then sonicated 5 times for 10 seconds each followed by centrifugation for 10 minutes at 4°C. Before immunoprecipitation, the supernatants were diluted 10x with ChIP dilution buffer and pre-cleared by the addition of protein A agarose/salmon sperm DNA (50% slurry) for 2 hours at 4°C. After centrifugation, the pre-cleared chromatin supernatant fraction was immunoprecipitated overnight at 4°C with 15 μl of specific antibodies or with control rabbit-IgG. After immunoprecipitation, protein A agarose/salmon sperm DNA (50% slurry) was added and the samples were incubated for 1 hour at 4°C to collect the antibody/histone complex. After centrifugation, the precipitates were washed sequentially before elution by 15 minute incubation in 250 μl of freshly prepared elution buffer (1 % SDS, 0.1 M NaHCO3). Eluates were heated at 65°C for 6 hours in the presence of 5 M NaCl to reverse cross-linking. The samples were then treated with proteinase K (10 mg/ml) for 1 hour at 45°C before purification using QIAquick PCR purification kit (QIAGEN, Valencia, CA) and collected in 50 μl of elution buffer (10 mM Tris-HCl, pH 8.5). Purified DNA was subjected to RT-qPCR analysis with appropriate primer pairs for human CXCR4 promoter region which spanned from 812 to 898 bp: 5’-GAGAGACGCGTTCCTAGCC-3’ (forward) and 5’-GGACCTCCCAGAGGCATTTC-3’ (reverse). The primer sequences used in our experiments were blasted against the human genome database and generated a 100% alignment with the promoter region of CXCR4. The reactions were run and analyzed as reported above using the input samples for normalization.
Statistical analysis
Each experiment was repeated 3 times, with the exception of the data generated with normal mast cells, which was repeated twice. Statistical differences between the control and experimental groups were analyzed using either the Mann-Whitney U test or the Student's t-test (GraphPad Prism, La Jolla, CA). Significant differences were defined as p < 0.05.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by a grant from the National Cancer Institute (CA131207) and the Cancer Prevention and Research Institute of Texas (RP120777). The histology, characterized cell line core, DNA methylation analysis core, and flow cytometry facilities at the MDACC are supported in part by a core grant from the NCI (CA16672). We thank Nasser Kazimi and Francesca Brugè for technical support and Dr Haiyan Li for help with the ChIP assays.
Abbreviations
- ChIP
Chromatin immunoprecipitation
- cPAF
carbamyl-Platelet-activating factor
- CXCR4
C-X-C chemokine receptor type 4
- CXCL12
Chemokine C-X-C motif ligand 12
- DNMT
DNA methyltransferase
- FcεRI
Fc epsilon receptor 1
- H3
histone H3
- HDAC
histone deacetylase
- PAF
platelet activating factor
- RT-qPCR
real time quantitative polymerase chain reaction
- UVB
UV radiation 290-320 nm
Footnotes
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
REFERENCES
- Adcock IM, Tsaprouni L, Bhavsar P, et al. Epigenetic regulation of airway inflammation. Curr Opin Immunol. 2007;19:694–700. doi: 10.1016/j.coi.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Albert DH, Magoc TJ, Tapang P, et al. Pharmacology of ABT-491, a highly potent platelet-activating factor receptor antagonist. Eur J Pharmacol. 1997;325:69–80. doi: 10.1016/s0014-2999(97)00109-x. [DOI] [PubMed] [Google Scholar]
- Aponte M, Jiang W, Lakkis M, et al. Activation of platelet-activating factor receptor and pleiotropic effects on tyrosine phospho-EGFR/Src/FAK/paxillin in ovarian cancer. Cancer Res. 2008;68:5839–48. doi: 10.1158/0008-5472.CAN-07-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya M, Shergill IS, Williamson M, et al. Basic principles of real-time quantitative PCR. Expert Rev Mol Diagn. 2005;5:209–19. doi: 10.1586/14737159.5.2.209. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Varier RA, Altaf M, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–71. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Biancone L, Cassoni P, et al. PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis. Am J Pathol. 2000;157:1713–25. doi: 10.1016/S0002-9440(10)64808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield JH, Weiler D, Dewald G, et al. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–55. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Limón-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol. 2008;180:4648–55. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EE, Bachman KE, Myohanen S, et al. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–7. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- Chacón-Salinas R, Chen L, Chávez-Blanco AD, et al. An essential role for platelet-activating factor in activating mast cell migration following ultraviolet irradiation. J Leukoc Biol. 2014;95:139–48. doi: 10.1189/jlb.0811409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón-Salinas R, Limón-Flores AY, Chávez-Blanco AD, et al. Mast cell-derived IL-10 suppresses germinal center formation by affecting T follicular helper cell function. J Immunol. 2011;186:25–31. doi: 10.4049/jimmunol.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–71. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Conte M, Dell'Aversana C, Benedetti R, et al. HDAC2 deregulation in tumorigenesis is causally connected to repression of immune modulation and defense escape. Oncotarget. 2014;6:886–901. doi: 10.18632/oncotarget.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts JL, Goodman JI. Alterations in DNA methylation may play a variety of roles in carcinogenesis. Cell. 1995;83:13–5. doi: 10.1016/0092-8674(95)90228-7. [DOI] [PubMed] [Google Scholar]
- Denizot Y, De Armas R, Caire F, et al. Platelet-activating factor and human meningiomas. Neuropathol Appl Neurobiol. 2006;32:674–8. doi: 10.1111/j.1365-2990.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- Denizot Y, Descottes B, Truffinet V, et al. Platelet-activating factor and liver metastasis of colorectal cancer. Int J Cancer. 2005;113:503–5. doi: 10.1002/ijc.20585. [DOI] [PubMed] [Google Scholar]
- Dy LC, Pei Y, Travers JB. Augmentation of ultraviolet B radiation-induced tumor necrosis factor production by the epidermal platelet-activating factor receptor. J Biol Chem. 1999;274:26917–21. doi: 10.1074/jbc.274.38.26917. [DOI] [PubMed] [Google Scholar]
- Feuerherm AJ, Jorgensen KM, Sommerfelt RM, et al. Platelet-activating factor induces proliferation in differentiated keratinocytes. Mol Cell Biochem. 2013;384:83–94. doi: 10.1007/s11010-013-1784-6. [DOI] [PubMed] [Google Scholar]
- Gong F, Miller KM. Mammalian DNA repair: HATs and HDACs make their mark through histone acetylation. Mutat Res. 2013;750:23–30. doi: 10.1016/j.mrfmmm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Gray SG, Teh BT. Histone acetylation/deacetylation and cancer: an “open” and “shut” case? Curr Mol Med. 2001;1:401–29. doi: 10.2174/1566524013363537. [DOI] [PubMed] [Google Scholar]
- Gul H, Marquez-Curtis LA, Jahroudi N, et al. Valproic acid exerts differential effects on CXCR4 expression in leukemic cells. Leuk Res. 2009;34:235–42. doi: 10.1016/j.leukres.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Hart PH, Grimbaldeston MA, Swift GJ, et al. Dermal mast cells determine susceptibility to Ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–53. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildmann C, Riester D, Schwienhorst A. Histone deacetylases--an important class of cellular regulators with a variety of functions. Appl Microbiol Biotechnol. 2007;75:487–97. doi: 10.1007/s00253-007-0911-2. [DOI] [PubMed] [Google Scholar]
- Jones PA. DNA methylation and cancer. Oncogene. 2002;21:5358–60. doi: 10.1038/sj.onc.1205597. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juremalm M, Hjertson M, Olsson N, et al. The chemokine receptor CXCR4 is expressed within the mast cell lineage and its ligand stromal cell-derived factor-1alpha acts as a mast cell chemotaxin. Eur J Immunol. 2000;30:3614–22. doi: 10.1002/1521-4141(200012)30:12<3614::AID-IMMU3614>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lehr HA, Weyrich AS, Saetzler RK, et al. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J Clin Invest. 1997;99:2358–64. doi: 10.1172/JCI119417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Niederkorn JY, Sadegh L, et al. Epigenetic regulation of CXCR4 expression by the ocular microenvironment. Invest Ophthalmo Vis Sci. 2013;54:234–43. doi: 10.1167/iovs.12-10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang L, Zhao K, et al. The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature. 2008;451:846–50. doi: 10.1038/nature06546. [DOI] [PubMed] [Google Scholar]
- Lorant DE, Patel KD, McIntyre TM, et al. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991;115:223–34. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hwang RF, Logsdon CD, et al. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res. 2013;73:3927–37. doi: 10.1158/0008-5472.CAN-12-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe GK, Johnson C, Billings SD, et al. Ultraviolet B Radiation Generates Platelet-activating Factor-like Phospholipids underlying Cutaneous Damage. J Biol Chem. 2005;280:35448–57. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Mori T, Kim J, Yamano T, et al. Epigenetic up-regulation of C-C chemokine receptor 7 and C-X-C chemokine receptor 4 expression in melanoma cells. Cancer Res. 2005;65:1800–7. doi: 10.1158/0008-5472.CAN-04-3531. [DOI] [PubMed] [Google Scholar]
- Pazin MJ, Kadonaga JT. What's up and down with histone deacetylation and transcription? Cell. 1997;89:325–8. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- Pei Y, Barber LA, Murphy RC, et al. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J Immunol. 1998;161:1954–61. [PubMed] [Google Scholar]
- Pitton C, Lanson M, Besson P, et al. Presence of PAF-acether in human breast carcinoma: relation to axillary lymph node metastasis. J Natl Cancer Inst. 1989;81:1298–302. doi: 10.1093/jnci/81.17.1298. [DOI] [PubMed] [Google Scholar]
- Prescott SM, Zimmerman GA, Stafforini DM, et al. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–45. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- Przybylski M, Kozlowska A, Pietkiewicz PP, et al. Increased CXCR4 expression in AsPC1 pancreatic carcinoma cells with RNA interference-mediated knockdown of DNMT1 and DNMT3B. Biomed Pharmacother. 2010;64:254–8. doi: 10.1016/j.biopha.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Puebla-Osorio N, Damiani E, Bover L, et al. Platelet-activating factor induces cell cycle arrest and disrupts the DNA damage response in mast cells. Cell Death Dis. 2015;6:e1745. doi: 10.1038/cddis.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos EA, Grochoski M, Braun-Prado K, et al. Epigenetic changes of CXCR4 and its ligand CXCL12 as prognostic factors for sporadic breast cancer. PloS one. 2011;6:e29461. doi: 10.1371/journal.pone.0029461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee I, Bachman KE, Park BH, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–6. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- Sahu RP, Turner MJ, DaSilva SC, et al. The environmental stressor ultraviolet B radiation inhibits murine antitumor immunity through its ability to generate platelet-activating factor agonists. Carcinogenesis. 2012;33:1360–7. doi: 10.1093/carcin/bgs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Matsubayashi H, Fukushima N, et al. The chemokine receptor CXCR4 is regulated by DNA methylation in pancreatic cancer. Cancer Biol Ther. 2005;4:70–6. doi: 10.4161/cbt.4.1.1378. [DOI] [PubMed] [Google Scholar]
- Shuto T, Furuta T, Oba M, et al. Promoter hypomethylation of Toll-like receptor-2 gene is associated with increased proinflammatory response toward bacterial peptidoglycan in cystic fibrosis bronchial epithelial cells. Faseb J. 2006;20:782–4. doi: 10.1096/fj.05-4934fje. [DOI] [PubMed] [Google Scholar]
- Sreevidya CS, Khaskhely NM, Fukunaga A, et al. Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res. 2008;68:3978–84. doi: 10.1158/0008-5472.CAN-07-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafforini DM, Prescott SM, Zimmerman GA, et al. Mammalian platelet-activating factor acetylhydrolases. Biochim Biophys Acta. 1996;1301:161–73. doi: 10.1016/0005-2760(96)00040-9. [DOI] [PubMed] [Google Scholar]
- Suarez-Alvarez B, Baragano Raneros A, Ortega F, et al. Epigenetic modulation of the immune function: a potential target for tolerance. Epigenetics. 2013;8:694–702. doi: 10.4161/epi.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KE, Reddy AB, Dietzmann K, et al. Epigenetic regulation of tumor necrosis factor alpha. Mol Cell Biol. 2007;27:5147–60. doi: 10.1128/MCB.02429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Berry D, Yao Y, et al. Ultraviolet B radiation of human skin generates platelet-activating factor receptor agonists. Photochem Photobiol. 2010;86:949–54. doi: 10.1111/j.1751-1097.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–9. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Rae V, Bruins-Slot W, et al. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–6. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.