Abstract
A national recipient hemovigilance system was introduced in the United States in 2010, when voluntary enrollment began as part of the National Healthcare Safety Network (NHSN) Hemovigilance Module. NHSN is a secure, web-based surveillance system operated by the Centers for Disease Control and Prevention and used by US healthcare facilities to report a variety of patient safety information. The Hemovigilance Module is used for comprehensive monitoring of transfusion-related adverse events. Participating facilities can utilize analytic tools available within the module to identify opportunities for enhancing transfusion safety, evaluate the effectiveness of interventions, and compare facility specific transfusion-related data to aggregate national estimates. Data may be voluntarily shared by facilities with external partners for patient safety improvement initiatives and to fulfill reporting mandates. We describe the key characteristics of the Hemovigilance Module, highlight the benefits for participating facilities, and discuss the use of reported data for establishing national estimates of transfusion-associated adverse events to identify gaps in transfusion safety and opportunities for interventions. National hemovigilance systems are essential to recognize gaps in transfusion safety and identify opportunities for interventions to improve patient safety and outcomes.
Keywords: hemovigilance, national healthcare safety network, blood safety
Graphical abstract
Introduction
With the emergence of HIV as a threat to blood safety in the 1980s, mitigating risks to transfusion recipients has been a worldwide public health priority.1,2 Hemovigilance, which includes surveillance activities encompassing the entire transfusion process, was implemented as a national program in France in 1994, followed by similar programs in other European countries, Canada, and elsewhere.1,3–6 In the United States, hospital transfusion services report different hemovigilance activities to federal, state, and non-governmental organizations to fulfill mandatory reporting requirements or as part of patient safety improvement initiatives.7–10 Although these hemovigilance activities provide valuable patient safety data, a national hemovigilance system was not introduced in the United States until recently.
In 2006, the Advisory Committee on Blood Safety and Availability (ACBSA) recommended that the Department of Health and Human Services (HHS) coordinate, support, and facilitate biovigilance in partnership with the private sector. The ACBSA defined biovigilance as a “comprehensive and integrated national patient safety program to collect, analyze, and report on the outcomes of collection and transfusion and/or transplantation of blood components and derivatives (i.e., hemovigilance), cells, tissues, and organs.”11 In 2007, to specifically address the hemovigilance aspect, the Centers for Disease Control and Prevention (CDC), in collaboration with transfusion medicine experts convened by AABB, developed the data requirements for a national recipient hemovigilance system as part of CDC’s National Healthcare Safety Network (NHSN).12
NHSN is a secure, web-based surveillance system used by more than 12,000 U.S. healthcare facilities to report healthcare-associated infections (HAIs), central line insertion practices, antimicrobial use and resistance, healthcare personnel influenza vaccination coverage, and other patient safety data. This system integrated previously existing CDC surveillance systems used to capture data on nosocomial infections (National Nosocomial Infections Surveillance System), occupational exposures and infections among healthcare personnel (National Surveillance System for Healthcare Workers), and HAIs among hemodialysis patients (Dialysis Surveillance Network). NHSN includes the Hemovigilance Module, which is used for comprehensive monitoring of transfusion-related adverse events in US healthcare facilities and is intended to assist facilities with internal blood utilization practices and transfusion safety monitoring while allowing facilities to compare adverse events trends to national level data through analytic and benchmarking features. Furthermore, the module is intended to reduce the reporting burden on facilities by facilitating data sharing with external groups for mandatory or voluntary quality improvement reporting requirements. The Hemovigilance Module was piloted by nine facilities in 2009 and was opened for voluntary national enrollment in 2010 (Figure 1).
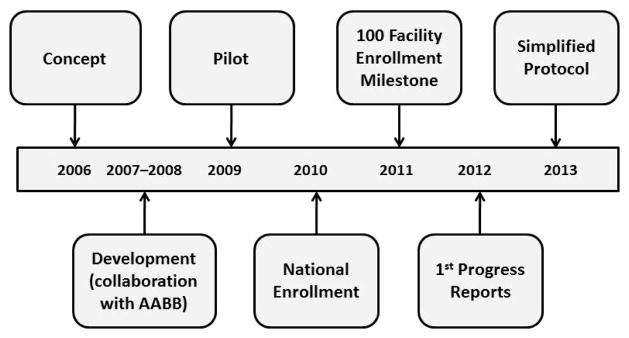
Figure 1. Development timeline of the NHSN Hemovigilance Module.
The concept for the Hemovigilance Module was introduced in 2006 with development beginning in 2007 in collaboration with expert groups convened by AABB. In 2009, the module was piloted in 9 facilities, and national enrollment began in January 2010. In 2011, the total number of facilities that enrolled in the module reached 100. The first progress reports were presented in 2012. In 2013, in an effort to increase participation, the protocol was simplified such that near-miss and no-harm incidents were no longer required.
NHSN Hemovigilance Module Data Reporting
Any transfusing healthcare facility in the U.S. may submit recipient hemovigilance data to CDC, and by enrolling, agrees to adhere to the Hemovigilance Module protocol for continuous surveillance for a minimum of 12 months. Participants submit an annual facility survey, individual reports on adverse transfusion reactions, incidents (i.e., errors and accidents) associated with adverse reactions, and monthly counts of transfused or discarded components and patient samples collected for cross-match (Figure 2). Data are submitted electronically to CDC through NHSN’s secure, web-based application. Additional optional fields are also available in the application and may be used to conduct facility-specific surveillance, though they are not included in national aggregate analysis by CDC.
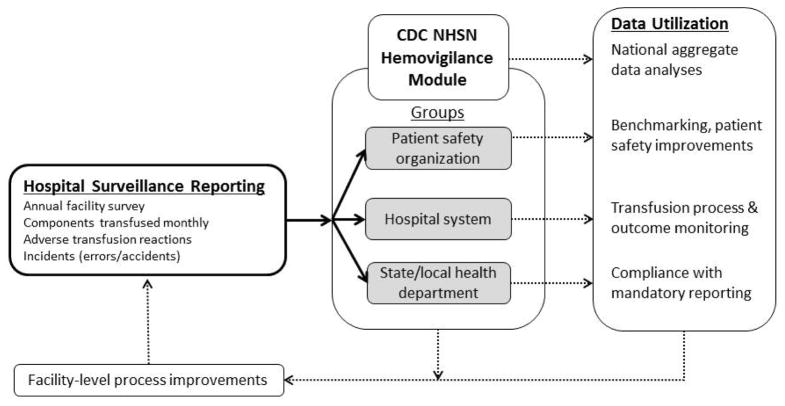
Figure 2. NHSN Hemovigilance Module Data Flow Chart.
Participating hospitals report an annual survey, components transfused monthly, adverse transfusion reactions, and incidents associated with reactions to the NHSN Hemovigilance Module. CDC conducts national aggregate data analyses. Facilities may also share their data with groups within NHSN. Groups may include patient safety organizations (PSO), hospital systems, and state and local health departments. These data can be used for benchmarking, allowing hospitals to monitor transfusion processes and outcomes, or fulfilling state and local mandatory reporting. Facilities may use the data for facility-level process improvements.
Annual Facility Survey
The annual facility survey is completed upon enrollment in the Hemovigilance Module and each calendar year thereafter. The survey collects facility characteristics such as hospital ownership, setting, and trauma level; the number of beds served by the transfusion service; hospital and laboratory accreditations; and the number of surgeries performed each year. Transfusion service characteristics and blood bank practices such as the number of transfusion service staff members employed; number of blood components transfused annually; inventory management systems in use; and cross-matching methods used are also captured in the survey. In addition to providing insight into blood banking practices, survey data allow CDC to classify facilities for comparisons in aggregate data analyses.
Monthly Denominators
Participating facilities report monthly totals of transfused or discarded components by component type (whole blood, red blood cell, platelet, plasma, and cryoprecipitate), collection method (apheresis or whole blood derived), modification methods (irradiation or leukocyte reduction), and unit size (full unit or aliquot). The number of patient samples collected and total crossmatch procedures performed are also reported monthly. Total patients transfused each month may be optionally reported.
Adverse Reactions
The protocol provides case definition criteria for 12 adverse reactions – transfusion-associated circulatory overload, transfusion-related acute lung injury, transfusion-associated dyspnea, allergic, hypotensive, febrile non-hemolytic, acute hemolytic, delayed hemolytic, delayed serologic, transfusion-associated graft vs. host disease, post-transfusion purpura, and transfusion-transmitted infection – and general criteria for patient events that can be classified as ‘unknown’ or ‘other’ reactions. Facilities use the ‘unknown’ category when the patient experienced transfusion-related symptoms, but the medical event could not be identified or classified within the specified categories. The ‘other’ category is used when the patient experienced a recognized transfusion-related adverse reaction that is not defined by the protocol such as transfusion-associated gut injury. Reactions are reported with an imputability designation, which is the likelihood that the reaction is attributable to the transfusion, and a severity grade (Figure 3). The adverse reaction record includes general demographic information, reaction details, clinical investigation results, patient outcome, and transfused component details. Adverse reactions are reported after an investigation has been completed in the hospital. Participants report all of the 12 CDC-defined adverse reactions that meet definite, probable, or possible imputability criteria that occur in their facility. Data reported optionally, such as non-severe allergic reactions, can be used by the reporting facility for their own purposes but are not included in national aggregate analyses by CDC.
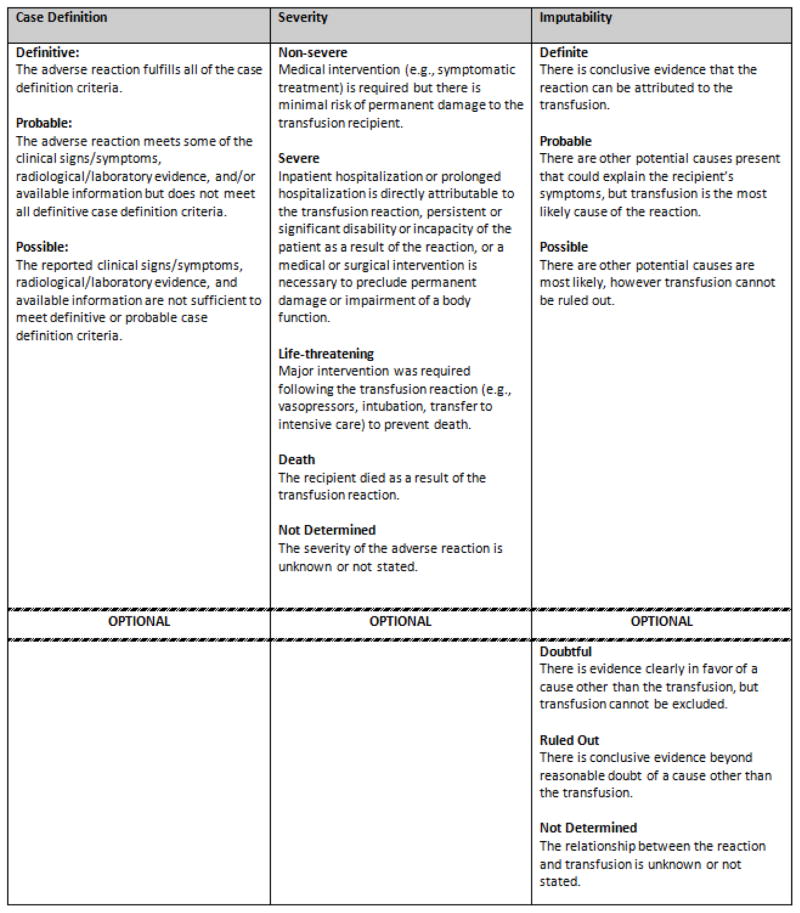
Figure 3. General case definition criteria for an adverse transfusion reaction to NHSN Hemovigilance Module.
The NHSN Hemovigilance Module protocol specifies the case definition for 12 adverse transfusion reactions based on the presence of signs, symptoms, and laboratory and radiographic data. Reactions are reported with a severity designation based on clinical outcomes. Imputability designations which specify the likelihood that reaction was associated with the transfusion event are also reported.
Incidents
More than 150 incident codes are provided in the surveillance protocol to describe transfusion-related errors and accidents that occur throughout the transfusion process. These codes were adapted from the US Medical Event Reporting System for Transfusion Medicine (MERS TM) and the Canadian Transfusion Errors Surveillance System (TESS) incident code sets.13,14 The incident record includes details on incident discovery, occurrence, and hospital investigation results. Facilities participating in the Hemovigilance Module must report incidents associated with adverse reactions. In addition, facilities may use the system to report incidents not associated with an adverse reaction for their own facility use and analysis.
Making use of Hemovigilance Data
NHSN offers analytic options to participants for their facility-specific transfusion practice and safety monitoring. For example, facilities can create monthly adverse reaction rate bar charts to establish baseline occurrence trends and then monitor the effect of prevention efforts. Regular monitoring of adverse reaction rates allow for the recognition of blood safety threats that may occur, as well. Participating facilities can create data tables of specific adverse reactions or all adverse reactions occurring per 10,000 transfused blood components. Rates may also be calculated for sample-related incidents occurring per 10,000 samples collected or component-related incidents occurring per 10,000 components transfused. These functionalities also allow facilities to compare their rates with aggregate adverse event rates published by CDC.
CDC will produce national estimates of transfusion-associated adverse events through aggregate analysis of submitted data to identify gaps in transfusion safety that can inform the development of public health interventions. The national data can then be used to evaluate the effectiveness of interventions as has occurred in other countries. For example, in response to deaths due to bacterial contamination reported to the French hemovigilance network, new guidelines for skin disinfection methods were introduced that resulted in a decrease in septic transfusion reactions.3 Similarly, in Quebec, transfusion-transmitted bacterial infection rates led to the implementation of diversion segments and post-collection culturing of platelets that resulted in a decline in bacterial infection transmissions, all of which were monitored through the national hemovigilance program.15 Furthermore, hemovigilance data reported to the UK Serious Hazards of Transfusion system revealed that post-transfusion purpura and transfusion-associated graft vs. host disease could be prevented through universal leukocyte reduction.16 These patient safety interventions are now common transfusion practice across industrialized countries.
Participating facilities can also share Hemovigilance Module data with external partners through a function built into NHSN to satisfy reporting requirements for patient safety improvement initiatives or reporting mandates without the added burden of entry into multiple systems. Using this function, facilities can share varying subsets of data with multiple groups for various purposes. One example is the recent mandate by the Massachusetts Department of Public Health (MDPH) that requires all licensed hospital blood banks and transfusion services in the state to report blood bank activity reports to the Hemovigilance Module, which is made immediately available to MDPH within the NHSN framework. These facilities can track their own transfusion safety indicators and contribute to national hemovigilance while also meeting their state reporting requirement with MDPH.7
Conclusion
The NHSN Hemovigilance Module is the only national transfusion safety surveillance system in the United States and also serves as a tool for facilities to monitor and improve their own transfusion safety practices. The system is free to use by any transfusing healthcare facility in the United States. Reporting requirements have been streamlined to make it easier for facilities to take advantage of system features. Facilities can use the analytic tools within the application to drive facility-level transfusion safety initiatives for safety improvements, evaluate the effectiveness of these initiatives, and compare their progress to others nationally.
Footnotes
Conflict of Interest: The authors have no conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of trade names is for identification purposes only and does not constitute endorsement by the U.S. Centers for Disease Control and Prevention or the Department of Health and Human Services.
References
- 1.Robillard P, Nawej KI, Jochem K. The Quebec hemovigilance system: description and results from the first two years. Transfus Apher Sci. 2004;31:111–22. doi: 10.1016/j.transci.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Strong D, AuBuchon J, Whitaker B, Kuehnert M. Biovigilance initiatives. ISBT Science Series. 2008;3:77–84. [Google Scholar]
- 3.Andreu G, Morel P, Forestier F, Debeir J, Rebibo D, Janvier G, Herve P. Hemovigilance network in France: organization and analysis of immediate transfusion incident reports from 1994 to 1998. Transfusion. 2002;42:1356–64. doi: 10.1046/j.1537-2995.2002.00202.x. [DOI] [PubMed] [Google Scholar]
- 4.Giampaolo A, Piccinini V, Catalano L, Abbonizio F, Hassan HJ. The first data from the haemovigilance system in Italy. Blood Transfus. 2007;5:66–74. doi: 10.2450/2007.0001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, Chapman CE, Davison K, Gerrard R, Gray A, Knowles S, Love EM, Milkins C, McClelland DB, Norfolk DR, Soldan K, Taylor C, Revill J, Williamson LM, Cohen H, Group SS. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–82. doi: 10.1016/j.tmrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Faber JC. Worldwide overview of existing haemovigilance systems. Transfus Apher Sci. 2004;31:99–110. doi: 10.1016/j.transci.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Commonwealth of Massachusetts Department of Public Health Clinical Laboratory Program. Blood Bank Revised Procedures for Reporting Blood Bank Activity [monograph on the internet] 2014 Available from: http://www.mass.gov/eohhs/gov/departments/dph/programs/hcq/labs/public-health-clinical-lab-blood-banks.html.
- 8.U.S. Centers for Disease Control and Prevention. National Notifable Diseases Surveillance System (NNDSS) [monograph on the internet] 2014 Available from: http://wwwn.cdc.gov/nndss/default.aspx.
- 9.U.S. Food and Drug Administration. Vaccines, Blood & Biologics, [monograph on the internet] 2014 Available from: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/default.htm.
- 10.Linden JV, Wagner K, Voytovich AE, Sheehan J. Transfusion errors in New York State: an analysis of 10 years’ experience. Transfusion. 2000;40:1207–13. doi: 10.1046/j.1537-2995.2000.40101207.x. [DOI] [PubMed] [Google Scholar]
- 11.Public Health Service Biovigilance Task Group. Biovigilance in the United States: efforts to bridge a critical gap in patient safety and donor health [monograph on the internet] 2009 Available from: http://www.hhs.gov/ash/bloodsafety/biovigilance/index.html.
- 12.U.S. Centers for Disease Control and Prevention. National Healthcare Safety Network [monograph on the internet] 2014 Available from: http://www.cdc.gov/nhsn/
- 13.Kaplan HS, Battles JB, Van der Schaaf TW, Shea CE, Mercer SQ. Identification and classification of the causes of events in transfusion medicine. Transfusion. 1998;38:1071–81. doi: 10.1046/j.1537-2995.1998.38111299056319.x. [DOI] [PubMed] [Google Scholar]
- 14.BC Provincial Blood Coordinating Office. Transfusion Error Surveillance System (TESS) [monograph on the internet] 2014 Available from: http://www.pbco.ca/index.php?option=com_content&task=view&id=61&Itemid=30.
- 15.Robillard P, Delage G, Itaj NK, Goldman M. Use of hemovigilance data to evaluate the effectiveness of diversion and bacterial detection. Transfusion. 2011;51:1405–11. doi: 10.1111/j.1537-2995.2010.03001.x. [DOI] [PubMed] [Google Scholar]
- 16.Williamson LM, Stainsby D, Jones H, Love E, Chapman CE, Navarrete C, Lucas G, Beatty C, Casbard A, Cohen H. The impact of universal leukodepletion of the blood supply on hemovigilance reports of posttransfusion purpura and transfusion-associated graft-versus-host disease. Transfusion. 2007;47:1455–67. doi: 10.1111/j.1537-2995.2007.01281.x. [DOI] [PubMed] [Google Scholar]