Abstract
High serum concentrations of polychlorinated biphenyls (PCBs) have been reported previously among residents of Anniston, Alabama, where a PCB production facility was located in the past. As the second of two cross-sectional studies of these Anniston residents, the Anniston Community Health Survey: Follow-Up and Dioxin Analyses (ACHS-II) will yield repeated measurements to be used to evaluate changes over time in ortho-PCB concentrations and selected health indicators in study participants. Dioxins, non-ortho PCBs, other chemicals, heavy metals, and a variety of additional clinical tests not previously measured in the original ACHS cohort will be examined in ACHS-II. The follow-up study also incorporates a questionnaire with extended sections on diet and occupational history for a more comprehensive assessment of possible exposure sources. Data collection for ACHS-II from 359 eligible participants took place in 2014, seven to nine years after ACHS.
Keywords: polychlorinated biphenyls, PCBs, dioxins, PCDDs, PCDFs, PBDEs, heavy metals, Anniston
Background
Anniston, Alabama, is a city where polychlorinated biphenyls (PCBs) were manufactured from 1929 until 1971. The city’s current population is about 23,000 (U.S. Census Bureau, 2010). The Anniston Community Health Survey (ACHS) was a cross-sectional study conducted in response to concerns among Anniston community members over whether exposure to polychlorinated biphenyls (PCBs) had increased their body burden of these contaminants and negatively affected their health. Data were collected for ACHS from 2005 to 2007.
Compared with the general U.S. population as described by the National Health and Nutrition Examination Survey (NHANES) 2003–4, the summed serum concentrations of 35 ortho-substituted PCBs were about three times higher for African-American ACHS participants and two times higher for White ACHS participants (Pavuk et al., 2014a). Additionally, associations with serum PCB concentrations were observed for hypertension, elevated blood pressure, diabetes, and serum lipid profiles (Aminov et al., 2013, Goncharov et al., 2010, 2011; Silverstone et al., 2012).
In a pilot study of 65 ACHS participants, highly elevated serum concentrations of non-ortho PCBs 126 and 169 were found (Pavuk et al., 2014b and 2014c). Median concentrations of both congeners in participants of ages 40 to 59 approached the 95th percentile reported for the same age group in the general U.S. population in the NHANES 2003–4. The median PCB 126 concentration for Anniston African-Americans was 105 pg/g lipid, five times higher than the median measurements from NHANES for the 40- to 59-year-old group (17.8 pg/g lipid) and three times higher than the median for the ≥60-year-old group (31.5 pg/g lipid); age-by-race group categories were not presented for the NHANES 2003–4 data. Concentrations in this ACHS pilot subsample at the 95th percentile were 4 to 5 times higher for PCB 126 and 2 to 3 times higher for PCB 169 than in NHANES 2003–4. In commercially produced Aroclor products, non-ortho PCBs generally have been found at trace to low (<1%) concentrations, when they were measured (ATSDR, 2000). The Anniston population may have been exposed to non-ortho PCBs through PCB-containing waste from manufacture of Aroclors, burning of trash, or other sources (Brown, 1995; EPA, 2013). We cannot exclude the possibility of recent exposure to these compounds, but the higher levels observed in the older age groups suggests that past exposures may be more relevant.
Objectives
The Anniston Community Health Survey: Follow-Up and Dioxin Analyses (ACHS-II) is follow-up study to ACHS, the second of two cross-sectional surveys and sample collection components combined to construct a longitudinal design. ACHS-II is a collaboration between the Agency for Toxic Substances and Disease Registry (ATSDR), National Institutes of Health (NIH), and University of Alabama at Birmingham (UAB).
We aim to use this new study to more fully assess exposures to both dioxin-like and non-dioxin-like PCBs and to complement them with measurements of polychlorinated dioxins, furans, polybrominated diphenyl ethers (PBDEs), and heavy and trace metals (lead, mercury, cadmium, manganese, and selenium). The addition of dioxins and dioxin-like analytes is supported by evidence from a small nested pilot study in the ACHS, in which high levels of non-ortho PCBs were found among a subset of 65 cohort members (Pavuk et al., 2014b and 2014c).
Anniston has been the site of extensive lead pipe production, long-term presence of ferro-manganese smelters, as well as mercury use and releases from PCB production (Love, 2007). The trace and heavy metals will be measured in members of the ACHS cohort for the first time in the current investigation.
A major goal of ACHS-II is to obtain repeated measures of the 35 ortho-PCB congeners analyzed in the ACHS in order to evaluate whether concentrations of these compounds decreased over time. The original ACHS PCB panel was selected because it represents the majority of steady-state and episodic PCB congeners by mass that are found in humans (Hansen, 2001) and did not include non-ortho PCBs.
Another main goal of ACHS-II is evaluation of changes in health indicators since ACHS, with continued emphasis on heart disease, obesity, diabetes, thyroid disease and autoimmune disease. Evaluation of health outcomes will also benefit from more comprehensive clinical tests (including extended lipid profiles, glycemic parameters, and cytokine measurements).
The follow-up study also collected more information about dietary and other practices that may have influenced exposure to the PCBs, dioxins, pesticides, and metals that were measured. Based on the analyses of ACHS data, questionnaires with additional detail on fat consumption and extended sections on local food consumption and occupational history were used to help expand understanding of possible exposure sources (Pavuk et al., 2014d).
ACHS-II eligibility
Surviving ACHS participants with valid PCB results were eligible to enroll in ACHS-II. Pregnant women and any persons who identified as being incarcerated (including house arrest) were excluded. Eligible respondents who were not medically able or willing to provide a blood specimen were allowed and encouraged to participate in questionnaire and body measurements parts of the study. In general, home interviews and blood draws requested by ACHS-II respondents were limited to those who resided within a one-hour drive from the study office in Anniston.
Sample size estimation
For detecting differences in mean PCB congener levels, we assumed a simple exponential decay model to describe the expected mechanism for human metabolism and excretion (Knobeloch et al. 2009; Seegal et al. 2011). We selected representative congeners with lower (PCB 118, five chlorines), high (PCB 153, six chlorines), and very high (PCB 206, nine chlorines) chlorination. PCB 118 is also mono-ortho substituted and partially dioxin-like, while PCB 153 is di-ortho substituted and non-dioxin-like. PCB 206 is a tetra-ortho substituted congener. The three congeners represent a spectrum of PCBs with different chlorination patterns, toxicities, and shorter to longer half-lives (Hansen, 2001).
For all three congeners, we assumed: 1) that the metabolism and elimination of each PCB congener follows an exponential decay model; 2) that additional individual exposure to PCB congeners since baseline is minimal; and 3) that the change in mean PCB congener concentration is log-normally distributed (Knobeloch et al. 2009). We also assumed a half-life (HL) of 14 years under the null hypothesis. We assumed t, the time since baseline to be 7 years and an alternative hypothesis of a HL of 20 years. In this model and with simplifying assumptions, C(t) represents the predicted mean ACHS-II serum concentration, C(0) represents the mean ACHS serum PCB concentration, K represents the decay constant, and t is the time interval between studies. Given K = ln(2)/HL it follows that C(t) can be estimated: C(t)=C(0) e−Kt.
Sample size and power estimations were performed using one-sided one-sample t-tests (Dudewicz and Mishra, 2008). To compare mean differences in PCB congener concentrations between the two study time points under the null and alternate hypotheses, with 80% power at a 5% level of significance, a sample size of 232 to 420 respondents was needed (Table 1). Although not taken into account in the above decay model assumptions, we recognize that factors such as body composition and weight (or weight change) may substantially alter elimination rates for PCBs (Chevrier et al., 2000; Ritter et al., 2011).
Table 1.
Mean ACHS concentrations of three representative PCB congeners and associated sample size required for ACHS-II.
| PCB Congener |
Congener characteristics |
Mean ACHS PCB Concentration C(0), in ng/g lipid |
Standard Deviation of ACHS PCB Concentration |
Mean change in PCB Concentration at t=7 years: H0: HL = 14 years |
Mean change in PCB Concentration at t=7 years: HA: HL = 20 years |
Sample Size at 80% Power and α=0.05* |
|---|---|---|---|---|---|---|
| 118 | 5 chlorines Mono-ortho substituted Partially dioxin-like |
70 | 177 | −20.5 | −15.1 | 420 |
| 153 | 6 chlorines Mono-ortho substituted Non-dioxin-like |
218 | 409 | −63.9 | −47.0 | 232 |
| 206 | 9 chlorines Tetra-ortho substituted Non-dioxin-like |
40 | 98 | −11.7 | −8.62 | 395 |
HO - null hypothesis, HA - alternative hypothesis, HL – half life.
For detecting differences in PCB levels by health outcomes, we used type 2 diabetes as an example. We estimated numbers of incident and prevalent diabetes cases in ACHS-II based on the number at risk after baseline (Silverstone et al. 2012), using US general population rates of diabetes and pre-diabetes (CDC 2012). There were 205 prevalent cases of diabetes (Silverstone et al., 2012) identified in ACHS (27% of 766). Since then, we estimated that about 66 cohort members were deceased. We planned to enroll up to 500 respondents of the surviving 700 cohort members (71.4%) in the follow-up study. Therefore, we assumed that proportionately 365 of 500 ACHS-II respondents (73%) would require assessment for incident diabetes since baseline. The national age-adjusted incidence rates for diabetes for 18- to 79-year-olds ranged between 9.8/1,000 and 13.0/1,000 per year for Black (average 11.1/1,000) and between 7.0/1,000 and 8.0 /1,000 per year for White (average 7.8/1,000) U.S. populations for the 2005–2010 period (CDC, 2012). Assuming our sample is half White and half African-American, we could use a combined average rate of 9.5/1,000 per year for those without diabetes. However, of those without diabetes at baseline (n=561), 169 subjects were found to have impaired fasting glucose and classified as pre-diabetics. These persons would likely have a higher rate of developing diabetes, estimated conservatively at 50/1,000 a year (Inzucchi and Sherwin, 2008). Proportionately, out of 365 persons without diabetes, 110 would likely be pre-diabetic and would have estimated to develop about 33 incident diabetes cases. Combined with an estimated 16 cases of incident diabetes for normoglycemic individuals (255 out of 365, average rate of 9.5/1,000 as derived above), we estimated to detect a total of 49 incident diabetes cases in the period of 2006–2013 in this sample of the Anniston population consisting of normoglycemic and pre-diabetic individuals. When enrolling 400 individuals, the number of estimated incident diabetes cases was 39 (Table 2).
Table 2.
Power to detect differences in ΣPCBs in incident and prevalent cases of diabetes.
| No. Enrolled | Incident Diabetes | Prevalent Diabetes* | ||
|---|---|---|---|---|
| No. with Diabetes Mellitus /No. Non-diabetic |
Power | No. with Diabetes Mellitus /No. Non-diabetic |
Power | |
| 200 | 20/126 | 54% | 40/160 | 77% |
| 300 | 29/190 | 68% | 60/240 | 90% |
| 400 | 39/253 | 79% | 80/320 | 96% |
| 500 | 49/316 | 87% | 100/400 | 98% |
Estimated prevalence of diabetes – 20%.
We used two-sample t-test power analyses (Machin et al., 1997) to calculate the power to detect significant differences in the sum of PCB concentrations between cases of diabetes and non-diabetics (Table 2). We reported that geometric mean levels of the sum of 35 PCB congeners in normoglycemic individuals were 6.31 ng/g wet weight and 7.71 ng/g wet weight for diabetics (, Silverstone et al., 2012). For pre-diabetics, the PCB levels were similar to normoglycemic individuals (6.16 ng/g wet weight). For this calculation, we used log-transformed, adjusted geometric mean levels of the sum of PCBs and standard deviations; a common mean total PCB level was used for all non-diabetics. Applying the above assumptions, we estimated 79% power to detect a statistically significant difference at alpha=0.05 level of confidence in PCB levels between 39 new cases of diabetes and 253 non-diabetics (Table 2). Preliminary analyses revealed 38 incident diabetes cases, so the sample size ultimately attained in ACHS-II (n=359) should provide sufficient power in statistical analyses.
For prevalent diabetes, the achieved power would be higher due to a higher number of included prevalent diabetes cases, even with the relatively smaller sample size. For modeling purposes and to provide conservative estimates, we assumed that the prevalence of diabetes would decrease from 27% in the 2005–2007 Anniston sample to 20% in the planned follow-up study sample. Other assumptions were the same as in estimating power based on differences in PCB levels in the incidence power calculations. The power to detect differences when enrolling 400 respondents with the diabetes prevalence of 20% (80 prevalent diabetes cases) would be 96%. When enrolling 300 respondents (60 prevalent diabetes cases), the power was estimated to be 90% (Table 2). Preliminary analyses showed 124 prevalent diabetes cases in ACHS-II (34.5%).
Tracking and recruitment
The ACHS-II protocol and data collection instruments were reviewed and approved by the Institutional Review Boards at the Centers for Disease Control and Prevention (CDC) and University of Alabama at Birmingham (UAB). Under the contract with ATSDR, UAB was responsible for the recruitment and data collection activities in Anniston. All UAB study staff and study interviewers and nurses recruited from the staff of Calhoun County Health Department (CCHD) were trained by UAB Survey Research Unit (SRU) or ATSDR staff to properly execute operational methods, to understand the goals and purposes of recruitment and enrollment, and to recognize the importance of the proper documentation of recruitment, consent, and enrollment procedures.
The length of the follow-up period – seven to nine years since baseline – called for the use of a multi-phase tracking and recruitment process that took place from late December 2013 through early August 2014. Several complementary methods were used to locate and confirm current contact information for and make contact with ACHS participants: two mailings, a public meeting, reference database searches, phone calls, and field visits to residences. A first mailing of recruitment letters was sent to all original ACHS participants at their addresses known as of ACHS (i.e., baseline). Within the first month of recruitment, a public meeting was held to provide study information to past participants and community members, as well as to recruit participants for follow-up enrollment; local news media covered the meeting and opening of the study office at CCHD in Anniston. In January and February 2014, three study staff members made direct calls to ACHS participants’ last known phone numbers in order to determine the need to search for updated contact information. Study staff searched the Social Security Death Index to check vital status, and they searched for new contact information in free (411.com) and subscription-based (ReferenceUSA, Lexis-Nexis Accurint) reference databases. A second mailing was sent in late February to those who had not yet participated in ACHS-II and not known to be deceased; this package was sent to all available known and updated addresses. Using the most recently available phone numbers, one study staff member continued making phone calls throughout the recruitment period in order to recruit participants and set appointments for them. This staff member called all ACHS participants not known to have deceased, moved outside of the study area, or already participated. Reminder phone calls were made on the day before each participant’s appointment. ACHS participants who were called received one to 22 calls per person, with a mean call length of 3.9 minutes. More than 1,300 calls were made with ACHS participants. Within January and February 2014, 171 (47.6%) ACHS-II participants enrolled; their participation can be attributed to the combination of mailings, reference database searches, and phone calls.
From March through June 2014, a team of two to five study staff members made field visits to last known and updated addresses to check contact information, inform ACHS participants about the follow-up study, recruit them to enroll in ACHS-II, and make appointments for data collection. Of 766 ACHS participants, field visits were made to addresses of 371 individuals (for an average of 92.8 individuals’ addresses visited per month). Some individuals had multiple address records and many addresses were visited multiple times, including on weekends. Of 359 ACHS-II participants, 188 (52.4%) had their appointments from March through the end of recruitment in August. Of these 188, 108 had received at least one field recruitment visit. Considering that two mailings had been sent and study staff had been making phone calls for months, it may be inferred that in-person field visits were the most influential factor for the enrollment of these 108 individuals (30.1% of 359 total participants). The combination of all methods - mailings, database searches, phone calls, and field visits – was crucial for finding and confirming ACHS participants' contact information, as well as in influencing participants’ decision to enroll.
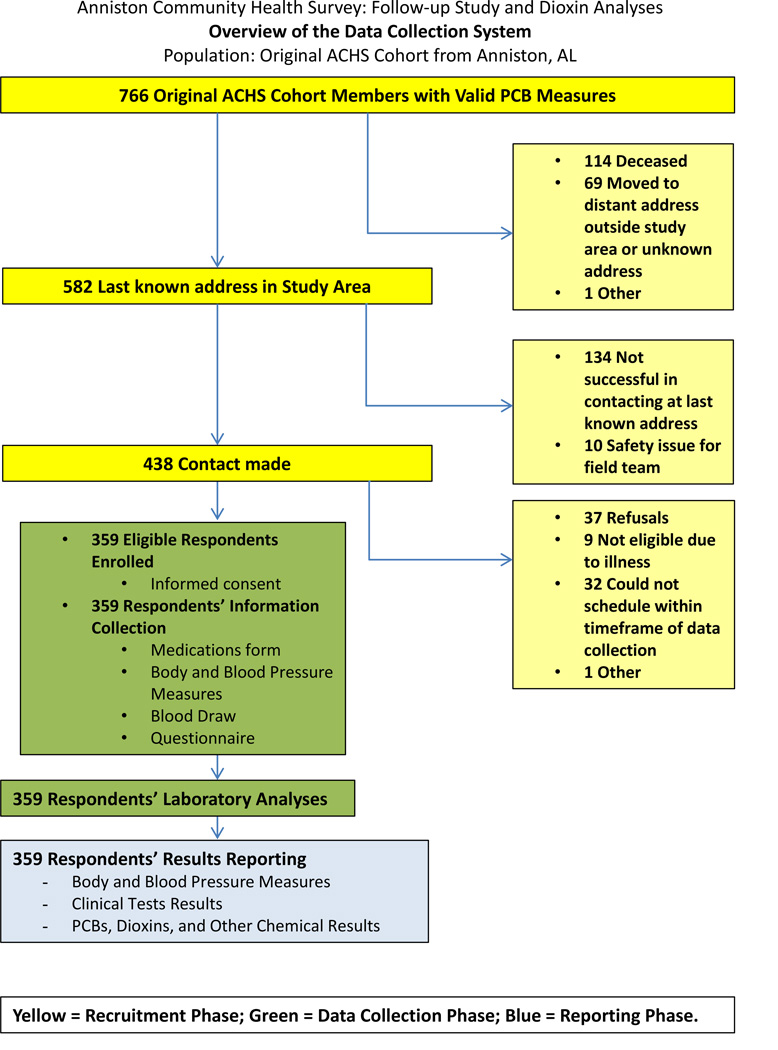
Overall, of the 766 ACHS participants with valid PCB measurements who were tracked for follow-up, 114 were found to be deceased, corresponding to a death rate of approximately 1.6–2.1% per year over 7–9 years, as opposed to an all-cause mortality rate of 1.23% per year estimated for Calhoun County (Djamba, 2011). Additionally, 69 had moved away to an address outside the study area (>1 hour drive outside Anniston) or to an unknown address. Out of the ACHS 582 living cohort members with last known addresses still inside the study area, 438 respondents were successfully contacted, and 359 eligible individuals enrolled in ACHS-II (response rate: 61.6%, cooperation rate among contacts: 82.0%). Ten ACHS participants had residences that presented safety issues for the recruitment field team and 134 could not be contacted at the most recent available address via phone calls or field visits. Thirty-seven ACHS participants refused to enroll in ACHS-II (refusal rate among contacts: 8.4%), whereas 9 were considered ineligible due to health conditions and 32 expressed interest in participation but could not be scheduled within the timeframe of the study (Figure 1).
Figure 1.
Overview of data collection system for ACHS-II.
The 359 ACHS-II participants were demographically similar to the ACHS participants with PCB measurements. The mean age of the ACHS participants was 54.9 years, compared to 55.2 at baseline among ACHS-II participants. Mean body mass index among ACHS participants was 31.2 kg/m2 and 31.6 kg/m2 among ACHS-II participants. About half the participants were African-American and a majority of participants was female (73%) (Table 3).
Table 3.
Selected demographic characteristics of ACHS and ACHS-II participants.
| Characteristic | ACHS participants, 2005–7 (n=765) |
ACHS-II participants, 2014 (n=359) |
|
|---|---|---|---|
| Mean ± standard error | |||
| Age in years at baseline (2005–7) | 54.9 ± 0.6 | 55.2 ± 0.7 | |
| BMI – kg/m2 | 31.2 ± 0.3 | 31.6 ± 0.4 | |
| N (%, non-missing) | |||
| Self-identified race | |||
| African-American | 353 (46.1%) | 190 (53.0%) | |
| White | 412 (53.6%) | 169 (47.1%) | |
| Female | 537 (70.2%) | 262 (73.0%) | |
Data collection
The research team collected data from January through August 2014 – an average of eight years after ACHS – onsite at the study office, which was set up at the CCHD in Anniston, or at homes of individual participants as needed. Of 359 participants, 96 (26.7%) had home visits for data collection. All participants gave written, informed consent prior to data collection. Participants were asked to bring current medications to the study office. Study staff (nurses from the CCHD or nursing faculty and students from Jacksonville State University (JSU)) recorded participants’ medications and then measured participants’ height, weight, waist circumference, and blood pressure. Nurses then determined which participants were eligible to provide fasting blood samples and drew 125mL blood from those participants (or a smaller amount from participants for whom the full quantity was deemed too burdensome). Venous blood samples were drawn between 7:30 and 11:00am, after participants’ overnight fast, by one of the nurses. Blood samples were centrifuged within 2 hours, and the collected serum samples were then frozen within 1 hour. The temperature in the sampling/blood processing room was kept constant at 72°F. Water and light snacks were provided following blood collection. Blood specimens collected at the study office or home visit were centrifuged at the study office to isolate serum and were processed by CCHD nurses. Whole blood and serum specimens were stored at the study office at −20°C until they were shipped weekly to CDC, where specimens were kept at −70°C until needed for analysis.
Study staff were trained to conduct interviews with participants using a questionnaire to gather information on a variety of topics, including demographics and lifestyle, diet, physical activity, occupational history, smoking, alcohol consumption, and physical activity. Extensive sections of the questionnaire focused on the diet, with an emphasis on local foods, frequency of consumption, and when they were consumed (e.g., specific decades relevant to PCB production). Interviewers also asked participants about self-reported health outcomes (e.g., change in diabetes or heart disease status since ACHS). The questionnaire included separate sections for health questions that were sex-specific and about participants’ children. Computer-assisted personal interviewing (CAPI) software was used for data entry. At the study office, a team of 4 to 6 nursing students from JSU, supervised by 2 JSU nursing faculty, was available to interview the participants; a senior UAB study staff member conducted interviews on home visits, accompanied by a CCHD nurse to collect blood as needed.
As a token of appreciation, each ACHS-II participant was offered $100 cash after participating in the interview and volunteering to provide a blood specimen (or $50 if they participated in only the interview).
Laboratory analyses
Serum and whole blood specimens are currently undergoing analysis at CDC and several collaborating laboratories. Staff and investigators at these laboratories are blinded to the identities of the participants, as the specimens are labeled only with identification numbers. The National Center for Environmental Health (NCEH) Division of Laboratory Sciences measured the exposure analytes: 2,3,7,8-substituted polychlorinated dioxins and furans, non-ortho PCBs (congeners 81, 126, 169), PBDEs (congeners 17, 28, 47, 66, 85, 99, 100, 153, 154, 183, 209), 35 ortho-substituted PCBs (congeners 28, 44, 49, 52, 66, 74, 87, 99, 101, 105, 110, 118, 128, 138–158, 146, 149, 151, 153, 156, 157, 167, 170, 172, 177, 178, 180, 183, 187, 189, 194, 195, 196–203, 199, 206, and 209; same congeners measured in the original ACHS), nine organochlorine pesticides (hexachlorobenzene (HCB), β-hexachlorocyclohexane (β-HCCH), γ-HCCH, o,p'-dichlorodiphenyltrichloroethane (DDT), p,p'-DDT, p,p'-dichlorodiphenyldichloroethene (DDE), oxychlordane, trans-nonachlor, and mirex; measured in ACHS), lead, cadmium, mercury, manganese, and selenium.
Serum from each participant also underwent a variety of clinical tests. Glycemic parameters (glucose, insulin, anti-GAD, anti-IA2) and the lipid panel (total cholesterol, triglycerides, LDL, HDL, phospholipids, free fatty acids) were measured at the Northwest Lipid Metabolism and Diabetes Research Laboratories at the University of Washington. The University of Southern California (USC) Endocrine Laboratories measured thyroid hormones and autoantibodies. Standard liver enzyme tests and several biomarkers of inflammation and/or liver damage and disease, including cytokeratin 18 (CK-18) M30 and M65, adiponectin, resistin, plasminogen activator inhibitor-1 (PAI-1), monocyte chemotactic protein 1 (MCP-1), TNF-α, interleukin 1β (IL-1β), IL-6, IL-8, leptin, antioxidant assay, hyaluronic acid, and endotoxin, were measured at the University of Louisville. Autoimmune parameters (including rheumatoid factor, anti-nuclear antibodies (ANA), ANA-titer), as well as additional cytokines and inflammation-related biomarkers, were measured at State University of New York (SUNY) Upstate Medical University. In the future, we plan to examine DNA methylation changes using reserve whole blood samples. The same laboratories and similar analytical methods as used in ACHS have been contracted where possible for the follow-up analyses in order to reduce the potential for inter-laboratory variability.
Study Limitations
The original ACHS sample was not intended to be representative of the demographics of Anniston or Calhoun County as a whole. For ACHS, Anniston residents were selected through a stratified random sample of housing units, with oversampling (two-thirds of all eligible) from west Anniston, which facilitated enrollment of more residents who lived closer to the plant and thus had higher potential for PCB exposure. However, the follow-up sample closely resembles the original ACHS cohort demographically, which suggests that tracking and recruitment may not have been biased. No external comparison group was used as part of the original design. As such, internal comparisons based on the distribution of PCBs, dioxins, and other chemicals will be used in statistical analyses of the follow-up sample.
Summary
Together, the two cross-sectional studies ACHS and ACHS-II make up a longitudinal design, which will allow repeated measurements to be used to evaluate changes over time in concentrations of ortho-PCBs and persistent organochlorine pesticides, as well as in selected health indicators, in Anniston residents who lived in the vicinity of a former PCB production facility. The follow-up study also provides the opportunity to take new measurements and collect additional data in order to assess exposure to non-ortho PCBs and other chemicals not previously measured in the full ACHS cohort, as well as to examine additional health indicators. Preliminary counts show that the 359 eligible individuals who participated in ACHS-II appear demographically similar to the original ACHS participants. ACHS-II data collection was completed in August 2014. Currently, collaborating laboratories are measuring analytes in serum and whole blood specimens, and data cleaning is underway for data collected in interviews. Laboratory test results will be reported to participants when completed. Results from ACHS and ACHS-II may clarify relationships between exposure to PCBs, dioxins, and other chemicals and effects on human health, particularly within populations with high non-occupational exposures.
Acknowledgements
We thank all study participants and community members who participated in the study.
The study was funded by the National Cancer Institute (Dr. Linda Birnbaum) through interagency agreements with the Centers for Disease Control and Prevention (CDC) (IAA#: 11-AT1-001-00; IAA#: 12-AT-12-ANNISTON) and by ATSDR. Dr. Pavuk, of ATSDR, is the study’s principal investigator. Data collection for this study was funded via contract from ATSDR to the University of Alabama at Birmingham (UAB) (CDC Contract No. 200-2011-40834; Prof. S. Mennemeyer, PI).
This project was supported in part by an appointment to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC/ATSDR.
We also thank the laboratories that provided expert chemical analyses for this study at NCEH Division of Laboratory Sciences (Dr. Andreas Sjödin and Dr. Kathy Caldwell), Northwest Lipid Metabolism and Diabetes Research Laboratories (Dr. Santica Marcovina), USC Endocrine Laboratories (Dr. Carole Spencer), SUNY Upstate Medical University (Dr. Frank Middleton and Dr. Allen Silverstone), and University of Louisville (Dr. Matthew Cave).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of ATSDR or CDC. The authors declare they have no actual or potential competing financial interests.
References
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Polychlorinated Biphenyls (PCBs) Atlanta: U.S. Department of Health and Human Services; 2000. [PubMed] [Google Scholar]
- Aminov Z, Haase RF, Pavuk M, Carpenter DO Anniston Environmental Health Research Consortium. Analysis of the effects of exposure to polychlorinated biphenyls and chlorinated pesticides on serum lipid levels in residents of Anniston, Alabama. Environ Health. 2013;12:108. doi: 10.1186/1476-069X-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Jr, Frame GM, II, Olson DR, Webb JL. The Sources of the Coplanar PCBs. Organohalogen Compounds. 1995;26:427–430. [Google Scholar]
- Chevrier J, Dewailly E, Ayotte P, Mauriège P, Després JP, Tremblay A. Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. Int. J. Obes. Relat. Metab. Disord. 2000;24:1272–1278. doi: 10.1038/sj.ijo.0801380. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Diabetes Data and Trends. Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. Available: http://apps.nccd.cdc.gov/DDTSTRS/default.aspx. [Google Scholar]
- Djamba YK. 2011 Population Data Sheet. Alabama, USA: Center for Demographic research, Auburn University at Montgomery; 2011. [Google Scholar]
- Dudewicz EJ, Mishra SN. Modern Mathematical Statistics. New York: John Wiley & Sons, Inc.; 1988. [Google Scholar]
- Environmental Protection Agency. [Accessed 24 Sept. 2014];Human Health. Wastes - Non-Hazardous Waste - Municipal Solid Waste. 2013 http://www.epa.gov/waste/nonhaz/municipal/backyard/health.htm.
- Goncharov A, Bloom M, Pavuk M, Birman I, Carpenter DO. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. J. Hypertension. 2010;28(10):2053–2060. doi: 10.1097/HJH.0b013e32833c5f3e. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Pavuk M, Foushee HR, Carpenter DO for the Anniston Environmental Health Research Consortium. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environ. Health Perspect. 2011;119(3):319–325. doi: 10.1289/ehp.1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. Identification of steady state and episodic PCB congeners from multiple pathway exposures. In: Robertson LW, Hansen LG, editors. PCBs: Recent Advances in Environmental Toxicology and Human Health Effects. Lexington, KY: University Press of Kentucky; 2001. pp. 47–56. [Google Scholar]
- Inzucchi SE, Sherwin RS. Type 2 Diabetes Mellitus. In: Goldman, Aussielo, editors. Cecil Medicine 23rd Edition. Philadelphia, PA: Saunders Elsevier; 2008. pp. 1748–1759. [Google Scholar]
- Knobeloch L, Turyk M, Imm P, Schrank C, Anderson H. Temporal changes in PCB and DDE levels among a cohort of frequent and infrequent consumers of Great Lakes sportfish. Environ Res. 2009;109(1):66–72. doi: 10.1016/j.envres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Love D. My city was gone: one American town's toxic secret, its angry band of locals, and a $700 million day in court. New York: Harper Perennial Publishers; 2007. [Google Scholar]
- Machin D, Campbell M, Fayers P, Pinol A. Sample Size Tables for Clinical Studies. 2nd Edition. Malden, MA: Blackwell Science; 1997. [Google Scholar]
- Pavuk M, Olson JR, Sjödin A, Wolff P, Turner WE, Shelton C, Dutton ND, Bartell S for the Anniston Environmental Health Research Consortium. Serum concentrations of polychlorinated biphenyls (PCBs) in participants of the Anniston Community Health Survey. Sci. Total Environ. 2014a;473–474:286–297. doi: 10.1016/j.scitotenv.2013.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuk M, Dutton N, Turner WE, Sjödin A, Bartell S for the Anniston Environmental Health Research Consortium. Dioxins, dibenzofurans, and non-ortho polychlorinated biphenyls in a subset of the Anniston Community Health Survey. 34th International Symposium on Halogenated Persistent Organic Pollutants (Dioxin 2014).2014b. [Google Scholar]
- Pavuk M, Dutton N, Turner WE, Sjödin A, Bartell S for the Anniston Environmental Health Research Consortium. Dioxins, dibenzofurans, and non-ortho polychlorinated biphenyls in a subset of the Anniston Community Health Survey. Organohalogen Compd. 2014c;76:1195–1198. ( http://www.dioxin20xx.org/pdfs/2014/1090.pdf). [Google Scholar]
- Pavuk M, Olson JR, Wattigney WA, Dutton ND, Sjödin A, Shelton C, Turner WE, Bartell SM Anniston Environmental Health Research Consortium. Predictors of serum polychlorinated biphenyl concentrations in Anniston residents. Sci Total Environ. 2014d;496:624–634. doi: 10.1016/j.scitotenv.2014.06.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter R, Scheringer M, MacLeod M, Moeckel C, Jones KC, Hungerbühler K. Intrinsic human elimination half-lives of polychlorinated biphenyls derived from the temporal evolution of cross-sectional biomonitoring data from the United Kingdom. Environ. Health Perspect. 2011;119(2):225–231. doi: 10.1289/ehp.1002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Fitzgerald EF, Hills EA, Wolff MS, Haase RF, Todd AC, Parsons P, Molho ES, Higgins DS, Facto SA, Marek KL, Seibyl JP, Jennings DL, McCaffrey RJ. Estimating the half-lives of PCB congeners in former capacitor workers measured over a 28-year interval. J Expo Sci Environ Epidemiol. 2011;21(3):234–246. doi: 10.1038/jes.2010.3. [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, Pavuk M. Polychlorinated Biphenyl (PCB) Exposure and Diabetes: Results from the Anniston Community Health Survey. Environ. Health Perspect. 2012;120(5):727–732. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. [Accessed 24 Sept. 2014];2010 Census. State & County Quickfacts. 2010 http://quickfacts.census.gov/qfd/states/01/0101852lk.html.