Summary
Enteric pathogens must overcome intestinal defenses to establish infection. In Drosophila, the ERK signaling pathway inhibits enteric virus infection. The intestinal microflora also impacts immunity but its role in enteric viral infection is unknown. Here we show that two signals are required to activate antiviral ERK signaling in the intestinal epithelium. One signal depends on recognition of peptidoglycan from the microbiota, particularly from the commensal Acetobacter pomorum, which primes the NF-kB-dependent induction of a secreted factor, Pvf2. However, the microbiota is not sufficient to induce this pathway; a second virus-initiated signaling involving release of transcriptional paused genes mediated by the kinase Cdk9 is also required for Pvf2 production. Pvf2 stimulates antiviral immunity by binding to the receptor tyrosine kinase PVR, which is necessary and sufficient for intestinal ERK responses. These findings demonstrate that sensing of specific commensals primes inflammatory signaling required for epithelial responses that restrict enteric viral infections.
Graphical Abstract
Introduction
Enteric viral pathogens are widespread. Humans are commonly infected with enteroviruses, and these infections are associated with a wide variety of clinical manifestations ranging from asymptomatic to meningitis (Abzug, 2014; Jubelt and Lipton, 2014; Muehlenbachs et al., 2014). Recent epidemiological evidence indicates that enteric viruses are the leading cause of foodborne disease in the USA and worldwide are a major group of waterborne disease agents (Atreya, 2004; Koo et al., 2010; Sair et al., 2002). Enteroviruses are a widespread class of picornaviruses that infect organisms from insects to humans. The picorna-like virus of Drosophila, Drosophila C virus, is a widespread pathogenic enterovirus of fruit flies (Jousset, 1976). Arthropod-borne viruses (arboviruses) are another group of viruses of global importance. Infection of the insect vector occurs orally during the blood meal, while infection of vertebrate hosts is through an insect bite (Attardo et al., 2005; Hansen et al., 2014; Raikhel and Dhadialla, 1992). Viruses within this blood meal infect intestinal epithelial cells to establish infection, as is the case for many enteric infections in mammals (Davis and Engstrom, 2012; Steinert and Levashina, 2011; Weaver and Barrett, 2004). Moreover, there has been a resurgence of vector-borne viral pathogens, which have become an increasing source of worldwide morbidity and mortality in humans and livestock. In particular, dengue virus (DENV), a member of the Flaviviridae family, is a re-emerging arbovirus that infects >300 million people and causes ~250,000 deaths annually (Bhatt et al., 2013).
It has long been recognized that the gut represents a formidable immune barrier against enteric viral infections in both vertebrates and insects. The high barrier presented by the gastrointestinal tract causes most studies on human enteric viruses in mice to rely on intraperitoneal injection (Bopegamage et al., 2005; Gill et al., 2011; Mossel and Ramig, 2002; Nagler-Anderson, 2001). Arboviruses within the blood meal must also overcome barrier immunity to establish infection in the insect (Weaver and Barrett, 2004). Experimentally, this infection barrier is well-described: oral infection of mosquitoes that are not the natural vector is usually non-productive; however, bypassing the gut by injecting the virus in the body cavity allows the virus to establish infection that can even be transmitted to vertebrates (Kingsolver et al., 2013; Tabachnick, 2013; Xu and Cherry, 2014). While the intestinal environment is clearly restrictive to viral infection from insects to humans, few molecular mechanisms are known.
The gut is a complex environment, housing an extensive microbiota that influences homeostasis and nutrient uptake. Recently, there has been an increasing appreciation that the commensals that inhabit the intestine are essential players in immunity across hosts (Buchon et al., 2013a; Charroux and Royet, 2012; Lee and Brey, 2013; Sommer and Backhed, 2013). Indeed, the microbiota and innate immune system are constantly engaged and impact infection in the gut (Cirimotich et al., 2011; Pang and Iwasaki, 2012; Ramirez et al., 2012; Schaffer et al., 1963; Xi et al., 2008). However, the molecular links between the microbiota and immunity are only beginning to be defined. Understanding the role of the microbiota in the context of viral infection may reveal strategies to restrict enteric infections.
To explore the mechanisms involved in oral acquisition of viral pathogens, we developed an oral model of viral infection using the genetically tractable organism Drosophila melanogaster (Xu et al., 2013). We found, as has been shown in vectors and murine systems, that the intestine is highly restrictive; however, loss of ERK signaling in the intestinal epithelium, specifically in enterocytes, significantly increases susceptibility to the Drosophila enteric picorna-like virus Drosophila C virus (Xu et al., 2013). We also tested human arboviruses from three different families that are orally acquired in insects and observed that ERK is restrictive. Importantly, we found that the intestinal epithelium rapidly responds to viral infection by inducing the ERK pathway (Xu et al., 2013). Since these viruses are diverse, but regulated similarly, our data suggest that the ERK pathway is broadly antiviral against orally acquired viruses.
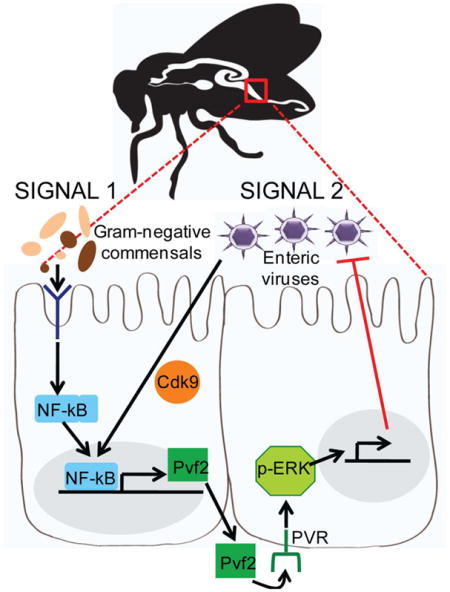
In this study, we set out to determine how the ERK pathway was regulated in the gut to control viral infection. We found that the ligand Pvf2 is induced upon viral infection and activates the receptor tyrosine kinase (RTK), PVR, which is required for activation of the antiviral ERK pathway in enterocytes. Moreover, we found that Pvf2 induction is regulated by the microbiota; gram-negative commensals are sensed by enterocytes priming NF-kB-dependent Pvf2 expression. In the absence of the microbiota, the animals are more susceptible to oral challenge and this can be overcome by ectopically expressing Pvf2 or by mono-association with Acetobacter pomorum, a gram-negative commensal which activates Pvf2, but not Lactobacillus brevis, a gram-positive commensal which does not induce Pvf2. A second signal is required which is dependent on sensing virus. We had previously defined a pausing-dependent transcriptional program in flies (Xu et al., 2012) and we now show that this pathway is also required for virus-dependent Pvf2 induction. Taken together, these results clearly demonstrate that sensing of specific components of the microbiota coupled with viral signals are integrated to play an essential role in the control of enteric viral infection of a broad range of viruses.
Results
PVR is required for antiviral defense
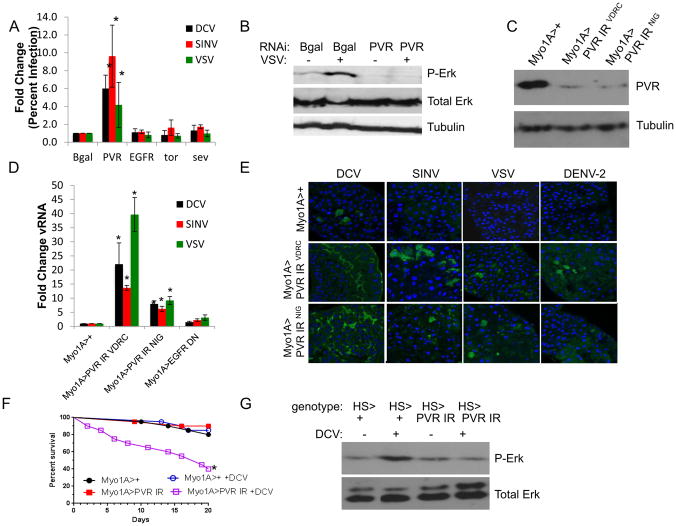
The canonical ERK signaling pathway is initiated by secreted factors binding to RTKs, which activates a three-tiered phosphorylation cascade, culminating with phosphorylation of ERK (Sundaram, 2013). The Drosophila genome encodes 21 RTKs (Sopko and Perrimon, 2013), and to determine the receptor responsible for activating the antiviral ERK pathway in the Drosophila intestine, we screened a panel of RTKs in vitro using RNAi for their role in antiviral defense against a panel of viruses that we previously found to be restricted by the ERK pathway. This included Sindbis virus (SINV), Vesicular stomatitis virus (VSV), and Drosophila C virus (DCV). VSV and SINV are arboviruses belonging to two disparate families (Alphaviridae and Rhabodoviridae, respectively). Their natural cycle involves transmission between insect vectors and vertebrate hosts, but they do not naturally infect Drosophila. DCV is a natural Drosophila enteric pathogen similar to picornaviruses (Jousset, 1976). We found that only when PVR (platelet derived growth factor and vascular endothelial growth factor receptor) was depleted, we observed a significant increase in infection with all three viruses in cell culture (Fig. 1A). We previously showed that virus infection is sensed in Drosophila leading to the activation of ERK signaling (Xu et al., 2013), and using an antibody that recognizes activated Drosophila ERK (phospho-ERK), we observed that the virus-induced increase in phospho-ERK was dependent on PVR in vitro (Fig. 1B, Fig. S1A).
Figure 1. PVR is required for antiviral defense in the Drosophila intestine.
(A) Drosophila cells treated with the indicated dsRNAs were challenged with the indicated viruses and monitored by automated microscopy and image analysis. Percent of infected cells was quantified and normalized to control for three independent experiments with mean ± SD shown; *p<0.05. (B) Representative immunoblot of Drosophila cells treated with the indicated dsRNAs and infected with VSV for 30 min. (C) Representative immunoblot analysis of 20 pooled intestines from control (Myo1A>+) or PVR-depleted guts. (D) Flies of the indicated genotypes were infected with the indicated viruses. RT-qPCR analysis of viral RNA normalized to rp49 and shown relative to control (Myo1A>+) from 15 pooled intestines 7 dpi with mean ± SD; n≥3; *p<0.05. (E) Representative confocal images of midguts from Myo1A>+ or two independent RNAi lines to deplete PVR (Myo1A>PVR IR VDRC or NIG) infected with the indicated viruses analyzed 7 dpi (DCV, SINV, and VSV) or 10 dpi (DENV-2) (40×; virus-green, nuclei-blue). (F) Percent survival of control (PBS-fed) or infected (DCV-fed) Myo1A>+ or Myo1A>PVR IR NIG flies (n=3, *p=0.0026, log-rank test). (G) Representative immunoblot analysis of 20 pooled intestines from control (HS>+) or PVR-depleted guts (HS>PVR IR NIG) at 2 hpi. See also Figure S1.
We next examined the requirement of PVR in the gut. The Drosophila intestine, similar to the mammalian intestine, is a tubular epithelium composed of a monolayer of cells with the absorptive enterocytes lining >95% of the surface area. We previously found that ERK is specifically required in enterocytes (Xu et al., 2013), which is the known target of many enteric viruses, including picornaviruses in humans and arboviruses in their insect vectors (Blair, 2011; Davis and Engstrom, 2012; Franz et al., 2015; Mordstein et al., 2010; Peterson and Artis, 2014). Using in vivo RNAi specifically expressing in the intestinal epithelial cells (Myo1A-GAL4 driver), we depleted PVR using two independent RNAi lines and verified efficient knockdown of PVR (Fig. 1C, Fig. S1B). We found that these flies had a normal lifespan (Fig. 1F) with no barrier dysfunction (Fig. S1C) (Rera et al., 2011). Upon challenge with DCV, SINV, VSV, or dengue (DENV) flies with PVR-depleted intestinal epithelial cells had increased viral infection in the intestine as measured by RT-qPCR (Fig. 1D, Fig. S1D) and confocal microscopy (Fig. 1E) with the infection largely in the posterior midgut (Fig. S1E–F). As a control, we expressed a dominant negative Epidermal Growth Factor Receptor (EGFR DN), another RTK endogenously expressed in the intestine (Buchon et al., 2010), which had no impact on viral infection (Fig. 1D). To further confirm these results, and bypass any developmental requirements, we used a heat shock inducible driver to deplete PVR only in adult animals prior to challenge. Upon heat shock, we found again that knockdown of PVR resulted in a significant increase in DCV infection in the intestine (Fig. S1G). Moreover, we found that loss of PVR in the intestinal epithelium had a major impact on immunity. First, PVR-depleted animals challenged with DCV now succumbed to infection converting a largely non-pathogenic infection into a lethal one (Fig. 1F). Second, we found that PVR was required for virus induced ERK signaling in the gut (Fig. 1G, Fig. S1H). Altogether, our data show that PVR is a receptor required for antiviral ERK signaling in the intestinal epithelium.
Pvf2 is required for antiviral defense
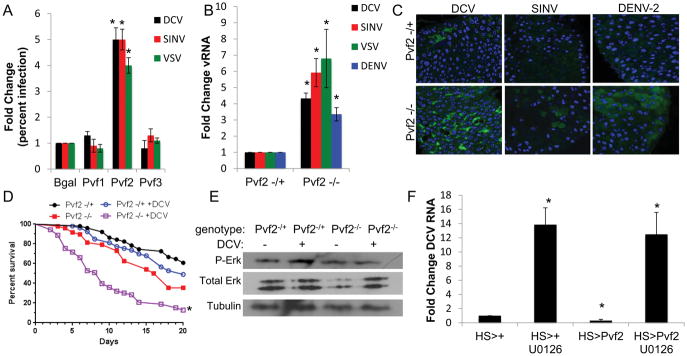
RTKs are activated by secreted ligands. PVR has three known ligands: Pvf1, Pvf2, and Pvf3 (Cho et al., 2002; Duchek et al., 2001) and RNAi in cell culture revealed that Pvf2 was required for antiviral defense against DCV, SINV and VSV (Fig. 2A). We orally challenged flies mutant for Pvf2 (Pvf2c06947) and found that they are more susceptible to DCV, SINV, VSV, and DENV infection as measured by RT-qPCR (Fig. 2B) and confocal microscopy (Fig. 2C). In contrast, flies mutant for Pvf1 (Pvf1EP1624) do not display a change in viral infection in the intestine (Fig. S2A). Moreover, Pvf2 mutants have increased lethality upon oral infection with DCV (Fig. 2D). Furthermore, we found that Pvf2 is required for DCV-induced phospho-ERK signaling in the intestine (Fig. 2E, Fig. S2B).
Figure 2. Pvf2 is required for antiviral defense in the Drosophila intestine.
(A) Drosophila cells treated with the indicated dsRNAs were challenged with the indicated viruses and monitored by automated microscopy and image analysis. Percent of infected cells was quantified and normalized to control with mean ± SD; n=3; *p<0.05. (B) Control (Pvf2-/+) or Pvf2 mutant (Pvf2-/-) flies were challenged with the indicated viruses. RT-qPCR analysis of viral RNA normalized to rp49 and shown relative to Pvf2 sibling controls from 15 pooled intestines 7 dpi (DCV, SINV, and VSV) or 10 dpi (DENV-2) with mean ± SD; n≥3; *p<0.05. (C) Representative images of midguts from sibling control or Pvf2 mutant flies infected with the indicated viruses analyzed 7 dpi (DCV and SINV) or 10 dpi (DENV-2) (40×; virus-green, nuclei-blue). (D) Percent survival of control (PBS-fed) or infected (DCV-fed) Pvf2 mutants or heterozygous sibling controls. (n=3; *p<0.01, log-rank test). (E) Representative immunoblot analysis of 20 pooled Pvf2 mutant or heterozygous sibling control intestines at 2 hpi. (F) RT-qPCR analysis of viral RNA normalized to rp49 and shown relative to control (HS>+) from 15 pooled intestines 7 dpi. Mean ± SD; n=4; *p<0.05. See also Figure S2.
Next, we tested whether Pvf2 induction was sufficient to induce antiviral ERK signaling in the gut. Here, we ectopically expressed Pvf2 in either the intestinal epithelium (Myo1A) or with a heat shock inducible driver (hs) and confirmed that expression of Pvf2 resulted in an increase in basal phospho-ERK levels (Fig. S2C–F). Next, we challenged these flies with DCV and observed decreased infection (Fig. 2F, Fig. S2G). We were unable to test the other viruses because we cannot detect infection in wild type flies. We next verified that Pvf2 is upstream of ERK, by challenging flies ectopically expressing Pvf2 in the presence and absence of the ERK inhibitor U0126 (Xu et al., 2013). Treatment with U0126 led to increased infection, which could not be suppressed by ectopic Pvf2 expression (Fig. 2F). Therefore, Pvf2 is necessary and sufficient to induce the antiviral ERK pathway in the intestine.
Pvf2 is induced by viral infection
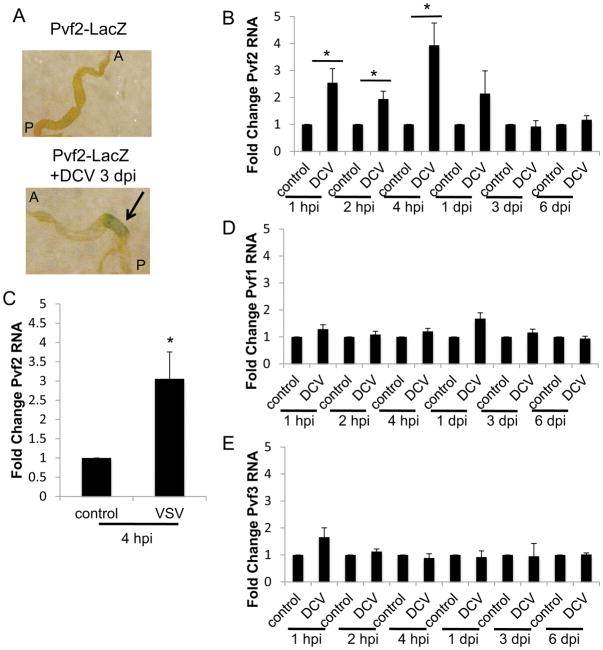
Since we observed induction of ERK signaling upon infection that was dependent upon Pvf2, we hypothesized that Pvf2 is regulated during viral infection. We first monitored Pvf2 levels using transgenic flies that carry a lacZ reporter downstream of the endogenous Pvf2 promoter (Choi et al., 2008). Upon oral infection, we observed induction of lacZ in the posterior midgut (Fig. 3A). This is the region of the gut where we observe the highest level of viral infection (Fig. S1E–F) and that is also the most responsive to the microbiota and bacterial infections (Bosco-Drayon et al., 2012; Broderick et al., 2014; Buchon et al., 2013a; Neyen et al., 2012).
Figure 3. Pvf2 expression is induced by viral infection.
(A) Flies carrying a Pvf2 promoter driven lacZ reporter (Pvf2-lacZ) were challenged with DCV and stained for beta-galactosidase activity. A representative image of the posterior midgut and arrow indicates DCV induced lacZ expression (A, anterior; P, posterior). (B–E) RT-qPCR analysis of Pvf2 (B–C), Pvf1 (D), or Pvf3 (E) mRNA normalized to rp49 and shown relative to control from 15 pooled intestines infected with DCV (B, D–E) or VSV (C) and isolated at the indicated time post infection. Mean ± SD; n≥3; *p<0.05.
Next, we monitored Pvf2 by RT-qPCR in the intestine following DCV infection over a wide time course. We observed a significant increase in Pvf2 mRNA 1 hpi that was highest at 4 hpi and returned to baseline by 24 hpi (Fig. 3B). Moreover, we observed a significant increase in Pvf2 when flies were orally challenged with VSV (Fig. 3C). This transcriptional induction was specific to Pvf2, since we did not observe a significant increase in Pvf1 or Pvf3 following oral challenge (Fig. 3D–E).
Pvf2 expression is regulated by the microbiota
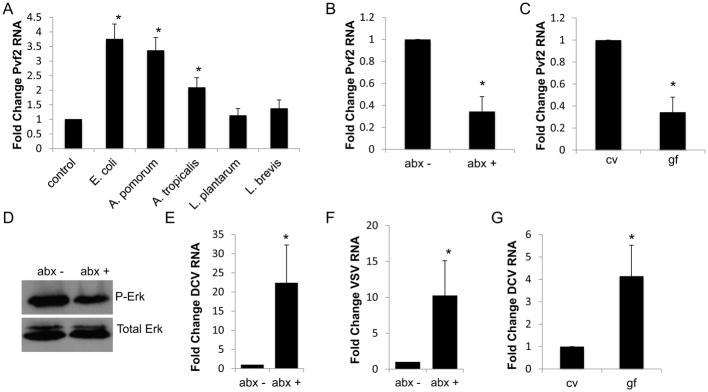
We set out to determine how Pvf2 is regulated in response to infection. It was previously shown that treatment with E. coli for 1hr can induce Pvf2 in cultured insect cells and that this was through the inflammatory Imd signaling pathway (Bond and Foley, 2009). This suggested that bacterial peptidoglycan was the stimulant, and therefore, we reasoned that the endogenous microflora might regulate intestinal Pvf2. First, we tested if bacterial products from Drosophila commensals could induce Pvf2 in vitro. The major commensals in the Drosophila intestine are: Acetobacter pomorum, Acetobacter tropicalis, Lactobacillus brevis, and Lactobacillus plantarum (Broderick and Lemaitre, 2012; Wong et al., 2011). We found that supernatants from E. coli and the gram-negative commensals A. pomorum and A. tropicalis strongly induced Pvf2 in cell culture, while the gram-positives L. plantarum and L. brevis did not (Fig. 4A). Moreover, the relative levels of Pvf2 induction by these commensals correlates with activation of the Imd pathway as measured by the production of the antimicrobial peptide mRNA diptericin (Fig. S3A).
Figure 4. The microbiota regulates Pvf2 expression and antiviral defense.
(A) Drosophila cells were treated for 1h with the supernatant of the indicated bacterial species and Pvf2 expression was monitored by RT-qPCR with mean ± SD; n=3; *p<0.05. (B) Flies fed vehicle or antibiotics were analyzed by RT-qPCR for Pvf2 mRNA normalized to rp49 and shown relative to control from 15 pooled intestines with mean ± SD; n≥3; *p<0.05. (C) Flies raised conventionally or germ-free were analyzed by RT-qPCR for Pvf2 mRNA normalized to rp49 and shown relative to control from 15 pooled intestines with mean ± SD; n=3; *p<0.05. (D) Representative immunoblot analysis of 20 pooled control or antibiotic treated guts. (E–F) Control or antibiotic treated flies were infected with (E) DCV or (F) VSV and RT-qPCR analysis of viral RNA was normalized to rp49 and shown relative to control from 15 pooled intestines 7 dpi. Mean ± SD; n=4; *p<0.05. (G) Conventionally reared or germ-free flies were infected with DCV and RT-qPCR analysis of DCV RNA normalized to rp49 is shown relative to control (conventionally reared flies) from 15 pooled intestines 7 dpi. Mean ± SD; n=6; *p<0.05. See also Figure S3.
This data suggested that the microbiota, and in particular gram-negatives, might be playing a role in Pvf2 regulation during viral infection. We set out to test this hypothesis by manipulating the endogenous microbiota. First, we used a cocktail of antibiotics to ablate the microbiota and observed a significantly decreased bacterial load (>2.9 log decrease in CFU/gut, Fig. S3B) and observed no defect in barrier function (Fig. S3C). Second, we raised germ-free flies, verified that these flies had no detectable bacteria (Fig. S3D) and observed normal barrier function (Fig. S3E). We measured the basal levels of Pvf2 in the microbiota-depleted intestine and observed decreased Pvf2 mRNA levels as measured by RT-qPCR (Fig. 4B–C). We also observed reduced basal phospho-ERK levels in antibiotic-treated intestines (Fig. 4D, Fig. S3F).
The microbiota is required for intestinal antiviral defense and A. pomorum is sufficient to confer intestinal antiviral immunity
If the microbiota regulates Pvf2, then loss of the microbiota would lead to increased enteric viral infection. First, we orally challenged antibiotic treated adult flies with DCV or VSV and measured viral replication and found that antibiotic-treated flies were more susceptible to viral infection (Fig. 4E–F). Second, we orally challenged germ-free flies (gf) and found that these flies displayed a significant increase in viral infection compared to conventionally reared animals (cv) (Fig. 4G).
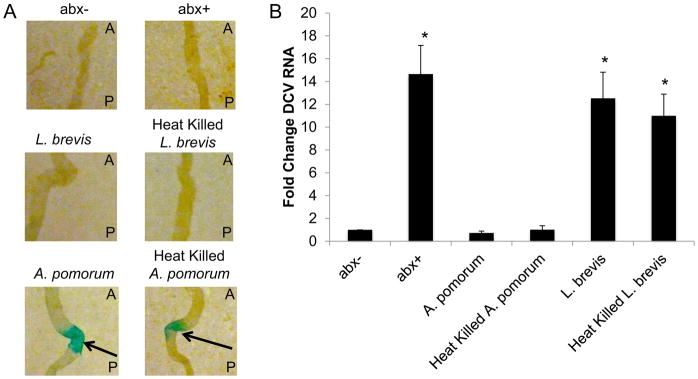
Therefore, the microbiota is required for antiviral defense and specific components of the microbiota efficiently activate Pvf2 expression in cell culture. These data suggest that the specific components of the microbiota that activate Pvf2 mediate this response. We thus hypothesized that A. pomorum, which potently induces Pvf2, would restore antiviral immunity to flies lacking a microbiota while L. brevis, which is a poor inducer of Pvf2, would not (Fig. 4A). To test this, we used a cocktail of antibiotics to ablate the microbiota and then monoassociated with A. pomorum or L. brevis. First, we determined the impact of monocolonization on Pvf2 induction in vivo. Consistent with our cell culture experiment, flies monoassociated with A. pomorum but not L. brevis had detectable Pvf2 expression in the intestine (Fig. 5A). Furthermore, we challenged the monoassociated flies with DCV. We found that A. pomorum but not L. brevis could completely restore antiviral immune function (Fig. 5B).
Figure 5. A. pomorum is sufficient to restore antiviral defense in the Drosophila intestine.
(A) Flies carrying a Pvf2 promoter driven lacZ reporter (Pvf2-lacZ) were associated with the indicated commensal and stained for beta-galactosidase activity. A representative image of the posterior midgut and arrows indicate induction of lacZ expression (A, anterior; P, posterior). (B) RT-qPCR analysis of DCV RNA normalized to rp49 and shown relative to control (w1118 abx-) from midguts of flies associated with the indicated commensal at 7 dpi. Mean ± SD; n=4; *p<0.05.
Since we used supernatants from the bacteria to induce Pvf2 in cell culture and a previous study found that peptidoglycan of E. coli was sufficient to induce Pvf2 in cell culture (Bond and Foley, 2009), we tested whether heat killed bacteria could mediate the antiviral response. Indeed, we observed that flies monoassociated with heat killed A. pomorum but not L. brevis had detectable Pvf2 expression in the intestine (Fig. 5A). Furthermore, we found that heat killed A. pomorum but not L. brevis could completely restore antiviral immune function (Fig. 5B).
The Imd pathway is required for antiviral defense and virus-induced Pvf2 expression
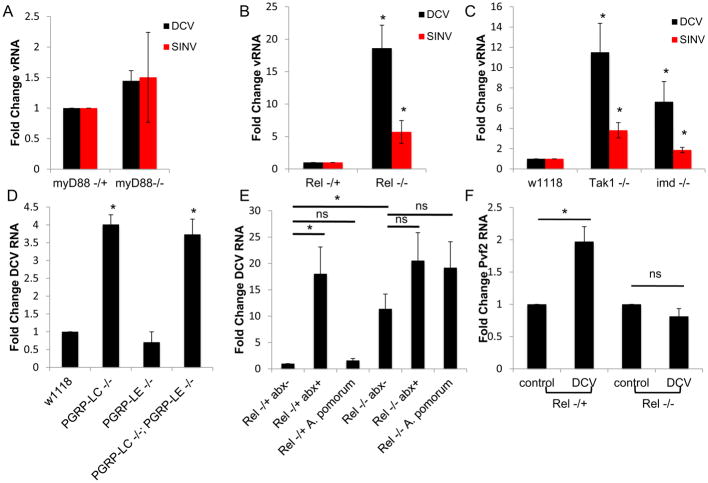
Since we found that heat killed bacteria could protect flies, our data suggested that a bacterial PAMP was mediating the antiviral activity. Insects encode the Toll and Imd pathways that sense microbes and converge on NF-kB activation, but studies suggest that the Imd but not the Toll pathway is active in the intestine (Lee and Brey, 2013). Thus, we tested if the Toll or Imd pathway plays a role in enteric viral infection. We challenged the Toll pathway mutant dMyD88 (dMyD88 EP(2)2133), which did not display altered susceptibility to infection (Fig. 6A). We also challenged three different mutants in the Imd pathway: imd (imd1 [the Drosophila homolog of FADD]), Tak1 (Tak12 [the Drosophila homolog of TAK1]), and Rel (RelE38 [the NF-kB transcription factor]). We found that all three mutants display a significant increase in viral infection in the intestine (Fig. 6B–C, Fig. S4A–B). Since the Imd component Tak1 induces the JNK pathway (Silverman et al., 2003; Takatsu et al., 2000), and others have found that JNK can regulate Pvf2 (Bond and Foley, 2009), we also challenged flies expressing a dominant negative form of JNK (bskDN). We expressed bskDN either in the intestinal epithelium or ubiquitously upon heat shock and found that inhibition of JNK signaling did not impact viral infection in the intestine (Fig. S4C–D), suggesting a specific role for Imd-dependent NF-kB activation in antiviral defense. These data are consistent with previous studies that suggested that the posterior midgut, where we observed Pvf2 expression, is the region of the gut most responsive to the Imd pathway (Bosco-Drayon et al., 2012; Broderick et al., 2014; Buchon et al., 2013a; Neyen et al., 2012). Since we found that the microbiota is signaling through the Imd pathway to activate Pvf2 expression, we next tested which of the peptidoglycan recognition protein receptors (PGRPs) in this pathway was involved. We challenged the two different PGRP receptor mutants, PGRP-LE (PGRP-LE112) and PGRP-LC (PGRP-LCΔE), along with the double mutant and observed increased DCV infection in the double mutant and PGRP-LC mutants, but not PGRP-LE mutants (Fig. 6D, Fig. S4B). These data suggest that peptidoglycans from the microbiota are sensed by PGRP-LC in the midgut to drive Pvf2 induction.
Figure 6. NF-kB is required for Pvf2 expression and antiviral defense in the Drosophila intestine.
(A–F) Flies of the indicated genotypes were infected with the indicated viruses. RT-qPCR analysis of viral (A–E) or Pvf2 (F) RNA normalized to rp49 and shown relative to control from 15 pooled intestines 7 dpi (A–E) or 4 hpi (F) with mean ± SD; n≥3; *p<0.05. See also Figure S4.
These data suggest that the microbiota, and in particular A. pomorum, is activating the Imd pathway to induce the antiviral ERK cascade. To directly test whether the antiviral activity of A. pomorum is dependent on the Imd pathway, we monoassociated control flies or flies mutant for the NF-kB gene Rel with A. pomorum. As expected, in control flies, A. pomorum is able to rescue antiviral function that is lost by ablation of the microbiota (Fig. 6E). However, in flies mutant for Rel, A. pomorum is no longer protective (Fig. 6E), and similar results were observed for flies mutant for Tak1 (Fig. S4E). Therefore, the antiviral activity of the microbiota is dependent on Imd signaling.
Next, we tested whether virus-dependent Pvf2 induction in the intestine was NF-kB-dependent. We found that flies mutant for Rel were unable to induce Pvf2 upon oral viral infection (Fig. 6F). We also tested the JNK pathway and found that expression of bskDN in the intestinal epithelium had no effect on virus-induced Pvf2 levels (Fig. S4F). These data suggest that activation of NF-kB downstream of the microbiota is required for antiviral defense in the intestine.
Virus-induced Pvf2 is microbiota and Cdk9-dependent
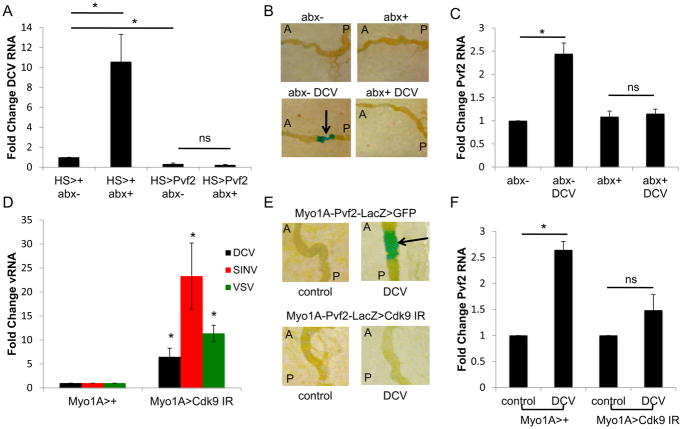
Altogether, these data suggest that virus-induced Pvf2 activation is downstream of the microbiota. Therefore, we tested whether we could rescue the microbiota-dependent antiviral activity by enforced Pvf2 expression. We transiently expressed Pvf2 in either conventionally reared or antibiotic treated flies using a heat shock inducible driver. As expected, antibiotic treatment of control flies led to increased infection, and expression of Pvf2 attenuated infection in conventional animals (Fig. 7A). Furthermore, we found that ectopic Pvf2 expression is sufficient to completely restore antiviral immunity to antibiotic-treated flies (Fig. 7A). These data demonstrate that Pvf2 is downstream of the microbiota.
Figure 7. Virus-induced Pvf2 expression is microbiota and Cdk9 dependent.
(A, C–D, F) RT-qPCR analysis of viral (A, D) or Pvf2 (C, F) RNA normalized to rp49 and shown relative to control for the indicated genotypes 7 dpi (A, D) or 4 hpi (C, F) from 15 pooled intestines. Mean ± SD; n=4; *p<0.05. (B) Flies carrying a Pvf2 promoter driven lacZ reporter (Pvf2-lacZ) were antibiotic treated, infected with DCV, and stained for beta-galactosidase activity at 3 dpi. A representative image of the posterior midgut and arrows indicate induction of lacZ expression (A, anterior; P, posterior). (E) Flies of the indicated genotypes were infected with DCV stained for beta-galactosidase activity at 3 dpi. A representative image of the posterior midgut and arrows indicate induction of lacZ expression (A, anterior; P, posterior). See also Figure S5.
Moreover, these data suggest that the microbiota is required for virus-induced Pvf2 expression. To test this directly, we challenged antibiotic treated flies with DCV and found that while conventional flies responded to viral infection by inducing Pvf2 as measured either by lacZ expression or RT-qPCR, loss of the microbiota prevented virus-induced Pvf2 expression (Fig. 7B–C).
Therefore, these data suggest that two signals are required for Pvf2 induction. We found that the microbiota, specifically gram-negative commensals, such as A. pomorum, along with a virus-dependent signal are required for full activation. We previously showed that transcriptional pausing primes virally induced genes to facilitate rapid induction of the antiviral response (Xu et al., 2012). Pol II is recruited to the promoter and engages in transcriptional initiation; however, due to its association with NELF (negative elongation factor) and DSIF (DRB-sensitivity factor), Pol II can only synthesize short, abortive transcripts. Upon viral sensing, Pol II is released from pausing by recruitment of P-TEFb (positive elongation factor) leading to the rapid production of functional antiviral mRNAs (Nechaev and Adelman, 2011; Xu et al., 2012). Indeed, we previously demonstrated in cell culture that virus-induced ERK activation is pausing dependent by depleting Cdk9, which is a component of P-TEFb (Xu et al., 2013). Therefore, we examined the role of transcriptional pausing in oral infection. We depleted Cdk9 in the intestinal epithelium, orally challenged with DCV, SINV, or VSV, and observed a significant increase in viral infection in the intestine as measure by RT-qPCR (Fig. 7D). We also used a heat shock driver to transiently deplete Cdk9 and again observed a significant increase in DCV infection (Fig. S5A). Next, we examined if transcriptional pausing is required for virus-induced Pvf2 activation using two assays. We found that virus-dependent Pvf2 expression is Cdk9-dependent both by monitoring Pvf2-lacZ expression (Fig. 7E) and by measuring Pvf2 by RT-qPCR (Fig. 7F). Both assays show that virus-dependent Pvf2 expression is dependent on Cdk9. Overall, we propose a model in which Pvf2 is induced by a virus-stimulated and microbiota-dependent NF-kB signaling cascade in the intestine, which activates epithelial antiviral ERK responses (Fig. S5B).
Discussion
Enteric viruses must overcome the intestinal barrier to establish infection within the organism. Here we demonstrate that the antiviral ERK pathway is activated in the Drosophila intestine by the Pvf2-PVR pathway and that Pvf2 expression is induced in the posterior midgut by viral infection (Fig. S5B). Additionally, the posterior midgut is the region of the gut that is most responsive to the microbiota (Broderick et al., 2014), and we found that induction of Pvf2 is dependent on microbiota signaling through the NF-kB pathway, which primes the antiviral response. In the absence of the microbiota, the animals are more susceptible to oral challenge and this can be overcome by ectopically expressing Pvf2 or by mono-association with A. pomorum, the commensal, which potently activates Pvf2, but not L. brevis, which does not induce Pvf2. However, the endogenous microbiota signaling through the Imd pathway is not sufficient to induce Pvf2, but requires a second signal. We found that transcriptional pausing is also required for Pvf2 induction. We previously found that transcriptional pausing is required for the induction of half of the virus-induced genes, (Xu et al., 2012) suggesting that a pausing-regulated gene is required for Pvf2 induction. Future studies will be directed toward understanding the mechanism of how transcriptional pausing cooperates with NF-kB to regulate Pvf2 expression.
A growing body of literature has shown that the microbiota can play a protective role in antiviral immunity against enteric viruses. Antibiotic-treated Aedes aegypti are more susceptible to DENV infection (Cirimotich et al., 2011; Ramirez et al., 2012; Xi et al., 2008). In mammalian intestinal cell culture, commensals were shown to block rotavirus infection (Varyukhina et al., 2012) and germ-free mice are more susceptible to coxsackievirus infection displaying an increase in virus-associated mortality (Pang and Iwasaki, 2012; Schaffer et al., 1963). Moreover, the microbiota is protective from influenza virus infection of the lung and from systemic lymphocytic choriomeningitis virus infection (Abt et al., 2012; Ichinohe et al., 2011). Our work further supports an essential role for the microbiota in maintaining the host’s health defenses against viral challenges and provides mechanistic insight into molecular pathways involved.
Further, by taking advantage of the simplified Drosophila system, we found a role for specific members of the community. We demonstrated that the endogenous gram-negative commensals are strong inducers of the Imd pathway and therefore activate NF-kB signaling to induce Pvf2 expression in the epithelium. In particular, we found that A. pomorum is protective from viral infection. This bacteria is sufficient to promote normal developmental time (Newell and Douglas, 2014) and for optimal growth of larvae on nutrient scarce diets (Shin et al., 2011). A. pomorum activates the insulin signaling pathway (Shin et al., 2011), which is known to activate ERK signaling (Kim et al., 2004; Lee et al., 2008). Therefore, A. pomorum may be modulating antiviral defense through two independent pathways. Interestingly, Acetobacter species are commonly associated with most laboratory raised and wild caught strains (Broderick and Lemaitre, 2012), suggesting that the protective role of Acetobacter could be acting in the wild.
Our finding that Pvf2 is induced in the midgut by commensals is consistent with previous studies that found that Pvf2 expression was dependent on PGRP-LC and Imd signaling (Bond and Foley, 2009). Furthermore, in addition to observing viral infection in enterocytes, we observed regional responsiveness: both virus-induced Pvf2 expression and viral infection was observed in the same region, the posterior midgut. From insects to mammals, the digestive tract is divided into distinct regions with distinct characteristics (Buchon et al., 2013b; Karasov et al., 2011). Moreover, intestinal pathologies tend to be region specific (Stainier, 2005) and therefore due to its relative simplicity and small size, the Drosophila intestine serves as an ideal model for examining compartmentalization and its role in disease states. Whether enteric viral infections in mammals are regionalized is unclear, but likely.
Activation of NF-kB signaling by viral infection is commonly observed. In mosquitoes, the Imd pathway controls DENV infection in the midgut (Sim et al., 2013). Moreover, the Imd pathway activates the NF-kB transcription factor Rel2, which is required for antiviral defense against orally acquired viruses in the blood meal of mosquitoes (Avadhanula et al., 2009; Cirimotich et al., 2011; Paradkar et al., 2014; Xi et al., 2008). We propose that some of this regulation may be downstream of the microbiota of mosquitoes, and that this regulation may require multiple inputs; NF-kB may be necessary but not sufficient for antiviral responses. In mammals, the intestinal epithelium senses infection of bacterial products through TLRs, including TLR2 and TLR4, which also induce NF-kB-dependent responses (Kumar et al., 2009; Takeuchi et al., 1999). Furthermore, TLR2 and TLR4 also activate ERK signaling (Banerjee and Gerondakis, 2007; Good et al., 2012), suggesting that there may be a functional conservation of the links between the microbiota, NF-kB activity, and antiviral response in the digestive tract. However, virus-intestinal interactions are clearly more complex, as in some cases enteric viruses associate with bacterial products to either stabilize them or to activate pro-viral NF-kB pathways (Kane et al., 2011; Kuss et al., 2011; Robinson et al., 2014). However, TLR2/4 pathways can restrict viral infection in some cases (Arpaia and Barton, 2011; Lester and Li, 2014) and thus may intersect with our findings that the microbiota influences the activation of the NF-kB signaling for antiviral immunity.
Recent studies have also suggested that enteric viruses can induce signals that intersect with the microbiota demonstrating unappreciated interrelationships between viral and microbiota-dependent responses (Kernbauer et al., 2014). Further, our identification of a secreted factor, Pvf2, which directly impacts enteric virus immunity in the intestine of Drosophila may have other parallels in mammals given the increasing appreciation of the roles of the epithelium in producing secreted factors that drive downstream immune functions (Gallo and Hooper, 2012; Peterson and Artis, 2014; Rescigno, 2011). Since we find that this microbiota-NF-kB-ERK pathway is active against divergent viruses, it may represent an ancient pathway that evolved to restrict infection at this mucosal surface. Since all enteric viruses come into contact with the microbiota prior to establishing infection, future studies will be directed toward understanding the mechanisms driving these interactions. Importantly, we also found that specific commensals drive different responses and a clearer understanding of how the particular commensals and the structure of the commensal population impacts immunity will inform mechanisms of dysbiosis driven pathologies.
Experimental Procedures
Fly Rearing and Infections
All fly stocks used in this study are Wolbachia-free and listed in Table S1. Flies were orally infected as previously described (Xu et al., 2013). In brief, seven to ten day old female flies of the indicated genotypes were orally infected with 10uL of each virus (DCV: 1×1012 IU/mL; VSV: 1×108 pfu/mL; SINV: 1×109 pfu/mL; DENV-2: 2×108 pfu/mL) in sucrose for three days and then transferred to virus-free food every 3 days or for the duration of the experiment. For survival, flies were scored daily for 20 days. Three independent replicates of 15 flies each were performed for each experiment. Heat shock flies were incubated at 37°C for 1 hour everyday for 3 days prior to infection. Once orally infected, flies were incubated at 37°C for 1 hour every other day for the duration of the experiment.
Cells and Viruses
Insect cells (DL1) were grown and maintained as described (Rose et al., 2011). VSV-GFP, SINV-GFP, DCV, and DENV-2 were grown as described (Sessions et al., 2009; Xu et al., 2012).
Cell Culture
Amplicons used are described at http://flyrnai.org. dsRNA were generated and used for RNAi for 3 days as previously described (Cherry et al., 2005). For Pvf2 induction in vitro, 3×106 DL1 cells were plated in a 6-well plate in complete media for 24 hours. Commensals and E. coli were grown and normalized to an OD of 0.1 and then 1 mL of bacteria was centrifuged at 13,000 rpm for 2 minutes. 30 μL of supernatant was added to the cells for 1 hour and then processed for RT-qPCR.
RNA and Quantitative Real-Time PCR
Total RNA was extracted from cells or 15 fly guts, using TRIzol (Invitrogen) according to manufacturer’s protocol and as previously described (Xu et al., 2013). cDNA was prepared using M-MLV reverse transcriptase (Invitrogen). cDNA was analyzed using Power SYBR Green PCR Master Mix (Applied Biosystems), along with gene specific primers in triplicate, for at least three independent experiments. Data was analyzed by relative quantification, by normalizing to rp49. Primers are listed in Table S2.
Immunoblotting
Cells or 20 fly guts were collected and lysed with NP40 buffer supplemented with a protease inhibitor cocktail. Samples were separated and blotted as previously described for three independent experiments (Xu et al., 2013).
X-Gal Staining
X-gal staining was performed as previously described (Choi et al., 2008). Three independent experiments were performed imaging at least 5 guts per condition.
Immunofluorescence and Confocal Microscopy
Guts were processed as previously described (Xu et al., 2013). Briefly, 5 guts per experiment were dissected in PBS, fixed in 4% formaldehyde solution for 30 minutes, rinsed 3 times in PBS, and blocked with 5% normal donkey serum for 45 minutes. Samples were incubated with primary antibody (DCV capsid 1:3000) or (Dengue 1:4000) overnight at 4°C, rinsed 3 times in PBT, and incubated with secondary antibody (1:1000) and Hoescht 33342 at room temperature for 1 hour 15 minutes. Samples were rinsed 3 times in PBT and mounted in Vectashield (Vector Laboratories). Guts were imaged on Leica TCS SPE confocal microscope at 10× or 40×. Three independent experiments were performed imaging.
Statistics and Data Analysis
For RT-qPCR studies, P values were obtained by comparing delta CT values for three independent experiments. For survival curves, pairwise comparisons of each experimental group with its control were carried out using a Mantel-Haenszel test. For other experiments, the Student’s two-tailed t test was used to measure the statistical significance in each experiment and then considered significant if p<0.05 in each of three independent experiments.
Supplementary Material
Acknowledgments
We would like to thank the following for fly stocks: Bloomington, VDRC, NIG, BDGP, DRSC, B. Stronach, N. Silverman, E. Baehrecke, and M. Yoo. We would like to thank M. Diamond and K. Bruckner for antibodies; J. Rose, R. Hardy, P. Christian and ATCC for viruses. We would like to thank the Cherry lab, C. Bartman, R. Bushman, M. Povelones, and S. Ross for helpful discussions and advice. This work was supported by National Institute of Health grants R01AI074951, U54AI057168, and R01AI095500 to SC and pilot funding from P30DK050306. SC is a recipient of the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abzug MJ. The enteroviruses: problems in need of treatments. The Journal of infection. 2014;68(Suppl 1):S108–114. doi: 10.1016/j.jinf.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Barton GM. Toll-like receptors: key players in antiviral immunity. Current opinion in virology. 2011;1:447–454. doi: 10.1016/j.coviro.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya CD. Major foodborne illness causing viruses and current status of vaccines against the diseases. Foodborne pathogens and disease. 2004;1:89–96. doi: 10.1089/153531404323143602. [DOI] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect biochemistry and molecular biology. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS pathogens. 2009;5:e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Gerondakis S. Coordinating TLR-activated signaling pathways in cells of the immune system. Immunology and cell biology. 2007;85:420–424. doi: 10.1038/sj.icb.7100098. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair CD. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future microbiology. 2011;6:265–277. doi: 10.2217/fmb.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond D, Foley E. A quantitative RNAi screen for JNK modifiers identifies Pvr as a novel regulator of Drosophila immune signaling. PLoS pathogens. 2009;5:e1000655. doi: 10.1371/journal.ppat.1000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopegamage S, Kovacova J, Vargova A, Motusova J, Petrovicova A, Benkovicova M, Gomolcak P, Bakkers J, van Kuppeveld F, Melchers WJ, et al. Coxsackie B virus infection of mice: inoculation by the oral route protects the pancreas from damage, but not from infection. The Journal of general virology. 2005;86:3271–3280. doi: 10.1099/vir.0.81249-0. [DOI] [PubMed] [Google Scholar]
- Bosco-Drayon V, Poidevin M, Boneca IG, Narbonne-Reveau K, Royet J, Charroux B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell host & microbe. 2012;12:153–165. doi: 10.1016/j.chom.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Broderick NA, Buchon N, Lemaitre B. Microbiota-Induced Changes in Drosophila melanogaster Host Gene Expression and Gut Morphology. mBio. 2014;5 doi: 10.1128/mBio.01117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick NA, Lemaitre B. Gut-associated microbes of Drosophila melanogaster. Gut microbes. 2012;3:307–321. doi: 10.4161/gmic.19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC biology. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nature reviews Microbiology. 2013a;11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- Buchon N, Osman D, David FP, Fang HY, Boquete JP, Deplancke B, Lemaitre B. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell reports. 2013b;3:1725–1738. doi: 10.1016/j.celrep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Charroux B, Royet J. Gut-microbiota interactions in non-mammals: what can we learn from Drosophila? Seminars in immunology. 2012;24:17–24. doi: 10.1016/j.smim.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Cherry S, Doukas T, Armknecht S, Whelan S, Wang H, Sarnow P, Perrimon N. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes & development. 2005;19:445–452. doi: 10.1101/gad.1267905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NK, Keyes L, Johnson E, Heller J, Ryner L, Karim F, Krasnow MA. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell. 2002;108:865–876. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich CM, Ramirez JL, Dimopoulos G. Native microbiota shape insect vector competence for human pathogens. Cell host & microbe. 2011;10:307–310. doi: 10.1016/j.chom.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Engstrom Y. Immune response in the barrier epithelia: lessons from the fruit fly Drosophila melanogaster. Journal of innate immunity. 2012;4:273–283. doi: 10.1159/000332947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Franz AW, Kantor AM, Passarelli AL, Clem RJ. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses. 2015;7:3741–3767. doi: 10.3390/v7072795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nature reviews Immunology. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill N, Wlodarska M, Finlay BB. Roadblocks in the gut: barriers to enteric infection. Cellular microbiology. 2011;13:660–669. doi: 10.1111/j.1462-5822.2011.01578.x. [DOI] [PubMed] [Google Scholar]
- Good DW, George T, Watts BA., 3rd Toll-like receptor 2 is required for LPS-induced Toll-like receptor 4 signaling and inhibition of ion transport in renal thick ascending limb. The Journal of biological chemistry. 2012;287:20208–20220. doi: 10.1074/jbc.M111.336255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Rodriguez SD, Drake LL. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Frontiers in physiology. 2014;5:103. doi: 10.3389/fphys.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousset FX. Host range of drosophila melanogaster C virus among diptera and lepidoptera (author’s transl) Annales de microbiologie. 1976;127:529–544. [PubMed] [Google Scholar]
- Jubelt B, Lipton HL. Enterovirus/picornavirus infections. Handbook of clinical neurology. 2014;123:379–416. doi: 10.1016/B978-0-444-53488-0.00018-3. [DOI] [PubMed] [Google Scholar]
- Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. Successful transmission of a retrovirus depends on the commensal microbiota. Science (New York, NY) 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov WH, Martinez del Rio C, Caviedes-Vidal E. Ecological physiology of diet and digestive systems. Annual review of physiology. 2011;73:69–93. doi: 10.1146/annurev-physiol-012110-142152. [DOI] [PubMed] [Google Scholar]
- Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;516:94–98. doi: 10.1038/nature13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Cho JY, Kim KS, Lee SJ, Lee KH, Choi KY. Drosophila PI3 kinase and Akt involved in insulin-stimulated proliferation and ERK pathway activation in Schneider cells. Cellular signalling. 2004;16:1309–1317. doi: 10.1016/j.cellsig.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Kingsolver MB, Huang Z, Hardy RW. Insect antiviral innate immunity: pathways, effectors, and connections. Journal of molecular biology. 2013;425:4921–4936. doi: 10.1016/j.jmb.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo HL, Ajami N, Atmar RL, DuPont HL. Noroviruses: The leading cause of gastroenteritis worldwide. Discovery medicine. 2010;10:61–70. [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochemical and biophysical research communications. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science (New York, NY) 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, Jung SA, Kim AK, You KH, Tatar M, Yu K. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nature cell biology. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Brey PT. How microbiomes influence metazoan development: insights from history and Drosophila modeling of gut-microbe interactions. Annual review of cell and developmental biology. 2013;29:571–592. doi: 10.1146/annurev-cellbio-101512-122333. [DOI] [PubMed] [Google Scholar]
- Lester SN, Li K. Toll-like receptors in antiviral innate immunity. Journal of molecular biology. 2014;426:1246–1264. doi: 10.1016/j.jmb.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. Journal of virology. 2010;84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossel EC, Ramig RF. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. Journal of virology. 2002;76:6502–6509. doi: 10.1128/JVI.76.13.6502-6509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbachs A, Bhatnagar J, Zaki SR. Tissue tropism, pathology and pathogenesis of enterovirus infection. The Journal of pathology. 2014 doi: 10.1002/path.4438. [DOI] [PubMed] [Google Scholar]
- Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nature reviews Immunology. 2001;1:59–67. doi: 10.1038/35095573. [DOI] [PubMed] [Google Scholar]
- Nechaev S, Adelman K. Pol II waiting in the starting gates: Regulating the transition from transcription initiation into productive elongation. Biochimica et biophysica acta. 2011;1809:34–45. doi: 10.1016/j.bbagrm.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell PD, Douglas AE. Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Applied and environmental microbiology. 2014;80:788–796. doi: 10.1128/AEM.02742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyen C, Poidevin M, Roussel A, Lemaitre B. Tissue- and ligand-specific sensing of gram-negative infection in drosophila by PGRP-LC isoforms and PGRP-LE. Journal of immunology (Baltimore, Md : 1950) 2012;189:1886–1897. doi: 10.4049/jimmunol.1201022. [DOI] [PubMed] [Google Scholar]
- Pang IK, Iwasaki A. Control of antiviral immunity by pattern recognition and the microbiome. Immunological reviews. 2012;245:209–226. doi: 10.1111/j.1600-065X.2011.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar PN, Duchemin JB, Voysey R, Walker PJ. Dicer-2-dependent activation of Culex Vago occurs via the TRAF-Rel2 signaling pathway. PLoS neglected tropical diseases. 2014;8:e2823. doi: 10.1371/journal.pntd.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nature reviews Immunology. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Dhadialla TS. Accumulation of yolk proteins in insect oocytes. Annual review of entomology. 1992;37:217–251. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- Ramirez JL, Souza-Neto J, Torres Cosme R, Rovira J, Ortiz A, Pascale JM, Dimopoulos G. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS neglected tropical diseases. 2012;6:e1561. doi: 10.1371/journal.pntd.0001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T, Jr, Jones DL, Walker DW. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell metabolism. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends in immunology. 2011;32:256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell host & microbe. 2014;15:36–46. doi: 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PP, Hanna SL, Spiridigliozzi A, Wannissorn N, Beiting DP, Ross SR, Hardy RW, Bambina SA, Heise MT, Cherry S. Natural resistance-associated macrophage protein is a cellular receptor for sindbis virus in both insect and mammalian hosts. Cell host & microbe. 2011;10:97–104. doi: 10.1016/j.chom.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sair AI, D’Souza DH, Moe CL, Jaykus LA. Improved detection of human enteric viruses in foods by RT-PCR. Journal of virological methods. 2002;100:57–69. doi: 10.1016/s0166-0934(01)00397-4. [DOI] [PubMed] [Google Scholar]
- Schaffer J, Beamer PR, Trexler PC, Breidenbach G, Walcher DN. Response of germ-free animals to experimental virus monocontamination. I. Observation on Coxsackie B virus. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 1963;112:561–564. doi: 10.3181/00379727-112-28105. [DOI] [PubMed] [Google Scholar]
- Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, et al. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science (New York, NY) 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, Maniatis T. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. The Journal of biological chemistry. 2003;278:48928–48934. doi: 10.1074/jbc.M304802200. [DOI] [PubMed] [Google Scholar]
- Sim S, Jupatanakul N, Ramirez JL, Kang S, Romero-Vivas CM, Mohammed H, Dimopoulos G. Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in dengue virus-refractory populations and identifies novel virus-vector molecular interactions. PLoS neglected tropical diseases. 2013;7:e2295. doi: 10.1371/journal.pntd.0002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nature reviews Microbiology. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Sopko R, Perrimon N. Receptor tyrosine kinases in Drosophila development. Cold Spring Harbor perspectives in biology. 2013;5 doi: 10.1101/cshperspect.a009050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY. No organ left behind: tales of gut development and evolution. Science (New York, NY) 2005;307:1902–1904. doi: 10.1126/science.1108709. [DOI] [PubMed] [Google Scholar]
- Steinert S, Levashina EA. Intracellular immune responses of dipteran insects. Immunological reviews. 2011;240:129–140. doi: 10.1111/j.1600-065X.2010.00985.x. [DOI] [PubMed] [Google Scholar]
- Sundaram MV. Canonical RTK-Ras-ERK signaling and related alternative pathways. WormBook : the online review of C elegans biology. 2013:1–38. doi: 10.1895/wormbook.1.80.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick WJ. Nature, nurture and evolution of intra-species variation in mosquito arbovirus transmission competence. International journal of environmental research and public health. 2013;10:249–277. doi: 10.3390/ijerph10010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu Y, Nakamura M, Stapleton M, Danos MC, Matsumoto K, O’Connor MB, Shibuya H, Ueno N. TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Molecular and cellular biology. 2000;20:3015–3026. doi: 10.1128/mcb.20.9.3015-3026.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Varyukhina S, Freitas M, Bardin S, Robillard E, Tavan E, Sapin C, Grill JP, Trugnan G. Glycan-modifying bacteria-derived soluble factors from Bacteroides thetaiotaomicron and Lactobacillus casei inhibit rotavirus infection in human intestinal cells. Microbes and infection / Institut Pasteur. 2012;14:273–278. doi: 10.1016/j.micinf.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nature reviews Microbiology. 2004;2:789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CN, Ng P, Douglas AE. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environmental microbiology. 2011;13:1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS pathogens. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Cherry S. Viruses and antiviral immunity in Drosophila. Developmental and comparative immunology. 2014;42:67–84. doi: 10.1016/j.dci.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Grant G, Sabin LR, Gordesky-Gold B, Yasunaga A, Tudor M, Cherry S. Transcriptional pausing controls a rapid antiviral innate immune response in Drosophila. Cell host & microbe. 2012;12:531–543. doi: 10.1016/j.chom.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hopkins K, Sabin L, Yasunaga A, Subramanian H, Lamborn I, Gordesky-Gold B, Cherry S. ERK signaling couples nutrient status to antiviral defense in the insect gut. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15025–15030. doi: 10.1073/pnas.1303193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.