Synopsis
Circadian (body clock) timing has a profound influence on mental health, physical health, and health behaviors. This review focuses on how light, melatonin and other melatonin receptor agonist drugs can be used to shift circadian timing in patients with misaligned circadian rhythms. A brief overview of the human circadian system is provided, followed by a discussion of patient characteristics and safety considerations that can influence the treatment of choice. The important features of light treatment, light avoidance, exogenous melatonin and other melatonin receptor agonists are reviewed, along with some of the practical aspects of light and melatonin treatment.
Keywords: advance, agonist, circadian, delay, light, melatonin
Introduction
Multiple varieties of light devices and multiple formulations of exogenous melatonin are commercially available without prescription in the United States. Light devices are most commonly used by patients with seasonal affective disorder, which represent about 1–2% of the North American general population [5]. Similarly, about 2% of US adults use exogenous melatonin, most typically as a sleep aid [6, 7]. There are also several melatonin receptor agonist formulations available via prescription in various countries around the world.
Light, melatonin, and other melatonin receptor agonists can significantly impact circadian (“body clock”) physiology, particularly the timing of circadian rhythms. Circadian timing in turn has a widespread and profound influence on mental and physical health (e.g. [8–10]). For example, there are projections from the central circadian clock to peripheral tissues [11, 12], and the circadian clock has a direct influence on sleep [13] and inflammatory processes [14]. The central circadian clock also influences circadian clocks in peripheral systems [15]. The focus of this review is on the use of light, melatonin and other melatonin receptor agonists to shift central circadian timing in patients in whom misaligned biological rhythms are thought to play a role, and the practical issues surrounding their use. Light can also suppress melatonin [16] and increase alertness [17] and exogenous melatonin can increase circulating levels of melatonin [18], but here we restrict our focus to circadian phase shifting as this is most often the aim of light and melatonin treatment. We intend for this review to complement rather than replace clinical recommendations on the use of light, melatonin and other melatonin receptor agonists to treat circadian rhythm sleep disorders ([19]), depression (e.g. [20, 21]) and/or insomnia (e.g. [22]). Below we provide a brief review of the human circadian system, followed by a summary of the patient characteristics and safety issues to consider before recommending light treatment, melatonin or other melatonin receptor agonists to patients. We then review the characteristics of light that are relevant to circadian physiology, and practical aspects of light treatment and conversely, light avoidance. The important features of exogenous melatonin and other melatonin receptor agonists are then described, followed by practical aspects of melatonin treatment and briefly, how melatonin can be combined with light treatment to increase shifts in circadian timing. We end with a consideration of how to evaluate patient outcomes post-treatment.
The Central Circadian System
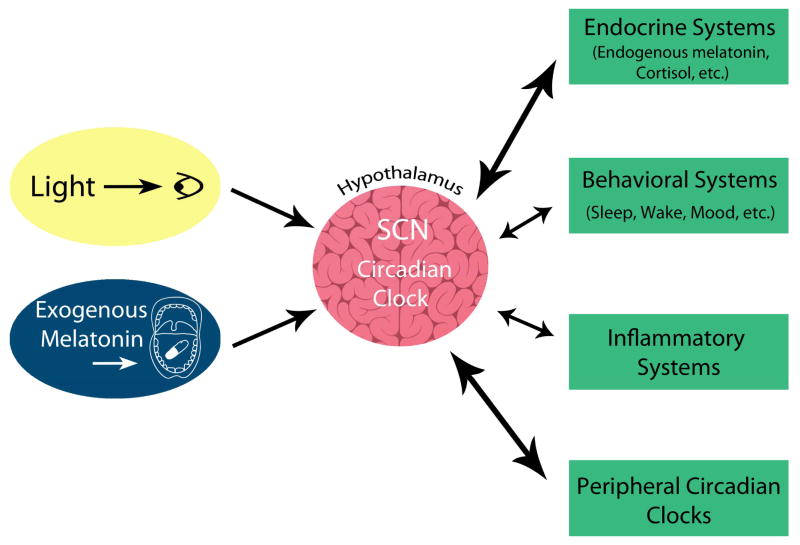
The central circadian system can be conceptualized as having 3 components: (1) input pathways that provide signals to synchronize the endogenous central clock to the external environment, (2) the central clock, which generates the rhythms, and (3) output pathways or rhythms that convey the central clock signal to other regulatory systems in the brain and body (Figure 1). In terms of input pathways, the strongest resetting agent is light. Light is captured by the five retinal photoreceptors (rods, blue cones, green cones, red cones, and the intrinsically photosensitive retinal ganglion cells [ipRGCs]) and the signal is transmitted to the central circadian clock [23]. Other “non-photic” stimuli, such as exogenous melatonin, can also be used to shift circadian timing. Here we refer to exogenous melatonin as melatonin that people typically ingest, after which it enters the circulation and is believed to shift circadian timing by binding to melatonin receptors on the central clock [24].
Figure 1.
The three components of the circadian system: (1) input pathways such as light and exogenous melatonin, which provide timing signals to the central circadian clock, (2) the central clock, suprachiasmatic nuclei (SCN) in the hypothalamus which generates the rhythms, and (3) the output rhythms which includes endogenous melatonin and molecular peripheral circadian clocks contained in most tissues. Many of these output rhythms can feedback to the central circadian clock.
The central circadian clock is located in the suprachiasmatic nuclei (SCN) in the hypothalamus [25]). More than 70% of humans have an endogenous period greater than 24 hours (on average ~24.2 h) [26, 27]. Thus, for most humans, their internal body clock takes more than 24 hours to complete one cycle, meaning that they have an endogenous tendency to drift later (“phase delay”) each day. This is perhaps most commonly seen in the later sleep times that often occur on the weekend or work-free days [28]. A gradual later drift in circadian timing is also seen in totally blind individuals, as light does not reach their internal circadian clock [29]. Thus, daily input signals are required to shift the clock earlier (“phase advance”) to synchronize the clock’s timing to the external 24-hour day.
The output circadian rhythm often measured to infer the timing of the central circadian clock in humans is the endogenous melatonin rhythm. We use the term endogenous melatonin, to refer to internally produced melatonin. The endogenous melatonin rhythm is believed to accurately represent the timing of the central circadian clock, as the secretion of melatonin from the pineal gland is controlled by the SCN [30]. Endogenous melatonin also likely feeds back to bind to the central clock and reinforces circadian timing [31]. Currently, the most reliable marker of circadian timing is the dim light melatonin onset (DLMO) [32], which is the time when endogenous melatonin levels begin to rise in dim light ~2–3 hours before habitual bedtime [33] (Figure 2). Endogenous melatonin must be measured in dim light, as light suppresses melatonin [16, 34]. The DLMO is most easily assessed via half-hourly or hourly saliva samples collected in the ~6 hours or so before habitual bedtime, which are later assayed for melatonin concentration [35].
Figure 2.
Individual melatonin profiles generated from saliva samples collected every 30 minutes in dim light. Baseline dim light melatonin profiles are shown in black circles. In both individuals, the dim light melatonin onset (DLMO) occurs ~3–3.5 hours before habitual bedtime. Note that the clock time of the DLMOs varies according to the clock time of habitual bedtime in each individual. On the left, the dim light melatonin profile shifts later in clock time, after exposure to evening bright light (phase delay). On the right the dim light melatonin profile shifts earlier in clock time, after exposure to morning bright light (phase advance).
In addition to the endogenous melatonin rhythm, it is important to note that the central circadian clock drives a whole host of other output rhythms, and impacts multiple other systems including, but not limited to: endocrine systems [36], metabolism [37], inflammation [14], mood [10, 38, 39] and behaviors including sleep [40]. Indeed, whether a direct or indirect influence, mistiming of circadian rhythms is also associated with an increase in negative health behaviors such as the excessive consumption of alcohol, caffeine and nicotine [41] and is also associated with unhealthy dietary habits [42]. It has been hypothesized that mistimed circadian rhythms play a role in mood disorders as well [39, 43]. As the importance of circadian timing to mental and physical health is increasingly recognized, we anticipate light and/or exogenous melatonin will be increasingly used as adjunctive treatments to other therapies. Light treatment in particular, as a nonpharmacological therapy, is likely to be attractive to many patients.
Patient Evaluation Overview and Safety Considerations
When considering light or exogenous melatonin treatment the clinician should first address whether the etiology of the presenting signs and/or symptoms may be at least partly due to a mistiming of the circadian system (Figure 3). In the case of circadian rhythm sleep disorders, this is fairly straightforward as the etiology is assumed to rest with a misalignment between the timing of the clock and the desired timing of sleep and wakefulness. However, in depression and insomnia, as well as circadian rhythm sleep disorders, it can often be difficult in practice to determine whether the etiology of a patient’s complaint is circadian in nature. For example, sleep onset insomnia can result from a circadian phase delay but might also be the direct result of medical or psychiatric illness, environmental disturbances, substances or a host of other factors. The picture is further complicated by the fact that a shift in the timing of the clock might be the result of another disorder (e.g., back pain might cause early morning awakening that in turn results in exposure to phase-advancing morning light). In this way, circadian resetting might be a perpetuating factor as opposed to a precipitating one. Nonetheless, symptoms of sleep onset insomnia and morning hypersomnolence or fatigue are at least suggestive of a phase delay in circadian timing while symptoms of late afternoon/early evening hypersomnolence or fatigue and early morning awakening are suggestive of a phase advance in circadian timing. Evaluation of symptoms of depression as they relate to the circadian system are beyond the scope of this review but suffice to say that both phase advances and phase delays have been hypothesized to play a role [21, 38].
Figure 3.
A flow diagram illustrating an example of clinical questions that should be asked when considering light and melatonin treatment. Note that as discussed in the text, light and melatonin can be used in combination to augment circadian phase advances. It has not yet been determined if the addition of melatonin to light can augment circadian phase delays.
Another important consideration is the consistency of the patient’s light/dark schedule, which is largely determined by the consistency of their sleep/wake schedule. In nature, the small variability in the timing of light and darkness from day to day (on the order of minutes) is easily accommodated by the circadian system. However, under conditions of artificial light, it is not uncommon to see variation in the light/dark cycle on the order of hours. This variability, most often greatest between work days and work-free days, can cause a misalignment between the timing of the central circadian clock and the desired timing of sleep and wakefulness (“social jet-lag”) [41]. Moreover, this variability in the light/dark cycle can confound the clinician’s efforts to reset the circadian pacemaker, as the prescribed resetting “signal” risks being partially lost in the “noise” of a variable light/dark schedule. For this reason, in patients with variable sleep/wake schedules, such as those with sleep onset and/or wake times that vary for example by more than 3 hours in a week, we recommend either first, or in conjunction with light and/or exogenous melatonin treatment, working with the patient to try to reduce the variability in sleep/wake timing and also the variability in light/dark timing.
Motivation for treatment should also be assessed as this can be a significant barrier to the use of light given the time involved and potential limitations on other activities during light therapy. The potential contraindications should also be considered (see bullets below). These contraindications are not based on large randomized clinical trials, but rather represent a cautious approach. Bright light is associated with some side effects, the most common being headache, eyestrain, nausea and agitation [44], but often these side effects spontaneously remit [44, 45], and patients rarely discontinue due to side effects [45]. Bright light is generally considered safe with no changes in extensive ophthalmologic examination observed after up to 6 years of daily use (in fall and winter months in patients with seasonal affective disorder) [46]. A rare but serious side effect of bright light therapy is mania in bipolar patients who overexpose themselves to the light [44, 45]. In our clinical practice and clinical trials no significant side effects or adverse events due to bright light treatment have occurred.
Potential Contraindications to Bright Light Treatment.
Existing eye disease
Migraine headaches (if elicited by light)
Phototoxic medication use
History of mania
Side effects, besides sleepiness, are infrequent with exogenous melatonin but increased depressive symptoms, headaches, hypertension and hypotension, and gastrointestinal upset have been associated with exogenous melatonin administration [3, 47]. There have been concerns about potential developmental effects in children (e.g., effects on growth hormone [1]) although one study found no effects on pubertal development [2]. Nonetheless, it is prudent to exercise caution in the administration of melatonin to pre-pubertal children unless the risk-benefit analysis strongly favors treatment (e.g., in children with significant developmental delay or children with non-24-hour sleep-wake schedule disorder). Although lower endogenous melatonin levels have been shown to be associated with increased risk of type II diabetes [48], one study found that exogenous melatonin (5 mg) when administered with food acutely impaired glucose tolerance [49]. More research investigating the effects of exogenous melatonin on glucose metabolism is required. It should be noted here that melatonin is not regulated by the FDA and we discuss issues of purity below.
Several melatonin agonists or prescription melatonin formulations are discussed below and carry their own potential side effect profiles including increased risk of liver injury with Agomelatine [50, 51]; headache, elevated liver enzymes, cardiac conduction changes, upper respiratory and urinary tract infections, and nightmares for Tasimelteon [52]; and headache, sleepiness, upper respiratory tract infections, gastrointestinal upset, dizziness and dysmenorrheal for Ramelteon [53]. Circadin is an extended release formulation of melatonin and has a similar side effect profile to melatonin [54].
Potential Contraindications to Exogenous Melatonin Treatment.
Using Light to Shift Circadian Timing
Timing of Light
One of the most complicated aspects of light treatment is that the timing of light treatment is critical to the resulting shift in circadian timing. Indeed, it is possible to generate completely opposite shifts in circadian timing with precisely the same light, depending solely on when the light is administered relative to the timing of the internal circadian clock. To help assist in predicting what the effect of light treatment is likely to have on circadian timing, mathematical functions called “phase response curves” (PRCs) have been developed (e.g. [55]). PRCs can be understood by imagining your participation in a laboratory experiment. You are first asked to maintain a consistent sleep/wake schedule with an 8-hour sleep opportunity for a week or more at home to stabilize your circadian timing. At the end of this baseline week, you arrive at the laboratory in the afternoon, are seated in dim light and every 30 minutes provide a saliva sample up until or even past your usual bedtime. Then, you sit in front of a light box for an hour, before sleeping in the laboratory. You awake the next morning and remain in the laboratory until later that afternoon you repeat the dim light saliva collection. The saliva samples are later assayed for melatonin and your individual dim light melatonin onset (DLMO, Figure 2) is calculated for each saliva collection session. Your first baseline DLMO is then compared to your second post-light treatment DLMO. The phase shift in the DLMO is plotted on a y-axis: positive number if the DLMO shifted earlier in time after light treatment (phase advance), negative number if the DLMO shifted later in time after light treatment (phase delay) or on zero line if no shift in DLMO. On the x-axis, the data point is plotted as the start of light treatment relative to your baseline DLMO, in this case it would probably be several hours after your baseline DLMO. This experiment is then repeated again and again, until there are many data points covering the breadth of the x-axis, reflecting that the light treatment was administered at all different circadian (DLMO) times. The data points are then curve fit with the resulting mathematical function revealing what phase shifts are likely to result when light is administered at different times relative to the DLMO. There are a variety of experimental protocols used to construct a PRC, but all follow the same basic pattern of assessment of circadian phase, exposure to a resetting stimulus, and then reassessment of circadian phase.
There are several limitations to PRCs that are important to keep in mind. First, PRCs represent a global average across the individuals who participated in the research protocol that generated the PRC. These individuals may have distinct individual differences in their response to the resetting stimulus, for example light. Thus, the average PRC is unlikely to accurately predict the effect of light treatment in an individual patient. Second, the protocols used to generate PRCs often include the unusual timing or shifting of sleep/dark, which may not generalize to the nocturnal sleep timing of many patients. Accordingly, we may not be as sensitive to light in the middle of day as the light PRC suggests us to be. Indeed, studies of either bright light or darkness in the afternoon found no changes in circadian timing [56, 57]. Third, most PRCs are referenced to the timing of the DLMO. To date the DLMO is not commonly measured in the clinic, although validated home procedures may become part of standard clinical practice in the future [58]. Currently, without access to the timing of the DLMO, the next best estimate of the PRC is to consider it relative to habitual sleep timing, as the DLMO on average occurs ~2.5 hours before habitual bedtime [59]. Note that throughout this review, we use the terms habitual bedtime and habitual wake time as proxies for habitual lights out and habitual lights on time. As there is substantial individual variability in the DLMO to bedtime interval [59], this re-referencing of the PRC from DLMO to habitual bedtime adds in substantial error. Furthermore, as noted above, there may be significant variability in an individual’s sleep schedule making DLMO predictions based on habitual bedtime even more fraught. Nonetheless, despite all these limitations, there is still useful information to gain from PRCs (Figure 4).
Figure 4.
The light and melatonin phase response curves re-referenced to habitual sleep timing. The light phase response curve is adapted from a phase response curve generated to a single 1 hour pulse of white broad spectrum bright light [55]. There is some debate as to whether humans are as sensitive to light during the day as this phase response curve suggests. The melatonin phase response curve is adapted from a phase response curve generated to 3 days of a daily dose of 0.5 mg of exogenous melatonin [96]. Accordingly, we have reduced the amplitude of the melatonin phase response curve by a factor of 3 to better estimate the effects of a single dose. This phase response curve may overestimate the phase shifting effects of exogenous melatonin as the phase shifts were measured in the absence of a fixed light-dark cycle. As discussed in the main text, phase response curves are useful general guides, but cannot be used to precisely predict phase shifts in circadian timing in individual patients.
The key principles evident from the PRC to light that are most relevant to light treatment include: (1) light in the evening prior to bedtime and in the first part of habitual sleep phase delays circadian timing, (2) light in the morning from a few hours before habitual wake time and for several hours after habitual wake time phase advances circadian timing, and (3) the effect of light switches from phase delays to phase advances on average, about 3 hours before habitual wake time (Figure 4). Thus, for most night-time sleeping individuals with an endogenous circadian period greater than 24 hours, this means that evening light (after sunset) should be minimized as it will only exacerbate the natural tendency to drift further out of sync with the 24-hour day. Conversely, morning light should be beneficial for most people as it provides a corrective phase advance to overcome the natural tendency to drift later. Lastly, one needs to be especially careful in administering morning light earlier than about 1 hour before habitual wake time, as light treatment earlier than this in an individual patient could in fact lead to phase delays instead of the desired phase advances. Keep in mind that the magnitude of the phase advances and phase delays following light treatment will depend on factors such as the intensity, duration and wavelength of light, as described below. Often large individual differences in phase shifts to the same bright light treatment are observed (e.g. [60]). On average, patients assigned to a daily white broad spectrum bright light treatment for ~1 hour before usual bedtime phase delay ~1 hour, whereas those assigned to the same light treatment but around habitual wake time, phase advance ~ 1 hour (e.g. [61]).
Intensity of Light
Most typically, light intensity is reported in units of lux, although this unit does not adequately reflect the wavelength sensitivities of the primary circadian photoreceptor, the ipRGCs [62]. Nonetheless, as a guide, very bright light, such as that experienced outside during a sunny cloudless day, can be as high as 100,000 lux. Outside light during rainy days can often be above ~1,000 lux. Bright light boxes are often marketed as emitting 10,000 lux but that intensity is measured at the light box itself, and when measured at the level of the patient’s eye about 2 feet from the light box, the lux level is closer to ~3,000–5,000 lux. Indoor light is typically only about 100–200 lux, although at night people typically experience less than 40 lux in their homes [63, 64].
Light of greater intensity is often associated with larger phase shifts, but importantly, the dose response relationship is nonlinear [65]. The most detailed dose response curve suggests maximum phase shifts in humans occur at about 1,000 lux, but importantly the subjects in this particular experiment were kept in the laboratory for days in very dim light (< 10 lux) before receiving the bright light. The sensitivity to light in humans can vary such that a history of dimmer light exposure can increase sensitivity to light, and conversely a history of bright light exposure (outdoor workers or beach vacation) can reduce sensitivity to light [66]. Accordingly, most patients who are exposed to some outdoor light every day probably show increasing phase shifts to light up to approximately 3,000 lux. Notably, the lens of the human eye yellows with age [67], leading to reduced responses to moderate light levels [68] in older subjects. Thus, the elderly are likely to require bright light for maximum circadian phase shifting.
Duration of Light
Generally, the longer the duration of light, the larger the phase shift in circadian timing. However, the dose response relationship between duration of light treatment and resulting phase shift is also nonlinear [69], such that the start of a light pulse can often have more of a phase shifting effect than the rest of the light pulse. The most detailed dose response curve suggests a 1 hour white broad spectrum bright light pulse does not maximize circadian phase delays [69]. Indeed, phase delays continue to grow in magnitude with a 4-hour white broad spectrum bright light pulse. However, most patients would likely struggle to complete a daily light treatment longer than 1 hour, and some may even comply better with a 30 minute daily treatment. In our clinical trials with patients we use a 1 hour bright light treatment as a practical duration that typically can produce significant circadian phase shifting effects of at least 1 hour after several days of treatment.
Color and Wavelength of Light
The primary circadian photoreceptor is considered to be the ipRGCs in the retina. Nonetheless, the rods and cones do contribute to circadian responses to light, particularly in dimmer light conditions [70, 71] such as that which occurs in the home in the evening after sunset [63, 64]. When it was discovered that the photopigment in the ipRGCs, melanopsin, is maximally sensitive to blue or short wavelength (~480nm) light [72, 73], researchers and clinicians wondered if larger phase shifts could be generated with blue light. However, to date there is no evidence that monochromatic blue light boxes can lead to greater phase shifts than white broad spectrum bright light boxes [74]. Similarly, white broad spectrum bright light boxes with additional blue photons (“blue-enriched”) produce similar phase advances and phase delays to white broad spectrum bright light boxes [75, 76]. The lack of an additional phase shift is likely due to the bright light boxes already saturating the retinal receptors, and thus phase shifts cannot be increased. Nonetheless, at dimmer light intensities that do not saturate the retinal receptors, blue light can produce larger phase shifts than white broad spectrum light [77].
Practical Aspects of Light Treatment
Light Outdoors
When morning bright light can be found outdoors, patients who need morning light treatment should consider a reorganization of their morning schedule (dog walking, outdoor exercise etc without sunglasses) to increase their morning light exposure. However, light devices are needed for when circumstances do not allow for this, such as with inclement weather, or when evening or night-time light treatment is required.
Light Boxes
Light boxes, in a variety of shapes and sizes, come on and off the market at various times. Often the choice of a particular light box will vary according to each individual patient’s circumstances and preference. In general, we prefer larger white broad spectrum bright light boxes as they generate a larger field of bright light which is easier to stay within, ensuring adequate light exposure. However, these larger light boxes are somewhat cumbersome to move and therefore are best for patients who will receive their light treatment in the same place each day (most typically at home). Smaller light boxes are much more portable and when battery operated, are particularly easy to take travelling internationally. But it is easier to move out of the range of light exposure emitted from smaller light boxes. As described above there is no evidence to date to suggest that blue monochromatic or blue-enriched white broad spectrum bright light is more effective than white broad spectrum bright light, but if some patients find the brighter light levels aversive they may prefer the dimmer devices that emit more blue wavelengths. If a smaller light box is used, it may be useful for patients to periodically check their faces in a mirror to confirm they are receiving sufficient light [78].
In our laboratory-based studies, we have found a single broad spectrum bright light box works well to shift circadian timing providing it is set up correctly. Figure 5 illustrates the use of such a light box (EnergyLight HF3318/60, Philips, Inc). A particular advantage of this model of light box is that it can tip forward towards the patient from its base. We tape a 2 feet long string to the base of the light box and the patient pulls the string out to their eyes during their light treatment to ensure they are sitting close enough. Note with one light box in front of the patient, the ideal activity during the light treatment is to either read or use an e-tablet, flat on the table in front of the subject in order to not diminish the amount of light reaching their eyes. We also ask patients to turn on their indoor lighting during the light treatment to maximum intensity to optimize the light treatment. Patients should not sleep immediately after a morning light treatment as this “dark pulse” can counteract the effects of the morning light.
Figure 5.
A member of our research staff demonstrates receiving light treatment from a white broad spectrum bright light box. A 2 feet long string attached to the base can remind patients of how close they need to sit to the light box to receive light of >3,000 lux.
In our home-based clinical research trials we find that more than half of the patients wish to either watch TV or use a computer during a daily 1 hour bright light treatment. To ensure adequate bright light still reaches both eyes, we then use 2 light boxes, that are set up to face the patient but are angled slightly to the right and left in order that the patient can view their computer screen or TV directly in front of them [79]. If the light set up occurs in the living room, side tables are brought in to support the light boxes, and the light set up often creates a considerable change to the layout of the room, potentially interfering with the use of the space by other family members. A better option when possible, is to set the light boxes up in a room separate from the living space, such as a spare bedroom or study.
Dawn Simulators and Light Masks
There has been some interest in shifting circadian timing with light administration during sleep. Commercially available dawn simulators are light boxes designed to be placed near the bed and set to start increasing light in the 30 minutes or so before usual wake time, sometimes remaining on in the first 30 minutes or so after habitual wake time. They are typically not as bright as standard light boxes, reaching lower peak intensities (e.g. ~250 lux). While dawn simulators can improve mood and alertness, the evidence on whether or not they can shift circadian timing is mixed. Some studies report that a single morning exposure, and a 2 week treatment with a dawn simulator did not shift circadian timing [80, 81]. However, others report a 3 week treatment produced small phase advances in circadian timing (~30 minutes) [82]. As only about 1–2% of the more potent blue/green light wavelengths passes through closed eyelids [83], the light from dawn simulators will be most effective when it wakes patients early, causing them to open their eyes while the light is on. Currently, there is not enough evidence to conclusively recommend dawn simulators for phase advancing, but they may be an option for patients who are otherwise unlikely to complete a daily morning light treatment.
Light masks, designed to administer light during sleep, have been developed [84, 85]. The masks are designed to emit bright light such that light of sufficient intensity still reaches the retina after passing through closed eyelids, without major disruption to sleep. There is preliminary evidence that light masks can shift circadian timing [84, 85] and they appear to be well tolerated. Nonetheless, more testing of light masks are required and to our knowledge no light masks are currently commercially available.
Light Visors and Light Glasses
A significant problem with light boxes is that the patient needs to sit in front of the light box for 30–60 minutes per day. One study that examined adherence to a daily light box treatment found that on average patients only self-administer about 59% of a prescribed light treatment [86]. An alternative to light boxes are head worn light devices. There is some evidence that light visors, in which the light source is positioned above the eyes, can phase shift circadian timing [87, 88]. One type of light glasses, the Re-timer (Re-time, Inc), has recently become commercially available (Figure 6). In this device, the light source is positioned below the eyes, and there is some evidence that an earlier prototype of these glasses could shift circadian timing [89]. Importantly, these head-worn light devices are highly portable, and permit the patient to engage in household activities during their light treatment. Thus, these light devices may improve compliance to light treatment, although this remains to be conclusively demonstrated.
Figure 6.
A member of our research staff demonstrates receiving light treatment from a Re-timer. Patients can freely move around while receiving light treatment, and can complete household activities.
Most light devices do not record their on/off times for later review by clinicians, despite evidence that patients’ reports of light treatment can overestimate duration of light treatment [86]. In our home based clinical trials we tape a photosensor (Actiwatch Spectrum, Philips, Inc) facing inwards on the bottom of the light box, near the stand, to record when the light box is turned on. Ideally in the future, light devices will record their on and off times, just as for example CPAP machines for sleep apnea record their usage. This information will help the clinician understand if a poor treatment response is due to potential minimal use or even use at the wrong time, or if the patient has been compliant to the prescribed light treatment.
Light Avoidance
To facilitate circadian phase shifts to either light treatment and/or exogenous melatonin, light exposure at inappropriate times can be avoided or reduced. For example, night shift workers wishing to phase delay, can wear dark sunglasses during their morning commute home to reduce their exposure to phase advancing morning light [90]. Conversely, patients needing to phase advance, can reduce their evening light exposure with the use of blue blocker orange glasses which block the blue wavelengths of light the circadian system is most sensitive to [91]. We have found that dimming indoor lights in the evening combined with evening use of blue blockers, phase advanced the DLMO by ~1 hour, as compared to maximizing indoor lighting in the evening [92]. On average, subjects wore the blue blockers for ~70% of the prescribed 4 hours, but there was considerable individual variability (10–98%). There is also much interest in reducing exposure to potentially phase delaying light-emitting electronics in the evening, as these devices emit blue wavelength light that the circadian system is most sensitive to [93]. This can be done by dimming the screen, using devices with smaller screens, wearing blue blockers and overall reducing evening use of electronic devices that are brought close to the eyes such as e-tablets and cell phones.
Light/Dark Scheduling
As noted above, it is not uncommon for patients to have irregular sleep/wake schedules that beget variable light/dark patterns. Light treatment and the strategic avoidance of light therefore have the potential to simply add to the existing light/dark variability. For this reason, it is important to ensure that light avoidance and/or light treatments occur at a consistent time from day to day. In our clinical practice we often recommend the use of alarms, device alerts and outlet timers (for non-battery powered electronic devices) to help patients develop a consistent light/dark schedule. Such aids are important for enforcing both regular lights on and lights out times. In such cases, it is important to make the distinction for the patient between a consistent light/dark schedule, that is under the patient’s control, and a consistent sleep/wake schedule which is often out of their control. Finally, many individuals will expose themselves to light when attempting stimulus control therapy, a mainstay of cognitive-behavioral therapy for insomnia that involves getting out of bed when unable to sleep. This also has the potential to cause circadian resetting especially in dark-adapted individuals.
Using Melatonin and other Melatonin Receptor Agonists to Shift Circadian Timing
Melatonin (N-Acetyl-5-methoxytryptamine) is a hormone secreted by the pineal gland. In both nocturnal and diurnal (day-active) animals it is secreted during the nighttime and as such can be thought of as a marker for the biological night. As first shown by Redman et al. [94], exogenous melatonin administration is capable of resetting the circadian pacemaker to both an earlier and later time (phase advance and phase delay respectively). Subsequent studies have shown this to be true in humans as well [31, 95, 96]. There are two melatonin receptor subtypes, MT1 and MT2 and there is evidence demonstrating that both help to mediate the circadian resetting effects of melatonin [97, 98].
Timing of Melatonin
Exogenous melatonin, like light, shows differential resetting of the circadian pacemaker based on the biological time of administration and, as with light, this can be represented graphically using a PRC. The same caveats noted above for light PRCs apply to melatonin PRCs as well. Namely, that PRCs average data across many subjects and may not accurately predict an individual’s response to exogenous melatonin and that habitual sleep timing provides only a rough approximation of circadian phase. One additional caveat that applies to the melatonin PRCs is that multiple daily doses were administered over 3–4 days in a manner analogous to earlier “3-pulse” light PRCs. With these caveats in mind, several PRCs have been published in humans (e.g. [31, 96]) and they generally show: (1) phase advances when exogenous melatonin is administered in the late afternoon and evening prior to habitual bedtime with maximum phase advances generally occurring about 5–7 hours before habitual bedtime, (2) phase delays when exogenous melatonin is administered in the late night and early morning with maximum phase delays generally occurring a couple hours after habitual wake time and (3) the effect of melatonin switches from phase advances to phase delays just before habitual bedtime (Figure 4). Thus, for most individuals sleeping at conventional times, melatonin administration causes maximal shifts in the biological clock when patients are habitually awake (i.e., either in the late afternoon for phase advances or the morning after awakening for phase delays). As discussed below, this can necessitate selection of a dose that does not result in soporific effects. Lastly, one should be aware that although the administration of exogenous melatonin just before or during the habitual sleep episode would be expected to cause phase delays (Figure 4), there exists potential the potential for phase advances during this period of time [96].
Dose of Melatonin
A variety of exogenous melatonin doses have been examined for circadian resetting [31, 96, 99, 100]. There is evidence of a dose response relationship at lower doses of 0.02 and 0.30 mg [100]. By contrast, when 0.5 mg and 3.0mg were compared across a range of administration times, maximum phase advance and phase delays were similar [96]. Even higher doses of exogenous melatonin (≥ 10 mg) may result in a smaller resetting effect [99, 101]. This initially counter-intuitive finding is likely due to the fact that increasing the dose of exogenous melatonin simultaneously increases the concentration of melatonin in the circulation and the duration of administration or exposure. Initially, increases in dose simply cause increased resetting effects [100] but as higher doses are employed, exogenous melatonin levels remain elevated in the circulation for longer periods of time. As a result, additional parts of the melatonin PRC may be stimulated resulting in less net circadian resetting (i.e., a less discrete time signal is provided). Such “spill over” [101] of melatonin onto the “wrong” portion of the melatonin PRC is possible, despite a half-life of just about an hour, because even 0.5–1.0 mg doses of melatonin can produce supra-physiological levels over several hours or more [31, 102]. Melatonin also has well demonstrated soporific effects [102–104] although, at least at 0.30 and 5.0 mg doses, this effect was confined to circadian times when endogenous melatonin levels were low (i.e., the biological day) [105]. Therefore, care should be taken to use the lowest dose possible when exogenous melatonin is taken during the habitual waking hours as is often necessary for maximal circadian resetting effects.
Formulation and Melatonin Agonists
Recent years have seen the introduction of a variety of approved melatonin receptor agonists and melatonin preparations, all of which are MT1 and MT2 melatonin receptor agonists. These include Ramelteon, for the treatment of insomnia; Agomelatine, which is also a serotonin 5-HT2c antagonist, approved in Europe and Australia for the treatment of depression, Tasimelteon, which was approved by the FDA in 2014 for the treatment of the circadian rhythm sleep disorder non-24-hour sleep-wake disorder (non-24), and Circadin, an extended release formulation of 2.0 mg melatonin approved in Europe and Australia for the treatment of primary insomnia. Of these four drugs, only Tasimelteon was developed with circadian resetting in mind and it should be noted that it is the first and only drug approved by the FDA for the treatment of a circadian rhythm sleep disorder [106]. Complete PRCs do not exist for any of these melatonin agonists although logic and the results of resetting trials suggest that the approximate timing of phase advances and phase delays is similar to that of exogenous melatonin [107, 108]. However, variability in the magnitude of phase shifts and in the timing of administration for advance and delay shifts might exist. There are no data demonstrating superior efficacy of one formulation over another for circadian resetting although intuitively, extended release formulations might be expected to be less effective due to the “spill over” effects discussed above.
Practical Aspects of Melatonin and other Melatonin Agonist Treatment
Melatonin Preparations
Exogenous melatonin has been classified by the FDA as a dietary supplement and as such is not subject to the regulation given to pharmaceuticals; the purity and accuracy of dose of exogenous melatonin formulations are not always carefully controlled [109]. There has been recent progress in this area and the purity and accuracy of doses may improve, particularly those from large well-established supplement manufacturers [110]. Meanwhile, choosing an over the counter formulation from a manufacturer who participates in the USP Verified Program for Dietary Supplements may help with this quality control problem [111]. Consumers can also pay to access the results of consumer group testing of various melatonin brands and formulations [112]. An important consideration for therapy with melatonin is the low cost (often <10 cents/pill [112]).
Issues of dose are discussed in more detail above but the clinician should be aware that commonly available doses (0.5 mg and 3 mg) demonstrate equivalent resetting effects [96] while evidence of diminished efficacy with higher doses (“spill over”) appears at doses of 10 mg and higher. With this in mind, the clinician should choose the lowest dose with demonstrated efficacy and lower it further if unwanted sedation occurs. Caution should be exercised with the first dose, as with a sedative-hypnotic, to ensure that excessive sedation does not occur. Finally, pharmacokinetic data shows that even doses as low as 0.5 mg to 1.0 mg may result in supra-physiologic levels of melatonin [31, 102].
Melatonin Administration
As with light administration, the clinician should ideally help the patient to adopt as consistent of a light/dark schedule as possible first. This may be therapeutic in its own right and it also allows for a better prediction of circadian phase based on habitual sleep times. It is important to make sure that melatonin is not in competition with either the self-selected light/dark cycle or light therapy. It should be noted that the most current melatonin PRC was constructed in the laboratory without competing light resetting [96] and, as might be expected, it showed somewhat greater phase shifts than a PRC based on melatonin administered while subjects lived at home [31]. If symptoms are improved but the timing of sleep remains delayed or advanced, the timing of administration can be slowly advanced or delayed an hour [113], or less, every several days, as tolerated by the patient, in concert with the timing of light and dark such that the relationship to habitual sleep/wake and light/dark times is maintained. Patients can be reminded to take melatonin with the use of alarms of various sorts, including setting a daily alarm on their cell phones.
A special case exists for the treatment of the circadian rhythm sleep disorder non-24 in which circadian phase drifts progressively later, and sometimes earlier, each day due to a lack of synchronization of the biological clock (typically due to a loss of photic input in totally blind individuals). The clinician must be especially mindful of timing the administration of melatonin or melatonin agonist in such a way as to promote a normal relationship between the timing of the clock and the desired timing of sleep (i.e. a DLMO ~2–3 hours before bedtime). Studies have shown that the final entrained DLMO can occur roughly 0 to 5 hours after the time of administration depending on the dose [100, 114]. Therefore, administration 3–8 hours before desired bedtime may be necessary. We recommend an initial administration time of 6 hours before the desired bedtime. If the patient shows evidence of being phase advanced, the time of administration can be delayed to a later time (and vice versa). A small minority of blind individuals drift to a progressively earlier time [29]. In these cases, administration of melatonin just after the desired wake time has been shown to appropriately set the biological clock relative to sleep [115].
Finally, potential interactions with other prescription medications and/or other over the counter dietary supplements should be considered (see potential contraindications above). Unfortunately, there are indications that less than half of the general population do so [7].
Melatonin and Light Combination Treatment
It has been shown that afternoon melatonin can increase the phase advances obtained with morning light administration [116–118]. It has yet to be shown that phase delays obtained with evening light treatment can be increased with morning melatonin. To our knowledge, there have been no studies to date on light treatment in combination with other melatonin receptor agonists.
Evaluation of Outcome
In practice, treatment response is largely assessed on the basis of symptom improvement in circadian rhythm sleep disorders since there are currently no FDA approved laboratory assessments of circadian phase (i.e. no current clinical DLMO test). Subjective sleep diary or, if available, more objective wrist actigraphy measures of sleep timing and relevant sleep variables such as sleep onset latency, total sleep time, and early morning awakening are useful. In the case of non-24 in the blind, spectral analysis of wrist actigraphy data has the potential to be a cost-effective method of assessing entrainment status and therefore treatment efficacy [29]. Similar to oral contraceptives, consistent timing in light and/or exogenous melatonin treatment is important for efficacy and this should be assessed as well (e.g., by diary or medication compliance bottles) before determinations of efficacy are made.
Summary
There is increasing recognition of the important influence circadian timing has on mental health, physical health and health behaviors. In this review, we provided a brief summary of the circadian system, followed by some of the initial considerations to make when considering treating a patient with suspected circadian misalignment. A consistent light/dark schedule should first be attempted, followed by a consideration of contradictions and safety concerns associated with light and exogenous melatonin treatment. If the patient has no contraindications for light treatment, decisions regarding the timing, intensity, duration, color of light and the possibility for complementary light avoidance need to be made. Patient input, particularly their motivation to engage in an often required daily treatment, should guide the choice of light device. If the patient has no contraindications for exogenous melatonin treatment, decisions regarding the timing, dose, slow or fast release formulation, purity, and the possible use of other melatonin receptor agonists needs to be made. Currently, treatment response is largely assessed based on symptom improvement.
Key Points.
Circadian timing has a profound influence on mental health, physical health and health behaviors
Individual patients suspected of misaligned circadian rhythms can vary in their suitability for light, melatonin and other melatonin receptor agonist treatment
Prescribing a relatively consistent light/dark cycle is often the first step in treatment
Key features of light treatment to consider include timing, intensity, duration, color, light avoidance, and choosing a light device to best accommodate patient motivation for treatment
Key features of exogenous melatonin and other melatonin receptor agonist treatment to consider include timing, dose, fast or slow release formulations and purity.
Acknowledgments
We thank Muneer Rizvydeen for his assistance in creating the figures. We thank Haein Sung for allowing us to include photos of her during light treatment. HJB is supported by grants from the National Center for Complementary and Integrative Health (AT007104), and National Institute on Alcohol Abuse and Alcoholism (AA021762). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure statement: The authors have nothing to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jonathan S. Emens, Email: emensj@ohsu.edu.
Helen J. Burgess, Email: Helen_J_Burgess@rush.edu.
References
- 1.Valcavi R, Zini M, Maestroni GJ, et al. Melatonin stimulates growth hormone secretion through pathways other than the growth hormone-releasing hormone. Clin Endocrinol (Oxf) 1993;39:193–9. doi: 10.1111/j.1365-2265.1993.tb01773.x. [DOI] [PubMed] [Google Scholar]
- 2.van Geijlswijk IM, Mol RH, Egberts TC, et al. Evaluation of sleep, puberty and mental health in children with long-term melatonin treatment for chronic idiopathic childhood sleep onset insomnia. Psychopharmacology (Berl) 2010;216:111–20. doi: 10.1007/s00213-011-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. The Cochrane Database Syst Rev. 2002;2:CD001520. doi: 10.1002/14651858.CD001520. [DOI] [PubMed] [Google Scholar]
- 4.Sheldon SH. Pro-convulsant effects of oral melatonin in neurologically disabled children. Lancet. 1998;351:1254. doi: 10.1016/S0140-6736(05)79321-1. [DOI] [PubMed] [Google Scholar]
- 5.Lam RW, Levitt AJ. Canadian consensus guidelines for the treatment of seasonal affective disorder. Canada: Clinical & Academic Publishing; 1999. p. 160. [Google Scholar]
- 6.National Sleep Foundation. Sleep in America poll. 2005 www.sleepfoundation.org.
- 7.Bliwise DL, Ansari FP. Insomnia associated with valerian and melatonin usage in the 2002 National Health Interview Survey. Sleep. 2007;30:881–4. doi: 10.1093/sleep/30.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright KP, Jr, Hull JT, Hughes RJ, et al. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18:508–21. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- 9.Scheer FA, Hilton MF, Mantzoros CS, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–58. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levandovski R, Dantas G, Fernandes LC, et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 2011;28:771–8. doi: 10.3109/07420528.2011.602445. [DOI] [PubMed] [Google Scholar]
- 11.Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J Biol Rhythms. 2001;16:196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- 12.Buijs RM, Scheer FA, Kreier F, et al. Organization of circadian functions: interaction with the body. Prog Brain Res. 2006;153:341–60. doi: 10.1016/S0079-6123(06)53020-1. [DOI] [PubMed] [Google Scholar]
- 13.Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005;20:279–90. doi: 10.1177/0748730405278292. [DOI] [PubMed] [Google Scholar]
- 14.Scheiermann C, Kunisaki Y, Lucas D, et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–85. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewy AJ, Wehr TA, Goodwin FK, et al. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–69. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 17.Gaggioni G, Maquet P, Schmidt C, et al. Neuroimaging, cognition, light and circadian rhythms. Front Syst Neurosci. 2014;8:126. doi: 10.3389/fnsys.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeMuro RL, Nafziger AN, Blask DE, et al. The absolute bioavailability of oral melatonin. J Clin Pharmacol. 2000;40:781–4. doi: 10.1177/00912700022009422. [DOI] [PubMed] [Google Scholar]
- 19.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleepwake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD) J Clin Sleep Med. doi: 10.5664/jcsm.5100. An update for 2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guaiana G, Gupta S, Chiodo D, et al. Agomelatine versus other antidepressive agents for major depression. Cochrane Database Syst Rev. 2013;12:CD008851. doi: 10.1002/14651858.CD008851.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewy AJ, Lefler BJ, Emens JS, et al. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–9. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neubauer DN. New and emerging pharmacotherapeutic approaches for insomnia. Int Rev Psychiatry. 2014;26:214–24. doi: 10.3109/09540261.2014.888990. [DOI] [PubMed] [Google Scholar]
- 23.Lucas RJ, Lall GS, Allen AE, et al. How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Prog Brain Res. 2012;199:1–18. doi: 10.1016/B978-0-444-59427-3.00001-0. [DOI] [PubMed] [Google Scholar]
- 24.Reppert SM, Weaver DR, Rivkees SA, et al. Putative melatonin receptors in a human biological clock. Science. 1988;242:78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- 25.Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic nucleus. Fed Proc. 1983;42:2783–89. [PubMed] [Google Scholar]
- 26.Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J Biol Rhythms. 2008;23:374–6. doi: 10.1177/0748730408318592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108 (Suppl 3):15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Sleep Foundation. Sleep in America poll. 2011 www.sleepfoundation.org.
- 29.Emens JS, Laurie AL, Songer JB, et al. Non-24-hour disorder in blind individuals revisited: variability and the influence of environmental time cues. Sleep. 2013;36:1091–100. doi: 10.5665/sleep.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore RY. Neural control of the pineal gland. Behav Brain Res. 1996;73:125–30. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- 31.Lewy AJ, Bauer VK, Ahmed S, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 32.Klerman EB, Gershengorn HB, Duffy JF, et al. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–93. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 33.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker of circadian phase position. J Biol Rhythms. 1999;14:227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 34.Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int. 2011;28:714–8. doi: 10.3109/07420528.2011.597531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicolaides NC, Charmandari E, Chrousos GP, et al. Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions. Ann N Y Acad Sci. 2014;1318:71–80. doi: 10.1111/nyas.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Challet E. Circadian clocks, food intake, and metabolism. Prog Mol Biol Transl Sci. 2013;119:105–35. doi: 10.1016/B978-0-12-396971-2.00005-1. [DOI] [PubMed] [Google Scholar]
- 38.Emens J, Lewy A, Kinzie JM, et al. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168:259–61. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Boivin DB. Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J Psychiatry Neurosci. 2000;25:446–58. [PMC free article] [PubMed] [Google Scholar]
- 40.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 41.Wittmann M, Dinich J, Merrow M, et al. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 42.Kanerva N, Kronholm E, Partonen T, et al. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int. 2012;29:920–7. doi: 10.3109/07420528.2012.699128. [DOI] [PubMed] [Google Scholar]
- 43.Van den Hoofdakker RH. Chronobiological theories of nonseasonal affective disorders and their implications for treatment. J Biol Rhythms. 1994;9:157–183. doi: 10.1177/074873049400900206. [DOI] [PubMed] [Google Scholar]
- 44.Pail G, Huf W, Pjrek E, et al. Bright-light therapy in the treatment of mood disorders. Neuropsychobiology. 2011;64:152–62. doi: 10.1159/000328950. [DOI] [PubMed] [Google Scholar]
- 45.Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: Efficacy, protocol, safety, and side effects. CNS Spectrums. 2005;10:647–63. doi: 10.1017/s1092852900019611. [DOI] [PubMed] [Google Scholar]
- 46.Gallin PF, Terman M, Reme CE, et al. Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. Am J Ophthal. 1995;119:202–10. doi: 10.1016/s0002-9394(14)73874-7. [DOI] [PubMed] [Google Scholar]
- 47.Buscemi N, Vandermeer B, Hooton N, et al. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J Gen Int Med. 2005;20:1151–8. doi: 10.1111/j.1525-1497.2005.0243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMullan CJ, Schernhammer ES, Rimm EB, et al. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309:1388–96. doi: 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubio-Sastre P, Scheer FA, Gomez-Abellan P, et al. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014;37:1715–9. doi: 10.5665/sleep.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freiesleben SD, Furczyk K. A systematic review of agomelatine-induced liver injury. J Mol Psychiatry. 2015;3:4. doi: 10.1186/s40303-015-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor D, Sparshatt A, Varma S, et al. Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ. 2014;348:g1888. doi: 10.1136/bmj.g1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM374385.pdf.
- 53.Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med. 2014;15:385–92. doi: 10.1016/j.sleep.2013.11.788. [DOI] [PubMed] [Google Scholar]
- 54.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000695/WC500026805.pdf.
- 55.St Hilaire MA, Gooley JJ, Khalsa SB, et al. Human phase response curve to a 1h pulse of bright white light. J Physiol. 2012;590:3035–45. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dumont M, Carrier J. Daytime sleep propensity after moderate circadian phase shifts induced with bright light exposure. Sleep. 1997;20:11–17. doi: 10.1093/sleep/20.1.11. [DOI] [PubMed] [Google Scholar]
- 57.Buxton OM, L’Hermite-Baleriaux M, Turek FW, et al. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol. 2000;278:R373–82. doi: 10.1152/ajpregu.2000.278.2.R373. [DOI] [PubMed] [Google Scholar]
- 58.Burgess HJ, Wyatt JK, Park M, et al. Home circadian phase assessments with measures of compliance yield accurate dim light melatonin onsets. Sleep. 2015;38:889–97. doi: 10.5665/sleep.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3:e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burgess HJ. Partial sleep deprivation reduces phase advances to light in humans. Journal Biol Rhythms. 2010;25:460–8. doi: 10.1177/0748730410385544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgess HJ, Fogg LF, Young MA, et al. Bright light therapy for winter depression - Is phase advancing beneficial? Chronobiol Int. 2004;21:759–75. doi: 10.1081/cbi-200025979. [DOI] [PubMed] [Google Scholar]
- 62.Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burgess HJ, Eastman CI. Early versus late bedtimes phase shift the human dim light melatonin rhythm despite a fixed morning lights on time. Neurosci Lett. 2004;356:115–8. doi: 10.1016/j.neulet.2003.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crowley SJ, Molina TA, Burgess HJ. A week in the life of full-time office workers: work day and weekend light exposure in summer and winter. Appl Ergon. 2015;46(Pt A):193–200. doi: 10.1016/j.apergo.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeitzer JM, Dijk DJ, Kronauer RE, et al. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hebert M, Martin SK, Lee C, et al. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weale RA. Age and the transmittance of the human crystalline lens. J Physiol. 1988;395:577–87. doi: 10.1113/jphysiol.1988.sp016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang AM, Santhi N, St Hilaire MA, et al. Human responses to bright light of different durations. J Physiol. 2012;590:3103–12. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lall GS, Revell VL, Momiji H, et al. Distinct contributions of rod, cone and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–28. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walmsley L, Hanna L, Mouland J, et al. Colour as a signal for entraining the Mammalian circadian clock. PLoS Biol. 2015;13:e1002127. doi: 10.1371/journal.pbio.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Revell VL, Molina TA, Eastman CI. Human phase response curve to intermittent blue light using a commercially available device. J Physiol. 2012;590:4859–68. doi: 10.1113/jphysiol.2012.235416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith MR, Eastman CI. Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int. 2009;26:709–25. doi: 10.1080/07420520902927742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009;10:287–94. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mottram V, Middleton B, Williams P, et al. The impact of bright artificial white and ‘blue-enriched’ light on sleep and circadian phase during the polar winter. J Sleep Res. 2011;20:154–61. doi: 10.1111/j.1365-2869.2010.00875.x. [DOI] [PubMed] [Google Scholar]
- 78.Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27:1469–92. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatrics Soc. 1993;41:829–36. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 80.Gabel V, Maire M, Reichert CF, et al. Effects of artificial dawn and morning blue light on daytime cognitive performance, well-being, cortisol and melatonin levels. Chronobiol Int. 2013;30:988–97. doi: 10.3109/07420528.2013.793196. [DOI] [PubMed] [Google Scholar]
- 81.Gimenez MC, Hessels M, van de Werken M, et al. Effects of artificial dawn on subjective ratings of sleep inertia and dim light melatonin onset. Chronobiol Int. 2010;27:1219–41. doi: 10.3109/07420528.2010.496912. [DOI] [PubMed] [Google Scholar]
- 82.Terman M, Terman JS. Circadian rhythm phase advance with dawn simulation treatment for winter depression. J Biol Rhythms. 2010;25:297–301. doi: 10.1177/0748730410374000. [DOI] [PubMed] [Google Scholar]
- 83.Moseley MJ, Bayliss SC, Fielder AR. Light transmission through the human eyelid: in vivo measurement. Opthal Physiol Opt. 1988;8:229–30. doi: 10.1111/j.1475-1313.1988.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 84.Cole RJ, Smith JS, Alcala YC, et al. Bright-light mask treatment of delayed sleep phase syndrome. J Biol Rhythms. 2002;17:89–101. doi: 10.1177/074873002129002366. [DOI] [PubMed] [Google Scholar]
- 85.Figueiro MG, Rea MS. Preliminary evidence that light through the eyelids can suppress melatonin and phase shift dim light melatonin onset. BMC Res Notes. 2012;5:221. doi: 10.1186/1756-0500-5-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michalak EE, Murray G, Wilkinson C, et al. A pilot study of adherence with light treatment for seasonal affective disorder. Psychiatry Res. 2007;149:315–20. doi: 10.1016/j.psychres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 87.Boulos Z, Macchi MM, Sturchler MP, et al. Light visor treatment for jet lag after westward travel across six time zones. Aviat Space Environ Med. 2002;73:953–63. [PubMed] [Google Scholar]
- 88.Paul MA, Miller JC, Gray G, et al. Circadian phase delay induced by phototherapeutic devices. Aviat Space Environ Med. 2007;78:645–52. [PubMed] [Google Scholar]
- 89.Wright HR, Lack LC, Partridge KJ. Light emitting diodes can be used to phase delay the melatonin rhythm. J Pineal Res. 2001;31:350–5. doi: 10.1034/j.1600-079x.2001.310410.x. [DOI] [PubMed] [Google Scholar]
- 90.Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6:407–20. [PubMed] [Google Scholar]
- 91.Sasseville A, Paquet N, Sevigny J, et al. Blue blocker glasses impede the capacity of bright light to suppress melatonin production. J Pineal Res. 2006;41:73–8. doi: 10.1111/j.1600-079X.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 92.Burgess HJ, Molina TA. Home lighting before usual bedtime impacts circadian timing: A field study. Photochem Photobiol. 2014;90:723–6. doi: 10.1111/php.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang AM, Aeschbach D, Duffy JF, et al. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112:1232–7. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Redman J, Armstrong S, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–91. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- 95.Arendt J, Bojkowski C, Folkard S, et al. In: Some effects of melatonin and the control of its secretion in humans, in Photoperiodism, Melatonin, and the Pineal. Evered D, Clark S, editors. Pitman; London: 1985. pp. 266–83. [DOI] [PubMed] [Google Scholar]
- 96.Burgess HJ, Revell VL, Molina TA, et al. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab. 2010;95:3325–31. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: cloning and classification of subtypes. Tips. 1996;17:100–2. doi: 10.1016/0165-6147(96)10005-5. [DOI] [PubMed] [Google Scholar]
- 98.Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8 (Suppl 3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 99.Sack RL, Brandes RW, Kendall AR, et al. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–7. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 100.Lewy AJ, Emens JS, Lefler BJ, et al. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol Int. 2005;22:1093–106. doi: 10.1080/07420520500398064. [DOI] [PubMed] [Google Scholar]
- 101.Lewy AJ, Emens JS, Sack RL, et al. Low, but not high, doses of melatonin entrained a free-running blind person with long circadian period. Chronobiol Int. 2002;19:649–58. doi: 10.1081/cbi-120004546. [DOI] [PubMed] [Google Scholar]
- 102.Dollins AB, Zhdanova IV, Wurtman RJ, et al. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91:1824–8. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.James SP, Mendelson WB, Sack DA, et al. The effect of melatonin on normal sleep. Neuropsychopharmacology. 1987;1:41–4. doi: 10.1016/0893-133x(87)90008-x. [DOI] [PubMed] [Google Scholar]
- 104.Rajaratnam SMW, Middleton B, Stone BM, et al. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in entended sleep opportunities in humans. J Physiol. 2004;561:339–51. doi: 10.1113/jphysiol.2004.073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wyatt JK, Dijk DJ, Ritz-de Cecco A, et al. Sleep-facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–18. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 106.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm384092.htm.
- 107.Rajaratnam SM, Polymeropoulos MH, Fisher DM, et al. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet. 2009;373:482–91. doi: 10.1016/S0140-6736(08)61812-7. [DOI] [PubMed] [Google Scholar]
- 108.Richardson GS, Zee PC, Wang-Weigand S, et al. Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J Clin Sleep Med. 2008;4:456–61. [PMC free article] [PubMed] [Google Scholar]
- 109.Hahm H, Kujawa J, Augsburger L. Comparison of melatonin products against USP’s nutritional supplements standards and other criteria. J Am Pharm Assoc (Wash) 1999;39:27–31. doi: 10.1016/s1086-5802(16)30412-0. [DOI] [PubMed] [Google Scholar]
- 110.http://well.blogs.nytimes.com/2015/03/30/gnc-to-strengthen-supplement-quality-controls/?_r=0>.
- 111.http://www.usp.org/usp-verification-services/usp-verified-dietary-supplements/participating-companies.
- 112.ConsumerLab.com. [Accessed August 13, 2012];Product Review: Melatonin Supplements. www.consumerlab.com/results/melatonin.asp.
- 113.Crowley SJ, Eastman CI. Melatonin in the afternoons of a gradually advancing sleep schedule enhances the circadian rhythm phase advance. Psychopharmacol (Berl) 2013;225:825–37. doi: 10.1007/s00213-012-2869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lewy AJ, Emens JS, Bernert RA, et al. Eventual entrainment of the human circadian pacemaker by melatonin is independent of the circadian phase of treatment initiation: clinical implications. J Biol Rhythms. 2004;19:68–75. doi: 10.1177/0748730403259670. [DOI] [PubMed] [Google Scholar]
- 115.Emens J, Lewy A, Yuhas K, et al. Melatonin entrains free-running blind individuals with circadian periods less than 24 hours. Sleep. 2006;29:A62. [Google Scholar]
- 116.Revell VL, Burgess HJ, Gazda CJ, et al. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocr Metab. 2006;91:54–9. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paul MA, Gray GW, Lieberman HR, et al. Phase advance with separate and combined melatonin and light treatment. Psychopharmacology (Berl) 2011;214:515–23. doi: 10.1007/s00213-010-2059-5. [DOI] [PubMed] [Google Scholar]
- 118.Burke TM, Markwald RR, Chinoy ED, et al. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep. 2013;36:1617–24. doi: 10.5665/sleep.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]