Synopsis
Cardiovascular disease, diabetes and obesity are highly prevalent diseases associated with reduce quality of life and life expectancy. In this manuscript, we discuss a novel risk factor for these cardiometabolic diseases: circadian disruption. Circadian disruption occurs when the internal circadian (~24-hour) rhythms are not in synchrony with the environment or each other. This “desynchrony” can occur when behaviors such as sleep and meals are not at an appropriate time relative to the central circadian clock or the external environment, particularly the light-dark cycle. This paper will review studies that examined cardiometabolic health of shift work, which often leads to circadian disruption. We also review studies that experimentally disrupted circadian rhythms to determine the effects on cardiometabolic function. We then review observational studies that examined sleep timing and behavioral chronotype. We end with a brief discussion of some potential mediators linking chronotype and shift work to circadian disruption and cardiometabolic health.
Keywords: Circadian rhythms, diabetes, cardiovascular disease, shift work
Introduction
Cardiovascular disease (CVD), diabetes and obesity affect millions of people worldwide and the rates of these cardiometabolic diseases are on the rise [1, 2]. Cardiometabolic diseases are associated with reduced quality of life, lower life expectancy and increased economic burden on both the individual and on society [3–6], and therefore thorough understanding of all the risk factors for these diseases could contribute to improvement in global health. In this manuscript, we discuss a potentially novel risk factor for cardiometabolic disease: circadian disruption.
Circadian disruption occurs when the endogenous circadian (~24-hour) rhythms are not in synchrony with either the environment or each other. This “desynchrony” can occur when behaviors such as wake, sleep and meals are not at an appropriate time relative to the timing of the central circadian clock, which is located in the hypothalamus, and/or relative the external environment, particularly the light-dark cycle. This paper will first review studies that examined cardiometabolic health of shift work, which typically leads to circadian disruption. We also review studies that experimentally disrupted circadian rhythms to determine the effects on cardiometabolic function. We then review observational studies that examined sleep timing and behavioral chronotype. Finally, we end with a brief discussion of a few potential mediators linking the chronotype and shift work to circadian disruption and cardiometabolic health.
Observational Studies of Shift Work
Shift work does not have a universal definition, but can refer to work shifts that occur always at night (permanent night shift) or rotate between different shifts (day, afternoon, night) across the month. Some studies also include work shifts that are simply outside the standard 9:00 a.m. to 5:00 p.m. on Monday through Friday. Any work shift that requires an individual to be awake at a time that their central circadian clock associates with sleep has the potential to disrupt that individual’s circadian rhythms.
Shift work has been associated with an increased risk of numerous cardiometabolic diseases and their risk factors. Several studies have reported that the risk of developing CVD is higher in shift workers compared to day workers [7–9]. Shift workers also often have higher blood pressure or rates of hypertension than day workers [10–12]. One study found that endothelial function, a marker of CVD risk, was reduced in shift workers [13] and another study reported abnormalities on the electrocardiogram in the shift workers [14]. Shift workers are also reported to have a higher prevalence or incidence of type 2 diabetes [15] and the longer the history of working as a shift worker the greater the risk of developing diabetes [16], and one study suggested that the risk of diabetes was mediated by body weight [17]. A meta-analyses of 12 studies with 226,652 total participants, including 14,595 diabetes patients found that having ever worked shift work was associated with increased prevalence of diabetes (Pooled odds ratio [OR] 1.09, 95% confidence interval [CI] 1.05 to 1.12) [18]. This meta-analyses also found significant sex differences in that the association was stronger in men (OR=1.37, 95% CI 1.20–1.56) than in women (OR=1.09, 95% CI 1.04–1.14).
There are several risk factors for cardiovascular disease, including overweight/obesity, dyslipidemia, insulin resistance and impaired beta-cell function in the pancreas. Individuals performing shift work often have larger body mass indices (BMI) or waist circumferences than those only working on day shifts [12, 19–24]. Several studies have found that shift workers have higher levels of either total cholesterol or triglycerides or lower levels of high density lipoprotein (HDL) cholesterol [12, 22, 25–29]. Other studies have reported alterations in markers of glucose metabolism, including hyperglycemia [29]. One study observed worse estimated beta-cell function but no differences in estimated insulin resistance in shift workers compared to day workers [30]. Finally, shift workers are also more likely to have the metabolic syndrome, which is a cluster of metabolic abnormalities that increase the risk of CVD and diabetes, including abdominal obesity, insulin resistance, high blood pressure and dyslipidemia. [31–33]
It is important to acknowledge that not all studies have reported significant differences between shift workers and day workers on some cardiometabolic measures or all subgroups studied [14, 28, 34–37]. Differences in results could be due to varying effects of age, sex, definition of shift work or the duration of shift work.
(Tags: shift work, circadian misalignment, circadian disruption, obesity, cardiovascular disease, diabetes)
Experimental Circadian Disruption
Several studies have experimentally manipulated circadian rhythms in healthy volunteers to determine the effect of circadian disruption on cardiometabolic functions (Table 1 summarizes these studies). In one such study, 10 participants underwent a 10-day forced desynchrony (FD) protocol in which they slept and consumed isocaloric meals during a recurring cycle of 28-hour day [38]. Blood samples were taken hourly to measure levels of leptin, insulin, glucose and cortisol, and blood pressure was measured four times during wake. When participants ate and slept 12 h from their habitual times, maximal circadian misalignment, glucose levels increased by 6%, which was mostly due to postprandial rather than fasting levels, and the glucose levels were in a prediabetic range in 3 of 8 participants. This increase in glucose occurred despite a 22% increase in insulin levels, suggesting decreased insulin sensitivity with insufficient β-cell compensation. In addition, the circadian rhythm of cortisol was reversed during circadian misalignment with higher levels at the end of wake episode and at the beginning of sleep episode, which could also contribute to hyperglycemia. Circadian misalignment was also associated with a 3% increase in mean arterial pressure during wakefulness. Finally, one hormone that is involved in appetite regulation is leptin, which is considered a satiety signal, and leptin levels decreased by 17% after circadian disruption. This study has demonstrated numerous changes in markers of cardiometabolic function and could explain some of the observed differences between shift workers and day workers.
Table 1.
Circadian disruption experiments in healthy volunteers with evaluation of cardiometabolic changes
| Study | N | Protocol | Assessments* | Results |
|---|---|---|---|---|
| Scheer et al 2009 [38] | 10 | 10-day forced desynchrony protocol of 28h day, consisted of 2 baseline days (8h habitual sleep) followed by 7 recurring 28-h sleep-wake cycles under dim light conditions, with 4 isocaloric meals during each cycle. The ratio of scheduled sleep to wake was maintained at 1:2. |
|
During circadian misalignment (subjects ate and slept 12 h from their habitual time):
|
| Buxton et al 2012 [39] | 21 (11 mean age 23y; and 10, mean age 60y) | 3 weeks of forced desynchrony (28h day) with 5.6 h sleep restriction, followed by 9 day recovery period (10h sleep opportunity/day) |
|
At the end of concurrent sleep restriction and circadian disruption:
|
| Leproult et al 2014 [40] | 26 (19 male) | A parallel design comparing 8-day of sleep restriction (5h) and sleep restriction combined with circadian misalignment (5h bedtime with 8.5 sleep onset delayed for 4 of 8 days) |
|
At the end of the experiment:
|
| McHill et al 2014 [41] | 14 | 6-day inpatient simulated nightshift protocol (3-day daytime schedule followed by 3-day nightshift schedule), conducted in a whole-room calorimeter |
|
During nightshifts:
|
| Morris et al 2015 [42] | 14 | Two 8-day of circadian aligned and circadian misaligned to evaluate the relative effects of behavioral cycle, endogenous circadian system and circadian misalignment on glucose and lipids metabolism |
|
|
Some studies had additional assessments. Please refer to references listed.
A second experimental study of circadian disruption also used the 28-hour day FD protocol but with concurrent sleep restriction (5.6 hours/day) for 3 weeks to explore the combined effects of sleep restriction and circadian disruption as commonly experienced by shift workers, followed by 9-day recovery period [39]. They enrolled 21 participants; 11 were younger (mean age 23) and 10 were older (mean age 60). Circadian disruption combined with sleep restriction was associated with an 8% increase in fasting glucose levels and a 14% increase in postprandial glucose levels in response to a standardized breakfast. There was an inadequate pancreatic β-cell response because fasting and postprandial peak insulin levels were significantly reduced (by 12% and 27%, respectively). Circadian disruption combined with sleep restriction also decreased the resting metabolic rate decreased by 8%. The 24-hour levels of leptin were slight lower after circadian disruption combined with sleep restriction, and ghrelin, which is an orexigenic hormone involved in appetite regulation, were slightly higher. These metabolic changes did not differ significantly between the older and younger participants. These results have provide further details on potential underlying mechanisms of increased diabetes and obesity risk in shift workers.
A third experimental study was designed to determine whether circadian disruption impairs cardiometabolic function independently from the effects from sleep loss using a parallel group design [40]. One group was allowed 5 hours in bed for 8 days with bedtimes always centered at 03:00h (circadian aligned) and the second group had 5 hours in bed but on 4 days the bedtimes were delayed by 8.5 hours (circadian misaligned). Both the circadian aligned and misaligned groups had significantly reduced insulin sensitivity without compensatory insulin response. Furthermore, in the men, the decrease in insulin sensitivity was twice as large during circadian misaligned compared to the circadian aligned group (there were too few women to examine separately). Furthermore, high sensitivity c-reactive protein (hsCRP), which is a marker of inflammation, increased in both groups, but increased substantially more in the circadian misaligned group (+146±103% vs. +64±63%, P=0.049,). The results of this experimental study support an independent effect of circadian disruption on glucose metabolism and cardiometabolic risk.
Eating at a circadian inappropriate time, i.e. at night in humans as is commonly seen in shift workers, may play a role in the obesity risk, one study simulated shiftwork in order to examine the effects on energy metabolism using a whole-room calorimeter [41]. This 6-day inpatient study simulated shiftwork in 14 adults by having two daytime shifts with 8-hour nocturnal sleep opportunity followed by the first night shift, which only allowed a brief 2-hour sleep opportunity and then two additional night shifts with 8-hour sleep opportunities during the day. Compared to baseline, total daily energy expenditure was 4% higher on the first night shift, but 3% lower on the 2 subsequent nightshifts. The thermic effect of feeding (i.e. energy expenditure after food intake) was lower in response to late dinner on the first night shift. Subjective appetite decreased during nightshifts despite a decrease in levels of leptin and peptide-YY, another anorexigenic hormone. The combination of decreased energy expenditure and lower thermal effect of feeding after late meals could explain increased obesity in shift workers who often eat at night.
A final experimental study was designed to distinguish the effects of the behavioral cycles (sleep/wake, fasting/feeding and activity), the endogenous circadian system, and circadian disruption on glucose metabolism [42]. The protocol involved two 8-day cross-over studies when the behavioral cycles were aligned or misaligned (12-h shift) with their endogenous circadian system. Glucose tolerance was assessed at 8 am and 8 pm in response to an identical mixed meal along with a measurement of lipids. Postprandial glucose levels were 17% higher in the biological evening than morning and the early phase postprandial insulin response was 27% lower in the evening, indicative of insufficient β-cell response. This endogenous circadian effect was much larger than that of the behavioral cycle effect (8% higher postprandial glucose and 14% lower insulin responses at dinner time compared to breakfast time). In addition, circadian misalignment (12-h behavioral cycle inversion) increased postprandial glucose levels by 6% despite a 14% higher late phase postprandial insulin response, suggesting reduced insulin sensitivity during misalignment. This study demonstrates the relative importance of the endogenous circadian system, the behavioral cycle and circadian misalignment on glucose metabolism.
In summary, these experimental studies have demonstrated the importance of the circadian system and the timing of behaviors such as eating in controlling metabolism, and have provided insights into the mechanisms linking circadian disruption to increased cardiometabolic disease risk.
(Tags: circadian misalignment, circadian disruption, glucose intolerance, energy expenditure, obesity, hypertension, cardiometabolic risk, diabetes)
Observational Studies of Milder Circadian Disruption
Shift work can be an extreme form of circadian disruption, but circadian disruption in milder forms can also be detrimental. For example, when people go to bed at a different time on work or school days than they do on free days or weekends this could lead to “social jet lag”, which may also be associated with cardiometabolic function. Also, the clock time that someone goes to bed, which can be a measure of “chronotype”, may be associated with cardiometabolic function. Finally, the time of day someone prefers to sleep versus be active, often called “circadian preference”, may be another characteristic of chronotype associated with cardiometabolic health. In this section, the association between cardiometabolic function and social jetlag and chronotype will be discussed.
Many individuals in modern society experience social jet lag because of obligations such as work or school that require a specific wake time, and this obligation is lifted on free days [43]. In a large epidemiological survey of more than 65,000 participants, greater social jetlag was associated with being overweight (BMI ≥25 kg/m2) [44]. In addition, among overweight participants, there was a positive correlation between social jetlag and BMI; those who slept at a later clock time had a higher BMI. Subsequent studies have demonstrated an association between social jetlag and adverse cardiometabolic profiles. In a study of 145 healthy participants, those with a social jetlag ≥2 h had significantly higher fasting morning cortisol and higher area-under-the-curve of cortisol levels collected over 5 hours starting in the morning [45]. Those with a social jetlag ≥2 h also had higher resting heart rate, shorter average sleep duration, and less physical activity. In a larger study of 815 non-shift workers, participants with greater social jetlag were more likely to be obese (OR 1.2, 95% CI: 1.0–1.5) and to have the metabolic syndrome (OR 1.3, 95% CI: 1.0–1.6) [46]. Furthermore, among those who were obese and had the metabolic syndrome, greater social jetlag was also associated with an increased odds of having elevated glycated hemoglobin (≥5.7%) and elevated inflammation (hsCRP levels >3 mg/L) [46].
Individuals with a later chronotype, i.e. those who sleep at a later clock time, often have a greater degree of circadian misalignment between behavioral rhythms and the endogenous central circadian clock, and they also often have greater social jetlag [43]. A later (evening) circadian preference and later chronotype have been associated with several cardiometabolic disorders and unhealthy behaviors (Table 2). In adolescents, large population studies have shown that those with evening circadian preference or later bed and wake times had higher BMI z-score, increased risk of being obese (OR 2.16), and less time spent in moderate-to-vigorous physical activity [47, 48]. An unhealthy diet may partly play a role in this association as those with evening preference were reported to have worse dietary habits, including frequent snacking, less fruits and vegetables consumption, increased caloric intake from fat and meal skipping [48–51]. In an 8-week prospective study of 159 college freshmen, students who were evening types gained more weight than those who were morning types [52].
Table 2.
Studies of the associations between chronotype and metabolic outcomes
| Study | Population | N | Chronotype assessments | Metabolic outcomes |
|---|---|---|---|---|
| Arora 2014 [48] | Young adolescents (aged 11–13) | 511 | Morningness eveningness questionnaire |
|
| Olds 2011 [47] | Adolescent (aged 9–16) | 2200 | Bedtime and wake time. Participants categorized into Early-bed/Early-rise, Early-bed/Late-rise, Latebed/Early-rise, and Latebed-Late-rise |
|
| Sato-Mito [49] | Young adults (aged 18–20) | 3304 | Midpoint of sleep |
|
| Culnan [52] | College freshmen (mean age 18) | 159 | Morningness eveningness questionnaire |
|
| Lucassen [51] | Obese adults with <6.5h of sleep | 119 | Morningness eveningness questionnaire |
|
| Nakanishi-Minami [93] | Adults | 32 healthy, and 74 T2DM | Bedtime and wake time |
|
| Merikanto [54] | Adults, aged 25–74 | 4589 | Modified Morningness eveningness questionnaire |
|
| Yu [53] | Adults, aged 47–59 | 1620 | Morningness eveningness questionnaire |
|
| Reutrakul [55] | Adults with T2DM | 194 | Mid sleep time on free day corrected for sleep debt |
|
| Iwasaki [56] | Adults with T2DM | 101 | Morningness eveningness questionnaire |
|
| Osonoi [57] | Adults with T2DM | 725 | Morningness eveningness questionnaire |
|
In addition to obesity, having a later chronotype is also associated with increased cardiovascular risk. For example, obese short sleepers with an evening chronotype had higher stress hormone levels (24h urinary epinephrine and plasma ACTH levels) and higher resting heart rate [51]. Two large population-based studies of more than 6000 participants revealed that evening chronotype was associated with increased odds of having type 2 diabetes (OR was 1.73 [53] and 2.5 in men and women combined [54]) Evening chronotype was also associated with increased odds of having arterial hypertension (OR 1.3) [54]. In addition, a clinic based study of 194 patients with type 2 diabetes, later chronotype based on bedtimes was associated with poorer glycemic control independently of sleep duration [55]. Subsequent studies in type 2 diabetes patients (total 826 participants) have confirmed the association between evening chronotype and poorer glycemic control [56, 57]. Evening chronotype in type 2 diabetes patients was also associated with higher triglycerides and lower HDL levels [57].
These studies suggest that milder forms of circadian disruption, and not only the more extreme circadian disruption observed in shift workers, are associated with adverse cardiometabolic function. Future research should explore whether interventions to reduce circadian disruption and/or advancing bed times can improve cardiometabolic health.
(Tags: circadian misalignment, circadian disruption, chronotype, jet lag, glucose intolerance, energy expenditure, obesity, hypertension, cardiometabolic risk, diabetes)
Potential Mediators Linking Evening Chronotype or Shiftwork and Cardiometabolic Disease
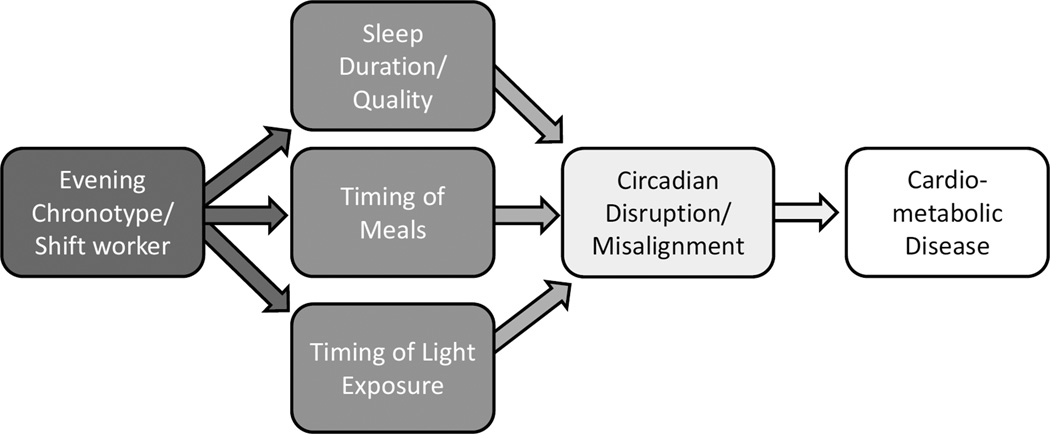
There are a few potential mediators linking evening chronotype and shift work to circadian disruption and ultimately to cardiometabolic disease (Figure). These include reduced sleep duration or quality, inappropriate timing of meals, and light at night. These potential mediators and their associations with cardiometabolic disease will be briefly reviewed below.
Figure.
Potential pathways leading from later chronotype or shift work to circadian disruption and cardiometabolic disease.
Sleep
Chronotype and shift work is often associated with reduced sleep duration and quality [16, 45]. Previous research has demonstrated that inadequate sleep durations, including short sleep, as well as poorer sleep quality are both associated with cardiometabolic disease. Several meta-analyses of existing studies have been conducted and have found that short sleep is associated with increased odds of prevalent obesity [58], prevalent metabolic syndrome [59], prevalent hypertension [60], incident type 2 diabetes [61], incident hypertension in those less than 65 years old [60], and increased risk of developing or dying of coronary heart disease [62]. Furthermore, poor sleep quality has also been associated with increased risk of incident type 2 diabetes [61]. The association between sleep and cardiometabolic disease has been reviewed extensively (see for example [63, 64]. Thus, impairments in sleep could partially mediate the association between shiftwork or chronotype and cardiometabolic disease.
Meal Timing
The timing of meals can impact internal circadian alignment because food metabolites serve as synchronizing signals for the clocks in many peripheral tissues and organs [65]. Exposure to food at an inappropriate time of day could lead to misalignment between central and peripheral clocks, which could impair metabolism and lead to weight gain [66]. Indeed, in an experimental model, mice fed at the wrong time of day gained more weight than mice with access to food at the appropriate circadian time despite similar food intake and physical activity [67].
Studies in humans have also observed a relationship between meal timing and altered metabolism. A randomized crossover study in 32 women compared the effects of eating an early lunch (13:00) to a late lunch (16:30). Compared to the early lunch, the late lunch was associated with decreased pre-meal resting-energy expenditure, decreased pre-meal carbohydrate oxidation, decreased thermal effect of food as well as decreased glucose tolerance to meal [68]. Decreased energy expenditure and decreased glucose tolerance are risk factors for weight gain and diabetes, and therefore these results provide evidence for a link between eating at a later clock time and metabolic disease. Another study found that more calories consumed after 20:00. was associated with higher BMI even after controlling for sleep timing and duration [69]. Studies of weight loss interventions have also demonstrated the importance of timing of food intake. In a 20-week weight loss study of 420 participants, those who consumed their main meal (lunch in this Mediterranean population) before 15:00 lost 2.2 kg more on average than those who ate after 15:00, despite consuming similar amount of calories [70]. In a second weight loss study, women were randomized to either a large proportion of calories earlier in the day (70% for breakfast, morning snack, and lunch and 30% for afternoon snack and dinner) or a more even distribution (55% for breakfast through lunch and 45% for afternoon snack and dinner) for 3 months [71]. Those who eat more earlier in the day lost significantly more weight (−8.2 vs −6.5 kg, p=.028), reduced waist circumference by more (−7 v −5 cm, p=.033), lost more fat mass (−6.8 vs −4.5 kg, p=.031) and improved their insulin sensitivity more. A qualitative study found that one strategy employed by individuals who maintained 10% weight loss for at least one year was eating small dinner, a strategy not employed by individuals who regained weight after an initial loss [72]. Finally, since glucose tolerance is known to be worse in the evening [73], late eating may also affect glycemic control in patients with diabetes. In fact, a study of patients with type 2 diabetes demonstrated that a greater amount of daily calories consumed at dinner was associated with poorer glycemic control, independently of chronotype [55]. Interestingly, a recent randomized crossover study in type 2 diabetes patients compared a hypoenergetic diet of two larger meals (breakfast and lunch) to six smaller meals in 54 patients for 12 weeks. Two larger meals resulted in a significantly greater reduction body weight, liver fat content, fasting plasma glucose, C-peptide and glucagon, and higher insulin sensitivity than the same caloric restriction split into six meals [74], indicating the timing of food intake has an important effect on metabolism.
Another potentially important meal pattern is breakfast skipping. There is overwhelming evidence that breakfast skipping is detrimental to health, including higher risks of overweight and obesity, increased visceral adiposity, insulin resistance, type 2 diabetes and dyslipidemia. For example, a longitudinal study of 2184 participants over 20 years found that those who skipped breakfast in both childhood and adulthood had significantly greater waist circumference and higher fasting insulin, total cholesterol and LDL cholesterol than those who consumed breakfast at both time points [75]. A study of 3,598 participants from the community-based Coronary Artery Risk Development in Young Adults (CARDIA) study found that relative to those with infrequent breakfast consumption (0–3 days/week), participants who reported eating breakfast daily gained 1.9 kg less weight over 18 years (P=0.001), along with significant reduction in the incidence of obesity, metabolic syndrome and hypertension [76]. Moreover, in a cohort of 26,902 American men, those who skipped breakfast had a 27% higher risk of coronary heart disease (CHD) compared with men who did not [77]. In addition men who ate after going to bed had a 55% higher CHD risk than men who did not. However, once adjusting for health factors, such as BMI, hypertension, hypercholesterolemia, and diabetes status, the associations were no longer significant. Another longitudinal study of 29,206 men reported that breakfast skipping was associated with a 21% increase in risk of developing type 2 diabetes, even after adjustment for BMI [78] and the Nurse’s Health study in women also observed a significant association between breakfast skipping and incident diabetes [79]. A recent meta-analysis of 106,935 participants found that breakfast skipping was associated with type 2 diabetes [80] [34]. In addition, in patients with type 2 diabetes, breakfast skipping was found to be associated with poorer glycemic control [50, 81, 82]. One recent study in Japan examined the combination of breakfast skipping and late night eating and found that a combination of breakfast skipping and late night eating (consumed dinner within 2 hours of bedtime ≥3 times/week) was associated with the presence of the metabolic syndrome (OR 1.17) while breakfast skipping or late night eating alone was not [83]. Finally, one weight-loss intervention studies found that eating breakfast was associated with greater weight loss despite similar caloric restriction [84], but a second intervention found no effect of breakfast eating recommendations [85]
Overall, the evidence suggests a relationship between meal timing or daily food distribution and cardiometabolic risk. While breakfast skipping and eating at night are associated with adverse cardiometabolic profiles, more interventional studies are needed to demonstrate whether manipulating meal timing will result in improved metabolism.
Light at night
Another potential mediator between late chronotype or shift work and cardiometabolic disease is exposure to artificial light at night. Light is the primary synchronizer of the central circadian clock, and therefore exposure to light during the biological night could lead to circadian disruption. There is some evidence from animal studies that exposure to light at night can impair metabolism. In these studies, male mice that were exposed to high fat diet and dim light at night had increased weight gain, reduced glucose tolerance, and altered insulin secretion as compared with mice that were not exposed to light at night, despite equivalent caloric intake [86]. Furthermore, the timing of the food intake was shifted when exposed to light [87], indicating that animals exposed to light at night may also beating more food at an inappropriate circadian time.
Another mechanism through which light at night could impair cardiometabolic function is through melatonin. Melatonin is a hormone primarily secreted by the pineal gland and its release is suppressed by light. Melatonin plays an important role in circadian physiology (See Burgess, this issue) and also plays a role in sleep promotion [88]. More recently, melatonin has been recognized as playing an important role in metabolism [89, 90]. Lower melatonin levels were associated with increased risk of incident diabetes in a large cohort study [91]. Thus, individuals who stay up later will be exposed to more artificial light, which will suppress melatonin and potentially reduce the total amount of melatonin secreted, putting them at risk of diabetes.
The role artificial light may play in health was recently recognized by the American Medical Association (AMA). In June, 2012, the AMA House of Delegates adopted a policy statement on nighttime lighting, and the Executive Summary states, “Other diseases that may be exacerbated by circadian disruption include obesity, diabetes” [92]. Thus, there is recognition that electric light could lead to circadian disruption, which in turn could impair human health.
(Tags: sleep duration, sleep quality, breakfast skipping, meal timing, light, circadian disruption, glucose intolerance, obesity, cardiometabolic risk, diabetes)
Summary
Circadian disruption is associated with impairments in cardiometabolic function and increased risk of obesity, diabetes and CVD. This association is not only among the most severe forms of circadian disruption, i.e. shift work, but is also observed with milder delays in the timing of sleep and meals. Future research should determine whether manipulating the timing of sleep, meals or light exposure can help to improve cardiometabolic health.
Key Points.
Circadian disruption can occur when sleep and/or meal timing occurs out of synchrony with the light-dark cycle (environment) or the central circadian clock (endogenous).
Circadian disruption is associated with increased risk of impaired cardiometabolic function and associated diseases, including obesity, diabetes and cardiovascular disease.
Shift work is associated with severe circadian disruption, but even milder delays in bed time or meals are associated with impaired cardiometabolic function.
Sleep, meal timing and light at night could link late chronotype and shift work to circadian disruption.
[text box] Circadian disruption contributes to increased cardiometabolic risks. Circadian misalignment results in
Impaired glucose tolerance as a result of decreased insulin sensitivity and inadequate β-cell response
Elevated inflammatory markers
Elevated mean arterial pressure
Decreased energy expenditure
Acknowledgments
Disclosure Statement: SR receives speaker honoraria from Sanofi Aventis, and research support from Merck. KLK is the National Sleep Foundation Poll Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swinburn BA, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 2.Seidell J. Obesity, insulin resistance and diabetes -- a worldwide epidemic. British Journal of Nutrition. 2000;83(Suppl 1):S5–S8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- 3.Ettaro L, et al. Cost-of-illness studies in diabetes mellitus. Pharmacoeconomics. 2004;22(3):149–164. doi: 10.2165/00019053-200422030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Franco OH, et al. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167(11):1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 5.Solomon CG, Manson JE. Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr. 1997;66(4 Suppl):1044S–1050S. doi: 10.1093/ajcn/66.4.1044S. [DOI] [PubMed] [Google Scholar]
- 6.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6(2):97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown DL, et al. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol. 2009;169(11):1370–1377. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawachi I, et al. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92(11):3178–3182. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 9.Knutsson A, et al. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 10.Sakata K, et al. The relationship between shift work and the onset of hypertension in male Japanese workers. J Occup Environ Med. 2003;45(9):1002–1006. doi: 10.1097/01.jom.0000085893.98441.96. [DOI] [PubMed] [Google Scholar]
- 11.Marqueze EC, Ulhoa MA, Moreno CR. Effects of irregular-shift work and physical activity on cardiovascular risk factors in truck drivers. Rev Saude Publica. 2013;47(3):497–505. doi: 10.1590/s0034-8910.2013047004510. [DOI] [PubMed] [Google Scholar]
- 12.Ha M, Park J. Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health. 2005;47(2):89–95. doi: 10.1539/joh.47.89. [DOI] [PubMed] [Google Scholar]
- 13.Amir O, et al. Brachial artery endothelial function in residents and fellows working night shifts. Am J Cardiol. 2004;93(7):947–949. doi: 10.1016/j.amjcard.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Murata K, et al. Effects of shift work on QTc interval and blood pressure in relation to heart rate variability. Int Arch Occup Environ Health. 2005;78(4):287–292. doi: 10.1007/s00420-004-0592-4. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson AK, et al. Work stress, sense of coherence, and risk of type 2 diabetes in a prospective study of middle-aged Swedish men and women. Diabetes Care. 2013;36(9):2683–2689. doi: 10.2337/dc12-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan A, et al. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroenke CH, et al. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165(2):175–183. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 18.Gan Y, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2015;72(1):72–78. doi: 10.1136/oemed-2014-102150. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa Y, et al. Effect of shift work on body mass index and metabolic parameters. Scand J Work Environ Health. 2007;33(1):45–50. doi: 10.5271/sjweh.1063. [DOI] [PubMed] [Google Scholar]
- 20.Barbadoro P, et al. Rotating shift-work as an independent risk factor for overweight Italian workers: a cross-sectional study. PLoS One. 2013;8(5):e63289. doi: 10.1371/journal.pone.0063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Amelsvoort L, Schouten E, Kok F. Duration of shiftwork related to body mass index and waist to hip ratio. International Journal of Obesity. 1999;23:973–978. doi: 10.1038/sj.ijo.0801028. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, et al. Shift work and risk factors for coronary heart disease in Japanese blue-collar workers: serum lipids and anthropometric characteristics. Occup Med (Lond) 1997;47(3):142–146. doi: 10.1093/occmed/47.3.142. [DOI] [PubMed] [Google Scholar]
- 23.Kim MJ, et al. Association between shift work and obesity among female nurses: Korean Nurses' Survey. BMC Public Health. 2013;13:1204. doi: 10.1186/1471-2458-13-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58(11):747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romon M, et al. Increased triglyceride levels in shift workers. Am J Med. 1992;93(3):259–262. doi: 10.1016/0002-9343(92)90230-9. [DOI] [PubMed] [Google Scholar]
- 26.Dochi M, et al. Shift work is a risk factor for increased total cholesterol level: a 14-year prospective cohort study in 6886 male workers. Occup Environ Med. 2009;66(9):592–597. doi: 10.1136/oem.2008.042176. [DOI] [PubMed] [Google Scholar]
- 27.Knutsson A, Akerstedt T, Jonsson BG. Prevalence of risk factors for coronary artery disease among day and shift workers. Scand J Work Environ Health. 1988;14(5):317–321. doi: 10.5271/sjweh.1913. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson BH, et al. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76(6):424–430. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 29.Nagaya T, et al. Markers of insulin resistance in day and shift workers aged 30–59 years. Int Arch Occup Environ Health. 2002;75(8):562–568. doi: 10.1007/s00420-002-0370-0. [DOI] [PubMed] [Google Scholar]
- 30.Esquirol Y, et al. Shiftwork and higher pancreatic secretion: early detection of an intermediate state of insulin resistance? Chronobiol Int. 2012;29(9):1258–1266. doi: 10.3109/07420528.2012.719959. [DOI] [PubMed] [Google Scholar]
- 31.Kawada T, Otsuka T. Effect of shift work on the development of metabolic syndrome after 3 years in Japanese male workers. Arch Environ Occup Health. 2014;69(1):55–61. doi: 10.1080/19338244.2012.732123. [DOI] [PubMed] [Google Scholar]
- 32.De Bacquer D, et al. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38(3):848–854. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- 33.Lin YC, Hsiao TJ, Chen PC. Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: a five-year follow-up. Chronobiol Int. 2009;26(4):740–755. doi: 10.1080/07420520902929029. [DOI] [PubMed] [Google Scholar]
- 34.Boggild H, et al. Shift work, social class, and ischaemic heart disease in middle aged and elderly men; a 22 year follow up in the Copenhagen Male Study. Occup Environ Med. 1999;56(9):640–645. doi: 10.1136/oem.56.9.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bursey RG. A cardiovascular study of shift workers with respect to coronary artery disease risk factor prevalence. J Soc Occup Med. 1990;40(2):65–67. doi: 10.1093/occmed/40.2.65. [DOI] [PubMed] [Google Scholar]
- 36.Fouriaud C, et al. Influence of socioprofessional conditions on blood pressure levels and hypertension control. Epidemiologic study of 6,665 subjects in the Paris district. Am J Epidemiol. 1984;120(1):72–86. doi: 10.1093/oxfordjournals.aje.a113876. [DOI] [PubMed] [Google Scholar]
- 37.Ika K, et al. Shift work and diabetes mellitus among male workers in Japan: does the intensity of shift work matter? Acta Med Okayama. 2013;67(1):25–33. doi: 10.18926/AMO/49254. [DOI] [PubMed] [Google Scholar]
- 38.Scheer FA, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buxton OM, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McHill AW, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(48):17302–17307. doi: 10.1073/pnas.1412021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris CJ, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(17):E2225–E2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wittmann M, et al. Social jetlag: misalignment of biological and social time. Chronobiology International. 2006;23(1–2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 44.Roenneberg T, et al. Social jetlag and obesity. Current Biology. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 45.Rutters F, et al. Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms. 2014;29(5):377–383. doi: 10.1177/0748730414550199. [DOI] [PubMed] [Google Scholar]
- 46.Parsons MJ, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (Lond) 2015;39(5):842–848. doi: 10.1038/ijo.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olds TS, Maher CA, Matricciani L. Sleep duration or bedtime? Exploring the relationship between sleep habits and weight status and activity patterns. Sleep. 2011;34(10):1299–1307. doi: 10.5665/SLEEP.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes (Lond) 2014 doi: 10.1038/ijo.2014.157. [DOI] [PubMed] [Google Scholar]
- 49.Sato-Mito N, et al. The midpoint of sleep is associated with dietary intake and dietary behavior among young Japanese women. Sleep Medicine. 2011;12(3):289–294. doi: 10.1016/j.sleep.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Reutrakul S, et al. The Relationship Between Breakfast Skipping, Chronotype, and Glycemic Control in Type 2 Diabetes. Chronobiology International. 2013 doi: 10.3109/07420528.2013.821614. [DOI] [PubMed] [Google Scholar]
- 51.Lucassen EA, et al. Evening Chronotype Is Associated with Changes in Eating Behavior, More Sleep Apnea, and Increased Stress Hormones in Short Sleeping Obese Individuals. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0056519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Culnan E, Kloss JD, Grandner M. A prospective study of weight gain associated with chronotype among college freshmen. Chronobiol Int. 2013;30(5):682–690. doi: 10.3109/07420528.2013.782311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu JH, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab. 2015;100(4):1494–1502. doi: 10.1210/jc.2014-3754. [DOI] [PubMed] [Google Scholar]
- 54.Merikanto I, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiology International. 2013;30(4):470–477. doi: 10.3109/07420528.2012.741171. [DOI] [PubMed] [Google Scholar]
- 55.Reutrakul S, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36(9):2523–2529. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwasaki M, et al. Morningness-eveningness questionnaire score correlates with glycated hemoglobin in middle-aged male workers with type 2 diabetes mellitus. Journal of Diabetes Investigation. 2013;4(4):376–381. doi: 10.1111/jdi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osonoi Y, et al. Morningness-eveningness questionnaire score and metabolic parameters in patients with type 2 diabetes mellitus. Chronobiol Int. 2014;31(9):1017–1023. doi: 10.3109/07420528.2014.943843. [DOI] [PubMed] [Google Scholar]
- 58.Cappuccio FP, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ju SY, Choi WS. Sleep duration and metabolic syndrome in adult populations: a meta-analysis of observational studies. Nutr Diabetes. 2013;3:e65. doi: 10.1038/nutd.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Q, et al. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res. 2012;35(10):1012–1018. doi: 10.1038/hr.2012.91. [DOI] [PubMed] [Google Scholar]
- 61.Cappuccio FP, et al. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009 doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cappuccio FP, et al. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European Heart Journal. 2011;32(12):1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 63.Ip M, Mokhlesi B. Sleep and Glucose Intolerance/Diabetes Mellitus. Sleep medicine clinics. 2007;2:19–29. doi: 10.1016/j.jsmc.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knutson KL. Does inadequate sleep play a role in vulnerability to obesity? American Journal of Human Biology: The Official Journal of the Human Biology Council. 2012;24:361–371. doi: 10.1002/ajhb.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annual Review of Physiology. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 66.Garaulet M, Gomez-Abellan P. Timing of food intake and obesity: A novel association. Physiology and Behavior. 2014 doi: 10.1016/j.physbeh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Arble DM, et al. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17(11):2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bandin C, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int J Obes (Lond) 2015;39(5):828–833. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 69.Baron KG, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 70.Garaulet M, et al. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37(4):604–611. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lombardo M, et al. Morning meal more efficient for fat loss in a 3-month lifestyle intervention. J Am Coll Nutr. 2014;33(3):198–205. doi: 10.1080/07315724.2013.863169. [DOI] [PubMed] [Google Scholar]
- 72.Karfopoulou E, et al. Behaviours associated with weight loss maintenance and regaining in a Mediterranean population sample. A qualitative study. Clin Obes. 2013;3(5):141–149. doi: 10.1111/cob.12028. [DOI] [PubMed] [Google Scholar]
- 73.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocrine Reviews. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 74.Kahleova H, et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: a randomised crossover study. Diabetologia. 2014;57(8):1552–1560. doi: 10.1007/s00125-014-3253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith KJ, et al. Skipping breakfast: longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am J Clin Nutr. 2010;92(6):1316–1325. doi: 10.3945/ajcn.2010.30101. [DOI] [PubMed] [Google Scholar]
- 76.Odegaard AO, et al. Breakfast frequency and development of metabolic risk. Diabetes Care. 2013;36(10):3100–3106. doi: 10.2337/dc13-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cahill LE, et al. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation. 2013;128(4):337–343. doi: 10.1161/CIRCULATIONAHA.113.001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mekary RA, et al. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr. 2012;95(5):1182–1189. doi: 10.3945/ajcn.111.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mekary RA, et al. Eating patterns and type 2 diabetes risk in older women: breakfast consumption and eating frequency. Am J Clin Nutr. 2013;98(2):436–443. doi: 10.3945/ajcn.112.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bi H, et al. Breakfast skipping and the risk of type 2 diabetes: a meta-analysis of observational studies. Public Health Nutr. 2015:1–7. doi: 10.1017/S1368980015000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kollannoor-Samuel G, et al. Determinants of fasting plasma glucose and glycosylated hemoglobin among low income Latinos with poorly controlled type 2 diabetes. J Immigr Minor Health. 2011;13(5):809–817. doi: 10.1007/s10903-010-9428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt LE, et al. The relationship between eating patterns and metabolic control in patients with noninsulin-dependent diabetes mellitus (NIDDM) Diabetes Educ. 1994;20(4):317–321. doi: 10.1177/014572179402000410. [DOI] [PubMed] [Google Scholar]
- 83.Kutsuma A, Nakajima K, Suwa K. Potential Association between Breakfast Skipping and Concomitant Late-Night-Dinner Eating with Metabolic Syndrome and Proteinuria in the Japanese Population. Scientifica (Cairo) 2014;2014:253581. doi: 10.1155/2014/253581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schlundt DG, et al. The role of breakfast in the treatment of obesity: a randomized clinical trial. Am J Clin Nutr. 1992;55(3):645–651. doi: 10.1093/ajcn/55.3.645. [DOI] [PubMed] [Google Scholar]
- 85.Dhurandhar EJ, et al. The effectiveness of breakfast recommendations on weight loss: a randomized controlled trial. Am J Clin Nutr. 2014;100(2):507–513. doi: 10.3945/ajcn.114.089573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fonken LK, et al. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology. 2013;154(10):3817–3825. doi: 10.1210/en.2013-1121. [DOI] [PubMed] [Google Scholar]
- 87.Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl 3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 89.Peschke E, Muhlbauer E. New evidence for a role of melatonin in glucose regulation. Best Pract Res Clin Endocrinol Metab. 2010;24(5):829–841. doi: 10.1016/j.beem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 90.Acuna-Castroviejo D, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McMullan CJ, et al. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309(13):1388–1396. doi: 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stevens RG, et al. Adverse health effects of nighttime lighting: comments on American Medical Association policy statement. Am J Prev Med. 2013;45(3):343–346. doi: 10.1016/j.amepre.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Nakanishi-Minami T, et al. Sleep-wake cycle irregularities in type 2 diabetics. Diabetol Metab Syndr. 2012;4(1):18. doi: 10.1186/1758-5996-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]