Abstract
The fibroblast-populated 3D collagen matrix has been used to model matrix contraction, cell motility, and general fibroblast biology. MCPIP1 (monocyte chemotactic protein-induced protein 1) has been shown to regulate inflammation, angiogenesis, and cellular motility. In the present study, we demonstrated induction of MCPIP1 in human fibroblasts embedded in the stress-released 3D collagen matrix, which occurred through activation of mitogen-activated protein kinases, phosphoinositide 3-kinase, and NF-κB. Furthermore, MCPIP1 induction was associated with inhibition of fibroblast migration out of the nested collagen matrix. MCPIP1 induction or ectopic expression also upregulated p53. RNA interference of p53 prevented the inhibition of migration produced by induction or ectopic expression of MCPIP1. Our findings suggest a new role for MCPIP1 as a molecular switch that regulates fibroblast migration in the nested collagen matrix model.
Introduction
Recently we observed (Chao et al., 2014) that foreskin fibroblasts preconditioned in a rigidly anchored collagen matrix migrated out of that matrix when it was re-embedded (“nested”) (Grinnell et al., 2006) in cell-free, anchored collagen, while fibroblasts preconditioned in a stress-released matrix had relatively poor motility under the same conditions (5% serum). This observation provided us with an opportunity to study mechanoregulation of fibroblast motility in the fibroblast-populated 3D collagen matrix (FPCM) model (Grinnell, 1994). A relevant signaling molecule was MCPIP1 (MCP-1-induced protein 1, also know as ZC3H12A), a 66 kDa protein identified in human peripheral blood monocytes and cardiomyocytes stimulated with MCP-1 (monocyte chemotactic protein-1) (Liu et al., 2015; Zhou et al., 2006). The known functions of MCPIP1 include: downregulation of inflammation through induction of apoptosis genes (Skalniak et al., 2013; Zhou et al., 2006); induction of angiogenesis in endothelial cells (HUVECs) (Niu et al., 2008); inhibition of Toll-like receptor signaling and macrophage activation (Huang et al., 2012); upregulation of adipogenesis independent of PPARγ (Younce et al., 2009); RNAse activity against viral DNA (Lin et al., 2013; Suzuki et al., 2011); inhibition of JNK and NF-κB (Liang et al., 2008; Liu et al., 2013); and protection against LPS-induced shock (Huang et al., 2013). MCPIP1-deficient mice developed a severe inflammatory syndrome with T-cell activation, increased cytokine production, and a 50% eight-week mortality (Miao et al., 2013).
MCPIP1 also was noted to promote migration in HUVECs (Niu et al., 2008), and MCP-1 knock-out mice demonstrated delayed wound re-epithelialization and angiogenesis (Low et al., 2001). So it seemed logical to test whether MCPIP1 participated in mechanoregulation of fibroblast healing functions, such as proliferation, contraction, and migration. Herein we report data demonstrating that MCPIP1 induction after stress-release of the FPCM inhibits fibroblast migration, working through a signaling pathway involving the mitogen-activated protein (MAP) kinases, NF-κB, and p53. This brake on fibroblast migration appears to be a new function for MCPIP1, and implicates this protein as a participant in the wound healing process.
Results
Mechanoregulation of migration in the restrained nested matrix; upregulation of MCPIP1 in the stress-released FPCM
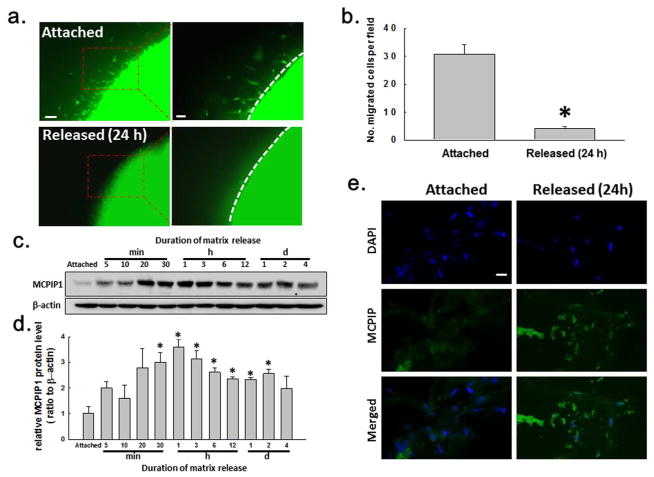
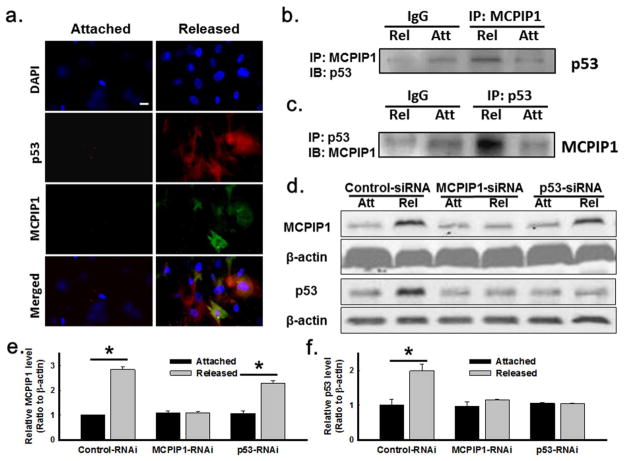
Fibroblast migration was assayed using serum-treated attached or stress-released collagen matrices populated with green fluorescent protein (GFP)-expressing human foreskin fibroblasts (HFFs) (Chao et al., 2014) in the restrained nested matrix (Supplementary Fig. S1A) (Miron-Mendoza et al., 2010). Fibroblast migration out of the nested stress-released matrix was decreased relative to the nested stressed (attached) matrix (Fig. 1A–B and S4F). Immunoblotting demonstrated that the MCPIP1 protein was upregulated after matrix release (Fig. 1C–D). The MCPIP1 signal reached a maximum at ~1 h and remained elevated for several days. Immunocytochemistry of MCPIP1 in attached vs. released matrices also demonstrated induction of this protein in the released state (Fig. 1E). The specificity of the MCPIP1 fluorescence in the released matrix was particularly impressive while focusing up and down through the 3D microscopy specimen.
Fig. 1. Effect of FPCM release on fibroblast migration and expression of MCPIP1.
(A) Migration of GFP-expressing fibroblasts out of nested matrices was decreased 24 hr after matrix release. Fibroblast migration shown at the interface between the nested matrix and the restrained cell-free matrix. Left scale bar = 200 μm, right scale bar= 80 μm. (B) Plot of migration (three separate experiments from panel A). (C) MCPIP1 induction after FPCM release. Whole cell lysates from attached or released matrices immunoblotted for MCPIP1 and β-actin. (D) MCPIP1 densitometry (four separate experiments from panel C). (E) MCPIP1 immunocytochemistry in the attached vs. released FPCM. Blue = DAPI; green = MCPIP1. Scale bar = 20 μm. Data are mean ± S.E.M.; *p < 0.05 vs. attached (unpaired t-test).
Effect of MCPIP1 RNAi on the mechanoregulation of FPCM contraction, matrix cell number, and fibroblast migration
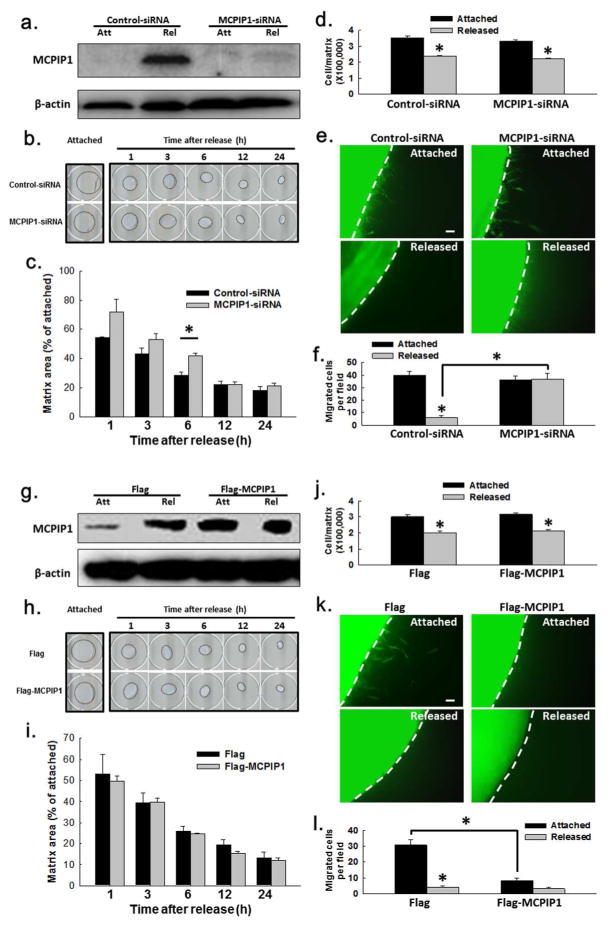
In order to determine whether MCPIP1 induction associated with FPCM stress-release was biologically relevant, the effect of MCPIP1 knockdown on FPCM contraction, matrix cell number, and fibroblast migration was determined in attached vs. released matrices. The efficiency of MCPIP1 RNA interference (RNAi) in the attached vs. released FPCM (72 hr after transfection, 24 hr after release) was near complete by immunoblotting (Fig. 2A). RNAi of MCPIP1 had minimal effect on contraction in the floating collagen matrix assay (“dermal equivalent” (Grinnell and Petroll, 2010)); see Fig. 2B–C. RNAi of MCPIP1 did not affect the decrease in matrix cell number (Fig 2D) known to occur after matrix stress-release (Carlson and Longaker, 2004). Using attached or stress-released matrices populated with GFP-expressing HFFs nested into restrained cell-free collagen, it was observed that MCPIP1 knockdown disinhibited migration out of the released, nested matrix (Fig. 2E–F). That is, the decrease in fibroblast migration (the inhibition) precipitated by release of the nested matrix was prevented (disinhibited) if MCPIP1 expression was blocked.
Fig. 2. Effect of MCPIP1 RNAi or ectopic expression on matrix contraction, matrix cell number, and fibroblast migration.
(A) Immunoblots of lysates from FPCMs expressing siRNA (MCPIP1 vs. nonsense). (B–C) Effect of MCPIP1 RNAi on FPCM contraction (well diameter=19mm); plot=three experiments. (D) Effect of MCPIP1 RNAi on FPCM cell number, one day post-release. (E–F) Effect of MCPIP1 RNAi on migration out of the released, nested FPCM (scale bar=80 μm); plot=three experiments. (G) Immunoblots of lysates from FPCMs expressing MCPIP1-Flag vs. Flag. (H–I) Effect of MCPIP1-Flag expression on FPCM contraction; plot=three experiments. (J) Effect of MCPIP1-Flag expression on FPCM cell number, one day post-release. (K) Effect of MCPIP1-Flag expression on migration out of the attached, nested FPCM (scale bar=80 μm); plot=three experiments. *p < 0.05, unpaired t-test.
Effect of MCPIP1 ectopic expression on the mechanoregulation of FPCM contraction, matrix cell number, and fibroblast migration
In order to corroborate the findings in Fig. 2, an analogous set of experiments was performed using plasmid-expressed Flag-tagged MCPIP1 (Fig. 2G–L). Confirmation of MCPIP1-Flag expression after plasmid transfection in the FPCM is shown in Fig. 2G and Supplementary Fig. S1B. Addition of the MAT-Tag-Flag sequence (see Methods) added 15 amino acids to the 599 amino acid sequence of MCPIP1, but no shift was seen on the immunoblots of MCPIP1 vs. MCPIP1-Flag. Subsequent ectopic expression of MCPIP1-Flag had no effect on contraction in the floating collagen matrix assay (Fig. 2H–I). Expression of MCPIP1-Flag also did not affect the decrease in matrix cell number which occurred after matrix stress-release (Fig. 2J). In experiments analogous to those in Fig. 2E–F, expression of MCPIP1-Flag inhibited GFP-HFF migration out of the attached matrix nested into restrained, cell-free collagen (Fig. 2K–L). Ectopic expression of MCPIP1-Flag in monolayer fibroblasts actually increased migration in a scratch assay (Supplementary Fig. 5A–B), i.e., the opposite effect to that observed in the 3D culture model.
Effect of FPCM release on phosphorylation of MAP kinases and Akt
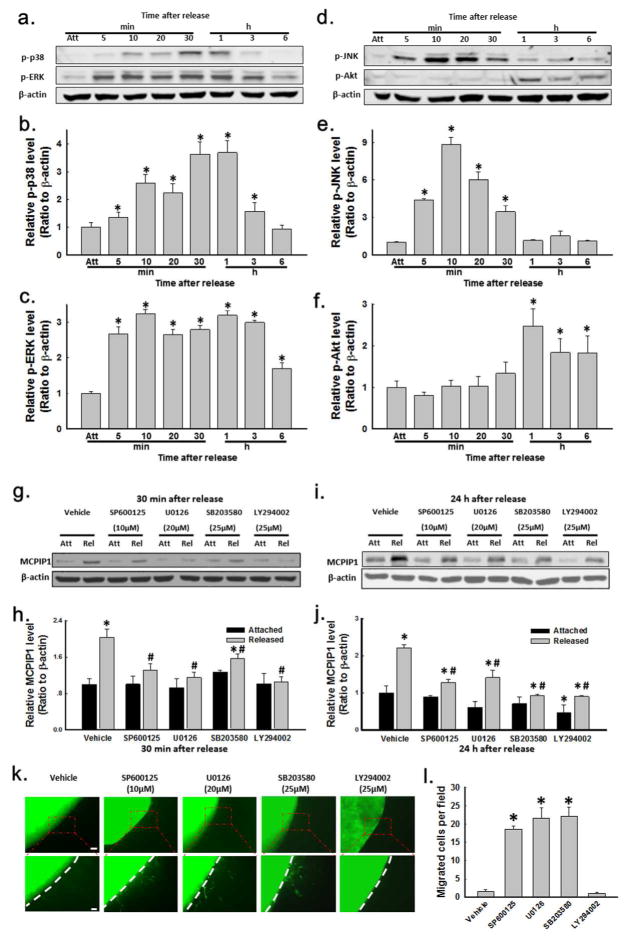
Previous reports have indicated that activation of MAP kinases and the phosphoinositide 3-kinase (PI3K)/Akt pathway both stimulate fibroblast migration (Clement et al., 2013; Li et al., 2004). In order to see if there was a link between these kinase pathways and MCPIP1-associated inhibition of cellular migration, phosphorylation of these kinases in the attached vs. released FPCM was evaluated first (Fig. 3). Within 5 min of matrix stress release, there was increased phosphorylation of Erk, which tapered off by 6 h (Fig. 3A, C). Within 5–10 min of release, p38 demonstrated increased phosphorylation, reaching a peak around 30–60 min and then tapering off (Fig. 3A, B). JNK also demonstrated a burst of activation from 5 to 30 min after release (Fig. 3D, E). The data of Fig. 3 was consistent with previous work of other investigators, who demonstrated Erk and p38 activation after stress-release of the FPCM under similar but not identical conditions (Lee et al., 2000).
Fig. 3. Kinase activity, inhibition, MCPIP1 expression, and migration in the FPCM.
(A–F) p38, Erk1/2, JNK, and Akt phosphorylation after FPCM release (immunoblots of whole lysates), with densitometry of n=4 experiments. (G–J) Effect of kinase inhibitors (JNK=SP600125, MEK=U0126, p38=SB203580, PI3K=LY294002) on FPCM MCPIP1, 30 min and 24 h post-release (whole lysate immunoblots), with densitometry (n=4 experiments each). *p < 0.05 vs. vehicle-attached (unpaired t-test); #p < 0.05 vs. vehicle-released (unpaired t-test). (K–L) Effect of kinase inhibitors on migration out of the released, nested matrix (upper scale bar= 200 μm, lower scale bar =80 μm); plot represents n=3 experiments/condition. *p < 0.05, unpaired t-test.
Akt also demonstrated a burst of activity 1 h after release, with tapering thereafter (Fig. 3D, F). Of note, previously-published reports demonstrated Akt was dephosphorylated during a longer time course (>6 h) of FPCM stress-release (Carlson et al., 2004; Tian et al., 2002; Xia et al., 2004). A long time course of Akt activity after matrix release was repeated for corroboration purposes in Supplementary Fig. S2A–B, which again demonstrated rapid and transient Akt phosphorylation, followed by gradual dephosphorlyation evident at 6–12 h (consistent with previously-published data).
Effect of pharmacological inhibition of MAP kinases or Akt on MCPIP1 induction and fibroblast migration after FPCM release
Pharmacological inhibition of the above kinases was used to determine whether the pathways of interest (JNK, ERK, p38, and PI3K/Akt) regulated (1) the expression of MCPIP1 and (2) fibroblast migration out of the nested matrix (Fig. 3G–L). Justification for the 30 min point was the relatively large increase in MCPIP1 expression in the released matrix present at this time; the 24 h time point was chosen because both MCPIP1 upregulation and migration inhibition were evident 24 h after release (Fig. 1). Treatment of FPCMs with commercially-available small molecules U0126 (MEK inhibitor), SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), or LY294002 (PI3K inhibitor) at the manufacturer-recommended dose decreased the stress-release-induced phosphorylation of the respective target kinase (see Supplementary Figure S2C–F).
Inhibitor pre-treatment for 2 hr prior to FPCM stress-release diminished the induction of MCPIP1 at both 30 min and 24 h after stress-release (Fig. 3G–J). At the 30 min time point, the MCPIP1 signal still appeared to be increased in the stress-released matrix in the presence of each kinase inhibitor, but this reached significance only with the p38 inhibitor (SB203580). At the 24 h time point, induction of MCPIP1 expression after stress-release was still blunted by each kinase inhibitor, but effects were less pronounced compared to the 30 min time point (Fig. 3J).
ANOVA and unpaired t-testing performed on the MCPIP1/actin expression ratios for the attached matrix in Fig. 3J (i.e., attached vehicle vs. attached SP600125 vs. attached U0126 vs. attached SB203580 vs. attached LY294002) revealed that the attached LY294002 ratio (indicated with an asterisk over that bar) was different from the attached vehicle ratio. So while kinase inhibition may have decreased MCPIP1 expression in the attached FPCM, our assay detected this only for the PI3K inhibitor. This observation might decrease the relevance of the decrease in MCPIP1 induction observed in the LY294002-treated released matrix; the absolute effect of the PI3K inhibitor in the released matrix, however, still was large compared to the effect in the attached matrix.
The effect of pharmacologic inhibition of kinase activity on fibroblast migration from stress-released matrices nested into restrained cell-free matrices then was evaluated (Fig. 3K–L). Pretreatment of the FPCM for 2 h prior to stress-release with the MEK, p38, or JNK inhibitor enhanced fibroblast migration out of the nested released matrix (i.e., resulted in disinhibition of fibroblast migration). Pretreatment of matrices with the PI3K inhibitor LY294002, however, did not have an effect; that is, fibroblast migration out of the nested released matrix was similar to vehicle treatment, meaning barely detectable. There was no significant effect of any of these inhibitors on fibroblast migration out of the nested attached matrix (Supplementary Fig. S3A–B). The data of Fig. 3 suggested that MAP kinase activation was upstream of MCPIP1 induction in a putative pathway that inhibited fibroblast migration after stress-release of the FPCM.
Involvement of NF-κB in the expression of MCPIP1 and inhibition of fibroblast migration after FPCM release
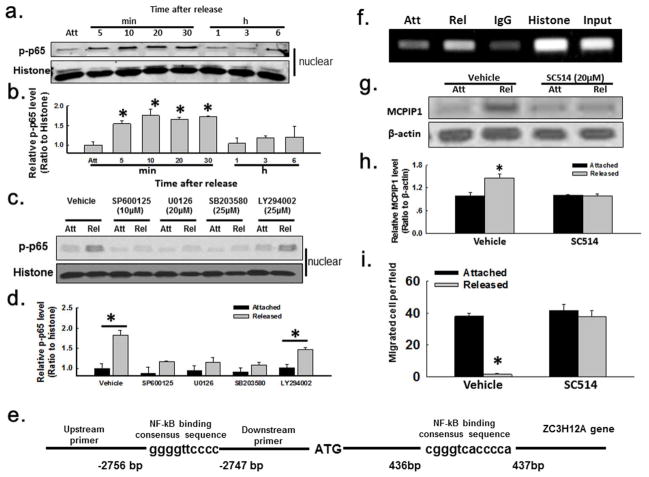
NF-κB activation has been documented in the contractile FPCM (Carlson et al., 2013; Xu and Clark, 1997; Xu et al., 1998), and NF-κB signaling has been implicated in the induction of both MCP-1 and MCPIP1 in endothelial cells (Qi et al., 2010; Yao et al., 2010). In addition, NF-κB activation can occur secondary to MAP kinase activation (Dhawan and Richmond, 2002; Dong et al., 2012; Troppmair et al., 1998). So it was hypothesized that MCPIP1 induction in the stress-released FPCM occurred as a consequence of MAP kinase-mediated NF-κB activation. During the 5–30 min interval after matrix release, there was an increase in the level of the phosphorylated p65 (p-p65) subunit of NF-κB in the nuclear fraction of the HFFs (Fig. 4A–B), with a concomitant loss of this subunit in the cytoplasm (Supplementary Fig. S4A), consistent with pilot data (Carlson et al., 2013). This increase of p-p65 after matrix release appeared to be abrogated if the matrices were pre-treated with pharmacologic inhibitors of Erk, p38, or JNK; pretreatment with the PI3K inhibitor had less effect (Fig. 4C–D). These results suggested that MAP kinase activation after FPCM release resulted in NF-κB activation.
Fig. 4. NF-κB signaling, MCPIP1 induction, and inhibition of migration in the FPCM.
(A–B) NF-κB p65 phosphorylation after FPCM release (nuclear fraction immunoblots); n=4 experiments for densitometry. (C–D) Effect of kinase inhibitors on p-p65 in attached vs. 20 min-released FPCMs; n=4 experiments for densitometry. (E) NF-κB p65 binding sequence, MCPIP1 promoter region. (F) ChIP of p65 binding to the MCPIP1 promoter, 20 min after FPCM release. (G–H) Effect of NF-κB activation inhibitor (SC-514) on MCPIP1 induction after FPCM release (24 h time point; whole cell immunoblots); n=4 experiments for densitometry. (I) Effect of SC-514 resulted on migration out of the nested released FPCM (immunofluorescent images in Supplementary Fig. S1C–D); n=3 experiments. *p<0.05, unpaired t-test.
The MCP-1 promoter has an NF-κB binding site (Yao et al., 2010); a chromatin immunoprecipitation (ChIP) assay was utilized to determine whether a similar site was present on the MCPIP1 promoter (Fig. 4E). Chromatin was cross-linked to protein in attached vs. 20 min stress-released FPCMs, and sonicated extracts were processed with a ChIP kit. Subsequent agarose gel electrophoresis of the amplified, immunoprecipitated DNA revealed much greater NF-κB binding in extracts from the released matrix compared to the attached (Fig. 4F). This was consistent with NF-κB binding to the MCPIP1 promoter in extracts from the stress-released FPCM.
In order to determine whether NF-κB activation after FPCM release regulated MCPIP1induction, matrices were pretreated with pharmacologic inhibitors of NF-κB activation (Fig. 4G–I, S1C–D, and S4D–F), including TPCK (N-p-tosyl-L-phenylalanine chloromethyl ketone) and SC-514 (4-Amino-[2,3″]bithiophenyl-5-carboxylic acid amide). TPCK, a serine protease inhibitor, will block NF-κB activation by preventing the proteolysis of IκB-α; SC-514, an ATP-competitive inhibitor selective for IκB kinase-2 (IKK-2), will block NF-κB activation by preventing phosphorylation of IκB-α (Ha et al., 2009; Henkel et al., 1993; Karin et al., 2004; Kishore et al., 2003). Compared to vehicle-treated matrices, MCPIP1 induction after release in matrices pretreated with SC-514 or TPCK or was not present at 24 h (Fig. 4G–H and S4D–E, respectively). Furthermore, FPCM pretreatment with either SC-514 or TPCK disinhibited HFF migration in the released matrix that was nested into a restrained, cell-free matrix (Fig. 4I, S1C–D, and S4F). In other words, pharmacologic inhibition of NF-κB allowed fibroblasts to migrate out of the collagen matrix under conditions in which they normally would not. The results thus far suggested that inhibition of fibroblast migration associated with FPCM release involved a pathway with sequential upregulation of MAP kinases, NF-κB, and MCPIP1.
Interaction of MCPIP1 with p53 after release of the FPCM
It has been shown that is p53 upregulated after release of the FPCM (Carlson et al., 2004; Carlson et al., 2009; Carlson et al., 2013). In order to determine whether there was co-localization of MCPIP1 and p53 after release of the FPCM, double-immunocytochemistry for these two antigens was performed (Fig. 5A and S3E). The orange-yellow coloring in the merged images indicated that there was some co-localization of MCPIP1 and p53 in the 1-day released matrix. Immunoprecipitation of either MCPIP1 or p53 followed by immunoblotting for the opposite antigen demonstrated an association of MCPIP1 and p53 in whole cell lysates from the released (but not attached) FPCM (Fig. 5B–C). Control immunoprecipitation experiments that used RNAi to knockdown either MCPIP1 or p53 prior to pull-down confirmed the specificity of the MCPIP1-p53 association (Supplementary Fig. S3C–D). The results from Fig. 5A–C suggested that there was a physical interaction between MCPIP1 and p53 after FPCM stress release.
Fig. 5. MCPIP1 and p53 association after FPCM release.
(A) MCPIP1 and p53 immunocytochemistry in attached vs. 1 day-released FPCM. Blue=DAPI; green=MCPIP1; red=p53; scale bar=20 μm. (B–C) MCPIP1 and p53 co-localization after FPCM release (whole lysates from attached vs. 1 day-released FPCMs immunoprecipitated for MCPIP1 or p53, then immunoblotted for the other; IgG used as immunoprecipitate control). (D–F) Effect of RNAi of MCPIP1 or p53 on p53 or MCPIP1 in 1 day-released FPCM (whole lysate immunoblots); n=4 experiments for densitometry. *p < 0.05 vs. attached (unpaired t-test).
In order to determine whether one of these proteins regulated the concentration of the other, RNAi of MCPIP1 or p53 was performed and protein levels were determined with immunoblotting (Fig. 5D–F). Fibroblasts transfected with siRNA against MCPIP1 did not have p53 upregulation 24 h after matrix release (Fig. 5F), even though the knockdown of MCPIP1 did not appear complete (Fig. 5E). Treatment with siRNA against p53 prevented most of the post-release increase in p53 (Fig. 5D, F). Dissimilar to the situation with MCPIP1 RNAi, however, partial knockdown of p53 did not affect the induction of MCPIP1 in the released matrix (Fig. 5D–E). These data suggested that MCPIP1 regulated p53 induction after FPCM release, i.e., that MCPIP1 was upstream of p53 in a putative pathway.
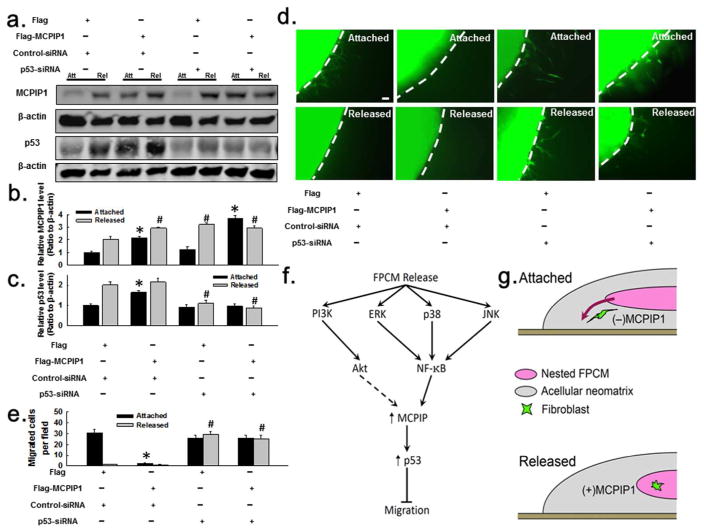
Further exploration of an MCPIP1–p53 relationship was done using ectopic expression of MCPIP1-Flag combined with p53 RNAi (Fig. 6). Expression of MCPIP1-Flag upregulated p53 in the attached FPCM, with no significant effect in the released matrix (Fig. 6A, C); as expected, MCPIP1-Flag inhibited cell migration out of the nested attached FPCM (Fig. 6D–E). Consistent with Fig. 5, RNAi of p53 did not affect induction of MCPIP1 associated with matrix release (Fig. 6A–B), but did result in disinhibition of cell migration that occurred in the nested released FPCM (Fig. 6D–E). Expression of MCPIP1-Flag could not overcome the effect of p53 RNAi on cell migration (Fig. 6D–E). In other words, MCPIP1 could inhibit the ability of fibroblasts to migrate out of the attached matrix that was nested into a restrained, cell-free matrix if p53 was not inhibited. If p53 was knocked down, however, then the fibroblasts could migrate out of either an attached or released matrix, regardless of the state of MCPIP1.
Fig. 6. MCPIP1 regulates cell migration through p53.
(A–C) Effect of MCPIP1-Flag expression and/or p53 RNAi on MCPIP1 and p53 in attached vs. 1 day-released FPCM (whole lysate immunoblots); n=4 experiments for densitometry. (D–E) Effect of MCPIP1-Flag expression and/or p53 RNAi on migration out of attached vs. 1 day-released FPCMs (scale bar=80 μm); plot represents n=3 experiments/condition. *p < 0.05 vs. control-attached (unpaired t-test); #p < 0.05 vs. control-released (unpaired t-test). (F–G) Putative pathway of MCPIP1-centric pathway regulating migration in the FPCM. Stress-release of the FPCM activates PI3K, ERK, p38, and JNK. Activated NK-κB induces expression of MCPIP1, which upregulates p53, which then inhibits fibroblast migration through unknown mechanisms. A pointed arrowhead head = stimulatory effect; flat arrowhead = inhibition.
MCPIP1 associated with USP10 (Ubiquitin Specific Peptidase 10) after genotoxic stress, enhancing the latter’s deubiquitinase activity, thereby producing deubiquitination of NEMO (IKK-γ) which resulted in inhibition of NF-κB activation (Niu et al., 2013). Genotoxic stress also produced a TANK-MCPIP1-USP10 complex that effected/enhanced deubiquitination of both TRAF6 (E3 Ubiquitin Protein Ligase) and NEMO, which in turn inhibited NF-κB activation (Wang et al., 2015). So in the setting of genotoxic stress, MCPIP1 appears to function as a facilitator of USP10 deubiquitinase, thereby acting as a “brake” (negative feedback mechanism) on NF-κB activation. In order to determine whether MCPIP1-facilitation of deubiquitination was relevant for MCPIP1-induced upregulation of the p53 protein, we performed FPCM experiments with fibroblasts transfected with MCPIP1(ΔZF), an MCPIP1 mutant that does not promote deubiquitination (Liang et al., 2010). Our data suggested that MCPIP1-facilitated deubiquitination is important for induction of p53 by MCPIP1 (Supplementary Fig. 4B–C), which suggested that the increase of the p53 protein after FPCM stress-release was mediated at least in part by a decrease in p53 ubiquitination. Whether MCPIP1 affects the ubiquitination status of p53 through USP10 will need further investigation.
The data of Fig. 6 were consistent with a pathway in which MCPIP1, acting upstream and through p53, effected inhibition of cell migration out of the nested released FPCM. Stress-release of the FPCM also has been shown both to increase apoptosis and inhibit the cell cycle in the resident fibroblasts (Carlson and Longaker, 2004; Carlson et al., 2013; Fluck et al., 1998; Grinnell et al., 1999; Hadjipanayi et al., 2009; Tian et al., 2002), which likely is an effect of p53 upregulation. Although cell survival and proliferation were not the focus of this study, we did demonstrate that the Bax protein increased after FPCM stress-release, and that this induction was abrogated by p53 knockdown (Supplementary Fig. 5C). This finding would be consistent with a p53-modulated increase in apoptosis associated with FPCM stress-release.
Discussion
The data suggested the existence of an MCPIP1-centric network which regulated fibroblast migration in the nested FPCM model (Fig. 6F–G). In this putative network, MCPIP1 in the nested attached FPCM was undetectable, allowing fibroblasts to migrate out of the FPCM and into the acellular neomatrix. If the matrix was released, however, phosphorylation of MAP kinases (Erk, p38, and JNK) ensued and produced NF-κB activation. The p65 subunit of NF-κB then bound to the promoter region of the MCPIP1 gene, followed by increased MCPIP1 expression. This induction of MCPIP1 upregulated p53, possibly through binding events between MCPIP1 and p53 (and perhaps some unspecified scaffolding proteins). Upregulation of p53 produced, through unidentified additional steps, inhibition of fibroblast migration out of the nested released matrix.
While we have implied that MCPIP1 induction after FPCM release was secondary to increased transcription and translation, we have not ruled out other protein turnover mechanisms, such as transcript stability or protein degradation. Our assumption of increased MCPIP1 transcription was based on two observations: (1) p65 NF-κB bound to the MCPIP1 promoter after FPCM release; and (2) inhibition of NF-κB activation abrogated both the induction of MCPIP1 and the inhibition of migration that occurred after matrix release (Fig. 6).
It is conceivable that other signaling events (e.g., involving the Rho GTPases (Raftopoulou and Hall, 2004)) may have contributed to the inhibition of migration associated with FPCM stress-release. Nevertheless, various “molecular switches” have been described in which a change in protein level and/or activity produced a large downstream event (Drees et al., 2005; Milburn et al., 1990; Murphy et al., 2004), so it is not inconceivable that increased MCPIP1 protein contributed to the inhibition of fibroblast migration. The effect of MAP kinase inhibition on MCPIP1 induction in Fig. 6 was partial, suggesting that the MAPK inhibitors may have had produced other downstream effects (e.g., on myosin light chain kinase (Huang et al., 2004)) that resulted in disinhibition of migration after matrix stress-release.
Previous reports have suggested that activation of oncogenic Ras, MAP kinases, and PI3K all may work to enhance fibroblast migration (Clement et al., 2013; Li et al., 2004; Menezes et al., 2008), in apparent contradiction to our report. These earlier reports all used one or more of the following conditions: (1) transformed cell lines with forced/sustained expression of various signaling proteins; (2) monolayer culture conditions; and (3) variable amounts of soluble growth factors. In contrast, our experimental system (1) did not utilize forced expression to make primary observations (Fig. 1 and 4), (2) did utilize a three-dimensional culture system, and (3) maintained a constant culture medium. These differences in experimental design might explain the discrepancy between the previous studies and our data. For example, we observed transient MAP kinase activation, the effect of which likely is different from activation secondary to forced expression (Marshall, 1995; Sabbagh Jr et al., 2001).
Our intent herein was to study fibroblast migration in a tractable 3D model which has morphologic and physiologic similarities to dermal wounds (Carlson and Longaker, 2004); clearly, these results may not perfectly translate to in vivo processes. It is interesting to note that forced expression of MCPIP1 in a monolayer assay of migration actually appeared to promote fibroblast motility; i.e., the opposite effect to what was observed in the 3D collagen matrix. This discordance of experimental results from monolayer vs. 3D culture systems suggests that it may be better to utilize 3D culture systems when studying cells whose natural environment is three-dimensional, not planar (Cukierman et al., 2001).
The FPCM model has been in continual use since the 1970s to study various phenomena related to wound healing, such as cellular migration, cell-mediated matrix contraction, synthesis and secretion of matrix proteins, cell death, cellular proliferation. Derivatives of the FPCM model also have been used to study tissue engineering (Carlson and Longaker, 2004; Grinnell and Petroll, 2010; Harunaga and Yamada, 2011; Kim et al., 2011). Continued progress in wound healing science likely will benefit from the use of (1) culture systems such as the FPCM and (2) in vivo models.
Materials and methods
Cell culture
The use of primary dermal fibroblasts derived from discarded human neonatal circumcision specimens was approved by the Research and Development Committee of the Omaha VA Medical Center and by the Institutional Review Board of the University of Nebraska Medical Center. Fibroblasts were cultured from explants of human neonatal foreskins, as previously described (Carlson et al., 2009). The collagen matrix model was utilized, as previously described (Carlson et al., 2009; Carlson et al., 2013; Grinnell et al., 1999). Lentiviral-transduced HFFs with stable expression of GFP was described in a separate report (Chao et al., 2014).
Nested matrix model and cell migration
The nested collagen matrix model was utilized as previously described (Chao et al., 2014; Grinnell et al., 2006; Liu et al., 2015; Miron-Mendoza et al., 2010) with some modifications; refer to Supplementary Fig. S1A and protocol in the Supplementary Information. For the nested attached matrix, a standard FPCM was incubated in the attached state for 72 h with 5% FBS in DMEM; the FPCM then was removed from the culture well, and placed onto a 60 μL aliquot of fresh acellular collagen matrix solution (neomatrix solution) that was centered inside a 12 mm-diameter score on the bottom of a new culture well. A 140 μL aliquot of neomatrix solution then was used to cover the newly-transferred FPCM. The neomatrix was allowed to polymerize for 1 h at 37°C and 5% CO2, and then 2 mL of DMEM with 5% FBS was added to the well. The same procedure was followed for the nested released matrix, except that the initial incubation of the FPCM was 48 h in the attached stated, followed by detachment, and then 24 h incubation in the released state (see Supplementary Fig. S1A). Cell migration out of the nested FPCM and into the acellular neomatrix was quantified 24 h after nesting with fluorescent microscopy, as described in the Supplementary Information. Cell number per matrix was determined using a Scepter™ Cell Counter (EMD Millipore), as previously described (Chao et al., 2014).
See Supplemental Information for details on reagents, transfections, contraction assays, immunoblotting, immunocytochemistry, immunoprecipitation, ChIP assay, and other methods.
Supplementary Material
Acknowledgments
This study is the result of work supported in part with resources and the use of facilities at the VA Nebraska-Western Iowa Health Care System. The Flag-MCPIP plasmids were kindly provided by Shilpa Buch of the University of Nebraska Medical Center. The authors would like to express their appreciation for helpful discussions with Amarnath Natarajan. The authors also would like to acknowledge the technical assistance of Chris Hansen and Dean Heimann. M.A.C. was supported by grants from the State of Nebraska, the United States Department of Defense, and the National Institutes of Health. J.C was supported by grants of The National Natural Science Foundation of China (81473263) and The Natural Science Foundation of Jiangsu Province, China (No. BK20141347 and No. BK20141497).
Abbreviations
- 3D
three-dimensional
- FPCM
fibroblast-populated collagen matrix
- HFF
human foreskin fibroblasts
- HUVEC
human umbilical vein endothelial cells
- MAPK
mitogen-activated protein kinase
- MEK
MAPK kinase
- MCPIP1
monocyte chemotactic protein-induced protein 1
Footnotes
Location where work was done: Omaha, Nebraska, USA, and Nanjing, Jiangsu, China
Competing Financial Interests
The authors state no conflict of interest.
References
- Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Rep Regen. 2004;12:134–47. doi: 10.1111/j.1067-1927.2004.012208.x. [DOI] [PubMed] [Google Scholar]
- Carlson MA, Longaker MT, Thompson JS. Modulation of FAK, Akt, and p53 by stress release of the fibroblast-populated collagen matrix. J Surg Res. 2004;120:171–7. doi: 10.1016/j.jss.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Carlson MA, Prall AK, Gums JJ, et al. Biologic variability of human foreskin fibroblasts in 2D and 3D culture: implications for a wound healing model. BMC Res Notes. 2009;2:229. doi: 10.1186/1756-0500-2-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MA, Smith LM, Cordes CM, et al. Attachment-regulated signaling networks in the fibroblast-populated 3D collagen matrix. Sci Rep. 2013;3:1880. doi: 10.1038/srep01880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Pena T, Heimann DG, et al. Expression of green fluorescent protein in human foreskin fibroblasts for use in 2D and 3D culture models. Wound Repair Regen. 2014;22:134–40. doi: 10.1111/wrr.12121. [DOI] [PubMed] [Google Scholar]
- Clement DL, Mally S, Stock C, et al. PDGFRalpha signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2–ERK1/2–p90RSK and AKT signaling pathways. J Cell Sci. 2013;126:953–65. doi: 10.1242/jcs.116426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, et al. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Richmond A. A novel NF-kB-inducing kinase-MAPK signaling pathway up-regulates NF-kB activity in melanoma cells. J Biol Chem. 2002;277:7920–8. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Ye X, Song N, et al. Urotensin II promotes the production of LTC4 in rat aortic adventitial fibroblasts through NF-kB-5-LO pathway by p38 MAPK and ERK activations. Heart Vessels. 2012:1–10. doi: 10.1007/s00380-012-0291-0. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, et al. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–15. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck J, Querfeld C, Cremer A, et al. Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile collagen gels. J Invest Dermatol. 1998;110:153–7. doi: 10.1046/j.1523-1747.1998.00095.x. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–61. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Rocha LB, Iucu C, et al. Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312:86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Zhu M, Carlson MA, et al. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res. 1999;248:608–19. doi: 10.1006/excr.1999.4440. [DOI] [PubMed] [Google Scholar]
- Ha KH, Byun MS, Choi J, et al. N-tosyl-L-phenylalanine chloromethyl ketone inhibits NF-kappaB activation by blocking specific cysteine residues of IkappaB kinase beta and p65/RelA. Biochemistry. 2009;48:7271–8. doi: 10.1021/bi900660f. [DOI] [PubMed] [Google Scholar]
- Hadjipanayi E, Mudera V, Brown RA. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med. 2009;3:77–84. doi: 10.1002/term.136. [DOI] [PubMed] [Google Scholar]
- Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363–8. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel T, Machleidt T, Alkalay I, et al. Rapid proteolysis of IkappaB-alpha is necessary for activation of transcription factor NF-kappaB. Nature. 1993;365:182–5. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–28. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- Huang S, Miao R, Zhou Z, et al. MCPIP1 negatively regulates toll-like receptor 4 signaling and protects mice from LPS-induced septic shock. Cell Signal. 2013;25:1228–34. doi: 10.1016/j.cellsig.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Qi D, Liang J, et al. The putative tumor suppressor Zc3h12d modulates toll-like receptor signaling in macrophages. Cell Signal. 2012;24:569–76. doi: 10.1016/j.cellsig.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Kim B-S, Park I-K, Hoshiba T, et al. Design of artificial extracellular matrices for tissue engineering. Prog Polymer Sci. 2011;36:238–68. [Google Scholar]
- Kishore N, Sommers C, Mathialagan S, et al. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–71. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Rosenfeldt H, Grinnell F. Activation of ERK and p38 MAP kinases in human fibroblasts during collagen matrix contraction. Exp Cell Res. 2000;257:190–7. doi: 10.1006/excr.2000.4866. [DOI] [PubMed] [Google Scholar]
- Li W, Fan J, Chen M, et al. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol Biol Cell. 2004;15:294–309. doi: 10.1091/mbc.E03-05-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Saad Y, Lei T, et al. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling. J Exp Med. 2010;207:2959–73. doi: 10.1084/jem.20092641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Wang J, Azfer A, et al. A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J Biol Chem. 2008;283:6337–46. doi: 10.1074/jbc.M707861200. [DOI] [PubMed] [Google Scholar]
- Lin RJ, Chien HL, Lin SY, et al. MCPIP1 ribonuclease exhibits broad-spectrum antiviral effects through viral RNA binding and degradation. Nucl Acids Res. 2013;41:3314–26. doi: 10.1093/nar/gkt019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhou Z, Huang S, et al. Zc3h12c inhibits vascular inflammation by repressing NF-kappaB activation and pro-inflammatory gene expression in endothelial cells. Biochem J. 2013;451:55–60. doi: 10.1042/BJ20130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fang S, Liu H, et al. Role of human pulmonary fibroblast-derived MCP-1 in cell activation and migration in experimental silicosis. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Low QE, Drugea IA, Duffner LA, et al. Wound healing in MIP-1alpha(−/−) and MCP-1(−/−) mice. Am J Pathol. 2001;159:457–63. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Menezes GC, Miron-Mendoza M, Ho CH, et al. Oncogenic Ras-transformed human fibroblasts exhibit differential changes in contraction and migration in 3D collagen matrices. Exp Cell Res. 2008;314:3081–91. doi: 10.1016/j.yexcr.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R, Huang S, Zhou Z, et al. Targeted disruption of MCPIP1/Zc3h12a results in fatal inflammatory disease. Immunol Cell Biol. 2013;91:368–76. doi: 10.1038/icb.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn MV, Tong L, Brunger A, et al. Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science. 1990;247:939–45. doi: 10.1126/science.2406906. [DOI] [PubMed] [Google Scholar]
- Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–35. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LO, MacKeigan JP, Blenis J. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Mol Cell Biol. 2004;24:144–53. doi: 10.1128/MCB.24.1.144-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Azfer A, Zhelyabovska O, et al. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) J Biol Chem. 2008;283:14542–51. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Shi Y, Xue J, et al. USP10 inhibits genotoxic NF-kappaB activation by MCPIP1-facilitated deubiquitination of NEMO. EMBO J. 2013;32:3206–19. doi: 10.1038/emboj.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Liang J, She ZG, et al. MCP-induced protein 1 suppresses TNFalpha-induced VCAM-1 expression in human endothelial cells. FEBS Lett. 2010;584:3065–72. doi: 10.1016/j.febslet.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Sabbagh W, Jr, Flatauer LJ, Bardwell AJ, et al. Specificity of MAP kinase signaling in yeast differentiation involves transient versus sustained MAPK activation. Mol Cell. 2001;8:683–91. doi: 10.1016/s1097-2765(01)00322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalniak L, Koj A, Jura J. Proteasome inhibitor MG-132 induces MCPIP1 expression. Febs J. 2013;280:2665–74. doi: 10.1111/febs.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki HI, Arase M, Matsuyama H, et al. MCPIP1 ribonuclease antagonizes dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol Cell. 2011;44:424–36. doi: 10.1016/j.molcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Tian B, Lessan K, Kahm J, et al. beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem. 2002;277:24667–75. doi: 10.1074/jbc.M203565200. [DOI] [PubMed] [Google Scholar]
- Troppmair J, Hartkamp J, Rapp UR. Activation of NF-KB by oncogenic Raf in HEK 293 cells occurs through autocrine recruitment of the stress kinase cascade. Oncogene. 1998;17:685–90. doi: 10.1038/sj.onc.1201981. [DOI] [PubMed] [Google Scholar]
- Wang W, Huang X, Xin HB, et al. TRAF Family Member-associated NF-kappaB Activator (TANK) Inhibits Genotoxic Nuclear Factor kappaB Activation by Facilitating Deubiquitinase USP10-dependent Deubiquitination of TRAF6 Ligase. J Biol Chem. 2015;290:13372–85. doi: 10.1074/jbc.M115.643767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Nho RS, Kahm J, et al. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004;279:33024–34. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- Xu J, Clark RA. A three-dimensional collagen lattice induces protein kinase C-zeta activity: role in alpha2 integrin and collagenase mRNA expression. J Cell Biol. 1997;136:473–83. doi: 10.1083/jcb.136.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zutter MM, Santoro SA, et al. A three-dimensional collagen lattice activates NF-kappaB in human fibroblasts: role in integrin alpha2 gene expression and tissue remodeling. J Cell Biol. 1998;140:709–19. doi: 10.1083/jcb.140.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Yang Y, Kim KJ, et al. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115:4951–62. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younce CW, Azfer A, Kolattukudy PE. MCP-1 (monocyte chemotactic protein-1)-induced protein, a recently identified zinc finger protein, induces adipogenesis in 3T3-L1 pre-adipocytes without peroxisome proliferator-activated receptor gamma. J Biol Chem. 2009;284:27620–8. doi: 10.1074/jbc.M109.025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Azfer A, Niu J, et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006;98:1177–85. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.