Abstract
A decrease in N-methyl-D-aspartate receptor (NMDAR) function is associated with age-related cognitive impairments. However, NMDAR antagonists are prescribed for cognitive decline associated with age-related neurodegenerative disease, raising questions as to the role of NMDAR activity in cognitive function during aging. The current studies examined effects of NMDAR blockade on cognitive task that are sensitive to aging. Young and middle-age rats were trained on the five-choice serial reaction time task (5-CSRTT) and challenged with MK-801 (0.025, 0.05, and 0.1 mg/kg or vehicle). Attention deficits were apparent in middle-age and performance of young and middle-age rats was enhanced for low doses of MK-801 (0.025 and 0.05). The beneficial effects on attention were reversed by the highest dose of MK-801. Older animals exhibited a delay-dependent impairment of episodic spatial memory examined on a delayed-matching to place water maze task. Similarly, a low dose of MK-801 (0.05 mg/kg) impaired performance with increasing delay and aged animals were more susceptible to disruption by NMDAR blockade. Despite MK-801 impairment of episodic spatial memory, MK-801 had minimal effects on spatial reference memory. Our results confirm that NMDARs contribute to rapidly acquired and flexible spatial memory and support the idea that a decline in NMDAR function contributes to the age-related impairments in cognition.
Keywords: aging, hippocampus, prefrontal cortex, NMDA receptor, episodic memory, attention, MK-801
Introduction
Cognitive aging is associated with weakening of episodic memory and executive function which, in the case of Alzheimer’s disease, can progress to more severe cognitive impairments and dementia (Albert, 2011; Alexander et al., 2012). In the case of aging, cognitive decline is associated with altered Ca2+ regulation and impaired synaptic function, including a decrease in the function of N-methyl-D-aspartate receptors (NMDARs) (Foster and Norris, 1997; Foster, 2007, 2012). The emergence of deficits in episodic memory and executive function are linked to decrease in the NMDAR-mediated synaptic responses in the hippocampus and prefrontal cortex (PFC), respectively (Foster and Norris, 1997; Foster, 2012; Kumar and Foster, 2013; Guidi et al., 2015). Similarly, β-amyloid (Aβ) impairs synaptic plasticity (Walsh et al., 2002; Shankar et al., 2007; Shankar et al., 2008) and rapidly decreases NMDAR responses (Snyder et al., 2005; Lacor et al., 2007; Dewachter et al., 2009), which may contribute to the onset of memory deficits associated with the early stages of Alzheimer’s disease.
While the onset of impaired episodic memory is associated with synaptic dysfunction, clinical dementia is more likely related to loss of connectivity throughout the brain, including cell death (DeKosky and Scheff, 1990; Terry et al., 1991; Scheff et al., 2007). Similarly, while a decline in NMDAR activation could underlie disruption of synaptic function early in Alzheimer’s disease, over activation of NMDARs may contribute to cell death. The idea that NMDAR activity contributes to neurotoxicity provides a basis for use of NMDAR antagonists to treat Alzheimer’s disease in order to limit the progression of cell loss (Rogawski and Wenk, 2003; Lipton, 2006). However, there is considerable confusion concerning the utility of NMDAR modulators to treat cognitive aging and Alzheimer’s disease. The low affinity activity-dependent NMDAR channel antagonist, memantine, has had mixed success in treating the symptoms of dementia and the progression of the disease (Schneider et al., 2011).
There are several reasons to predict that NMDAR blockade will have detrimental effects on cognition in older animals. In young animals, NMDAR antagonists impair the rapid acquisition and retention of flexible spatial information (i.e. episodic spatial memory) and an age-related impairment in episodic memory is associated with the decline in NMDAR function (Foster, 2012). Further, a decline in NMDAR function may contribute to the progression of neurodegeneration and cognitive decline. Synaptic NMDAR activity induces the expression of genes involved in the maintenance of neuronal health, which could protect cells from neurodegenerative processes (Papadia et al., 2008; Hardingham and Bading, 2010; Gleichmann et al., 2012). In the same way, a decline in NMDAR activity could underlie the decreased expression of synaptic and neuroprotective genes in aged-memory impaired animals (Blalock et al., 2003; Aenlle and Foster, 2010). Indeed, due to a decline NMDAR function with advancing age, it might be expected that older subjects are more susceptible to impairments following NMDAR blockade (Ingram et al., 1992).
Interestingly, some studies have reported that activity-dependent NMDAR channel antagonists at low doses have little effect or impair memory and yet improve attention and executive function in humans (Rammsayer, 2001; Ferris et al., 2007; Riepe et al., 2007; Wroolie et al., 2009; Nakamura et al., 2014) and in animals (Higgins et al., 2005; Smith et al., 2011; Pehrson et al., 2013; Benn and Robinson, 2014). Given that attention is an important factor in multiple cognitive processes that decline with aging, including executive function and memory, an understanding the dose dependent effect of NMDAR blockade with regard to age-sensitive cognitive processes is a critical question with clinical significance.
In the current study, low doses of the activity-dependent NMDAR channel blocker, MK-801, were used to examine spatial episodic memory and attention, cognitive processes that are sensitive to aging. We confirmed that performance on an attention task, which depends on the PFC, is impaired in middle-age rats (Guidi et al., 2015). NMDAR blockade produced a dose-dependent, biphasic enhancement in measures of attention for conditions that tax attentional demand. This enhancement was reversed by a higher dose of MK-801. An age-dependent impairment in retention of rapidly acquired episodic spatial memory was observed for the delayed-matching to place (DMTP) task, as the retention delay was increased beyond 30 min. MK-801 (0.05 mg/kg) impaired spatial episodic memory for a 30 min delay and aged animals were more susceptible to disruption. Finally, NMDAR blockade had minimal effects on spatial reference memory. The results indicate differential involvement of NMDARs in cognitive processes that depend on the PFC and hippocampus. NMDAR blockade and aging interact to impair hippocampal-dependent episodic memory, a form of memory that declines early in aging and Alzheimer’s disease. In contrast, NMDARs are not essential for spatial reference memory.
2. Methods
2.1 Subjects
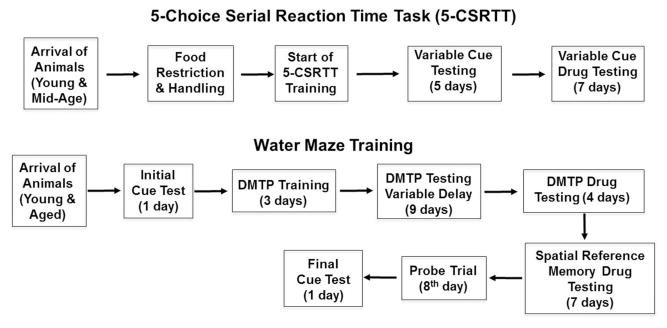
For study 1, involving the 5-choice serial reaction time task (5-CSRTT), young (3–6 months, n = 14) and middle-age (10–14 months, n = 23) male Fisher 344 rats were employed. For study 2, involving spatial memory on the water maze, young (4–6 months, n = 10) and aged (18–20 months, n = 32) male Fisher 344 rats were employed. Five aged animals were unable to complete training on the cue or spatial tasks and were not included in the analysis. Animals were obtained from the National Institute on Aging colony (Taconic) through the University of Florida Animal Care and Service facility. All procedures involving animal subjects were reviewed and approved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. The experimental timeline for behavioral characterization and drug testing is depicted in Figure 1.
Figure 1.
Schematic of the experimental Timeline. Young and middle-age animals were behaviorally tested on the 5-CSRTT which involved food restriction, training to respond to the light cue, training on a variable cue duration paradigm, and finally testing the effects of drug treatment on the variable cue duration paradigm. For the water maze, young and aged animals were initially tested on the cue discrimination version of the task. This was followed by training on a DMTP task, including training with a variable delay (1, 30, or 120 min) between trial 1 and trial 2. For drug testing on the DMTP task, animals were injected 30 min before trial 1. At the end of DMTP task, a subset of animals was assigned to receive MK-801 (0.05 mg/kg) or vehicle 30 min before training on a reference memory task. Injection and training continued for 7 days. This was followed by a probe trial on day 8, 30 min after injections. A final cue discrimination task was then introduced.
2.2 Behavioral Tests
2.2.1 5-Choice Serial Reaction Time Task (5-CSRTT)
Apparatus
Behavioral training of animals on 5-CSRTT was conducted as previously published (Guidi et al., 2015). Testing was conducted in two identical standard rat testing chambers (30.5 × 25.4 × 30.5 cm; Coulbourn Instruments, Allentown, PA) with metal front and back walls, and transparent Plexiglas sidewalls. Each testing chamber was housed in a sound-attenuating cubicle, illuminated by a 3W house light. Each cage was equipped with a recessed food pellet delivery trough, located approximately 2 cm above the grid floor, in the center of the back wall. Each trough was fitted with a photobeam sensor to detect head entries and food reward pellet retrieval (45 mg grain based; Test Diet, Richmond, IN). A 1.12W light was used to illuminate the food trough. Five evenly spaced circular apertures, each containing 6 tricolored cue LED lights and a photobeam sensor, were set into the front metal wall 2 cm above the grid floor. The behavioral experiments were controlled through a computer interfaced with the testing chambers, and equipped with Graphic State v3.03 software (Coulbourn Instruments, Whitehall, PA).
Training Procedures
Rats were food restricted to 85% of their free-feeding weight and were maintained at this weight throughout the behavioral experiments (Fig 1). Each rat was tested in the same chamber throughout the experiment. For the first phase of training, rats were placed in the conditioning chambers for 15 min with the house-light on and the food magazine was filled with 10 food pellets to familiarize the animals with the training box. In the second phase, rats received two 30 min food magazine training sessions, during which 100 food pellets were delivered using a variable time schedule (mean = 60s). In the third phase of training, all nose poke openings were accessible to the animal and were illuminated for the entire 30 min session. Each time the rat placed its nose into the illuminated hole, a food pellet was delivered to the animal. This training was continued until the rats made at least 50 nose pokes during a single session.
Once trained to elicit a nose poke response for a food reward, the rats were then trained to nose poke in response to a brief 30-second visual stimulus that was presented pseudorandomly in only one of the five possible locations. At the beginning of each training/test session, the cage house light was illuminated and a single food pellet was delivered to the magazine without requiring a nose poke. The first trial was initiated when the rat collected the food pellet from the food magazine. After a fixed delay (inter-trial interval, ITI = 5 sec), one of the 5 holes was illuminated for a given stimulus duration. The rat was rewarded with a food pellet if it made a response to the illuminated hole or a response in that particular hole during a fixed period of time after the light stimulus was turned off (the limited hole hold period = 5 sec). This response was recorded as a correct response. The next trial was initiated when the rat retrieved the food pellet. Responses in a non-illuminated hole during the signal period (i.e. the stimulus duration + the limited hole hold period) were recorded as incorrect responses and failures to respond within the limited hold period were recorded as omissions. These resulted in a 5 sec period of darkness (time-out). A response in any hole during the ITI was recorded as a premature response and was followed by a time-out period as well. After a time-out, the next trial was initiated when the rat placed its nose into the empty, lit food magazine. The daily testing sessions consisted either of 100 trials or was terminated after 30 min of testing. During a 100-trial session, the light stimulus was presented an equal number of times in each of the five aperture positions.
Animals progressed to training on the next, shorter cue duration interval once performance at the previous cue duration met criterion (50 completed trials for two consecutive training sessions). The cue duration intervals used for training included 0.25, 0.5, 2.5, 10, and 30 sec. Finally, rats were trained on the variable cue paradigm in which the optical stimuli were presented at various durations (0.25, 0.5, 2.5, 10, and 30s) during a single testing session. Animals were trained for 5 consecutive days on the variable cue paradigm and stable performance, with >50% choice accuracy was observed for 0.5 sec cue duration (Fig 2).
Figure 2.
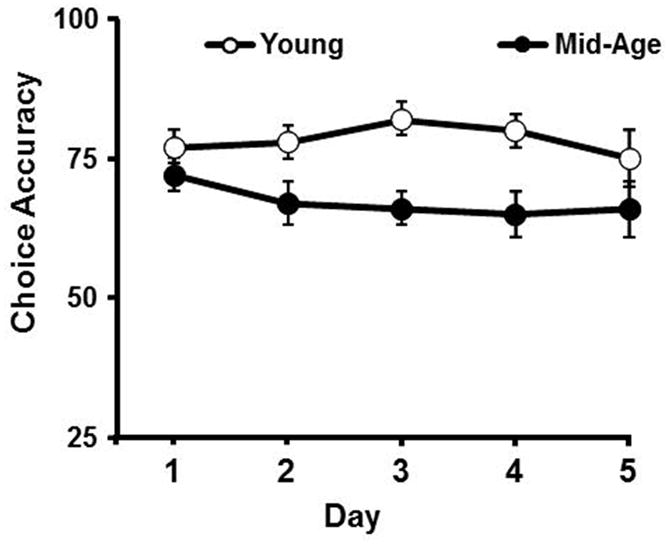
Stable choice accuracy was observed for the 0.5 sec cue duration during testing on the variable cue duration paradigm task. The data are presented for five consecutive days of testing prior to drug or vehicle treatment.
Following training on the variable cue paradigm, rats received a systemic intraperitoneal injection of MK-801 (0.025, 0.05, and 0.1 mg/kg) or vehicle 30 min prior to entering the operant conditioning chamber for testing on the variable cue paradigm. Injections occurred every other day, and a washout day was implemented in between each drug treatment session to ensure there was no lingering drug affects from the previous day. Injections were randomized using a within subject design, such that each animal received each MK-801 treatment over the course of eight days. The doses of MK-801 (0.025, 0.05, and 0.1 mg/kg) employed were found to have minimal effect on motor function (Wozniak et al., 1990; Carey et al., 1998).
Direct measures of attention were assessed in terms of choice accuracy in detecting the light stimulus, expressed as a percentage of the total number of correct and incorrect trials (correct responses/total number of correct and incorrect responses). The number of omissions was expressed as a percentage (number of omissions/total number of trials). Premature responses (number of premature responses/total number of trials) were used as an index of inhibitory response control. The latency to respond to the cue and latency to collect the reward were also recorded.
2.2.2 Water Maze
Apparatus
Methods for training of animals on the water maze task have previously been described (Foster, 2012; Foster et al., 2012; Kumar et al., 2012; Lee et al., 2012; Kumar and Foster, 2013; Guidi et al., 2014). Animals were trained in a black tank, 1.7 m in diameter, positioned in a well-lit room containing (when appropriate) an assortment of 2- and 3-dimensional cues. Water (27 ± 2° C) was maintained at a level approximately 8 cm below the surface of the tank. Across all water maze tasks, training and probe trials were limited to 60 sec. Behavioral data were acquired with Noldus EthoVision computer tracking software (Noldus Information Technology, Leesburg, VA) and included cumulative path-length during training trials and time in quadrant and platform (12 cm diameter) crossing during probe trials.
Cue Discrimination Training
Rats were first trained on the cue discrimination version of the water escape task (Fig 1). Animals were habituated to the pool by allowing for a 30 sec free swim and 4-guided attempts to climb onto the escape platform from the 4 different cardinal directions. The platform was extended approximately 1 cm above the water level and a white Styrofoam flag was attached. Training consisted of five blocks comprised of three trials with all training massed into one day. Inter-trial intervals were 30 sec and inter-block intervals were approximately 15 minutes. For each trial, a rat was placed in the water in one of four equally spaced start locations (N, S, E, and W) and was allowed 60 sec to escape onto the platform. If an animal did not escape the water maze within the allotted time, the rat was gently guided to the platform. Rats remained on the platform between trials. After each trial block, the rats were placed in home cages under warming fans in order to prevent hypothermia. The platform position and start locations were randomized and relocated prior to the start of each subsequent trial. The distance traveled to find the platform was recorded and averaged for each block. Rats that failed to find the visible platform at least two times during the last three trials were removed from the study and were not included in the analysis.
Delayed-Matching to Place (DMTP) Task
Three days following cue discrimination, animals were trained on a modified version of the DMTP task, which is sensitive to NMDAR function and aging (Means and Kennard, 1991; Steele and Morris, 1999; Bizon et al., 2009; Morris et al., 2013). The platform was lowered to just beneath the water surface and animals were trained to find a hidden platform (3 trials per day), using a different location each day. The start position was always distal from the platform. Rats remained on the platform for 20 sec and then were moved to a holding chamber for the inter-trial interval (ITI) delay. For the first 3 days of the task, a 30 sec ITI was imposed between the trials to acclimate the rats to the spatial working memory task procedures. During the nine days of testing, the ITI between the first trial (the information trial) and the second trial (retention trial) was varied between 1, 30, or 120 min, such that each delay occurred three times. Rats remained on the platform for 30 sec and then were placed in home cages under warm air during the delay. The ITI between the second and third trial was set at 1 min. The distance traveled to find the platform was recorded for each trial and a savings score was calculated for trials two and three as the difference between the initial acquisition trial and subsequent testing trials (e.g. distance trial 1 – distance trial 2). The savings scores were averaged for three days of testing and animals that failed to exhibit a decrease in swim distance for the 30 min ITI were removed.
Following the initial characterization, animals were tested for the effects of MK-801 on the DMTP task using the 1 and 30 min ITI between the information and retention trials. Animals were randomized using a within subject design, such that each animal received systemic intraperitoneal injections of MK-801 (0.05 mg/kg) or vehicle, 30 min prior to the first training trial each day. Thus, each animal was tested four times, with a trial 2 delay of 1 and 30 min following an injection of MK-801 or vehicle. The injection days were separated by two days. Again, savings scores were calculated and used for analysis.
Spatial Reference Memory Task
Following testing on the DMTP task, animals were randomized to receive eight days of injections of MK-801 (0.05 mg/kg) or vehicle 30 min prior to spatial reference memory training using standard techniques (Guidi et al., 2014). For this task, the platform remained in the same location across days of training. The animals received four trials per day with each trial separated by 30 sec. Training continued for seven days. The distance traveled to find the platform was recorded and averaged for each day. On day eight, animals were injected with MK801 or vehicle 30 min prior to a probe trial in which the platform was removed. The time, the animal spent searching the goal and the opposite quadrant was recorded, as well as the number of platform crossings.
2.3 Statistical Analysis
Analyses of variance (ANOVAs) for repeated measures were carried out using StatView 5.0 (SAS Institute Inc, NC) and used to determine significant main effects and interactions. Post hoc ANOVAs and Fisher’s protected least significant difference comparisons, with the p-value set at 0.05, were used to further localize differences. Finally, in order to confirm learning on the water maze, one group student t-tests were used to determine if savings scores and discrimination index scores were different from that expected by chance.
3. Results
3.1 Dose Dependent Biphasic Effect of MK-801 on Performace of the 5-CSRTT
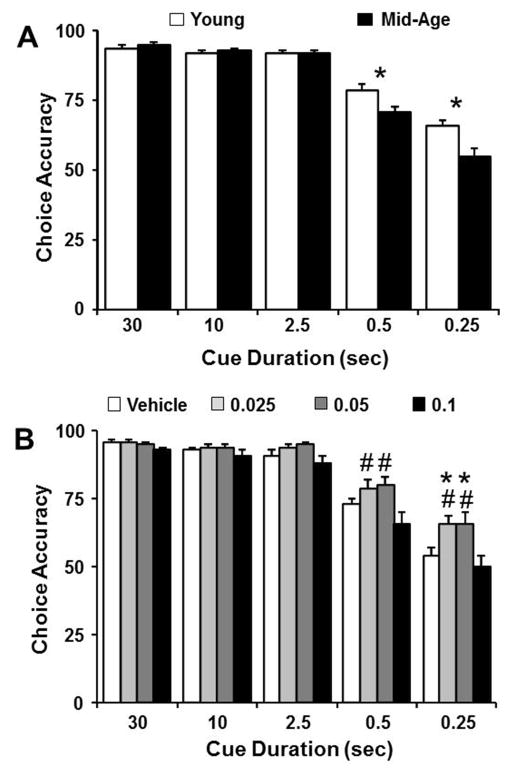
Following stable performance on the variable cue training (Fig 2), rats underwent a within-subject drug study, assessing the effects of systemic injections of MK-801 (0.025, 0.05, 0.1 mg/kg, and vehicle) on performance in the variable cue duration paradigm. A two-factor (age and drug dose) ANOVA, repeated across cue durations, performed on the choice accuracy indicated significant main effects of cue duration [F(4,560) = 196.25, p < 0.0001], age [F(1,140) = 4.74, p < 0.05], and MK-801 dose [F(3,140) = 6.72, p < 0.0005]. We confirmed a cue duration by age interaction [F(4,560) = 8.59, p < 0.0001] and post hoc tests collapsed across all drug doses indicated that the difference was primarily driven by better performance of young rats at the 0.5 and 0.25 sec cue durations (Fig. 3A). An interaction was also observed between cue duration and drug dose [F(4,560) = 2.43, p < 0.005]. Subsequent ANOVAs performed for each cue duration, and collapsed across age indicated that choice accuracy performance is significantly influenced by MK-801 for cue durations of 0.25 sec [F(3,140) = 5.86, p < 0.001] and 0.5 sec [F(3,140) = 4.33, p < 0.01] (Fig. 3B). Post hoc examination for these two cue durations revealed that the effect of drug dose on choice accuracy performance was largely due to a significant increase in accuracy for the two intermediate MK-801 doses compared to the 0.1 mg/kg dose. Additionally, the 0.025 and 0.05 mg/kg dose improved attentional performance at the 0.25 sec cue duration, relative to vehicle treatment. Interestingly, no age by drug dose interaction was observed for these biphasic effects.
Figure 3.
For short cue durations, choice accuracy is impaired in middle-age rats and influenced by MK-801 in a dose-dependent manner. The bars represent the mean (+SEM) choice accuracy for each cue duration. A) Data are collapsed across doses to illustrate that choice accuracy declines as cue duration decreases and the reduction in accuracy is greater for middle-age animals (filled bars), relative to young (open bars). B) Data are collapsed across age groups to illustrate the dose dependent effects of MK-801 (open = vehicle, light gray = 0.025, dark gray = 0.05, and filled = 0.1 mg/kg MK-801) on choice accuracy. Asterisks in A indicate an age difference (p <0.05). Asterisks and pound sign in B indicate a significant difference (p <0.05) relative to vehicle and the 0.1 mg/kg dose of MK-801, respectively.
The results indicate that biphasic effects were observed for shorter cue durations, which tax the attentional demands and for which age-related impairments were observed. In order to determine if the higher dose impaired performance, post hoc comparisons within each age group were performed by comparing choice accuracy for the vehicle and 0.1 mg/kg dose. A significant (p < 0.05) decrease in choice accuracy was observed only for middle-age animals at the longer cue durations of 30 sec (vehicle: 97 ± 1, 0.1 mg/kg MK-801: 92 ± 1.7) and 10 sec (vehicle: 96 ± 1, 0.1 mg/kg MK-801: 90 ± 2.3).
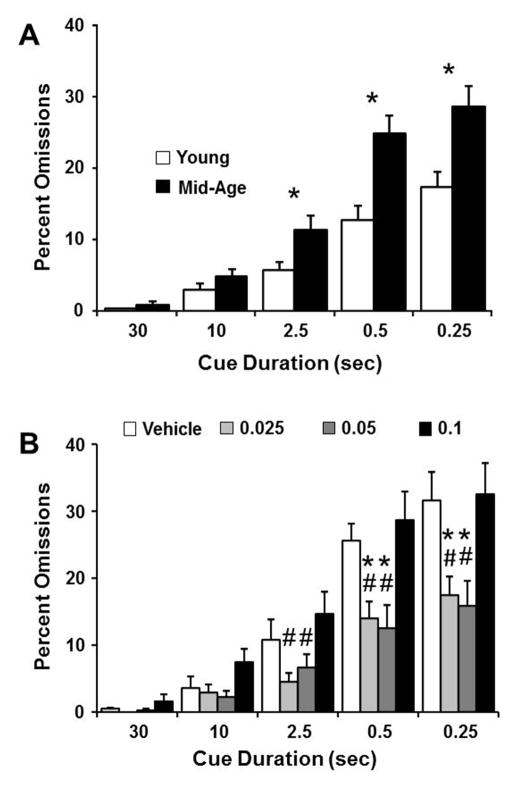
A similar pattern of results was obtained for measures of omissions (Fig. 4). A two-factor repeated measures ANOVA confirmed main effects of cue duration [F(4,560) = 98.37, p < 0.0001], age [F(1,140) = 8.81, p < 0.005], and dose of MK-801 [F(3,140) = 4.61, p < 0.005]. Moreover, a significant interaction was observed between cue duration and age [F(4,560) = 7.60, p < 0.0001]. Again, the age effect was primarily due to an increase in the percentage of omissions by middle-age rats for the lower cue durations and post hoc tests collapsed across all drug doses indicated better performance of young rats at the 2.5, 0.5, and 0.25 sec cue durations (Fig. 4A). A significant interaction was also detected between cue duration and MK-801 dose [F(12,560) = 3.61, p < 0.0001]. Subsequent ANOVAs performed for each cue duration, and collapsed across age, indicated that the percent omissions is influenced by the dose of MK-801 at 0.25 sec [F(3,144) = 5.18, p < 0.005], 0.5 sec [F(3,144) = 5.30, p < 0.005], 2.5 sec [F(3,144) = 3.15, p < 0.05]. Furthermore, post hoc examination revealed improved performance (decreased omissions) for the two intermediate doses, 0.025 and 0.05 mg/kg, relative to vehicle for the shortest cue durations (0.25 and 0.5 sec). Additionally, the percent omissions was decreased for the 0.025 and 0.05 mg/kg doses relative to the 0.1mg/kg dose at the 2.5, 0.5 and 0.25 sec cue durations. No age by dose interaction was observed for these cue durations. In order to determine if the higher dose impaired performance, post hoc comparisons within each age group were performed by comparing omissions for the vehicle and 0.1 mg/kg dose at each cue duration. A significant (p < 0.05) increase in the number of omissions was observed only for young animals at the 10 sec cue duration (vehicle: 1.7 ± 1, 0.1 mg/kg MK-801: 7.8 ± 2.2). The results indicate a biphasic effect of MK-801 on measures of attention with improved performance for lower doses at the shortest cue durations, where attentional deficits are observed. The higher dose reversed the benefits observed for lower doses and impairments for the highest dose of MK-801 were limited to longer cue durations that normally do not exhibit age-differences.
Figure 4.
The percent omissions is impaired in middle-age rats and influenced by MK-801 in a dose-dependent manner. The bars represent the mean (+SEM) percent omissions for each cue duration. A) The data are collapsed across drug doses to show that the percentage of omissions increases as the cue duration decreases for both age groups, and the increase is greater for middle-age (filled bars), relative to young rats (open bars). B) Data are collapsed across age groups to illustrate the dose dependent effects of MK-801 (open = vehicle, light gray = 0.025, dark gray = 0.05, and filled = 0.1 mg/kg MK-801) on percent omissions. Asterisks in A indicate an age difference (p <0.05). Asterisks and pound sign in B indicate a significant difference (p <0.05) relative to vehicle and the 0.1 mg/kg dose of MK-801, respectively.
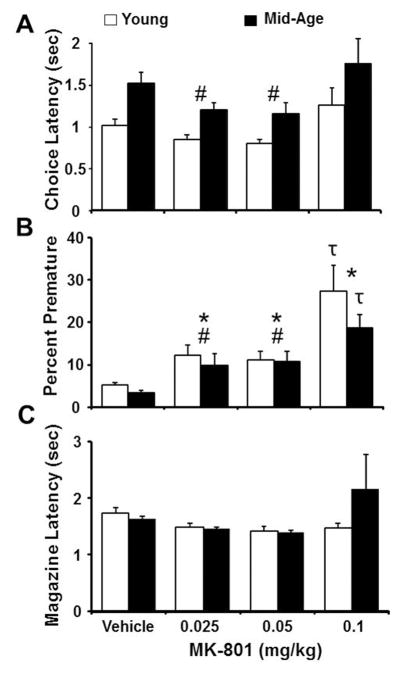
A two-factor ANOVA for choice latency indicated an effect of age [F(1,140) = 8.83, p < 0.005] and MK-801 dose [F(3,140) = 3.69, p < 0.05] in the absence of an interaction (Fig. 5A). The age effect for choice latency, collapsed across doses, was due to an increase in latency for middle-age animals (Young: 0.99 ± 0.06 sec, Middle-age: 1.41 ± 0.09 sec) consistent with previous reports (Guidi et al., 2015). For MK-801 effects, post hoc tests indicated that choice latency decreased for the two intermediate doses of MK-801 relative to the 0.1 mg/kg dose. Post hoc test of choice latency limited to for the vehicle and 0.1 mg/kg dose within each age group were not significant. An MK-801 dose effect [F(3,140) = 14.00, p < 0.0001], in the absence of an age difference or an age by dose interaction, was observed for premature responses, such that the percent premature responses increased as a function of drug dose (Fig. 5B). Post hoc test of premature responses limited to the vehicle and 0.1 mg/kg dose indicated a significant (p < 0.05) increase in premature responses for both age groups (Fig. 5B). In order to ensure that alterations in performance were not due to motor disturbances, we assessed the effect of MK-801 on magazine latency (Fig. 5C). No effect of age or drug dose was observed for this measure, indicating that MK-801, and the regimen of doses used, did not substantially influence the rodents’ ability to move between the two ends of the testing chamber. Finally, no age or dose effects were observed for the number of initiated trials.
Figure 5.
Effects of MK-801 on choice latency and premature responses measured during 5-CSRTT performance. The bars represent the mean (+SEM) for A) choice latency, B) percent premature responses, and C) magazine latency for middle-age (filled bars) and young rats (open bars). In general, middle-age rats exhibit an increase in choice latency relative to young; however, across age groups, choice latency was reduced by 0.025 and 0.05 mg/kg MK-801 relative to the 1.0 mg/kg dose. MK-801 also increased the percent of premature responses. In contrast, no drug effects were observed for the magazine latency. Pound signs and asterisks indicate a significant difference (p <0.05) relative to the 0.1 mg/kg dose of MK-801 and vehicle, respectively. The τ signs indicate a difference for the 0.1 mg/kg dose relative to the vehicle with in the separate age groups.
Examination of performance on the three washout days between each drug treatment session confirmed age and cue duration interactions for choice accuracy [F(4,196) = 9.43, 0.0001] and post hoc test indicated increased accuracy of young relative to middle-age for the 0.25 sec cue duration (Fig 6). No effect of previous drug treatment was observed.
Figure 6.
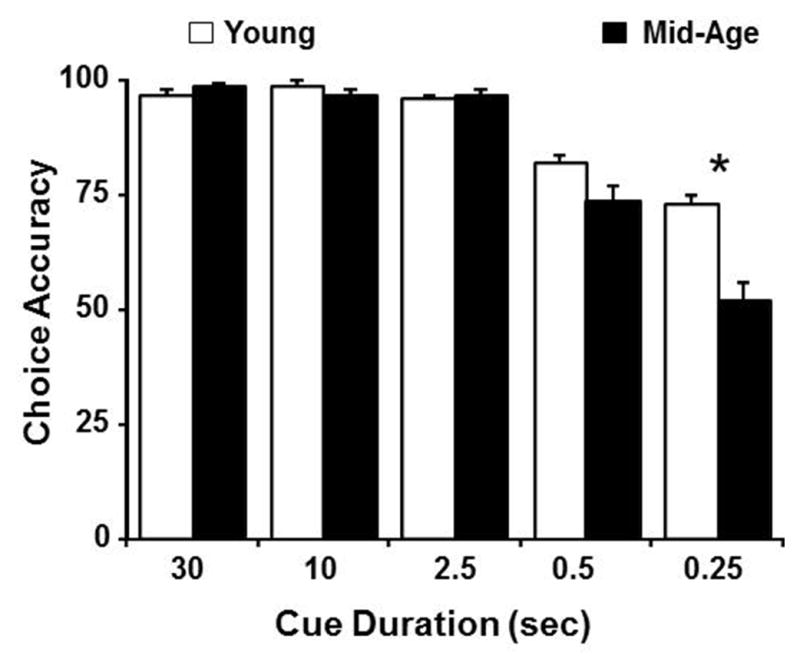
No effect of previous drug treatment was observed for choice accuracy performance examined during drug washout days. Data are collapsed across the three drug washout days and emphasizes that accuracy declines as cue duration decreases and the reduction in accuracy is greater for middle-age animals (filled bars) relative to young (open bars). Asterisk indicates an age difference (p < 0.05).
3.2 Low Dose of MK-801 Impairs Spatial Episodic Memory
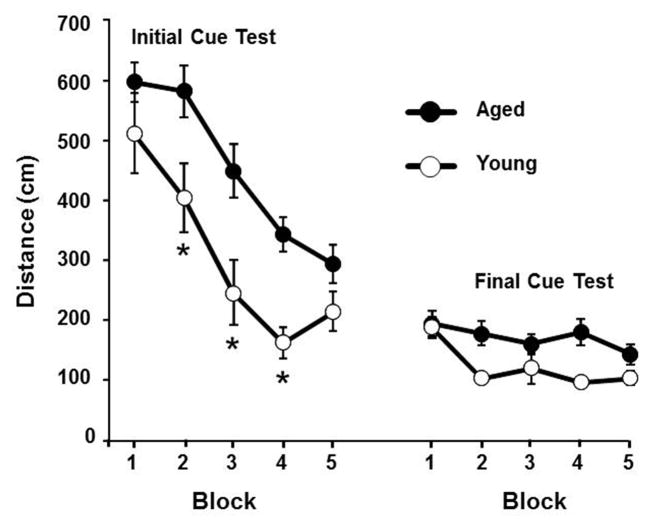
For examination of age and MK-801 effects on the spatial water maze, young and aged rats were first tested on the cue discrimination task. Following testing on the DMTP and reference memory versions of the tasks (see below), a second session of cue discrimination testing was conducted for a subset of animals (young = 7, aged = 24). For the initial training, a main effect of age [F(1,4) = 15.71, p < 0.0005] and training [F(4,140) = 18.71, p < 0.0001] was observed (p < 0.05) due to superior performance by young during training blocks 2–4 (Fig 7). However, no difference was observed for the initial training block or the final training block. For the final cue test, an effect of training [F(4,116) = 3.36, p < 0.05] was observed in the absence of an age effect. The results confirm that aged animals initially exhibit slower procedural learning during water maze training but, over the course of training, aged animals were able to acquire the procedural aspects of the task to the same extent as the young rats (Foster, 2012).
Figure 7.
Aging influences the rate of learning of the procedural aspects of the water maze. Mean (±SEM) escape distance for aged (filled circles) and young rats (open circles). Both groups exhibit a decrease in the distance to escape to a visible platform over five blocks of initial training on the cue discrimination task. Following testing on the DMTP task and reference memory task, animals were subsequently re-tested on the cue discrimination task. The age-related difference observed during the initial training was not evident following extensive water maze experience. Asterisks indicate an age difference (p < 0.05).
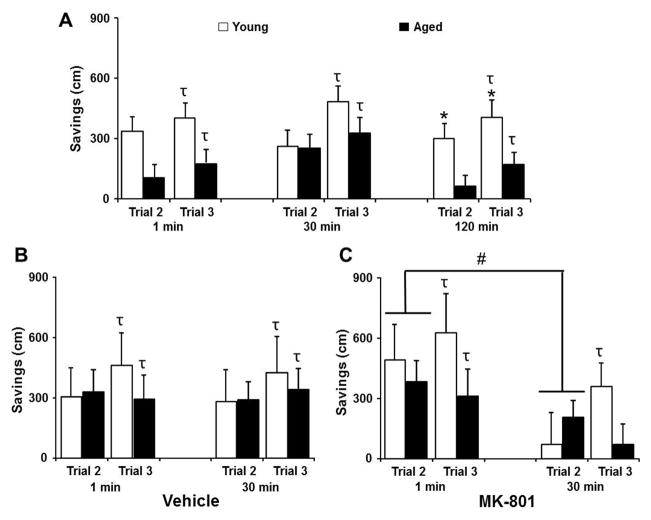
Three days following the initial cue discrimination test, animals were trained on the DMTP task. For each set of trial delays (1, 30, 120 min) following the acquisition trial (trial 1), savings scores were calculated for trials 2 and 3 relative to the acquisition trial, and the savings for each trial were averaged across 3 days of testing (Fig 8A). One group t-tests were employed to determine if performance on trial 3 was different from trial 1 (i.e. savings score different from 0). All groups exhibited learning across the three trials such that trial 3 savings scores were greater than 0. An ANOVA across saving scores for trials 2 and 3 confirmed an effect of training [F(1,108) = 11.23, p < 0.0001] due to increased savings across the short delay (1 min) between trials 2 and 3. In addition, an age difference [F(1,108) = 9.39, p < 0.005] was observed and post hoc comparisons indicated an age difference in savings scores for trial 2 [F(1, 105) = 5.25, p < 0.05] and trial 3 [F(1, 105) = 8.08, p < 0.01] due to increased savings for young animals. Previous work indicates that older animals exhibit increased forgetting of spatial episodic information, such that impairments in retention are observed for delays beyond 30 min (Means and Kennard, 1991; Mabry et al., 1996; Bizon et al., 2009). Post hoc comparisons for each delay time confirmed impaired retention with advanced age, such that an age difference in savings scores [F(1, 35) = 6.06, p < 0.05] was observed only for the 2 hr delay between trials 1 and 2. Similarly, comparisons of trial 3 savings scores for each delay time indicated an age difference [F(1, 35) = 4.33, p < 0.05] for the 2 hr delay.
Figure 8.
Effect of age and MK-801 on episodic spatial memory measured on the DMTP task. Bars represent the mean (+SEM) savings distance to escape to a hidden platform on trials 2 and 3 for aged (filled bars) and young rats (open bars). A) Savings scores for each trial were averaged over three days of training during the initial training sessions for each delay of 1, 30 or 120 min, which was inserted between trials 1 and 2. Age-related impairment was noted for the longest delay (120 min) between the acquisition trial and trial 2. B) No age or delay dependent effects were observed when vehicle was injected 30 min prior to trial 1. C) MK-801 (0.05 mg/kg) injected 30 min prior to trial 1 resulted in a decrease in savings scores on trial 2 when the delay was 30 min relative to a 1 min delay. Note across all conditions, a significance savings was observed for trial 3 except for aged animals treated with MK-801, with a 30 min delay. The τ signs indicate that trial 3 is different from trial 1 (i.e. savings score different from 0), asterisks in A indicate an age difference in savings scores, and the pound sign indicates a difference associated with the delay.
NMDAR antagonists disrupt retention of novel spatial information as retention intervals increase (Steele and Morris, 1999; Lee and Kesner, 2002; McDonald et al., 2005). Therefore, due to the age-related impairment in retention for the 120 min delay, only the 1 and 30 min time points were employed for examination of the effect of vehicle and MK-801 injections. For the vehicle treatment condition, t-tests comparing savings scores on trial 3 to chance, indicated that all groups learned for each delay condition. Indeed, an ANOVA across trials indicated no further improvement from trial 2 to trial 3, no effect of age, and no effect of trial delay (Fig 8B). Thus, in the vehicle condition, young and aged animals exhibited similar learning and memory for the 1 and 30 min delays. In contrast, an injection of MK-801 resulted in impaired learning for aged animals in the 30 min delay condition, such that this group did not exhibit significant savings on trial 3 (Fig 8C). An ANOVA indicated an interaction of age and training [F(1,70) = 7.68, p < 0.01] and a tendency for a trial delay effect (p = 0.05). ANOVAs within each age group to localize the age and training interaction, confirmed impairment in aged animals such that an increase in the savings scores was observed from trial 2 to trial 3 for young [(F(1,18) = 9.08, p < 0.01], but not for aged animals. Post hoc comparisons for trial 2 indicated a trial delay effect due to increased savings for trials with a 1 min delay, relative to the 30 min delay [(1, 70) = 4.89, p < 0.05) (Fig 8C), consistent with NMDAR involvement in the retention of novel spatial information (Steele and Morris, 1999; Lee and Kesner, 2002; McDonald et al., 2005). Thus, for both young and aged animals, MK-801 impaired retention on trial 2 as the delay was increased from 1 to 30 min. However, only young animals were able to utilize information from trial 2 to recover from this initial deficit, such that young animals exhibited improved performance on trial 3.
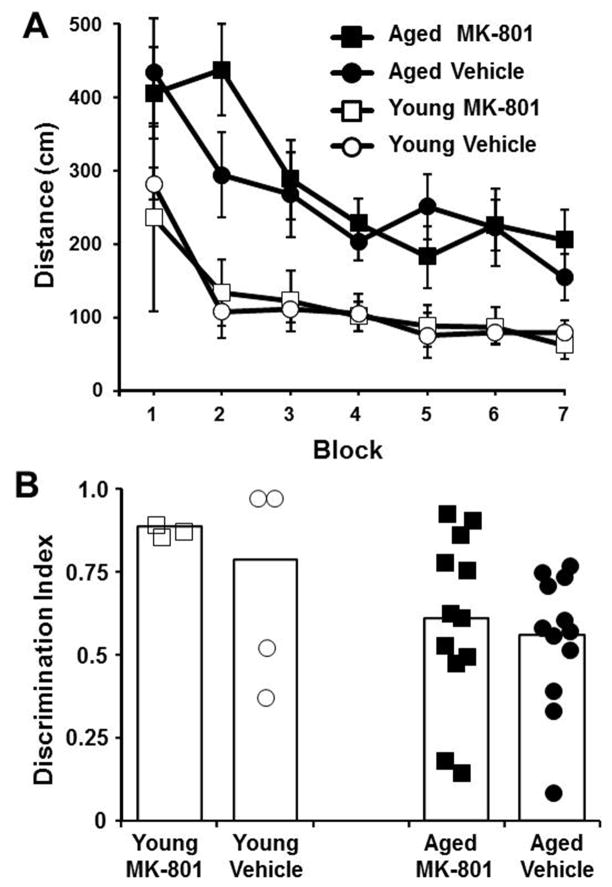
A subset of the animals were tested on acquisition of a spatial reference memory and were randomly assigned to receive MK-801 (0.05 mg/kg, Aged: n = 12, Young: n = 3) or vehicle (Aged: n = 12, Young: n = 4) 30 min before each day of training (Fig 9). A repeated measures ANOVA indicated an effect of age [F(1,27) = 11.43, p < 0.005] and training [F(6,162) = 5.59, p < 0.00001] in the absence of a drug treatment effect. An ANOVA for each day indicated that age effects were observed for days 2 and 4. We suspected that NMDAR blockade may have interacted with age to impair memory consolidation on day 2, following the initial acquisition on day 1. Previous work indicates age-related impairment of memory consolidation examined 24 hr after a single episode of water maze training (Aitken and Meaney, 1989; Foster et al., 1991; Norris and Foster, 1999; Foster et al., 2001; Driscoll et al., 2006; Foster and Kumar, 2007), and memory consolidation of novel spatial information is disrupted by NMDAR blockade (Kesner and Dakis, 1995; Holahan et al., 2005; McDonald et al., 2005). To determine whether NMDAR blockade may have contributed to an age-related impairment in consolidation, we examined age and drug effects for each day. In this case, the age difference was limited to MK-801 treated animals [(1,13) = 5.53, p < 0.05] on day 2, 24 hr after the initial training, consistent with the idea that NMDAR blockade contributed to a consolidation deficit in older animals.
Figure 9.
MK-801 does not impair spatial reference memory on the water maze. A) Mean path length (±SEM) for aged (filled symbols) and young rats (open symbols) treated with vehicle (circles) or MK-801 (squares) during seven days of training on the reference memory task. Age related difference in escape path length was observed in the absence of a drug effect. B) Discrimination index for a probe trial on day 8, 30 min after an injection of MK-801 or vehicle. The individual symbols represent the discrimination index scores for individual aged (filled symbols) and young rats (open symbols) treated with vehicle (circles) or MK-801 (squares).
A retention probe trial was delivered on day 8, 30 min after injection of MK-801 or vehicle. One group t-tests indicated that all groups exhibited a discrimination index above chance indicating that all groups acquired a spatial search strategy. Despite performing above chance, an age difference [F(1,26) = 5.88, p < 0.05] in the discrimination index was observed in the absence of an effect of drug treatment (Fig 9B). No effect of age or drug treatment was observed for the number of platform crossings (young vehicle = 5.0 ± 0.4, young MK-801 = 4.7 ± 2.0, aged vehicle = 3.0 ± 0.4, aged MK-801 = 4.0 ± 0.6).
4. Discussion
Neuronal survival and excitotoxicity depend on the level of NMDAR activity. Currently, the NMDAR channel blocker, memantine, is employed as a therapeutic treatment for cognitive decline associated with aging and Alzheimer’s disease. However, memantine has had mixed success in treating the symptoms of dementia and the progression of the disease (Schneider et al., 2011). The benefits or impairments associated with memantine treatment may relate to its low affinity for NMDARs and interaction with other transmitter systems. Furthermore, NMDAR activity is important for normal cognition. A decline in NMDAR function is associated with the emergence of cognitive impairment (Kumar and Foster, 2013; Guidi et al., 2015), suggesting that NMDAR blockade could disproportionately impair cognition during aging. To address the role of NMDARs in age-related cognitive decline, the current study examined the effects of a specific and high affinity NMDAR channel blocker, MK-801, on episodic memory and attention, cognitive processes that decline with advanced age and in Alzheimer’s disease.
4.1 NMDAR Involvement in Attention during Aging
We confirmed an age-dependent impairment in attention on the variable cue duration version of the 5-CSRTT. The deficits were limited to the shorter cue durations, when attentional load is increased (Jones et al., 1995; Muir et al., 1999; Harati et al., 2011; Guidi et al., 2015). In addition, we found a biphasic dose response curve for MK-801 effects on attention, with low doses of MK-801 improving choice accuracy and reducing omissions. The biphasic influence of MK-801 is consistent with previous work, which indicates that a high dose of MK-801 (≥ 0.1 mg/kg) impaired performance on the 5-CSRTT and low doses of MK-801 (≤ 0.05 mg/kg) or a GluN2B subunit selective antagonist can produce modest benefits, increasing correct choices and reducing omissions (Higgins et al., 2005; Smith et al., 2011; Pehrson et al., 2013; Benn and Robinson, 2014).
The beneficial effects observed for the high affinity NMDAR specific antagonist, MK-801, may be relevant to understanding the mechanisms for beneficial effects of memantine, which exhibits less specificity and low affinity for the NMDAR. Memantine has been reported to increase attention and executive function in postmenopausal women (Wroolie et al., 2009), elderly humans with memory complaints (Ferris et al., 2007), and people with probable Alzheimer’s disease (Riepe et al., 2007; Nakamura et al., 2014). It has been suggested that the beneficial effects may be related to the ability of memantine to act on other receptor systems including serotonin, nicotine, and dopamine receptors (Rammes et al., 2001; Aracava et al., 2005; Seeman et al., 2008). Indeed, for the typical dose of memantine (20 mg/day), the level of drug in the cerebrospinal fluid is considerably below the IC50 for blocking NMDARs (Kornhuber and Quack, 1995; Frankiewicz et al., 1996). Importantly, the current finding that low doses of MK-801, a high affinity and specific NMDAR antagonist, improved attention suggests that memantine effects on attention are likely due to NMDAR blockade.
It is possible that the increase in performance on the 5-CSRTT for the low MK-801 doses was due to activation of the PFC following NMDAR blockade in other brain regions that are more sensitive to NMDAR blockade. The 0.05 mg/kg dose which improved 5-CSRTT performance, impaired hippocampal-dependent episodic memory. Similarly, in most cases in which memantine was reported to improve attention and executive function, the drug either had little effect on memory or impaired learning and memory (Rammsayer, 2001; Ferris et al., 2007; Riepe et al., 2007; Wroolie et al., 2009; Nakamura et al., 2014). Previous work suggests that impaired hippocampal function or a decrease in hippocampal activation can result in recruitment of the PFC (Cabeza et al., 2004; Zelikowsky et al., 2013). Moreover, infusion of MK-801 into the hippocampus increases the activity of pyramidal cells in the PFC (Jodo et al., 2005). Thus, for low doses of MK-801, PFC functions may have been enhanced as compensation for or as a direct result of disruption of NMDARs in the hippocampus or other brain regions.
Alternatively, the biphasic response may have been due to differential effects on interneurons and pyramidal cells in the PFC. In this case, the most active neurons may be more readily influenced by NMDAR channel blockers such that channel blockers initially act on the fast spiking interneurons, reducing inhibitory circuits. This idea is consistent with observed differences in NMDAR function between interneurons and pyramidal cells of the PFC and work demonstrating that NMDAR blockade results in a decrease in inhibition (Jackson et al., 2004; Homayoun and Moghaddam, 2007; Xi et al., 2009; Povysheva and Johnson, 2012). Importantly, the age-related impairment in attention is thought to result from a decline in NMDAR function on dendritic spines on PFC pyramidal cells (Bloss et al., 2011; Morrison and Baxter, 2012; Guidi et al., 2015). Thus, if MK-801 is initially acting on interneurons to improve attention, and NMDAR function of interneurons is not altered with age, this could explain the finding that the beneficial effects of MK-801 were not age specific and were prominent in both age groups as attentional demand increased.
In contrast to the lower doses of MK-801, the highest dose of MK-801 impaired measures of attention and abolished the benefits observed for the lower doses. There are several reasons to think that the effect of MK-801 may represent an interaction with an age-related NMDAR hypofunction of principal neurons. First, it is important to note that the reversal of beneficial effects was mainly observed for the short duration cues where age-related deficits were also prevalent. Indeed, the effect of the highest dose for the short duration cues mimicked aging effects; decreasing choice accuracy, increasing omissions, and increasing choice latency. However, for the shorter cue durations, no age difference in the MK-801 mediated impairment was observed, suggesting possible floor effects. In this case, the age-related decline in NMDAR function could mediate impairment in attention, such that a further decrease in NMDAR function following antagonist treatment cannot further impair behavior. In contrast, age-relevant impairment for the highest MK-801 dose was observed for the longer cue durations, where performance was asymptotic and baseline age differences were not evident. For example, we confirm that no age difference is observed for the longest cue durations; however, middle-age animals were more sensitive to NMDAR disruption such that the highest MK-801 dose impaired choice accuracy in middle-age animals for these long duration cues.
The detrimental effects of NMDAR antagonists on attention provide a basis for the idea that NMDAR hypofunction in the PFC contributes to neuropsychological problems involving attention and executive function, including age-related impairments (Kotecha et al., 2002; Turic et al., 2004; Gaspar et al., 2009; Guidi et al., 2015). As noted above, the age-related impairment in attention is thought to result from NMDAR hypofunction of PFC pyramidal cells (Bloss et al., 2011; Morrison and Baxter, 2012; Guidi et al., 2015). There are several reasons to believe that the impairments observed for the higher MK-801 dose are mediated by blockade of NMDARs on pyramidal cells in the PFC. The increase in omissions and premature responses, observed for the highest dose of MK-801, is consistent with previous research indicating that direct infusion of MK-801 into PFC increases omissions (Pehrson et al., 2013) and premature responses (Benn and Robinson, 2014), suggesting that the highest dose of MK-801 was influencing PCF neurons. In contrast to benefit observed for the low affinity antagonist, memantine, impaired executive function and altered activity of PFC pyramidal neurons is routinely observed following treatment with high affinity NMDAR antagonists (Honey et al., 2003; Gouzoulis-Mayfrank et al., 2005; Homayoun and Moghaddam, 2007; Wang et al., 2013; Molina et al., 2014), suggesting that greater NMDAR antagonism influenced PFC pyramidal neurons. In fact, behavioral deficits are observed when the dose of memantine is increased to a level that is neuroprotective against NMDA induced neurotoxicity (Creeley et al., 2006), indicating that significant NMDAR blockade, even by a low affinity antagonist, impairs cognition. Together, the results indicate that antagonism of NMDARs located on pyramidal cell in the PFC results in impaired attention. The results are consistent with the idea that NMDAR hypofunction of PFC principal cells contributes to age-related impairment in attention (Guidi et al., 2015).
4.2. NMDAR Involvement in Episodic Memory During Aging
NMDAR channel blockers are employed to delay cognitive decline associated with Alzheimer’s disease. However, an early symptom of cognitive aging and Alzheimer’s disease is impaired episodic memory. Our previous work indicates that an age-related impairment in spatial episodic memory is linked to a decline in hippocampal NMDAR synaptic function (Foster, 2012; Kumar and Foster, 2013; Lee et al., 2014). In the current study, we confirmed that aged rats exhibit increased forgetting of episodic spatial information, observed as impairments on the retention trial for delays beyond 30 min (Means and Kennard, 1991; Mabry et al., 1996; Bizon et al., 2009).
Similar to effects of aging, we observed that NMDAR blockade impaired the retention of spatial episodic memory. In the current study, pre-treatment with a low dose of MK-801 (0.05 mg/kg) impaired retention on trial 2, as the delay increased from 1 to 30 min, regardless of age. The results support work that suggest NMDAR antagonists, delivered prior to acquisition, disrupt the retention of novel spatial information as retention intervals increase (Steele and Morris, 1999; Lee and Kesner, 2002; McDonald et al., 2005). Thus, both aging and NMDAR blockade are associated with impaired retention/consolidation of novel, rapidly acquired spatial information.
Due to the decline in NMDAR function with age, we hypothesized that MK-801 would impair behavior to a greater extent in older animals. Despite impaired retention over a 30 min interval, young animals treated with MK-801 were able to exhibit improved performance between trial 2 and 3. In contrast, aged animals did not exhibit an improvement and no savings was observed, indicating that aged animals were more susceptible to impairment following NMDAR blockade, possibly due to an initial NMDAR hypofunction. The increased susceptibility to NMDAR blockade in aged animals is also supported by the reference memory task in which aged-MK-801 treated animals exhibited impaired memory consolidation on day 2, 24 hr after the initial training episode.
Interestingly, while most aged animals exhibited impaired retention of an episodic spatial memory, observed as a lack of savings for the 120 min delay, all aged animals exhibited discrimination scores above chance for the reference memory task. The work supports the idea that the impairment of acquisition and retention of novel information is a more sensitive measure of age-related cognitive decline, which emerges earlier, and the proportion of animals with impaired episodic memory increases with advancing age (Foster, 2012; Kumar and Foster, 2013). Previous work in mutant mice with selective knockout of hippocampal NMDARs indicate NMDAR involvement in novel spatial memory acquired during a single episode, but not in the acquisition or recall of spatial reference memory acquired during distributed training (Nakazawa et al., 2003; McHugh et al., 2007; Bannerman et al., 2008; von Engelhardt et al., 2008). Certainly, any evidence for a drug effect on reference memory was limited to the second day of training for aged animals, again pointing to a possible influence of NMDAR antagonism interacting with an age-related NMDAR hypofunction to impair the acquisition or consolidation of novel spatial information. Indeed, in the current study, MK-801 impaired one trial episodic memory, but all groups acquired a spatial reference memory, supporting the idea for differential involvement of NMDARs in episodic and reference memory. The absence of a deficit in reference memory supports the idea that age-related impairment in the incremental acquisition of a reference memory represents a more serious progression of cognitive deficits, which is observed in a subset of the oldest animals, and is due to mechanisms other than or in addition to the decline in NMDAR function (Foster, 2012; Foster et al., 2012).
In conclusion, low doses of MK-801 improved measures of attention on the 5-CSRTT for both age groups and the highest doses (0.1 mg/kg) mimicked age effects, decreasing choice accuracy and increasing omissions. Our results for hippocampal-dependent spatial memory confirm that NMDARs are involved in spatial episodic memory, but are not essential for spatial reference memory. Aged animals were more susceptible to MK-801 mediated disruption on the DMTP task consistent with the idea that the decline in NMDAR function in the hippocampus contributes to the age-related impairment in episodic memory.
Highlights.
Lower doses of MK-enhanced attention under conditions that were age-sensitive.
Beneficial effects on attention were reversed by the highest dose of MK-801.
MK-801 produced delay-dependent impairment of spatial episodic memory.
MK-801 had minimal effects on spatial reference memory.
Aged rats were more susceptible to MK-801 mediated disruption of episodic memory.
Acknowledgments
Financial support by National Institutes of Aging Grant R01AG037984 and R37AG036800, and the Evelyn F. McKnight Brain Research Foundation is highly appreciated. Thanks to Dr. Jennifer Bizon for discussions about the behavioral tasks.
Footnotes
Authors do not have any competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aenlle KK, Foster TC. Aging alters the expression of genes for neuroprotection and synaptic function following acute estradiol treatment. Hippocampus. 2010;20:1047–1060. doi: 10.1002/hipo.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken DH, Meaney MJ. Temporally graded, age-related impairments in spatial memory in the rat. Neurobiology of aging. 1989;10:273–276. doi: 10.1016/0197-4580(89)90062-6. [DOI] [PubMed] [Google Scholar]
- Albert MS. Changes in cognition. Neurobiology of aging. 2011;32(Suppl 1):S58–63. doi: 10.1016/j.neurobiolaging.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Ryan L, Bowers D, Foster TC, Bizon JL, Geldmacher DS, Glisky EL. Characterizing cognitive aging in humans with links to animal models. Front Aging Neurosci. 2012;4:21. doi: 10.3389/fnagi.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. The Journal of pharmacology and experimental therapeutics. 2005;312:1195–1205. doi: 10.1124/jpet.104.077172. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, Sanderson DJ, Cottam J, Sprengel R, Seeburg PH, Kohr G, Rawlins JN. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn A, Robinson ES. Investigating glutamatergic mechanism in attention and impulse control using rats in a modified 5-choice serial reaction time task. PloS one. 2014;9:e115374. doi: 10.1371/journal.pone.0115374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiology of aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Dai H, Gui J. Effects of dizocilpine (MK-801) on motor activity and memory. Psychopharmacology. 1998;137:241–246. doi: 10.1007/s002130050616. [DOI] [PubMed] [Google Scholar]
- Creeley C, Wozniak DF, Labruyere J, Taylor GT, Olney JW. Low doses of memantine disrupt memory in adult rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:3923–3932. doi: 10.1523/JNEUROSCI.4883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Annals of neurology. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Dewachter I, Filipkowski RK, Priller C, Ris L, Neyton J, Croes S, Terwel D, Gysemans M, Devijver H, Borghgraef P, Godaux E, Kaczmarek L, Herms J, Van Leuven F. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiology of aging. 2009;30:241–256. doi: 10.1016/j.neurobiolaging.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: a multi-level analysis in the rat. Neuroscience. 2006;139:1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Ferris S, Schneider L, Farmer M, Kay G, Crook T. A double-blind, placebo-controlled trial of memantine in age-associated memory impairment (memantine in AAMI) International journal of geriatric psychiatry. 2007;22:448–455. doi: 10.1002/gps.1711. [DOI] [PubMed] [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca(2)(+) channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96:283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem. 2007;87:522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Defazio RA, Bizon JL. Characterizing cognitive aging of spatial and contextual memory in animal models. Front Aging Neurosci. 2012;4:12. doi: 10.3389/fnagi.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Barnes CA, Rao G, McNaughton BL. Increase in perforant path quantal size in aged F-344 rats. Neurobiology of aging. 1991;12:441–448. doi: 10.1016/0197-4580(91)90071-q. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankiewicz T, Potier B, Bashir ZI, Collingridge GL, Parsons CG. Effects of memantine and MK-801 on NMDA-induced currents in cultured neurones and on synaptic transmission and LTP in area CA1 of rat hippocampal slices. British journal of pharmacology. 1996;117:689–697. doi: 10.1111/j.1476-5381.1996.tb15245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar PA, Bustamante ML, Silva H, Aboitiz F. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. Journal of neurochemistry. 2009;111:891–900. doi: 10.1111/j.1471-4159.2009.06325.x. [DOI] [PubMed] [Google Scholar]
- Gleichmann M, Zhang Y, Wood WH, 3rd, Becker KG, Mughal MR, Pazin MJ, van Praag H, Kobilo T, Zonderman AB, Troncoso JC, Markesbery WR, Mattson MP. Molecular changes in brain aging and Alzheimer’s disease are mirrored in experimentally silenced cortical neuron networks. Neurobiology of aging. 2012;33:205 e201–218. doi: 10.1016/j.neurobiolaging.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar KA. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- Guidi M, Kumar A, Foster TC. Impaired attention and synaptic senescence of the prefrontal cortex involves redox regulation of NMDA receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:3966–3977. doi: 10.1523/JNEUROSCI.3523-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi M, Kumar A, Rani A, Foster TC. Assessing the emergence and reliability of cognitive decline over the life span in Fisher 344 rats using the spatial water maze. Front Aging Neurosci. 2014;6:2. doi: 10.3389/fnagi.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harati H, Majchrzak M, Cosquer B, Galani R, Kelche C, Cassel JC, Barbelivien A. Attention and memory in aged rats: Impact of lifelong environmental enrichment. Neurobiology of aging. 2011;32:718–736. doi: 10.1016/j.neurobiolaging.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nature reviews Neuroscience. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Enderlin M, Haman M, Kemp JA. Evidence for improved performance in cognitive tasks following selective NR2B NMDA receptor antagonist pre-treatment in the rat. Psychopharmacology. 2005;179:85–98. doi: 10.1007/s00213-005-2203-9. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Taverna FA, Emrich SM, Louis M, Muller RU, Roder JC, McDonald RJ. Impairment in long-term retention but not short-term performance on a water maze reversal task following hippocampal or mediodorsal striatal N-methyl-D-aspartate receptor blockade. Behav Neurosci. 2005;119:1563–1571. doi: 10.1037/0735-7044.119.6.1563. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey RA, Turner DC, Honey GD, Sharar SR, Kumaran D, Pomarol-Clotet E, McKenna P, Sahakian BJ, Robbins TW, Fletcher PC. Subdissociative dose ketamine produces a deficit in manipulation but not maintenance of the contents of working memory. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28:2037–2044. doi: 10.1038/sj.npp.1300272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Garofalo P, Spangler EL, Mantione CR, Odano I, London ED. Reduced density of NMDA receptors and increased sensitivity to dizocilpine-induced learning impairment in aged rats. Brain Res. 1992;580:273–280. doi: 10.1016/0006-8993(92)90954-8. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Suzuki Y, Katayama T, Hoshino KY, Takeuchi S, Niwa S, Kayama Y. Activation of medial prefrontal cortex by phencyclidine is mediated via a hippocampo-prefrontal pathway. Cerebral cortex. 2005;15:663–669. doi: 10.1093/cercor/bhh168. [DOI] [PubMed] [Google Scholar]
- Jones DN, Barnes JC, Kirkby DL, Higgins GA. Age-associated impairments in a test of attention: evidence for involvement of cholinergic systems. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:7282–7292. doi: 10.1523/JNEUROSCI.15-11-07282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Dakis M. Phencyclidine injections into the dorsal hippocampus disrupt long- but not short-term memory within a spatial learning task. Psychopharmacology. 1995;120:203–208. doi: 10.1007/BF02246194. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Quack G. Cerebrospinal fluid and serum concentrations of the N-methyl-D-aspartate (NMDA) receptor antagonist memantine in man. Neuroscience letters. 1995;195:137–139. doi: 10.1016/0304-3940(95)11785-u. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–1122. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Linking redox regulation of NMDAR synaptic function to cognitive decline during aging. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:15710–15715. doi: 10.1523/JNEUROSCI.2176-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiology of aging. 2012;33:828 e821–817. doi: 10.1016/j.neurobiolaging.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nature neuroscience. 2002;5:162–168. doi: 10.1038/nn790. [DOI] [PubMed] [Google Scholar]
- Lee WH, Kumar A, Rani A, Foster TC. Role of antioxidant enzymes in redox regulation of N-methyl-D-aspartate receptor function and memory in middle-aged rats. Neurobiology of aging. 2014;35:1459–1468. doi: 10.1016/j.neurobiolaging.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Kumar A, Rani A, Herrera J, Xu J, Someya S, Foster TC. Influence of viral vector-mediated delivery of superoxide dismutase and catalase to the hippocampus on spatial learning and memory during aging. Antioxid Redox Signal. 2012;16:339–350. doi: 10.1089/ars.2011.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nature reviews Drug discovery. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Mabry TR, McCarty R, Gold PE, Foster TC. Age and stress history effects on spatial performance in a swim task in Fischer-344 rats. Neurobiol Learn Mem. 1996;66:1–10. doi: 10.1006/nlme.1996.0038. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Hong NS, Craig LA, Holahan MR, Louis M, Muller RU. NMDA-receptor blockade by CPP impairs post-training consolidation of a rapidly acquired spatial representation in rat hippocampus. Eur J Neurosci. 2005;22:1201–1213. doi: 10.1111/j.1460-9568.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Means LW, Kennard KJ. Working memory and the aged rat: deficient two-choice win-stay water-escape acquisition and retention. Physiol Behav. 1991;49:301–307. doi: 10.1016/0031-9384(91)90047-r. [DOI] [PubMed] [Google Scholar]
- Molina LA, Skelin I, Gruber AJ. Acute NMDA receptor antagonism disrupts synchronization of action potential firing in rat prefrontal cortex. PloS one. 2014;9:e85842. doi: 10.1371/journal.pone.0085842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Steele RJ, Bell JE, Martin SJ. N-methyl-d-aspartate receptors, learning and memory: chronic intraventricular infusion of the NMDA receptor antagonist d-AP5 interacts directly with the neural mechanisms of spatial learning. Eur J Neurosci. 2013;37:700–717. doi: 10.1111/ejn.12086. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nature reviews Neuroscience. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Fischer W, Bjorklund A. Decline in visual attention and spatial memory in aged rats. Neurobiology of aging. 1999;20:605–615. doi: 10.1016/s0197-4580(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kitamura S, Homma A, Shiosakai K, Matsui D. Efficacy and safety of memantine in patients with moderate-to-severe Alzheimer’s disease: results of a pooled analysis of two randomized, double-blind, placebo-controlled trials in Japan. Expert opinion on pharmacotherapy. 2014;15:913–925. doi: 10.1517/14656566.2014.902446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Norris CM, Foster TC. MK-801 improves retention in aged rats: implications for altered neural plasticity in age-related memory deficits. Neurobiol Learn Mem. 1999;71:194–206. doi: 10.1006/nlme.1998.3864. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nature neuroscience. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Bondi CO, Totah NK, Moghaddam B. The influence of NMDA and GABA(A) receptors and glutamic acid decarboxylase (GAD) activity on attention. Psychopharmacology. 2013;225:31–39. doi: 10.1007/s00213-012-2792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povysheva NV, Johnson JW. Tonic NMDA receptor-mediated current in prefrontal cortical pyramidal cells and fast-spiking interneurons. Journal of neurophysiology. 2012;107:2232–2243. doi: 10.1152/jn.01017.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neuroscience letters. 2001;306:81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Effects of pharmacologically induced changes in NMDA-receptor activity on long-term memory in humans. Learning & memory. 2001;8:20–25. doi: 10.1101/lm.33701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepe MW, Adler G, Ibach B, Weinkauf B, Tracik F, Gunay I. Domain-specific improvement of cognition on memantine in patients with Alzheimer’s disease treated with rivastigmine. Dementia and geriatric cognitive disorders. 2007;23:301–306. doi: 10.1159/000100875. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS drug reviews. 2003;9:275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Dagerman KS, Higgins JP, McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Archives of neurology. 2011;68:991–998. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- Seeman P, Caruso C, Lasaga M. Memantine agonist action at dopamine D2 High receptors. Synapse. 2008;62:149–153. doi: 10.1002/syn.20472. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature medicine. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, Malik N, Tricklebank M. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology. 2011;217:255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nature neuroscience. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Annals of neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Turic D, Langley K, Mills S, Stephens M, Lawson D, Govan C, Williams N, Van Den Bree M, Craddock N, Kent L, Owen M, O’Donovan M, Thapar A. Follow-up of genetic linkage findings on chromosome 16p13: evidence of association of N-methyl-D aspartate glutamate receptor 2A gene polymorphism with ADHD. Molecular psychiatry. 2004;9:169–173. doi: 10.1038/sj.mp.4001387. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Doganci B, Jensen V, Hvalby O, Gongrich C, Taylor A, Barkus C, Sanderson DJ, Rawlins JN, Seeburg PH, Bannerman DM, Monyer H. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron. 2008;60:846–860. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Olney JW, Kettinger L, 3rd, Price M, Miller JP. Behavioral effects of MK-801 in the rat. Psychopharmacology. 1990;101:47–56. doi: 10.1007/BF02253717. [DOI] [PubMed] [Google Scholar]
- Wroolie TE, Kenna HA, Williams KE, Powers BN, Holcomb M, Lazzeroni L, Rasgon NL. Cognitive effects of memantine in postmenopausal women at risk of dementia: a pilot study. Acta neurologica Scandinavica. 2009;119:172–179. doi: 10.1111/j.1600-0404.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- Xi D, Zhang W, Wang HX, Stradtman GG, Gao WJ. Dizocilpine (MK-801) induces distinct changes of N-methyl-D-aspartic acid receptor subunits in parvalbumin-containing interneurons in young adult rat prefrontal cortex. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2009;12:1395–1408. doi: 10.1017/S146114570900042X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9938–9943. doi: 10.1073/pnas.1301691110. [DOI] [PMC free article] [PubMed] [Google Scholar]