Abstract
Hyperoxia is still broadly used in clinical practice in order to assure organ oxygenation in critically ill patients, albeit known toxic effects. In this present study, we hypothesize that lysophosphatidic acid (LPA) mediates NKT cell activation in a mouse model of hyperoxic lung injury. In vitro, pulmonary NKT cells were exposed to hyperoxia for 72 h, and the induction of the ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP-2) was examined and production of lysophosphatidic acid (LPA) was measured. In vivo, animals were exposed to 100 % oxygen for 72 h and lungs and serum were harvested. Pulmonary NKT cells were then incubated with the LPA antagonist Brp-LPA. Animals received BrP-LPA prior to oxygen exposure. Autotaxin (ATX, ENPP-2) was significantly up-regulated on pulmonary NKT cells after hyperoxia (p < 0.01) in vitro. LPA levels were increased in supernatants of hyperoxia-exposed pulmonary NKT cells. LPA levels were significantly reduced by incubating NKT cells with LPA-BrP during oxygen exposure (p < 0,05) in vitro. Hyperoxia-exposed animals showed significantly increased serum levels of LPA (p ≤ 0,05) as well as increased pulmonary NKT cell numbers in vivo. BrP-LPA injection significantly improved survival as well as significantly decreased lung injury and lowered pulmonary NKT cell numbers. We conclude that NKT cell-induced hyperoxic lung injury is mediated by pro-inflammatory LPA generation, at least in part, secondary to ENPP-2 up-regulation on pulmonary NKT cells. Being a potent LPA antagonist, BrP-LPA prevents hyperoxia-induced lung injury in vitro and in vivo.
Keywords: Oxygen, Immune response, Pulmonary inflammation
Introduction
Invariant natural killer T (iNKT) cells, a distinct lymphocyte lineage expressing semi-invariant T cell receptors, bridge innate and adaptive immune responses by recognizing lipid antigens that are presented by the non-classical Major Histocompatibility Complex molecule CD1d. NKT cells seem to regulate their broad range of immune responses by reacting to so called self-antigens such as lysophosphatidic acid (LPA) presented by CD1d antigen presenting cells (APCs) in the absence of any external antigen [1, 2].
Different lung disease models such as asthma and more recently hyperoxia-induced lung injury could be linked to NKT cell-induced immune responses [1, 3–7]. Hyperoxia remains a mainstay in clinical patient care in order to improve oxygen delivery to crucial organs in critically ill patients suffering from acute lung injury or during cardiac surgery to name a few [3]. The toxic effects of high-level oxygen are well known, with hyperoxia-induced lung injury being one of the most clinically relevant. Acute lung injury can be caused by different mechanical, infectious, or environmental pathogens or mechanisms. In addition, prolonged exposure to inhaled high oxygen levels (>50 % of inspired oxygen) can lead to acute lung injury. Hyperoxic lung injury is typically characterized by alveolar epithelial as well as endothelial damage. These effects result in a capillary leak syndrome followed by inflammatory cell recruitment [4–6].
We recently found that hyperoxia-induced lung injury can be NKT cell-induced and mediated by CD39 purinergic signaling mechanisms. Hyperoxia-exposed wild type mice developed severe lung injury with pulmonary NKT cell proliferation and showed a nearly 100 % mortality rate after 72 h while CD39 deficient animals survived with low NKT cell proliferation and significantly less lung injury [7]. The mechanism as to how NKT cells undergo activation and exhibit these detrimental effects in hyperoxic injury remains unclear.
One of the suggested mechanisms includes the ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP-2) also known as autotaxin (ATX) that catalyzes the production of lysophosphatidic acid (LPA) using lysophosphatidylcholine (LPC) as substrate in extracellular fluids [8]. Autotaxin was first discovered as a tumor motility protein secreted by cells as a type II membrane protein [9]. LPA and LPC [10] can evoke inflammatory growth factor-like responses generally including stimulation of cell proliferation and chemotaxis [11]. Significantly, LPA can also trigger NKT cell proliferation and activation [12, 13].
In this study, we hypothesized that up-regulation of ENPP-2 on pulmonary NKT cells triggers increased production of lysophosphatidic acid (LPA), which leads to NKT cell activation and proliferation in a murine model of hyperoxia-induced acute lung injury. In addition, we aimed to show that inhibition of the NKT-LPA axis was protected against hyperoxic damage.
Material and methods
Reagents and antibodies
The following antibodies were purchased from eBioscience: PECy7-conjugated anti-NK1.1, FITC-conjugated anti-CD3, PE-conjugated anti-GR-1, and FITC-conjugated anti-F4/80. α-Galactosylceramide (α-GalCer) was purchased from Toronto Research Chemicals, Inc. (Toronto, ON). Bromomethylene phosphonate LPA (BrP-LPA) was purchased from Echelon®.
Flow cytometry
Mononuclear cells were extracted from lung tissue using Ficoll gradient and stained with PECy7-conjugated anti-NK1.1 and FITC-conjugated anti-CD3. Natural killer T- (NKT) cells were defined as CD3low/NK1.1 low cells or CD1d-tetramer binding (as below) on flow cytometry [1, 14, 15]. Pulmonary mononuclear cells (PMNs) were extracted and stained with PE-conjugated anti-GR-1 and FITC-conjugated anti-F4/80. PMNs were defined as GR-1+/F4/80- cells [7].
Animals and animal model
Animals were housed in accordance with the guidelines from the American Association for Laboratory Animal Care. The protocol was approved by the institutional review committee at BIDMC. C57/BL6 mice (Taconic, Germantown, NJ) were studied. Littermate mice were used for the control groups. Separate sets of animals were studied for different experimental outcome groups.
Eight- to 10-week-old mice were placed in cages in a customized airtight plexiglass chamber, and exposure to 100 % inhaled oxygen was conducted for 72 h, as described [16].
Animals in the BrP-LPA group received a single intraperitoneal (i.p.) injection of the autotaxin inhibitor pan LPA receptor antagonist BrP-LPA (3 mg/kg, using H2O as vehicle) immediately prior to oxygen exposure [9].
Histopathological analysis
The whole lungs were harvested from hyperoxia treated and untreated mice, and sections from all mice were stained with hematoxylin and eosin by standard methods to assess overall histopathology.
iINKT cell cultures and BrP-LPA
Lungs were harvested and iNKT cells were extracted using CD1d-tetramer-sorting by FACS [17]. iNKT cells were cultured and exposed to room air (21 % oxygen) or to 95 % oxygen/5 % CO2 for 72 h [15].
NKT cells were incubated with 5 uM BrP-LPA using H2O as vehicle [12].
Thin Layer chromatography (TLC)
LPA and LPC extraction
Blood was collected in a plastic tube containing l/10 volume of heparin.
Plasma lipids were extracted after adjusting the plasma to pH 3.0 with HCI. The combined chloroform phases were dried under a stream of nitrogen.
Separation of LPA and LPC by TLC
The plasma phospholipids were dissolved in a chloroform/methanol mixture (2:1 v/v) and spotted on a silica gel plate. The plate was developed with a solvent system of chloroform/methanol/28 % ammonium hydroxide (60:40:10 by volume) in the dark. The LPA and LPC were detected by exposing the plate to iodine vapor. Standard LPA and LPC (Sigma) migration was used as markers.
Expression of P2X7 receptors on iNKT cells (reverse-transcription polymerase chain reaction)
RNA from iNKT cells was reverse transcribed to complementary DNA using the Reverse Transcription Kit (Applied Biosystems, Foster City, CA) [18].
Statistical analyses
Results are expressed as the median range and mean +/−standard error of mean (SEM). For statistical analyses, the Student t test was used. Significance was defined as p < 0.05 [19].
Results
ENPP-2 is up-regulated in hyperoxia-exposed pulmonary iNKT cells on a transcriptional level
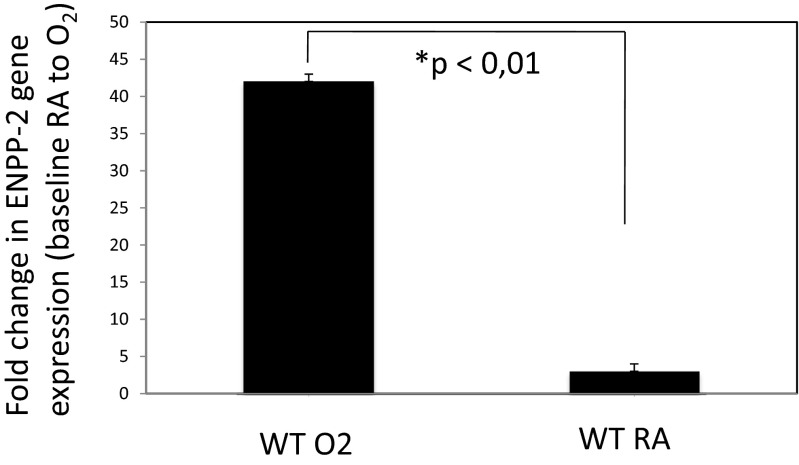
Pulmonary NKT cells were harvested, cultured, and exposed to 95 % oxygen/5 % CO2 for 72 h as described [7]. RT-PCR analysis revealed a significant and approximately 20-fold up-regulation of the ectonucleotide pyrophosphatase/phosphodiesterase 2 (Autotaxin, ENPP-2) on the NKT cells after exposure to hyperoxia when compared to NKT cells exposed to room air (p < 0.01) (Fig. 1).
Fig. 1.
Transcriptional up-regulation of ENPP-2 in hyperoxia-exposed pulmonary iNKT cells. Pulmonary NKT cells were harvested, cultured, and exposed to 95 % oxygen/5 % CO2 for 72 h. RT-PCA analysis showed a significant up-regulation of the ectonucleotide pyrophosphatase/phosphodiesterase 2 (Autotaxin, ENPP-2) on the NKT cells after exposure to hyperoxia when compared to NKT cells exposed to room air (p < 0.01)
Oxygen increases lysophosphatidic acid levels in pulmonary NKT cells, LPA levels decrease when cells are incubated with BrP-LPA in vitro
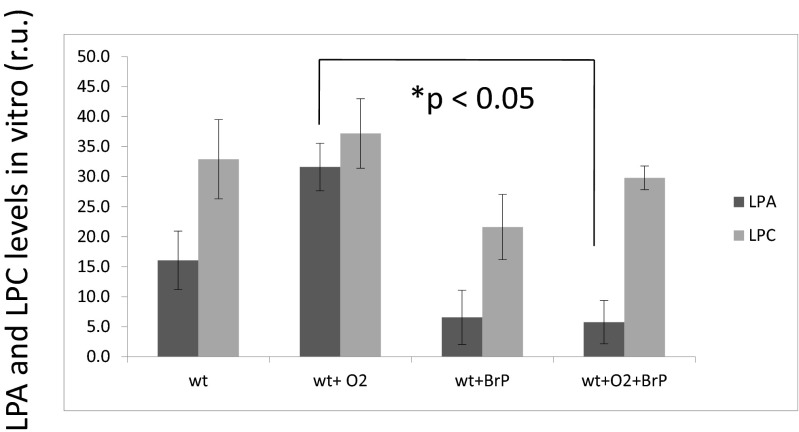
Pulmonary iNKT cells were cultured and exposed to 95 % O2/5 % CO2 for 72 h with and without addition of the ATX-inhibitor and LPA antagonist BrP-LPA. Levels of lysophosphatidic acid (LPA) were measured in the supernatants. LPA levels were higher with NKT cells after oxygen exposure. LPA levels in hyperoxia-exposed cells were significantly lower when cells were incubated with the pan-LPA antagonist BrP-LPA during hyperoxia (p < 0.05) (Fig. 2).
Fig. 2.
Hyperoxia increases lysophosphatidic acid (LPA) levels in pulmonary NKT cells in vitro. BrP-LPA diminishes level of LPA. Pulmonary iNKT cells were cultured and exposed to 95 % O2/5 % CO2 for 72 h with and without addition of the pan-LPA antagonist BrP-LPA. LPA levels in hyperoxia-exposed cells were significantly lower when cells were incubated with the pan LPA antagonist BrP-LPA during hyperoxia (p < 0.05). r.u. relative units (relative to the background-reaction in the absence of weight)
Lysophosphatidylcholine levels did not show a significant change after oxygen exposure or after administration of BrP-LPA
Animals that receive the LPA antagonist BrP-LPA prior to oxygen exposure show improved survival rates and less severe lung injury after hyperoxia
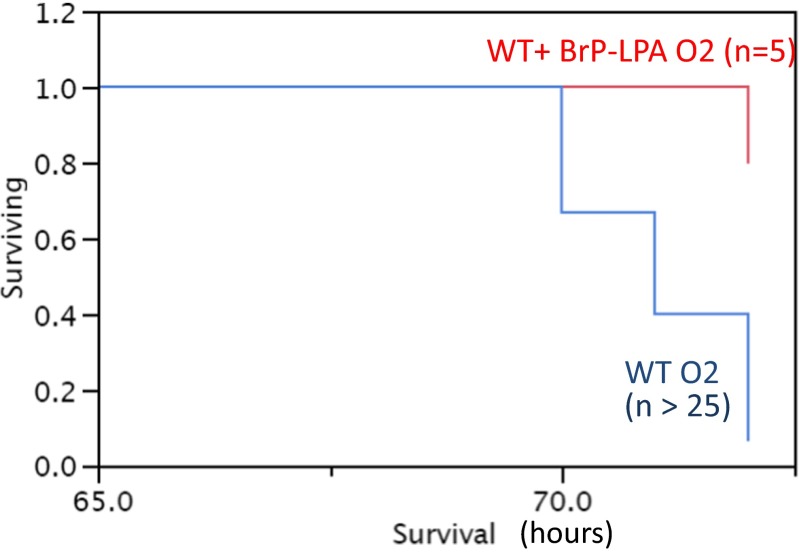
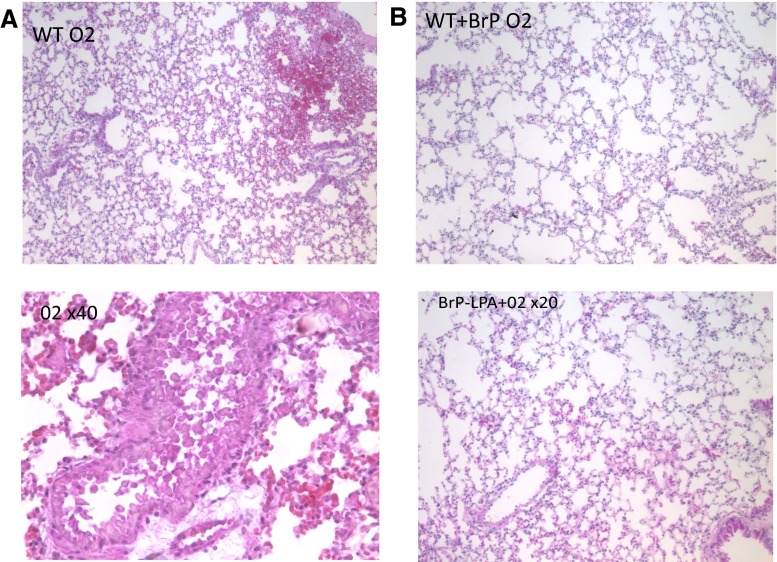
Animals showed severe systemic signs of illness such as lethargy, hypothermia, and ruffling of the fur after 72 h of 100 % oxygen exposure and underwent euthanasia (n > 25) (Fig. 3). Lungs from these mice with hyperoxia-induced lung injury showed large areas of hemorrhage, pronounced interstitial edema, and complete destruction of the bronchial epithelium (Fig. 4). To determine whether inhibition of ATX and LPA receptors leads to improved lung injury and survival, mice were treated with BrP-LPA.
Fig. 3.
Kaplan–Meier survival curves for (n > 25) and plus BrP-LPA animals after 72 h in 100 % oxygen demonstrate a clear survival benefit of those animals that received a BrP-LPA injection prior to oxygen exposure
Fig. 4.
Histological assessment of hyperoxic lung injury. a H&E staining of control lung tissue and b plus BrP-LPA injection prior to hyperoxia. Animals were exposed to 100 % oxygen for 72 h. The hyperoxia-exposed lungs show severe injury with pulmonary edema and hemorrhage, whereas the BrP-LPA injected animals express milder injury
BrP-LPA-treated mice (n = 5) remained healthier with excellent survival (Fig. 3) and only minimal lung injury after hyperoxia exposure (Fig. 4).
Mice that received the autotaxin antagonist BrP-LPA showed decreased pulmonary iNKT cell populations and decreased pulmonary MN/granulocyte infiltration after hyperoxia
In order to determine whether the ATX antagonist BrP-LPA has an impact on NKT cell infiltration in oxygen-exposed lungs, we then analyzed the lungs extracted from oxygen-exposed mice with and without BrP-LPA treatment. Baseline iNKT cell populations in the lung did not differ between BrP-LPA treated and untreated mice under normoxic conditions (<0.5 % of all mononuclear cells) (Fig. 5a). CD3/NK1.1 double intermediate positive cells were defined as iNKT cells as previously described [7]. After 72 h of 100 % exposure, animals show significant increases of iNKT cells compared to their baseline (0.23 versus 4.7 % of all pulmonary mononuclear cells) (Fig. 5a). BrP-LPA-treated animals exhibit small increase of pulmonary iNKT cells (0.89 versus 1.4 % of all pulmonary mononuclear cells) in response to hyperoxia (Fig. 5a).
Fig. 5.
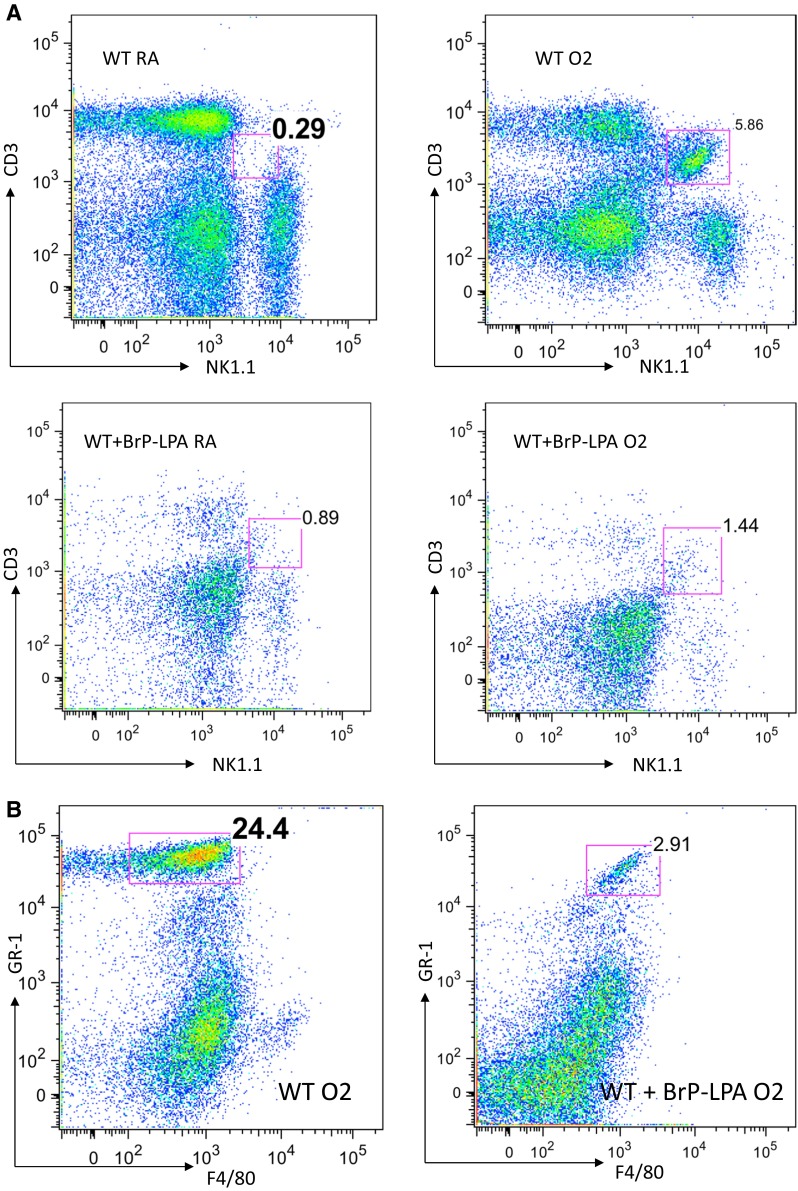
Flow cytometry analysis of pulmonary NKT cell and PMN populations after hyperoxia. a Lungs extracted from oxygen-exposed mice with and without BrP-LPA treatment were analyzed. Baseline iNKT cell populations in the lung did not differ between BrP-LPA treated and untreated wild type mice under normoxic conditions. After 72 h of 100 % exposure, animals show significant increases of iNKT cells compared to their baseline. After BrP-LPA treatment, animals exhibit only a minimal increase of pulmonary iNKT cells in response to hyperoxia. b Populations of GR-1+/F4/80- PMN-cells increase massively after oxygen exposure in lungs. When animals were injected with BrP-LPA prior to oxygen exposure PMN-cells only increased marginally
Flow cytometry confirmed significantly increased populations of GR-1+/F4/80- PMN-cells in oxygen-exposed lungs (24 % of all pulmonary mononuclear cells) (Fig. 5b) compared to the BrP-LPA-treated group (2.9 % of all pulmonary mononuclear cells).
Discussion
In this study, we demonstrate that LPA plays a crucial role in the development of iNKT cell-induced hyperoxic lung injury in a murine model. We have previously shown that hyperoxia-induced lung injury is mediated by increased pulmonary NKT cell proliferation [7]. We now show that pulmonary NKT cells produce large amounts of pro-proliferatory LPA in response to up-regulation of ENPP-2 when exposed to hyperoxia. In addition, hyperoxia-exposed mice also produce significant amounts of plasma LPA before they die from massive lung injury. Incubating NKT cells with the LPA antagonist BrP-LPA during oxygen exposure (a clinically relevant approach) diminishes LPA production. BrP-LPA-injected animals demonstrate improved survival rates and are ultimately protected from hyperoxic lung injury.
LPA is a phospholipid derivate that is generated from LPC by autotaxin (ectonucleotide pyrophosphatase/phosphodiesterase 2, ENPP-2), which has lysophospholipase D activity. LPA is known to evoke growth factor-like responses including stimulation of chemotaxis and cell proliferation. We previously found that pulmonary NKT cells proliferate in response to hyperoxia and exert massive pro-inflammatory effects that ultimately lead to lung injury and death. In the present study, we demonstrated up-regulation of ENPP-2 (autotaxin) on pulmonary NKT cells in response to hyperoxia. Autotaxin shows a broad tissue distribution in adult mice with high mRNA levels in the lung [20]. Increased autotaxin levels have been demonstrated in other disease models and could be found in synovial fluid of arthritis patients as well as human astrocytes following brain injury suggesting its role in inflammation [21]. Autotaxin expression has been studied in BLM-induced pulmonary inflammation where increased autotaxin levels were found in murine BALFs [22]. Bronchial epithelial cells as well as inflammatory macrophages showed the highest expression of autotaxin found in the aforementioned BALFs. Genetic depletion of autotaxin in bronchial epithelial cells and bronchial macrophages resulted in decreased disease severity [20]. In our study, we found that not only is ENPP-2 up-regulated in pulmonary NKT cells after hyperoxia, but that the downstream product LPA also increases after hyperoxic injury. Autotaxin as previously described seems to play a role in pulmonary inflammation and seems to be released in a hyperoxic environment. LPA acts as an NKT self-antigen and inflammatory mediator and seems to have a direct effect on NKT activation and the consequent hyperoxic lung injury in this present study.
Natural Killer T cells are distinct lymphocytes that recognize lipid antigens presented by the non-MHC-molecule CD1d. NKT cells have been known to exert potent pro-inflammatory responses and release large amounts of cytokines in the absence of prior antigen priming [23, 24]. Increasing evidence suggests that recognition of so-called self-antigens by NKT cells not only plays an important role in their development but also in their regulation of immune responses [1, 23–25]. Zeissig et al. recently demonstrated that NKT cell activation through endogenous antigenic lipids, including lysophospholipids generated by Hepatitis-B-virus-induced secretory phospholipases, plays an important role in acquired Hepatitis B immunity [25].
NKT cell self-antigens such as betaGalCer, iGb3, but also LPA have been shown to activate NKT cells in vitro [22]. In our study, we demonstrate that NKT cells that were activated with αGalCer and exposed to hyperoxia started to produce large amounts of LPA, which per se has pro-inflammatory properties and could in turn lead to maintenance of the inflammatory NKT cell-mediated cascade. In our model, LPA release from NKT cells in response to hyperoxia stimulates NKT cells and induces proliferation that ultimately leads to hyperoxia-induced lung injury as described. Interestingly, since LPA can function as a self-antigen for human NKT cells, Georas et al. showed that LPA levels are increased in BAL samples of patients with allergic airway inflammation [26]. A connection between LPA and NKT cells was not made in this study, but a link between allergic asthma and NKT cell involvement had previously been postulated by several authors [14, 27–29]. The findings of this present study, in addition to the previously described importance of NKT cells in human lung disease, could have important clinical implications.
The presented findings need to be translated into the clinical setting where patients depend on high levels of inspiratory oxygen for organ function, but subsequently suffer from the deleterious consequences of hyperoxic lung injury. One of the potential treatment options, that has been successful in this animal study for lung protection, could include the temporary administration of the pan-LPA antagonist BrP-LPA during hyperoxia exposure.
Hyperoxic lung injury remains a serious clinical problem in critically ill patients. As previously described, NKT cells as a bridge between innate and adaptive immunological responses play a crucial role in the development of hyperoxia-induced lung injury in mice. We have now identified LPA, a potent mediator and self-antigen for NKT cells, as the source for NKT cell activation during hyperoxic lung injury in our mouse model. Potential treatment options arise from successful use and protection from hyperoxia-induced lung injury through the pan LPA antagonist BrP-LPA.
Acknowledgments
Compliance with Ethical Standards
ᅟ
Funding
This study was partially funded grant by NIH T32 GM007592-32 (PI Keith Miller) through M Nowak-Machen.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Author contributions
MNM and SCR designed the study; AU, SW, and ML performed TLC experiments and analyzed the data; MNM performed the experiments; MNM and SCR analyzed the data and wrote the manuscript; and MAE advised on the project and edited the manuscript.
References
- 1.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 2.Lappas CM, Day Y-J, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203(12):2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhandari V, Choo-Wing R, Lee CG, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12(11):1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baleeiro CEO, Wilcoxen SE, Morris SB, Standiford TJ, Paine R. Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol. 2003;171(2):955–963. doi: 10.4049/jimmunol.171.2.955. [DOI] [PubMed] [Google Scholar]
- 5.Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- 6.Davis WB, Rennard SI, Bitterman PB, Crystal RG. Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med. 1983;309(15):878–883. doi: 10.1056/NEJM198310133091502. [DOI] [PubMed] [Google Scholar]
- 7.Nowak-Machen M, Schmelzle M, Hanidziar D, et al. Pulmonary natural killer T cells play an essential role in mediating hyperoxic acute lung injury. Am J Respir Cell Mol Biol. 2013;48(5):601–609. doi: 10.1165/rcmb.2012-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakanaga K, Hama K, Aoki J. Autotaxin—an LPA producing enzyme with diverse functions. J Biochem. 2010;148(1):13–24. doi: 10.1093/jb/mvq052. [DOI] [PubMed] [Google Scholar]
- 9.Schleicher SM, Thotala DK, Linkous AG, et al. Autotaxin and LPA receptors represent potential molecular targets for the radiosensitization of murine glioma through effects on tumor vasculature. Bauer JA, ed. PLoS ONE. 2011;6(7):e22182. doi: 10.1371/journal.pone.0022182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sevastou I, Kaffe E, Mouratis M-A, Aidinis V. Lysoglycerophospholipids in chronic inflammatory disorders: the PLA(2)/LPC and ATX/LPA axes. Biochim Biophys Acta. 2013;1831(1):42–60. doi: 10.1016/j.bbalip.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3(1-2):171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox LM, Cox DG, Lockridge JL, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. Cerundolo V, ed. PLoS Biol. 2009;7(10):e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellicci DG, Clarke AJ, Patel O, et al. Recognition of β-linked self glycolipids mediated by natural killer T cell antigen receptors. Nat Immunol. 2011;12(9):827–833. doi: 10.1038/ni.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rijavec M, Volarevic S, Osolnik K, Kosnik M, Korosec P. Natural killer T cells in pulmonary disorders. Respir Med. 2011;105(S1):S20–S25. doi: 10.1016/S0954-6111(11)70006-3. [DOI] [PubMed] [Google Scholar]
- 15.Molling JW, Moreno M, van der Vliet HJJ, et al. Generation and sustained expansion of mouse spleen invariant NKT cell lines with preserved cytokine releasing capacity. J Immunol Methods. 2007;322(1-2):70–81. doi: 10.1016/j.jim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Waxman AB, Einarsson O, Seres T, et al. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest. 1998;101(9):1970–1982. doi: 10.1172/JCI1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9(5):582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 18.Beldi G, Banz Y, Kroemer A, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51(5):1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmelzle M, Eisenberger CF, am Esch JS, Matthaei H, Krausch M, Knoefel WT. Non-colorectal, non-neuroendocrine, and non-sarcoma metastases of the liver: resection as a promising tool in the palliative management. Langenbeck’s Arch Surg. 2010;395(3):227–234. doi: 10.1007/s00423-009-0580-y. [DOI] [PubMed] [Google Scholar]
- 20.Boutin JA, Ferry G. Autotaxin. Cell Mol Life Sci. 2009;66(18):3009–3021. doi: 10.1007/s00018-009-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savaskan NE, Rocha L, Kotter MR, et al. Autotaxin (NPP-2) in the brain: cell type-specific expression and regulation during development and after neurotrauma. Cell Mol Life Sci. 2007;64(2):230–243. doi: 10.1007/s00018-006-6412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oikonomou N, Mouratis M-A, Tzouvelekis A, et al. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47(5):566–574. doi: 10.1165/rcmb.2012-0004OC. [DOI] [PubMed] [Google Scholar]
- 23.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2(8):557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 24.Gapin L, Godfrey DI, Rossjohn J. Natural Killer T cell obsession with self-antigens. Curr Opin Immunol. 2013;25(2):168–173. doi: 10.1016/j.coi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeissig S, Murata K, Sweet L, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18(7):1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georas SN, Berdyshev E, Hubbard W, et al. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37(3):311–322. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 27.Holtzman MJ. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J Clin Invest. 2012;122(8):2741–2748. doi: 10.1172/JCI60325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwamura C, Nakayama T. Role of NKT cells in allergic asthma. Curr Opin Immunol. 2010;22(6):807–813. doi: 10.1016/j.coi.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Meyer EH, DeKruyff RH, Umetsu DT. T cells and NKT cells in the pathogenesis of asthma. Annu Rev Med. 2008;59(1):281–292. doi: 10.1146/annurev.med.59.061506.154139. [DOI] [PubMed] [Google Scholar]