Abstract
ATP consumption during intense neuronal activity leads to peaks of both extracellular adenosine levels and increased glucose uptake in the brain. Here, we investigated the hypothesis that the activation of the low-affinity adenosine receptor, the A2B receptor (A2BR), promotes glucose uptake in neurons and astrocytes, thereby linking brain activity with energy metabolism. To this end, we mapped the spatiotemporal accumulation of the fluorescent-labelled deoxyglucose, 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG), in superfused acute hippocampal slices of C57Bl/6j mice. Bath application of the A2BR agonist BAY606583 (300 nM) triggered an immediate and stable (>10 min) increase of the velocity of 2-NBDG accumulation throughout hippocampal slices. This was abolished with the pretreatment with the selective A2BR antagonist, MRS1754 (200 nM), and was also absent in A2BR null-mutant mice. In mouse primary astrocytic or neuronal cultures, BAY606583 similarly increased 3H-deoxyglucose uptake in the following 20 min incubation period, which was again abolished by a pretreatment with MRS1754. Finally, incubation of hippocampal, frontocortical, or striatal slices of C57Bl/6j mice at 37 °C, with either MRS1754 (200 nM) or adenosine deaminase (3 U/mL) significantly reduced glucose uptake. Furthermore, A2BR blockade diminished newly synthesized glycogen content and at least in the striatum, increased lactate release. In conclusion, we report here that A2BR activation is associated with an instant and tonic increase of glucose transport into neurons and astrocytes in the mouse brain. These prompt further investigations to evaluate the clinical potential of this novel glucoregulator mechanism.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-015-9474-3) contains supplementary material, which is available to authorized users.
Keywords: Glucose uptake, Adenosine A2B receptor, Hippocampus, Striatum, Frontal cortex, 2-NBDG, Lactate, Glycogen
Introduction
The energy homeostasis of the brain is tightly dependent on energetic glucose metabolism occurring either through anaerobic or oxidative pathways [1, 2]. The glucose-derived ATP is consumed by glutamatergic signalling and by the associated activity of Na+/K+-ATPases (NKAs) in neurons and astrocytes [3]. Increased neuronal activity thus triggers a physiological accumulation of extracellular adenosine fed by ATP release [4] and ATP consumption by Na+/K+-ATPases [5]. Hence, when the circuitry is under heavy load and oxidizes more glucose, peaks of extracellular adenosine may serve as a paracrine and/or autocrine adaptive signal to stimulate glucose metabolism via activating one of its membrane-bound metabotropic receptors [6]. Additionally, pathophysiological conditions, such as transient cerebral ischemia, were shown to exacerbate ATP conversion into adenosine [7, 8]. In fact, the assumption has been toyed with for decades that adenosine generated under energetic crisis protects from cellular damage by restoring energy balance in the brain [9–11].
Accordingly, both of the two most abundant adenosine receptors in the brain, the A1 and A2A subtypes [12], have been documented to impact on cerebral glucose metabolism besides being important modulators of neuronal activity [13, 14]. There are two additional cloned adenosine receptors, the A2B and the A3 receptors [12], but their roles have been less explored in the brain. Notably, A2BR have been implicated in peripheral glucose homeostasis [15, 16] and have been proposed to control astrocytic glycogen metabolism [17, 18]. Furthermore, we recently observed that A2BRs control A1 receptor (A1R)-mediated responses in hippocampal glutamatergic synapses [19]. These observations prompt the attractive hypothesis that A2BR may be associated with cerebral glucoregulation, especially when a metabolic boost is needed. The therapeutic potential of such (patho)physiological role would be vast.
Here, we measured spatiotemporal and/or quantitative changes in the uptake of different glucose analogues, the synthesis of glycogen and the release of lactate, in frontocortical, hippocampal, and striatal slices, as well as in primary neuronal and astrocytic cultures of C57Bl/6 mice. Overall, we concluded that A2BRs regulate glucose metabolism in the mouse brain.
Materials and methods
Animals
All studies were carried out in accordance with the EU (2010/63/EU) and FELASA guidelines, and they were approved by the Ethical Committee of the Center for Neuroscience and Cell Biology. A total of 50 animals (29 males and 15 dams) were used in the experiments described here. We used 8–10-week-old male A2BR null-mutant (knockout, KO) mice with a C57Bl/6 background [20] kindly donated by Drs. Akio Ohta and Michael Sitkovsky (New England Inflammation and Tissue Protection Institute, Northeastern University, Boston, MA, USA) and wild-type C57Bl/6 mice, as well as E17-C57Bl/6 mouse embryos. The adult animals were group housed under controlled temperature (23 ± 2 °C) and subjected to a fixed 12-h light/dark cycle, with free access to food and water. All efforts were made to reduce the number of animals used and to minimize their stress and discomfort. The animals used to perform the in vitro studies were deeply anesthetized with halothane before sacrifice (no reaction to handling or tail pinching while still breathing).
Real-time fluorescent measurement of deoxyglucose uptake in hippocampal slices
Mice were killed, and their brains were quickly removed and placed in ice-cold Krebs-N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) solution (in mM NaCl 113, KCl 3, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, NaHCO3 25, glucose 5.5, HEPES 1.5, and pH 7.4). Coronal slices (300 μm thickness) were cut with a Vibratome 1500 (Leica, Germany), left to recover for 1 h at room temperature in Krebs-HEPES solution gassed with 5 % CO2 and 95 % O2, then gently mounted on coverslips with the hippocampus in the center, and placed in a RC-20 superfusion chamber on a PH3 platform (Warner Instruments, Harvard, UK). The slices were superfused with gassed Krebs-HEPES solution at a rate of 0.5 mL/min in a closed circuit, and they were then photographed with a CoolSNAP digital camera (Roper Scientific, Trenton, NJ, USA) every 30 s over the following 30 min, using a 5× Plan Neofluar objective (NA 0.25) on an inverted Axiovert 200-M fluorescence microscope (Carl Zeiss, Germany), coupled to a Lambda DG-4-integrated 175-W light source and wavelength switching excitation system (Sutter Instrument Company, Novato, CA, USA) to allow real-time video imaging. The data were band-pass filtered for excitation (470/40) and emission (525/50).
After recording six images for autofluorescence (to establish the baseline), 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) (30 μM) was applied through the reservoir of the closed superfusion circuit. As within 10 min, the increase of 2-NBDG signal reached linearity (Supplementary Fig. 1), we recorded a 10-min predrug period following the first 10 min of 2-NBDG application. Subsequently, at 20 min, the slices were challenged with the A2BR agonist BAY606583 (300 nM) or its vehicle, dimethyl sulfoxide (DMSO) (0.1 % v/v), and another 10-min period was recorded at every 30 s. To antagonize A2BRs, MRS1754 (200 nM) was applied 3 min before 2-NBDG. All measurements were obtained in duplicate from each animal (see Supplementary Material for additional details and calculation).
Cell culture preparation
Neuronal and astrocytic primary cultures were prepared as described elsewhere [21, 22]. The neocortex or hippocampi from E17 male and female C57Bl/6 mouse embryos were digested for 15 min with 0.125 % trypsin (type II-S, from porcine pancreas, Sigma-Aldrich, Sintra Portugal) and 50 μg/mL deoxyribonuclease (DNAse) (Sigma-Aldrich) in Hank’s balanced salt solution without calcium and magnesium (in mM NaCl 137, KCl 5.36, KH2PO4 0.44, NaHCO3 4.16, Na2HPO4 0.34, D-glucose 5, and pH 7.2). After dissociation, astrocytes were grown in plastic Petri dishes for 14 days in Dulbecco’s modified Eagle’s medium (DMEM) with 10 % fetal bovine serum, 50 U/mL penicillin, and 50 μg/mL streptomycin (Sigma-Aldrich), at 37 °C in an atmosphere of 95 %/5 % air/CO2, replacing half of the medium after 7 days. The cells were then trypsinized and plated onto poly-D-lysine- (100 μg/mL, Sigma-Aldrich) and laminin-coated (10 μg/mL, Sigma-Aldrich) 24-well culture plates at a cell density of 150,000/cm2. Uptake assays were performed 24 h later. For neuronal cultures, cells were plated directly onto poly-D-lysine- and laminin-coated 24-well culture plates, at the same cell density, in DMEM plus 10 % fetal bovine serum, 50 U/mL penicillin, and 50 μg/mL streptomycin. Two hours after seeding, the medium was replaced by Neurobasal medium with 2 % B27 supplement (GIBCO, Life Technologies), 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mM glutamine (Sigma-Aldrich), and cells were grown at 37 °C in an atmosphere of 95 %/5 % air/CO2 until they were used 14 days later. The medium was partially (40 %) replaced every 4 days.
3H-deoxyglucose uptake in neuron and astrocyte cultures
We used six different cerebrocortical astrocyte cultures, as well as five cerebrocortical and four hippocampal neuronal cultures, obtained from E17 mouse embryos of 11 pregnant dams, respectively. After 15 days in culture, the culture medium was replaced with 250-μL Krebs-HEPES assay solution (pH 7.4; 37 °C) (see above), containing either the vehicle alone (0.1 % DMSO, in 14 wells of the 24-well plate), or the A2BR antagonist, MRS1754 (200 nM, in the rest of the plate). The cultures were incubated for 60 min at 37 °C, and subsequently, the glucose transporter blocker, cytochalasin B (final concentration, 10 μM; 4 wells/plate) or BAY606583 (final concentration, 300 nM; in five DMSO-pretreated wells), or BAY606583 in combination with MRS1754 (in five MRS1754-pretead wells), or the vehicle for BAY606583 (DMSO) alone (5 wells) or in combination with MRS1754 (the last 5 wells) were gently added in a volume of 250 μL, together with the radioactive glucose analogue 3H-2-deoxyglucose (3HDG; 16.6 nM, final concentration). After a further 20-min incubation at 37 °C, the plates were transferred to ice and aliquot of 182 μL was taken from each well to determine the exact 3HDG concentration. The cells were then washed gently three times with 1-mL ice-cold Krebs-HEPES and were subsequently dissolved with 300 μL NaOH (0.5 M) on a shaker for 1 h to recover the proteins and the 3HDG taken up by the cells. The tritium content of the samples was measured in a Tricarb β-counter (PerkinElmer, Portugal, ILC Ltd., Lisbon), and these values were adjusted for protein quantity and the 3H concentrations in each individual well and multiplied by 3.3 × 105 (the ratio between 3HDG and D-glucose in the medium).
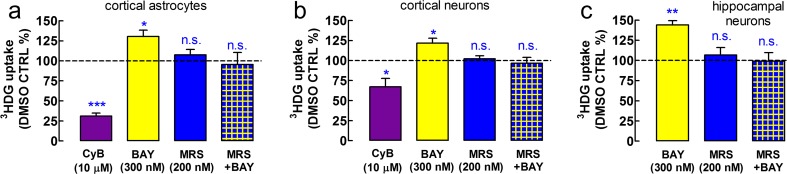
We validated this protocol by measuring the glucose uptake in the presence of cytochalasin B (CyB; 10 μM), which inhibits some glucose transporters, including glucose transporter type 1 (GLUT1), in astrocytes [23, 24]. CyB largely inhibited the uptake of glucose in astrocytes (n = 5, P < 0.001 vs. DMSO control; Fig. 2), and as expected, CyB had a much smaller but still significant effect on neuronal glucose transport (n = 4, P < 0.05; Fig. 3), which indicates a gain-of-function for GLUT1 in neuronal culture [25].
Fig. 2.
A2BR activation stimulates glucose uptake in cerebrocortical astrocytes as well as in cerebrocortical and hippocampal neurons in vitro. The A2BR-selective agonist BAY606583 (BAY; 300 nM; n = 6) stimulated glucose uptake in primary cultures of a cerebrocortical astrocytes, b cerebrocortical neurons, and c hippocampal neurons, obtained from E17-C57Bl/6 mouse embryos, as assessed using the 20-min 3HDG uptake assay. One-hour pretreatment with the A2BR-selective antagonist, MRS1754 (MRS; 200 nM; n = 5, astrocytes; n = 4, neurons), prevented the action of the A2BR agonist, while it had no effect on uptake per se (n = 5, astrocytes; n = 4, neurons). D-glucose uptake is calculated according to [21] and is expressed in nanomole per milligram protein, mean ± SEM of “n” cultures in quadruplicates for the cerebrocortical cultures (4 wells/treatment/culture) and triplicates for the hippocampal cultures. Cytochalasin B (CyB; 10 μM) inhibited the uptake of glucose in five astrocytic cultures stronger than in four cerebrocortical neuronal cultures, because neuronal glucose uptake is moderately dependent on GLUT1 transporters in culture, while GLUT1 is the primary glucose transporter in astrocytes [20]. CyB was not tested in hippocampal neurons. After 60-min preincubation at 37 °C in the assay medium, 3HDG was co-administered for 20 min with BAY, CyB, or their vehicle, DMSO. When used, MRS1754 was present since the beginning of the preincubation period. *P < 0.05, ***P < 0.001 versus DMSO control; n.s. not significant
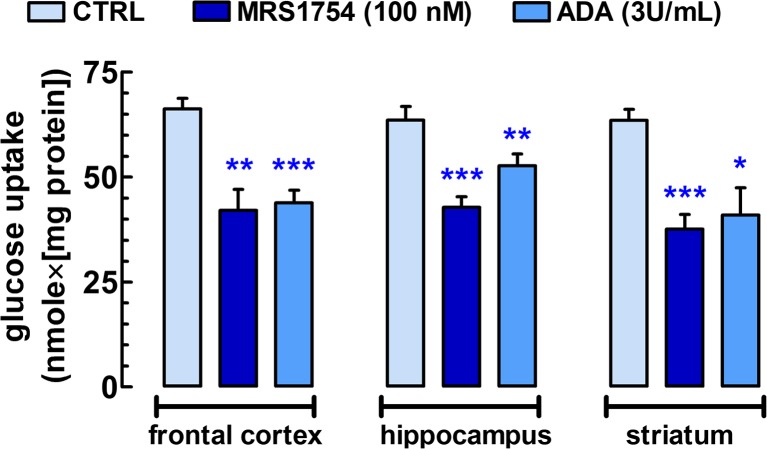
Fig. 3.
Endogenously active A2BR tonically stimulate glucose uptake in acute 400-μm-thick coronal frontocortical, transversal hippocampal, and rostrocaudal striatal slices from C57Bl/6j mice. Brain slices were divided into four groups, and they were incubated in the assay solution at 37 °C under continuous gassing with a mixture of 95 % O2 and 5 % CO2. One chamber with a group of slices from the three brain areas was exposed to MRS1754, another group was exposed to adenosine deaminase (ADA; 3 U/mL), while the remaining two groups served as control. After 60-min pretreatment, 3HDG (2 nM) was bath applied to trace the course of glucose uptake for a 30-min period. Total D-glucose uptake (nmol/mg protein) was determined for each group of slices (for further details, see Materials and Methods and [26]. Bars represent the mean ± SEM of individual measurements from n = 5–14 mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus control
In vitro 3H-deoxyglucose uptake in brain slices
To better characterize the endogenous glucoregulator role of A2BR in different brain areas typically affected by neuropsychiatric disorders, we moved to an in vitro glucose uptake protocol previously optimized in acute hippocampal slices, which allows simultaneously comparing the effect of various treatments [26]. Mice were anesthetized with halothane, sacrificed (around 2:00 P.M. each experimental day to reduce potential circadian hormonal effects), and their brain was immediately placed in ice-cold Krebs-HEPES assay solution (see above). The pairs of frontal cortices, hippocampi, and striata were rapidly dissected and cut in 400-μm-thick transverse slices with the help of a McIlwain tissue chopper, and the slices were gently separated in ice-cold assay solution (carboxygenated with 95 % O2 and 5 % CO2), then transferred and maintained at 37 °C in a multichamber slice incubator with 50 mL of carboxygenated assay solution until the end of the experiment. Each container had separated nylon-mesh bottom wells to keep three cortical or five hippocampal or three striatal slices from each of the three animals per experiment; i.e., each container had approximately 7.2 mg protein in 50-mL assay medium. MRS1754 (100 nM) or its vehicle, DMSO (0.1 %), or adenosine deaminase (3 U/mL) were bath applied from the beginning of the batch incubation period; that is, the 60 min of recovery period was used for the pretreatment as well. After 60 min of preincubation, the radioactive glucose analogue, 3HDG (final concentration of 2 nM), was applied to the bath for 30 min. The slices were washed twice in ice-cold assay solution for 5 min and collected in 1 mL NaOH (0.5 M) to dissolve the slices. An aliquot of 800 μL was then assayed for 3H in a Tricarb β-counter, while the remaining solution was used to quantify the protein with a bicinchoninic acid assay (Merck Biosciences, Germany). Tritium uptake was multiplied by a factor of 2.75 × 106 to estimate the total glucose uptake (corresponding to the concentration difference between D-glucose and 3HDG in the uptake medium [26]).
In vitro measurement of glycogen accumulation and lactate release in brain slices
The experimental layout was essentially the same as for the 3HDG uptake with slight modifications: since MRS1754 (100 nM) strongly reduced 3HDG uptake in the bathed slices at 37 °C (Fig. 3), we selected this A2BR antagonist to modulate glycogen synthesis and lactate release. We used the frontal cortex of eight mice and the striata of six mice for this assay as these brain areas provide more tissue and hence better signal-to-noise ratio. MRS1754 or its vehicle, DMSO, was co-administered in the bath with D-glucose labelled at all the six carbon atoms (14C6-D-glucose) at the concentration of 50 nM from the beginning of the 90-min incubation period. This protocol allowed us to monitor the recovery of glycogen stores which become depleted when sacrificing the animals [36]. Glycogen was separated from the tissue as before [26]. Upon completion of the 90-min incubation period, a sample was taken from the bath to determine lactate release. The 14C concentration in the bath as well as total 14C retention in the slices and 14C incorporation in glycogen were determined with the β-counter, while lactate levels were measured with a lactate dehydrogenase kit (Sigma-Aldrich) and expressed as nanomole per mg of tissue protein.
Data presentation and statistical analysis
All data are expressed as the means ± SEM of the number of independent observations (n), each averaged from duplicates, quadruplicates, or quintuplicates as indicated. These data were normalized to the respective DMSO control, and tested for normality with the Kolmogorov-Smirnov normality tests. Statistical significance was calculated by one sample t test against the hypothetical value of zero or with one-way analysis of variance (ANOVA) with Dunett’s post hoc test when more than two groups were compared and a value of P < 0.05 was accepted as significant.
Chemicals
3HDG (specific activity 60 Ci/mmol) was obtained from American Radiolabeled Chemicals, Inc. (Saint Louis, MO, USA). 14C6-D-glucose (14C-glucose; specific activity 0.36 Ci/mmol) was bought from PerkinElmer Portugal (ILC Lda., Lisbon, Portugal). 2-NBDG was acquired from Invitrogen (Carslbad, CA, USA). Non-organic reagents, adenosine deaminase (ADA), cytochalasin B, HEPES, and DMSO were purchased from Sigma-Aldrich Portugal (Sintra, Portugal) or Merck Biosciences (Darmstadt, Germany). BAY606583 and MRS1754 were obtained from Tocris Bioscience (Bristol, UK). All non-water soluble substances and 2-NBDG were reconstituted in DMSO at 1000× the final concentration and stored in aliquots at −20 °C.
Results
A2BR activation rapidly enhances glucose uptake in hippocampal slices
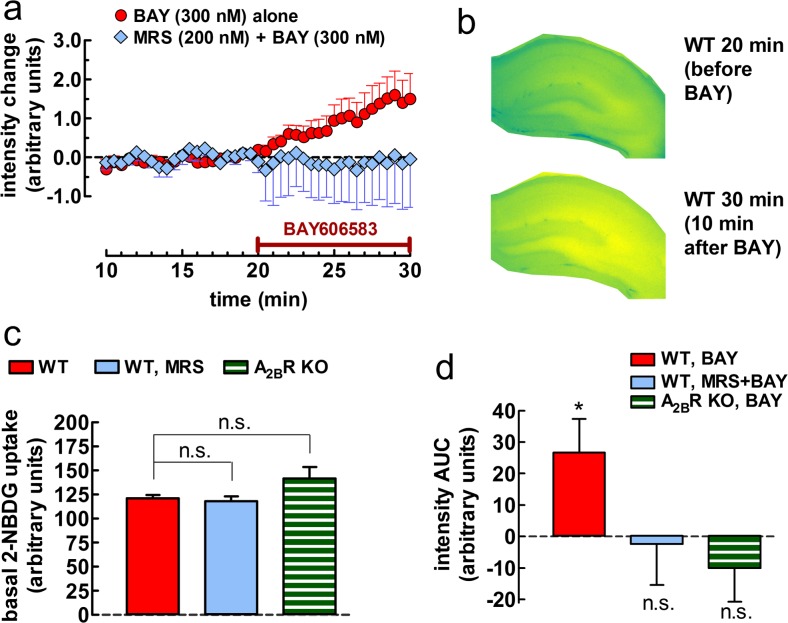
First, we assessed whether A2BR activation affects glucose uptake in superfused hippocampal slices by monitorizing the rate of fluorescence-labelled deoxyglucose (2-NBDG) accumulation. The basal accumulation of 2-NBDG was most evident in astrocyte-rich areas of transverse hippocampal slices, i.e., in the stratum lacunosum, followed by the strata radiatum and moleculare, and the smallest signal was found in the strata pyramidale and granulare (Fig. 1a, b), consistent with previous findings [27]. The pharmacological activation of A2BR with BAY606583 (300 nM, i.e., at a concentration selective for this receptor) rapidly increased the velocity of 2-NBDG accumulation in these hippocampal slices prepared from the wild-type (WT) mice (n = 7 in duplicate, P < 0.05), as gauged from the fluorescence intensity in the whole surface of the transversal slice (Fig. 1b) and compared to the average response in 2-NBDG accumulation in the pair of DMSO-treated slices (Fig. 1a). Pretreatment of the slices with the selective A2BR antagonist MRS1754 (200 nM) for 23 min prevented BAY606583 from stimulating 2-NBDG uptake (n = 4, P > 0.05; Fig. 1e). Additionally, BAY606583 also failed to stimulate 2-NBDG uptake in the hippocampal slices prepared from A2BR knockout (KO) mice (n = 6, P > 0.05; Fig. 1e). Importantly, 2-NBDG accumulation was not statistically different from the WT control neither after pretreatment with MRS1754 (n = 4, P > 0.05; Fig. 1d) nor in A2BR KO mice (n = 6, P > 0.05; Fig. 1d), even though the uptake velocity was tendentiously greater in A2BR KO mice (117.3 ± 9.8 % of WT control, P > 0.05). See Supplementary Material for additional details and calculation.
Fig. 1.
Time course and regional variation of the effect of A2BR activation on the uptake of the fluorescent glucose analogue, 2-NBDG, in 300-μm-thick transversal hippocampal slices of young C57Bl/6j male mice. a The A2BR agonist BAY606583 (BAY; 300 nM) rapidly increased the accumulation of 2-NBDG throughout the hippocampal slices of the wild-type (WT) mice. The x axis denotes the time of exposure to 2-NBDG (30 μM) and indicates that BAY606583 was applied 20 min after the addition of the tracer 2-NBDG. The y axis indicates that the values on the graph are the mean ± SEM of net changes (n = 7 for BAY606583 alone and n = 4 for BAY606583+MRS1754 (200 nM, an A2BR antagonist)), after the subtraction of the mean intensity values of the respective DMSO controls in each experiment. b Representative images taken from a WT hippocampal slice immediately before and 10 min after the beginning of BAY606583 superfusion. The false color scale illustrating the distribution of the fluorescence signal ranges from blue (low signal) through green (moderate signal), up to yellow (strong signal). c The bar graph summarizes the rate of basal 2-NBDG accumulation, expressed in arbitrary intensity units, under control condition (left, red bar; n = 7) or after ~23 min of preincubation with MRS1754 (middle, light blue bar; n = 4) in slices from WT mice, and under control condition in slices from A2BR KO (−/−) mice (right, striped green bars; n = 6). Each bar represents the mean ± SEM of signal intensities in quadruplicates in four to seven animals at the linear phase, i.e., 19.5 min after the addition of 2-NBDG; n.s. not significant, as assessed with one-way ANOVA followed by Bonferroni’s multiple comparison’s post hoc test. d Effect of drugs normalized to the respective DMSO controls (dashed line crossing the zero value). Values represent the mean ± SEM of the individual measurements in duplicates, as obtained with the area under the curve (AUC) method for BAY606583 versus DMSO application in the absence (n = 7) or in the presence of MRS1754 (n = 4) in the WT mice, as well as in the absence of A2BR (A2BR KO; n = 6). *P < 0.05. See Supplementary Material for additional details and calculation
A2BR activation stimulates glucose transport in cultured astrocytes and neurons
Since the uptake of 2-NBDG underrepresents neuronal glucose transport [27], we next measured the effect of acute in vitro A2BR activation on glucose uptake in primary mouse astrocytes and neurons, using a 3HDG transport assay. Glucose uptake during the 20-min assay amounted to 36.2 ± 5.8 nmol/mg protein in astrocytes (n = 6 in quadruplicates), 25.9 ± 6.6 nmol/mg protein in the cerebrocortical neurons (n = 5), and 10.3 ± 5.5 nmol/mg protein (n = 4 in triplicates) in the hippocampal neurons. BAY606583 (300 nM) stimulated the uptake of glucose by 30.4 ± 7.9 % (n = 6; P < 0.05 vs. DMSO control) in astrocytes (Fig. 2a), by 21.8 ± 5.9 % (n = 5; P < 0.05) in cerebrocortical neurons (Fig. 2b), and by 43.9 ± 10.7 % (n = 4; P < 0.01 vs. DMSO control) in hippocampal neurons (Fig. 2c). The pretreatment with the A2BR antagonist, MRS1754 (200 nM), did not affect the uptake of glucose indicating a lack of endogenous tone in the cultured cells (Fig. 2). However, MRS1754 prevented BAY606583 from stimulating glucose uptake in both cell types (Fig. 2), indicating the involvement of A2BR.
The inhibition of endogenous A2BR activity strongly decreases glucose uptake in brain slices
Next, we asked if A2BRs can also function as an endogenous glucoregulator in different brain areas. To this end, we batch-incubated acute frontocortical, hippocampal, or striatal slices from C57Bl/6 mice at 37 °C under continuous oxygenation. The control resting glucose uptake in the subsequent 30-min period was not different among the three brain regions investigated (n = 11–14, P > 0.05) (Fig. 3). We previously saw in rat hippocampal slices that the glutamate reuptake inhibitor, DL-TBOA (10 μM), significantly reduces resting 3HDG uptake by 23 % in a similar assay (unpublished data), indicating that glutamate recycling is responsible for almost one fourth of resting glucose uptake via the stimulation of Na+/K+-ATPases [28]. This is expected to contribute to extracellular adenosine accumulation. Indeed, 1-h pretreatment and 30-min treatment with MRS1754 (100 nM) reduced glucose uptake in the three brain areas by ~33–41 % (n = 6, P < 0.01) as compared to control (Fig. 3). This suggest that extracellular adenosine accumulation in these bathed slices at 37 °C is sufficient to activate A2BRs. Certainly, the reduction of extracellular adenosine levels with 1-h pretreatment and 30-min treatment with adenosine deaminase (3 U/mL) similarly diminished glucose uptake by 17–36 % in slices from the three brain areas (n = 5–6, P < 0.05) (Fig. 3).
The effect of A2BR blockade on glycogen synthesis and lactate release
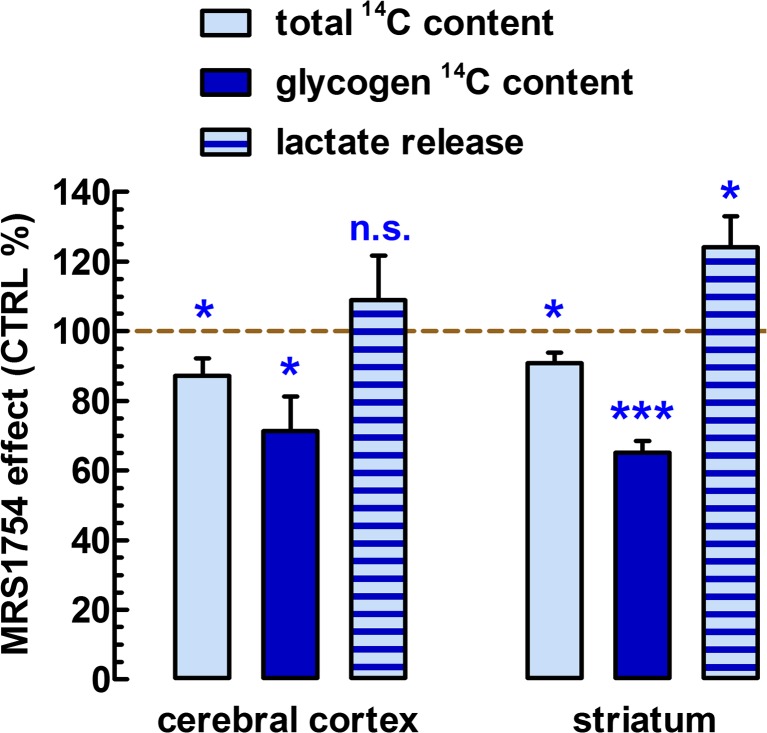
Glycogen levels and lactate release are major parameters reflecting the modulation of glucose metabolism [28, 29]. Hence, with the help of the metabolizable glucose analogue 14C6-glucose, we measured total glucose uptake and 14C incorporation into glycogen in frontocortical and striatal slices in the 90-min recovery period. Total glucose 14C retention by the striatal slices during the 90-min recovery period (78.8 ± 13.2 nmol/mg protein) was significantly smaller (by 9.2 ± 3.1 %, n = 6, P < 0.05) in the presence of MRS1754 (100 nM) (Fig. 4), which was a considerably lesser effect amplitude than what we measured with 3HDG after 1-h recovery. Moreover, 20 % of the above total retention value represented 14C6-glucose incorporation into glycogen (15.4 ± 3.2 nmol/mg protein). As expected, MRS1754 also greatly decreased striatal glycogen levels by 34.9 ± 3.4 % (P < 0.05) (Fig. 4). Last but not least, lactate loss from the control striatal slices to the medium amounted to 621 ± 78 nmol/mg protein. MRS1754 significantly stimulated lactate release by 24.1 ± 8.9 % (n = 6, P < 0.05) (Fig. 4).
Fig. 4.
Mapping the role of A2BRs on glycogen synthesis and lactate release. In acute 400-μm-thick coronal frontocortical and rostrocaudal striatal slices from C57Bl/6j mice, brain slices were divided into two groups and they were submerged in an assay solution containing the metabolizable glucose analogue, 14C6-glucose (50 nM), at 37 °C under continuous gassing with a mixture of 95 % O2 and 5 % CO2. One chamber with a group of slices from the three brain areas was exposed to MRS1754; the other group was exposed to its vehicle, DMSO (0.1 %, control). After 90-min incubation, the slices were washed three times in ice-cold assay solution, collected in 2 mL NaOH (0.5 M). After dissolving the slices, 200 μL of these samples was used to count total 14C retention in the slices and protein quantities; the rest of the 1800 μL was used to precipitate glycogen and count its 14C content as before [26]. Lactate content in the bath at the end of the 90-min incubation period was assessed with a lactic acid assay kit. Bars represent the mean ± SEM of individual measurements from n = 6–8 mice. *P < 0.05, ***P < 0.001 versus control
Frontocortical total 14C retention amounted to 87.9 ± 9.8 nmole/mg protein (n = 8). Glycogen synthesis was 18.2 ± 4.0 nmol/mg protein (20.7 % of total 14C retention), and lactate loss to the bath was 504 ± 56 nmol/mg protein (n = 8); i.e., none of these three parameters were statistically different from those in the striatum (P > 0.05). A2BR blockade reduced both 14C retention by 12.8 ± 4.0 % (P < 0.05) and 14C-labelled glycogen content by 38.6 ± 9.9 % (P < 0.05) but did not affect lactate loss to the medium (P > 0.05) (Fig. 4).
Discussion
The present study provides a direct pharmacological demonstration that the activation of adenosine A2B receptors (A2BR) triggers a rapid and sustained glucose uptake in the mouse brain. This effect appears to occur both in neurons and astrocytes. Hence, A2BR may link extracellular adenosine peaks with increased glucose uptake, thus allowing energy metabolism to meet the demands of neural activity. Such mechanism would be essential to avoid an hypoenergetic crisis, which might lead to cell death. Indeed, A2BR activation has been linked with increased survival of hippocampal and cortical cells under oxygen-glucose deprivation [30–32]. Hence, A2BRs seem to generally assist the homeostasis of brain cells in a manner similar to that occurring in the periphery.
Thus, it is well-known that A2BRs have widespread direct and indirect roles in the control of blood glucose levels and systemic energy metabolism. Indirect mechanisms involve the modulation of peripheral insulin sensitivity and tissue inflammation [15, 16, 33, 34]. Apart from this, A2BRs also can directly regulate glucose handling by the three major organs involved in peripheral glucoregulation, i.e., the liver, the white adipose tissue, and the skeletal muscle [35].
In the brain, compelling evidence supports that glycogen is a major glucose storage with a buffer function in astrocytes to mitigate the potential energetic crisis under prolonged intense activity of the circuitry [36, 37]. Importantly, the activation of A2BRs can promote Akt phosphorylation [16, 38], which in turn can stimulate glycogen synthesis. Furthermore, lactate production can be partly supported by glycogenolysis in astrocytes, and during activity, neurons and thin astrocytic processes in the synapse mostly rely on the oxidation of lactate rather than that of glucose [1, 37]. We indirectly demonstrated here that A2BR activation is positively linked with glycogen synthesis and negatively to lactate output in the striatal slice. Overall, these observations prompt the following scenario: during strong activity of the circuitry, high extracellular levels of adenosine are generated, which in turn will stimulate the replenishment of astrocytic glycogen stores, via A2BR activation. This is probably associated with a dampening effect on lactate release from astrocytes. This can be regarded as a counterregulatory process—a metabolic brake that protects astrocytes from glucose storage depletion and can also reduce extracellular acidification.
There is ample evidence for the presence of A2BRs in both neurons and astrocytes throughout the mammalian brain [39]; thus, A2BRs are well-positioned to modulate glucose metabolism in both brain cell types. In our neuronal cultures, glutamatergic cells were the likely candidates to respond to A2BR activation with increased glucose uptake. It is because hippocampal and cerebrocortical cultures at this age contain predominantly excitatory neurons [40, 41]. The principal neurons of the cerebral cortex and hippocampus are the glutamatergic pyramidal cells. This predicts that A2BR activation can regulate neuronal besides astrocytic metabolism in those brain areas. Normally, glutamatergic neurons use either glucose or lactate [42], so if A2BR activation increases glucose use in pyramidal cells, those neurons would concomitantly take up less lactate. Consequently, A2BR blockade would tip the balance to greater lactate uptake. The simultaneous stimulation of lactate release in astrocytes and lactate oxidation in pyramidal in cells should result in no net change in lactate outflow to the medium, in accordance with our observations. In contrast, A2BR blockade did not affect lactate turnover in the same way in the striatal slices. From the culture data, it is not easy to disentangle if A2BR activation has any effect on glucose or lactate use in striatal neurons, because these cells are predominantly GABAergic, and there is no evidence for glutamatergic neurons whatsoever [43]. This is a probable reason for the difference in frontocortical and striatal lactate loss to the medium.
Besides the direct regulation of glucose transport, the modulation of glutamatergic signalling will also affect glucose use [1]. This is because glutamatergic neurotransmission is a costly process, being responsible for the majority of energetic glucose metabolism in the brain [3]. In concert with this, we recently found that A2BRs modulate glutamatergic synaptic transmission in the hippocampus [19]. Not only neurons but also astrocytes are equipped with A2BR [39, 44], and both cell types are capable of releasing glutamate [45]. Therefore, A2BRs are well-positioned in the tripartite synapse to coordinate glucose uptake according to metabolic demands.
The present pharmacological study is based on the use of a selective A2BR agonist, BAY606583, and an antagonist of this adenosine receptor, MRS1754, together with A2BR KO mice. Hence, the lack of effect of BAY606583 either in the presence of MRS1754 or in the absence of A2BR provides compelling evidence for the involvement of A2BR in the action of BAY606583. Interestingly, there was a tendency for an increased 2-NBDG influx in the hippocampal slices from A2BR KO mice, which contradicts what one would expect, i.e., a decreased glucose uptake in the absence of A2BR. However, A2BR KO mice exhibit an apparent systemic metabolic dysregulation [34] that may also have an impact on cerebral glucoregulation—an additional confounding factor to be taken into account when the cerebral glucoregulator role of A2BR is investigated in future studies.
Glucose availability in the brain regulates cognition and memory in both healthy humans and dementia patients [46–48]. Although normally metabolic boosters (nootropics) do not mitigate the outcome of neurodegenerative disorders, A2BR agonists eventually possess multiple beneficial actions including boosting cerebral glucose metabolism and increasing cell survival apart from effects on energy metabolism [30–32]. Therefore, further studies are invited to evaluate the clinical potential of A2BR agonists in both animals and man.
Electronic supplementary material
(DOC 281 kb)
Acknowledgments
This study was financially supported by NARSAD, Santa Casa da Misercórdia, DARPA (09-68-ESR-FP-010), CAPES-FCT, and CNPq (Ciência sem Fronteiras) and co-funded by FEDER (QREN), through Programa Mais Centro under project CENTRO-07-ST24-FEDER-002006 and through Programa Operacional Factores de Competitividade—COMPETE and National funds (PTDC/SAU-OSM/105663/2008, EXPL/NEU-NMC/0671/2012 and Pest-C/SAU/LA0001/2013-2014) via FCT—Fundação para a Ciência e a Tecnologia.
Author Contributions
A.K., R.A.C., and D.R. conceived and designed the work. A.K., B.S.P., and C.L. carried out the experiments. R.O.B., J.M.M., and R.J.R. were responsible for the cell cultures. A.K. performed data analysis and wrote the first draft. A.K., C.L., R.A.C., and D.R. interpreted results and contributed to the writing and the edition of the manuscript. R.A.C., R.J.R., and D.R. supported the costs of the study. All authors have reviewed and commented on the manuscript.
Abbreviations
- A2BR(s)
Adenosine A2B receptor(s)
- 2-NBDG
2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose
- A1R
Adenosine A1 receptor
- FELASA
Federation for Laboratory Animal Science Associations
- KO
Knockout
- HEPES
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)
- DMSO
Dimethyl sulfoxide
- DNAse
Deoxyribonuclease
- DMEM
Dulbecco’s modified Eagle’s medium
- HDG
3H-2-deoxyglucose
- CyB
Cytochalasin B
- GLUT1
Glucose transporter type 1
- SEM
Standard error of the mean
- ANOVA
Analysis of variance
- ADA
Adenosine deaminase
- DL-TBOA
DL-threo-β-Benzyloxyaspartic acid
- GABA
γ-aminobutyric acid
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interests to report and received no financial support or compensation from any individual or corporate entity over the past 3 years for research or professional services, and there are no personal holdings that could be perceived as constituting a potential conflict of interest.
References
- 1.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivanov AI, Malkov AE, Waseem T, Mukhtarov M, Buldakova S, Gubkina O, Zilberter M, Zilberter Y. Glycolysis and oxidative phosphorylation in neurons and astrocytes during network activity in hippocampal slices. J Cereb Blood Flow Metab. 2014;34:397–407. doi: 10.1038/jcbfm.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int. 2008;52:65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Sims RE, Dale N. Activity-dependent adenosine release may be linked to activation of Na+-K+ ATPase: an in vitro rat study. PLoS ONE. 2014 doi: 10.1371/journal.pone.0087481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/S0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 7.Hagberg H, Andersson P, Butcher S, Sandberg M, Lehmann A, Hamberger A. Blockade of N-methyl-D-aspartate-sensitive acidic amino acid receptors inhibits ischemia-induced accumulation of purine catabolites in the rat striatum. Neurosci Lett. 1986;68:311–316. doi: 10.1016/0304-3940(86)90508-2. [DOI] [PubMed] [Google Scholar]
- 8.Onodera H, Iijima K, Kogure K. Mononucleotide metabolism in the rat brain after transient ischemia. J Neurochem. 1986;46:1704–1710. doi: 10.1111/j.1471-4159.1986.tb08487.x. [DOI] [PubMed] [Google Scholar]
- 9.Newby AC, Worku Y, Holmquist CA. Adenosine formation. Evidence for a direct biochemical link with energy metabolism. Adv Myocardiol. 1985;6:273–284. [PubMed] [Google Scholar]
- 10.Mori M, Nishizaki T, Okada Y. Protective effect of adenosine on the anoxic damage of hippocampal slice. Neuroscience. 1992;46:301–307. doi: 10.1016/0306-4522(92)90052-4. [DOI] [PubMed] [Google Scholar]
- 11.Huber M, Kittner B, Hojer C, Fink GR, Neveling M, Heiss WD. Effect of propentofylline on regional cerebral glucose metabolism in acute ischemic stroke. J Cereb Blood Flow Metab. 1993;13:526–530. doi: 10.1038/jcbfm.1993.68. [DOI] [PubMed] [Google Scholar]
- 12.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta. 2011;1808:1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Nehlig A, Daval JL, Boyet S. Effects of selective adenosine A1 and A2 receptor agonists and antagonists on local rates of energy metabolism in the rat brain. Eur J Pharmacol. 1994;258:57–66. doi: 10.1016/0014-2999(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 15.Rüsing D, Müller CE, Verspohl EJ. The impact of adenosine and A2B receptors on glucose homoeostasis. J Pharm Pharmacol. 2006;58:1639–1645. doi: 10.1211/jpp.58.12.0011. [DOI] [PubMed] [Google Scholar]
- 16.Johnston-Cox H, Koupenova M, Yang D, Corkey B, Gokce N, Farb MG, LeBrasseur N, Ravid K. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magistretti PJ, Hof PR, Martin JL. Adenosine stimulates glycogenolysis in mouse cerebral cortex: a possible coupling mechanism between neuronal activity and energy metabolism. J Neurosci. 1986;6:2558–2562. doi: 10.1523/JNEUROSCI.06-09-02558.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allaman I, Lengacher S, Magistretti PJ, Pellerin L. A2B receptor activation promotes glycogen synthesis in astrocytes through modulation of gene expression. Am J Physiol Cell Physiol. 2003;284:C696–C704. doi: 10.1152/ajpcell.00202.2002. [DOI] [PubMed] [Google Scholar]
- 19.Gonçalves FQ, Pires J, Pliassova A, et al. Adenosine A2b receptors control A1 receptor-mediated inhibition of synaptic transmission in the mouse hippocampus. Eur J Neurosci. 2015;41:876–886. doi: 10.1111/ejn.12851. [DOI] [PubMed] [Google Scholar]
- 20.Hua X, Kovarova M, Chason KD, et al. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med. 2007;204:117–128. doi: 10.1084/jem.20061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Matos M, Augusto E, Santos-Rodrigues AD, Schwarzschild MA, Chen JF, Cunha RA, Agostinho P. Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia. 2012;60:702–716. doi: 10.1002/glia.22290. [DOI] [PubMed] [Google Scholar]
- 23.Klip A, Pâquet MR. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990;13:228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- 24.Barros LF, Bittner CX, Loaiza A, Ruminot I, Larenas V, Moldenhauer H, Oyarzún C, Alvarez M. Kinetic validation of 6-NBDG as a probe for the glucose transporter GLUT1 in astrocytes. J Neurochem. 2009;109(S1):94–100. doi: 10.1111/j.1471-4159.2009.05885.x. [DOI] [PubMed] [Google Scholar]
- 25.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemos C, Valério-Fernandes A, Ghisleni GC, Ferreira SG, Ledent C, de Ceballos ML, Köfalvi A. Impaired hippocampal glucoregulation in the cannabinoid CB1 receptor knockout mice as revealed by an optimized in vitro experimental approach. J Neurosci Methods. 2012;204:366–373. doi: 10.1016/j.jneumeth.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Jakoby P, Schmidt E, Ruminot I, Gutiérrez R, Barros LF. Deitmer JW (2014) Higher transport and metabolism of glucose in astrocytes compared with neurons: a multiphoton study of hippocampal and cerebellar tissue slices. Cereb Cortex. 1991;24:222–231. doi: 10.1093/cercor/bhs309. [DOI] [PubMed] [Google Scholar]
- 28.Pellerin L, Magistretti PJ. Glutamate uptake stimulates Na+, K+-ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J Neurochem. 1997;69:2132–2137. doi: 10.1046/j.1471-4159.1997.69052132.x. [DOI] [PubMed] [Google Scholar]
- 29.Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- 30.Moidunny S, Vinet J, Wesseling E, Bijzet J, Shieh CH, van Ijzendoorn SC, Bezzi P, Boddeke HW, Biber K. Adenosine A2B receptor-mediated leukemia inhibitory factor release from astrocytes protects cortical neurons against excitotoxicity. J Neuroinflammation. 2012;9:198. doi: 10.1186/1742-2094-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu L, Huang B, Shen W, Gao L, Ding Z, Wu H, Guo J. Early activation of nSMase2/ceramide pathway in astrocytes is involved in ischemia-associated neuronal damage via inflammation in rat hippocampi. J Neuroinflammation. 2013;10:109. doi: 10.1186/1742-2094-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molz S, Olescowicz G, Kraus JR, Ludka FK, Tasca CI. Purine receptors are required for DHA-mediated neuroprotection against oxygen and glucose deprivation in hippocampal slices. Purinergic Signal. 2015;11:117–126. doi: 10.1007/s11302-014-9438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS, Kim JK, LaNoue KF, Linden J. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60:669–679. doi: 10.2337/db10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csóka B, Koscsó B, Töro G, Kókai E, Virág L, Németh ZH, Pacher P, Bai P, Haskó G. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014;63:850–866. doi: 10.2337/db13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonioli L, Blandizzi C, Csóka B, Pacher P, Haskó G. Adenosine signalling in diabetes mellitus-pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2015;11:228–241. doi: 10.1038/nrendo.2015.10. [DOI] [PubMed] [Google Scholar]
- 36.Hutchins DA, Rogers KJ. Physiological and drug-induced changes in the glycogen content of mouse brain. Br J Pharmacol. 1970;39:9–25. doi: 10.1111/j.1476-5381.1970.tb09551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pellerin L, Bouzier-Sore A-K, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 38.Schulte G, Fredholm BB. The GS-coupled adenosine A2B receptor recruits divergent pathways to regulate ERK1/2 and p38. Exp Cell Res. 2003;290:168–176. doi: 10.1016/S0014-4827(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 39.Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- 40.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- 41.de Lima AD, Voigt T. Identification of two distinct populations of gamma-aminobutyric acidergic neurons in cultures of the rat cerebral cortex. J Comp Neurol. 1997;388:526–540. doi: 10.1002/(SICI)1096-9861(19971201)388:4<526::AID-CNE2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Castro MA, Beltrán FA, Brauchi S, Concha II. A metabolic switch in brain: glucose and lactate metabolism modulation by ascorbic acid. J Neurochem. 2009;110:423–440. doi: 10.1111/j.1471-4159.2009.06151.x. [DOI] [PubMed] [Google Scholar]
- 43.Reid CB, Walsh CA. Evidence of common progenitors and patterns of dispersion in rat striatum and cerebral cortex. J Neurosci. 2002;22:4002–4014. doi: 10.1523/JNEUROSCI.22-10-04002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verkhratsky A, Burnstock G. Purinergic and glutamatergic receptors on astroglia. Adv Neurobiol. 2014;11:55–79. doi: 10.1007/978-3-319-08894-5_4. [DOI] [PubMed] [Google Scholar]
- 45.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Branconnier RJ. The efficacy of the cerebral metabolic enhancers in the treatment of senile dementia. Psychopharmacol Bull. 1983;19:212–219. [PubMed] [Google Scholar]
- 47.Messier C. Glucose improvement of memory: a review. Eur J Pharmacol. 2004;490:33–57. doi: 10.1016/j.ejphar.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 48.Watson GS, Craft S. Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer’s disease. Eur J Pharmacol. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 281 kb)