Abstract
Elevated plasma levels of homocysteine (Hcy) are associated with the development of coronary artery disease (CAD), peripheral vascular disease, and atherosclerosis. Hyperhomocysteinemia is likely related to the enhanced production of pro-inflammatory cytokines including IL-1β. However, the mechanisms underlying the effects of Hcy in immune cells are not completely understood. Recent studies have established a link between macrophage accumulation, cytokine IL-1β, and the advance of vascular diseases. The purpose of the present study is to investigate the effects of Hcy on IL-1β secretion by murine macrophages. Hcy (100 μM) increases IL-1β synthesis via enhancement of P2X7 expression and NF-ĸB and ERK activation in murine macrophages. In addition, the antioxidant agent N-acetylcysteine (NAC) reduces NF-κB activation, ERK phosphorylation, and IL-1β production in Hcy-exposed macrophages, indicating the importance of ROS in this pro-inflammatory process. In summary, our results show that Hcy may be involved in the synthesis and secretion of IL-1β via NF-ĸB, ERK, and P2X7 stimulation in murine macrophages.
Keywords: Homocysteine, Macrophages, IL-1β, P2X7 receptor
Introduction
Pathological serum levels of homocysteine (Hcy) are associated to cerebral and peripheral vascular disease, thrombosis, and atherosclerosis development [1–4]. High plasma levels of Hcy are likely related to the enhanced production of pro-inflammatory cytokines, including IL-1β [5–7]. However, the mechanisms underlying the effects of Hcy on immune cells are not completely understood. Moreover, an increase in the oxidative stress production of vascular cells and macrophages exposed to Hcy has been attributed to inflammatory substances release [8–10].
Macrophages are key cells in the inflammatory process and the most important source of IL-1β among immune cells. These cells are characterized by a marked heterogeneity in activation, depending on the microenvironmental stimulation [11–15]. In the last few years, studies have established a connection between IL-1β and the advance of chronic diseases [16–18]. Furthermore, clinical trials using therapeutic strategies against IL-1β have demonstrated a decrease in cardiovascular disease progression [19, 18].
The significant proteolytic process regulates the release of mature IL-1β and is dependent on the P2X7 receptor, an ionotropic purinergic receptor and is expressed in several types of immune cells. It is well established that extracellular ATP, acting via P2X7, induces proteolytic maturation of IL-1β by means of NLRP3/caspase 1 complex activation [20–23]. However, IL-1β production does involve another step: the synthesis of pro-IL-1β beyond the proteolytic cleavage of pro-IL-1β by NLRP3/caspase 1 to form the mature cytokine [24]. In this context, the NF-κB pathway is a central element involved in IL-1β synthesis [25]. Previous data has shown that acute hyperhomocysteinemia increases NF-κB/p65 subunit expression in rat hippocampi, as well as the serum levels of TNF-α, IL-1β, IL-6, and CCL2 (MCP-1) [26, 5]. Furthermore, ERK activation in murine macrophages has been associated with the pathological effects of Hcy, such as an increase in metalloproteinase 9 production [27].
Therefore, the aim of the present study was to investigate the different effects of physiological and pathological concentrations of Hcy on IL-1β secretion by murine macrophages. The results show an increase in IL-1β release from macrophages exposed to 100 μM of Hcy and enhanced IL-1β synthesis via ERK, NF-ĸB, and P2X7.
Materials and methods
Animals
Male C57BL/6 WT and C57BL/6 P2X7−/− (6–8 weeks, 25–30 g) were used in our study. C57/BL6 mice were obtained from Universidade Federal de Pelotas (Pelotas, RS, Brazil) and P2X7−/− mice were a kind gift from Dr. Robson Coutinho-Silva, Federal University of Rio de Janeiro (UFRJ, Rio de Janeiro, Brazil). P2X7 knockout mice (originally from the Jackson Laboratory, USA) were generated using the method developed by Dr. James Mobley (PGRD, Pfizer Inc., Groton, CT, USA). P2X7 receptor-deficient mice used in our study are on a C57BL/6 inbred background.
The animals were maintained under a standard 12-h light–dark cycle (lights on at 7:00 a.m.) at a room temperature of 22 ± 2 °C. Mice had free access to standard laboratory rodent chow and tap water. The animal handling and experiments were performed in accordance with international guidelines in compliance with the Federation of Brazilian Societies for Experimental Biology and were approved by the local Animal Ethics Committee (protocol number: 10/00206).
Macrophage preparation and Hcy treatment
Peritoneal macrophages were collected by lavage of the peritoneal cavity, as described by Zanin et al. [28]. Macrophages were evaluated under a microscope after May-Grunwald and Giemsa staining, indicating macrophage purity higher than 80 %, which was confirmed by CD11b Ab staining.
To test the effects of Hcy, macrophages were treated 30 min after attachment to the plates with 10, 50, and 100 μM of l,d-homocysteine (Sigma Chemical Co., St. Louis, MO, USA - LPS free) for 24 h in replaced complete medium (RPMI 10 % fetal bovine serum, FBS). The concentration of 10 μM is considered physiological whereas 50 and 100 μM correspond to 25 and 50 μM of l-homocysteine, respectively, and these concentrations are considered to be hyperhomocysteinemic [10].
TLR4 −/− Macrophages
Macrophages were derived from the bone marrow of C57BL/6 WT mice and C57BL/6 TLR4−/− according to Lopes et al. [29]. The macrophages were then exposed as described in “Macrophage preparation and Hcy treatment.” The bone marrow mice were kindly donated by Dr. Cristina Beatriz Bonorino, Pontifícia Universidade Católica of Rio Grande do Sul (PUC-RS, Rio Grande do Sul, Brazil).
Measurement of IL-1β
IL-1β was measured in the supernatant and in the macrophage lysates. For this purpose, peritoneal macrophages were primed for 24 h with 10, 50, or 100 μM Hcy. After washing with PBS, the macrophages were stimulated with 2 mM of ATP for 30 min to induce the release of pro-IL-1β cytokines. In some experiments, ammonium pyrrolidinedithiocarbamate (PDTC, NF-κB inhibitor), PD98059 (ERK inhibitor), N-1330 (capase1 inhibitor), N-acetylcysteine (NAC, ROS inhibitor), and KN62 and A438079 (P2X7 antagonists) were added to macrophages media (30 min or 2 h prior as described in the figure legends) before the addition of ATP or Hcy. The IL-1β secreted from macrophages was quantified by ELISA following the manufacturer’s protocol (DuoSet Kit; R&D Systems, Minneapolis, MN, USA). The results were normalized by protein.
IL-1β from lysate macrophages was measure after exposing the cells to 100 μM Hcy for 24 h. After the treatment, macrophages were washed with PBS (1×) and the total protein extracted by freezing/thawing (2×) with a lysis buffer (10 mM Tris–HCl, pH 7.5, 1 mM MgCl2, 1 mM methylenediamine-tetraacetic acid [EDTA], 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 5 mM 2-mercaptoethanol, 0.5 % 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate [CHAPS], and 10 % glycerol). Protein concentrations were estimated using the Qubit (Invitrogen) equipment. This process was used to ensure that the same quantities of protein were analyzed. IL-1β was quantified by ELISA following the manufacturer’s protocol (DuoSet Kit; R&D Systems, Minneapolis, MN, USA).
Western blotting
For extract preparation, cells (5 × 105, 70–80 % confluence) were washed with cold PBS and incubated for 40 min on ice with lysis buffer (10 mM Tris–HCl, pH 7.5, 1 mM MgCl2, 1 mM methylenediamine-tetraacetic acid [EDTA], 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 5 mM DTT, 0.5 % 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate [CHAPS], and 0.2 % NP-40) mixing every 10 min. The homogenate was centrifuged (13,000×g, 30 s), and the supernatants containing the cytoplasmic extracts were stored at −80 °C. The nuclear pellet was suspended in 100-μL ice-cold hypertonic extraction buffer [10 mmol/L HEPES (pH 7.9), 0.42 M NaCl, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.5 mmol/L phenylmethylsulfonyl fluoride] and centrifuged at 13,000 rpm for 1 h at 4 °C and supernatants containing nuclear proteins utilized. Protein concentration was estimated using the Qubit (Invitrogen) equipment. Proteins (10 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on a 10 % (wt/vol) acrylamide and 0.275 % (wt/vol) bisacrylamide gels and electro-transferred onto nitrocellulose membranes. Membranes were incubated in TBS-T [20 mmol/L Tris–HCl, pH 7.5, 137 mmol/L NaCl, 0.05 % (vol/vol) Tween 20] containing 1 % (wt/vol) non-fat milk powder for 1 h at room temperature. Subsequently, the membranes were incubated for 2 h with the appropriate primary antibody, rinsed with TBS-T, and exposed to horseradish peroxidase-linked anti-IgG antibodies (Invitrogen) for 2 h at room temperature. Chemiluminescent bands were detected using X-ray films, and densitometry analyses were performed using Image-J software. Anti-lamin B (1:500) and anti-p65 NFκB (1:500) antibodies were purchased from Santa Cruz Biotechnologies and Millipore, respectively.
Flow cytometry
We evaluated the expression of P2X7 by flow cytometry using a rabbit polyclonal anti-mouse P2X7 (APR-004; Alomone Labs, Jerusalem, Israel) and anti-CD11b PE antibodies (BD Pharmingen). Briefly, before staining, the macrophage Fc receptors were blocked by incubating with Fc receptor blocking solution (Fc block: CD16/32, clone 2.4G2 from ATCC - HB-197) for 30 min on ice. The cells were then incubated for 30 min with the above primary antibodies diluted in PBS, 1 % FBS, and 0.1 % sodium azide (PFA) and, when necessary, with secondary Alexa 488-conjugated goat anti-rabbit IgG Ab (Invitrogen) for 30 min, with a minimum of two washes with PFA after each incubation. Cell surface fluorescence was measured using a FACSCalibur Flow Cytometer (BD Biosciences).
A separate set of experiments was performed to determine the activation status of phospho-ERK using the BD phosflow protocol for adherent cells. Macrophages were stimulated with 100 μM Hcy for 30 min in RPMI (supplemented with 0.5 % FBS, or not). For one set of experiments, cells were pre-treated with a selective ERK inhibitor (PD98059 20 μM) or a NF-ĸB inhibitor (PDTC 25 μM). Briefly, cells were fixed in Phosflow Buffer I for 10 min at 37 °C. After washing, cells were permeabilized using phosflow perm buffer II for 30 min on ice. The cells were then washed and stained with APC anti-phospho-ERK 1/2 antibody (BD Biosciences) for 30 min on ice. Finally, cell fluorescence was measured using a FACSCalibur Flow Cytometer (BD Biosciences).
Statistical analysis
Data are expressed as the mean ± SD and subjected to one-way or two-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test (for multiple comparisons) or an unpaired Student’s t test. Differences between mean values are considered significant when P < 0.05.
Results
P2X7 is involved in IL-1β release by macrophages exposed to Hcy
First, we examined the effects of Hcy on IL-1β secretion by macrophages. We observed that macrophages exposed to Hcy display a significant increase in IL-1β release only when given at pathological concentrations (50 and 100 μM) after treatment with 2 mM ATP (Fig. 1a). It is important to note that there is no difference between the two pathological Hcy concentrations tested. We then chose the pathological concentration of 100 μM Hcy for all other experiments.
Fig. 1.
Macrophages stimulated with Hcy plus ATP have increased IL-1β release and P2X7 expression. a Macrophages were treated with 10, 50, and 100 μM Hcy for 24 h and stimulated with 2 mM ATP for 30 min. After 60 min, supernatant IL-1β levels were then analyzed by ELISA as described in “Materials and Methods.” Data are representative of three independent experiments with pooled macrophages from 2 to 4 mice per experiment. ***p < 001 indicates a significant difference in relation to the respective controls and analyzed by a one-way ANOVA. b Flow cytometry analysis of surface P2X7 expression in macrophages treated with 100 μM Hcy. Mean fluorescence intensity (MFI) of P2X7 expression. Data are representative of three independent experiments with pooled macrophages from 2 to 4 mice per experiment. **p < 0.01 indicates a significant difference between macrophages treated with Hcy in relation to untreated controls by an unpaired Student’s t test. c IL-1β released by Hcy-primed macrophages as analyzed by ELISA and illustrated in the experimental design. IL-1β released in the absence or presence of Apyrase (Apy; 2U) or P2X7 antagonists (25 μM A438079 or 3 μM KN-62) and a caspase 1 inhibitor (200 μM N-1330). Data are representative of least three independent experiments and are expressed as the mean ± SD. ***p < 0.001 indicates a significant difference in relation to the other groups. Data were analyzed by a two-way ANOVA with a Tukey’s post hoc test. d IL-1β released from macrophages from P2X7-knockout mice (P2X7−/−). Data are representative of least three independent experiments and expressed as the mean ± SD. ***p < 0.001 indicates a significant difference in relation to the other groups. Data were analyzed by a two-way ANOVA with Tukey’s post hoc test
It is well known that P2X7 is a key receptor associated with the release of IL-1β from macrophages [20, 21, 23]. We therefore investigated whether P2X7 expression and functionality are altered in macrophages treated with a pathological concentration of Hcy (100 μM). Figure 1b shows that P2X7 protein expression increases in macrophages exposed to Hcy in relation to control. In addition, we found that P2X7 activation is involved in the secretion of mature IL-1β. P2X7 antagonists (KN-62 and A438079) and an ATP scavenger (Apyrase) prevent IL-1β secretion stimulated by ATP (Fig. 1c). Furthermore, N-1330, a caspase1 inhibitor, also prevents IL-1β secretion by macrophages treated with 100 μM of Hcy and stimulated with ATP. Additionally, macrophages obtained from P2X7−/− mice and treated with Hcy (100 μM) following ATP stimulation do not induce IL-1β secretion when compared to WT animals (Fig. 1d). It is interesting to note that IL-1β from P2X7−/− macrophage lysates is increased in a similar manner as the WT after HCY (100 μM) incubation for 24 h (data not shown), suggesting that intracellular Hcy signaling is not been impaired.
Hcy increases IL-1β production via ERK/NF-κB
The results presented in Fig. 1a show that Hcy (50 and 100 μM) alone is unable to induce Il-1β secretion when compared to controls or to the group that received only ATP stimulation. However, Hcy-exposed cells stimulated with 2 mM ATP have a significant increase in IL-1β secretion. We then decided to investigate which mechanisms could be contributing to IL-1β secretion by macrophages exposed to 100 μM of Hcy.
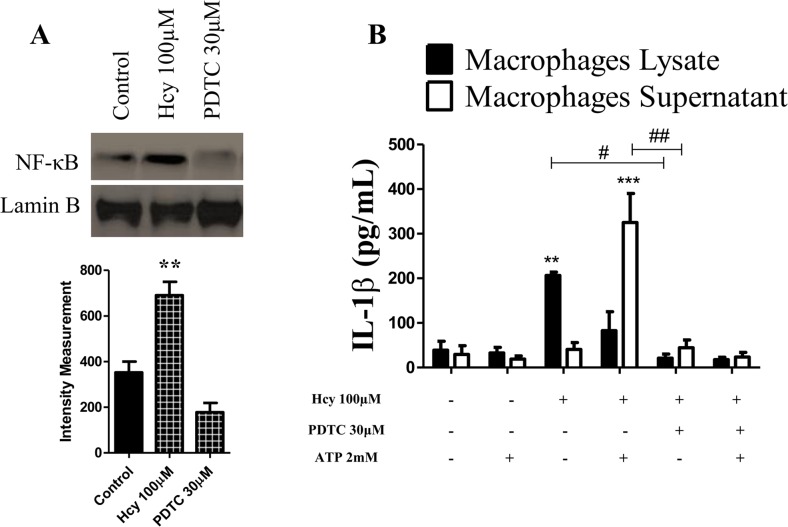
NF-κB is a central transcription factor that regulates the expression of multiple inflammatory genes including IL-1β [25]. The results presented in Fig. 2a show that Hcy (100 μM) treatment increases the nuclear NF-κB immune content. Furthermore, ammonium pyrrolidinedithiocarbamate PDTC, a potent NF-ĸB inhibitor, significantly decreases IL-1β secretion stimulated by ATP (Fig. 2b). Of note, our results reveal an increase in the intracellular IL-1β production in macrophage lysates treated only with Hcy (100 μM) in relation to the control and the NF-κB inhibitor treated groups. These results indicate that Hcy (100 μM) exposure induces IL-1β synthesis (increasing pro-IL-1β), which is dependent on NF-ĸB activation.
Fig. 2.
Hcy increases IL-1β production via NF-ĸB activation. a NF-ĸB nuclear protein assessed by Western blot from total cell extracts of macrophages treated for 24 h as described in “Materials and Methods.” The values represent means ± SD and data are representative of three independent experiments performed in triplicate. b IL-1β production by macrophages treated with Hcy for 24 h in the presence or absence of PDTC (an NF-ĸB inhibitor, 30 μM) 30 min before Hcy stimulation. The macrophages were then stimulated with 2 mM ATP for 30 min, and finally after 60 min, the macrophage supernatants and lysates were prepared as described in the “Materials and Methods,” and IL-1β concentration analyzed by ELISA. **p < 0.01 indicates a significant difference in relation to the respective controls from the macrophages lysate. ***p < 0.001 indicates a significant difference in relation to the respective controls from macrophage supernatants. # p < 0.05, ##p < 0.01 indicates a significant difference among the indicated groups. Data were analyzed by a two-way ANOVA with Tukey’s post hoc test
To determine whether ERK is involved in IL-1β synthesis induced by Hcy, we assessed ERK phosphorylation in our samples. We observed that 100 μM Hcy increases ERK phosphorylation in macrophages (Fig. 3a). It is noteworthy that ERK inhibition by PD98059 does not completely reduce IL-1β secretion from macrophages exposed to Hcy (100 μM) and stimulated with ATP (Fig. 3b), suggesting that ERK activation is only partially necessary to induce IL-1β secretion. However, the ERK inhibitor PD98059 (Fig. 3b) reduces intracellular IL-1β levels after Hcy (100 μM) treatment alone. It is important to note that the pathways used by NF-κB and ERK to produce IL-1β are independent of TLR4 activation as shown in Fig. 3c.
Fig. 3.
Hcy increases IL-1β production via ERK/NF-ĸB by murine macrophages. a Macrophages were pre-treated for 2 h with PD98059 (an ERK inhibitor, 25 μM) stimulated with 100 μM Hcy for 30 min and stained for phosphorylated ERK. Data are representative of two independent experiments performed in triplicate for each sample and expressed as the mean ± SD. ***p < 0.001 indicates a significant difference between the group treated with Hcy in relation to untreated controls as found by an unpaired Student’s t test. b IL-1β production by macrophages treated with Hcy for 24 h in the presence or absence of PD98059 (an ERK inhibitor, 25 μM) 120 min before Hcy stimulation. The macrophages were then stimulated with 2 mM ATP for 30 min, and finally after 60 min, the macrophage supernatants and lysates were prepared and IL-1β concentrations analyzed by ELISA as described in the “Materials and Methods.” The values represent means ± SD, and data are representative of three experiments performed in triplicate. **p < 0.01 indicates a significant difference in relation to the control macrophage lysates. ***p < 0.001 indicates a significant difference in relation to the control macrophage supernatants. #, ## p < 0.05 indicates a significant difference between the indicated groups. Asterisk indicates a significant difference between the p < 0.05 groups. Data were analyzed by a two-way ANOVA with a Tukey’s post hoc test. c IL-1β production from macrophages derived from the bone marrow of wild-type (WT) and TLR4− mice treated with Hcy for 24 h and stimulated with 2 mM ATP for 30 min, and finally after 60 min, the macrophage supernatants were analyzed by ELISA as described in “Materials and Methods.” Data are representative of two independent experiments performed in triplicate for each sample
Taken together, these results show that macrophages exposure to 100 μM of Hcy increases IL-1β synthesis through NF-κB and ERK activation. Moreover, IL-1β secretion induced by Hcy (100 μM) and ATP is partially dependent upon ERK activation.
Antioxidant pre-treatment reduces Hcy-induced ERK/NF-κB activation and IL-1β production
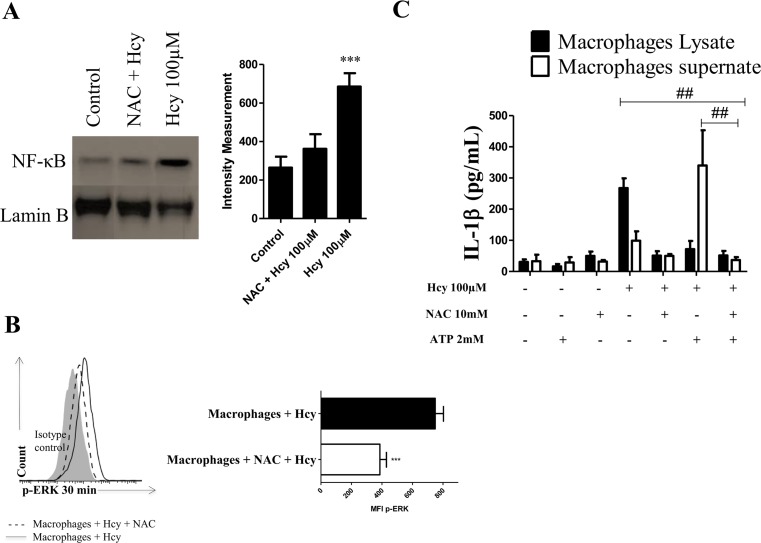
Next, we evaluated whether the Hcy effects we observed are related to ROS generation. NF-κB and ERK activation decreases in macrophages after N-acetylcysteine (NAC) pre-treatment, indicating the involvement of ROS in the effects of Hcy (100 μM) treatment (Fig. 4a, b). Figure 4c shows that pre-treatment with NAC decreases IL-1β synthesis (macrophage lysates) and release (macrophage supernatants) stimulated with Hcy (100 μM).
Fig. 4.
NAC inhibits IL-1β production via ERK/NF-ĸB in murine macrophages. a Macrophages were pre-treated 30 min in the presence or absence of NAC (10 mM) and stimulated with 100 μM Hcy for 24 h. Nuclear NF-ĸB protein was assessed by Western blot in nuclear cell extracts as described in “Materials and Methods. The experiments were repeated at least three times. ***p < 0.01 indicates a significant difference between the NAC treated group and the controls. Data were analyzed by a two-way ANOVA with a Tukey’s post hoc test. b Macrophages were pre-treated for 30 min in the presence or absence of NAC (10 mM) and stimulated with 100 μM Hcy for 30 min and immunostained for phosphorylated protein ERK. Phosphorylation of ERK is presented as MFI (shown in histograms). ***p < 0.01 indicates a significant difference between the group treated with NAC and the untreated controls as found from an unpaired Student’s t test. c IL-1β production from macrophages pre-treated with or without NAC (10 mM) and then treated with Hcy for 24 h and stimulated with 2 mM ATP for 30 min. After 60 min, the macrophage supernatants and lysates were prepared as described in the “Materials and Methods” and analyzed for IL-1β concentration by ELISA. The values represent means ± SD, and data are representative of three independent experiments performed in triplicate. ## p < 0.01 indicates a significant difference between the indicated groups. Data were analyzed by a two-way ANOVA with a Tukey’s post hoc test
Discussion
Increased circulating levels of Hcy are considered a risk factor for cardiovascular disease. Atherosclerosis is a chronic inflammation characterized by lipoprotein accumulation and monocyte recruitment and differentiation into macrophages. In the present study, we report an increase in IL-1β production in macrophages treated with a pathological Hcy concentration [5, 7, 12]. Although this effect is observed in macrophages in culture, it is suggestive that this pro-inflammatory process may contribute to the development of chronic inflammation such as atherosclerosis.
IL-1β is a classic pro-inflammatory cytokine that is essential for host–defense response to infection and injury. Monocytes and macrophages are pivotal cells that secrete IL-1β during pathological conditions. The process of IL-1β secretion is regulated by transcriptional and posttranscriptional controls [24, 25]. In this study, we demonstrate that the treatment of macrophages with Hcy at pathological concentrations (50 and 100 μM) induces IL-1β production, but not IL-1β secretion. In other words, the IL-1β secretion in response to Hcy exposure requires second stimuli such as ATP (Fig. 1).
Studies have shown that NLRP3 expression is a limiting factor for NLRP3/caspase 1 complex activation, suggesting this as an important step to the secretion of mature IL-1β. P2X7 stimulation by ATP is a well-known route for NLRP3/caspase 1 complex activation [24, 20, 21]. Accordingly, our results show an increase in P2X7 expression by macrophages in response to Hcy exposure, suggesting an important role for this receptor in this process (Fig. 2a). Moreover, the results obtained from the pharmacological inhibition or gene deletion of P2X7 confirm the involvement of this receptor in IL-1β secretion under our experimental conditions (Fig. 2b, c). Additionally, Gicquel et al. [30] have shown a connection between P2X7 and the NLRP3 inflammasome pathway in the release of IL-1β from ATP-stimulated human macrophages.
We also found that both the ERK and NF-κB signaling pathways are important for IL-1β synthesis in macrophages exposed to Hcy. Bauernfeind et al. [25] have also shown that NF-κB is a key element for regulating NLRP3 expression and consequent ATP-NLRP3/caspase 1 activation, resulting in secretion of mature IL-1β. Therefore, transcriptional (NF-κB and ERK activation and an increase in P2X7 expression) and a posttranscriptional signals (P2X7 stimulation) are related to IL-1β release from macrophages in response to Hcy exposure.
Other studies have shown that the injury associated with Hcy could be due to the increased rates of oxidative stress [31–33, 8]. In mammalian cells, redox status is related to cell signaling, which triggers the activation of protein kinase pathways and results in gene expression control, proliferation, and modulation of immune cell functions [34, 35]. Our results show that NAC, a free radical scavenger, decreases IL-1β secretion in macrophages exposed to Hcy, suggesting an imbalance redox during this process. In addition, activation of NF-κB is known to be regulated by ROS [36–38]. We found that NAC treatment reverses NF-κB activation to control levels in response to Hcy, but only partially decreases ERK activation. In agreement with our findings, other studies have shown an increase in NF-κB activation and ERK following Hcy exposure [26, 27]. Hence, the redox disequilibrium may be a mechanism whereby Hcy induces NF-κB and ERK activation and, consequently, IL-1β production in macrophages.
In conclusion, we show that the mechanisms involved in IL-1β production by macrophages exposed to Hcy are dependent upon NF-ĸB and ERK activation through a P2X7 trigger. This mechanism may not be the only one involved in IL-1β release during Hcy exposure, but it is also important to note that these may represent potential therapeutic alternatives for new pharmacological treatments for diseases associated with Hcy.
Acknowledgments
This work was supported by CNPq-Brazil. R.F. Zanin was recipient of a CNPq PhD fellowship. We would like to thank Dr. C. Bonorino for use of the FACSanto II machine and Dr. Maria M. Campos for kindly donating some of the reagents.
References
- 1.Finch JM, Joseph J. Homocysteine, cardiovascular inflammation, and myocardial remodeling. Cardiovasc Hematol Disord Drug Targets. 2010;10:241–245. doi: 10.2174/187152910793743887. [DOI] [PubMed] [Google Scholar]
- 2.Ueland PM, Clarke R. Homocysteine and cardiovascular risk: considering the evidence in the context of study design, folate fortification, and statistical power. Clin Chem. 2007;53:807–809. doi: 10.1373/clinchem.2007.085480. [DOI] [PubMed] [Google Scholar]
- 3.Undas A, Brozek J, Szczeklik A. Homocysteine and thrombosis: from basic science to clinical evidence. Thromb Haemost. 2005;94:907–915. doi: 10.1160/TH05-05-0313. [DOI] [PubMed] [Google Scholar]
- 4.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Cunha AA, Ferreira AG, Wyse AT. Increased inflammatory markers in brain and blood of rats subjected to acute homocysteine administration. Metab Brain Dis. 2010;25:199–206. doi: 10.1007/s11011-010-9188-8. [DOI] [PubMed] [Google Scholar]
- 6.Holven KB, Aukrust P, Retterstol K, Hagve TA, Morkrid L, et al. Increased levels of C-reactive protein and interleukin-6 in hyperhomocysteinemic subjects. Scand J Clin Lab Invest. 2006;66:45–54. doi: 10.1080/00335510500429821. [DOI] [PubMed] [Google Scholar]
- 7.Gori AM, Corsi AM, Fedi S, Gazzini A, Sofi F, et al. Aproinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr. 2005;82:335–341. doi: 10.1093/ajcn.82.2.335. [DOI] [PubMed] [Google Scholar]
- 8.da Cunha AA, Scherer E, da Cunha MJ, Schmitz F, Machado FR, et al. Acute hyperhomocysteinemia alters the coagulation system and oxidative status in the blood of rats. Mol Cell Biochem. 2012;360:205–214. doi: 10.1007/s11010-011-1058-0. [DOI] [PubMed] [Google Scholar]
- 9.Papatheodorou L, Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. 2007;9:1941–1958. doi: 10.1089/ars.2007.1750. [DOI] [PubMed] [Google Scholar]
- 10.Perla-Kajan J, Twardowski T, Jakubowski H. Mechanisms of homocysteine toxicity in humans. Amino Acids. 2007;32:561–572. doi: 10.1007/s00726-006-0432-9. [DOI] [PubMed] [Google Scholar]
- 11.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 12.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13. [PubMed] [Google Scholar]
- 16.Fearon WF, Fearon DT. Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation. 2008;117:2577–2579. doi: 10.1161/CIRCULATIONAHA.108.772491. [DOI] [PubMed] [Google Scholar]
- 17.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 18.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 22.Martins JP, Silva RB, Coutinho-Silva R, Takiya CM, Battastini AM, et al. The role of P2X7 purinergic receptors in inflammatory and nociceptive changes accompanying cyclophosphamide-induced haemorrhagic cystitis in mice. Br J Pharmacol. 2012;165:183–196. doi: 10.1111/j.1476-5381.2011.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bours MJ, Dagnelie PC, Giuliani AL, Wesselius A, Di Virgilio F. P2 receptors and extracellular ATP: a novel homeostatic pathway in inflammation. Front Biosci (Schol Ed) 2011;3:1443–1456. doi: 10.2741/235. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Cunha AA, Horn AP, Hoppe JB, Grudzinski PB, Loureiro SO, et al. Evidence that AKT and GSK-3beta pathway are involved in acute hyperhomocysteinemia. Int J Dev Neurosci. 2012;30:369–374. doi: 10.1016/j.ijdevneu.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Lee YS, Seo KW, Bae JU, Kim GH, et al. Homocysteine enhances MMP-9 production in murine macrophages via ERK and Akt signaling pathways. Toxicol Appl Pharmacol. 2012;260:89–94. doi: 10.1016/j.taap.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Zanin RF, Braganhol E, Bergamin LS, Campesato LF, Filho AZ, et al. Differential macrophage activation alters the expression profile of NTPDase and ecto-5’-nucleotidase. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes RL, Borges TJ, Araújo JF, et al. Extracellular mycobacterial DnaK polarizes macrophages to the M2-like phenotype. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gicquel T, Victoni T, Fautrel A, et al. Involvement of purinergic receptors and NOD-like receptor-family protein 3-inflammasome pathway in the adenosine triphosphate-induced cytokine release from macrophages. Clin Exp Pharmacol Physiol. 2014;41:279–286. doi: 10.1111/1440-1681.12214. [DOI] [PubMed] [Google Scholar]
- 31.Matte C, Stefanello FM, Mackedanz V, Pederzolli CD, Lamers ML, et al. Homocysteine induces oxidative stress, inflammatory infiltration, fibrosis and reduces glycogen/glycoprotein content in liver of rats. Int J Dev Neurosci. 2009;27:337–344. doi: 10.1016/j.ijdevneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Werstuck GH, Lhotak S, de Koning AB, Sood SK, et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemicapolipoprotein E-deficient mice. Circulation. 2004;110:207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- 33.Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest. 1996;98:5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truong TH, Carroll KS (2013) Redox regulation of protein kinases. Crit Rev BiochemMolBiol. [DOI] [PMC free article] [PubMed]
- 36.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/S0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 37.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J Immunol. 2005;175:5423–5429. doi: 10.4049/jimmunol.175.8.5423. [DOI] [PubMed] [Google Scholar]