Abstract
Amyotrophic lateral sclerosis (ALS) is a disease leading to neuromuscular transmission impairment. A2A adenosine receptor (A2AR) function changes with disease stage, but the role of the A1 receptors (A1Rs) is unknown and may have a functional cross-talk with A2AR. The role of A1R in the SOD1(G93A) mouse model of ALS in presymptomatic (4–6 weeks old) and symptomatic (12–14 weeks old) phases was investigated by recording endplate potentials (EPPs), miniature endplate potentials (MEPPs), and quantal content (q.c.) of EPPs, from Mg2+ paralyzed hemidiaphragm preparations. In presymptomatic mice, the A1R agonist, N6-cyclopentyladenosine (CPA) (50 nM), decreased mean EPP amplitude, MEPP frequency, and q.c. of EPPs, an effect quantitatively similar to that in age-matched wild-type (WT) mice. However, coactivation of A2AR with CGS 21680 (5 nM) prevented the effects of CPA in WT mice but not in presymptomatic SOD1(G93A) mice, suggestive of A1R/A2AR cross-talk disruption in this phase of ALS. DPCPX (50 nM) impaired CGS 21680 facilitatory action on neuromuscular transmission in WT but not in presymptomatic mice. In symptomatic animals, CPA only inhibited transmission if added in the presence of adenosine deaminase (ADA, 1 U/mL). ADA and DPCPX enhanced more transmission in symptomatic mice than in age-matched WT mice, suggestive of increase in extracellular adenosine during the symptomatic phase of ALS. The data documents that at the neuromuscular junction of presymptomatic SOD1(G93A) mice, there is a loss of A1R-A2AR functional cross-talk, while in symptomatic mice there is increased A1R tonic activation, and that with disease progression, changes in A1R-mediated adenosine modulation may act as aggravating factors during the symptomatic phase of ALS.
Keywords: Adenosine, ALS, Neuromuscular transmission, SOD1(G93A) mice
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that impairs motor neuron function resulting in muscle weakness and atrophy. After symptomatic onset, disease progression lasts 4–5 years with patients ultimately suffering from bulbar failure. The majority of the cases relate to unknown genetic mutations (sporadic ALS) with a few (5–10 %) having a known genetic background in specific proteins (familial ALS) [1]. The SOD1 encodes for the superoxide dismutase 1 enzyme and relates to ≈20 % of familial cases [2]. It was the first gene associated with ALS and used to create the SOD1(G93A) mouse model, which became the most intensely studied and well-characterized rodent model for the disease [3].
Adenosine is a key modulator of neuromuscular transmission, fine-tuning acetylcholine release by acting on both inhibitory A1 (A1R) and excitatory A2A (A2AR) adenosine receptors [4]. Early changes in neuromuscular transmission in the SOD1(G93A) mouse appear before motor symptoms onset [5, 6]. On the other hand, A1Rs and A2ARs interact in the control of synaptic transmission with potential implications in neurodegenerative diseases [7–10]. However, the function of A1Rs on neuromuscular transmission in ALS remains to be known.
In the present work, we explore the role of A1Rs on neuromuscular transmission in both the presymptomatic (4–6-week-old mice) and symptomatic (12–14-week-old mice) SOD1(G93A) mice and if these receptors are under control of A2ARs at the presymptomatic phase. The results showed that at presymptomatic ALS mice neuromuscular junctions, the A1R/A2AR functional cross-talk is disrupted, and that in the symptomatic phase, there is an exacerbated inhibitory A1R tonic activation.
Methods
Ethics statement
The study herein reported was performed in accordance with the European Community guidelines (Directives 86/609/EU and 2010/63/EU, Recommendation 2007/526/CE, European Convention for the Protection of Vertebrate Animals used for Experimental or Other Scientific Purposes ETS 123/Appendix A) and Portuguese Laws on Animal Care (Decreto-Lei 129/92, Portaria 1005/92, Portaria466/95, Decreto-Lei 197/96, Portaria 1131/97). Every protocol within this study was under approval of the Portuguese National Authority (General Direction of Veterinary) and the Ethics Committee of the Instituto de Medicina Molecular of the Faculty of Medicine, University of Lisbon, Lisbon, Portugal.
Animals
Transgenic B6SJL-TgN (SOD1-G93A)1Gur/J males (Jackson Laboratory, No. 002726) (G93A) [3] and wild-type B6SJLF1/J females were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred at IMM rodent facilities where a colony was established. A B6SJL background was maintained by breeding SOD1(G93A) transgenic males with non-transgenic females in a rotational scheme. F1 offspring was used in all experiments. Progeny was no longer used in breeding to avoid mSOD1 gene copy number loss and deviation from typical ALS phenotype [3]. The study used presymptomatic (4–6 weeks old) and symptomatic (12–14 weeks old) SOD1(G93A) mice and 4–6- and 12–14-week-old wild-type (WT) animals that served as age-matched controls. Both male and female mice were used in equal proportions as in previous works, since no gender influences over the intrinsic features of neuromuscular transmission have been detected in the B6SJL-Tg(SOD1-G93A)1Gur/J strain [5, 6].
Littermates were identified by dermal ear punching and separated into cages according to gender. Ear tissue was used in the genotyping protocol through polymerase chain reaction (PCR) [2]. Animals were housed four to five mice/cage, under a 12-h light/12-h dark cycle, receiving food and water ad libitum.
Electrophysiological recordings
Rodents were anesthetized using isoflurane and immediately decapitated. Right and left phrenic-nerve-attached hemidiaphragm muscles were then isolated. The phrenic nerve-hemidiaphragm preparation was used because it is the nerve-muscle preparation where adenosinergic modulation is better characterized. The phrenic-diaphragm has a large margin of safety, thus being one of the last muscles to be affected by ALS progression [1]. Variation in the safety margin of neuromuscular transmission, however, does not reflect in the present work since in our experimental conditions, we eliminated safety margin of neuromuscular transmission by blocking muscle contraction with high Mg2+ (see “Drugs and solutions” section). In each experiment, one phrenic nerve-hemidiaphragm preparation was stretched in a 3-mL volume Perspex chamber continuously perfused via a roller pump (3 mL min−1) with a physiologic saline solution (Krebs and Henseleit solution, see “Drugs and solutions” section) continuously oxygenated. The other preparation was immersed in a beaker with an oxygenated saline solution before being set up in the recording chamber. No functional differences were found between right and left phrenic nerve-hemidiaphragm preparations, allowing different protocols to be tested per animal.
Intracellular recordings were performed in the conventional way [11–13]. The phrenic nerve was stimulated supramaximally through a suction electrode (Cu/Cu2+) connected to a S48 square pulse stimulator (Grass Tecnologies, West Warwick, RI, USA). Stimuli were applied in a low frequency of 0.5 Hz with a current duration of 20 μs. The reference electrode was an Ag-AgCl pellet immersed in the bath. After checking for nerve-evoked contractions, muscle twitching in response to nerve stimulation was prevented by increasing the Mg2+ concentration in the bath (see “Drugs and solutions” section) and then a glass microelectrode filled with KCl (3 M) and 15–40 MΩ resistance was inserted into the motor endplate for electrophysiological recordings. Data acquisition was performed by a Digidata 1440A digitizer (Molecular Devices, Sunnyvale, CA, USA), designed to work with the Axoclamp 2B amplifier (Molecular Devices, Sunnyvale, CA, USA). Continuous monitoring and digital storage of evaluated parameters were performed with adequate software (pCLAMP 10.3, Molecular Devices, Sunnyvale, CA, USA).
Experiments were performed in endplates with a resting membrane potential between −65 and −85 mV. Throughout all experiments, the membrane potentials varied less than 5 % of its baseline control value. Endplate potential (EPP) amplitude was assessed as the average amplitude of 60 consecutive EPPs (with amplitudes ranging between 1 and 5 mV). To quantify the effect of a drug, the mean averaged EPP amplitudes in the last 10 min before adding that drug (taken as baseline control) was compared with the mean averaged EPP amplitudes from the last 10 min of drug perfusion. The quantal content (q.c.) of EPPs was calculated as the ratio between the mean EPP amplitude and the mean miniature endplate potential (MEPP) amplitude acquired during the same period. MEPPs were recorded in gap-free intervals of 100 s before initiating and at the end of drug perfusion. MEPP amplitude in these animals exhibits a normal Gaussian distribution allowing to set a MEPP detection threshold between 0.2 and 1 mV [5], while spontaneous events above 1 mV were considered as “giant” and were not considered for analysis. This allows to calculate q.c. of EPPs and MEPP frequency with a reduced margin of error [5, 6]. MEPP amplitude was defined as the mean of the amplitude of all spontaneous events and the frequency as the number of events registered during the 100 s. Offline analysis of evoked activity was performed with Clampfit software (Molecular Devices, Sunnyvale, CA, USA), while spontaneous events were analyzed with Mini-Analysis software (Synaptosoft Inc., Decatur, GA, USA).
Drugs and solutions
The bathing solution was adapted from Krebs and Henseleit [14] (NaCl 117 mM, KCl 5 mM, NaHCO3 25 mM, NaH2PO4 1.2 mM, glucose 11 mM, CaCl2 2.5 mM, MgCl2 1.2 mM, pH 7.4) continuously bubbled with 95 % O2 and 5 % CO2 maintained at room temperature (22–25 °C). Muscle contraction was prevented by raising [Mg2+] to 18.5–19.5 mM in 4–6-week-old animals and 20.0–22.0 mM in 12–14-week-old mice, thus reducing the q.c. of EPPs but preserving the main features of neuromuscular transmission [6, 11].
Drugs used were N6-cyclopentyladenosine (CPA), 2-p-(2-carboxyethyl) phenethylamino]-5′-N-ethylcarboxamido adenosinehydrochloride (CGS 21680), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), 5-amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo(4,3-e)-1,2,4-triazolo(1,5-c) pyrimidine (SCH 58261), adenosine deaminase type VI, 1803 U/mL, EC 3.5.44 (ADA), and 2-chloroadenosine (CADO). Stock solutions (5 mM) of CPA, CGS 21680, SCH 58261, DPCPX, and CADO were made in dimethyl sulfoxide. Aliquots were kept frozen at −20 °C until used. At the maximal concentration applied to the preparation (1/100,000 v/v), dimethyl sulfoxide was devoid of effect as now tested and previously reported [11].
Statistical analysis
Data are reported as mean ± standard error of the mean in each group, with n corresponding to the number of animals used (one endplate per hemidiaphragm).
Statistical comparison of drug effects between two groups was done using Student’s t test for independent samples (unpaired t test). Student’s t test for paired samples (paired t test) was applied to data obtained before and after adding a drug (e.g., mean MEPP frequency before drug perfusion and at the end of this perfusion). Values of p < 0.05 indicated statistically significant differences.
Results
In presymptomatic SOD1(G93A) mice, the functional interaction between A1R/A2AR is impaired
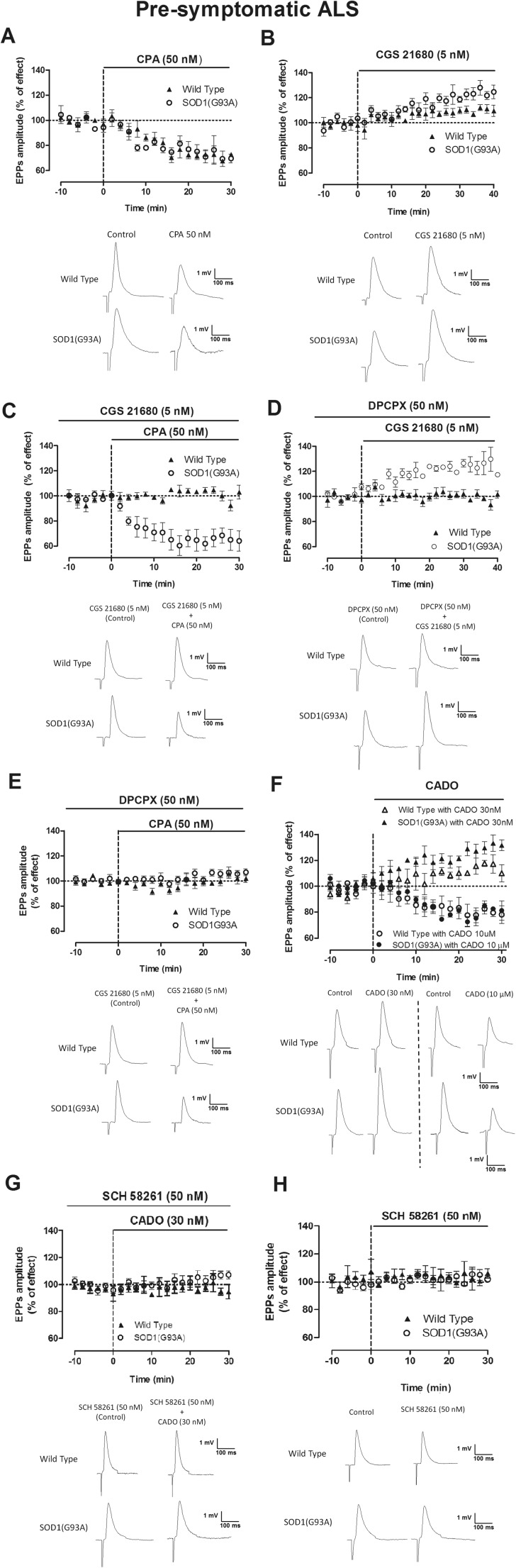
As illustrated in Fig. 1a, the A1R selective agonist CPA (50 nM) decreased (p < 0.05 paired t test) the mean EPP amplitude in presymptomatic SOD1(G93A) mice (−28.6 ± 2.8 %, n = 6), as it did in age-matched WT animals (−25.2 ± 4.7 %, n = 5), but these effects were not significantly different between groups (p > 0.05 unpaired t test). The A2AR agonist CGS 21680 (5 nM) increased mean EPP amplitude in both groups, with the effect being significantly larger (p < 0.05 unpaired t test) in presymptomatic mice (23.5 ± 3.0 %, n = 13) than in healthy controls (8.1 ± 2.5 %, n = 14) (Fig. 1b), as previously described [6]. Prior activation of A2AR with CGS 21680 (5 nM) greatly impaired the inhibitory effect of CPA (50 nM) in mean EPP amplitude in WT mice, but not in presymptomatic SOD1(G93A) mice (Fig. 1c). An attenuation of the inhibitory action of A1R by coactivation of A2AR is known to occur at the neuromuscular junction [15] as well as in the central nervous system synapses [9, 16] of young mice. Our data with coapplication of receptor agonists indicates that this functional interaction is disrupted in presymptomatic SOD1(G93A) mice. Another hallmark of A1R/A2AR interaction in young mice is the ability of A1R receptor antagonists to prevent facilitatory actions of A2AR agonists [11, 16]. To further assess the A1R/A2AR cross-talk in presymptomatic SOD1(G93A) mice, we then evaluated the influence of an A1R antagonist, DPCPX (50 nM) over the facilitatory action of the A2AR agonist, CGS 21680 (5 nM) on neuromuscular transmission. As shown in Fig. 1d, the facilitatory action of CGS 21680 (5 nM) on neuromuscular transmission of presymptomatic mice remains in spite of the presence of DPCPX (50 nM), therefore further supporting the hypothesis that in presymptomatic mice, the functional cross-talk between A1R and A2AR is impaired. By itself, DPCPX (50 nM) was virtually devoid of effect on mean EPP amplitude both in presymptomatic SOD1(G93A) mice (2.5 ± 3.0 %, n = 6) or in age-matched WT mice (1.5 ± 2.2 %, n = 9). As expected, DPCPX (50 nM) effectively blocked the inhibitory effect of CPA (50 nM) in either presymptomatic SOD1(G93A) mice (n = 4) or age-matched WT mice (n = 3) (Fig. 1e).
Fig. 1.
A1R-mediated modulation of neuromuscular transmission and its action on presymptomatic SOD1(G93A) mice as compared with age-matched WT mice. Note that in 4–6-week-old WT mice, the A1AR-mediated action on mean EPP amplitude is impaired by the coaction of the A2AR agonist, while the action of the A2AR agonist is blocked by coapplication of an A1R antagonist. a CPA at 50 nM (n = 5, WT, n = 5, SOD1(G93A)), b CGS at 5 nM (n = 10, WT, n = 10, SOD1(G93A)). c CPA (50 nM) after prior application of CGS 21680 (5 nM) (n = 4, WT; n = 5, SOD1(G93A)), d CGS 21680 at 5 nM after previous blockade of A1R by DPCPX at 50 nM (n = 4, WT, n = 5, SOD1(G93A)), e DPCPX (50 nM) blockade of CPA (50 nM) effect on mean EPP amplitude (n = 4, WT, n = 5, SOD1(G93A)), f mean EPP changes and representative EPP recording upon perfusion of CADO at 30 nM (n = 5, WT; n = 3, SOD1(G93A)) and 10 μM (n = 3, WT; n = 3, SOD1(G93A)), g SCH 58261 at 50 nM when coapplied with CADO at 30 nM (n = 4, WT, n = 3, SOD1(G93A)) in presymptomatic rodents, and h SCH (50 nM) in wild-type (n = 5) and SOD1(G93A) mice (n = 5)
To evaluate the q.c. of EPPs, EPPs and MEPPs were recorded simultaneously. The mean MEPP amplitude was not significantly changed in all experiments, confirming that A1Rs and A2ARs act as presynaptic modulators [17]. After perfusion of CPA (50 nM), MEPP frequency was decreased in presymptomatic SOD1(G93A) mice as in age-matched WT mice (p < 0.05 paired t test). The q.c. of EPPs was also decreased by CPA (50 nM) in both groups of animals (Table 1). As it occurred with mean EPP amplitude, the inhibitory effect of CPA (50 nM) on the q.c. of EPPs but not on MEPP frequency was prevented in WT mice but not presymptomatic mice when applied in the presence of the A2AR agonist, CGS 21680 (5 nM) (Table 1). The A1R antagonist, DPCPX (50 nM), significantly impaired A2AR activation by CGS 21680 (5 nM) in WT mice, but not in age-matched presymptomatic SOD1(G93A) mice (Table 1). As a whole, therefore, data obtained while evaluating q.c. of EPPs and frequency of MEPPs relates to the data obtained while recording EPP amplitude, further confirming the presynaptic nature of the effects.
Table 1.
A1R receptor activation decreased MEPP frequency in both groups even after A2AR activation, while the results in the facilitation of the q.c. of EPPs indicate the presence of a functional activation/blockade interaction between A1R and A2AR in 4–6-week-old WT mice that is not present in SOD1(G93A) rodents
| 4–6 weeks old | ||||
|---|---|---|---|---|
| MEPP frequency (% of effect) | Quantal content of EPPs (% of effect) | |||
| Wild type | SOD1(G93A) | Wild type | SOD1(G93A) | |
| CPA 50 nM | −22.9 ± 4.2# (n = 10) | −24.5 ± 3.4# (n = 11) | −26.2 ± 2.6# (n = 6) | −24.7 ± 5.0# (n = 6) |
| CPA 50 nM (in the presence of CGS 21680 5 nM) | −23.2 ± 7.6# (n = 4) | −20.7 ± 7.0# (n = 4) | −2.3 ± 5.3*CPA50nM (n = 4) | −21.7 ± 4.1#*WT (n = 4) |
| CGS 21680 5 nM (in the presence of DPCPX 50 nM) | 2.3 ± 0.9 (n = 6) | −5.2 ± 8.3*CGS 21680 (n = 5) | 0.0 ± 2.2*CGS 21680 (n = 6) | 18.3 ± 4.6#*WT (n = 5) |
| DPCPX 50 nM | −4.9 ± 6.8 (n = 6) | 7.1 ± 3.6 (n = 6) | 1.2 ± 2.8 (n = 8) | 6.0 ± 1.1 (n = 5) |
| CGS 21680 5 nM | −4.2 ± 7.7 (n = 10) | 43.3 ± 19.9#*WT (n = 9) | 8.3 ± 2.3# (n = 12) | 24.0 ± 4.1#*WT (n = 13) |
| CADO 30 nM | 8.4 ± 6.6 (n = 4) | 33.1 ± 6.4 #*WT (n = 4) | 13.8 ± 1.1# (n = 4) | 28.3 ± 5.0#*WT (n = 4) |
| CADO 10 μM | −22.5 ± 8.7#*CADO30nM (n = 3) | −18.1 ± 5.1#*CADO30nM (n = 3) | −22.9 ± 4.2#*CADO30nM (n = 5) | −24.3 ± 2.0#*CADO30nM (n = 3) |
| CADO 30 nM (in the presence of SCH 58261 50 nM) | −3.6 ± 4.2 (n = 3) | 3.3 ± 5.4*CADO30nM (n = 3) | −0.7 ± 2.0*CADO30nM (n = 3) | 1.8 ± 4.3*CADO30nM (n = 3) |
| SCH 58261 50 nM | 2.4 ± 2.3 (n = 5) | −0.6 ± 3.2 (n = 5) | −1.9 ± 3.2 (n = 4) | 3.2 ± 1.1 (n = 5) |
The values are mean ± SEM of % of drug effect. Results from presymptomatic SOD1(G93A) animals were compared with age-matched WT mice and with each other. 100 % represents MEPP frequency or q.c. of EPPs before beginning drug perfusion of the drug under test
*p < 0.05 unpaired t test vs similar parameters and drug in WT
#p < 0.05 paired t test vs before drug perfusion
Since in presymptomatic mice, there is a clear A2AR-mediated facilitatory action of neuromuscular transmission ([6] and present work) together with a clear A1R-mediated inhibitory action that becomes unaffected by A2AR activation (Fig. 1a–d), we assessed the action of a receptor agonist, CADO, which does not discriminate between both receptors, therefore mimicking the action of adenosine with the advantage of not being metabolized or taken up. As shown in Fig. 1f, CADO at a nanomolar concentration (30 nM) caused a clear facilitatory action on EPP amplitude, whereas at higher concentrations (10 μM), it caused a clear inhibitory action on EPP amplitude. Similarly, it caused a facilitation of MEPP frequency and q.c. of EPPs at the nanomolar concentration but an inhibitory action at the micromolar concentration (Table 1). Facilitatory actions of CADO on mean EPP amplitude in aged animals are due to an action over A2AR [17]. Similarly, the facilitatory action of CADO (30 nM) on mean EPP amplitude in presymptomatic mice could be attributed to A2AR activation, since it was prevented by the A2AR antagonist, SCH 58261 (50 nM) (0.3 ± 4.2 %, n = 3, p > 0.05 paired t test; Fig. 1g). Also depicted in Fig. 1h, the A2AR antagonist by itself was virtually devoid of effect on EPP amplitude (4.5 ± 2.1 %, n = 5, p > 0.05 paired t test).
Altogether, the above results indicate that in presymptomatic ALS mice, there is a disruption of the cross-talk between A1R and A2AR, and that the inhibitory A1R-mediated action is preserved in ALS mice. In contrast, as previously reported [6], the A2AR-mediated facilitation is exacerbated.
Symptomatic SOD1(G93A) mice have an exacerbated tonic A1R activation on neuromuscular transmission
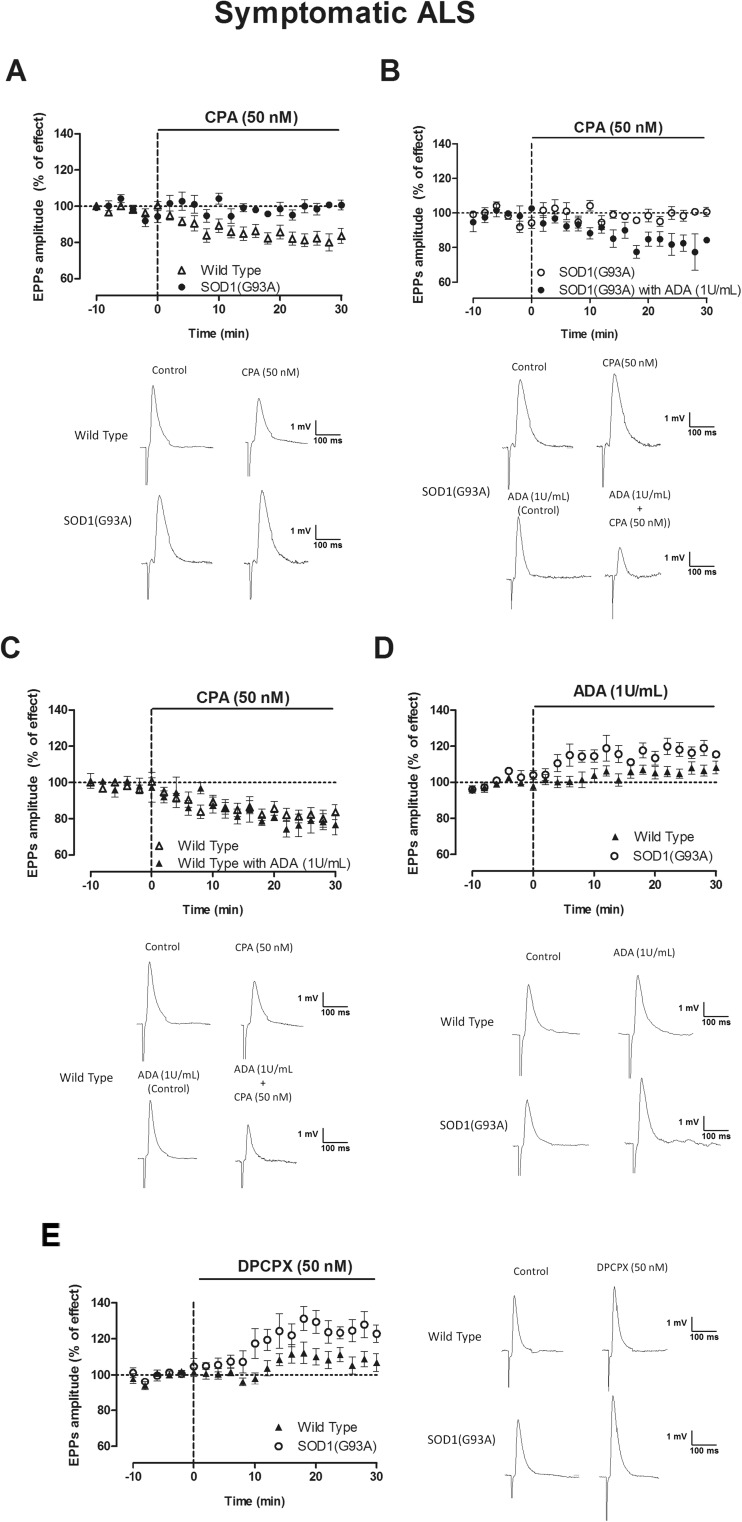
Symptomatic SOD1(G93A) mice do not display A2AR-mediated effects on neuromuscular transmission [6], and therefore, we decided not to explore A1-A2AR functional interactions at this stage and to focus our attention on the influence of A1Rs in these animals. CPA (50 nM) decreased the mean EPP amplitude in WT, but not in symptomatic animals (p < 0.05 unpaired t test) (Fig. 2a). Remarkably, in the presence of ADA (1 U/mL), known to hydrolyze extracellular adenosine into inosine (which has virtually no affinity for A1R or A2ARs), the inhibitory effect of CPA (50 nM) on mean EPP amplitude in symptomatic SOD1(G93A) mice was rescued (p < 0.05 paired t test) (Fig. 2b), while the presence of ADA did not influence the inhibitory action of CPA in age-matched WT mice (Fig. 2c). ADA (1 U/mL) (Fig. 2d) and DPCPX (50 nM) (Fig. 2e) had a higher effect upon EPP amplitude in symptomatic mice than in age-matched controls (p < 0.05 unpaired t test) (Fig. 2d, e).
Fig. 2.
Symptomatic SOD1(G93A) mice have a higher A1R tonic modulation of mean EPP amplitude when compared to 12–14-week-old WT mice; note also that removal of endogenous adenosine rescues the inhibitory effect of the A1AR agonist in symptomatic mice. a Representative EPP recording and changes of mean EPP amplitude caused by CPA 50 nM (n = 17, WT, n = 19, SOD1(G93A)) and with prior ADA (1 U/mL) application in b SOD1(G93A) (n = 11) and c WT mice (n = 5). Effect of d ADA (1 U/mL) (n = 14, WT, n = 6, SOD1(G93A)) and e the A1R antagonist DPCPX (50 nM) (n = 7, WT, n = 5, SOD1(G93A))
Altogether, these data suggest that the levels of adenosine are increased at the synaptic cleft in symptomatic animals, thus resulting in a higher tonic A1R activation in this group, which occludes the action of exogenously applied agonists. MEPP frequency and q.c. of EPPs detected were consistent with mean EPP amplitude data, confirming the presynaptic nature of the effects. This MEPP frequency and the q.c. of EPPs (Table 2) were decreased in WT mice by CPA (50 nM) (p < 0.05 paired t test), while the A1R agonist was virtually devoid of effect in SOD1(G93A) animals (p > 0.05 paired t test). As it occurred with mean EPP amplitude, the inhibitory action of CPA (50 nM) on MEPP frequency and q.c. of EPPs was rescued in the presence of ADA (1 U/mL) (Table 2). By itself, ADA (1 U/mL) increased MEPP frequency and q.c. of EPPs in both groups (p < 0.05 paired t test), the effect in the q.c. of EPPs being significantly higher in SOD1(G93A) mice than in age-matched WT mice (Table 2) (p < 0.05 unpaired t test). The facilitatory action of DPCPX (50 nM) on the q.c. of EPPs was also higher in SOD1(G93A) mice than in age-matched WT controls (p < 0.05 unpaired t test).
Table 2.
Symptomatic SOD1(G93A) mice have a higher A1R tonic modulation of MEPP frequency and q.c. of EPPs when compared to 12–14-week-old WT mice
| 12–14 weeks old | ||||
|---|---|---|---|---|
| MEPP frequency (% of effect) | Quantal content of EPPs (% of effect) | |||
| Wild type | SOD1(G93A) | Wild type | SOD1(G93A) | |
| CPA 50 nM | −23.0 ± 3.1# (n = 17) | −0.8 ± 4.4*WT (n = 20) | −18.6 ± 2.1# (n = 18) | −6.3 ± 2.1*WT (n = 21) |
| CPA 50 nM (in the presence of ADA, 1 U/mL) | −25.0 ± 4.7# (n = 5) | −41.0 ± 9.2#*CPA50nM (n = 6) | −19.3 ± 4.1# (n = 4) | −16.3 ± 3.5#*CPA50nM (n = 11) |
| ADA 1 U/mL | 29.3 ± 6.3# (n = 8) | 28.0 ± 8.8# (n = 9) | 7.0 ± 1.7# (n = 14) | 15.5 ± 2.8# *WT (n = 7) |
| DPCPX 50 nM | 26.1 ± 10.0# (n = 8) | −8.4 ± 6.4*WT (n = 12) | 12.1 ± 2.1# (n = 8) | 25.3 ± 2.5# *WT (n = 6) |
The values are mean ± SEM of % of drug effect. Results from SOD1(G93A) animals were compared with age-matched WT mice (indicated with WT symbol next to the statistical significance symbol) and with each other (indicated with drug name symbol next to the statistical significance)
*p < 0.05 unpaired t test
#p < 0.05 paired t test (as compared with control value before drug perfusion; control corresponds to 100 % in all cases)
Comparison between the effect of adenosine on A1R activation at SOD1(G93A) neuromuscular junctions upon disease progression
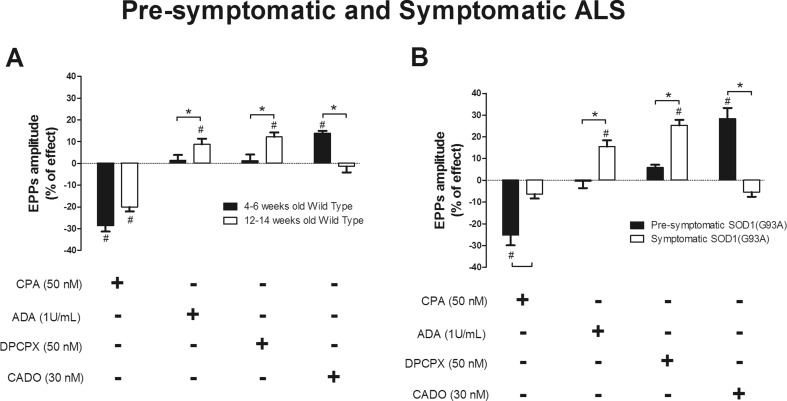
To allow the assessment of the differences in A1R modulation throughout disease progression, we compared the effects between presymptomatic and symptomatic animals, and this comparison is summarized in Fig. 3.
Fig. 3.
Twelve- to 14-week-old mice have higher levels of endogenous adenosine than 4–6-week-old mice with the A1R-agonist losing its effect on neuromuscular transmission in symptomatic when compared to presymptomatic mice. Representation of the mean EPP amplitude changes in the last 10 min of drug perfusion from a 4–6- and 12–14-week wild-type mice (CPA 50 nM n = 5, 4–6-week-old WT, n = 17, 12–14-week-old WT; ADA 1 U/mL n = 8, 4–6-week-old WT, n = 15, 12–14-week-old WT; DPCPX 50 nM n = 8, 4–6-week-old WT, n = 8, 12–14-week-old WT; CADO 30 nM n = 4, 4–6-week-old WT, n = 9, 12–14-week-old WT) and b presymptomatic and symptomatic rodents (CPA 50 nM n = 6, presymptomatic, n = 21, symptomatic; ADA 1 U/mL n = 8, presymptomatic, n = 7, symptomatic; DPCPX 50 nM n = 6, presymptomatic, n = 6, symptomatic; CADO 30 nM n = 4, presymptomatic, n = 7, symptomatic). *p < 0.05 unpaired t test; #p < 0.05 paired t test (as compared with control value before drug perfusion)
The decrease in mean EPP amplitude caused by CPA (50 nM) in 4–6- and 12–14-week-old WT mice was similar (Fig. 3a). In contrast, the A1R agonist was devoid of effect in symptomatic mice but not in the presymptomatic SOD1(G93A) mice (p < 0.05 unpaired t test) (Fig. 3b). ADA (1 U/mL) and DPCPX (50 nM) caused a greater dysinhibition (p < 0.05 unpaired t test) of mean EPP amplitude in symptomatic than in presymptomatic SOD(G93A) mice. This difference also emerged while comparing age-matched WT mice. The same pattern applies for the excitatory action of CADO (30 nM), which clearly enhanced (p < 0.05 unpaired t test) mean EPP amplitude in presymptomatic but not symptomatic mice. This facilitatory action of CADO was more robust in SOD1(G93A) mice than in healthy controls.
MEPP frequency was decreased by CPA (50 nM) in presymptomatic but not in symptomatic mice (p < 0.05 unpaired t test), while in age-matched WT mice, A1R activation caused a similar inhibition of MEPP frequency. A1R blockade (by DPCPX 50 nM) increased spontaneous release frequency in the 12–14-week-old WT group but not in the 4–6-week-old one (p < 0.05 unpaired t test). The decrease in the q.c. of EPPs promoted by CPA (50 nM) in presymptomatic mice was not present when symptoms appeared (p < 0.05 unpaired t test). ADA (1 U/mL) and DPCPX (50 nM) had a higher excitatory effect in both groups of animals with 12–14 weeks of age when compared to 4–6-week-old animals (p < 0.05 t test). Thirty nanomolars of CADO increased the q.c. of EPPs in both groups of 4–6-week-old mice when compared to 12–14-week-old rodents (p < 0.05 unpaired t test).
Discussion
Major findings in the present work strongly indicate that the A1R/A2AR functional cross-talk is lost in ALS and that symptomatic mice display a higher A1R tonic activation than age-matched controls.
In infant rats, it was observed that A2AR facilitatory actions on neuromuscular transmission were blocked in the presence of an A1R antagonist [11], while in young adult rats, A2AR activation impairs A1R-mediated inhibitory actions at the neuromuscular junction [17]. This A1/A2AR interaction is also present in the hippocampus and cerebral cortex of young adults [9, 16] but not of aged rats [16]. In rat astrocytes in culture [18] and in transfected HEK-293T cells [19], A1R and A2AR receptors form heterodimers on the cell surface, reciprocally influencing the signaling mediated by A1R and A2AR ligands [10]. Indeed, G-protein-coupled receptor heterodimerization can affect receptor function [8]. Whether the loss of functional interaction between A1R and A2ARs at the neuromuscular junction of SOD1(G93A) mice reflects abnormal receptor-receptor interaction awaits further clarification.
The loss of the functional cross-talk between adenosine receptors has been associated with ageing or disease [8, 10, 16, 20]. Indeed, an early maturation or compensatory mechanisms on neuromuscular transmission in SOD1(G93A) mice may delay disease progression [5, 6]. The absence of interaction between A1R/A2AR in presymptomatic mice, clearly identified in the present work, implies an early dysfunction at adenosine signaling even before disease onset. Thus, in presymptomatic mice, A2AR receptors could be activated by a low concentration of CADO suggesting that adenosine at low concentrations may trigger an overactivation of the A2AR downstream pathway. A2AR activation results in an increase in the levels of intracellular Ca2+ [21], whereas A1R activation decreases it [22]. Interestingly, intracellular Ca2+ homeostasis is dysfunctional in SOD1(G93A) mice motor neurons [23]. One may then speculate that dysfunctional A1R and A2AR signaling may play a role in Ca2+ in ALS. An exacerbated A2AR-mediated action [6], along with the presently reported loss of an A1/A2AR-mediated fine-tuning of transmission, could render neuromuscular transmission towards a hyperexcitable adenosinergic tonus that could contribute to the Ca2+-mediated excitotoxicity in presymptomatic ALS. Indeed, recent evidence showed that partial genetic ablation of A2AR in the SOD1(G93A) mouse delayed disease progression [24], further highlighting a role for these receptors in disease progression.
In symptomatic mice, we observed that A1R had a more pronounced tonic activation attributed to higher levels of extracellular adenosine when compared to age-matched controls. Increase in the levels of extracellular adenosine can result from muscle or neuronal degeneration, mitochondrial dysfunction, inflammatory processes, or even abnormalities in adenosine modulation (e.g., adenosine transporters or ATP release and cleavage reactions) [25]. For example, in the SOD1(G93A) mouse model, it was recently reported that activation of the adenosine 5′-monophosphate-activated protein kinase (AMPK) is increased throughout disease progression [26]. AMPK activation is dependent on high AMP/ATP ratios [27], thus suggesting that the increase in intracellular levels of AMP, which can be converted into adenosine, could result in higher endogenous levels of the nucleoside at the cleft since adenosine transporters operate according to the concentration gradient [28].
A1R function is kept during disease progression though the influence of the exogenous ligand could only be into evidence when recording extracellular adenosine. Accordingly, A1R blockade markedly increases neuromuscular transmission in 12–14-week-old SOD1(G93A) mice. In lymphocytes from ALS patients, A1R expression does not change as opposite of A2ARs [29].
Previous studies on purinergic signaling in ALS focused only on A2AR [6, 30, 31]. To our knowledge, this is the first study focusing on A1R in ALS. Whereas A2AR activation increases synaptic transmission by positively modulating Ca2+-dependent release mechanisms [21, 32], A1R activation at the prejunctional site decreases the release of acetylcholine by reducing the levels of intraterminal Ca2+ [22]. Symptomatic SOD1(G93A) presents abnormal Ca2+ buffering resulting in deficient Ca2+ handling during neuronal firing [23]; therefore, the reported increase in A1R tonus in symptomatic mice may act as a compensatory mechanism to delay Ca2+-mediated excitotoxicity in later stages. A1R can increase synchronization of acetylcholine release at mammalian neuromuscular junctions which could help to preserve synaptic fidelity in high redox situations such as in ALS [33]. Whether the loss of A2AR function and change in the A1R tonus could be a result of disease progression or a means to decrease excitotoxicity is open to debate.
In conclusion, the work herein documents the loss of a functional cross-talk between A1R and A2AR at the neuromuscular junction of presymptomatic ALS mice and the enhanced A1R tonic activation in the symptomatic stage of the SOD1(G93A) mouse model. The results thus suggest that A1R and A2AR dysfunction is implicated in ALS. While the loss of A1/A2AR comparatively in early disease stages may push the balance towards enhanced excitotoxicity, the enhanced tonic activation of A1R in late disease states may contribute to neuromuscular transmission failure.
Acknowledgments
This study was supported by FCT, PTDC/SAU-FAR/118787/2010.
References
- 1.Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol. 2014;10(11):661–670. doi: 10.1038/nrneurol.2014.184. [DOI] [PubMed] [Google Scholar]
- 2.Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364(6435):362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- 3.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 4.Correia-de-Sá P, Sebastião AM, Ribeiro JA. Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve ending of the rat. Br J Pharmacol. 1991;103(2):1614–1620. doi: 10.1111/j.1476-5381.1991.tb09836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha MC, Pousinha PA, Correia AM, Sebastião AM, Ribeiro JA. Early changes of neuromuscular transmission in the SOD1(G93A) mice model of ALS start long before motor symptoms onset. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0073846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nascimento F, Pousinha PA, Correia AM, Gomes R, Sebastião AM, Ribeiro JA. Adenosine A2A receptors activation facilitates neuromuscular transmission in the pre-symptomatic phase of the SOD1(G93A) ALS mice, but not in the symptomatic phase. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebastião AM, Ribeiro JA. Triggering neurotrophic factor actions through adenosine A2A receptor activation: implications for neuroprotection. Br J Pharmacol. 2009;158(1):15–22. doi: 10.1111/j.1476-5381.2009.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferre S, Ciruela F, Quiroz C, Lujan R, Popoli P, Cunha RA, Agnati LF, Fuxe K, Woods AS, Lluis C, Franco R. Adenosine receptor heteromers and their integrative role in striatal function. Sci World J. 2007;7:74–85. doi: 10.1100/tsw.2007.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha RA, Johansson B, van der Ploeg I, Sebastião AM, Ribeiro JA, Fredholm BB. Evidence for functionally important adenosine A2a receptors in the rat hippocampus. Brain Res. 1994;649(1–2):208–216. doi: 10.1016/0006-8993(94)91066-9. [DOI] [PubMed] [Google Scholar]
- 10.Sheth S, Brito R, Mukherjea D, Rybak LP, Ramkumar V. Adenosine receptors: expression, function and regulation. Int J Mol Sci. 2014;15(2):2024–2052. doi: 10.3390/ijms15022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pousinha PA, Correia AM, Sebastião AM, Ribeiro JA. Predominance of adenosine excitatory over inhibitory effects on transmission at the neuromuscular junction of infant rats. J Pharmacol Exp Ther. 2010;332(1):153–163. doi: 10.1124/jpet.109.157255. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro JA, Sebastião AM. On the role, inactivation and origin of endogenous adenosine at the frog neuromuscular junction. J Physiol. 1987;384:571–585. doi: 10.1113/jphysiol.1987.sp016470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro JA, Walker J. The effects of adenosine triphosphate and adenosine diphosphate on transmission at the rat and frog neuromuscular junctions. Br J Pharmacol. 1975;54(2):213–218. doi: 10.1111/j.1476-5381.1975.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs HA, Henseleit K. Untersuchungen uber die Harnstoffbildung im Tierkoper. Hoppe-Seyler’s Z Physiol Chem. 1932;210:33–37. doi: 10.1515/bchm2.1932.210.1-2.33. [DOI] [Google Scholar]
- 15.Correia-de-Sá P, Timoteo MA, Ribeiro JA. Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J Neurophysiol. 1996;76(6):3910–3919. doi: 10.1152/jn.1996.76.6.3910. [DOI] [PubMed] [Google Scholar]
- 16.Lopes LV, Cunha RA, Ribeiro JA. Cross talk between A(1) and A(2A) adenosine receptors in the hippocampus and cortex of young adult and old rats. J Neurophysiol. 1999;82(6):3196–3203. doi: 10.1152/jn.1999.82.6.3196. [DOI] [PubMed] [Google Scholar]
- 17.Pousinha PA, Correia AM, Sebastião AM, Ribeiro JA. Neuromuscular transmission modulation by adenosine upon aging. Neurobiol Aging. 2012;33(12):2869–2880. doi: 10.1016/j.neurobiolaging.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Cristovao-Ferreira S, Navarro G, Brugarolas M, Perez-Capote K, Vaz SH, Fattorini G, Conti F, Lluis C, Ribeiro JA, McCormick PJ, Casado V, Franco R, Sebastião AM. A1R-A2AR heteromers coupled to Gs and G i/0 proteins modulate GABA transport into astrocytes. Purinergic Signalling. 2013;9(3):433–449. doi: 10.1007/s11302-013-9364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26(7):2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA. Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition. Neuroscience. 2002;112(2):319–329. doi: 10.1016/S0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 21.Palma AG, Muchnik S, Losavio AS. Excitatory effect of the A2A adenosine receptor agonist CGS-21680 on spontaneous and K +-evoked acetylcholine release at the mouse neuromuscular junction. Neuroscience. 2011;172:164–176. doi: 10.1016/j.neuroscience.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 22.De Lorenzo S, Veggetti M, Muchnik S, Losavio A. Presynaptic inhibition of spontaneous acetylcholine release induced by adenosine at the mouse neuromuscular junction. Br J Pharmacol. 2004;142(1):113–124. doi: 10.1038/sj.bjp.0705656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs A, Kutterer S, Muhling T, Duda J, Schutz B, Liss B, Keller BU, Roeper J. Selective mitochondrial Ca2+ uptake deficit in disease endstage vulnerable motoneurons of the SOD1G93A mouse model of amyotrophic lateral sclerosis. J Physiol. 2013;591(Pt 10):2723–2745. doi: 10.1113/jphysiol.2012.247981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng SK, Higashimori H, Tolman M, Yang Y. Suppression of adenosine 2a receptor (AR)-mediated adenosine signaling improves disease phenotypes in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2015;267:115–122. doi: 10.1016/j.expneurol.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastião AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handb Exp Pharmacol. 2009;193:471–534. doi: 10.1007/978-3-540-89615-9_16. [DOI] [PubMed] [Google Scholar]
- 26.Coughlan KS, Mitchem MR, Hogg MC, Prehn JH. “Preconditioning” with latrepirdine, an adenosine 5′-monophosphate-activated protein kinase activator, delays amyotrophic lateral sclerosis progression in SOD1 mice. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271(44):27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro JA, Cunha RA, Correia-de-Sá P, Sebastião AM. Purinergic regulation of acetylcholine release. Prog Brain Res. 1996;109:231–241. doi: 10.1016/S0079-6123(08)62107-X. [DOI] [PubMed] [Google Scholar]
- 29.Vincenzi F, Corciulo C, Targa M, Casetta I, Gentile M, Granieri E, Borea PA, Popoli P, Varani K. A2A adenosine receptors are up-regulated in lymphocytes from amyotrophic lateral sclerosis patients. Amyotrophic Lateral Sclerosis Frontotemporal Degeneration. 2013;14(5–6):406–413. doi: 10.3109/21678421.2013.793358. [DOI] [PubMed] [Google Scholar]
- 30.Potenza RL, Armida M, Ferrante A, Pezzola A, Matteucci A, Puopolo M, Popoli P. Effects of chronic caffeine intake in a mouse model of amyotrophic lateral sclerosis. J Neurosci Res. 2013;91(4):585–592. doi: 10.1002/jnr.23185. [DOI] [PubMed] [Google Scholar]
- 31.Yanpallewar SU, Barrick CA, Buckley H, Becker J, Tessarollo L. Deletion of the BDNF truncated receptor TrkB.T1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Correia-de-Sá P, Timoteo MA, Ribeiro JA. A(2A) adenosine receptor facilitation of neuromuscular transmission: influence of stimulus paradigm on calcium mobilization. J Neurochem. 2000;74(6):2462–2469. doi: 10.1046/j.1471-4159.2000.0742462.x. [DOI] [PubMed] [Google Scholar]
- 33.Tsentsevitsky A, Kovyazina I, Nikolsky E, Bukharaeva E, Giniatullin R. Redox-sensitive synchronizing action of adenosine on transmitter release at the neuromuscular junction. Neuroscience. 2013;248:699–707. doi: 10.1016/j.neuroscience.2013.05.065. [DOI] [PubMed] [Google Scholar]