Abstract
Extracellular adenosine triphosphate (ATP) regulates pancreatic duct function via P2Y and P2X receptors. It is well known that ATP is released from upstream pancreatic acinar cells. The ATP homeostasis in pancreatic ducts, which secrete bicarbonate-rich fluid, has not yet been examined. First, our aim was to reveal whether pancreatic duct cells release ATP locally and whether they enzymatically modify extracellular nucleotides/sides. Second, we wished to explore which physiological and pathophysiological factors may be important in these processes. Using a human pancreatic duct cell line, Capan-1, and online luminescence measurement, we detected fast ATP release in response to pH changes, bile acid, mechanical stress and hypo-osmotic stress. ATP release following hypo-osmotic stress was sensitive to drugs affecting exocytosis, pannexin-1, connexins, maxi-anion channels and transient receptor potential cation channel subfamily V member 4 (TRPV4) channels, and corresponding transcripts were expressed in duct cells. Direct stimulation of intracellular Ca2+ and cAMP signalling and ethanol application had negligible effects on ATP release. The released ATP was sequentially dephosphorylated through ecto-nucleoside triphosphate diphosphohydrolase (NTPDase2) and ecto-5′-nucleotidase/CD73 reactions, with respective generation of adenosine diphosphate (ADP) and adenosine and their maintenance in the extracellular medium at basal levels. In addition, Capan-1 cells express counteracting adenylate kinase (AK1) and nucleoside diphosphate kinase (NDPK) enzymes (NME1, 2), which contribute to metabolism and regeneration of extracellular ATP and other nucleotides (ADP, uridine diphosphate (UDP) and uridine triphosphate (UTP)). In conclusion, we illustrate a complex regulation of extracellular purine homeostasis in a pancreatic duct cell model involving: ATP release by several mechanisms and subsequent nucleotide breakdown and ATP regeneration via counteracting nucleotide-inactivating and nucleotide-phosphorylating ecto-enzymes. We suggest that extracellular ATP homeostasis in pancreatic ducts may be important in pancreas physiology and potentially in pancreas pathophysiology.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-015-9472-5) contains supplementary material, which is available to authorized users.
Keywords: Pannexin, CD39, Ecto-nucleotidase, Adenylate kinase, UTP, VNUT, Connexin, Pancreatitis
Introduction
Extracellular adenosine triphosphate (ATPe) is an important signalling molecule, which regulates a wide range of cellular functions including cell proliferation, apoptosis, immune response, neurotransmission and, focus of our interest, the epithelial ion transport. In order to achieve these effects, ATP is released from cells in response to a number of stimuli including transmitter or hormone stimulation, shear stress, cell volume changes, hypoxia and release from damaged cells. Several ATP release mechanisms have been proposed to explain transport of ATP across the plasma membrane. One of the ATP releasing pathways is the regulated vesicular exocytosis, which occurs in neurons, endocrine cells and some exocrine cells, and constitutive vesicular transport [1]. Vesicular ATP accumulation is achieved by the vesicular nucleotide transporter (VNUT, SLC17A9), first detected in enterochromaffin cells but now demonstrated in a number of other cells [1, 2]. Other models of ATP release pathways include anion channels and transporters (e.g. maxi-anion channels, volume-regulated anion channels, cystic fibrosis transmembrane conductance regulator (CFTR)), multidrug resistance proteins, pannexin-1 and pannexin-1 in conjunction with the P2X7 receptor and connexin hemichannels [1, 3, 4].
Once released, the extracellular nucleotide concentrations are regulated by a number of extracellular enzymes (ecto-enzymes). Ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases) catalyse sequential ATP degradation via adenosine diphosphate (ADP) into adenosine monophosphate (AMP), and ecto-nucleotide pyrophosphatase/phosphodiesterases (E-NPPs) hydrolyse ATP directly into AMP [5, 6]. Subsequently, AMP can be hydrolysed to adenosine by ecto-5′-nucleotidase (CD73). Another group of counteracting enzymes includes adenylate kinases (AKs) and the nucleoside diphosphate kinase (NDPK/NME) family, which via phosphate group transfer lead to ATP generation [5, 6]. These enzymes are important in maintaining and regulating extracellular nucleotide concentrations and therefore modulate receptor activation or termination of signalling events.
Pancreatic exocrine secretion is a joined effort of secretion from pancreatic acini and pancreatic ducts, and purinergic signalling regulates or co-regulates secretion at several points [7, 8]. Pancreatic acini release ATP into ductal lumen in response to cholinergic, hypo-osmotic or mechanical stimulation [9, 10]. Importantly, ATP is accumulated into zymogen granules by a VNUT-dependent mechanism [9, 11]. The released ATP, and other nucleotides/sides, activate P2 receptors and/or adenosine receptors and regulate pancreatic duct functions such as epithelial secretion. For example, P2 receptors regulate Ca2+-activated K+ and Cl− channels (KCa3.1 and ANO1) as well as CFTR [12]. Nevertheless, there are hydrolytic enzymes released into pancreatic juice, exosomal NTPDase1/CD39 and ecto-5′-nucleotidase/CD73 [13, 14], and it may be questionable whether the more distal ducts would be exposed to sufficient acinar ATP for P2 receptor activation. Pancreatic ducts are not just passive conduits for enzyme secretion, but they produce HCO3−-rich fluid that is essential for normal pancreatic function and digestion [15, 16].
Therefore, in the present study, we asked the question whether pancreatic ducts themselves can release ATP and thus could provide immediate local source of ATP for P2 receptor function. Duct cells could be exposed to several stimuli that are associated with secretory processes including mechanical forces, such as shear stress of flowing fluid, distension of the duct swollen with secretion and perhaps cell volume changes. Furthermore, since pancreatic ducts secrete HCO3−-rich fluid (and correspondingly acidify interstitium), one would expect that pH changes could affect ATP release as they do, for example, in intestinal epithelia [17]. In addition to physiological stimuli by neurotransmitters and hormones, it is known that the patency of pancreatic ducts is important for overall exocrine function, and various agents such as bile acids and ethanol can lead to increased ductal secretion, if present in low concentrations. However, in high concentrations, they can cause duct (and acinar) disruption and pathology associated with pancreatitis [15].
Therefore, the aim of our study was to evaluate basic physiological and potential pathophysiological stimuli that pancreatic ducts may encounter and determine whether these could lead to ATP release. Furthermore, we quested to decipher which ATP release mechanisms operate in pancreatic ducts. And finally we addressed the role of ecto-enzymes in the local nucleotide/side homeostasis. For this purpose, we used human duct cell lines, in particular Capan-1 cells, which have been established as a human pancreatic duct epithelia model as they can be grown as polarised epithelium and exhibit ion channels and transporters consistent with secretion [12, 18, 19]. We found that Capan-1 cells release ATP in response to pH changes as well as to mechanical and hypo-osmotic stress and bile acid but not to ethanol stimulation. Capan-1 cells expressed transcripts for several connexins as well as pannexin-1, and VNUT, and these were involved in cell volume-induced ATP release. Exposure of the duct cells to UTP increased extracellular ATP formation, which was mainly due to the transfer of phosphate groups catalysed by NDPK. In addition, we demonstrated the ability of pancreatic ducts to regulate directionally extracellular purine homeostasis via selective network of ecto-enzymes, including nucleotide-inactivating NTPDase2 and ecto-5′-nucleotidase/CD73, as well as counteracting nucleotide-phosphorylating enzymes AK and NDPK.
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich unless otherwise specified. In the present study, following chemicals in final concentrations were used: UTP (0.1–100 μM), UTPγS (10 μM), UDP (10 μM), UDPβS (10 μM), UDP-glucose (10 μM), guanosine triphosphate (GTP) (10 μM), inosine triphosphate (ITP) (10 μM), ADP (10 μM), which was treated with hexokinase to eliminate possible ATP contamination [20], ionomycin (5 μM), forskolin (50 μM), chenodeoxycholic acid (0.25 mM), ethanol, EtOH (10 and 100 mM), GSK1016790A (300 nM), ATP kit SL 144-041 (BioThema), ATPLite assay kit (PerkinElmer, Groningen, The Netherlands), NDPK from baker’s yeast Saccharomyces cerevisiae (0.4 U/ml), adenosine deaminase (ADA, type IX from calf spleen, 0.3 U/ml), bacterial purine nucleoside phosphorylase (PNP, 0.3 U/ml), microbial xanthine oxidase (XO, 0.2 U/ml), horseradish peroxidase (HRP) (1 U/ml), Amplex Red reagent (60 μM, Invitrogen, Molecular Probes), [2,8-3H]ADP (PerkinElmer), [2-3H]AMP (Quotient Bioresearch, GE Healthcare), Cell Counting kit-8 (CCK-8, DOJINDO) and TOX7 in vitro toxicology assay kit (Sigma-Aldrich). Cells were pre-treated/incubated with one of the following inhibitors: HC064047 (10 μM, Tocris), gadolinium chloride (Gd3+, 50 μM), probenecid (250 μM), pannexin inhibitor 10Panx (100 μM, Tocris), N-ethylmaleimide (NEM, 250 μM), bafilomycin A1 (0.1 μM), glyoxylate (100 μM), BAPTA (25 μM), methyl-β-cyclodextrin (MβCD, 1 mM), P1,P5-Di(adenosine-5′) pentaphosphate pentasodium salt (Ap5A, 60 μM) and α,β-methylene-ADP (AMPCP, 60 μM).
Cell cultures
Pancreatic human duct cell line Capan-1 (HTB-79) was obtained from ATTC (Manassas, VA) and cultured according to recommended procedures. For selected experiments, we also used CFPAC-1 cells which lack functional CFTR channel (CRL-1918) and transformed “normal” human pancreatic duct epithelial (HPDE) cell line [21]. For online luminescence recordings of ATP release, 50,000 Capan-1, 35,000 CFPAC-1 and HPDE cells were cultured in 96-well white plate to 80 % confluence. For thin layer chromatographic (TLC) analysis and purine-sensing assays, 135,000 Capan-1 cells were cultured in 24-well plate for 24 h. All experiments were conducted at 37 °C.
Online measurement of ATP release
ATPe was monitored using luciferin/luciferase luminescence reaction in extracellular medium. All experiments were conducted in duplicates in FLUOstar Optima (BMG, Labtech). Capan-1 and other pancreatic duct cell lines (CFPAC-1, HPDE) were washed and allowed to rest in 65 μl of physiological buffer that contained (in mM) 140 NaCl, 1 MgCl2·6H2O, 1.5 CaCl2, 0.4 KH2PO4, 1.6 K2HPO4·3H2O, 10 HEPES and glucose concentrations corresponding to the culture conditions, pH = 7.4. After 30 min, 25 μl of 2× concentrated luciferin/luciferase mix from BioThema was added into the wells. Relative luminescence (RLU) was measured to monitor stability of cell preparations/basal ATP levels. Various stimuli, and physiological buffer as a control, were added gently with a pipette and care was taken to avoid mechanical disturbances. To study hypo-osmolar-induced ATP release, 45 μl of water containing 1 mM MgCl2 and 1.5 mM CaCl2 was added into wells, and this decreased the osmolality by 33 % (from 310 to 205 mOsm/kg). For pH-stimulated ATP release, 10 μl of HCl or NaOH diluted in physiological solution (pH 1.5 and 12) was added into well with cells incubated in 90 μl of physiological buffer of pH 7.4.
Measurements were made using plate mode (20-s sampling rate with 1-s integration). For experiments with mechanical stimulation, cells were incubated in 50 μl of the physiological buffer, subsequently 50 μl of 1× concentrated luciferin/luciferase was added, and 50 μl of the buffer was added using an injection pump of the instrument at the indicated speeds (100–420 μl/s) and relative luminescence was monitored using well mode (1-s sampling rate with 1-s integration). For each experiment, basal ATP was determined (from 5 to 15 s for mechanical stimulation and for the rest of measurements from 20 to 100 s before stimulation) and peak ATP values were determined from average measurements of 30 s after 10 s of mechanical stimulation and for other stimuli during 80 s after 1 min of stimulation. In some experiments with slower kinetics, plateau-like levels were determined as indicated in the results. For summary graphs, delta ATP values are given (i.e. stimulated–basal ATPe). The effect of all applied stimuli and inhibitors on luciferase/luciferin assay activity was tested independently, and for each experimental protocol, standard curves were made with ATP concentrations ranging from 10−9 to 10−5 M. Cell number was determined by cell counting kit in the parallel wells with similar cell seeding in the same plate, and ATP concentrations were corrected for 106 cells per 1 ml, unless otherwise specified.
Extracellular pH measurement
For extracellular pH measurements, Capan-1 cells were prepared in 96-well plates, and experiments were conducted at the same time as online ATP release measurement. After changing media and letting the cells rest in 90 μl of physiological buffer (without HEPES), 10 μl of buffer (pH 7.4) and buffer with HCl (pH 1.5) or NaOH (pH 12) solutions were added into the wells. Changes of the pH were measured every 5 min during first 15 min and then every 10 min using micro pH electrode (Hanna HI 1093B).
Transfection
Transfection of CFPAC-1 cells with CFTR was made using FuGENE transfection kit (Promega) according to manufacturer’s protocol. In short, 5,000 of CFPAC-1 cells were plated into 96-well plate in 100 μl of Iscove’s modified Dulbecco’s media (IMDM) with 10 % fetal bovine serum (FBS). After 24 h, cells were transfected for 2 days with CFTR plasmid (pCNDA3-CFTR), a kind gift from Prof. J. Hanrahan. The day before experiments, media with transfection reagent were changed for the culture media to limit a possible cytotoxic effect. The transfection efficiency was assessed with a GFP plasmid transfection.
Reverse transcription PCR
RNA was isolated using RNeasy Mini Kit (QIAGEN 74104) followed the manufacturer’s instructions. RT-PCR was analysed with QIAGEN OneStep RT-PCR Kit (210212) with amplification parameters as follows: one cycle at 50 °C for 30 min and one cycle at 95 °C for 15 min followed by 37 cycles at 95 °C for 30 s, 58 °C for 30 s, 72 °C for 40 s and one final cycle at 72 °C for 10 min. The primers (Table 1) used in the study were designed for human proteins using Primer BLAST and used for amplification. All primers were synthesised by TAG Copenhagen A/S (Denmark).
Table 1.
Primer sequences and expected sizes
| Name | Forward primer 5′–3′ | Reverse primer 5′–3′ | Product size (bp) |
|---|---|---|---|
| Connexin 26 NM_004004.5 |
TCCCGACGCAGAGCAAAC | AAAGTCGGCCTGCTCATCTC | 186 |
| Connexin 30 NM_001110219.2 |
AGGACTCAGGGATAAACCAGC | GCAGTGTGTTGCAGACGAAG | 193 |
| Connexin 43 NM_000165.4 |
TTAAGCAAAAGAGTGGTGCCC | AGCGCACATGAGAGATTGGG | 257 |
| Pannexin-1 NM_015368.3 |
TTCTTGCTGAAGGAGCCCAC | GTAGGGGAAAAACTTATGCAGCC | 291 |
| TRPV4 NM_001177431.1 |
TCCCATTCTTGCTGACCCAC | AGGGCTGTCTGACCTCGATA | 217 |
| VNUT-1 NM_022082.3 |
CGCCGTCTGAGCACCCCAAG | AGCTGCTGAGCACGATGCCG | 229 |
| VNUT-2 NM_001302643.1 |
ACCACCCTGTGTATGCATGACCCT | AGCTGCTGAGCACGATGCCG | 203 |
| AK1β XM_005251786.1 |
GCAGCAGTGTGGGCTGTC | GACCATGGGCTGCTGCTC | 398 |
| NME1 NM_198175.1 |
GTAGTTGCCATGGTCTGGGAG | GTTCCTCAGGGTGAAACCACA | 199 |
| NME2 NM_001018139.2 |
CATTCAGGTTGGCAGGAACATC | TGCTGTTGTGTCCACCTCTT | 151 |
Confocal microscopy
ATP stores were visualised using protocols described earlier [10]. Briefly, Capan-1 cells were loaded with 1–5 μM quinacrine dihydrochloride for 5–15 min, and fluorescence was detected at 490–540 nm with 476 nm excitation. In some experiments, cells were incubated with 20 μM MANT-ATP for 3 h prior to quinacrine loading. Upon excitation with 364 nm, the fluorescence signal was detected at 430–480 nm. Images were recorded in Leica SP1 CLSM (Leica Microsystems Heidelberg) with 63 × 1.2 NA PL APO and 20 × 1.7 NA HC PL APO objectives.
TLC analysis of extracellular purine-converting pathways
For determination of enzymatic activities, Capan-1 cells were seeded overnight in 24-well plate in complete medium, washed and incubated in a final volume of 220 μl of RPMI-1640 supplemented with 1 % heat-inactivated foetal bovine serum and 4 mM β-glycerophosphate in the following ways: (1) for ADPase/NTPDase and ecto-5′-nucleotidase assays, cultured cells were incubated for 45 min with 400 μM [3H]ADP and 300 μM [3H]AMP, respectively, and (2) backward AK and NDPK activities were assayed by incubating the cells with 400 μM [3H]AMP (for 45 min) or [3H]ADP (for 15 min) as respective phosphorus acceptors in the presence of 600 μM γ-phosphate-donating ATP. Catalytic reactions were terminated by applying aliquots of the mixture onto Alugram SIL G/UV254 TLC sheets (Macherey-Nagel, Duren, Germany). 3H-labelled nucleotides and nucleosides were separated by TLC using appropriate solvent mixture and then quantified by scintillation β-counting, as described elsewhere [13, 22].
Quantification of extracellular ATP, ADP and adenosine—offline analysis
For analysing the concentrations of extracellular purines, Capan-1 cells were washed and incubated with basal salt solution (BSS) buffer containing 130 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1 mM MgSO4, 25 mM HEPES, 20 mM glucose and 0.1 % FBS. After 15 min of rest, the cells were stimulated mechanically (using pipette flow) or with ATP (10 μM). Supernatant samples were collected after 1 h (stimulation with ATP) or after 1 min (following mechanical stimulation and taking into account responses detected in online ATP measurement) or every 20 min (to study the kinetics of extracellular nucleotides changes). After heat inactivation at 65 °C for 5 min, ATP, ADP and adenosine were quantified using the enzyme-coupled assays described in detail by Helenius and coworkers [23] and briefly given below. For each experimental protocol, standard curves were made using samples in a range of concentrations 0.04–0.6 μM (for ATP and ADP) and from 0.08 to 1.2 μM (for adenosine). All purine concentrations were corrected for 106 cells per 1 ml.
Luminometric measurement of extracellular ATP and ADP
Extracellular ADP concentrations were determined based on ADP transphosphorylation into ATP during incubation with a mixture of UTP (40 μM) and NDPK for 15 min in 96-well white plate in final volume of 130 μl. Subsequently, to all samples, 50 μl of ATP-monitoring reagent (ATPLite assay kit, PerkinElmer) was added. Luminescence was measured with Tecan Infinite M200 microplate reader (Salzburg, Austria).
Fluorometric measurement of extracellular adenosine
Each sample of BSS buffer collected from stimulated cells was divided into two aliquots: the first for analysing the total content of adenosine and the second for analysing inosine concentration, presence of which may affect adenosine measurements. In 96-well plate, samples were incubated with the mixture of ADA, PNP and XO (for adenosine determination) or PNP and XO (for inosine content) for at least 30 min at 37 °C in final volume 130 μl. After enzymatic treatment, 30 μl of H2O2 detecting mixture containing HRP and Amplex Red reagent was added to all samples. Fluorescence was measured immediately at the emission and excitation wavelengths of 545 and 590 nm, respectively, using Tecan Infinite M200 microplate reader.
Western blot
Capan-1 cells were seeded overnight in 24-well plate in IMDM, washed and incubated in 500 μl of physiological buffer for 1.5 h. To determine the presence of soluble forms of NTPDase2, samples of supernatant were collected and centrifuged for 10 min in 5,000×g at 4 °C to remove potential cells and cell debris. Subsequently, supernatants were ultracentrifuged for 1 h in 70,000 rpm (Beckman Ultracentrifuge Ti 70.1 Rotor TLA-100.3) to obtain the microsomal/particulate fraction (Capan-1 PF.). The pellet was dissolved in 50 μl of lysis buffer (50 mM Tris Base, 0.25 M NaCl, 5 mM EDTA, 1 % Triton X-100 and 4 mM NaF) containing protease inhibitor. Cell protein lysates (Capan-1 L) were prepared by adding lysis buffer and centrifuging samples at 15,000×g for 15 min at 4 °C, and the supernatant was collected. Western blot samples were denatured by heating to 37 °C in 50 mM dithiothreitol for 30 min and run on precast gels from Invitrogen. The membranes were blocked overnight at 4 °C in 0.5 % milk powder and 1 % BSA. Primary antibody for NTPDase2 (1:1,600 rabbit; Centre de Recherche du CHUL—ectonucleotidases-ab.com) was added in blocking buffer overnight. The goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:2500) was added in blocking buffer, for 1 h. EZ-ECL chemiluminescence detection kit for HRP (BI, Biological Industries) was added, and blots were viewed on Fusion FX Vilber Lourmat.
Lactate dehydrogenase assay
Cell viability after mechanical stimulation was assessed by In Vitro Toxicology Assay Kit, Lactic Dehydrogenase based (TOX7) according to manufacturer’s protocol. Briefly, Capan-1 cells were cultured and stimulated mechanically by pump injections with physiological buffer (260 and 420 μl/s speed) to release ATP as described above. Samples of 75 μl buffers were transferred to a new 96-well white plate, and lactate dehydrogenase (LDH) substrate solution was added in 1:2 ratio. After 30 min of incubation at room temperature, reaction was stopped by adding 1 M HCl. Amount of released LDH was measured as absorbance at 490 nm.
Statistics
All samples are measured in duplicates and averaged. The number of n represents separate experiments. Data are shown as the mean values ± S.E.M. To test the statistical significance between two conditions, unpaired two-tail Student’s t test was applied. For multiple conditions, one-way ANOVA with Bonferroni’s Multiple Comparison Test was used. P < 0.05 was considered statistically significant.
Results
Effect of pH on ATP release
Capan-1 cells are a well-established model of pancreatic duct epithelia and can be cultured on membranes as polarised monolayer [12, 18]. However, detection of released ATP from epithelial monolayers using offline measurements is inaccurate due to the dilution of ATP in high volume of buffer (at least 300 μl) needed for transwells and the expression of extracellular ATP-hydrolysing enzymes [24] (also see below). Therefore, we decided to conduct study on ATP release in 96-well plate, which allowed fast online measurement of extracellular ATP concentration in lower volume.
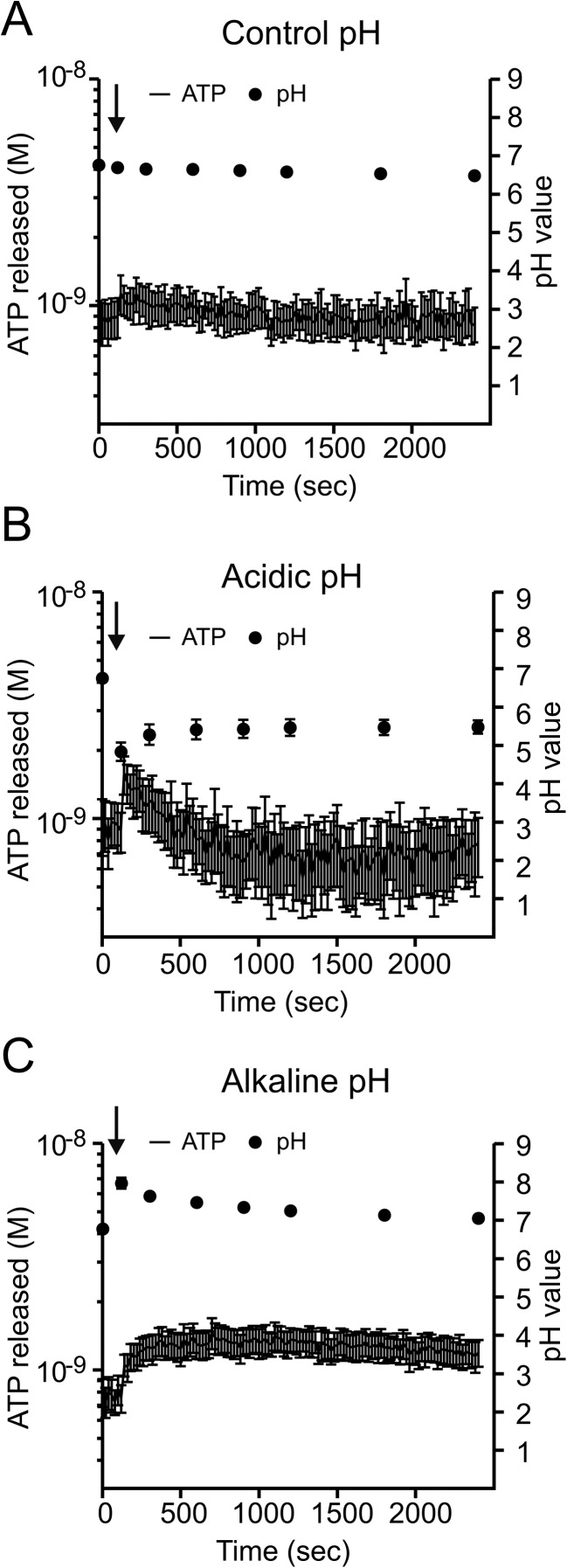
Pancreatic duct cells can be exposed to a wide range of pH values ranging from acid to alkaline pH when stimulated to secrete a NaHCO3-rich fluid. Therefore, we tested whether pH changes affect ATP release in duct cells. At physiological pH, basal ATPe seems to remain stable at around 0.9 ± 0.1 nM (Fig. 1a). After cells were exposed to low extracellular pH ~5 (Fig. 1b), there was a biphasic ATP response compared to control (Fig. 1a). First, there was a fast increase in ATPe (from 0.92 ± 0.22 to 1.37 ± 0.29 nM, n = 8), and then it decreased to below basal. In contrast, alkalisation to an extracellular pH of ~8 (Fig. 1c) caused a monophasic ATPe increase with peak values, reaching from 0.8 ± 0.1 to 1.3 ± 0.2 nM (n = 8). The effect of pH changes on ATPe was relatively small compared to other stimuli and could be due to ATP release and/or effects on ecto-enzymes (see below). Interestingly, extracellular pH changes were transient (Fig. 1b, c), indicating that duct epithelium could potentially modify it by secreting acid/bases.
Fig. 1.
The pH effect on ATP release. The original time course traces of ATP released from Capan-1 cells after stimulation with extracellular pH changes: physiological (a), acidic 0.45 ± 0.11 (b) and alkaline 0.51 ± 0.09 (c), respectively (n = 8). The left axis shows the ATP concentration values corrected per 106 cells in 1 ml. The right axis represents the values of extracellular pH showed as grey dots. Arrows indicate addition of acid/base solutions
Effect of ionomycin, forskolin, CDCA and ethanol
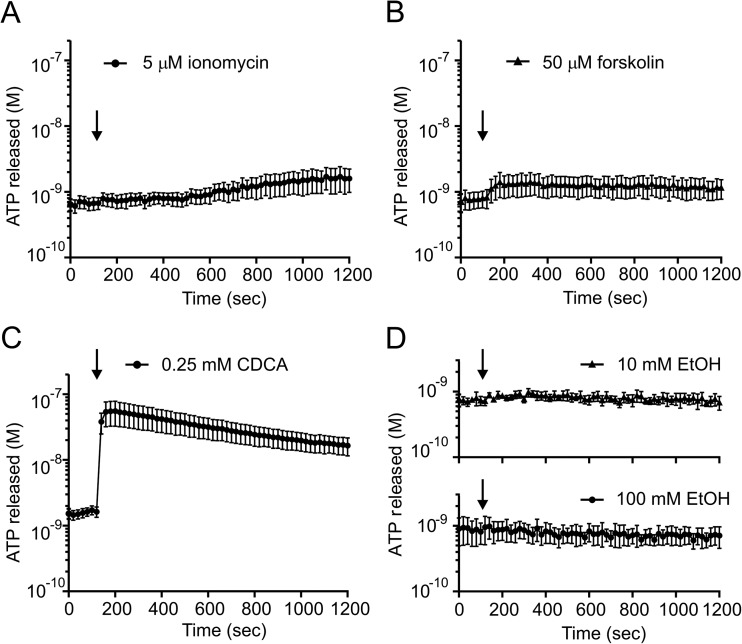
Ionomycin and forskolin bypass plasma membrane receptors and increase intracellular Ca2+ and cAMP concentrations, respectively. These are the two main signalling pathways regulating ductal secretion [8]. Figure 2a shows that ionomycin (5 μM) had minimal effects on ATPe; the basal values were 0.67 ± 0.12 and after a longer incubation period (1,000–1,200 s) they were 1.64 ± 0.49 nM (n = 6). HPDE cells also showed slow increase in ATPe (data not shown). Forskolin (50 μM) also had minimal effects on ATPe (Fig. 2b); basal values were 0.77 ± 0.16 nM and stimulated values were 1.32 ± 0.31 nM (n = 6). Bile acids and ethanol are potential factors involved in pathophysiology of pancreatitis [25]. Here, we show in Fig. 2c that one bile acid (chenodeoxycholic acid, CDCA) in 0.25 mM concentration considered as non-lytic [26] caused a large and fast ATP release from basal values of 0.67 ± 0.06 up to peak values of 52 ± 22 nM, (n = 5). Interestingly, ethanol at concentrations 10 and 100 mM had no effect on ATPe (n = 4) (Fig. 2d).
Fig. 2.
The effect of ionomycin, forskolin, ethanol and CDCA on ATP release. The time courses of the ATP release from Capan-1 after stimulation of the cells with (a) ionomycin (5 μM, n = 6) and (b) forskolin (50 μM, n = 6), (c) bile acid chenodeoxycholic acid (CDCA, 0.25 mM, n = 5) and (d) ethanol (10 and 100 mM, n = 4). The left axis shows the ATP concentration values corrected per 106 cells in 1 ml. The arrow indicates the time of adding the stimulants
Hypo-osmotic stress causes ATP release
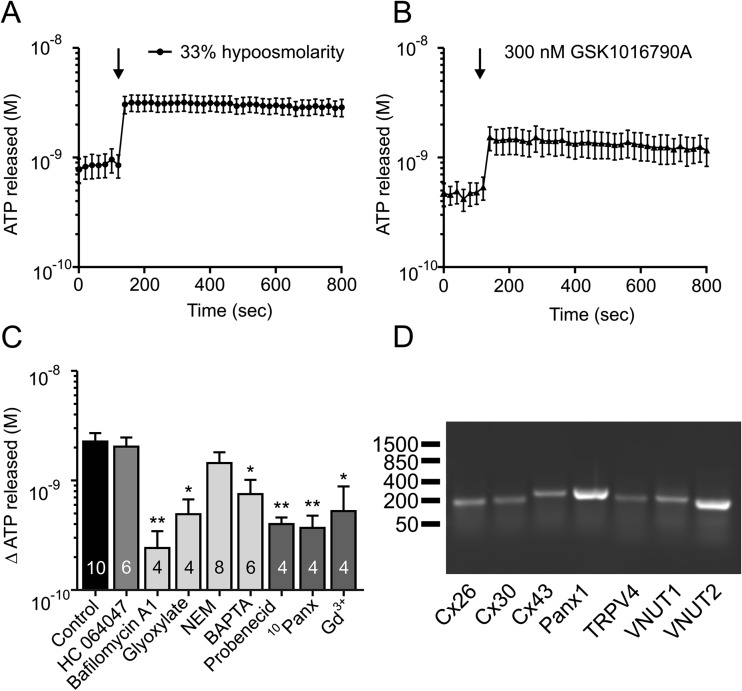
One of the well-known stimuli for ATP release is hypo-osmotic stress. Cell swelling is accompanied with release of ATP, and P2 receptor stimulation can mediate ion and water efflux from cells and contribute to cell volume regulation [24, 27]. Figure 3a shows that in Capan-1 cells, a decrease in solution osmolality by 33 % (i.e. from 310 to 205 mOsm/kg) caused fast ATP increase from the basal level of 0.87 ± 0.21 to 3.14 ± 0.53 nM within 20 s (n = 10). Addition of the same volume of physiological buffer caused no ATP release (Supplementary Fig. S1).
Fig. 3.
Hypo-osmotic effects on ATP release from Capan-1 cells. a Hypo-osmotic stress (33 %) caused fast ATP release from the duct cells (n = 10). b The TRPV4 activator GSK 1016790A (300 nM) evoked ATP release from Capan-1 cells (n = 6). c The TRPV4 inhibitor HC 064047 (10 μM) did not have an effect on hypo-osmotic stimulated ATP release from the cells (n = 6). The vesicle fusion inhibitor N-ethylmaleimide (NEM, 250 μM, n = 8) appeared to decrease ATP release, but no significance was reached; Ca2+ chelator BAPTA decreased the release (25 μM, n = 6). Vacuolar-type H+-ATPase inhibitor bafilomycin A1 (0.1 μM, n = 4), VNUT inhibitor glyoxylate (50 μM, n = 4), probenecid (50 μM; n = 4), gadolinium chloride (Gd3+, 50 μM; n = 4) and mimetic pannexin peptide (10 Panx, 100 μM; n = 4) inhibited hypo-osmotically induced ATP released from Capan-1 cells. d Capan-1 express connexin 26, 30 and 43, pannexin-1, TRPV4, VNUT-1 and VNUT-2 as detected by RT-PCR. Results are given as mean net values ± SEM. * = P < 0.05, ** = P < 0.01. The left axis shows the ATP concentration values corrected per 106 cells in 1 ml. The arrow indicates the time of adding the stimulants
One of mechano/osmotic sensor upstream of ATP release is the cation channel transient receptor potential cation channel subfamily V member 4 (TRPV4) [28, 29], and its role might be to transduce mechanosensing to Ca2+-dependent ATP release [30]. Here, we observed that the specific agonist (GSK 1016790A, 300 nM) induced ATP release from Capan-1 cells from 0.46 ± 0.1 to 1.45 ± 0.39 nM (n = 6, Fig. 3b). However, the inhibitor of the TRPV4, HC064047 (10 μM) did not decrease ATP release in hypo-osmotic conditions (Fig. 3c). In contrast, incubation of the cells with bafilomycin A1, a V-ATPase inhibitor, and glyoxylate, a potential VNUT inhibitor [31], significantly reduced the hypo-osmotic ATP release by 0.24 ± 0.1 and 0.49 ± 0.18 nM compared with the control without the inhibitors, i.e. 2.27 ± 0.43 nM (n = 4, 4, 10) (Fig. 3c). In addition, we observed significant reduction of released ATP (0.75 ± 0.26 nM, n = 6) from Capan-1 cells after pre-incubation with BAPTA, which binds intracellular Ca2+ ions. ATP release in cells pre-treated with NEM was not significantly affected compared to control (1.44 ± 0.36 nM, n = 8). We also studied the role of non-vesicular pathways of ATP release in Capan-1 cells in hypo-osmotic conditions. ATP release was significantly diminished in the presence of probenecid (0.39 ± 0.06 nM, n = 4), an inhibitor of pannexin and connexin hemichannels; Gd3+ (0.52 ± 0.36 nM, n = 4), an inhibitor of maxi-anion channel and connexins; and pannexin-1 mimetic inhibitory peptide 10Panx (0.37 ± 0.11 nM, n = 4), a pannexin inhibitor.
Above results indicate the role of vesicular and non-vesicular pathways in hypo-osmolar-induced ATP release. However, the expression of the transporters/channels mediating ATP release was never examined in Capan-1 cells or any pancreatic duct cells. Therefore, we used the RT-PCR on Capan-1 cells and show that they express transcripts for connexins 26, 30 and 43, pannexin-1, TRPV4 and VNUT (Fig. 3d).
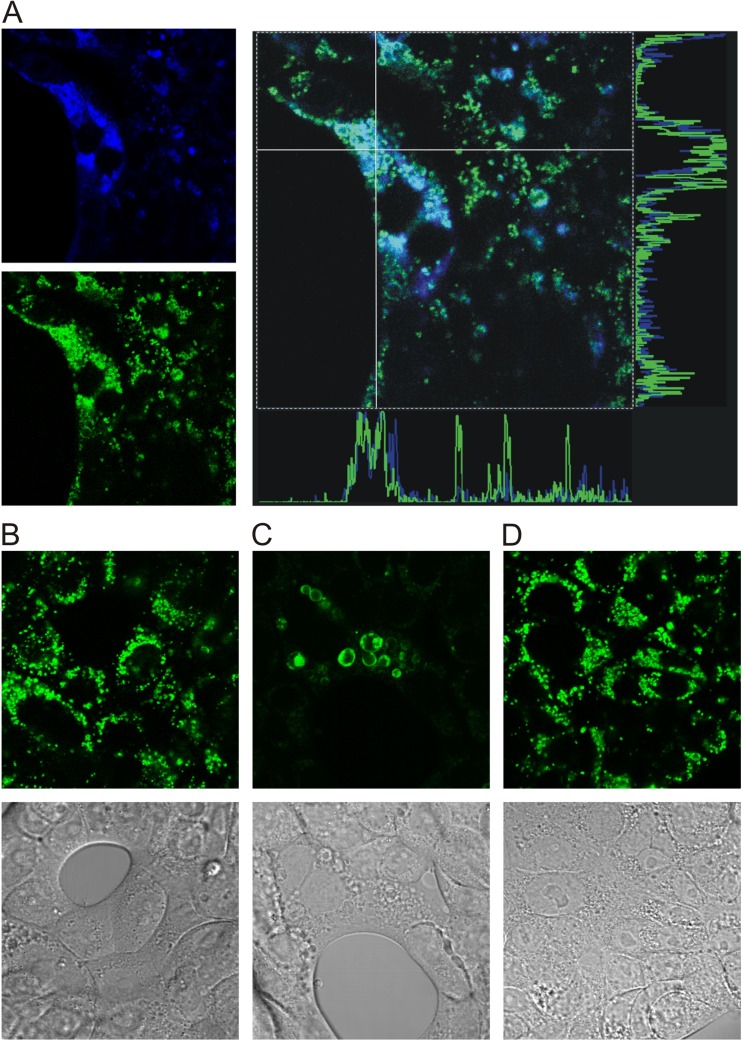
Similar to our previous studies on pancreatic acinar cells expressing VNUT containing secretory vesicles [10, 11], we investigated whether Capan-1 cells expressed vesicular ATP stores. Imaging experiments showed that Capan-1 cells loaded with quinacrine, putative ATP store marker, as well as fluorescent ATP derivative MANT-ATP and the two signals colocalised into vesicles of 2–3 μm in diameter (Fig. 4a). Pre-incubation of cells with bafilomycin A (1 μM for 1 h) altered vesicle appearance, they became less numerous and distended and quinacrine loading was diminished compared to control cells (Fig. 4b, c). Pre-treatment of cells with the inhibitor of protein traffic from the ER to Golgi, brefeldin A (5 μg/ml for 2 h), did not have any pronounced effect on cell morphology and vesicular loading of quinacrine (Fig. 4d). These experiments indicate that Capan-1 cells contain secretory-type vesicles that store ATP.
Fig. 4.
Intracellular ATP stores in Capan-1 cells. a Capan-1 cells accumulate quinacrine (green) and MANT-ATP (blue) in intracellular vesicles. The right panels show that fluorescence of the two signals overlap. b Transmission and quinacrine image of control Capan-1 cells. Effect of various inhibitors on quinacrine vesicles in Capan-1 cells: bafilomycin A (c) and brefeldin A (d). Representative images from three to four independent experiments
Mechanical stimulation induces ATP release
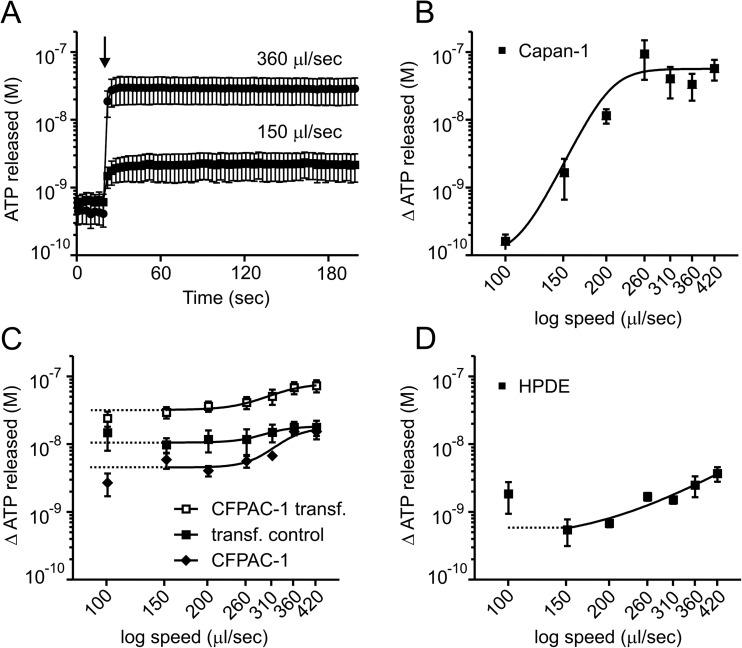
In pancreas that is stimulated to secrete with acinar or ductal agonists, ducts can be exposed to mechanical stress imposed by fluid flow and duct distension, the latter can be quite significant during duct obstruction with calcified stones. In the following series of experiments, we studied ATP release in Capan-1 cells exposed to mechanical stimulation of luminometer injection pump with speeds 100–420 μl/s (50 μl into 100 μl). The mechanical stress (shear and turbulence) is not defined in these open-chamber experiments; however, basic mechanosensitivity of cells can be easily investigated in online ATP detection of fast responses. The sample trace is shown in Fig. 5a. We observed fast and speed-dependent ATP release with the maximum increase in ATPe concentration by 94.1 ± 55.1 nM (n = 7) with 260 μl/s speed. Figure 5b shows speed-dependent change in ATP concentrations.
Fig. 5.
Mechanically induced ATP release from Capan-1, CFPAC and HPDE cells. a The representative time course of the fast ATP release from Capan-1 cells after mechanical stimulation using pump injections, here 150 and 360 μl/s (n = 7, 7). The arrow indicates the time of physiological buffer injections. Dose-dependent release of ATP from Capan-1 b and in response to a range of pump injection speeds as follows: 100, 150, 200, 260, 310, 360 and 420 μl/s (n = 6–7). c Transfection CFPAC-1 cells with CFTR plasmid. The values of ATP released from the CFPAC-1 cells transfected with CFTR plasmid (full squares) (n = 5) in response to mechanical stimulation (100–420 μl/s) were significantly increased compare to control cells treated with transfection reagent (open squares) (n = 5). Between untreated cells (full diamonds) and transfection control cells (open squares), there were no significant differences. d Effect of mechanical stimulation on ATP release from HPDE cells after pump injection (100–420 μl/s, n = 5). The left axis shows the ATP concentration values corrected per 106 cells in 1 ml. Results are given as mean net values ± SEM
To identify the mechanisms of mechanically stimulated ATP release, we incubated cells with a wide range of inhibitors. Inhibitors for vesicular release pathway (bafilomycin A1, NEM, glyoxylate, brefeldin A), caveolin-1 (MβCD) and non-vesicular release (probenecid, Gd3+, 10Panx) did not significantly inhibit this mechanically induced ATP release from Capan-1 cells in the used setup (see Supplementary Fig. S2a–c). Since we were unable to inhibit the mechanical ATP release, we checked whether an increase in ATPe was not due to cell death/lysis. This was done by quantifying the release of cellular LDH as a marker of cell viability. We observed that mechanical stimulation of Capan-1 cells with pump injection of a physiological buffer with speed of 260 and 420 μl/s had detectable but minimal effects on LDH release compared to control unstimulated cells. This relatively low number of leaky or lysed cells is much lower than that needed to account for mechanically induced ATP release, should that occur by cell lysis. Also trypan blue exclusion confirmed cell viability (see Supplementary Fig. S2d–f).
In addition, since CFTR is thought to be involved in mechanically induced ATP release [32], we tested CFPAC-1 cells, which have ΔF508-CFTR mutation in CFTR (Fig. 5c). Although their response to mechanical stimulation was less pump speed-dependent, transfection with functional CFTR did not make the cells more mechanosensitive, just elevated the overall release of ATP to all pump speeds as shown in Fig. 5c. Another cell line HPDE which expresses functional CFTR also showed modest mechanical stress dependency using the pump protocols (Fig. 5d). These data indicate that Capan-1 cells are sensitive to this type of mechanical stress, and they have multiple/complex mechanisms for mechanically induced ATP release, which application of a single inhibitor at a time could not eliminate.
Effect of UTP and UDP analogues on extracellular ATP
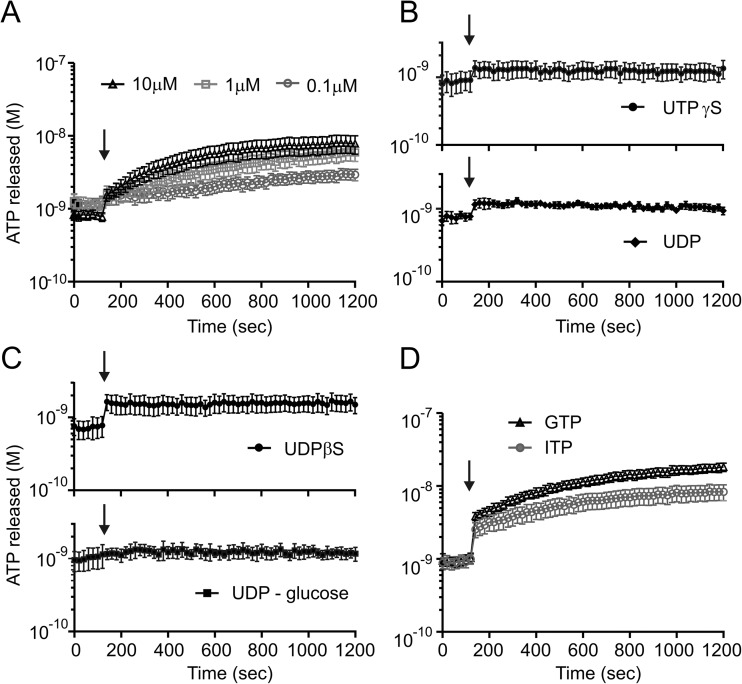
Previous studies on Schwann cells and osteocytes have shown that UTP can enhance the ATPe concentration [33, 34]. UTP could be released together with ATP from acinar zymogen granules or from duct cells, as it has been shown for airway epithelia and astrocytoma cells [35]. Therefore, we aimed to investigate whether UTP had any effect on ATPe in duct cells. Exposure of Capan-1 cells to UTP (0.1–10 μM) (Fig. 6a) induced a concentration-dependent increase in extracellular ATP. This increase in ATPe was slower than that induced with other stimuli (see above), and the increase in ATPe detected after 20 min with 10 μM UTP was 7.1 ± 2.2 nM (n = 10).
Fig. 6.
Effect of nucleotides on ATP in Capan-1 cells. a The extracellular ATP increase in Capan-1 cells after incubation with 0.1 (n = 5), 1 (n = 5) and 10 μM (n = 10) of UTP. b, c Stimulation of P2Y receptors with 10 μM of enzymatically stable agonists as follow: UTPγS (n = 7), UDP-glucose (n = 5), UDPβS (n = 9) and ADP (n = 4) did not increase the extracellular concentration of ATP in Capan-1 cells. In contrast triphosphates d, GTP and ITP (10 μM, n = 4, 7), show a similar ATP response as UTP. The left axis shows the ATP concentration values corrected per 106 cells in 1 ml. The arrows indicate the time of adding the stimulants
In the next step, we aimed to elucidate whether the observed augmentation of ATPe could be due to possible stimulation of P2Y receptors causing ATP release, as has been proposed for urothelial cells [36]. Since UTP can also be degraded to UDP and activate a range of P2Y receptors, we used selective and metabolically stable agonists. Addition of UTPγS, the agonist of P2Y2 and P2Y4 receptors, had very small initial effect on ATP release (Fig. 6b), but there was no build up of ATP with kinetics observed with UTP (Fig. 6a). Exposing duct cells to UDP (Fig. 6b) again slightly increased initial ATPe levels, but thereafter, ATPe remained relatively stable. Since UDP is an agonist for P2Y6 and P2Y14 receptors, we selectively activated P2Y6 receptor with UDPβS and P2Y14 receptor with UDP-glucose (Fig. 6c). Stimulation of the P2Y6 receptor induced a small but significant immediate increase of ATPe. Stimulation of the P2Y14 receptor had no significant effect on ATPe. Nevertheless, none of these UTP/UDP enzymatically stable analogues reproduced the kinetic of slow increase in ATPe as observed with UTP. Therefore, we considered the role of extracellular enzymes in ATP generation.
Evidence for enzymes generating ATP in duct cells
NDPK does not have a substrate preference and catalyses the exchange of γ-phosphate between nucleotide di- and triphosphates. To investigate possible role of NDPK in extracellular ATP increase, Capan-1 cells were stimulated with other triphosphates including GTP and ITP (10 μM) (Fig. 6d). Both the γ-phosphate donors increased ATPe concentrations to the similar levels and with similar kinetics as UTP. Notably, to date, there are no identified human receptors activated by either GTP or ITP. Therefore, these data indicate that ATP is likely generated extracellularly rather than slowly released, if the appropriate substrate is present.
The next series of experiments investigated the activity of ecto-ATP generating enzymes in Capan-1 cells using online luminescence measurements of plateau levels of ATPe generated between 30 and 90 min. Exposing cells to increasing concentrations of UTP (0.001–100 μM), as a donor of phosphate groups in the presence of ADP as an acceptor (10 μM), increased proportionally the extracellular concentration of ATP (Fig. 7a). This phenomenon was not observed when UDP was added to compete with ADP as a substrate for the NDPK reaction (Fig. 7b). That is, treating cells with the buffer containing UTP (10 μM), ADP (1 μM) and increasing concentrations of UDP (0.001–1,000 μM), as the second acceptor of γ-phosphates groups, dose-dependently inhibited the ATP-generating effect (Fig. 7b). Since UDP competed with ADP for the phosphate transferred from UTP, ATP decreased and UTP increased. Interestingly, when the cells were treated with UTP (10 μM) together with ADP in a range of concentration (0.001–100 μM, Fig. 7c), the generation of ATP did not follow the same kinetics as observed for varying UTP concentrations (Fig. 7a). This effect could be due to the concerted action of two enzymes, NDPK and AK. The former enzyme transfers phosphate from UTP to ADP, while AK may additionally contribute to ADP conversion to ATP through reversible phosphotransfer reaction [5].
Fig. 7.
Effect of phosphate donors/acceptor on ATP generation. a Capan-1 cells generate extracellular ATP proportionally to the concentration of applied UTP from 0.001 to 100 μM in the presence of 10 μM ADP (n = 3). This effect was gradually decreased b, when the cells were stimulated with donor of phosphate groups, UTP (10 μM) and two different acceptors of phosphate groups ADP (1 μM) and increasing concentration of UDP (0.001–1,000 μM) (n = 3). c Extracellular ATP was generated in Capan-1 cells in presence of increasing concentration of acceptor ADP (0.001–100 μM) and 10 μM concentration of UTP. The left axis shows the ATP concentration values corrected per 106 cells in 1 ml. Results are given as mean net values ± SEM
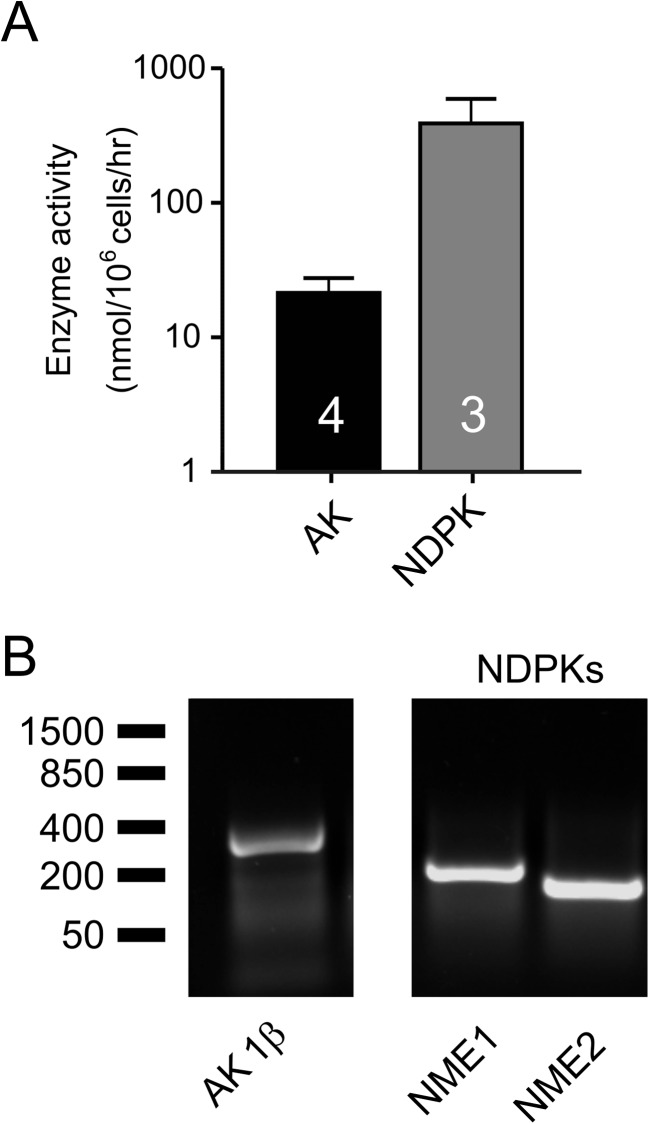
In order to determine the pattern of nucleotide metabolism in Capan-1 cells, we employed TLC enzymatic assay with 3H-labelled and unlabelled substrates. Quantitative analysis confirms the capability of Capan-1 cell enzymes to generate high-energy 3H-phosphoryls after incubation of cells with [3H]AMP (AK) and especially [3H]ADP (NDPK) in the presence of γ-phosphate-donating ATP (Fig. 8a). The activities of AK and NDPK were measured, respectively, as 22 ± 6 nmol/106 cells/h (n = 4) and 389 ± 205 nmol/106 cells/h (n = 3). Pre-treatment of the cells with specific AK inhibitor Ap5A (60 μM) inhibited by ~90 % (to about 0.5 nmol/106 cells/h, n = 2; data not shown) their capability to transphosphorylate [3H]AMP and ATP. Furthermore, we confirmed the presence of both ATP-generating enzymes in Capan-1 cells using RT-PCR method (Fig. 8b). We designed the primers based on the study conducted by Collavin and colleagues [37] for an AK1β isoform, which was shown to be localised to cell surface [38]. For NDPK, the primers were designed to detect NME1 and NME2, which are the genes required for catalytic active NDPK [39].
Fig. 8.
The expression and activity of NDPK and AK1β in Capan-1 cells. a The activity of extracellular enzymes: nucleoside diphosphate kinase (NDPK, n = 3) and adenylate kinase (AK, n = 4) in Capan-1 cells. NDPK and AK were assayed by TLC with ATP and ADP (400 μM) and the labelled tracer [3H]ADP and AMP (400 μM), ATP (500 μM) and [3H]AMP as respective substrates. Enzymatic activities are presented as bar graph expressed as nanomoles of phosphorylated 3H-substrates by 106 cells per 1 h. The left axis shows the ATP concentration values corrected per 106 cells in 1 ml. Results are given as mean net values ± SEM. b The RT-PCR of extracellular membrane-bound adenylate kinase isoform 1β (AK1β) and NDPK domains (NME1, 2) in Capan-1 cells
Evidence for nucleotide-hydrolysing enzymes
The above studies indicate that pancreatic duct cells can both release ATP to extracellular milieu, as well as generate ATPe by kinases from other purine and pyrimidine nucleotides. Since purinergic receptors expressed on duct cells are activated not only by ATP, but also by the products of its hydrolysis, we wanted to elucidate mechanisms for the extracellular ATP degradation pathways.
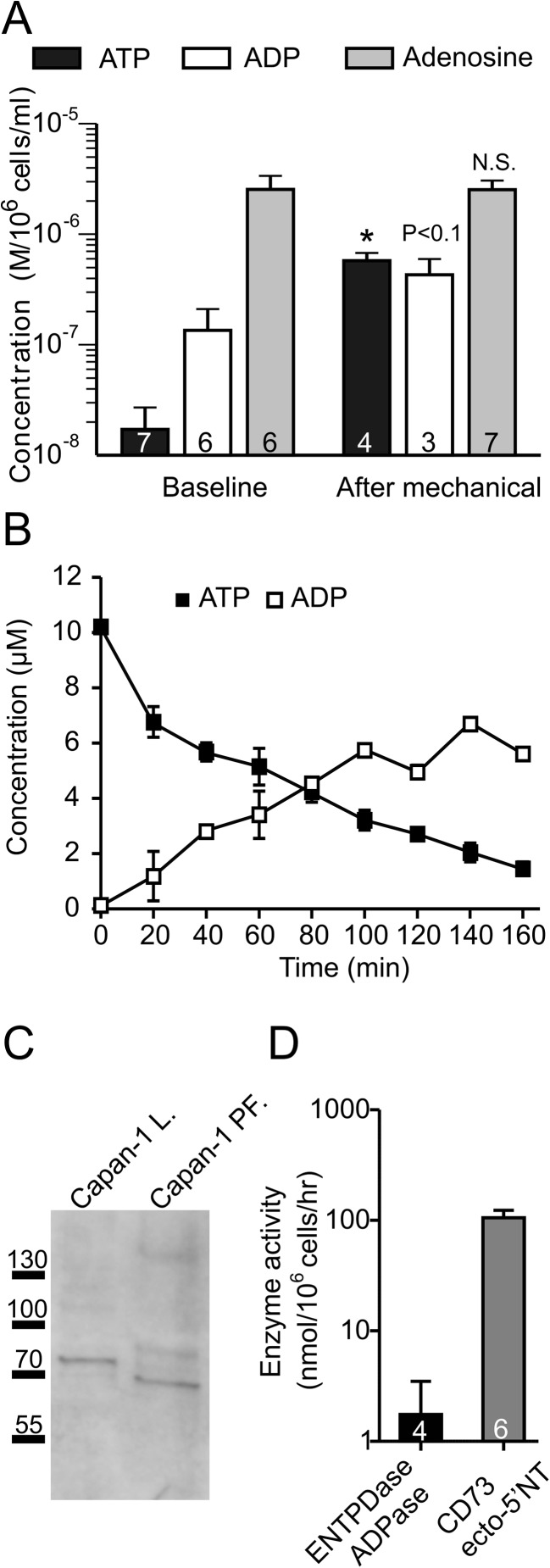
Using offline luminometric/fluorometric assay, we analysed the extracellular concentration of adenine derivatives in supernatants collected from Capan-1 cells in two different conditions. We observed that in unstimulated baseline conditions, the extracellular ATP concentration was relatively low (20 ± 11 nM, n = 6) compared to ADP (135 ± 76 nM, n = 6) and adenosine (2,550 ± 827 nM, n = 6) (Fig. 9a). Furthermore, after 1 min of mechanical stimulation, the concentration of ATPe significantly increased to 578 ± 100 nM. ADP concentration appeared slightly higher (431 ± 166 nM, n = 3) compared to basal conditions (Fig. 9a), but no statistical significance was reached. Interestingly, there was no significant changes in concentration of extracellular adenosine after mechanical stimulation, i.e. it was 2,535 ± 535 (n = 7). These observations lead to the following interpretations. Firstly, ATP is released by mechanical stimulation. Secondly, ADP and adenosine levels are relatively stable before and after about 1 min of mechanical stimulation. For the following experiments, we looked at longer time intervals and determined ATP and ADP concentrations. Capan-1 cells were exposed to 10 μM ATP, and Fig. 9b shows that after the incubation (0–180 min), the cells were able to degrade ATP to ADP, and ADP levels remained relatively stable after 80 min. Furthermore, we hypothesised that the high and stable concentration of ADP may be due to the expression of NTPDase2 combined with low ADPase/NTPDase activity. Therefore, we tested this hypothesis by performing western blot for NTPDase2 and TLC analysis of ADP/NTPDase activity. Figure 9c shows the expression of glycosylated NTPDase2 (75 kDa) in the total cell lysate (Capan-1 L). In addition, the analysis of ultracentrifuged samples from the extracellular solution collected from Capan cells showed NTPDase2 expression, suggesting that it was present in a microsomal/particulate fraction (Capan-1 PF) containing secreted microvesicles/exosomes.
Fig. 9.
The extracellular concentration of adenine derivatives and activity of nucleotide-hydrolysing enzymes in Capan-1 cells. a Analysis of extracellular concentrations of ATP, ADP and adenosine in unstimulated conditions (n = 6–8) and after mechanical stimulation (n = 3–7) in Capan-1 cells. b The net values of concentrations of extracellular ATP (full squares) and ADP (empty squares) released from Capan-1 cells during 160 min after stimulation of Capan-1 with 10 μM of ATP (n = 2). c Western blot analysis of expression NTPDase2 in Capan-1 cells (Capan-1 lysate) and secreted form present in particulate fraction. d The rates of nucleotides hydrolyses were determined by TLC using 400 μM [3H]ADP (NTPDase/ADPase, n = 4) and 300 μM [3H]AMP (AMPase/CD73, n = 6) as initial substrates. The enzyme activities are presented as bar graph expressed as nanomoles of 3H-nucleotides hydrolysed by 106 cells per 1 h. Results are given as mean net values ± SEM
The activity of ADPase/NTPDase was measured by incubation of the cells with ADP as a substrate for the enzymes and with 3H-labelled ADP tracer. After 45 min, only one out of four samples showed a small hydrolysing activity of the enzyme, while others had almost no activity; together the total enzyme activity was calculated as 1.7 ± 1.7 nmol/106 cells/h (n = 4) (Fig. 9d). In contrast, exposing duct cells to [3H]AMP resulted in a marked generation of extracellular adenosine to 106 ± 18 nmol/106 cells/h (n = 6), which can be prevented after pre-treatment the cells with CD73 inhibitor AMPCP (to about 7 nmol/106 cells/h, n = 2; data not shown), thereby suggesting the presence relatively high ecto-5′-nucleotidase/CD73 activity on the studied cells.
Discussion
The role of purinergic receptors in regulation of pancreatic duct epithelial function is relatively well established. However, whether duct cells can release ATP and how this might be regulated has not been investigated until now. In the present study, we show that ATP is released from pancreatic duct cells in response to different stimuli including pH changes, mechanical and hypo-osmotic stress. Capan-1 cells express transcripts of a number of proteins/transporters potentially involved in ATP release and regulation including VNUT, pannexin-1, connexins 26, 30, and 43 and TRPV4. We also show that these pancreatic duct cells are able to hydrolyse extracellular ATP, as well as regenerate it, and that Capan-1 expresses active enzymes including NDPK and AK1β and NTPDase2. In the following paragraphs, we discuss how these findings contribute to our understanding of exocrine pancreas physiology.
Pancreatic juice can vary in pH depending on the mode of stimulation and the contributing epithelium. Pancreatic acini secrete enzyme-rich fluid which may be potentially acidic, although this is not fully settled [16, 40]. Pancreatic ducts are exposed to acinar fluid, and they themselves can be stimulated to produce of HCO3−-rich fluid with pH ~8. Therefore, it was important to establish whether acid or alkaline pH had any effect on extracellular/surface ATP, as proposed for duodenal epithelium where alkaline phosphatase contributes to ATP hydrolysis [17]. In the present study, we show that acid and alkaline pH caused a small but significant initial increase in ATPe, most likely due to ATP release, and after about 10 min there was suppression of ATPe (acid pH) and increase in ATPe (alkaline pH) (Fig. 1b, c), possibly due to concerted action of ecto-nucleotidases and nucleotide kinases co-expressed on the cell surface (see below).
Pancreatic secretagogues, secretin and acetylcholine, act via cAMP and Ca2+ signalling pathways [15, 41]. Using direct stimulants of these pathways, forskolin and ionomycin, respectively, had only minimal effects on ATP release on their own (Fig. 2a, b). This was somewhat surprising as the Capan-1 epithelium expresses CFTR and Ca2+-activated Cl− channel ANO1 [12, 42], and from other studies, it is known that CFTR is essential in adrenaline- and forskolin-induced ATP release in epididymis [43] and in cAMP-stimulated retinal epithelial cells [44]. Ethanol at low concentrations potentiates pancreatic duct secretion, while at high concentrations, it is damaging and associated with chronic pancreatitis [15, 45, 46]. We predicted that ethanol would cause cell volume changes and cell damage and ATP release. To our surprise, it had no effect on ATP release whatsoever. In contrast, bile acids, which are pancreatic duct stimulants at low concentrations [15, 41], had pronounced effect on ATP release (Fig. 2c). The bile acid-induced ATP release was investigated in more detail elsewhere, and it was shown to involve vesicular and non-exocytotic ATP release mechanisms [26].
Hypo-osmotic stress leading to cell volume changes is one of the well-studied stimuli evoking ATP release. Potentially, solute/water transporting epithelial cells could undergo similar cell volume changes (without being exposed to hypo-osmotic stimulus). Here, we show that Capan-1 cells release significant amount of ATP in response to 33 % decrease in osmolality (Fig. 3a). Cell swelling and regulation of the cell volume involve various ion channels and transporters [47], which could be involved in ATP release. For instance, maxi-anion channels are involved in hypo-osmotically induced ATP release in mouse astrocytes [48], while in fibrosarcoma cells also, pannexin 1 contributed to ATP release [49]. In airway epithelia, pannexin-1 is the main contributor to hypo-osmotic ATP release as established by inhibitor, pannexin-1 knockdown and pannexin-1 knockout mice [50]. Our data shows that hypo-osmotic ATP release in Capan-1 cells has several components. Firstly, it is inhibited by pannexin-1, connexin and maxi-anion channel blockers (Fig. 3c), and the cells express several connexins and pannexin-1 (Fig. 3d). Secondly, ATP release was stimulated by specific TRPV4 activator GSK1016790A and partially inhibited by BAPTA (Fig. 3b, c), indicating that increased Ca2+ influx through this channel may be transducing cell membrane stretch to ATP release, similar to airway epithelia and keratinocytes [30, 50]. Though, it is not clear why inhibitor of TRPV4 did not have any effect in Capan-1 cells. Thirdly, ATP release in Capan-1 cells was significantly inhibited by vesicular/exocytosis inhibitors (Fig. 3c). Furthermore, these cells express VNUT1 and VNUT2 (Fig. 3d). In support of the vesicular ATP release and ATP stores are the imaging studies, which show that Capan-1 cells have very pronounced vesicular ATP stores, which decreased significantly if cells were pre-incubated with bafilomycin A (Fig. 4). Similar hypo-osmotic stimulation of vesicular ATP release was observed in biliary cells, which have similar epithelial transporters as pancreatic duct cells [51]. In summary, these studies show that ATP release from duct cells with hypo-osmotic stimulus involves several release mechanisms.
Another type of stimulus commonly used to elicit ATP release is the mechanical (pressure, stretch or shear) stress, and again several pathways could be involved. It was shown that mechanical stimuli on keratinocytes and biliary epithelia increased intracellular Ca2+ and triggered ATP release [52, 53], which can be vesicular as shown in smooth muscle cells and epidermal keratinocytes [54, 55]. Other findings from renal tubules and endothelial cells indicate the key role of connexin hemichannels in shear stress-induced ATP release [56–58], or pannexin-1 and P2X7 receptor in periodontal ligament cells [59], or the involvement of both vesicular and non-vesicular pathways in which alveolar cells [60]. Furthermore, recent studies based on cell surface-attached firefly luciferase showed that shear stress-induced ATP release from endothelial cells is associated with the caveolin-1-rich regions in cell membranes [61]. Also very early on CFTR has been proposed as a channel of mechanically induced ATP release [62]. This, however, has been opposed as these channels have no detectable ATP currents [63, 64]. Therefore, CFTR has been instead considered as an important regulator or mechanosensor [65], as for example, in red blood cells, CFTR is required for ATP release [32]. Our study showed that Capan-1 cells are very sensitive to mechanical stress and release ATP proportionally to the applied stress pump speeds (Fig. 5b). This stress stimulus is not easily defined in terms of shear/stretch components. However, it might approximate some situations occurring in pancreatic ducts, which are exposed to both fluid flow and as well as distension during secretion. We used a wide range of inhibitors for ATP release mechanisms, and neither of them inhibited the ATP release significantly (although a small inhibitory tendency with vesicular inhibitors was observed, see Supplementary Fig. S2a–c). To investigate if the mechanical stimulation may be caused by cell lysis, we performed analysis of LDH release, showing that an insignificant number of cells released their LDH (Supplementary Fig. S2d–f). Therefore, we conclude that the lack of clear pharmacological profile response indicates more complex/multiple mechanisms involved in mechanically induced ATP release in these cells. Interestingly, the same setup was used to study ATP release from HPDE and CFPAC-1 cells, and pump-induced mechanical stimulation caused very modest increases in ATP release compared to Capan-1 cells. Furthermore, expression of functional CFTR in knockin CFPAC-1 cells and CFTR-expressing HPDE cells (Fig. 5c, d) or CFTR-172inh (Fig. S2) had no pronounced effects on extracellular ATP as a function of mechanical stress. Potentially, several ATP releasing or yet undefined pathways could be involved. Alternatively, also ecto-enzyme released by shear stress, such as from vascular endothelial cells [66], could influence ATPe composition as discussed below.
One the physiological modulators of extracellular ATP could be UTP. UTP and (UDP, UDP sugars) are co-released together with ATP in epithelia [1, 35, 67, 68], and this could be the case for pancreatic acini and/or ducts. Activation of P2Y receptors can stimulate ATP release from urothelium and osteocytes [34, 36]. We indeed observed an increase of ATPe after duct cell exposure to different concentrations of UTP (Fig. 6a). Notably, this had slower kinetics than ATP release evoked by stimuli discussed above. Based on our experiments with specific and enzymatically stable P2Y receptor agonists (Fig. 6b, c), we concluded that the UTP activation of P2Y receptors is a pathway of minor importance in increasing ATPe in our cells. A minor involvement of P2Y6-stimulated exocytosis can however not be excluded [34]. Applying enzyme assays to our preparation, data strongly suggest that UTP as well as other nucleotide triphosphates can act as donors of phosphate groups to produce extracellular ATP in presence of ATP-generating enzymes. Indeed, we showed the expression and activity of NDPK and AK1β in Capan-1 cells, based on TLC analysis and RT-PCR (Fig. 8). Additionally, luminescence measurements showed that ATPe concentration increased proportionally to the amount of donor phosphates groups, as the result of the enzyme activities, and that could be inhibited by the presence of another phosphate acceptor than ADP (Fig. 7b). Therefore, we postulate that Capan-1 cells maintain a high extracellular ADP level, which in turn can act as an acceptor of phosphates groups from nucleotide triphosphates (NTPs). Indeed, we only observed an increase of generated ATPe after incubating cells with concentrations of ADP higher than 10 nM (Fig. 7c), and this is the ADP level in Capan-1 cells under unstimulated conditions (Fig. 9a). Regarding the localisation of enzymes, although many AK isoforms are located in the cytosol, AK1β isoform is localised on the cell surface [38]. Also NDPK (NME1, 2) are expressed on the plasma membrane or as soluble enzymes [5], and previously, NDPK was identified on membrane fraction from rat pancreas [69].
Interestingly, analysis of extracellular ATP, ADP and adenosine in basal conditions showed that ATP level was relatively low, while ADP was about tenfold higher and adenosine was about 100-fold higher. These results are basically consistent with our previous studies showing the ability of different vascular endothelial, lymphoid and tumour cells to maintain basal extracellular ATP, ADP and adenosine as certain nanomolar steady-state levels [23]. Mechanical stimulation which caused ATP release and increase in ATPe showed that, first of all, ATP was hydrolysed and this is dependent on ATPase activity, presumably ATPase/NTPDase (as ADPase/NTPDase was low; Fig. 9c), and there was a buildup and ADP that remained relatively stable (Fig. 9b). Second, there was a high level of adenosine, which was independent of mechanical stimulation (Fig. 9c). However, it is not clear whether this nucleoside is primarily derived via ecto-enzymatic conversion of ATP through concerted action of NTPDases, AK and CD73 or, alternatively, there is another source of adenosine, such as adenosine release and/or transport via equilibrative nucleoside transporter, ENT [70].
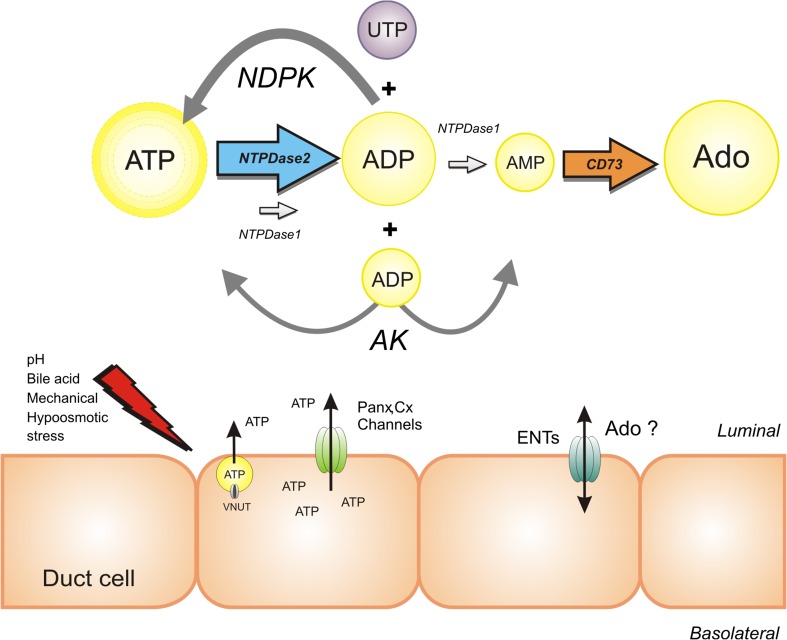
The question remains—how do these ecto-nucleotidases/kinases correlate with what is known in pancreas physiology? Pancreatic juice collected from the main pancreatic duct of rodents contained about 1 nM ATP [14, 71]. We know that rat pancreatic acini, and possibly ducts, express NTPDase1/CD39, which is released into pancreatic juice in microvesicles/exosomes [14]. Rat pancreatic juice also contains CD73 activity, as well as modest activity of NDPK and AK [13]. One study shows that human pancreatic juice is also low in ATP, and contains ecto-ATPase activity, though enzyme histochemistry and immunostaining with NTPDase1/CD39 antibodies revealed inactive enzyme in human as opposed to rat pancreatic ducts (70). Also in the mouse pancreas, duct epithelium appeared negative for NTPDase1 antibody but reacted with the NTPDase2 antibody [72]. Although there might be species differences in NTPDase expression in pancreatic ducts, our study on Capan-1 cells indicates expression of NTPDase2, as we observed fast ATP hydrolysis into ADP, and ADP concentrations remained relatively stable after 80 min. These data are consistent with properties of NTPDase2 [6]. We also found NTPDase2 protein in Capan-1 cells, as well as secreted extracellular enzyme detected in a particulate fraction, which suggests microvesicular secretion. Such microvesicular secretion of ecto-nucleotidases has been proposed also for the rat pancreas and submandibular glands [14, 73]. Overall, it appears that pancreatic ducts express ecto-enzymes somewhat different to acini and to pancreatic juice arising from stimulation of acinar by cholecystokinin. That is, ducts have active enzyme consistent with NTPDase 2 and very active ATP-generating enzymes, which are modifying extracellular fluid.
In summary, this study shows that our duct cell model (Fig. 10) has multiple mechanisms to keep extracellular ATP high—by ATP release and ATP regeneration. Thus, pancreatic ducts do not need to rely on ATP delivered form acini, but can regulate it locally, and therefore can activate a number of ATP-preferring receptors to regulate ion/fluid transport. Even though Capan-1 cells form epithelial monolayers and exhibit ion transport consistent with normal pancreatic duct epithelium and are a good functional model for human duct epithelium, they are an adenocarcinoma cell line. Several ATP-hydrolysing and generating enzymes are upregulated in various cancers [5], and potential role of these pathways in pancreas duct pathophysiology is yet to be investigated. One study indicates that soluble plasma CD73 is predictive for acute pancreatitis [74], while CD39 is upregulated in chronic pancreatitis and pancreas cancer, though immunohistochemistry indicates that the enzyme is preferentially associated with the vasculature and stromal elements [75].
Fig. 10.
Proposed model of purinergic signalling cascade in Capan-1 cells. Capan-1 cells release ATP in response to several stimuli via vesicular and non-vesicular pathways. Subsequent, released ATP can possibly be hydrolysed by NTPDase2 (ENTPDase/ATPase) to ADP; ADP concentration seems to be high and relatively stable due to a very low activity of NTPDase1 (ENTPDase/ADPase). Capan-1 cells express functional CD73 characterised by relatively high activity. In addition, high extracellular adenosine concentration was detected, which most likely does not originate from ATP hydrolysis but could be mediated by ENTs. The presence of extracellular ADP and UTP, possibly released from the pancreatic cells, may increase the extracellular ATP in reaction catalysed by highly active NDPK. Alternatively, ATP could be generated from ADP via a reverse reaction catalysed by AK. The sizes of letters indicate predicted purine concentrations and arrows indicate detected activities of enzymes. Following abbreviations are used: nucleoside triphosphate diphosphohydrolase 1,2 (NTPDase1, 2), ecto-5′-nucleotidase (CD73), nucleoside diphosphate kinase (NDPK), adenylate kinase (AK), pannexin-1 (Panx), connexin chemichannels (Cx) and equilibrative nucleoside transporters (ENTs)
In conclusion, this study illustrates for the first time that pancreatic duct (model) exhibits a complex regulation of extracellular ATP. First, it involves multiple release pathways which cause fast increase in ATPe and mode of release depends on eliciting stimulus (Fig. 10). Furthermore, ecto-kinases and ecto-phosphohydrolases regulate nucleotide/side homeostasis, and it can be postulated that some stimuli can also have influence on these enzymes, either their activity and or release from cells to the medium/interstitium. Purinergic signalling and P2 and adenosine receptors are important for pancreatic duct ion transport, and here, we present that ATP can also be released locally and be transformed by extracellular enzymes, increasing the possible ways of ductal regulation.
Electronic supplementary material
(PDF 338 KB)
Acknowledgments
We appreciate assistance of M. Zuccarini in autoradiographic assays and opportunity to use some of the facilities of S. Jalkanen’s laboratory. We are grateful to Prof. J. Hanrahan for providing us with the CFTR plasmid, Prof. Jean Sevigny for providing anti-NTPDase2 antibody and Prof. P. A. Pedersen and D. Sørensen for use of their facilities. The technical assistance of P. Roshof is greatly appreciated. Imaging experiments were done the Center for Advanced Bioimaging (CAB), University of Copenhagen, Denmark.
Author contribution
JMK performed all experiments and analysis on ATP release and enzyme activity. Enzyme assays were performed in collaboration with GY. IN performed imaging studies. The project was planned by IN, JMK and GY. The manuscript was written jointly by JMK and IN. All authors were involved in critically revising and approved the final version of the manuscript.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baroja-Mazo A, Barbera-Cremades M, Pelegrin P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta. 2013;1828:79–93. doi: 10.1016/j.bbamem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010 doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol. 2014;49:473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novak I. Purinergic signalling in epithelial ion transport: regulation of secretion and absorption. Acta Physiol (Oxf) 2011;202:501–522. doi: 10.1111/j.1748-1716.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G, Novak I. Purinergic signalling in the pancreas in health and disease. J Endocrinol. 2012;213:123–141. doi: 10.1530/JOE-11-0434. [DOI] [PubMed] [Google Scholar]
- 9.Haanes KA, Kowal JM, Arpino G, Lange SC, Moriyama Y, Pedersen PA, Novak I. Role of vesicular nucleotide transporter VNUT (SLC17A9) in release of ATP from AR42J cells and mouse pancreatic acinar cells. Purinergic Signal. 2014 doi: 10.1007/s11302-014-9406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem. 2001;276:32925–32932. doi: 10.1074/jbc.M103313200. [DOI] [PubMed] [Google Scholar]
- 11.Haanes KA, Novak I. ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J. 2010;429:303–311. doi: 10.1042/BJ20091337. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Haanes KA, Novak I. Purinergic regulation of CFTR and Ca2+-activated Cl− channels and K+ channels in human pancreatic duct epithelium. Am J Physiol Cell Physiol. 2013;304:C673–C684. doi: 10.1152/ajpcell.00196.2012. [DOI] [PubMed] [Google Scholar]
- 13.Yegutkin GG, Samburski SS, Jalkanen S, Novak I. ATP-consuming and ATP-generating enzymes secreted by pancreas. J Biol Chem. 2006;281:29441–29447. doi: 10.1074/jbc.M602480200. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen CE, Amstrup J, Rasmussen HN, Ankorina-Stark I, Novak I. Rat pancreas secretes particulate ecto-nucleotidase CD39. J Physiol. 2003;551:881–892. doi: 10.1113/jphysiol.2003.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegyi P, Petersen OH. The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol. 2013;165:1–30. doi: 10.1007/112_2013_14. [DOI] [PubMed] [Google Scholar]
- 16.Novak I, Haanes KA, Wang J. Acid–base transport in pancreas—new challenges. Front Physiol. 2013;4:380. doi: 10.3389/fphys.2013.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaunitz JD, Akiba Y. Purinergic regulation of duodenal surface pH and ATP concentration: implications for mucosal defence, lipid uptake and cystic fibrosis. Acta Physiol (Oxf) 2011;201:109–116. doi: 10.1111/j.1748-1716.2010.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Novak I. Ion transport in human pancreatic duct epithelium, Capan-1 cells, is regulated by secretin, VIP, acetylcholine, and purinergic receptors. Pancreas. 2013;42:452–460. doi: 10.1097/MPA.0b013e318264c302. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Barbuskaite D, Tozzi M, Giannuzzo A, Sorensen CE, Novak I. Proton pump inhibitors inhibit pancreatic secretion: role of gastric and Non-gastric H+/K+-ATPases. PLoS One. 2015;10:e0126432. doi: 10.1371/journal.pone.0126432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarowski ER, Paradiso AM, Watt WC, Harden TK, Boucher RC. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proc Natl Acad Sci U S A. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouyang H, Mou L, Luk C, Liu N, Karaskova J, Squire J, Tsao MS. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol. 2000;157:1623–1631. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yegutkin GG, Henttinen T, Jalkanen S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- 23.Helenius M, Jalkanen S, Yegutkin G. Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim Biophys Acta. 2012;1823:1967–1975. doi: 10.1016/j.bbamcr.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27:113–120. doi: 10.1016/j.tips.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Kowal JM, Haanes KA, Christensen NM, Novak I. Bile acid effects are mediated by ATP release and purinergic signalling in exocrine pancreatic cells. Cell Commun Signal. 2015;13:28. doi: 10.1186/s12964-015-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudreault F, Grygorczyk R. Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol. 2004;561:499–513. doi: 10.1113/jphysiol.2004.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci. 2005;118:2435–2440. doi: 10.1242/jcs.02372. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Gao X, Brown RC, Heller S, O’Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 30.Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011;589:3471–3482. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiasa M, Togawa N, Miyaji T, Omote H, Yamamoto A, Moriyama Y (2014) Essential role of vesicular nucleotide transporter in vesicular storage and release of nucleotides in platelets. Physiol Rep 10.14814/phy2.12034 [DOI] [PMC free article] [PubMed]
- 32.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 33.Liu GJ, Werry EL, Bennett MR. Secretion of ATP from Schwann cells in response to uridine triphosphate. Eur J Neurosci. 2005;21:151–160. doi: 10.1111/j.1460-9568.2004.03831.x. [DOI] [PubMed] [Google Scholar]
- 34.Kringelbach TM, Aslan D, Novak I, Schwarz P, Jorgensen NR. UTP-induced ATP release is a fine-tuned signalling pathway in osteocytes. Purinergic Signal. 2014;10:337–347. doi: 10.1007/s11302-013-9404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazarowski ER, Harden TK. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br J Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timoteo MA, Carneiro I, Silva I, Noronha-Matos JB, Ferreirinha F, Silva-Ramos M, Correia-de-Sa P. ATP released via pannexin-1 hemichannels mediates bladder overactivity triggered by urothelial P2Y6 receptors. Biochem Pharmacol. 2014;87:371–379. doi: 10.1016/j.bcp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Collavin L, Lazarevic D, Utrera R, Marzinotto S, Monte M, Schneider C. wt p53 dependent expression of a membrane-associated isoform of adenylate kinase. Oncogene. 1999;18:5879–5888. doi: 10.1038/sj.onc.1202970. [DOI] [PubMed] [Google Scholar]
- 38.Ruan Q, Chen Y, Gratton E, Glaser M, Mantulin WW. Cellular characterization of adenylate kinase and its isoform: two-photon excitation fluorescence imaging and fluorescence correlation spectroscopy. Biophys J. 2002;83:3177–3187. doi: 10.1016/S0006-3495(02)75320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacombe ML, Milon L, Munier A, Mehus JG, Lambeth DO. The human Nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr. 2000;32:247–258. doi: 10.1023/A:1005584929050. [DOI] [PubMed] [Google Scholar]
- 40.Behrendorff N, Floetenmeyer M, Schwiening C, Thorn P. Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology. 2010;139:1711–1720. doi: 10.1053/j.gastro.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 41.Novak I, Praetorius J (2015) Fundamentals of bicarbonate secretion in epithelia. In: Kirk L. Hamilton, Daniel C. Devor, Brian J. Harvey (eds) Ion channels and transporters of epithelia in health and disease. Springer
- 42.Sauter DR, Novak I, Pedersen SF, Larsen EH, Hoffmann EK. ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC) Pflugers Arch. 2014 doi: 10.1007/s00424-014-1598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruan YC, Shum WW, Belleannee C, Da SN, Breton S. ATP secretion in the male reproductive tract: essential role of CFTR. J Physiol. 2012;590:4209–4222. doi: 10.1113/jphysiol.2012.230581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reigada D, Mitchell CH. Release of ATP from retinal pigment epithelial cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol. 2005;288:C132–C140. doi: 10.1152/ajpcell.00201.2004. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto A, Ishiguro H, Ko SB, Suzuki A, Wang Y, Hamada H, Mizuno N, Kitagawa M, Hayakawa T, Naruse S. Ethanol induces fluid hypersecretion from guinea-pig pancreatic duct cells. J Physiol. 2003;551:917–926. doi: 10.1113/jphysiol.2003.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maleth J, Balazs A, Pallagi P, Balla Z, Kui B, Katona M, Judak L, Nemeth I, Kemeny LV, Rakonczay Z, Jr, Venglovecz V, Foldesi I, Peto Z, Somoracz A, Borka K, Perdomo D, Lukacs GL, Gray MA, Monterisi S, Zaccolo M, Sendler M, Mayerle J, Kuhn JP, Lerch MM, Sahin-Toth M, Hegyi P. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology. 2015;148:427–439. doi: 10.1053/j.gastro.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pedersen SF, Kapus A, Hoffmann EK. Osmosensory mechanisms in cellular and systemic volume regulation. J Am Soc Nephrol. 2011;22:1587–1597. doi: 10.1681/ASN.2010121284. [DOI] [PubMed] [Google Scholar]
- 48.Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res. 2008;18:558–565. doi: 10.1038/cr.2008.49. [DOI] [PubMed] [Google Scholar]
- 49.Islam MR, Uramoto H, Okada T, Sabirov RZ, Okada Y. Maxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cells. Am J Physiol Cell Physiol. 2012;303:C924–C935. doi: 10.1152/ajpcell.00459.2011. [DOI] [PubMed] [Google Scholar]
- 50.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, Feranchak AP. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem. 2011;286:25363–25376. doi: 10.1074/jbc.M111.232868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M, Inoue K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380:329–338. doi: 10.1042/bj20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl- transport in biliary epithelial cells through a PKCzeta-dependent pathway. J Physiol. 2008;586:2779–2798. doi: 10.1113/jphysiol.2008.153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahara N, Ito S, Furuya K, Naruse K, Aso H, Kondo M, Sokabe M, Hasegawa Y. Real-time imaging of ATP release induced by mechanical stretch in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2014;51:772–782. doi: 10.1165/rcmb.2014-0008OC. [DOI] [PubMed] [Google Scholar]
- 55.Inoue K, Komatsu R, Imura Y, Fujishita K, Shibata K, Moriyama Y, Koizumi S. Mechanism underlying ATP release in human epidermal keratinocytes. J Invest Dermatol. 2014;134:1465–1468. doi: 10.1038/jid.2013.516. [DOI] [PubMed] [Google Scholar]
- 56.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol. 2009;20:1724–1732. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomes P, Srinivas SP, Van DW, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 58.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanjanamekanant K, Luckprom P, Pavasant P. P2X7 receptor-Pannexin1 interaction mediates stress-induced interleukin-1 beta expression in human periodontal ligament cells. J Periodontal Res. 2014;49:595–602. doi: 10.1111/jre.12139. [DOI] [PubMed] [Google Scholar]
- 60.Grygorczyk R, Furuya K, Sokabe M. Imaging and characterization of stretch-induced ATP release from alveolar A549 cells. J Physiol. 2013;591:1195–1215. doi: 10.1113/jphysiol.2012.244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci. 2011;124:3477–3483. doi: 10.1242/jcs.087221. [DOI] [PubMed] [Google Scholar]
- 62.Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, Cantiello HF. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- 63.Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- 64.Grygorczyk R, Tabcharani JA, Hanrahan JW. CFTR channels expressed in CHO cells do not have detectable ATP conductance. J Membr Biol. 1996;151:139–148. doi: 10.1007/s002329900065. [DOI] [PubMed] [Google Scholar]
- 65.Zhang WK, Wang D, Duan Y, Loy MM, Chan HC, Huang P. Mechanosensitive gating of CFTR. Nat Cell Biol. 2010;12:507–512. doi: 10.1038/ncb2053. [DOI] [PubMed] [Google Scholar]
- 66.Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tatur S, Kreda S, Lazarowski E, Grygorczyk R. Calcium-dependent release of adenosine and uridine nucleotides from A549 cells. Purinergic Signal. 2008;4:139–146. doi: 10.1007/s11302-007-9059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol. 2003;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- 69.Blevins GT, Jr, van de Westerlo EM, Williams JA. Nucleoside diphosphate kinase associated with rat pancreatic membranes regulates CCK receptor affinity. Am J Physiol. 1994;267:G866–G874. doi: 10.1152/ajpgi.1994.267.5.G866. [DOI] [PubMed] [Google Scholar]
- 70.Chu S, Xiong W, Zhang D, Soylu H, Sun C, Albensi BC, Parkinson FE. Regulation of adenosine levels during cerebral ischemia. Acta Pharmacol Sin. 2013;34:60–66. doi: 10.1038/aps.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kordas KS, Sperlagh B, Tihanyi T, Topa L, Steward MC, Varga G, Kittel A. ATP and ATPase secretion by exocrine pancreas in rat, guinea pig, and human. Pancreas. 2004;29:53–60. doi: 10.1097/00006676-200407000-00056. [DOI] [PubMed] [Google Scholar]
- 72.Kittel A, Pelletier J, Bigonnesse F, Guckelberger O, Kordas K, Braun N, Robson SC, Sevigny J. Localization of nucleoside triphosphate diphosphohydrolase-1 (NTPDase1) and NTPDase2 in pancreas and salivary gland. J Histochem Cytochem. 2004;52:861–871. doi: 10.1369/jhc.3A6167.2004. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez DA, Egido P, Balcarcel NB, Hattab C, van Haaster MM, Pelletier J, Sevigny J, Ostuni MA. Rat submandibular glands secrete nanovesicles with NTPDase and 5′-nucleotidase activities. Purinergic Signal. 2015;11:107–116. doi: 10.1007/s11302-014-9437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maksimow M, Kyhala L, Nieminen A, Kylanpaa L, Aalto K, Elima K, Mentula P, Lehti M, Puolakkainen P, Yegutkin GG, Jalkanen S, Repo H, Salmi M. Early prediction of persistent organ failure by soluble CD73 in patients with acute pancreatitis*. Crit Care Med. 2014;42:2556–2564. doi: 10.1097/CCM.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 75.Kunzli BM, Berberat PO, Giese T, Csizmadia E, Kaczmarek E, Baker C, Halaceli I, Buchler MW, Friess H, Robson SC. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am J Physiol Gastrointest Liver Physiol. 2007;292:G223–G230. doi: 10.1152/ajpgi.00259.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 338 KB)