Abstract
The effect of garlic’s aqueous extract (GAE) during refrigerated storage of the restructured products from Pangasius (Pangasianodon hypophthalmus) was evaluated. Protein and lipid oxidation, protein pattern on SDS-PAGE, TPC as well as WHC, gelling properties, texture profiles and whiteness of the surimi gel was evaluated periodically during a refrigerated storage period of 20 days. Increase of water holding capacity in GAE added gels indicated stronger protein network formation, whereas, decrease of protein solubility suggested formation of protein aggregates during gelation process. Lipid oxidation decreased in treated samples but the rate of increase varied, depending upon the concentration of GAE. Protein carbonyl content increased during storage, but slow increase in treated samples. Gel strength in treated samples increased and accompanied by thickening of myofibrillar head chain. Hardness, adhesiveness and gumminess parameters affected most due to addition of GAE. Sensory analysis revealed that the RP with 1 % GAE preferred most and control was acceptable upto 12 days.
Keywords: Pangasius, Restructured fish products, Garlic, Texture profile analysis, Protein oxidation, Lipid oxidation, SDS-PAGE
Introduction
Restructuring yields fish products with high commercial value, considered as a way to expand the potential for muscle foods in the market place. It is of great interest to develop restructured products with high economic and nutritive value from non-commercial fish species. The physico-functional properties of restructured fish products (RP) such as gelling and textural characteristics as well as water holding capacities are considered to be significant in respect of consumers’ acceptability. However, two most important hazards during refrigerated storage of RPs from surimi gel of fatty fish are lipid oxidation and microbial propagation. Lipids oxidation is responsible for reduction in nutritional quality as well as changes in flavour (Aguirrezabal et al. 2000; Frankel 2005) of products developed out of surimi especially from fatty fish, while microbial contamination can precipitate major public health hazards and economic loss in terms of food poisoning and meat spoilage.
Antioxidants are therefore necessary to incorporated in order to increase storage stability, sensory quality and nutritional value of fish products. Beneficial effects of suitable agents possessing both antioxidant and antimicrobial activities for maintaining meat quality, extending shelf-life and preventing economic loss has been reported (Yin and Cheng 2003). Since, addition of synthetic antioxidants has been restricted because of their health risks and toxicity (Linderschmidt et al. 1986), attention has been diverted towards the potential of herbs, spices as antioxidative additives in food products leading to novel combinations of antioxidants and the development of novel food products (Perumalla and Hettiarachchy 2011).
Garlic (Allium sativum L.) has been cultivated and used for culinary and medicinal purposes by many cultures for centuries. Garlic is rich in organosulfur compounds, which are responsible for its flavor, aroma, and potential health benefits. Apart from it, garlic has a wide spectrum of actions which include antibacterial, antifungal, antioxidant and beneficial effects on the cardiovascular and immune system of human (Mah et al. 2009; Gafar et al. 2012). Garlic contains many sulphur containing compounds and the most important of which is alliin (Chukwu et al. 2012). Polyphenolics present in both edible and inedible plants and herbs and spices act as reducing agents, free radical scavengers and Fe2+chelators or quenchers in the formation of singlet oxygen (Juntachote et al. 2006; Pizzale et al. 2002). Recently, plant phenolics have got an increasing interest in the food industry because they can retard the oxidative degradation of lipids and thereby improve the quality and nutritional value of food (Wojdylo et al. 2007).
For restructured products, the technique mostly used for obtaining a good gel depends on solubilizing and extracting myofibrillar proteins with 2 to 3 g/100 g salt. The solubilized expanding proteins form a continuous matrix and then undergo thermal aggregation, cross-linking and develop into fine three dimensional solid-like networks resulting in elastic gel (Gordon and Barbut 1992). The cross-linking of myosin is promoted by an endogenous transglutaminase (TGase) contained in fish muscle which catalyses an acyl transfer reaction between γ-carboxyamide groups of glutaminyl residues in proteins. Fish with high content of lipid and myoglobin results difficulties in making high quality surimi (Chen 2002).
To enhance the gel strength of surimi or fish mince, various food-grade ingredients and cross-linking enzymes such as microbial transglutaminase have been used (Benjakul et al. 2004). Recently, use of plant phenolics as protein cross-linking agent has been paid increasing attention for the surimi industry. The interactions between phenolic compounds and proteins play a very important role in the processing of certain food products. The formation of rigid molecular structures by reactions of ortho-quinones with proteins has been demonstrated by Strauss and Gibson (2004). Interactions of different phenolic acids and flavonoids with soy proteins were reported by Rawel et al. (2002). Significant increase in the gel strength of bigeye snapper surimi was found when oxidised phenolic compounds were added (Balange and Benjakul 2009).
Thai pangas (Pangasianodon hypophthalmus) is extensively cultured in India and Bangladesh. The fish has a great aquaculture potential due to its very high growth rate compared to other popular major carps. The abundant catch of Thai pangas might be utilized as an alternative source of surimi raw material for development of restructured products. This study aims to determine the effects of garlic’s aqueous extract on textural properties of RP as well as its inhibitory potential on lipid and protein oxidation and microbial growth during refrigerated storage.
Materials and methods
Preparation of aqueous extract of garlic (GAE)
To prepare aqueous extract of locally available garlic (Allium sativum), the garlic was peeled, cut into pieces and dried in an hot air oven at temperature 40 ± 2 °C. It was then ground using an electric blender. Twenty grams of the ground material was soaked in 100 ml of hot sterile water and allowed to stand for 48 h. The crude extracts were obtained by filtration. The process was repeated twice and all the filtrates were collected and subjected to evaporation at 50 °C in rotary vacuum evaporator (Osaka J.P. Selecta, Spain). The powdered aqueous extract of garlic was kept in aluminium pouch and stored at −20 °C for future use.
Preparation of surimi
Fresh pangasius for the study was collected from the local fish farm at Lembucherra, Tripura. Length and weight range of fish were 37–49 cm and 636–809 g respectively. Fishes were washed with chilled water, gutted, dressed, filleted by hand and minced by employing a mechanical meat mincer with a 3 mm-hole plate. Washing of the minced meat was performed in wash tanks maintaining a water temperature of 10 °C using a fish mince to water ratio of 1:4 (w/v) for three times with ten min duration of each wash (twice with potable water and last wash with 0.1 % NaCl solution to facilitate dewatering). The slurry was stirred for 6 min and allowed to settle for 4 min before water was decanted. Final dewatering was carried out using a screw press (Deb Enterprise, India). Sorbitol (4 g), sucrose (4 g) and polyphosphate (0.3 g) were added to 100 g of dewatered mince as cryprotective agents and then mixed for 5 min in a silent cutter (Sunlabz Equipments, Chennai, India) at a temperature below 10 °C.The washed mince (surimi) was packed in low density polyethylene (LDPE) pouches (500 g per pouch) and quickly frozen at −35 °C for 2 h in air blast freezer (Sanyo, Japan) and stored at −20 °C in a deep freezer (Vest Frost, Denmark) for development of restructured products within a week.
Preparation of surimi gel
Frozen surimi was tempered for about 2 h at 20 ± 2 °C until it reached 5 ± 1 °C, followed by chopping for 1 min at high speed in a silent cutter. Moisture of surimi was adjusted to 80 % by using ice water. Salt (NaCl) was added at the rate of 2.5 % and mixed in silent cutter for five min. Aqueous extract of garlic was added to each 500 g part at the rate of 0.5, 1.0, 1.5 and 2.0 % and designated the treatments as PSgl-0.5, PSgl-1.0, PSgl-1.5 and PSgl-2.0 respectively. Throughout the mixing operation temperature of surimi sol was kept below 10 °C. The control (CON) was made without addition of garlic extract. The surimi paste was stuffed into vinylidene chloride casing (10 cm length, 2.0 cm diameter). Thermal setting was done according to the two-step heating method suggested by Luo et al. (2008). The casings were immersed in water bath at 40 °C for 30 min followed by immersion in another water bath at 85 °C for 30 min. After cooking, the casings were immediately removed, placed in iced water, and cooled to 4–5 °C for 30 min. The gels were stored overnight at 4 °C in a refrigerator. For storage study, the gels were stored at 4 °C in a refrigerator for 20 days and storage changes were analysed at 4 days interval.
Analyses of moisture, ash, protein, fat content and pH
Moisture, ash, protein and fat content of Thai pangas, surimi and RP were determined according to AOAC (2000). For determination of the pH, 10 g of sample was homogenized with 50 ml distilled water and pH value was measured by a digital pH-meter (Sartorius, PB-20).
Determination of protein solubility and WHC
Gel (0.5 g) were homogenised in 10 ml of 0.6 M KCl in 50 mM pH 7.4 tris–HCl buffer for 1 min in a tissue homogenizer (IKA, Germany). The homogenate was centrifuged at 10,000 rpm for 10 min at 4 °C (Remi, India). The supernatant was diluted ten-fold with 0.6 M KCl and protein determination was performed by Biuret method (Gornall et al. 1949). Analyses were performed in triplicate and the solubility was expressed in mg of soluble protein/100 mg of gel.
WHC was evaluated by the technique outlined by Barrera et al. (2002). A portion of 5 g of each gel was weighed and placed on 8 layers of filter paper (Whatman No. 1). Samples were placed in 50 ml centrifuge tubes and centrifuged at 5000×g at 4 °C for 15 min. Immediately after centrifugation, the gels were removed and re-weighed. WHC was expressed as the weight of the centrifuged gels relative to the original weight of samples.
Where, W1 represents the weight of the gel before centrifugation and W2 represents the weight of the gel after centrifugation.
Determination of thiobarbituric acid reactive substances (TBARS)
The 2-thiobarbituric acid (TBA) assay was carried out according to the procedure of Schmedes and Holmer (1989). RP sample (10 g) was mixed with 25 ml of trichloroacetic acid solution (200 g/l of TCA in 135 ml/l phosphoric acid solution) and homogenized in a blender for 30 s. After filtration, 2 ml of the filtrate were added to 2 ml TBA solution (3 g/l) in a test tube. The test tubes were incubated at room temperature in the dark for 20 h; then the absorbance was measured at 532 nm by using UV–VIS spectrophotometer (Shimadzu, Japan). A standard curve was constructed using malondialdehyde (MDA), and results were expressed as mg malondialdehyde per kg of RP.
Determination of protein carbonyls
Protein carbonyls were estimated according to the method suggested by Levine et al. (1994). RP sample (0.5 g) was homogenised in 10 ml 50 mM tris-buffer (pH: 7.4) containing 1 mM EDTA and 0.01 % BHT. 200 μl of the homogenate was precipitated with 50 μl of TCA (100 %) followed by centrifugation (10,000 × g) for 5 min and the pellet was incubated in the dark for 1 h with 500 μl of 10 mM dinitrophenylhydrazine (DNPH) in 2 M HCl. A blank without added DNPH was made in 2 M HCl and incubated in similar way. The samples were precipitated with 50 μl TCA (100 %) and centrifuged (10,000 × g) for 5 min followed by washing of the pellets three times with 1 ml ethanol/ethyl acetate 1:1 (v/v). The pellet was re-dissolved in 1 ml of 6 M guanidine chloride in 20 mM KH2PO4. The carbonyl content of the sample was estimated by measuring the absorbance at 370 and 280 nm against guanidine as blank. Carbonyl content was calculated using the molar absorption coefficient of 22,000 M−1 cm−1 relative to protein concentration and results are expressed in nmoles carbonyl per mg of protein.
SDS-polyacrylamide gel electrophoresis
Gel electrophoresis was carried out according to the method of Laemmli (1970) using 10 g/100 ml, acrylamide separating gel and 4 g/100 ml stacking gel. About 3 g sample in 27 ml of 20 mmol/l Tris–HCl buffer (pH 8.0) containing 10 g/100 mL SDS was homogenized at 10,000 r/min for 30 s, and then incubated at 85 °C for 30 min. The mixture was centrifuged at 10,000 × g for 5 min to remove undissolved pieces after vigorous agitation using a vortex mixer (Remi, India). An aliquot taken from the middle layer of the supernatant was heated at 100 °C for 5 min in Tris-glycine buffer (pH 8.8) containing 1 g/100 ml SDS and 1 ml/100 ml L-mercaptoethanol. Electrophoresis (Bio-RAD Mini Protein System, USA) was carried out at a constant voltage of 95 V using an electrophoretic buffer of Tris-glycine containing 0.1 g/100 ml SDS.
Determination of total plate count (TPC)
RP sample (10 g) was homogenized with 90 ml of sterile peptone water (1 g/l) in a stomachar (Seward, UK) and serial dilutions were prepared. 0.1 ml of each dilution was spread with a bent sterile glass rod on duplicate plates of pre-poured and dried standard plate count agar (Hi-Media, India). After 48-h incubation at 30 ± 2 °C, colonies were counted and results were expressed as log10 cfu/g of sausage sample.
Sensory evaluation
Sensory evaluation was performed by a panel of 6 judges. The panel evaluated each treatment within each replication in triplicate, and the evaluation was performed with the samples at room temperature. The panel judges were trained on the attributes of the restructure fish products such as appearance, flavour, taste and texture. Based on those attributes they were instructed to evaluate acceptability using ten-point Hedonic Scale (like extremely-9, like very much-8, like moderately-7, like slightly-6, neither like nor dislike-5, dislike slightly-4, dislike very much-3, dislike moderately-2, dislike slightly-1). A score below 6 was considered as rejected.
Statistical analysis
The data obtained were analyzed using analysis of variance (ANOVA), and when significant differences were found, comparisons among means were carried out by using Duncan’s Multiple Comparison Test (p < 0.05) by Statistical Package for Social Sciences (SPSS, version 11.0 for windows).
Results and discussion
Proximate analyses of fish muscle and surimi
The principal biochemical constituents of raw material fish Pangasius (Pangasianodon hypophthalmus) were moisture (740.4 ± 2.5 g kg−1), protein (163.9 ± 3.4 g kg−1), fat (75.7 ± 1.4 g kg−1) and ash (10.9 ± 0.2 g kg−1). The proximate analysis showed that the fish had low moisture and high protein and moderate fat content. Lower moisture and higher lipid content in pangas muscle was also reported by Hossain et al. (2004). Silverstein et al. (2000) reported the proximate composition of channel catfish as moisture (74–76 %), protein (17–19 %) and fat (3–6.9 %). Whereas, in another study, proximate composition of catfish (Clarias gariepinus) was reported moisture, protein and fat content as 71.85 %, 19.51 % and 14.28 % respectively (Chukwu and Shaba 2009). There are influences of various factors such as nutrition, living area, and fish size, catching season, seasonal and sexual variation as well as other environmental condition on the proximate composition of fish species (Sankar and Ramachandran 2001). The surimi had moisture (795.7 ± 1.8 g kg−1), protein (146.8 ± 2.7 g kg−1), fat (13.3 ± 0.4 g kg−1) and ash (33.6 ± 1.4 g kg−1). Washing reduced total protein content which may be explained as the removal of sarcoplasmic protein during washing which makes up to 20 % to 25 % of total protein of fish muscles (Negbenebor et al. 1999; Taskaya et al. 2003). Majumdar et al. (2012) also reported a significant decrease of protein content after washing of silver carp mince. Removal of sarcoplasmic proteins may result increased concentration of myofibrillar proteins followed by improved water holding capacity of meat, because of increased hydration of protein due to removal of blood, pigments, proteins etc. during washing (Lin and Park 1997).
Changes in biochemical properties during refrigerated storage
Changes in pH, WHC and protein solubility
Changes in pH, water holding capacity (WHC) and protein solubility (PS) during refrigerated storage are given in Table 2. The initial pH value ranged from 7.65 (in control sample) to 7.13–7.6 (in treated samples). Significant (p < 0.05) reduction of pH in GAE treated samples may be attributed to the interaction of organosulphur/phenolic compounds of garlic with fish muscle proteins. In all GAE added formulations, storage had a significant (p < 0.05) effect on the pH values, which tended to increase with storage time.
Table 2.
Change s in pH, WHC and protein solubility of GAE incorporated sausages from Pangasius surimi during refrigerated storage#
| Treatments* | Day 1 | Day 4 | Day 8 | Day 12 | Day 16 | Day 20 | |
|---|---|---|---|---|---|---|---|
| pH | CON | 7.65 ± 0.03Aa | 7.69 ± 0.01Aa | 7.78 ± 0.03Ab | 7.86 ± 0.04Ac | 7.93 ± 0.02Acd | 7.96 ± 0.05Ad |
| PSgl-0.5 | 7.56 ± 0.04Ba | 7.72 ± 0.09Ab | 7.79 ± 0.05Abc | 7.84 ± 0.03Acd | 7.92 ± 0.04Ad | 8.15 ± 0.08Be | |
| PSgl-1.0 | 7.6 ± 0.02ABa | 7.68 ± 0.03Ab | 7.69 ± 0.06Bb | 7.72 ± 0.04Bbc | 7.77 ± 0.02Bc | 7.87 ± 0.03Cd | |
| PSgl-1.5 | 7.55 ± 0.05Ba | 7.57 ± 0.02Bab | 7.58 ± 0.05Cab | 7.6 ± 0.02Cab | 7.65 ± 0.05Cbc | 7.69 ± 0.03Dc | |
| PSgl-2.0 | 7.13 ± 0.02Ca | 7.14 ± 0.01Ca | 7.15 ± 0.02Dab | 7.19 ± 0.02Dbc | 7.21 ± 0.04Cc | 7.24 ± 0.04Ec | |
| Water holding capacity (WHC) | CON | 71.8 ± 0.49Aa | 71.3 ± .21Aab | 70.4 ± 0.58Abc | 70.1 ± 0.32Ac | 69.4 ± 0.51Acd | 68.7 ± 1.1Ad |
| PSgl-0.5 | 73.8 ± 1.66Aa | 73.2 ± 0.95Bab | 72.7 ± 0.6Bab | 72.1 ± 0.78Babc | 71.6 ± 0.9Bbc | 70.3 ± 0.8Ac | |
| PSgl-1.0 | 85.4 ± 0.79Ba | 85.2 ± 1.1Cab | 84.6 ± 0.61Cab | 84.2 ± 0.32Cabc | 83.5 ± 0.98Cbc | 82.7 ± 1.46Bc | |
| PSgl-1.5 | 87.6 ± 0.43Ca | 87.3 ± 0.8Da | 86.9 ± 0.75Dab | 86.5 ± 1.29Dab | 86.1 ± 1.3Dab | 85.1 ± 0.55Cc | |
| PSgl-2.0 | 89.2 ± 1.61Ca | 88.9 ± 0.72Ea | 88.7 ± 0.43Eab | 88.5 ± 0.7Eab | 88.1 ± 0.98Eab | 87.2 ± 0.95Dc | |
| Protein solubility (%) | CON | 82.5 ± 0.74Aa | 81.8 ± 0.52Aab | 81.4 ± 0.87Aabc | 80.6 ± 0.52Abc | 79.8 ± 0.55Ac | 77.3 ± 0.38Ad |
| PSgl-0.5 | 82.7 ± 0.66Aa | 82.3 ± 0.41Aa | 82.1 ± 0.56Aa | 80.7 ± 0.43ABab | 80.2 ± 0.62ABab | 79.2 ± 0.42Bb | |
| PSgl-1.0 | 81.5 ± 0.43Aa | 81.1 ± 0.67Aa | 80.2 ± 0.47Aa | 79.8 ± 0.26ABa | 79.6 ± 0.47ABa | 78.9 ± 0.84Ba | |
| PSgl-1.5 | 80.8 ± 0.88Aa | 80.5 ± 0.38Aa | 80.5 ± 0.28Aa | 79.1 ± 0.44ABa | 78.9 ± 0.39ABa | 77.3 ± 0.51Ba | |
| PSgl-2.0 | 80.1 ± 0.29Aa | 79.7 ± 0.27Aa | 79.5 ± 0.42Aa | 78.4 ± 0.62Ba | 77.1 ± 0.51Ba | 76.6 ± 0.77Ba |
*CON = mince without garlic extract, PSgl-0.5, PSgl-1.0, PSgl-1.5 and PSgl-2.0 surimi gel with 0.5, 1.0, 1.5 and 2.0 % garlic extract respectively
# = mean ± SD
Indices such as water holding capacity, is often used to assess the textural quality of the restructured fish products and it also indicates the deterioration of protein quality during low temperature storage. Water holding capacity increased proportionately with the addition of GAE. The increases in WHC in day-1 in treated RPs may be explained as the formation of stronger network induced by GAE might imbibed more water. However, the variation in WHC between the treatments may be due to differences in the concentration of phenolics in different treatments. From day-4 onwards, the differences between the treatments were significant (p < 0.05). This may be explained as the result of protein denaturation induced by refrigerated storage leading to low affinity for water and it was accompanied by gradual loss of protein solubility. Moreover, modification of protein-phenolics interaction with the gradual denaturation and/or degradation of protein during storage may also be responsible for changes in WHC of protein as observed in this study. The increases in WHC were in accordance with the increased breaking force and deformation of GAE added surimi gels (Table 1). The texture of gel is also dependent on WHC which affect or influence sensory acceptability. So WHC is important to maintain at higher level during the storage period for better sensory quality.
Table 1.
Gelling properties of garlic’s (Allium sativum) aqueous extract incorporated sausages from Pangasius surimi (mean ± SD)
| Treatments* | Breaking force (g) | Breaking deformation (cm) | Gel strength (g*cm) |
|---|---|---|---|
| CON | 293.53 ± 33.66a | 0.64 ± 0.006a | 188.79 ± 21.02a |
| PSgl-0.5 | 385.43 ± 88.42ab | 0.62 ± 0.01ab | 238.54 ± 51.6ab |
| PSgl-1.0 | 424.01 ± 11.65b | 0.63 ± 0.015ab | 265.77 ± 11.95b |
| PSgl-1.5 | 417.29 ± 82.27b | 0.61 ± 0.02b | 253.46 ± 42.16b |
| PSgl-2.0 | 351.87 ± 24.33ab | 0.63 ± 0.005ab | 222.76 ± 13.53ab |
*CON = mince without garlic extract, PSgl-0.5, PSgl-1.0, PSgl-1.5 and PSgl-2.0 surimi gel with 0.5, 1.0, 1.5 and 2.0 % garlic extract respectively. # = mean ± SD
The decrease in solubility suggests the formation of protein aggregates during gelation process. During heating, proteins underwent denaturation and aggregation to form a three dimensional structure. The alteration of protein extractability is a useful factor which may be used to determine the textural quality of fish muscle, as protein aggregation is accompanied by a significant decrease in their solubility (Badii and Howell 2002). In the present study, protein solubility was found to be decreased significantly (P < 0.05) in all the groups in Day-1 as well as the storage progressed (Table 2) indicating the formation of protein aggregates. The formation of disulphide bond which results in the aggregation of proteins (Lim and Haard 1984) might have contributed to low solubility of proteins. Hydrogen bonds might involve in the interactions between hydroxyl groups of phenolic compounds and the nitrogen or oxygen of amino acids. From the result, the decreased protein solubility over the storage period indicated the aggregation as well as denaturation of proteins caused by low temperature.
Changes in thiobarbituric acid reactive substances (TBARS)
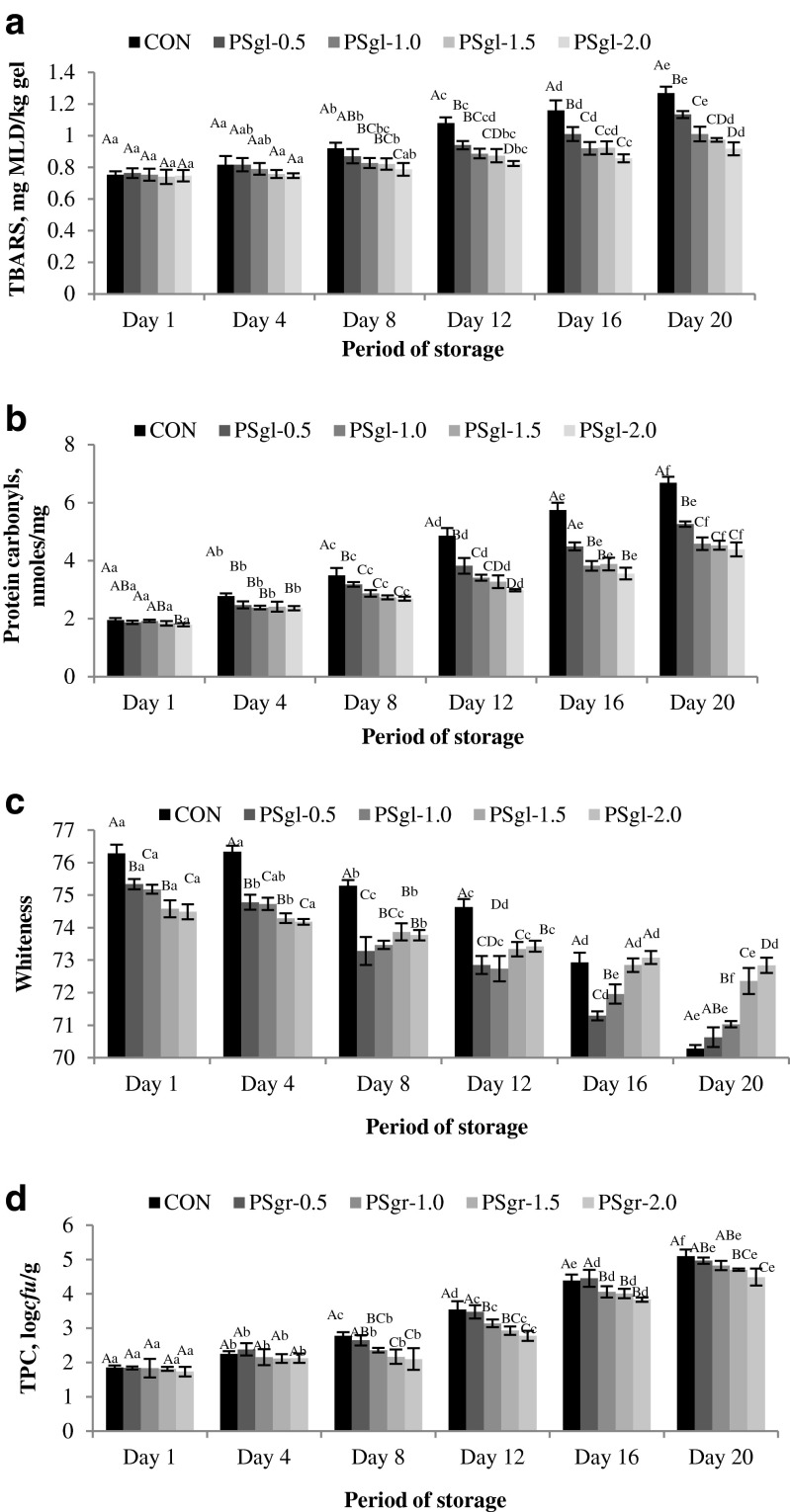
Figure 1 depicts the effect of different concentrations of GAE on TBARS value of surimi sausage during refrigerated storage. There was no significant (p > 0.05) differences in TBARS value in Day-1 and Day-4 between the control and GAE treated groups. As the storage time increased, there was significant (p < 0.05) differences in TBARS value between GAE added samples and control. Although, with the progress of storage period, the TBARS value increased in all the treatments. But the rate of increase varied, depending upon the concentration of GAE. At the end of the storage period, the increase of TBARS value from the initial value was in the order of CON > PSgl-0.5 > PSgl-1.0 > PSgl-1.5 > PSgl-2.0. Lipid oxidation occurred during refrigerated storage might cause the denaturation of proteins. Many lipid degradation products are also capable of cross-linking polypeptides and thus are responsible for the generation of insoluble protein aggregate (Buttkus 1966). This possibly resulted in the loss in solubility of protein, especially when the storage time increased. Aldehydes formed during lipid oxidation can interact with amino groups of protein to form Schiff base products (Zamora et al. 1999)
Fig. 1.
Changes in TBARS (a), protein carbonyls (b), whiteness (c) and TPC (d) of GAE incorporated sausages from Pangasius surimi during refrigerated storage
Lipid oxidation represented by TBARS was reduced with the higher concentrations of GAE. This result was in accordance with that of Yang et al. (1993), who noted that the antioxidant activity of several compounds of garlic and garlic extracts was concentration dependent. The investigated lipid oxidation preventive activity of the five different treatments followed the order PSgl-2.0 > PSgl-1.5 > PSgl-1.0 > PSgl-0.5 > CON. Fresh garlic or garlic powder has been reported to have more physiological activity as compared to garlic oil, aged garlic and steam-distilled garlic since they do not contain significant amounts of alliin or allicin, but contain various products of allicin transformation (Lawson and Gardner 2005). Whole garlic and aqueous garlic extract exhibit direct antioxidant effects by efficiently scavenging exogenously generated hydroxyl radicals in a dose dependent fashion (Kumaraguruparan et al. 2005). Antioxidant compounds such as alliin, diallyl sulphide, allyl sulphide and propyl sulphide present in garlic have been reported to be responsible for significant reduction of lipid oxidation in fish mince during chilled storage (Kumaraguruparan et al. 2005). Kumolu-Johnson and Ndimele (2011) also reported the ability of fresh garlic to inhibit the increase of peroxide and TBA values on hot-smoked catfish during the 28 days of storage period (Fig. 2).
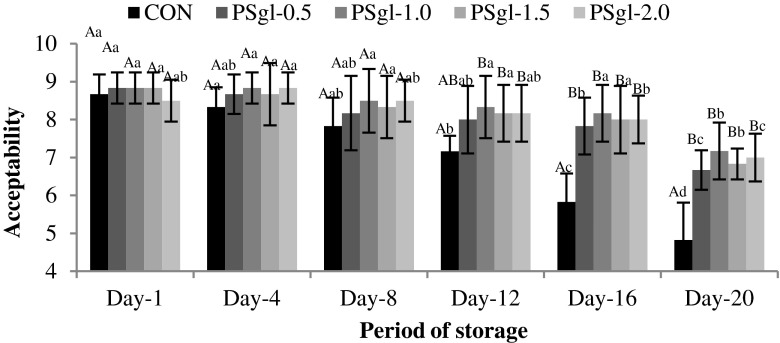
Fig. 2.
Changes in acceptability of GAE incorporated sausages from Pangasius surimi during refrigerated storage
Changes in carbonyl compounds
Recently the impact of protein oxidation on protein functionality and muscle food quality has received more attention (Baron et al. 2007). In oxidising environments proteins are very susceptible to chemical modification, such as amino acid destruction, peptide scission and formation of protein–lipid complexes (Saeed and Howell 2002). Different secondary compounds including radicals and hydroperoxides derived from lipid oxidation react with protein leading to the loss of protein functionality (Zamora et al. 1999).
Estimation of carbonyl compounds indicates most important changes taking place during the oxidation of proteins. Carbonyl compounds are usually formed by major amino acids, such as lysine, histidine, proline and arginine yield and therefore the measuring concentration of such compounds is a meaningful indicator of the oxidative status of muscle proteins (Dalle-Donne et al. 2003). Changes in the protein carbonyls of different treatments during refrigerated storage are shown in Fig. 1b. Except treatment with 2.0 % GAE (PSgl-2.0), there was no significant difference with the control in Day-1. As storage progressed protein oxidation was evident by a significant (P < 0.05) increase in the protein carbonyl content. But the treated gel showed significant difference (P < 0.05) with control in all sampling days. At the end of the storage period, control showed the highest and the surimi gel with 2.0 % GAE showed lowest levels of carbonyl content. The order of protection offered against the formation of carbonyl compounds was PSgl-2.0 > PSgl-1.5 > PSgl-1.0 > PSgl-0.g > control. These results are consistent with lipid oxidation products emphasizing a possible relationship between the protein carbonyl formation and lipid peroxidation.
The lowest carbonyl contents in GAE treated restructured products suggests a protective role of this extract against oxidation of proteins. Certain phenolic acids have been found to inhibit formation of protein carbonyls in meat products as measured by DNPH method (Vuorela et al. 2005). Likewise, in our study also the phenolic rich extracts of garlic prevented the formation of protein carbonyls and thereby protein oxidation. Phenolic compounds have been suggested to inhibit the oxidation of proteins either by retarding the lipid oxidative reactions or by binding to the proteins or by forming complex with them (Siebert et al. 1996).
Changes in whiteness
Whiteness of the gels was found to be reduced (P < 0.05) upon addition of GAE (Fig. 1c). That may be due to sunset yellow colour of GAE. These results are in agreement with O’Connell and Fox (2001) who reported that phenolic compounds were responsible for darkening in cheese products. Besides this, there was possibility of interaction of garlic’s organosulphur compounds with the muscle pigments leading to reduction of the whiteness of the gel. Also there was significant reduction (P < 0.05) of whiteness during refrigerated storage of all the treatments. The control showed higher decreasing rate of whiteness, compared to the treated groups. Moreover, rate of decrease of whiteness was in the order of increase of concentration of GAE in gel. Such observation may be related with the formation of compounds following lipid oxidation which was more in control and lower in treated groups. The differences in colour alteration were possibly caused by the different in pigment content in muscle. The result also indicates that the surimi treated with GAE prevented the oxidation of heme proteins present in the gel which are red in their reduced form and brown in their oxidized ferric form.
Effect of GAE on protein patterns
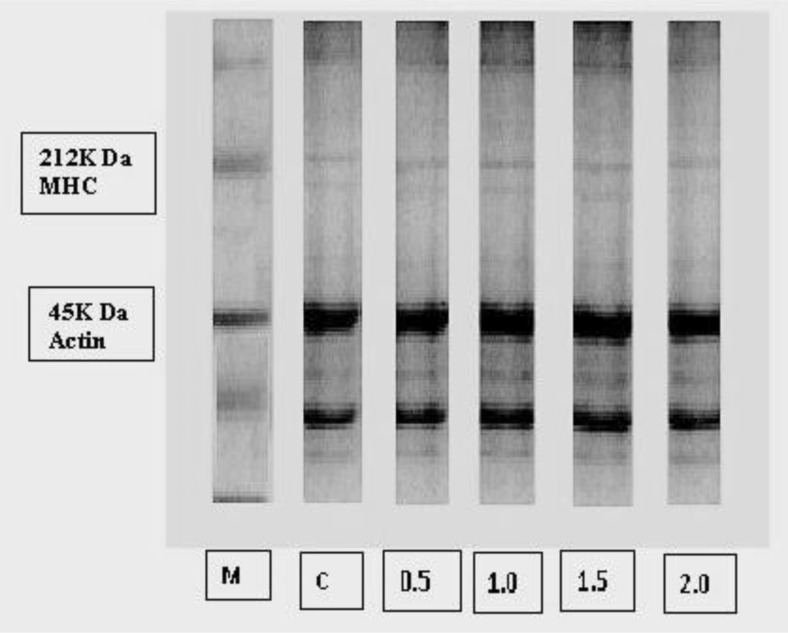
Protein patterns of gel in control and GAE treated groups are depicted in Fig. 3. Breaking force as well as gel strength increased in the treated groups compared to control. The thickness of the myofibrillar head chain (MHC) was found to be more in treated samples and also tended to increase with increasing concentration of GAE. Actin was found to be the dominant protein in the gel, suggesting that actin was more resistant to proteolysis or could not be polymerised during gelation. The result was in agreement with Benjakul et al. (1997) who reported that actin in Pacific whiting muscle was more resistant to proteolysis than MHC. The result suggests that GAE might be able to inhibit the degradation of MHC to some extent, as evidenced by the more retained MHC in treated RPs. Protein cross-links might be more resistant to proteolysis caused by indigenous proteases. Phenolics present in GAE might have exerted some protective activity against certain proteases. Kroll et al. (2003) reported that the interactions between phenolic compounds and proteins may result in inhibiting certain proteases.
Fig. 3.
Protein patterns of GAE treated surimi gel in day-1*. *M = Protein ladder, C = surimi without garlic extract, 0.5, 1.0, 1.5 and 2.0 are surimi gel with 0.5, 1.0, 1.5 and 2.0 % garlic extract respectively
Gelling properties of restructured fish products
Breaking force, breaking deformation and work of penetration (gel strength) in control and GAE treated gel samples are presented in Table 1. Although gel strength (attributed to both breaking force and breaking deformation) of treated groups were higher than the control, but, amongst the treated groups gel containing 1.0 and 1.5 % GAE showed highest gel strength (P < 0.05), but gel strength decreased at higher percentage of GAE. Garlic is a source of various biologically active phytomolecules, including organosulfur compounds, phenolic acids, allyl thiosulfinates, flavonoids, and many phenolic phytochemicals (Nuutila et al. 2003) which contain sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with the proteins and other macromolecules. Balange and Benjakul (2009) reported a significant increase in the gel strength of big eye snapper surimi when oxidised phenolic compounds were added. In the present study, increased gel strength of GAE added surimi gel may be because of cross linking effect of phenolic acids resulting more protein-protein interaction.
The higher MHC content of PSgl-1.0 and PSgl-1.5 (Fig. 3) might be associated with higher gel forming ability because it has been established that myofibrillar proteins, mainly MHC, contribute to gel formation (Benjakul and Visessanguan 2003). Additionally, the cross-links mainly contributed to the increases in gel strength of surimi added with GAE at optimal level. In this study, the optimal level of GAE was found to be 1.0 to 1.5 % and gel strength decreased at 2.0 % level. Cao et al. (2007) reported the polymerisation of protein molecules as a possible subsequent reaction of different proteins with phenolic substances. The decreased gel strength at higher concentrations (2.0 %) of GAE in the present study might be associated with self-aggregation of phenolic compounds, leading to the loss in capability of protein cross-linking. The lower solubility of large phenolic compounds at high concentration causes the difficulty to interact with proteins (De Freitas and Mateus 2001). It is also possible that the size of the phenolic compound can decrease its conformational flexibility in protein–phenolic compound interactions.
Changes in texture profiles
Amongst the textural attributes; hardness, adhesiveness, springiness, gumminess and cohesiveness were measured periodically and presented in Table 3. Hardness, adhesiveness and gumminess of RPs were affected most due to addition of GAE. Hardness, peak force required for the first compression, increased (P < 0.05) with increase of GAE upto 1.0 %, but decreased thereafter in RPs having 1.5 and 2.0 % GAE. Maximum hardness was found to be in RP containing 1.0 % GAE. This may be explained that more cross links following more GAE might reduce the flexibility of protein aggregates. This also supports the observation by Ngapo et al. (1996) i.e., more interactions or crosslinks restrict the flexibility of the protein aggregates, and the gels become less springy and more rigid. As the storage period increased, the hardness decreased with varying rate. On Day-20, the hardness of the RPs was found to be in the order of PSgl-2.0 > PSgl-0.5 > PSgl-1.5 > PSgl-1.0 > CON. Such behaviour of RPs may be because of protein denaturation during low temperature storage and this was also found to be associated with decrease of WHC and protein solubility.
Table 3.
Changes in texture profile of garlic’s (Allium sativum) aqueous extract incorporated sausages from Pangasius surimi during refrigerated storage#
| Treatments* | Day 1 | Day 4 | Day 8 | Day 12 | Day 16 | Day 20 | |
|---|---|---|---|---|---|---|---|
| HRD (gf)# | CON | 812.9 ± 20.2Aa | 786.0 ± 37.9Aa | 735.8 ± 30.9Aa | 672.2 ± 50.7Aab | 538.9 ± 23.7Abc | 441.8 ± 22.2Ac |
| PSgl-0.5 | 834.5 ± 42.5Aa | 800.7 ± 35.7Aab | 759.7 ± 34.3Abc | 733.9 ± 30.5Acd | 693.7 ± 24.6Cd | 691.1 ± 27.1Bd | |
| PSgl-1.0 | 920.0 ± 17.1Ba | 786.9 ± 29.3Ab | 759.1 ± 24.6Ab | 739.9 ± 45.8Abc | 653.64 ± 17.9Bcd | 580.8 ± 35.0Cd | |
| PSgl-1.5 | 775.9 ± 13.1Cb | 842.2 ± 25.06Aa | 774.5 ± 63.6Aab | 718.6 ± 38.6Ab | 690.2 ± 18.2Cbc | 626.7 ± 23.4Cc | |
| PSgl-2.0 | 855.1 ± 24.1ABa | 826.0 ± 29.3Aab | 781.9 ± 37.3Abc | 744.9 ± 40.5Ac | 717.4 ± 49.3Dc | 771.1 ± 45.4Dc | |
| ADH (gf)# | CON | −16.4 ± 15.2Aa | −26.8 ± 18.8Aab | −26.6 ± 15.9A ab | −33.5 ± 5.02A ab | −35.8 ± 11.1Aab | −44.2 ± 7.8Ab |
| PSgl-0.5 | −15.3 ± 9.1Aa | −35.9 ± 10.1Aab | −34.0 ± 5.02Aab | −32.0 ± 5.3Aab | −40.1 ± 12.3Ac | −50.0 ± 23.7Ac | |
| PSgl-1.0 | −21.3 ± 15.3Aa | −35.6 ± 11.4Aa | −24.8 ± 14.9Aa | −34.0 ± 6.5Aa | −43.7 ± 16.2Aa | −37.2 ± 10.3Aa | |
| PSgl-1.5 | −34.5 ± 8.7Aa | −40.8 ± 15.2Aa | −28.5 ± 11.1Aa | −32.0 ± 6.4Aa | −43.3 ± 12.1Aa | −45.0 ± 4.8Aa | |
| PSgl-2.0 | −34.2 ± 18.6Aa | −26.4 ± 19.5Aa | −37.4 ± 5.7Aa | −37.7 ± 7.2Aa | −22.4 ± 18.9Aa | −48.2 ± 8.9Aa | |
| SPR (mm)# | CON | 0.98 ± 0.03Aa | 0.97 ± 0.01Aa | 0.97 ± 0.01Aa | 0.97 ± 0.01Aa | 0.96 ± 0.02Aa | 0.93 ± 0.01Aa |
| PSgl-0.5 | 0.98 ± 0.01Aa | 0.96 ± 0.01Aa | 0.97 ± 0.01Aa | 0.97 ± 0.01Aa | 0.96 ± 0.01Aa | 0.96 ± 0.02Aa | |
| PSgl-1.0 | 0.98 ± 0.02Aa | 0.98 ± 0.02Aa | 0.97 ± 0.02Aa | 0.97 ± 0.02Aa | 0.96 ± 0.01Aa | 0.97 ± 0.02Aa | |
| PSgl-1.5 | 0.98 ± 0.01Aa | 0.97 ± 0.01Aab | 0.97 ± 0.01Aab | 0.97 ± 0.01 Aab | 0.96 ± 0.01A ab | 0.96 ± 0.01Ab | |
| PSgl-2.0 | 0.95 ± 0.02Aa | 0.98 ± 0.01Aa | 0.96 ± 0.01Aa | 0.97 ± 0.01Aa | 0.96 ± 0.01Aa | 0.95 ± 0.02Aa | |
| GUM (gf)# | CON | 705.6 ± 66.7Aa | 652.0 ± 55.6 Aab | 641.6 ± 54.0A abc | 593.71 ± 34.0 Aabc | 527.05 ± 29.5Abc | 490.35 ± 37.4Ac |
| PSgl-0.5 | 735.6 ± 61.1Aa | 694.3 ± 48.2ABab | 661.1 ± 54.4Aabc | 642.74 ± 44.7Aabc | 618.89 ± 42.0Bbc | 591.19 ± 45.7BCc | |
| PSgl-1.0 | 711.5 ± 84.2Ab | 810.7 ± 47.6Aa | 659.4 ± 51.1Abc | 584.36 ± 34.4Ac | 680.49 ± 41.0ABbc | 485.03 ± 42.4BCd | |
| PSgl-1.5 | 696.8 ± 38.3Aab | 737.3 ± 35.1ABa | 675.4 ± 52.5Aabc | 609.52 ± 34.5Abcd | 579.53 ± 34.7ABcd | 541.69 ± 32.9ABd | |
| PSgl-2.0 | 842.3 ± 62.7Aa | 805.2 ± 39.7Bab | 682.2 ± 40.5Abc | 660.70 ± 29.8Abc | 613.48 ± 39.9Bc | 658.22 ± 55.4Cbc | |
| COH (gf)# | CON | 0.88 ± 0.03Aa | 0.86 ± 0.04Aa | 0.87 ± 0.04Aa | 0.83 ± 0.04Aab | 0.82 ± 0.04Aab | 0.78 ± 0.01Ab |
| PSgl-0.5 | 0.86 ± 0.03Aa | 0.89 ± 0.03Aa | 0.88 ± 0.03Aa | 0.87 ± 0.02ABa | 0.85 ± 0.04ABa | 0.87 ± 0.04Ba | |
| PSgl-1.0 | 0.88 ± 0.04Aab | 0.89 ± 0.03Aab | 0.89 ± 0.03Aa | 0.89 ± 0.03Ba | 0.90 ± 0.03Bab | 0.83 ± 0.04ABb | |
| PSgl-1.5 | 0.87 ± 0.04Aab | 0.87 ± 0.03Aab | 0.88 ± 0.03Aa | 0.89 ± 0.03Ba | 0.86 ± 0.03ABab | 0.80 ± 0.05ABb | |
| PSgl-2.0 | 0.88 ± 0.02Aa | 0.87 ± 0.01Aa | 0.88 ± 0.03Aa | 0.87 ± 0.02ABa | 0.85 ± 0.03ABa | 0.85 ± 0.04ABa |
*CON = mince without garlic extract, PSgl-0.5, PSgl-1.0, PSgl-1.5 and PSgl-2.0 surimi gel with 0.5, 1.0, 1.5 and 2.0 % garlic extract respectively
# = mean ± SD
Adhesiveness, the negative force area of the first compression bite, increased (P < 0.05) in treated groups in Day-1 and gradually increased (P > 0.05) in all the gels as the storage period progressed. Similarly, gumminess and cohesiveness decreased during the refrigerated storage of surimi gels, whereas no significant (P > 0.05) change was observed in case of springiness. Such changes of texture profile was presumed to be due to the increased denaturation of proteins, induced by extended refrigerated storage accompanied by loss of WHC and protein solubility.
Changes in TPC
Total plate count increased in all the groups during the period of refrigerated storage, but difference between control and GAE treated RPs was not significant upto day-4 (Fig. 1d). There were no significant differences (P > 0.05) with respect to TPC between the samples and ranged from 1.73 to 1.85 log cfu/g. Whereas, on 20th day, the TPC of control increased to 5.09 log cfu/g which significantly (P < 0.05) differed from the treated groups, wherein the count ranged from 4.49 to 4.97 log cfu/g with lowest recorded from sample treated with 2 % GAE. The antimicrobial activity of garlic and garlic-derived organosulfur compounds was widely investigated against both food spoilage bacteria and food-borne pathogens (Leuschner and Ielsch 2003). Although the antimicrobial activity of garlic and garlic derived organosulfur compounds were reported in culture media, few reports are available on its effect in meat products (Yin and Cheng 2003). However, observation in this study supports the work of Sallam et al. (2004) on chicken sausages and Gheisari and Ranjbar (2012) on camel meat, wherein, they reported that fresh garlic extract was more potential antimicrobial than garlic powder or garlic oil.
Acceptability
The acceptability scores of the samples were assigned based on the attributes such as appearance, flavour, taste and texture. The acceptability of the product over the storage time as affected by different concentration of garlic’s aqueous extract is presented in Fig. 2. All the treated groups were acceptable upto the end of the storage, however, they were scored in the order of PSgl-1.0 > PSgl-2.0 > PSgl-1.5 > PSgl-0.5. The control was found acceptable only upto Day-12 and sample PSgl-0.5 and PSgl-1.5 scored between slightly liked and moderate at the end of storage period. The consumers’ acceptability is usually based on the cumulative effect of all the sensory qualities, viz., appearance, texture, flavour, taste etc. Textural properties of sausage type of products are regarded as an important criterion for the consumer’s acceptability is concerned. In this study, the gel strength as well as hardness of treatment PSgl-1.0 estimated to be highest amongst the samples. Next to texture, the sensory attribute which attracts the consumer more is flavour. Garlic has got some pungency in its flavour and too much garlic flavour is not accepted by the consumers. The result indicated that minimum garlic flavour in treatment with 1.0 % GAE was highly accepted compared to others.
Conclusion
The study revealed that addition of garlic’s aqueous extract in surimi sausage reduced lipid and protein oxidation and microbial growth during refrigerated storage. Moreover, the textural quality of the surimi sausage also improved with incorporation of garlic’s aqueous extract. Therefore, for development of sausages from freshwater fish like pangasius with inherent low gelling capacity, garlic in the form of aqueous extract may be used for safety of the product as well as for making the product as health food due to its enrichment with antioxidants from garlic.
Acknowledgments
Authors thank Department of Biotechnology, Govt. of India, New Delhi for funding project (Grant No. BT/379/NE/TBP/2012 Dated, November 30, 2012) under which this work was undertaken. The assistances rendered by the technical staff of the Department of Fish Processing Technology is sincerely acknowledged.
References
- Aguirrezabal MM, Mateo J, Dominguez MC, Zumalacarregui JM. The effect of paprika, garlic and salt on rancidity in dry sausages. Meat Sci. 2000;54:77–81. doi: 10.1016/S0309-1740(99)00074-1. [DOI] [PubMed] [Google Scholar]
- AOAC (2000) Official Methods of Analysis. In: Association of Official Analytical Chemists, 15thed edn, Washington
- Badii F, Howell NK. Effect of antioxidants, citrate, and cryoprotectants on protein denaturation and texture of frozen cod (gadus morhua) J Agric Food Chem. 2002;50:2053–2061. doi: 10.1021/jf010824f. [DOI] [PubMed] [Google Scholar]
- Balange A, Benjakul S. Enhancement of gel strength of bigeye snapper (priacanthus tayenus) surimi using oxidised phenolic compounds. Food Chem. 2009;113:61–70. doi: 10.1016/j.foodchem.2008.07.039. [DOI] [Google Scholar]
- Baron C P, Kjaersgard IVH, Jessen F, Jacobsen C (2007) Protein and lipid oxidation during frozen storage of rainbow trout (oncorhynchus mykiss). J Agric Food Chem 8118–8125. [DOI] [PubMed]
- Barrera AM, Ramirez JA, Gonzalez-Cabriales JJ, Vazquez M. Effect of pectins on the gelling properties of surimi from silver carp. Food Hydrocoll. 2002;16:441–447. doi: 10.1016/S0268-005X(01)00121-7. [DOI] [Google Scholar]
- Benjakul S, Seymour TS, Morrissey MT, An H. Physicochemical changes in pacific whiting muscle proteins during iced storage. J Food Sci. 1997;62:729–733. doi: 10.1111/j.1365-2621.1997.tb15445.x. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W. Transglutaminase-mediated setting in big eye snapper surimi. Food Res Int. 2003;36:253–266. doi: 10.1016/S0963-9969(02)00167-9. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Chantarasuwan C. Cross-linking activity of sarcoplasmic fraction from bigeye snapper (priacanthus tayenus) muscle. LWT Food Sci Technol. 2004;37:79–85. doi: 10.1016/S0023-6438(03)00137-3. [DOI] [Google Scholar]
- Buttkus H. Preparation and properties of trout myosin. J Fish Res Board Can. 1966;23(4):563–573. doi: 10.1139/f66-047. [DOI] [Google Scholar]
- Cao N, Fu Y, He J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannic acid. Food Hydrocoll. 2007;21:575–584. doi: 10.1016/j.foodhyd.2006.07.001. [DOI] [Google Scholar]
- Chen HH. Decoloration and gel-forming ability of horse mackerel mince by air-flotation washing. J Food Sci. 2002;67:2970–2975. doi: 10.1111/j.1365-2621.2002.tb08847.x. [DOI] [Google Scholar]
- Chukwu O, Shaba IM. Effects of drying methods on proximate compositions of catfish (Clarias gariepinus) World J Agricul Sci. 2009;5(1):114–116. [Google Scholar]
- Chukwu OOC, Odu CE, Chukwu ID, Chidozie VN, Onyimba IA, Bala Z. Carrot (daucus carrota), garlic (allium sativum) and ginger (zingiber officinale) extracts as bacteria selective agents in culture media. Afr J Microbiol Res. 2012;6(2):219–224. [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl group as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1–2):23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- De Freitas V, Mateus N. Structural features of procyanidin interactions with salivary proteins. J Agric Food Chem. 2001;49:940–945. doi: 10.1021/jf000981z. [DOI] [PubMed] [Google Scholar]
- Frankel EN. Lipid oxidation. 2nd. UK: The Oily Press; 2005. [Google Scholar]
- Gafar MK, Itodo AU, Warra AA, Abdullahi L. Extraction and physicochemical determination of garlic(allium sativum L) oil. Int J Food Sci Nutr. 2012;1(2):4–7. [Google Scholar]
- Gheisari HR, Ranjbar VR. Antioxidative and antimicrobial effects of garlic in ground camel meat. Turk J Vet Anim Sci. 2012;36(1):13–20. [Google Scholar]
- Gordon A, Barbut S. Effect of chloride salts on protein extraction and interfacial protein film formation in meat batters. J Sci Food Agric. 1992;58:227–238. doi: 10.1002/jsfa.2740580211. [DOI] [Google Scholar]
- Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- Hossain MI, Kamal MM, Shikha FH, Hoque MS. Effect of washing and salt concentration on the gel forming ability of two tropical fish species. Int J Agric Biol. 2004;6(5):762–766. [Google Scholar]
- Juntachote T, Berghofer E, Bauer F, Siebenhandl S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int J Food Sci Technol. 2006;41:121–133. doi: 10.1111/j.1365-2621.2005.00987.x. [DOI] [Google Scholar]
- Kroll J, Rawel HM, Rohn S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci Technol Res. 2003;9:205–218. doi: 10.3136/fstr.9.205. [DOI] [Google Scholar]
- Kumaraguruparan R., Chandra Mohan K. V., Abraham S. K. Attenuation of N-methyl-N0-nitro-N-nitrosoguanidine induced genotoxicity and oxidative stress by tomato and garlic combination. Life Sci. 2005;76:2247–2255. doi: 10.1016/j.lfs.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Kumolu-Johnson C A, Ndimele PE. The antioxidative and antifungal effects of fresh garlic on the shelf-life of Hot Smoked Catfish (Clarias gariepinus, Burchell,1822) World Appl Sci J. 2011;13(7):1628–1634. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson LD, Gardner CD (2005) Composition, stability, and bioavailability of garlic products used in a clinical trial. J Agric Food Chem 6254–6261. [DOI] [PMC free article] [PubMed]
- Leuschner RG, Ielsch V. Antimicrobial effects of garlic, clove and red hot chili on Listeria monocytogenes in broth model systems and soft cheese. Int J Food Sci Nutr. 2003;54(2):127–133. doi: 10.1080/0963748031000084070. [DOI] [PubMed] [Google Scholar]
- Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/S0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- Lim HK, Haard NF. Protein insolubilization in frozen Greenland halibut (reinhardtius hippoglossoides) J Food Biochem. 1984;8:163–187. doi: 10.1111/j.1745-4514.1984.tb00323.x. [DOI] [Google Scholar]
- Lin T, Park J. Effective washing condition reduce water usage for surimi processing. J Aqua Food Prod Technol. 1997;6:65–79. doi: 10.1300/J030v06n02_06. [DOI] [Google Scholar]
- Linderschmidt R, Trylka A, Goad M, Witschi H. The effects of dietary butylated hydroxytoluene on liver and colon tumour development in mice. Toxicology. 1986;38:151–160. doi: 10.1016/0300-483X(86)90116-2. [DOI] [PubMed] [Google Scholar]
- Luo YK, Shen HX, Pan DD, Bu GH. Gel properties of surimi from silver carp (hypophthalmichthys molitrix) as affected by heat treatment and soy protein isolate. Food Hydrocoll. 2008;22:1513–1519. doi: 10.1016/j.foodhyd.2007.10.003. [DOI] [Google Scholar]
- Mah JH, Kim YJ, Hwang HJ. Inhibitory effects of garlic and other spices on biogenic amine production in myeolchi-jeot. Kor Salted Fermented Anchovy Product, Food Control. 2009;20:449–454. [Google Scholar]
- Majumdar RK, Deb S, Dhar B, Priyadarshini BM (2012) Chemical changes in washed mince of silver carp (Hypophthalmichthys molitrix) during frozen storage at -20 °C with or without cryoprotectants. J Food Process Preserv (On line publication: doi 10.1111/j.1745-4549.2012-00741.x.)
- Negbenebor CA, Godiya AA, Igene JO. Evaluation of clarias anguillaris treated with spice (piper guineense) for washed mince and kamaboko-type products. J Food Compos Anal. 1999;12:315–322. doi: 10.1006/jfca.1999.0824. [DOI] [Google Scholar]
- Ngapo TM, Wilkinson BHP, Chong R. 1, 5-glucono-d-lactone-induced gelation of myofibrillar protein at chilled temperatures. Meat Sci. 1996;42(1):3–13. doi: 10.1016/0309-1740(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Nuutila AM, Puupponen-Pimia R, Aarni M, Oksman-Caldentey K. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003;81(4):485–493. doi: 10.1016/S0308-8146(02)00476-4. [DOI] [Google Scholar]
- O’Connell JE, Fox PF. Significance and applications of phenolic compounds in the production and quality of milk and dairy products. Int Dairy J. 2001;11:103–120. doi: 10.1016/S0958-6946(01)00033-4. [DOI] [Google Scholar]
- Perumalla AVS, Hettiarachchy NS. Green tea and grape seed extracts – potential applications in food safety and quality. Food Res Int. 2011;44(4):827–839. doi: 10.1016/j.foodres.2011.01.022. [DOI] [Google Scholar]
- Pizzale L, Bortolomeazzi R, Vichi S, Uberegger E, Conte LS. Antioxidant activity of sage (salvia officinalis and S. fructicosa) and oregano (Origanum onites and O. indercedens) extracts related to their phenolic compound content. J Agric Food Chem. 2002;82:1645–1651. doi: 10.1002/jsfa.1240. [DOI] [Google Scholar]
- Rawel HM, Czajka D, Rohn S, Kroll J. Interactions of different phenolic acids and flavonoids with soy proteins. Int J Biol Macromol. 2002;30:137–150. doi: 10.1016/S0141-8130(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Saeed S, Howell NK. Effect of lipid oxidation and frozen storage on muscle proteins of Atlantic mackerel (Scomber scombrus) J Sci Food Agric. 2002;82:579–586. doi: 10.1002/jsfa.1080. [DOI] [Google Scholar]
- Sallam KI, Ishioroshi M, Samejima K. Antioxidant and antimicrobial effect of garlic on chicken sausage. Lebensm Wiss Technol. 2004;37(8):849–855. doi: 10.1016/j.lwt.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar TV, Ramachandran A. Changes in biochemical composition in Indian major carp in relation to size. Fish Technol. 2001;38(1):22–27. [Google Scholar]
- Schmedes A, Holmer G. A new thiobarbituric acid (TBA) method for determination of free malonaldehyde (MDA) and hydroperoxides selectivity as a measure of lipid peroxidation. J Am Oil Chem Soc. 1989;66:813–817. doi: 10.1007/BF02653674. [DOI] [Google Scholar]
- Siebert KJ, Troukhanova NV, Lynn PY. Nature of polyphenol - protein interactions. J Agric Food Chem. 1996;44:80–85. doi: 10.1021/jf9502459. [DOI] [Google Scholar]
- Silverstein JT, Wolters WR, Shimizu M, Dickhoff WW. Bovine growth hormone treatment of channel catfish: strain and temperature effects on growth, plasma IGF-I levels, feed intake and efficiency and body composition. Aquaculture. 2000;160:77–88. doi: 10.1016/S0044-8486(00)00387-2. [DOI] [Google Scholar]
- Strauss G, Gibson SM. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocoll. 2004;18:81–89. doi: 10.1016/S0268-005X(03)00045-6. [DOI] [Google Scholar]
- Taskaya L, Cakli S, Kisla D, Kilinc B. Quality changes of fish burger from rainbow trout during refrigerated storage. J Fish Aqua Sc. 2003;20:147–154. [Google Scholar]
- Vuorela S, Salminen H, Makela M, Kivikari R, Karonen M, Heinonen M. Effect of plant phenolics on protein and lipid oxidation in cooked pork meat patties. J Agric Food Chem. 2005;53:8492–8497. doi: 10.1021/jf050995a. [DOI] [PubMed] [Google Scholar]
- Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- Yang GC, Yasaei PM, Page SW. Garlic as antioxidant and free radical scavengers. J Food Drug Anal. 1993;1:357–364. [Google Scholar]
- Yin MC, Cheng WS. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci. 2003;63:23–28. doi: 10.1016/S0309-1740(02)00047-5. [DOI] [PubMed] [Google Scholar]
- Zamora R, Alaiz M, Hidalgo FJ. Modification of histidine residues by 4, 5- epoxy-3-alkenals. Chem Res Toxicol. 1999;12:654–660. doi: 10.1021/tx980218n. [DOI] [PubMed] [Google Scholar]