Abstract
Whole grains consumption promotes health benefits, but demonstrates controversial impacts from phytic acid in meeting requirements of good health. Therefore, this study was aimed to determine the nutrient bioaccessibility and antioxidant properties of rice cultivars named “Adan” or “Bario” and deduce the nutritional impact of phytic acid. Majority of the dehusked rice in the collection showed an acceptable level of in-vitro starch digestibility and in-vitro protein digestibility, but were poor in antioxidant properties and bioaccessibility of minerals (Ca, Fe and Zn). The drawbacks identified in the rice cultivars were due to relatively high phytic acid content (2420.6 ± 94.6 mg/100 g) and low phenolic content (152.39 ± 18.84 μg GAE/g). The relationship between phytic acid content and mineral bioaccessibility was strongest in calcium (r = 0.60), followed by iron (r = 0.40) and zinc (r = 0.27). Phytic acid content did not significantly correlate with in-vitro starch digestibility and in-vitro protein digestibility but showed a weak relationship with antioxidant properties. These suggest that phytic acid could significantly impair the mineral bioaccessibility of dehusked rice, and also act as an important antioxidant in non-pigmented rice. Bario rice cultivars offered dehusked rice with wide range of in-vitro digestibility of starch and protein, and also pigmented rice as a good source of antioxidants. However, there is a need to reduce phytic acid content in dehusked rice for improved mineral bioaccessibility among Bario rice cultivars.
Keywords: Phytic acid, Antinutrients, Antioxidants, Bario rice, Dehusked rice, Sarawak
Introduction
Rice is a major cereal contributing to the worlds’ calories consumption (Subudhi et al. 2006). World production of rice was 740 million tons paddy rice in 2014, mainly contributed by China and India. Malaysia produced 2.6 million tons paddy rice in 2012, with 9.1 % (237,111 t) contributed by the state of Sarawak. It is consumed primarily in milled or polished form, containing only the starchy endosperm. Dehusked rice is commonly recognized as peasant food, and only consumed by the elderly among Asians (Dipti et al. 2012). In Malaysia, increasing epidemiological evidence and growing diet diversification had promoted increasing whole grains consumption (Norhaizan and Nor Faizadatul Ain 2009).
Dehusked rice, a well-recognized health promoting food, contains substantial amounts of phytic acid in the bran layer (Liang et al. 2008). Phytic acid (myo-inositol (1,2,3,4,5,6) hexakisphosphate, InsP6) has a polydenate structure that can bind to more than one coordination site of a metal atom, and is highly capable of binding divalent and trivalent cations to form stable complexes (Bohn et al. 2008; Kumar et al. 2010). The chelator also interacts with protein and starch by electrostatic bonding, by salt bridges and hydrogen bonding (Thompson 1993). The bonding of phytic acid with food components raised the controversy of promoting whole grain consumption.
The primary concern regarding the presence of phytic acid is the anti-nutritional properties resulting from strong negative charges under gastro-intestinal conditions (Kumar et al. 2010). This is often reported in mineral availability due to the formation of stable phytate complexes and the inhibition of phytase enzymatic actions (Maenz et al. 1999). Additionally, the binding of proteins by phytic acid in insoluble binary and ternary structures makes proteins unavailable for digestion (Kies et al. 2006). The ability of phytic acid to bind proteins also leads to inhibition of α-amylase enzyme, which leads to the incomplete digestion of starch, and thus reduces starch digestibility (Yoon et al. 1983).
Phytic acid is found to be beneficial despite the common drawbacks reported. The binding of iron promotes antioxidative activity by reducing lipid peroxidation and free radical generation (Bohn et al. 2008). Phytic acid-zinc interactions reduce non-specific deoxyribose nucleic acid (DNA) synthesis and lower serum cholesterol by lowering the zinc/copper ratio, which could prevent cancer and coronary heart diseases (Norazalina et al. 2010). Phytic acid also reduces the calcification process that can prevent renal stone formation (Grases et al. 2006). The influence of phytic acid on starch benefits diabetic patients and promotes colon health by lowering the blood glucose response and slowing down gastric emptying (Yoon et al. 1983).
Bario rice is one of the traditional premium rice grown in the Kelabit highlands (Bario), which is also widely adopted by lowland rice growers’ in Sarawak. It is a low amylose rice famous for its soft texture and fine elongated grains (medium slender grain with elongation ratio of 1.85). The premium quality of Bario rice dictates the geographical indication award from Intellectual Property Corporation of Malaysia (MyIPO). Bario rice cultivars collected from Bario highlands are a good source of protein and thiamine, moderate in glycemic index and also low in fat content (Nicholas et al. 2014). Nevertheless, no information is available on the bioaccessibility of nutrients and antioxidant properties of Bario rice cultivars in relation to their high phytic acid content (18.20 to 32.36 g/kg) (Lee et al. 2014). Nutrient bioaccessibility refers to the nutrient fraction released from the food matrix, in contrast to nutrient bioavailability that refers to nutrients available for utilization under physiological conditions (Parada and Aguilera 2007).
The benefits and drawbacks of phytic acid in dehusked rice is still a debatable issue. Dehusked rice, which is widely consumed for health benefits, falls in the high phytic acid content group. The effort of one-sided promoting or demonizing phytic acid often does not provide a clear picture of its effects in the food system. This study aims to elucidate the relationship between phytic acid content, nutrient bioaccessibility and antioxidant properties in dehusked rice, thus deducing the nutritional impact of phytic acid using correlation and multivariate regression analyses. The information on these aspects was first profiled in Bario rice cultivars and will be an invaluable guide for utilization of dehusked rice of Bario cultivars in development of products for nutritional programmes.
Materials and methods
Samples
The rice cultivars named as “Adan” or “Bario” were collected between March to July 2010 in paddy form from Limbang, Miri and Bintulu divisions in northern Sarawak, Malaysia. There were two highland and five lowland sampling sites in the area. These samples are generally referred to Bario rice cultivars in this study, due to potential distribution of the original Bario rice from the Bario highlands. Genotypic and grain morphological information of these rice cultivars were obtained from previous study (Lee et al. 2014). Rice bran colors (Fig. 1) were determined based on the rice standard evaluation system (IRRI 2002). The collected paddy samples were stored in −20 °C freezer until further processing.
Fig. 1.
Dehusked rice of Bario rice cultivars: a Pigmented rice – SH03 (Red), SH04 (variable purple) and SL17 (Red); b Non-pigmented rice – remaining cultivars with light brown bran color
Chemicals
The phytic acid standard was sodium phytate from rice (Sigma, P0109-25G, Sigma-Aldrich Co. LLC., St. Louis MO, USA). The ferric thiocyanate solution was prepared by mixing a 5X concentration of ammonium thiocyanate with ferric chloride for a final ferric ion concentration of 100 μg/mL in the mixture. The dinitrosalicylic acid reagent was prepared with 250 mg dinitrosalicylic acid, 7.5 g sodium potassium tartrate and 5 mL of 2 N sodium hydroxide in a total volume of 25 mL. Other analytical standards used were D-maltose monohydrate (Aldrich, 11,256-9, Sigma-Aldrich Co. LLC., St. Louis MO, USA), gallic acid (Aldrich, 147915, Sigma-Aldrich Co. LLC., St. Louis MO, USA), L-ascorbic acid (Merck, 1831 Merck KGaA, Darmstadt, Germany), certified pure grade single element standards in 2 % HNO3 (Perkin Elmer, N9303763 (Ca); N9303771 (Fe); N9300178 (Zn), Shelton, CT06484, USA), ethylenediaminetetraacetic acid (EDTA) standard (Merck, 324503, Merck KGaA, Darmstadt, Germany), bovine serum albumin (Vivantis Technologies, USA) and sodium phytate from rice (Sigma, P0109-25G, Sigma-Aldrich Co. LLC., St. Louis MO, USA).
Samples preparation
The paddy was dehusked manually, pulverized using a stainless steel electrical blender and passed through a 425 μm sieve. Manual dehusking was performed to avoid adventitious contamination from the rice dehusker. The moisture content was determined using a moisture analyzer (AND, MX50, A&D Company Limited, Tokyo, Japan). The moisture analyzer was weight and temperature calibrated before use. All reported values were adjusted by the moisture correction factor, calculated from a ratio of dry sample weight to wet sample weight.
Phytic acid content
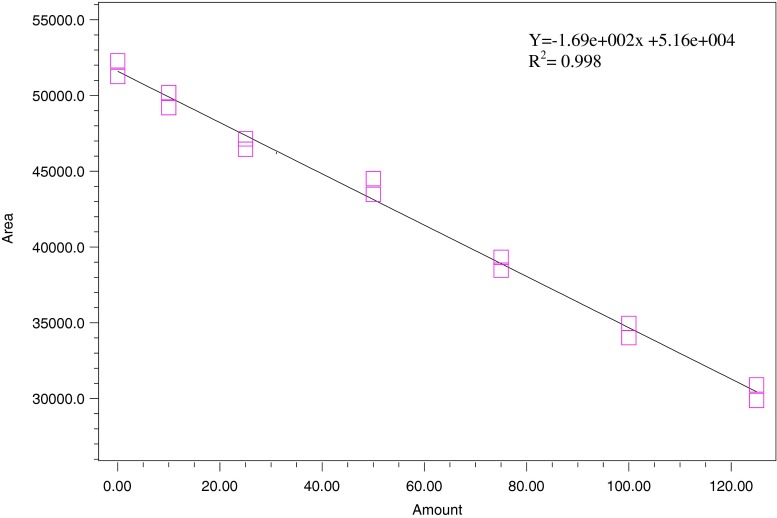
The phytic acid content was determined as described by Dost and Tokul (2006). The extraction was performed by shaking 200 mg of rice flour in 10 mL hydrochloric acid (500 mM) for 2 h. A colorimetric analysis was performed based on the complexometric replacement of ferric ion by phytic acid from the ferric thiocyanate solution. The sample supernatant was filtered and injected into HPLC system which was equipped with a CN3 analytical column (5 μm; 4 × 150 mm, Inertsil GL Science Inc., Torrance CA, USA). The chromatogram (Fig. 2) was monitored at 460 nm using a photodiode array (PDA) detector with a mobile phase of acetonitrile and water with 0.1 M nitric acid at a ratio of 30:70. The phytic acid content was quantified against the standard curve of sodium phytate (Fig. 3).
Fig. 2.
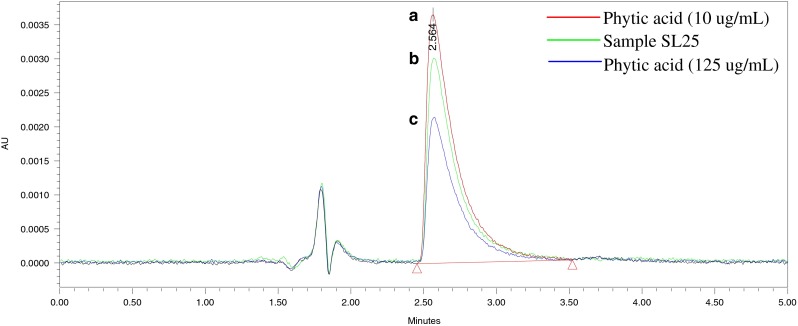
The chromatograms of iron(III)thiocyanate (100 μg/mL) after treatment with a 10 μg/mL phytic acid standard, b sample extract of Bario Merah (SL25) and c 125 μg/mL phytic acid standard
Fig. 3.
Standard calibration curve of phytic acid (10–125 μg/mL)
Nutrient bioaccessibility
Iron, zinc, and calcium contents were determined by atomic absorption spectrophotometer (AAS, AAnalyst 800, Perkin Elmer, Massachusetts, USA), followed by an estimation of the mineral’s bioaccessibility based on the mole ratio of phytic acid to the respective mineral (Norhaizan and Nor Faizadatul Ain 2009). The wavelengths monitored for these elements were 248.3 nm (Fe), 213.9 nm (Zn) and 422.7 nm (Ca). The amount of each element was quantified against a standard calibration curve prepared from certified pure grade standards. Phytic acid to mineral mole ratio was expressed as mole of phytic acid to mole of respective mineral (Norhaizan and Nor Faizadatul Ain 2009). The mole ratios were at critical levels for absorption of minerals when phytic acid to calcium mole ratio exceeded 0.24, the phytic acid to iron mole ratio exceeded 1, and the phytic acid to zinc mole ratio exceeded 15.
The in-vitro starch digestibility (IVSD) was determined based on sugar released under amylase incubation according to Lee et al. (2010). The sugar released was quantified at 0 min and after 2 h incubation with α-amylase (200 U/mL) using maltose as standard. The absorbance at 540 nm was recorded against a standard curve after mixing with the dinitrosalicylic acid reagent and heating in a boiling water bath. The in-vitro starch digestibility was calculated from the subtracted product of the sugar content at 2 h and at 0 min, and expressed as milligrams of sugar released per gram of sample (mg sugar/g).
The in-vitro protein digestibility (IVPD) was determined as described by Gbadamosi et al. (2012). Rice samples were digested with pepsin (0.1 mg/mL) and pancreatin (0.5 mg/mL) to obtain indigestible pellets. Protein content was determined on the rice samples before digestion (total protein content) and the indigestible pellets (non-available protein content) according to the Lowry method. The absorbance at 650 nm was recorded against a standard curve prepared from bovine serum albumin using an UV-vis spectrophotometer. The available protein content was calculated by subtracting the non-available protein content from the total protein content. The in-vitro protein digestibility was calculated as a percentage of available protein to total protein content.
Antioxidant assays
The total phenolic content was determined according to Butsat and Siriamornpun (2010) using Folin-Ciocalteu’s phenol reagent. Methanolic extracts (80 %) were mixed with Folin-Ciocalteu’s phenol reagent (0.2 N) in a ratio of 1:5. Sodium carbonate (10 %) was added into the mixtures, topped up to 1 mL and incubated for 2 h. The reaction mixture was then read at 760 nm against a standard curve of gallic acid and reported as milligrams of gallic acid equivalent per gram of samples (mg GAE/g).
The 2,2-diphenyl-1-picryhydrazyl (DPPH) scavenging activity was determined according to Sharma et al. (2012). Methanolic extracts (80 %) were mixed with 1.9 mL DPPH solution (0.1 mM) and incubated at room temperature for 30 min in the dark. Absorbance was recorded at 515 nm at 0 and 30 min of incubation and was compared to L-ascorbic acid after 0 and 30 min incubations. The DPPH scavenging activity was expressed as a percentage (%) of absorbance inhibition within 30 min to the initial absorbance at 0 min.
The chelating activity was measured as described by Zhao et al. (2008). Methanolic extracts (80 %) were mixed with ferrous chloride (2 mM) and allowed to stand at room temperature for 5 min. Ferrozine (5 mM) was added and topped up to 3 mL using 80 % methanol. The mixtures were mixed well and left to stand at room temperature for 10 min. Chelating activity was monitored at absorbance of 562 nm and compared to the ethylenediaminetetraacetic acid (EDTA) standard. Chelating activity was calculated as a percentage (%) of the absorbance change to the absorbance of the control.
The reducing power was measured as described by Zhao et al. (2008). Methanolic extracts (80 %) were mixed with phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide (1 %) and then incubated in a water bath at 50 °C for 20 min. After 20 min, trichloroacetic acid (10 %) was added and centrifuged at 10,000×g for 10 min. The supernatant was pipetted into a test tube, and added with ferric chloride (0.1 %) and water. The absorbance at 700 nm was recorded against the standard curve of ascorbic acid for determination of reducing power, which was expressed as milligrams of ascorbic acid equivalent per gram samples (mg AAE/g).
Inhibition on lipid peroxidation assays were performed according to Zhu et al. (2011a). Methanolic extracts (80 %) were mixed with linoleic acid (2.5 %) in ethanol, phosphate buffer (0.05 M, pH 7.0) and water, and incubated at 40 °C in the dark. Total peroxide released from the mixtures was determined every 24 h. Aliquots were taken from the mixtures and mixed with ethanol (75 %), ammonium thiocyanate (30 %) and ferrous chloride (20 mM). The absorbance was monitored at 500 nm and presented as percentage (%) of the absorbance change to the absorbance of the control on the day when the maximum absorbance of the control was obtained. Ascorbic acid was used as a standard antioxidant for comparison.
Statistical analysis
Statistical differences between rice cultivars in all parameters were estimated from the analysis of variance (ANOVA). The parameters on nutrient bioaccessibility and antioxidant properties were correlated to phytic acid content by Pearson’s bivariate correlation and partial correlation tests. Multivariate regression analysis was performed to elucidate the influence of phytic acid on nutritional profiles with concurrent nutrient interactions. The data reported are the means of five sample replicates at statistical significance of P < 0.05. IBM© SPSS© Statistics version 2.0 was used to perform the above analyses.
Results and discussion
Phytic acid content
The phytic acid content, nutrient bioaccessibility and antioxidant properties of 30 Bario rice cultivars are presented (Tables 1 and 2; Fig. 4). The phytic acid content was significantly different among cultivars, with a range of 1826.7 to 3235.6 mg/100 g. The phytic acid content was relatively higher compared to Chinese brown rice (Liang et al. 2007). The high level of phytic acid was common in dehusked form, due to dense distribution of phytate globoids in the aleurone and bran layer (Liang et al. 2008). However, the differences in phytic acid content from various studies could be due to differences among rice cultivars and ecological environments of rice cultivation (Goufo and Trindade 2014).
Table 1.
Phytic acid content and nutrients bioaccessibility of 30 Bario rice cultivars
| Code | Cultivar name | PA (mg/100 g) |
Minerals content (mg/100 g) | Mole ratio | TP | IVPD | IVSD | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Zn | Ca | Phy/Fe | Phy/Zn | Phy/Ca | (mg/g) | (%) | (mg sugar/g) | |||
| SH01 | Adan Halus | 2266.8 ± 85.7 | 2.5 ± 0.1 | 1.1 ± 0.0 | 12.4 ± 0.1 | 81.5 ± 2.2 | 216.7 ± 12.6 | 11.6 ± 0.6 | 48.2 ± 1.8 | 55.7 ± 2.0 | 158.4 ± 8.6 |
| SH02 | Adan Sederhana | 2165.9 ± 86.8 | 2.1 ± 0.1 | 1.3 ± 0.0 | 12.9 ± 0.2 | 85.9 ± 2.3 | 163.8 ± 3.6 | 10.0 ± 0.2 | 57.8 ± 3.2 | 66.5 ± 1.5 | 72.6 ± 6.3 |
| SH03 | Merah | 1819.7 ± 53.6 | 1.2 ± 0.0 | 0.9 ± 0.0 | 13.3 ± 0.1 | 169.9 ± 3.8 | 264.9 ± 15.9 | 10.5 ± 0.4 | 47.5 ± 0.4 | 48.3 ± 0.5 | 83.5 ± 10.9 |
| SH04 | Hitam | 2092.1 ± 56.9 | 1.6 ± 0.1 | 1.5 ± 0.0 | 20.2 ± 0.4 | 143.0 ± 9.0 | 180.8 ± 3.6 | 8.1 ± 0.1 | 64.4 ± 3.2 | 51.6 ± 1.3 | 158.5 ± 15.3 |
| SH05 | Pulut | 2261.4 ± 72.5 | 1.5 ± 0.0 | 1.5 ± 0.0 | 13.2 ± 0.1 | 166.1 ± 5.0 | 192.9 ± 1.7 | 13.7 ± 0.3 | 65.2 ± 1.9 | 60.6 ± 0.8 | 301.4 ± 48.1 |
| SH06 | Tuan | 2277.0 ± 80.9 | 1.3 ± 0.0 | 1.2 ± 0.0 | 10.8 ± 0.2 | 130.9 ± 4.5 | 168.5 ± 8.3 | 11.3 ± 0.8 | 52.6 ± 3.4 | 69.2 ± 1.1 | 135.2 ± 10.3 |
| SH07 | Adan Halus | 2566.5 ± 155.3 | 1.1 ± 0.1 | 0.5 ± 0.0 | 10.1 ± 0.0 | 205.9 ± 41.8 | 497.6 ± 74.1 | 15.9 ± 2.1 | 56.1 ± 2.4 | 60.0 ± 0.4 | 141.3 ± 8.6 |
| SH08 | Adan | 2238.8 ± 103.2 | 0.9 ± 0.0 | 0.6 ± 0.0 | 10.1 ± 0.0 | 170.1 ± 7.1 | 297.1 ± 17.0 | 11.4 ± 0.8 | 46.8 ± 3.1 | 58.9 ± 3.4 | 147.0 ± 7.4 |
| SH09 | Adan Merah | 2515.6 ± 88.4 | 1.1 ± 0.1 | 0.6 ± 0.0 | 11.0 ± 0.3 | 166.0 ± 3.6 | 366.5 ± 23.8 | 12.2 ± 0.6 | 55.0 ± 2.2 | 69.7 ± 0.6 | 139.9 ± 7.9 |
| SL10 | Bario Pendek | 2527.5 ± 79.7 | 1.1 ± 0.0 | 1.1 ± 0.0 | 10.1 ± 0.0 | 140.1 ± 8.0 | 161.9 ± 8.0 | 10.3 ± 0.3 | 46.6 ± 1.1 | 64.4 ± 0.5 | 124.1 ± 13.6 |
| SL11 | Bario Banjal | 2804.7 ± 109.7 | 1.3 ± 0.1 | 1.2 ± 0.0 | 8.2 ± 0.2 | 187.1 ± 6.1 | 232.9 ± 15.8 | 21.4 ± 1.4 | 66.0 ± 0.3 | 75.3 ± 0.1 | 30.7 ± 0.3 |
| SL12 | Adan Halus | 3235.6 ± 166.0 | 1.2 ± 0.1 | 1.1 ± 0.0 | 9.7 ± 0.1 | 186.0 ± 18.3 | 220.3 ± 7.0 | 15.8 ± 0.7 | 64.9 ± 3.6 | 72.8 ± 1.1 | 92.3 ± 5.0 |
| SL13 | Adan Sederhana | 2327.4 ± 71.0 | 1.8 ± 0.0 | 1.0 ± 0.0 | 8.4 ± 0.1 | 124.7 ± 6.5 | 253.5 ± 18.3 | 18.7 ± 1.3 | 66.3 ± 2.8 | 74.3 ± 0.7 | 349.2 ± 21.1 |
| SL14 | Bario | 2429.3 ± 104.0 | 2.0 ± 0.1 | 1.7 ± 0.0 | 12.5 ± 0.1 | 119.2 ± 13.3 | 161.0 ± 12.6 | 13.4 ± 1.0 | 59.3 ± 1.5 | 74.4 ± 0.5 | 383.0 ± 12.5 |
| SL15 | Bario (A) | 2402.2 ± 107.9 | 2.2 ± 0.0 | 1.8 ± 0.1 | 13.3 ± 0.1 | 68.8 ± 9.5 | 99.0 ± 12.0 | 8.2 ± 1.1 | 68.8 ± 0.6 | 71.0 ± 0.2 | 312.1 ± 20.9 |
| SL16 | Bario (B) | 2593.2 ± 75.4 | 2.2 ± 0.1 | 1.8 ± 0.1 | 13.1 ± 0.0 | 88.6 ± 2.9 | 125.6 ± 2.8 | 10.6 ± 0.4 | 70.1 ± 0.6 | 72.7 ± 0.2 | 358.5 ± 2.2 |
| SL17 | Bario Merah | 2544.3 ± 50.5 | 2.4 ± 0.1 | 1.5 ± 0.0 | 16.4 ± 0.0 | 79.6 ± 2.3 | 147.2 ± 2.7 | 8.2 ± 0.1 | 78.0 ± 2.3 | 63.3 ± 0.8 | 309.5 ± 15.6 |
| SL18 | Bario Sederhana | 2812.3 ± 125.1 | 2.5 ± 0.1 | 1.4 ± 0.0 | 13.1 ± 0.2 | 73.9 ± 9.9 | 154.6 ± 15.5 | 10.0 ± 1.1 | 62.7 ± 1.9 | 67.7 ± 0.2 | 247.2 ± 3.7 |
| SL19 | Bario Brunei | 2232.6 ± 96.0 | 2.0 ± 0.0 | 1.3 ± 0.0 | 17.9 ± 0.0 | 95.4 ± 8.4 | 167.8 ± 12.9 | 7.5 ± 0.6 | 75.9 ± 2.1 | 69.0 ± 0.5 | 226.1 ± 11.1 |
| SL20 | Bario Pendek | 2162.1 ± 59.1 | 1.7 ± 0.0 | 1.7 ± 0.1 | 16.7 ± 0.1 | 114.2 ± 6.8 | 139.4 ± 15.6 | 8.3 ± 0.4 | 65.5 ± 0.9 | 71.5 ± 0.4 | 272.2 ± 4.6 |
| SL21 | Bario Pendek | 1864.2 ± 106.2 | 1.7 ± 0.0 | 1.2 ± 0.0 | 15.2 ± 0.2 | 116.6 ± 8.4 | 194.4 ± 11.9 | 9.4 ± 0.7 | 97.7 ± 4.3 | 80.3 ± 0.6 | 239.4 ± 5.1 |
| SL22 | Bario Pendek | 1826.7 ± 87.3 | 1.7 ± 0.1 | 1.4 ± 0.0 | 12.5 ± 0.3 | 113.4 ± 4.0 | 159.7 ± 6.7 | 11.0 ± 0.5 | 90.2 ± 1.1 | 77.5 ± 0.4 | 181.6 ± 12.3 |
| SL23 | Bario Brunei | 2780.0 ± 74.5 | 1.6 ± 0.0 | 1.3 ± 0.0 | 13.0 ± 0.3 | 142.4 ± 3.2 | 214.6 ± 2.8 | 12.8 ± 0.6 | 84.3 ± 14.8 | 79.9 ± 2.0 | 197.8 ± 5.5 |
| SL24 | Bario Merah | 2848.6 ± 69.8 | 2.2 ± 0.1 | 2.0 ± 0.1 | 14.6 ± 0.3 | 101.8 ± 7.2 | 131.6 ± 9.5 | 10.8 ± 0.6 | 90.3 ± 15.1 | 74.4 ± 2.6 | 248.2 ± 3.7 |
| SL25 | Bario Merah | 2079.3 ± 94.2 | 2.3 ± 0.1 | 1.7 ± 0.0 | 13.6 ± 0.1 | 122.5 ± 8.8 | 191.1 ± 4.8 | 14.3 ± 0.2 | 98.9 ± 9.1 | 71.0 ± 2.0 | 187.9 ± 5.4 |
| SL26 | Bario Pendek | 2666.7 ± 59.2 | 1.9 ± 0.0 | 1.2 ± 0.0 | 15.1 ± 0.2 | 114.6 ± 6.4 | 204.4 ± 6.5 | 10.2 ± 0.2 | 78.5 ± 2.2 | 74.2 ± 0.4 | 211.9 ± 3.1 |
| SL27 | Bario Pendek | 2826.4 ± 87.2 | 1.7 ± 0.1 | 1.5 ± 0.0 | 16.4 ± 0.1 | 115.8 ± 6.7 | 159.7 ± 6.5 | 8.7 ± 0.4 | 93.1 ± 1.7 | 73.9 ± 0.2 | 185.6 ± 12.1 |
| SL28 | Bario Selepin | 2339.5 ± 137.6 | 2.2 ± 0.0 | 1.3 ± 0.0 | 17.0 ± 0.0 | 94.4 ± 6.1 | 190.2 ± 11.0 | 8.7 ± 0.4 | 90.3 ± 1.8 | 72.7 ± 0.4 | 157.7 ± 4.2 |
| SL29 | Bario Tinggi | 2774.5 ± 140.2 | 2.2 ± 0.1 | 1.4 ± 0.0 | 13.8 ± 0.1 | 110.0 ± 10.5 | 197.1 ± 15.5 | 12.5 ± 1.0 | 103.0 ± 0.8 | 73.4 ± 0.2 | 101.2 ± 5.5 |
| SL30 | Bario Tinggi | 2337.6 ± 155.1 | 1.8 ± 0.1 | 1.4 ± 0.0 | 14.6 ± 0.3 | 133.1 ± 3.8 | 204.8 ± 4.9 | 11.9 ± 0.1 | 91.7 ± 2.6 | 76.1 ± 0.2 | 152.8 ± 8.7 |
Values reported are means ± standard error; Abbreviations used are shown in parenthesis: PA (Phytic Acid); IVSD (In-vitro starch digestibility); IVPD (In-vitro protein digestibility); TP (Total protein content); Phy/elements (mole ratios of phytic acid to respective mineral element)
Table 2.
Phytic acid content and antioxidant properties of 30 Bario rice cultivars
| Cultivars | Cultivar name | PA (mg/100 g) |
TPC (μg GAE/g) |
DPPH (%) |
RP (mg AAE/g) |
CA (%) |
LP (%) |
|---|---|---|---|---|---|---|---|
| SH01 | Adan Halus | 2266.8 ± 85.7 | 61.0 ± 1.0 | 11.5 ± 0.7 | 0.8 ± 0.0 | 42.5 ± 1.2 | 100.0 ± 0.0 |
| SH02 | Adan Sederhana | 2165.9 ± 86.8 | 100.0 ± 8.7 | 19.9 ± 1.0 | 1.1 ± 0.0 | 49.3 ± 0.6 | 100.0 ± 0.0 |
| SH03 | Merah | 1819.7 ± 53.6 | 685.3 ± 12.7 | 90.3 ± 0.3 | 1.9 ± 0.0 | 41.0 ± 0.5 | 100.0 ± 0.0 |
| SH04 | Hitam | 2092.1 ± 56.9 | 382.7 ± 5.4 | 77.5 ± 0.4 | 1.7 ± 0.0 | 40.6 ± 0.5 | 100.0 ± 0.0 |
| SH05 | Pulut | 2261.4 ± 72.5 | 102.7 ± 1.5 | 21.5 ± 0.5 | 1.4 ± 0.0 | 56.7 ± 0.8 | 100.0 ± 0.0 |
| SH06 | Tuan | 2277.0 ± 80.9 | 96.0 ± 1.0 | 19.6 ± 1.2 | 1.7 ± 0.0 | 36.9 ± 0.1 | 100.0 ± 0.0 |
| SH07 | Adan Halus | 2566.5 ± 155.3 | 62.7 ± 1.5 | 16.7 ± 0.4 | 0.9 ± 0.0 | 43.3 ± 0.2 | 100.0 ± 0.0 |
| SH08 | Adan | 2238.8 ± 103.2 | 86.0 ± 2.1 | 13.4 ± 0.1 | 1.2 ± 0.0 | 49.8 ± 0.8 | 100.0 ± 0.0 |
| SH09 | Adan Merah | 2515.6 ± 88.4 | 68.7 ± 2.3 | 17.3 ± 1.9 | 1.1 ± 0.0 | 67.5 ± 0.3 | 100.0 ± 0.0 |
| SL10 | Bario Pendek | 2527.5 ± 79.7 | 98.3 ± 3.3 | 22.1 ± 0.4 | 1.3 ± 0.0 | 50.6 ± 0.4 | 100.0 ± 0.0 |
| SL11 | Bario Banjal | 2804.7 ± 109.7 | 134.3 ± 3.0 | 15.3 ± 0.9 | 1.2 ± 0.0 | 50.4 ± 0.6 | 100.0 ± 0.0 |
| SL12 | Adan Halus | 3235.6 ± 166.0 | 52.7 ± 1.5 | 11.8 ± 0.6 | 0.9 ± 0.0 | 56.4 ± 0.7 | 100.0 ± 0.0 |
| SL13 | Adan Sederhana | 2327.4 ± 71.0 | 105.3 ± 1.5 | 14.4 ± 0.8 | 1.5 ± 0.0 | 44.2 ± 0.3 | 100.0 ± 0.0 |
| SL14 | Bario | 2429.3 ± 104.0 | 91.7 ± 1.7 | 20.5 ± 1.4 | 1.4 ± 0.2 | 56.5 ± 1.4 | 100.0 ± 0.0 |
| SL15 | Bario (A) | 2402.2 ± 107.9 | 117.7 ± 4.3 | 18.0 ± 1.1 | 1.7 ± 0.0 | 50.3 ± 0.6 | 100.0 ± 0.0 |
| SL16 | Bario (B) | 2593.2 ± 75.4 | 118.7 ± 3.0 | 8.5 ± 1.0 | 1.6 ± 0.0 | 42.7 ± 0.6 | 100.0 ± 0.0 |
| SL17 | Bario Merah | 2544.3 ± 50.5 | 852.0 ± 12.4 | 83.6 ± 0.5 | 5.0 ± 0.1 | 25.4 ± 0.7 | 100.0 ± 0.0 |
| SL18 | Bario Sederhana | 2812.3 ± 125.1 | 91.3 ± 1.7 | 18.6 ± 0.5 | 1.5 ± 0.0 | 48.8 ± 0.2 | 100.0 ± 0.0 |
| SL19 | Bario Brunei | 2232.6 ± 96.0 | 105.3 ± 6.7 | 20.1 ± 0.3 | 1.4 ± 0.0 | 55.8 ± 0.1 | 76.8 ± 5.1 |
| SL20 | Bario Pendek | 2162.1 ± 59.1 | 79.7 ± 1.7 | 14.7 ± 0.7 | 1.3 ± 0.0 | 47.1 ± 0.4 | 85.8 ± 2.8 |
| SL21 | Bario Pendek | 1864.2 ± 106.2 | 84.7 ± 1.7 | 24.4 ± 1.0 | 1.6 ± 0.0 | 46.0 ± 0.7 | 80.6 ± 0.4 |
| SL22 | Bario Pendek | 1826.7 ± 87.3 | 86.0 ± 1.0 | 17.1 ± 0.9 | 1.6 ± 0.0 | 49.6 ± 0.8 | 84.4 ± 0.7 |
| SL23 | Bario Brunei | 2780.0 ± 74.5 | 101.3 ± 1.7 | 17.5 ± 1.2 | 1.5 ± 0.0 | 49.4 ± 0.3 | 88.9 ± 4.1 |
| SL24 | Bario Merah | 2848.6 ± 69.8 | 108.7 ± 0.7 | 21.3 ± 1.7 | 1.9 ± 0.0 | 45.8 ± 0.2 | 83.5 ± 0.6 |
| SL25 | Bario Merah | 2079.3 ± 94.2 | 272.0 ± 13.5 | 12.5 ± 1.7 | 4.3 ± 0.0 | 59.6 ± 0.6 | 63.7 ± 1.6 |
| SL26 | Bario Pendek | 2666.7 ± 59.2 | 78.0 ± 5.8 | 10.9 ± 1.0 | 2.1 ± 0.0 | 51.3 ± 0.4 | 73.0 ± 0.7 |
| SL27 | Bario Pendek | 2826.4 ± 87.2 | 86.7 ± 1.7 | 11.5 ± 1.6 | 1.5 ± 0.0 | 52.2 ± 0.2 | 84.0 ± 0.6 |
| SL28 | Bario Selepin | 2339.5 ± 137.6 | 82.7 ± 3.9 | 15.0 ± 0.2 | 1.7 ± 0.0 | 59.1 ± 1.1 | 64.9 ± 0.0 |
| SL29 | Bario Tinggi | 2774.5 ± 140.2 | 84.3 ± 0.7 | 24.3 ± 0.3 | 1.7 ± 0.0 | 64.0 ± 0.6 | 69.8 ± 4.9 |
| SL30 | Bario Tinggi | 2337.6 ± 155.1 | 95.3 ± 1.5 | 24.6 ± 0.9 | 1.7 ± 0.0 | 76.3 ± 0.5 | 55.4 ± 0.4 |
Values reported are means ± standard error; Abbreviations used are shown in parenthesis: PA (Phytic Acid); TPC (Total Phenolic Content); DPPH (DPPH scavenging activity); RP (Reducing Power); CA (Chelating Activity) and LP (Inhibition of Lipid Peroxidation)
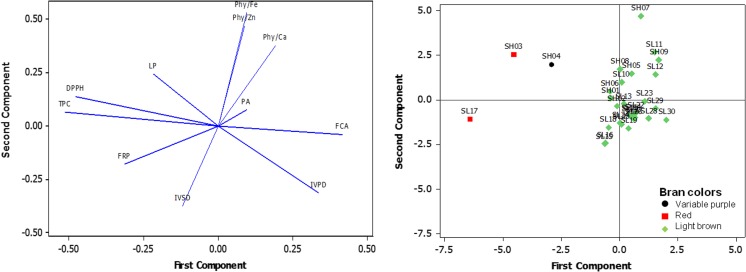
Fig. 4.
Factor loading plot (left) and score plot (right) of first and second components describing the variation of nutritional value among the Bario rice cultivars
Nutrient bioaccessibility
The rice cultivars showed a wide range of in-vitro starch digestibility (30.7 to 383.0 mg sugar/g) and in-vitro protein digestibility (48.3 to 80.3 %). The digestibility of starch and protein were generally higher in lowland adapted cultivars (Fig. 4; SL coded samples) and differed among the genetically close cultivars. Physicochemical properties of rice starch, such as gelatinization temperature, amylograph consistency, crystallinity, and also the length of the amylose and amylopectin chains could contribute to the observed differences (Syahariza et al. 2013). Lower in-vitro starch digestibility of rice was often reported in rice with high levels of amylose, resistant starch and dietary fiber (Zhu et al. 2011b). In-vitro protein digestibility of rice cultivars was dependent on protein content (r = 0.65; p < 0.01) and undigested protein content (r = − 0.42; p < 0.05), which was similarly reported in previous studies (Gilani et al. 2012).
The wide range of in-vitro starch digestibility and in-vitro protein digestibility offered dehusked rice suitable for both diabetic and infant diets. Bario Banjal (SL11) with low in-vitro starch digestibility showed good potential for blood glucose control in diabetic patients. Evaluation of blood glucose responses under in-vivo models relate to the ingestible fractions in dehusked rice would further clarify its potential as a low glycemic index food. Bario (SL14) and Bario Pendek (SL21) with high in-vitro digestibility of starch and protein could be an easily digestible ingredient for development of infant food.
However, the mineral bioaccessibility of the Bario rice cultivars were poor, with phytic acid to iron, zinc and calcium mole ratios exceeding 1, 15 and 0.24, respectively. The iron, zinc and calcium bioaccessibility of the rice cultivars may be impaired by phytic acid due to the high level of phytic acid content in the dehusked rice. Iron and calcium bioaccessibility were also impaired by phytic acid in commercial rice from Malaysia and China, but the inhibition of zinc bioaccessibility by phytic acid was not consistently reported (Liang et al. 2007; Ma et al. 2007; Norhaizan and Nor Faizadatul Ain 2009).
Antioxidant properties
Antioxidant properties of the rice cultivars were low, except for three pigmented rice cultivars (Figs. 1 and 4). Pigmented rice gave higher total phenolic content (382.7–852.0 μg GAE/g) than the remaining non-pigmented rice (52.7–272.0 μg GAE/g). The pigmented rice cultivars also showed higher levels of DPPH scavenging activity (77.5–90.3 %) and reducing power (1.7–5.0 mg AAE/g), which were strongly correlated with total phenolic content (Table 3). DPPH scavenging activity and total phenolic content of the rice collection followed the hierarchy of white rice < variable purple rice < red rice, which could be explained by high level of protocatechuic and syringic acids in red rice (Goufo and Trindade 2014). These suggest that phenolic compounds are abundantly present in pigmented rice and participate actively in the DPPH scavenging and ferric ion reducing mechanisms (Rattanachitthawat 2010; Zhang et al. 2010). Phenolic compounds, a diverse group of chemicals with hydroxyl groups attached to aromatic ring, are derived from the phenylpropanoid pathway in the rice grains. These compounds could be classified by number of carbons and give maximum absorption at 280 and 320 nm based on the structure of carbon skeleton (Vermerris and Nicholson 2008; Goufo and Trindade 2014).
Table 3.
Correlation between phytic acid content and antioxidant properties
| Phytic acid content | Total phenolic content | DPPH scavenging activity | Reducing power | Chelating activity | Inhibition of lipid peroxidation | |
|---|---|---|---|---|---|---|
| Phytic acid content | ||||||
| Whole collection (n = 30) | 1.00 | −0.03 | −0.02 | 0.17 | 0.29** | −0.35** |
| Pigmented rice (n = 3) | 1.00 | −0.90** | −0.69* | −0.62 | 0.55 | 0.00 |
| Non-pigmented rice (n = 27) | 1.00 | 0.34 | 0.04 | 0.35** | 0.32** | −0.36** |
| Antioxidant properties (n = 30) | ||||||
| Total phenolic content | 1.00 | 0.90** | 0.70** | −0.51** | 0.16 | |
| DPPH scavenging activity | 1.00 | 0.44** | −0.45** | 0.19 | ||
| Reducing power | 1.00 | −0.27* | −0.27* | |||
| Chelating activity | 1.00 | −0.56** | ||||
| Inhibition of lipid peroxidation | 1.00 | |||||
Values with single asterisk (*) and double asterisk (**) are significant at P level < 0.05 and P level < 0.01, respectively
The inhibition of lipid peroxidation were also higher in pigmented rice, but did not significantly correlated with total phenolic content. The chelating activity of the rice cultivars ranged from 25.4 to 76.3 %, with the higher content in non-pigmented rice (36.9–76.3 %). Antioxidants other than phenolic compounds such as γ-oryzanol, tocotrienols, tocopherols and phytic acid could be present in the dehusked rice and contribute to the inhibition of lipid peroxidation and chelating activity. The pigmented Bario rice could be a good source of antioxidants for protection against chronic diseases.
Impact of phytic acid content on nutrient bioaccessibility
Phytic acid content of the rice collection showed no significant relationship with in-vitro starch digestibility and in-vitro protein digestibility. In-vitro digestibility of starch and protein could be strongly affected by the physicochemical properties rather than be inhibited by phytic acid (Syahariza et al. 2013). Besides, mineral elements such as iron and zinc could also influence starch physicochemical properties and influence in-vitro digestibility of starch (Table 4). In-vitro protein digestibility of rice cultivars could be largely determined by protein content and undigested protein content.
Table 4.
Correlation between phytic acid content and nutrients bioaccessibility
| Phytic acid content | Minerals content | Phytic acid to mineral mole ratio | In-vitro starch digestibility | Total protein content | In-vitro protein digestibility | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Zn | Ca | Fe | Zn | Ca | ||||||
| Phytic acid content | |||||||||||
| Pearson correlation | 1.00 | 0.10 | 0.19 | 0.06 | 0.40** | 0.27** | 0.60** | −0.06 | 0.41** | 0.17 | |
| Partial correlation | – | – | – | 0.89** | 0.71** | 0.93** | −0.18 | – | 0.06 | ||
| Nutrients bioaccessibility | |||||||||||
| Minerals content | Fe | 1.00 | 0.64** | 0.44* | −0.83** | −0.54** | −0.31** | 0.41** | 0.44** | 0.20* | |
| Zn | 1.00 | 0.47** | −0.55** | −0.79** | −0.27* | 0.50** | 0.48** | 0.34** | |||
| Ca | 1.00 | −0.44** | −0.41** | −0.73** | 0.19 | 0.44** | −0.07 | ||||
| Phytic acid to minerals mole ratio | Fe | 1.00 | 0.73** | 0.62** | −0.48** | −0.27** | −0.19 | ||||
| Zn | 1.00 | 0.51** | −0.40** | −0.29** | −0.26* | ||||||
| Ca | 1.00 | −0.21* | 0.10 | 0.16 | |||||||
| In-vitro starch digestibility | 1.00 | 0.14 | 0.25* | ||||||||
| Total protein content | 1.00 | 0.64** | |||||||||
| In-vitro protein digestibility | 1.00 | ||||||||||
Values with single asterisk (*) and double asterisk (**) are significant at P level < 0.05 and P level < 0.01, respectively
Phytic acid content in the rice cultivars was positively correlated with phytic acid to mineral mole ratios. The inhibitory effect of phytic acid was highest in calcium (r = 0.60; p < 0.01), followed by iron (r = 0.40; p < 0.01) and zinc (r = 0.27; p < 0.01). The elimination of covariance in the partial correlation also showed strong relationships between phytic acid content and mineral bioaccessibility in a dose dependent manner (Table 4). Nevertheless, phytic acid content gave a greater impact on iron bioaccessibility when co-occurrence of nutrients and their interactions were considered in multivariate regression analysis. A regression equation, Phy/Fe = 4.08 Phytic Acid – 50.45 Fe + 168.07 with good linearity (R2 = 0.998) was obtained for iron bioaccessibility, which highlighted phytic acid as a predominant inhibitor of iron bioaccessibility.
The greater influence of phytic acid on calcium and iron was in congruence to previous studies (Ma et al. 2005; Norhaizan and Nor Faizadatul Ain 2009). These could be due to the different localization of these minerals in rice grains and dominant elements in the natural phytate globoids of dehusked rice. Phosphorus, iron and calcium were co-localized in the aleurone layer and formed phytates, but zinc was broadly distributed in the inner endosperm as phytate and other forms (Iwai et al. 2012). However, the calcium and iron phytates are relatively easier to be broken down by phytase enzyme (Maenz et al. 1999), indicating the potential utilization of these bounded minerals through phytase supplementation.
Impact of phytic acid content on antioxidant properties
Phytic acid content in dehusked rice significantly contributed to ferrous chelating activity, especially in non-pigmented rice (Table 3). The polydenate structure of phytic acid enabled the molecule to bind on to ferrous ion in a dose-dependent manner (Bohn et al. 2008; Sakac et al. 2010). However, weak correlation between phytic acid content and ferrous chelating activity suggests that other chelating components may have also contributed to ferrous chelating activity. Fiber from cereal bran could also bind cations (Ekholm et al. 2003).
Phytic acid content in the dehusked rice showed a negative relationship for inhibition of peroxide formation from linoleic acid (Table 3). This suggests that a mechanism other than Fenton reaction was involved in the generation of peroxides from linoleic acid. Similar to Sakac et al. (2010), phytic acid did not inhibit thermal oxidation but exerted antioxidant effects only on iron-catalyzed oxidation of hydroperoxyde-enriched soybean oil.
Phytic acid content in dehusked rice did not contribute to the DPPH scavenging activity and reducing power of the dehusked rice based on Pearson’s correlation. The lack of DPPH scavenging activity was commonly reported in extracted phytic acid (Norhaizan et al. 2011). However, contrasting results have been reported on the reducing power of phytic acid extracted from rice bran, suggesting that the reducing power of phytic acid was not consistent (Norhaizan et al. 2011; Canan et al. 2012). Several studies have shown that phenolic compounds are important antioxidants in DPPH radical scavenging and ferric reducing power (Rattanachitthawat 2010; Zhang et al. 2010). Phenolic compounds in dehusked rice offer a wider range of antioxidant protections than phytic acid.
Conclusion
The majority of dehusked rice in the collection showed acceptable levels in in-vitro starch digestibility and in-vitro protein digestibility but were poor in mineral bioaccessibility and antioxidant properties. High level of phytic acid could significantly impair the mineral bioaccessibility of dehusked rice, and also contribute to antioxidant properties of non-pigmented dehusked rice. Bario rice cultivars offered dehusked rice suitable for both infant and diabetic diets. However, there is a need to reduce phytic acid content in dehusked rice for improved mineral bioaccessibility among Bario rice cultivars. Pigmented rice containing high level of phenolic compounds appeared as a good source of antioxidants.
Acknowledgments
The authors gratefully acknowledge the research funding provided by the Ministry of Education, Malaysia (FRGS 01-01-13-1246FR), financial support from the National Paddy and Rice Board, Malaysia (64279) and generous assistance from the Sarawak Department of Agriculture. Lee HH also wishes to thank the Ministry of Science, Technology and Innovation, Malaysia on the National Science Fellowship provided. We thank Dr. Rajan Amartalingam and ScienceDocs. Inc for the English revision and editorial services.
Footnotes
Research highlights
1. High level of InsP6 in dehusked rice impaired Ca, Fe and Zn bioaccessibility.
2. InsP6 showed the strongest relationship with calcium bioaccessibility.
3. InsP6 content contributed to antioxidant properties of non-pigmented rice.
4. Phenolic compounds in dehusked rice exhibit wider antioxidant properties than InsP6.
5. Breakdown of InsP6 is needed for efficient mineral absorption from Bario rice.
References
- Bohn L, Meyer AS, Rasmussen SK. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ Sci B. 2008;9:165–191. doi: 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butsat S, Siriamornpun S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem. 2010;119:606–613. doi: 10.1016/j.foodchem.2009.07.001. [DOI] [Google Scholar]
- Canan C, Delaroza F, Casagrande R, et al. Antioxidant capacity of phytic acid purified from rice bran. Acta Sci Technol. 2012;34:457–463. doi: 10.4025/actascitechnol.v34i4.16358. [DOI] [Google Scholar]
- Dipti SS, Bergman C, Indrasari SD, et al. The potential of rice to offer solutions for malnutrition and chronic diseases. Rice (N Y) 2012;5:16. doi: 10.1186/1939-8433-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dost K, Tokul O. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anal Chim Acta. 2006;558:22–27. doi: 10.1016/j.aca.2005.11.035. [DOI] [Google Scholar]
- Ekholm P, Virkki L, Ylinen M, Johansson L. The effect of phytic acid and some natural chelating agents on the solubility of mineral elements in oat bran. Food Chem. 2003;80:165–170. doi: 10.1016/S0308-8146(02)00249-2. [DOI] [Google Scholar]
- Gbadamosi SO, Abiose SH, Aluko RE. Amino acid profile, protein digestibility, thermal and functional properties of Conophor nut (Tetracarpidium conophorum) defatted flour, protein concentrate and isolates. Int J Food Sci Technol. 2012;47:731–739. doi: 10.1111/j.1365-2621.2011.02901.x. [DOI] [Google Scholar]
- Gilani GS, Chao WX, Cockell KA. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr. 2012;108:S315–S332. doi: 10.1017/S0007114512002371. [DOI] [PubMed] [Google Scholar]
- Goufo P, Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci Nutr. 2014;2:75–104. doi: 10.1002/fsn3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grases F, Costa-Bauza A, Prieto RM. Renal lithiasis and nutrition. Nutr J. 2006;5:23. doi: 10.1186/1475-2891-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRRI (2002) Standard Evaluation System for Rice (SES). International Rice Research Institute (IRRI)
- Iwai T, Takahashi M, Oda K, et al. Dynamic changes in the distribution of minerals in relation to phytic-acid accumulation during rice seed development. Plant Physiol. 2012;160:2007–2014. doi: 10.1104/pp.112.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kies AK, De Jonge LH, Kemme PA, Jongbloed AW. Interaction between protein, phytate, and microbial phytase. In vitro studies. J Agric Food Chem. 2006;54:1753–1758. doi: 10.1021/jf0518554. [DOI] [PubMed] [Google Scholar]
- Kumar V, Sinha AK, Makkar HPS, Becker K. Dietary roles of phytate and phytase in human nutrition: a review. Food Chem. 2010;120:945–959. doi: 10.1016/j.foodchem.2009.11.052. [DOI] [Google Scholar]
- Lee KY, Lee S, Lee HG. Effect of the degree of enzymatic hydrolysis on the physicochemical properties and in vitro digestibility of rice starch. Food Sci Biotechnol. 2010;19:1333–1340. doi: 10.1007/s10068-010-0190-z. [DOI] [Google Scholar]
- Lee HH, Bong CFJ, Loh SP, et al. Genotypic, grain morphological and locality variation in rice phytate content and phytase activity. Emirates J Food Agric. 2014;26:844–852. [Google Scholar]
- Liang J, Han B, Han L, et al. Iron, zinc and phytic acid content of selected rice varieties from China. J Sci Food Agric. 2007;87:504–510. doi: 10.1002/jsfa.2747. [DOI] [Google Scholar]
- Liang J, Li Z, Tsuji K, et al. Milling characteristics and distribution of phytic acid and zinc in long-, medium- and short-grain rice. J Cereal Sci. 2008;48:83–91. doi: 10.1016/j.jcs.2007.08.003. [DOI] [Google Scholar]
- Ma G, Jin Y, Piao J, et al. Phytate, calcium, iron, and zinc contents and their molar ratios in foods commonly consumed in China. J Agric Food Chem. 2005;53:10285–10290. doi: 10.1021/jf052051r. [DOI] [PubMed] [Google Scholar]
- Ma G, Li Y, Jin Y, et al. Phytate intake and molar ratios of phytate to zinc, iron and calcium in the diets of people in China. Eur J Clin Nutr. 2007;61:368–374. doi: 10.1038/sj.ejcn.1602513. [DOI] [PubMed] [Google Scholar]
- Maenz DD, Engele-Schaan CM, Newkirk RW, Classen HL. The effect of minerals and mineral chelators on the formation of phytase-resistant and phytase-susceptible forms of phytic acid in solution and in a slurry of canola meal. Anim Feed Sci Technol. 1999;81:177–192. doi: 10.1016/S0377-8401(99)00085-1. [DOI] [Google Scholar]
- Nicholas D, Hazila KK, Chua HP, Rosniyana A. Nutritional value and glycemic index of Bario rice varieties. J Trop Agric Food Sci. 2014;42:1–8. [Google Scholar]
- Norazalina S, Norhaizan ME, Hairuszah I, Norashareena MS. Anticarcinogenic efficacy of phytic acid extracted from rice bran on azoxymethane-induced colon carcinogenesis in rats. Exp Toxicol Pathol. 2010;62:259–268. doi: 10.1016/j.etp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Norhaizan ME, Nor Faizadatul Ain A. Determination of phytate, iron, zinc, calcium contents and their molar ratios in commonly consumed raw and prepared food in malaysia. Malays J Nutr. 2009;15:213–222. [PubMed] [Google Scholar]
- Norhaizan ME, Ng S, Norashareena M, Abdah MA. Antioxidant and cytotoxicity effect of rice bran phytic acid as an anticancer agent on ovarian, breast and liver cancer. Malays J Nutr. 2011;17:367–375. [PubMed] [Google Scholar]
- Parada J, Aguilera JM. Food microstructure affects the bioavailability of several nutrients. J Food Sci. 2007;72:R21–R32. doi: 10.1111/j.1750-3841.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Rattanachitthawat S. Phenolic content and antioxidant activities in red unpolished Thai rice prevents oxidative stress in rats. J Med Plants Res. 2010;4:796–801. [Google Scholar]
- Sakac M, Canadanovic-Brunet J, Misan A, et al. Antioxidant activity of phytic acid in lipid model system. Food Technol Biotechnol. 2010;48:524–529. [Google Scholar]
- Sharma P, Gujral HS, Singh B. Antioxidant activity of barley as affected by extrusion cooking. Food Chem. 2012;131:1406–1413. doi: 10.1016/j.foodchem.2011.10.009. [DOI] [Google Scholar]
- Subudhi PK, Sasaki T, Khush GS. Rice. In: Kole C, editor. Genome Mapp. Mol. Breed. Plants, Cereal. Millets. Verlag: Springer; 2006. pp. 264–270. [Google Scholar]
- Syahariza ZA, Sar S, Hasjim J, et al. The importance of amylose and amylopectin fine structures for starch digestibility in cooked rice grains. Food Chem. 2013;136:742–749. doi: 10.1016/j.foodchem.2012.08.053. [DOI] [PubMed] [Google Scholar]
- Thompson LU. Potential health benefits and problems associated with antinutrients in foods. Food Res Int. 1993;26:131–149. doi: 10.1016/0963-9969(93)90069-U. [DOI] [Google Scholar]
- Vermerris W, Nicholson R (2008) Phenolic compound biochemistry. Springer Science+Business Media B.V
- Yoon JH, Thompson LU, Jenkins DJ. The effect of phytic acid on in vitro rate of starch digestibility and blood glucose response. Am J Clin Nutr. 1983;38:835–842. doi: 10.1093/ajcn/38.6.835. [DOI] [PubMed] [Google Scholar]
- Zhang MW, Zhang RF, Zhang FX, Liu RH. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J Agric Food Chem. 2010;58:7580–7587. doi: 10.1021/jf1007665. [DOI] [PubMed] [Google Scholar]
- Zhao H, Fan W, Dong J, et al. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem. 2008;107:296–304. doi: 10.1016/j.foodchem.2007.08.018. [DOI] [Google Scholar]
- Zhu K-X, Lian C-X, Guo X-N, et al. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011;126:1122–1126. doi: 10.1016/j.foodchem.2010.11.144. [DOI] [Google Scholar]
- Zhu L-J, Liu Q-Q, Wilson JD, et al. Digestibility and physicochemical properties of rice (Oryza sativa L.) flours and starches differing in amylose content. Carbohydr Polym. 2011;86:1751–1759. doi: 10.1016/j.carbpol.2011.07.017. [DOI] [Google Scholar]