Abstract
Glycerol contributes to the beverage body and fullness. Moreover, it also influences the flavor intensity. As a major byproduct, glycerol not only serves critical roles in yeast osmoregulation and redox balancing, but also acts as the carbon competitor against ethanol in alcoholic fermentation. Therefore, increasing glycerol yield benefits both the flavor and ethanol reduction for the fermented beverages. Glycerol yield has been elevated either by fermentation optimization or by yeast genetic modification. The fermentation optimizations reached maximum 14 g/L glycerol through screening yeast strains and optimizing fermentation parameters. Meanwhile the yeast overexpressing GPD1 (encoding glycerol-3-phosphate dehydrogenase) produced up to 6 folds more glycerol for beer and wine. Except for glycerol improvement, the genetically modified yeasts accumulated dramatically undesirable compounds such as acetaldehyde, acetate and acetoin which are detrimental for beverage flavor. In comparison, the natural high glycerol producers showed strain-specific manner on the yeast-derived aroma compounds like volatile acids, fusel alcohols, esters, and aldehydes. Temperature, sugar concentration, nitrogen composition, oxygen and pH-value, which influence glycerol biosynthesis, also obtained various effects on the production of aromatic compounds. In the current review, we firstly deliberate the organoleptic contributions of glycerol for fermented beverages. Furthermore, glycerol optimization strategies are discussed regarding to the yield improvement, the genes expressions, the overall flavor impacts and the feasibilities in beverage applications. Lastly, for improving beverage flavor by glycerol optimization, a high-throughput platform is proposed to increase the screening capacity of yeast strains and parameters in the processing of fermented beverages.
Keywords: Glycerol, Yeast, Fermentation, Flavor, Beverage, High throughput screening (HTS)
Introduction
As a viscous polyalcohol with slight sweet taste, glycerol mainly contributes to the body for fermented beverage (Scanes et al. 1998). Additionally, it has been also reported to enhance the flavor intensity (Jones et al. 2008; Omori et al. 1995), suppress the perceived roughness (Jones et al. 2008; Peleg et al. 1999), increase the worty off-flavor retention and influence on the aroma volatility (Jones et al. 2008; Perpete and Collin 1999; Perpete and Collin 2000). Glycerol was ranging from 1 to 3 g/L against its claimed threshold of 10 g/L in beers (Briggs et al. 2004; Nykänen and Suomaleinen 1983; Parker and Richardson 1970). In wine, the general concentration was across 4 to 15 g/L opposing to the thresholds at 5.2 and 9 g/L (Nieuwoudt et al. 2002; Noble and Bursick 1984; Nykänen and Suomaleinen 1983). For other fermented beverages, approximate 2 to 6 g/L could be found in Asian rice wines, cider and barley shochu, a Japanese spirit (Chen and Xu 2010; del Campo et al. 2003; Liu et al. 2014; Omori et al. 1995).
Glycerol serves two vital physiological roles during yeast fermentation. The accumulation of osmolyte glycerol allows yeast to re-adjust osmotic gradient across the cell membrane to remain cellular functions under osmotic stress condition (Albertyn et al. 1994; Ansell et al. 1997). Glycerol is also involving in the maintenance of cytosolic redox balance for that the surplus NADH can be reoxidized via glycerol synthesis by serving as the reaction cofactor (Albers et al. 1996). Interestingly, more glycerol formation also means that less carbon source will be diverted to ethanol synthesis in alcoholic fermentation.
Numerous efforts have been devoted to redirect the glycolysis flow towards glycerol for beverage flavor improvements and ethanol reduction. The major strategy is by optimizing the yeast culture conditions. Up to 14 g/L has been observed by screening yeast strains followed by optimizations on the fermentation parameters and substrates (Berovič et al. 2007; Erasmus et al. 2004; Nieuwoudt et al. 2002). Primarily, the prolonged or increased transcription level of GPD1, which encodes NAD-dependent glycerol-3-phosphate dehydrogenase (GPDH), was responsible for the elevated glycerol level under the optimized conditions (Du et al. 2011; Kajiwara et al. 2000). However, except for glycerol, the optimized conditions also impacted on the yield of fermentative flavor compounds. The higher temperature and higher sugar concentration, which were in favor of glycerol formation, led to over accumulation of acetic acid, acetaldehyde and higher alcohols. Even worse, inadequate optimizations also impaired yeast growth and further affected the fermentation kinetics and the overall flavor balance (Csutorás et al. 2014; Du et al. 2011; Liu et al. 2014; Piddocke et al. 2009; Yu et al. 2012).
Glycerol overproduction has been realized by the yeast overexpressing genes involved in glycerol synthesis, disruption or deletion of the genes in glycolysis and fermentation pathways (Eglinton et al. 2002; Ehsani et al. 2009; Nevoigt et al. 2002; Nevoigt and Stahl 1996; Varela et al. 2012). Alternatively, fermentative glycerol has been also overproduced by raising the surplus intracellular NADH and by “opening” the flux facilitator (Overkamp et al. 2000; Varela et al. 2012). The genetically modified yeasts promoted glycerol yield up to 5.6 and 6.1 folds and decrease ethanol concentration by 18 % and 21 % under beer and wine fermentations individually (Nevoigt et al. 2002; Varela et al. 2012). Nonetheless, the engineered strains accumulated dramatic amounts of detrimental flavors such as acetaldehyde, acetate and acetoin in addition to the impaired growth of the yeast and poor social acceptance for their application (Cambon et al. 2006; Eglinton et al. 2002; Lopes et al. 2000; Nevoigt et al. 2002; Remize et al. 1999; Varela et al. 2012).
In order to realize beverage flavor improvement by glycerol optimization, we present this review firstly, to discuss the flavor impacts of glycerol for fermented beverages based on the analytical and sensory studies. Secondly, strategies for glycerol optimization are evaluated respecting to the effectiveness, side effects and feasibilities for beverage application. Lastly, we propose a high-throughput platform for optimizing glycerol yield with flavor consideration in the processing of fermented beverages.
Sensory contribution of glycerol to fermented beverages
Glycerol has favorable impacts on the quality of beer, wine and spirit (Langstaff et al. 1991; Liu et al. 2014; Nieuwoudt et al. 2002; Omori et al. 1995; Omori et al. 1996). Large surveys showed that obvious higher level of glycerol was present in wines of superior grade than that of ordinary (Nieuwoudt et al. 2002; Nykänen and Suomaleinen 1983). Nevertheless, flavor profile is unique for each wine. Individual wine, as well as other beverage, has debating benefits from glycerol.
Wide agreement on the body contribution
It is widely accepted for the contribution of glycerol to beverages body and fullness. Glycerol was reported to contribute to the “mouth-feel”, “viscosity”, “density”, “smoothness” and “roundness”. These sensory terms were closely correlated in subordinate or synonymic manner (Gawel et al. 2007; Jones et al. 2008; Langstaff et al. 1991; Nurgel and Pickering 2005). In beers, glycerol was found significant correlated to the density and viscosity which were categorized as the mouth-feel attributes (Langstaff et al. 1991). Alcohol-free beer benefited from glycerol addition across a range from 0.3 % to 2.0 % with an improved body and fullness (Sohrabvandi et al. 2010). For wines, although the majority research has reached an agreement on the mouth-feel contribution (Gawel et al. 2007; Jones et al. 2008; Noble and Bursick 1984; Nurgel and Pickering 2005), the effective levels of glycerol varied even contradicted. Over 25 g/L glycerol was required for a noticeable effect in the perceived viscosity while the decrease trend was observed in the range of 0–15 g/L in the wine (Noble and Bursick 1984; Nurgel and Pickering 2005). However, recent researches revealed that practical level of glycerol at around 10 g/L was already enough to produce a noticeable improvement of the perceived viscosity (Gawel et al. 2007; Jones et al. 2008).
Beverage body benefits from glycerol in certain concentrations or ranges. It might because of that the viscosity and density of glycerol are higher than most of the fermented beverages. In addition, by balancing the alcoholic strength and astringency, glycerol conferred a degree of “smoothness” and “roundness” on the palate (Jones et al. 2008). Glycerol was also found in the total extract of beverages and might participate in the fullness contribution in associated with high extract content (Nieuwoudt et al. 2002). Nevertheless, the body contribution was highly dependent on the individual wine (Gawel et al. 2007; Jones et al. 2008). It was rather less consistent for sweet wines than that for the dry wines. For example, glycerol has a doubtful contribution on the mouth-feel of ice wine since the high contents of alcohol and residual sugars tended to deliver greater impacts on the viscosity and body (Erasmus et al. 2004). This observation partly explains the controversial concentrations of glycerol for perceived viscosity.
Debate on the flavor impact
Glycerol influences beverage flavor. Glycerol addition above or below the threshold (10 g/L) was found to modify the beer flavor (Parker and Richardson 1970). The overall flavor intensity was positively influenced by glycerol (10 g/L) especially when the volatile concentration was low in wine (Jones et al. 2008). Glycerol together with other components were indicated to responsible for the delicate tastes and flavors of Chinese rice wine (Chen and Xu 2010; Liu et al. 2014). The flavor compounds of shochu was enhanced by varying glycerol concentration in the mash (Omori et al. 1994). Except for the impacts on overall flavor profile, glycerol also affected the perception of specific flavors. The sweetness threshold of glycerol in wine was revealed at 5.2 g/L in earlier study (Noble and Bursick 1984). However, no obvious effect on sweetness perception was observed in the model white wine by 10 g/L, in Riesling wines by 10.2 g/L and in ice wines by 13.8 g/L in recent investigations (Erasmus et al. 2004; Gawel et al. 2007; Jones et al. 2008). Neither acidity was affected (Jones et al. 2008). For the bitterness, glycerol was reported to suppress bitter and roughness perception of a model wine (Jones et al. 2008; Noble 1996). Notice that, the perception of individual flavor is highly depend on the overall beverage flavor matrix. In addition, the changed sensations may also due to the increased viscosity. For that reduced perception on astringency and flavor intensity were reported in the high viscous solution although no measurable changes were provided by the analytical results (Hollowood et al. 2002; Peleg et al. 1999).
Glycerol may have an obscure and indirect role on the aroma volatility. Results from both sensory and analytical investigations denied the detectable effect of glycerol on the perception of overall aroma intensity of wines (Gawel et al. 2007; Jones et al. 2008; Lubbers et al. 2001). However, at lower level of volatiles, ethanol seemed have enhanced the overall aroma in the presence of glycerol while suppressed it in its absence (Jones et al. 2008). Additionally, the volatility of two fruity aroma (3-methyl butyl acetate and ethyl hexanoate) were positively influenced by glycerol concentration ranging from 0 to15 g/L although no overall aroma impacts have been claimed for the wine (Lubbers et al. 2001). The above observations highlight the flavor-binding effect of glycerol (Jones et al. 2008; Lubbers et al. 2001; Nawar 1971). 50 % glycerol was reported to change headspace concentration of the volatiles such as 2-heptanone and 2-pentanone (Nawar 1971). The esters concentrations in distillate were also affected by the amounts of glycerol in the mash (Omori et al. 1995; Omori et al. 1994). The isoamyl acetate concentration in the barley shochu was highest at 10 g/l of glycerol, while that of β-phenylethyl acetate increased gradually when increased glycerol from 6 to 14 g/L in the mash (Omori et al. 1995). The concentration of octenol, 2-ethylhexanol, β-phenylethyl alcohol and some fatty acid ethyl esters were influenced by the glycerol concentration in the mash as well (Omori et al. 1995; Omori et al. 1994; Omori et al. 1996).
Glycerol was found to increase the worty off-flavor retention for the non-alcoholic beer (Perpete and Collin 1999; Perpete and Collin 2000). 3-methylbutanal and 2-methylbutanal were described as the predominant compounds responsible for the worty taste in alcohol-free beer (Perpete and Collin 1999; Sohrabvandi et al. 2010). In regular beers, ethanol restored the thresholds of higher aldehyde flavors. However, both the absence of ethanol and the higher residual sugar could strengthen worty off-flavor in the low−/non-alcoholic beers. Glycerol seemed to have an ethanol-mimic effect which was able to increase the aldehydes retention as low as the level of 0.5 % and 4.5 % of glycerol retained up to 40 % of the investigated aldehydes (Perpete and Collin 2000).
So far, the flavor impacts of glycerol, especially the optimal ranges of such impacts, still remain as a topic for debate. By varying glycerol and ethanol concentrations in 20 wines, the highest desirability were obtained at 6.19 g/L (Kaur et al. 2013). The preference shows that glycerol in certain concentration may conduct an optimum sensory impacts for the fermented beverages. This range is highly depending on the beverage type, on the production methods and most of all on the overall flavor matrix. Further sensory investigations should be conducted to clarify the optimal concentration range of glycerol in specific fermented beverages, especially in beers which are comparably lacking of attention. Moreover, the sensory research in the low−/non- alcoholic beverages tends to be more attractive considering the body and flavor contributions of glycerol.
Increasing glycerol by fermentation optimization
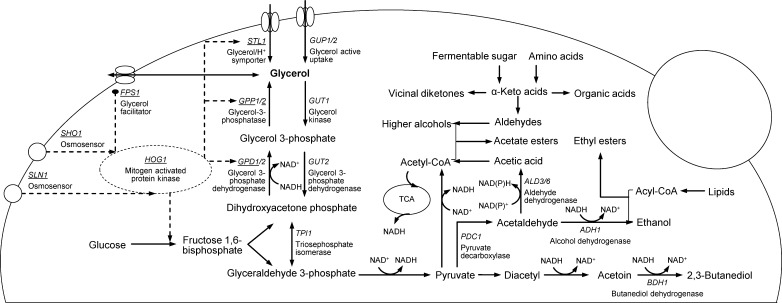
Glycerol is the third largest fermentation product after ethanol and carbon dioxide. As illustrated in Fig. 1, the phosphorylated glucose is converted into dihydroxyacetone phosphate (DHAP) and glyceroladehyde-3-phosphate (GAP) in equimolar amounts. However, most of the DHAP is isomerized into GAP which is further converted towards ethanol. On the other side, the rest of DHAP is transformed into glycerol via two steps of enzymatic reactions. The first step transforms the DHAP into glycerol-3-phosphate (G3P) with NADH as a reaction cofactor. This step was reported as the rate limiting reaction for glycerol synthesis. The second step is to phosphate G3P into glycerol (Albertyn et al. 1994; Ansell et al. 1997; Cordier et al. 2007). Two pairs of isogenes are involved in glycerol synthesis: GPD1/GPD2 encoding GPDH and GPP1/GPP2 encoding the paralogs of glycerol-3-phosphatase. The isogenes work in a cross combination manner and serve distinct physiological roles. The GPD1 follows by GPP2 are essential for the growth under osmotic stress conditions while their expressions are regulated by high osmolarity glycerol (HOG) response pathway, whereas the GPD2 and GPP1 are primarily functioned in maintaining the redox balance under anaerobic conditions (Albertyn et al. 1994; Ansell et al. 1997; Påhlman et al. 2001; Remize et al. 1999).
Fig. 1.
Glycerol biosynthesis in respond to osmotic stress and redox statue and the interrelationship with yeast-derived aroma compounds in S. cerevisiae NADH: reduced form of nicotinamide adenine dinucleotide; TCA: tricarboxylic acid cycle; Acety-CoA: acetyl-coenzyme A; Acyl-CoA: acyl-coenzyme A; Underline genes: the expressions regulated by osmotic stress; Solid line: metabolic reaction(s); Dash line: schematic osmoregulatory signal transduction cascade (HOG signaling pathway); Many reactions, isogenes, ATP, Pi, CO2 and CoA are omitted in the figure for clarity. The organoleptic impression of the aroma-active metabolites in associate with glycerol sensory contributions and yield optimizations (Briggs et al. 2004): vicinal diketones: diacetyl (buttery), acetoin (musty), 2,3-butanediol (slightly bitter, relative neutral); Organic acids: acetic, malic, lactic and citric acids (sour), succinic acid (bitter-salty); Aldehydes: acetaldehyde (grassy), 3-methylbutanal (malty, chocolate-like), 2-methylbutanal (malty, cheesy); Higher alcohols: octenol (earthy, mushroom-like), 2-ethylhexanol (mushroom-like, sweet fruity), β-phenylethyl alcohol (rosy, honey-like); Acetate esters: ethyl acetate (solvent-like), isoamyl acetate or 3-methyl butyl acetate (banana-like), β-phenyl ethyl acetate (rosy); Fatty acid ethyl ester: ethyl hexanoate (apple-like)
Glycerol biosynthesis is predominantly strain-dependent
Variances of industry strains in glycerol and flavor compounds production
The predominant role of yeast strains is confirmed by the results that the strains which were initially selected as high or low glycerol producers remained their ability in glycerol biosynthesis in different cultivation conditions (Du et al. 2011; Erasmus et al. 2004; Radler and Schütz 1982; Remize et al. 2000). The trancriptomic study showed that, except for the difference in intracellular glycerol level, the six wine strains showed remarkably differences in the transcription level of the genes GPD1, GPD2, STL1 (encoding a glycerol-proton symporter), FPS1 (encoding the aquaglyceroporin, a plasma membrane channel involved in the influx and efflux of glycerol) and GUP1 (encoding the plasma membrane protein involving in active glycerol uptake) (Fig. 1) ( Jiménez-Martí et al. 2011). High activity of GPDH was found in the high glycerol producers. In comparison, for the strains formed very little glycerol, GPDH was hardly detectable though exceptions existed (Radler and Schütz 1982). Furthermore, the high glycerol producing strains tended to acquire higher ability for the adaption of osmotic stress or lower ability to retain glycerol as osmolyte in the cytoplasm (Erasmus et al. 2004). Meanwhile, higher ratio of glycerol to ethanol was observed in those strains although the alcohol dehydrogenase (ADH) activity seemed not correlated with yeast ability of glycerol production (Chen and Xu 2010; Radler and Schütz 1982). In addition, unlike the engineered strains, the natural high glycerol producers did not show collectively higher concentration of acetic acid, succinic acid, acetaldehyde and acetoin (Du et al. 2011; Erasmus et al. 2004; Remize et al. 2000). The formation of the yeast derived flavor-active compounds were not related with glycerol. To some extent, the productions of higher alcohols, esters and organic acids were also strain-dependent (Chen and Xu 2010; Du et al. 2011; Erasmus et al. 2004; Estévez et al. 2004; Radler and Schütz 1982; Remize et al. 2000). Therefore, screening yeast strains seems of special importance for flavor improvement of fermented beverages by glycerol optimization.
The main difference in glycerol productivity was observed in non-proliferating growth phase of yeast whereat over 70 % glycerol was produced (Du et al. 2011; Kajiwara et al. 2000; Kukec et al. 2003; Michnick et al. 1997; Remize et al. 2000). In the exponential growth phase, yeast possessed the highest GPDH activity and the highest level of GPD1 expression but not glycerol yield (Du et al. 2011; Remize et al. 2000). In comparison, the ethanol yield, ADH activity and expression level of ADH1 all achieved peaks in the middle fermentation phase (Du et al. 2011). The significance of non-proliferating phase in glycerol production are also confirmed by the fact that sugars are mainly consumed in this stage during beverage fermentations. Besides, glycerol was suspected to be mainly synthesized during the early phase of fermentation because of the osmotic stress but released in the later stage due to cellular lysis and higher membrane permeability (Orlic et al. 2010).
Higher initial cell density is slightly in favor of glycerol formation for beer and wine yeasts depending on the strains (Gehlhoff and Piendl 1973; Radler and Schütz 1982; Verbelen et al. 2009; Yalçιn and Özbaş 2006). Ultra-high cell pitching numbers led to faster glycerol formation and higher end concentration but no obvious influences was observed under the practical inoculum range (Gehlhoff and Piendl 1973; Radler and Schütz 1982). However, maximum glycerol concentration (8.6 g/L) was also obtained at the low inoculum of the wine yeast when varying the inoculum size between volume ratio of 2.5 to 12.5 % (Yalçιn and Özbaş 2006). For glycerol optimization, the improper inoculation strongly disturbed fermentation kinetics and further led to imbalanced flavor formation (Radler and Schütz 1982; Verbelen et al. 2009; Yalçιn and Özbaş 2006). Consequently, the cell pitching rate should be optimized together with other parameters such as sugar, nitrogen and oxygen in the processing of fermented beverages.
Temperature shocks stimulate excessive glycerol production
Cold and heat shocks triggered glycerol accumulation by activating the HOG pathway via different branches of osmosensors as shown in Fig. 1. The down shift of temperature into 0–12 °C for 4–24 h caused the accumulation of intracellular glycerol, trehalose and heat shock proteins to protect the cells from freeze injury (Panadero et al. 2006). The cold shock has been investigated with great interest for baker’s yeasts in coping with the frozen dough technology. However, rare attention has been put in the beverage fermentation process concerning the risk of stuck and sluggish fermentation. The pre-adaptation of the active dry wine yeast strains at low temperature (13 °C) was proved to increase glycerol formation together with improved fermentation performance in terms of shortening fermentation period, effective uptake of ammonium and clear reduction of acetaldehyde, acetic acid and fusel alcohols but again the benefits were depend on strains (Csutorás et al. 2014; Llaurado et al. 2005). Moreover, some strains, which hybridized from S. cerevisiae and the cryotolerant species S. eubayanus, S. uvarum as well as S. kudriavzevii, adapt effectively to the cold temperature in beer, wine and cider fermentations. Those strains may have better chance to overproduce glycerol in respond to the cold shock ( Arroyo-López et al. 2010; Gibson et al. 2013).
The heat shocks have been applied to beverage fermentation and up to two folds more glycerol has been produced by the brewing, wine, sake, shochu and whisky yeasts (Berlot and Berovič 2011; Berovič et al. 2007; Kajiwara et al. 2000; Omori et al. 1996; Omori et al. 1997). The heat shocks often perform during cell pre-culture process at the temperature of 30–50 °C for 20–120 min one or multiple times. In addition to the hyperosmotic stress induced glycerol accumulation, the heat shock also initiated the changes in redox status where glycerol over-synthesis was responded to resume the redox homeostasis (Berlot and Berovič 2011; Kukec et al. 2003). Both glycerol production and GPDH activity were strongly but transiently induced by heat shock treatment. The prolonged time of intensive transcription of GPD1 was responsible for the excessive glycerol production of shochu yeast (Kajiwara et al. 2000). Moreover, the heat exposure tended to create the short memory effect in the yeast and it was used to enhance glycerol production by repeating the moderate heat shocks (Berlot and Berovič 2011; Berovič et al. 2007; Kukec et al. 2003).
Except for the impact on glycerol biosynthesis, heat shock treatment caused a transient delay in growth but followed by more biomass production and even complete sugar consumption after cell recovery (Berlot and Berovič 2011; Berovič et al. 2007; Kajiwara et al. 2000; Petropoulos et al. 2010). No consistent changes on ethanol and acceptable level of variations on organic acids were found after heat shock treatments (Berlot and Berovič 2011; Berovič et al. 2007; Omori et al. 1996; Petropoulos et al. 2010). While the yields of isoamyl acetate and the fatty acid ethyl esters were significant increase in the heat shock mutants overproducing glycerol (Omori et al. 1997). Hence, heat shock was claimed as an effective treatment for increasing glycerol without negative impacts on beverage flavors (Berlot and Berovič 2011; Omori et al. 1996). Further improvement can be made by carefully choosing the proper strains since the heat shock response is also strongly strain-dependent (Omori et al. 1996; Petropoulos et al. 2010). The heat shock temperature, duration and interval should also be optimized.
Environmental parameters regulate glycerol yield
High temperature is in favor of glycerol synthesis
High fermentation temperature is in favor of glycerol synthesis of S. cerevisiae ( Arroyo-López et al. 2010; Scanes et al. 1998; Yalçιn and Özbaş 2008). 1.2–1.7 folds increase of glycerol were observed by optimizing fermentation temperatures for various beverage yeasts (Chen and Xu 2010; Du et al. 2011; Yalçιn and Özbaş 2008). The enhanced level of GPD1 expression and GPDH activity at higher temperature was responsible for such increases ( Arroyo-López et al. 2010; Du et al. 2011). Generally, glycerol presents at higher level in ale beer and red wine than that in lager beer and white wine. This is probably due to that the former beverages are fermented at higher temperature regardless other variables. Except for the increase in glycerol yield, higher temperature also resulted in obvious increase in fermentation rate and decrease of yeast viability. Meanwhile it imputed negative impacts for the overall flavor developments (Csutorás et al. 2014; Liu et al. 2014). Acetic acid and succinic acid were found increase with temperature (Du et al. 2011; Liu et al. 2014; Llaurado et al. 2005). Controversially, ethanol was higher at low temperature but not ADH activity and ADH1 expression (Du et al. 2011). Besides, glycerol production can also benefit from complete sugar consumption when fermented at relative low temperature. The fermentation temperature should be optimized with great care due to the impacts on either flavor development or the risk of sluggish fermentation.
Sugar concentration is positively related to glycerol yield
Within the tolerance range of osmotic stress, more glycerol were produced with the increasing of sugar concentration ( Arroyo-López et al. 2010; Erasmus et al. 2004; Jiménez-Martí et al. 2011; Orlic et al. 2010; Piddocke et al. 2009; Radler and Schütz 1982). As illustrated in Fig. 1, the HOG signaling cascade is activated when the cells sense the hyperosmotic stress environment. On one hand, the Hog1 kinase appear to stimulate the glycolytic flux and increase the transcription of the genes involved in glycerol synthesis (GPD1 and GPP2) and glycerol import (STL1); On the other hand, it may also participate in the closure the channel aquaglyceroporin (FPS1) to prevent glycerol efflux (Hohmann et al. 2007). Notice that, under wine fermentation conditions, the HOG pathway was reported to only partly control glycerol formation. Glycerol yield was mainly meditated by the GPDH activity and the expression level of GPD1 (Pigeau and Inglis 2005; Remize et al. 2003). Moreover, the channel protein Fps1p seemed play a critical role for the overall glycerol yield (Remize et al. 2001).
The sugar composition also impact glycerol yield (Myers et al. 1997; Orlic et al. 2010; Piddocke et al. 2009). Higher proportion of fermentable sugar enhanced glycerol formation (Myers et al. 1997). Glycerol was higher in the fermentation of glucose rich medium than in medium with maltose addition (Orlic et al. 2010; Piddocke et al. 2009). The reason could be that more DHAP and NADH were generated in high monosaccharide condition. The drawbacks of the high sugar fermentation lies at that it often leads to lower growth rate, longer lag phase, incomplete sugar utilization and strongly accumulations of acetaldehyde and acetate esters ( Jiménez-Martí et al. 2011; Piddocke et al. 2009; Yu et al. 2012). More than two folds increase in ethyl acetate and isoamyl acetate was accumulated and the disproportional changes of flavor-active metabolites have resulted in the over-fruity character of beer (Piddocke et al. 2009). Interestingly, yeast viability in the high sugar fermentation process was improved by the pre-adaptation in osmotic stress media ( Jiménez-Martí et al. 2011). The pre-adaptations of yeast have great potential to enhance glycerol production in the main process of beverage fermentations.
Effects of other fermentation parameters
The quantity and variety of the nitrogen source have a pronounced effect on yeast fermentation performance and flavor development in beverage fermentations. However, the nitrogen concentration seemed only have little influences on glycerol formation (Orlic et al. 2010; Remize et al. 2000). Some amino acids may be of special importance for glycerol synthesis considering that the omission of thiamine in the medium lowered one third of the overall glycerol yield (Radler and Schütz 1982). The ammonium grown culture led to double amounts of glycerol than the cultures on amino acids or the mixtures as nitrogen source. This was probably due to the surplus NADH generated in de novo synthesis of amino acids (Albers et al. 1996; Michnick et al. 1997; Remize et al. 2000; Scanes et al. 1998). Ethanol yield, on the other hand, was found higher in amino acids media but was strain-dependent (Albers et al. 1996).
For the impacts of agitation, two controversy results have been obtained on glycerol yield (Bjorkqvist et al. 1997; Gardner et al. 1993; Petropoulos et al. 2010; Radler and Schütz 1982; Remize et al. 2000). Glycerol is dominantly formed under anaerobic conditions to consume the excessive NADH during yeast alcoholic fermentation. Nevertheless, considerable amount of glycerol can be also accumulated under the osmotic stress condition. Under such condition, GPD1 and GPP2 tend to be overexpressed out of the need of osmoregulation (Albertyn et al. 1994). The difference in the transcription level of GPD1 might be in charge of the controversial impacts of agitation on glycerol yield in previous studies (Ansell and Adler 1999; Bjorkqvist et al. 1997).
The high pH-value has slightly positive effect on glycerol formation in beverage fermentation process ( Arroyo-López et al. 2010; Nykänen and Suomaleinen 1983; Scanes et al. 1998; Yalçιn and Özbaş 2008). The positive effect was probably because of the increased activity of aldehyde dehydrogenase at higher pH-value. Aldehyde dehydrogenase catalyze the oxidation of acetaldehyde into acetic acid. This step generates extra NADH which require reoxidization via glycerol synthesis in the absence of oxygen (Fig. 1) (Wang et al. 2001). However, for beverage fermentation, low pH-value is often adopted for its inhibition on the growth of spoilage microorganisms and enhancement on flavor development (Briggs et al. 2004; Jackson 2008).
Small addition of sulphur dioxide (SO2) in the medium resulted in elevated glycerol yield (Gardner et al. 1993; Nykänen and Suomaleinen 1983; Radler and Schütz 1982; Scanes et al. 1998). SO2 is a natural fermentation byproduct presented in beer and wine. It can be also added to beer (up to 20 mg/L) to protect oxidative flavor deterioration and to must (50–100 mg/L) to inhibit wild yeast and spoilage bacteria (Briggs et al. 2004; Jackson 2008). The positive effect of SO2 is based on that the bisulfites ion binds the acetaldehyde which results in over-accumulation of cytosolic NADH and further shifts the carbon flow towards glycerol (Wang et al. 2001). Disputed glycerol yield was reported when varying SO2 addition. Additionally, this method seems not applicable anymore for the trend to limit SO2 addition in beverage industry.
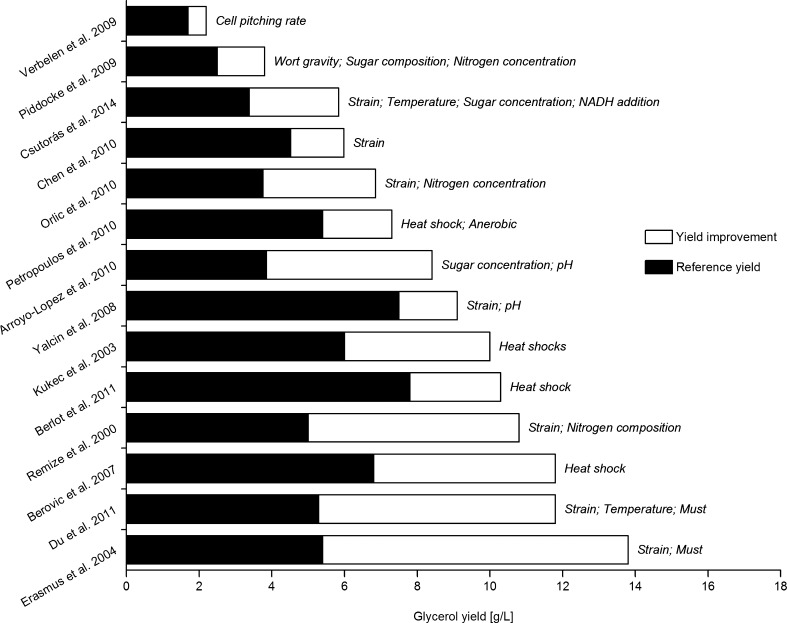
Sulfate at alkaline pH, sodium chloride (NaCl), potassium chloride (KCl) and sodium carbonate (Na2CO3) have been exploited as the selective agent (stress) for adaptive evolution to create high glycerol mutants (Kutyna et al. 2010). However, those mutants are often non-stable in addition to the acetic acid overproduction and prolonged fermentation time. On grape must, S. cerevisiae yeast produced more glycerol compare with that on the synthetic medium (Du et al. 2011; Erasmus et al. 2004; Radler and Schütz 1982). This indicates that the nutrients complexity may also influence glycerol biosynthesis in beverage fermentation (Ansell and Adler 1999). In Fig. 2, we depict the yield improvement of glycerol by various optimizations in the process of beverage fermentation. To summarize, yeast glycerol production is influenced by many growth, process and environmental factors but primarily it is strain-dependent. By the carefully choosing the yeast strains and further optimizing the fermentation conditions, beverage flavor could be improved by elevating glycerol yield.
Fig. 2.
Glycerol yield improvement by the optimizations of various parameters during beverage fermentations The results were reported under beer (Piddocke et al. 2009; Verbelen et al. 2009), wine ( Arroyo-López et al. 2010; Berlot and Berovič 2011; Berovič et al. 2007; Csutorás et al. 2014; Du et al. 2011; Erasmus et al. 2004; Kukec et al. 2003; Orlic et al. 2010; Petropoulos et al. 2010; Remize et al. 2000; Yalçιn and Özbaş 2008) and rice wine (Chen and Xu 2010) fermentation conditions. Some of the results are estimated from the published figures if not provided directly
Overproducing glycerol by genetically modified yeast
Promote glycerol biosynthesis by overexpressing GPD and GPP genes
The two homologous genes GPD1 and GPD2 are crucial for glycerol synthesis. The single deletions of GPD1 or GPD2 only slightly reduced glycerol formation. While the gpd1Δ and gpd2Δ double mutant did not produce detectable glycerol and was highly osmosensitive meanwhile failed to grow under anoxic conditions (Ansell et al. 1997; Bjorkqvist et al. 1997). In comparison, mutants lacking of GPP1 and GPP2 genes were devoid of glycerol-3-phosphatase activity but still produced a small amount of glycerol. Moreover, the two GPP genes substituted well with each other for that the single mutant remained unaffected by the high osmolality stress (Påhlman et al. 2001).
Glycerol overproduction has been realized by the adoption of yeasts overexpressing GPD and GPP genes for beverage fermentations (Cambon et al. 2006; Eglinton et al. 2002; Lopes et al. 2000; Nevoigt et al. 2002; Remize et al. 2003; Remize et al. 1999; Varela et al. 2012). Depending on the strains and fermentation environments, the GPD1 overexpression has yielded up to 6 folds more glycerol compared with 2.6 times increase of the GPD2 overexpression (Eglinton et al. 2002; Varela et al. 2012). The single overexpression of GPP genes did not significant changed glycerol yield whereat only 1.2 times increase was observed (Påhlman et al. 2001). Mimic the beer fermentation conditions, the GPD1 overexpressing lager yeast yielded 8.73 g/L glycerol compared with 1.57 g/L of the reference strain (Nevoigt et al. 2002). For wine fermentation, the GPD1 mutant produced 34.3 g/L glycerol that was 6 folds more compared with that of the wild type (Varela et al. 2012). To a less extent, 1.5 to 3.8 folds increase in glycerol yield has been lifted up by the overexpression of GPD1 in various commercial strains (Table 1) (Cambon et al. 2006; Remize et al. 1999). In comparison, the lab strains (such as V5, W303-1A and OL1) overexpressing GPD1 seemed to respond stronger in glycerol synthesis even some of them were unable to complete the sugar consumption (Cambon et al. 2006; Cordier et al. 2007; Lopes et al. 2000; Nevoigt and Stahl 1996; Remize et al. 1999). The overexpression of GPD2, GPP1 and GPP2 are less predominant for glycerol overproduction but have similar side effects under beverage fermentation conditions (Påhlman et al. 2001; Remize et al. 2001). The engineered strains produced lower biomass and decreased ethanol formation (Nevoigt et al. 2002). Minor changes in higher alcohols, esters and fatty acids were reported. However, strongly accumulation of acetate, succinate, acetaldehyde, acetoin and 2,3-butanediol were observed but also strain-dependent (Table 1) (Cambon et al. 2006; Cordier et al. 2007; Nevoigt et al. 2002; Nevoigt and Stahl 1996; Remize et al. 1999). The effects for other organic acids such as malic, citric, tartaric and lactic acids were not obvious (Lopes et al. 2000; Remize et al. 2001). The “engineered beer” was described as sherry-like due to the imbalance flavor development. Majority of the sensory panel members were able to distinguish the “engineered wine” but did not fond of it (Lopes et al. 2000; Nevoigt et al. 2002).
Table 1.
Glycerol and byproducts yield changes of the engineered yeast strains adopted in beverage fermentations a
| Genetic modification | Fermentation | Glycerol [g/L] |
Ethanol [g/L] |
Acetate [g/L] |
Succinate [g/L] |
Acetaldehyde [g/L] |
Acetoin [g/L] |
2,3-Butanediol [g/L] |
Ref. |
|---|---|---|---|---|---|---|---|---|---|
| GPD1 | Beer (Lager strain) |
8.7 (1.6) |
30.4 (37.0) |
0.45 (0.03) | 0.27 (0.06) |
0.23 (0.01) |
1.30 (0.003) |
0.21 (0.16) |
(Nevoigt et al. 2002) |
| GPD1 | Wine (Ind. strains) |
18.4 ± 6.6 (7.5 ± 1.3) |
85.9 ± 4.8 (96.5 ± 3.1) |
1.5 ± 1.0 (0.4 ± 0.2) |
0.9 ± 0.3 (0.5 ± 0.1) |
0.08 ± 0.06 (0.02 ± 0.01) |
1.48 ± 2.12 (0) | 2.80 ± 1.28 (0.53 ± 0.46) |
(Cambon et al. 2006; Remize et al. 1999; Varela et al. 2012) |
| GPD1 | Wine (Lab. strains) |
27.3 ± 2.0 (8.3 ± 2.4) |
72.9 ± 9.4 (88.3 ± 6.5) |
2.1 ± 0.7 (1.1 ± 0.5) |
0.4 ± 0.1 (0.3 ± 0.1) |
0.22 (<0·1) |
5.31 ± 1.03 (0.03 ± 0.06) |
2.60 ± 1.50 (0.76 ± 0.34) |
(Michnick et al. 1997; Remize et al. 2001; Remize et al. 1999) |
| GPD1 ald6Δ | Wine | 32.5 ± 7.1 (7.5 ± 1.4) |
89.9 ± 12.4 (110.7 ± 14.1) |
0.5 ± 0.2 (0.3 ± 0.2) |
2.7 ± 2.8 (2.7 ± 3.1) |
0.23 ± 0.07 (0.03 ± 0.01) |
4.65 ± 3.08 (0.02 ± 0.01) |
2.27 ± 2.48 (0.52 ± 0.59) |
(Cambon et al. 2006; Ehsani et al. 2009; Varela et al. 2012) |
| GPD1 ald6Δ BDH1 | Wine | 32.2 ± 1.7 (7.9 ± 1.0) |
101.9 ± 5.1 (121.2 ± 3.6) |
0.5 ± 0.2 (0.4 ± 0.2) |
2.0 ± 1.2 (1.8 ± 1.6) |
0.14 ± 0.03 (0.02 ± 0.01) |
0.33 ± 0.26 (0.01 ± 0.01) |
3.39 ± 4.35 (0.46 ± 0.51) |
(Ehsani et al. 2009; Varela et al. 2012) |
aData are expressed as average value ± standard deviation for the same genetic modifications in different investigations (Ref.) on either industry (Ind.) or laboratory (Lab.) strains; the data in round brackets are calculated from the yields of the wide types (no genetic modifications) while the data outside the brackets are the yields of the mutants: GPD1, BDH1 overexpression and ald6Δ deletion; Fermentations were held on either on wort (11.38 °P) or on grape must (synthetic must); Sugar concentrations of the must were 200, 240 or 246 g/L to simulate wine fermentations; Some of the results are evaluated from the published figures if not provided directly
To combat the over accumulation of acetic acid and 2,3-butanediol, ALD6 encoding aldehyde dehydrogenase and BDH1 encoding the 2,3-butanediol dehydrogenase (which catalyze the conversion of rancid acetoin to the innocuous 2,3-butanediol) were modified in the GPD1/2 overexpression mutants (Cambon et al. 2006; Cordier et al. 2007; Eglinton et al. 2002; Ehsani et al. 2009; Varela et al. 2012). The multiple engineered strains performed similarly in glycerol and ethanol formation compared with GPD1 mutants (Table 1). The GPD1 ald6Δ mutants showed lower level of acetate, but the increase of acetaldehyde, diacetyl and acetoin yield were even stronger. The accumulations of acetoin and diacetyl were further decreased in the GPD1 ald6Δ BDH1 mutant (Cambon et al. 2006; Eglinton et al. 2002; Ehsani et al. 2009; Varela et al. 2012).
Direct carbon flux towards glycerol by other genetic modifications
The indirect genetic modifications have been also performed to increase glycerol production by deletion of TPI1, which encodes triose phosphate isomerase, deletion of PDC2, regulatory gene involved in the up-regulation of transcription factor of PDC1/5 encoding pyruvate decarboxylase and deletion of ADH1 in S. cerevisiae yeasts (Cordier et al. 2007; Nevoigt and Stahl 1996; Varela et al. 2012). Other genetic modification strategies such as increasing the cytosolic NADH proportion by the deletion of GUT2 and NDE1/2 (involving in mitochondrial oxidation of cytosolic NADH) and “opening” the glycerol efflux facilitator by truncation of Fps1p were also applied to promote glycerol overproduction (Cordier et al. 2007; Overkamp et al. 2000; Remize et al. 2001; Varela et al. 2012). Although these modifications resulted in dramatic increase in glycerol yield even up to 18 times more in some synthetic medium. The mutants showed significantly impaired growth ability and accumulated large amounts of pyruvate, acetate and acetaldehyde because of the diverted carbon flow (Cordier et al. 2007; Nevoigt and Stahl 1996; Varela et al. 2012). The yields of esters and higher alcohols were slightly decreased or remained unchanged. Even no negative sensory effects was claimed for wine quality, the prolonged fermentation time and poor social acceptance still hinder the application of those genetically modified yeasts in beverage industry (Cambon et al. 2006; Eglinton et al. 2002; Ehsani et al. 2009; Varela et al. 2012).
Conclusion
Glycerol yield has been optimized intensively for improving beverage flavor and ethanol reduction by the strategies such as screening of yeast strains, optimization of fermentation parameters and yeast genetic modifications. However, in both the sensory research and fermentation optimization of glycerol, the following aspects deserve more attention. Firstly, obvious gap exists between the actual glycerol level and the sensory beneficial concentration. Whereas, the beneficial concentration (range) remains unclear for beer as well as other fermented beverage. Additionally, glycerol was suspected to contribute better for the non- and low-alcoholic beverage considering the reported organoleptic impacts and it needs further confirmation. Secondly, yeast glycerol production is primarily strain-dependent and yeast strains have been screened for their glycerol productivity. Nevertheless, the screening was restricted within dozens of strains which seemed inadequate to get favorable yeast for flavor improvement. Besides, fermentation parameters have been optimized to improve glycerol yield. However, the variables and effects have been only partly considered. In the process of fermented beverages, the change of single parameter would inevitably affect the whole fermentation kinetics and further impact on glycerol yield and the overall flavor developments. Therefore, comprehensive optimization with the multiple inputs (strains, environmental and process parameters) and multiple outputs (glycerol, important flavor compounds and beverage sensory impression) should be investigated in the upcoming research. To facilitate the screening of large number of strains and optimization of multiple variables, we propose a platform constructed by the high throughput screening (HTS) technology in combination with the design of experiments (DoE) methods. The cell-based HTS often adopt the microtiter plates to miniaturize the fermentation and has been widely applied in the screening of mutants and secondary metabolites in recent years. The application of DoE methods could further enhance the effectiveness for glycerol optimization in the processing of fermented beverages.
Acknowledgments
The first author is funded by China Scholarship Council.
Footnotes
Highlights: • Glycerol and major beverage flavor interactions
• Impacts of glycerol optimizations on the genes transcription level
• Changes of aroma compounds formation during glycerol yield optimizations.
• Glycerol yield improvements in beverage fermentations
References
- Albers E, Larsson C, Lidén G, Niklasson C, Gustafsson L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol. 1996;62:3187–3195. doi: 10.1128/aem.62.9.3187-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertyn J, Hohmann S, Thevelein JM, Prior BA. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol. 1994;14:4135–4144. doi: 10.1128/MCB.14.6.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell R, Adler L. The effect of iron limitation on glycerol production and expression of the isogenes for NAD+ − dependent glycerol 3-phosphate dehydrogenase in Saccharomyces cerevisiae. FEBS Lett. 1999;461:173–177. doi: 10.1016/S0014-5793(99)01456-8. [DOI] [PubMed] [Google Scholar]
- Ansell R, Granath K, Hohmann S, Thevelein JM, Adler L. The two isoenzymes for yeast NAD+ − dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J. 1997;16:2179–2187. doi: 10.1093/emboj/16.9.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-López FN, Pérez-Torrado R, Querol A, Barrio E. Modulation of the glycerol and ethanol syntheses in the yeast Saccharomyces kudriavzevii differs from that exhibited by Saccharomyces cerevisiae and their hybrid. Food Microbiol. 2010;27:628–637. doi: 10.1016/j.fm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Berlot M, Berovič M. Saccharomyces cerevisiae inoculum heat shock treatment - new method for enhanced glycerol production in wine. Chem Biochem Eng Q. 2011;25:241–245. [Google Scholar]
- Berovič M, Pivec A, Košmerl T, Wondra M, Čelan Š. Influence of heat shock on glycerol production in alcohol fermentation. J Biosci Bioeng. 2007;103:135–139. doi: 10.1263/jbb.103.135. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist S, Ansell R, Adler L, Liden G. Physiological response to anaerobicity of glycerol-3-phosphate dehydrogenase mutants of Saccharomyces cerevisiae. Appl Environ Microbiol. 1997;63:128–132. doi: 10.1128/aem.63.1.128-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing science and practice. Abington Hall, Abington: Woodhead Publishing Limited; 2004. [Google Scholar]
- Cambon B, Monteil V, Remize F, Camarasa C, Dequin S. Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl Environ Microbiol. 2006;72:4688–4694. doi: 10.1128/AEM.02975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xu Y. The influence of yeast strains on the volatile flavour compounds of Chinese rice wine. J I Brewing. 2010;116:190–196. doi: 10.1002/j.2050-0416.2010.tb00417.x. [DOI] [Google Scholar]
- Cordier H, Mendes F, Vasconcelos I, Francois JM. A metabolic and genomic study of engineered Saccharomyces cerevisiae strains for high glycerol production. Metab Eng. 2007;9:364–378. doi: 10.1016/j.ymben.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Csutorás C, Hudák O, Rácz K, Rácz L. Technological experiments for the enhancement of glycerol content in high quality wines. J Agric Chem Environ. 2014;03:48–52. [Google Scholar]
- del Campo G, Santos JI, Berregi I, Velasco S, Ibarburu I, Dueñas MT, Irastorza A. Ciders produced by two types of presses and fermented in stainless steel and wooden vats. J I Brewing. 2003;109:342–348. doi: 10.1002/j.2050-0416.2003.tb00608.x. [DOI] [Google Scholar]
- Du G, Zhan JC, Li JY, You YL, Zhao Y, Huang WD. Effect of fermentation temperature and culture medium on glycerol and ethanol during wine fermentation. Am J Enol Vitic. 2011;63:132–138. doi: 10.5344/ajev.2011.11067. [DOI] [Google Scholar]
- Eglinton JM, Heinrich AJ, Pollnitz AP, Langridge P, Henschke PA, de Barros Lopes M. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast. 2002;19:295–301. doi: 10.1002/yea.834. [DOI] [PubMed] [Google Scholar]
- Ehsani M, Fernández MR, Biosca JA, Julien A, Dequin S. Engineering of 2,3-butanediol dehydrogenase to reduce acetoin formation by glycerol-overproducing, low-alcohol Saccharomyces cerevisiae. Appl Environ Microbiol. 2009;75:3196–3205. doi: 10.1128/AEM.02157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus DJ, Cliff M, van Vuuren HJJ. Impact of yeast strain on the production of acetic acid, glycerol, and the sensory attributes of icewine. Am J Enol Vitic. 2004;55:371–378. [Google Scholar]
- Estévez P, Gil ML, Falqué E. Effects of seven yeast strains on the volatile composition of Palomino wines. Int J Food Sci Technol. 2004;39:61–69. doi: 10.1046/j.0950-5423.2003.00755.x. [DOI] [Google Scholar]
- Gardner N, Rodrigue N, Champagne CP. Combined effects of sulfites, temperature, and agitation time on production of glycerol in grape juice by Saccharomyces cerevisiae. Appl Environ Microbiol. 1993;59:2022–2028. doi: 10.1128/aem.59.7.2022-2028.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawel R, Sluyter SV, Waters EJ. The effects of ethanol and glycerol on the body and other sensory characteristics of Riesling wines. Aust J Grape Wine R. 2007;13:38–45. doi: 10.1111/j.1755-0238.2007.tb00070.x. [DOI] [Google Scholar]
- Gehlhoff R, Piendl A. Glycerin im Bier. Teil II: Beeinflussung durch technologische Maßnahmen. Monatsschr Brauwiss. 1973;26:183–188. [Google Scholar]
- Gibson BR, Storgards E, Krogerus K, Vidgren V. Comparative physiology and fermentation performance of Saaz and Frohberg lager yeast strains and the parental species Saccharomyces eubayanus. Yeast. 2013;30:255–266. doi: 10.1002/yea.2960. [DOI] [PubMed] [Google Scholar]
- Hohmann S, Krantz M, Nordlander B. Yeast osmoregulation. Methods in enzymology. 2007;428:29–45. doi: 10.1016/S0076-6879(07)28002-4. [DOI] [PubMed] [Google Scholar]
- Hollowood TA, Linforth RST, Taylor AJ. The effect of viscosity on the perception of flavour. Chem Senses. 2002;27:583–591. doi: 10.1093/chemse/27.7.583. [DOI] [PubMed] [Google Scholar]
- Jackson RS. Fermentation. In: Jackson RS, editor. Wine science. 3rd. San Diego: Academic Press; 2008. pp. 332–417. [Google Scholar]
- Jiménez-Martí E, Gomar-Alba M, Palacios A, Ortiz-Julien A, del Olmo M-l (2011) Towards an understanding of the adaptation of wine yeasts to must: relevance of the osmotic stress response. Appl Microbiol Biotechnol 89:1551–1561. doi:10.1007/s00253-010-2909-4 [DOI] [PubMed]
- Jones PR, Gawel R, Francis IL, Waters EJ. The influence of interactions between major white wine components on the aroma, flavour and texture of model white wine. Food Qual Prefer. 2008;19:596–607. doi: 10.1016/j.foodqual.2008.03.005. [DOI] [Google Scholar]
- Kajiwara Y, Ogawa K, Takashita H, Omori T. Enhanced glycerol production in Shochu yeast by heat-shock treatment is due to prolonged transcription of GPD1. J Biosci Bioeng. 2000;90:121–123. doi: 10.1016/S1389-1723(00)80046-8. [DOI] [PubMed] [Google Scholar]
- Kaur M, Sharma HK, Patil S, Shitandi A. Optimization of ethanol concentration, glycerol concentration and temperature conditions of grape-mahua wine to maximize the quality and overall acdeptability. JMBFS. 2013;2:2426–2430. [Google Scholar]
- Kukec A, Berovic M, Wondra M, Celan S, Kosmerl T. Influence of temperature shock on the glycerol production in cv. Sauvignon blanc fermentation Vitis. 2003;42:205–206. [Google Scholar]
- Kutyna DR, Varela C, Henschke PA, Chambers PJ, Stanley GA. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci Technol. 2010;21:293–302. doi: 10.1016/j.tifs.2010.03.004. [DOI] [Google Scholar]
- Langstaff SA, Guinard JX, Lewis MJ. Instrumental evaluation of the mouthfeel of beer and correlation with sensory evaluation. J I Brewing. 1991;97:427–433. doi: 10.1002/j.2050-0416.1991.tb01081.x. [DOI] [Google Scholar]
- Liu D, et al. Effect of temperature on Chinese rice wine brewing with high concentration presteamed whole sticky rice. Biomed Res Int. 2014;2014:8. doi: 10.1155/2014/426929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaurado JM, Rozes N, Constanti M, Mas A. Study of some Saccharomyces cerevisiae strains for winemaking after preadaptation at low temperatures. J Agric Food Chem. 2005;53:1003–1011. doi: 10.1021/jf049324n. [DOI] [PubMed] [Google Scholar]
- Lopes MdB, ATA ur-Rehman, Gockowiak H, Heinrich AJ, Langridge P, Henschke PA. Fermentation properties of a wine yeast over-expressing the Saccharomyces cerevisiae glycerol 3-phosphate dehydrogenase gene (GPD2) Aust J Grape Wine R. 2000;6:208–215. doi: 10.1111/j.1755-0238.2000.tb00181.x. [DOI] [Google Scholar]
- Lubbers S, Verret C, Voilley A. The effect of glycerol on the perceived aroma of a model wine and a white wine. Lebensm Wiss Technol. 2001;34:262–265. doi: 10.1006/fstl.2001.0766. [DOI] [Google Scholar]
- Michnick S, Roustan J-L, Remize F, Barre P, Dequin S. Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomyces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast. 1997;13:783–793. doi: 10.1002/(SICI)1097-0061(199707)13:9<783::AID-YEA128>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Myers DK, Lawlor DTM, Attfield PV. Influence of invertase activity and glycerol synthesis and retention on fermentation of media with a high sugar concentration by Saccharomyces cerevisiae. Appl Environ Microbiol. 1997;63:145–150. doi: 10.1128/aem.63.1.145-150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawar W. Variables affecting composition of headspace aroma. J Agric Food Chem. 1971;19:1057–1059. doi: 10.1021/jf60178a003. [DOI] [Google Scholar]
- Nevoigt E, Stahl U. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD+] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast. 1996;12:1331–1337. doi: 10.1002/(SICI)1097-0061(199610)12:13<1331::AID-YEA28>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Nevoigt E et al. (2002) Genetic engineering of brewing yeast to reduce the content of ethanol in beer. FEMS Yeast Res 2:225–232. doi:S1567-1356(02)00076-4 [DOI] [PubMed]
- Nieuwoudt HH, Prior BA, Pretorius IS, Bauer FF. Glycerol in South African table wines: an assessment of its relationship to wine quality. S Afr J Enol Vitic. 2002;23:22–30. [Google Scholar]
- Noble AC. Taste–aroma interactions. Trends Food Sci Technol. 1996;7:439–444. doi: 10.1016/S0924-2244(96)10044-3. [DOI] [Google Scholar]
- Noble AC, Bursick GF. The contribution of glycerol to perceived viscosity and sweetness in white wine. Am J Enol Vitic. 1984;35:110–112. [Google Scholar]
- Nurgel C, Pickering G. Contribution of glycerol, ethanol and sugar to the perception of viscosity and density elicited by model white wines. J Texture Stud. 2005;36:303–323. doi: 10.1111/j.1745-4603.2005.00018.x. [DOI] [Google Scholar]
- Nykänen L, Suomaleinen H. Aroma of beer, wine and distilled alcoholic beverages. Berlin: Akademie-Verlag; 1983. [Google Scholar]
- Omori T, Iwata T, Umemoto Y, Tsujimoto S, Shimoda M, Hatate Y. Effects of glycerol in mash on flavor components of barley Shochu. J Brew Soc Jpn. 1994;89:726–731. doi: 10.6013/jbrewsocjapan1988.89.726. [DOI] [Google Scholar]
- Omori T, Iwata T, Umemoto Y, Shimoda M. Improvement of barley shochu flavor by controlling the glycerol concentration in the mash. J Ferment Bioeng. 1995 [Google Scholar]
- Omori T, Ogawa K, Umemoto Y, Yuki K, Kajihara Y, Shimoda M, Wada H. Enhancement of glycerol production by brewing yeast (Saccharomyces cerevisiae) with heat shock treatment. J Ferment Bioeng. 1996;82:187–190. doi: 10.1016/0922-338X(96)85048-3. [DOI] [Google Scholar]
- Omori T, Umemoto Y, Ogawa K, Kajiwara Y, Shimoda M, Wada H. A novel method for screening high glycerol- and ester-producing brewing yeasts (Saccharomyces cerevisiae) by heat shock treatment. J Ferment Bioeng. 1997;83:64–69. doi: 10.1016/S0922-338X(97)87329-1. [DOI] [Google Scholar]
- Orlic S, Arroyo-Lopez FN, Huic-Babic K, Lucilla I, Querol A, Barrio E. A comparative study of the wine fermentation performance of Saccharomyces paradoxus under different nitrogen concentrations and glucose/fructose ratios. J Appl Microbiol. 2010;108:73–80. doi: 10.1111/j.1365-2672.2009.04406.x. [DOI] [PubMed] [Google Scholar]
- Overkamp KM, Bakker BM, Kotter P, van Tuijl A, de Vries S, van Dijken JP, Pronk JT. In vivo analysis of the mechanisms for oxidation of cytosolic NADH by Saccharomyces cerevisiae mitochondria. J Bacteriol. 2000;182:2823–2830. doi: 10.1128/JB.182.10.2823-2830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Påhlman AK, Granath K, Ansell R, Hohmann S, Adler L. The yeast glycerol 3-phosphatases gpp1p and gpp2p are required for glycerol biosynthesis and differentially involved in the cellular responses to osmotic, anaerobic, and oxidative stress. J Biol Chem. 2001;276:3555–3563. doi: 10.1074/jbc.M007164200. [DOI] [PubMed] [Google Scholar]
- Panadero J, Pallotti C, Rodriguez-Vargas S, Randez-Gil F, Prieto JA. A downshift in temperature activates the high osmolarity glycerol (HOG) pathway, which determines freeze tolerance in Saccharomyces cerevisiae. J Biol Chem. 2006;281:4638–4645. doi: 10.1074/jbc.M512736200. [DOI] [PubMed] [Google Scholar]
- Parker WE, Richardson PJ. The quantitative determination of glycerol in beer by gas–liquid chromatography. J I Brewing. 1970;76:191–198. doi: 10.1002/j.2050-0416.1970.tb03282.x. [DOI] [Google Scholar]
- Peleg H, Gacon K, Schlich P, Noble AC. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J Sci Food Agric. 1999;79:1123–1128. doi: 10.1002/(SICI)1097-0010(199906)79:8<1123::AID-JSFA336>3.0.CO;2-D. [DOI] [Google Scholar]
- Perpete P, Collin S. Contribution of 3-methylthiopropionaldehyde to the worty flavor of alcohol-free beers. J Agric Food Chem. 1999;47:2374–2378. doi: 10.1021/jf9811323. [DOI] [PubMed] [Google Scholar]
- Perpete P, Collin S. Influence of beer ethanol content on the wort flavour perception. Food Chem. 2000;71:379–385. doi: 10.1016/S0308-8146(00)00179-5. [DOI] [Google Scholar]
- Petropoulos S, Grbin P, Jiranek V. Influence of heat shock-treated cells on the production of glycerol and other metabolites in alcoholic fermentation. Int J Wine Res. 2010;2:115–122. doi: 10.2147/IJWR.S12988. [DOI] [Google Scholar]
- Piddocke M, Kreisz S, Heldt-Hansen H, Nielsen K, Olsson L. Physiological characterization of brewer’s yeast in high-gravity beer fermentations with glucose or maltose syrups as adjuncts. Appl Microbiol Biotechnol. 2009;84:453–464. doi: 10.1007/s00253-009-1930-y. [DOI] [PubMed] [Google Scholar]
- Pigeau GM, Inglis DL. Upregulation of ALD3 and GPD1 in Saccharomyces cerevisiae during Icewine fermentation. J Appl Microbiol. 2005;99:112–125. doi: 10.1111/j.1365-2672.2005.02577.x. [DOI] [PubMed] [Google Scholar]
- Radler F, Schütz H. Glycerol production of various strains of Saccharomyces. Am J Enol Vitic. 1982;33:36–40. [Google Scholar]
- Remize F, Roustan JL, Sablayrolles JM, Barre P, Dequin S. Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl Environ Microbiol. 1999;65:143–149. doi: 10.1128/aem.65.1.143-149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remize F, Sablayrolles JM, Dequin S. Re-assessment of the influence of yeast strain and environmental factors on glycerol production in wine. J Appl Microbiol. 2000;88:371–378. doi: 10.1046/j.1365-2672.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Remize F, Barnavon L, Dequin S. Glycerol export and glycerol-3-phosphate dehydrogenase, but not glycerol phosphatase, are rate limiting for glycerol production in Saccharomyces cerevisiae. Metab Eng. 2001;3:301–312. doi: 10.1006/mben.2001.0197. [DOI] [PubMed] [Google Scholar]
- Remize F, Cambon B, Barnavon L, Dequin S. Glycerol formation during wine fermentation is mainly linked to Gpd1p and is only partially controlled by the HOG pathway. Yeast. 2003;20:1243–1253. doi: 10.1002/yea.1041. [DOI] [PubMed] [Google Scholar]
- Scanes KT, Hohmann S, Prior BA. Glycerol production by the yeast Saccharomyces cerevisiae and its relevance to wine: a review. S Afr J Enol Vitic. 1998;19:17–24. [Google Scholar]
- Sohrabvandi S, Mousavi SM, Razavi SH, Mortazavian AM, Rezaei K. Alcohol-free beer: methods of production, sensorial defects, and healthful effects. Food Rev Int. 2010;26:335–352. doi: 10.1080/87559129.2010.496022. [DOI] [Google Scholar]
- Varela C, et al. Evaluation of gene modification strategies for the development of low-alcohol-wine yeasts. Appl Environ Microbiol. 2012;78:6068–6077. doi: 10.1128/AEM.01279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbelen PJ, Dekoninck TML, Saerens SMG, Mulders SE, Thevelein JM, Delvaux FR. Impact of pitching rate on yeast fermentation performance and beer flavour. Appl Microbiol Biotechnol. 2009;82:155–167. doi: 10.1007/s00253-008-1779-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhuge J, Fang H, Prior BA. Glycerol production by microbial fermentation: a review. Biotechnol Adv. 2001;19:201–223. doi: 10.1016/S0734-9750(01)00060-X. [DOI] [PubMed] [Google Scholar]
- Yalçιn SK, Özbaş ZY. Production of glycerol by two endogenic wine yeast strains at different inoculum size. Food Technol Biotechnol. 2006;44:525. [Google Scholar]
- Yalçιn SK, Özbaş ZY. Effects of PH and temperature on growth and glycerol production kinetics of two indigenous wine strains of Saccharomyces cerevisiae from Turkey. Braz J Microbiol. 2008;39:325–332. doi: 10.1590/S1517-83822008000200024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Zhao H, Li H, Zhang Q, Lei H, Zhao M. Selection of Saccharomyces pastorianus variants with improved fermentation performance under very high gravity wort conditions. Biotechnol Lett. 2012;34:365–370. doi: 10.1007/s10529-011-0780-8. [DOI] [PubMed] [Google Scholar]