Abstract
Peel is a major by-product during processing of mango fruit into pulp. Recent report indicates that the whole peel powder ameliorated diabetes. In the present study, ethanolic extract of mango peel was analysed for its bioactive compounds, evaluated for α-amylase and α-glucosidase inhibitory properties, oral glucose tolerance test, antioxidant properties, plasma insulin level and biochemical parameters related to diabetes. In addition to gallic and protocatechuic acids, the extract also had chlorogenic and ferulic acids, which were not reported earlier in mango peel extracts. The peel extract inhibited α-amylase and α-glucosidase activities, with IC50 values of 4.0 and 3.5 μg/ml. Ethanolic extract of peel showed better glucose utilization in oral glucose tolerance test. Treatment of streptozotocin-induced diabetic rats with the extract decreased fasting blood glucose, fructosamine and glycated hemoglobin levels, and increased plasma insulin level. Peel extract treatment decreased malondialdehyde level, but increased the activities of antioxidant enzymes significantly in liver and kidney compared to diabetic rats. These beneficial effects were comparable to metformin, but better than gallic acid treated diabetic rats. The beneficial effects of peel extract may be through different mechanism like increased plasma insulin levels, decreased oxidative stress and inhibition of carbohydrate hydrolyzing enzyme activities by its bioactive compounds. Thus, results suggest that the peel extract can be a potential source of nutraceutical or can be used in functional foods and this is the first report on antidiabetic properties of mango peel extract.
Keywords: Mango peel extract, α-Amylase and α-glucosidase inhibition, Antioxidants, Mangifera indica, Antidiabetic properties
Introduction
Diabetes mellitus (DM) is a metabolic disorder that affected several million people worldwide. The number of people in the world with diabetes is projected to rise to 439 million by 2030 (Chen et al. 2012). There are two types of DM, type I and type II diabetes. Type I or insulin dependent diabetes is common in children and young adults, and is characterized by body’s inability to produce enough insulin due to the destruction of β-cells in pancreas, which causes insulin deficiency. Type II or non-insulin dependent diabetes results from body’s inability to respond to the action of insulin (Sutharson et al. 2003). Increased oxidative stress reported to be a major factor for the development and progression of diabetes and its complications. Further, DM causes increased production of free radicals or impaired antioxidant defences (Baynes and Thorpe 1999; Saxena et al. 1993). The excessive production of free radicals damage cellular proteins, membrane lipids and nucleic acids, and leads to cell death (Baynes 1991; Giugliano et al. 1996). DM can be controlled by different approaches like scavenging of free radicals or improving the antioxidant enzyme activities. Another approach to control postprandial glucose levels could be by inhibition of intestinal α-glucosidase and pancreatic α-amylase (Kwon et al. 2007).
Chemical hypoglycemic agents like acarbose, metformin, sulphonylurea and meglitinides are used to control DM. However, these drugs are reported to cause side effects such as increase in appetite and weight gain, increase in occurrence of cardiovascular risk, gastrointestinal disturbances, hypersensitivity reactions and abdominal pain, diarrhoea and severe hepatotoxicity (Feinglos and Bethel 1999; Sheehan 2003; Bastaki 2005; Kelle 1995; Stumvoll et al. 1995; Bolen et al. 2007; Carrascosa et al. 1997). Therefore, there is a growing demand by diabetic patients to use natural products for the management of diabetes and its complications (Visnagri et al. 2014). Generally, plant products are considered to be less toxic with fewer side effects than synthetic compounds. WHO (2002) also recommended rational use of traditional and natural indigenous medicines for the treatment of diabetes mellitus.
Phytochemicals ameliorate imbalanced glucose homeostasis in diabetes status by different mechanisms, such as inhibition of starch hydrolysing enzyme activities, stimulation of insulin secretion from the pancreatic β-cells, modulation of glucose release from liver, activation of insulin receptors and glucose uptake in the insulin-sensitive tissues, and modulation of hepatic glucose output (Iwai et al. 2006; Iwai 2008; Cabrera et al. 2006; Jung et al. 2007). Several studies suggest that diets rich in anti-oxidants ameliorate diabetes and its complications by scavenging free radicals and also by improving the antioxidant enzyme levels (Johansen et al. 2005). Extracts of soy (Orgaard and Jensen 2008), grape (Zunino 2009), apple (Boyer and Liu 2004) and several herbs (Hui et al. 2009) were reported to control diabetes in animal models.
Mango is one of the widely consumed tropical fruits in the world. Different parts of mango tree are shown to have medicinal properties (Guevara Garcia et al. 2004). The flour of fruit pulp, the extracts of fruit kernel, leaves and stem bark were reported to exhibit various health benefits. Mango pulp flour and leaf extracts of mango produced significant hypoglycemic effect in STZ-induced diabetic animals (Sharma et al. 1997; Aderibigbe et al. 1999; Perpetuo and Salgado 2003). The aqueous extract of mango stem bark exhibited antiinflammatory and analgesic properties (Garrido et al. 2001). These extracts of mango fruit pulp also showed immunomodulatory activity (Naveed et al. 2005), antimutagenic property (Botting et al. 1999), anticancer activity (Percival et al. 2006).
During mango processing, peel is one of the major by-products, which is being wasted. Earlier studies indicated that acetone extract of peel is a good source of antioxidants such as polyphenols, carotenoids, vitamin E and C, besides dietary fibre and the extract exhibited antioxidant properties (Ajila et al. 2007; Ajila and Prasada Rao 2008). Hence, peel has potential to control diabetes and other diseases. Recently, it has been shown that whole mango peel powder ameliorates diabetes in streptozotocin-induced diabetic rats (Gondi et al. 2014) and also possesses antiproliferative properties of cancer cell lines (Kim et al. 2010). As whole peel contains a variety of compounds including proteins, carbohydrates, minerals and dietary fibre apart from bioactive compounds, we have extracted the nutraceuticals with aqueous ethanol, which is generally recognized as safe compared to acetone, and investigated the effect of ethanol extract on inhibition of α-amylase and α-glucosidase activities, and amelioration of diabetes in STZ-induced diabetic rats.
Materials and methods
Plant material
Freshly processed Badami variety mango peel was collected (single batch) from Global Industries company, mango processing industry, washed with water and the underlying pulp from the peel was removed using a knife. Cleaned peel was spread in trays and dried at 45 ± 2 °C using a cross flow drier (Model PTD-48E, Premium Industries Ltd., Ahmadabad, India).
Preparation of extract
The mango peel powder was extracted with 80 % ethanol, extract obtained was filtered through Whatman No.1 filter paper, filtrate obtained was concentrated under reduced pressure using a rotator evaporator at 40 °C, and was lyophilized. The lyophilized powder was stored at 4 °C until further use. The peel extract powder was dissolved in distilled water and used for the in vitro and in vivo studies.
Determination of bioactive compounds and antioxidant activity of the peel extract
Peel extract powder was dissolved in 80 % ethanol and subjected for the determination of various bioactive compounds and antioxidant activity. Total polyphenol content was assayed according to the method of Singleton and Rossi (1965) using gallic acid as a standard. Total carotenoid content in 80 % mango peel ethanol extract was estimated using colorimetric method reported by Lichtenthaler (1987). Anthocyanin content in 80 % mango peel ethanol extract was determined by using the method described by Wolfe et al. (2003). Flavonoid content in 80 % mango peel ethanol extract was estimated by the method of Xu and Chang (2007). Antioxidant activity of the 80 % mango peel ethanol extract was determined using DPPH radical scavenging method (Brand-Williams et al. 1995) as well as reducing power assay (Yen and Chen 1995). Free phenolic acids were identified in 80 % mango peel ethanol extract using appropriate standards using RP-HPLC (Girish et al. 2012).
α-Amylase and α-glucosidase inhibitory properties of ethanolic extract of mango peel
The α-amylase and α-glucosidase inhibitory effect of mango peel extract was determined according to the method described by Kim et al. (2000). The α-amylase inhibitory assay was performed with slight modification. α-Amylase solution (1 unit ml−1) was mixed with extract at different concentrations, incubated for 15 min and 180 μl of 1 % starch solution in 20 mM sodium phosphate buffer (pH 6.9) was added to start the reaction. The reaction was carried out at room temperature for 5 min. The reaction was terminated by the addition of 500 μl of the DNS reagent, placed in a boiling water bath for 15 min, cooled to room temperature and absorbance was measured at 540 nm using a UV-visible spectrophotometer. The α-glucosidase enzyme inhibition assay reaction mixture contained 50 μl of p-nitrophenyl-α-D-glucopyranoside (10 mg in 2 ml phosphate buffer), different concentrations of extract (inhibitor; 10 μl) and the reaction mixture was made up to 2.98 ml with phosphate buffer (pH 6.8; 50 mM). The reaction was initiated by adding 20 μl of α-glucosidase enzyme (2 mg in 1 ml of phosphate buffer; 5.7 U/mg). The reaction was monitored by increase in absorption at 405 nm. The IC50 value was defined as the concentration of inhibitor required to inhibit 50 % of the enzyme activity. For all tests, inhibition assay was performed in triplicate.
Oral glucose tolerance test (OGTT)
Oral glucose tolerance test was performed using overnight (16 h) starved normal albino Wistar rats. The rats were randomly divided into six groups (n = 6). Initially the rats were administered with mango peel extract or metformin or gallic acid at different concentration as shown below and after 30 min, all the animals were further administered with glucose [2 g/kg body weight (b.w.)] (Bonner-weir 1988). Blood was withdrawn from the tail vein at 0, 30, 60, 90,120 and 150 min. Blood glucose levels were evaluated by the GOD-POD kit. Water was used as a vehicle as all the samples were dissolved in water.
Group I: Rats treated with vehicle only
Group II: Rats treated with mango peel extract (100 mg/kg b.w.)
Group III: Rats treated with mango peel extract (150 mg/kg b.w.)
Group IV: Rats treated with mango peel extract (200 mg/kg b.w.)
Group V: Rats treated with metformin (10 mg/kg b.w.)
Group VI: Rats treated with gallic acid (10 mg/kg b.w.)
Induction of diabetes
The study had the approval of the CFTRI Animal Ethical Committee. Diabetes was induced in the overnight fasted male albino Wistar rats by a single intraperitoneal injection (i.p.) of streptozotocin (45 mg/kg body weight) dissolved in 0.1 M citrate buffer (pH 4.5). Normal rats received only citrate buffer as vehicle. After 3 days of induction of diabetes using STZ, blood samples were collected from the retro-orbital plexus of the rat eyes and plasma glucose levels were determined. The animals confirmed diabetic by the elevated plasma glucose levels (200 mg/dl) were used for the study. The rats were divided into seven groups with six rats in each group. Rats were administered with respective sample using intragastric tube, once a day for 60 days, continuously as described below (Arunachalam and Parimelazhagan 2013).
Group I: Normal control rats administered with water only–SFC
Group II: Diabetic control rats administered with water only–SFD
Group III: Tested rats administered with mango peel extract, 100 mg/kg b,w.–SFDM-100
Group IV: Tested rats administered with mango peel extract, 150 mg/kg b.w.–SFDM-150
Group V: Tested rats administered with mango peel extract, 200 mg/kg b.w.–SFDM-200
Group VI: Tested rats administered with metformin, 10 mg/kg b.w.–SFDMet-10
Group VII: Tested rats administered with gallic acid, 10 mg/kg b.w.–SFDGA-10
Determination of basic diabetic parameters
Diabetic parameters like fasting blood sugar, urine volume and urine sugar were determined in control, diabetic and mango peel extract administered diabetic rats. Urine sugar was measured by 3,5-dinitrosalicylic acid method as described by Miller (1959). At the end of the experiment, blood was drawn by sacrificing the rats under mild ether anesthesia. Blood sugar was measured by glucose oxidase/peroxidase (GOD/POD) method using commercially available kit. Albumin in urine was measured by using Albumin Blue 580 reagent (Kessler et al. 1997). Creatinine was estimated in urine and serum by Jaffes method described by Bowers (1980). Glomerular filtration rate (GFR) was determined after estimating creatinine levels in urine and serum using the formula given by Yokozawa et al. (1996). Fructosamine assay was carried out by the method described by Roger (1982). Lipid peroxides were assayed by measuring the malondialdehyde (MDA) concentration as thiobarbituric acid reactive substances (TBARS) according to the method described earlier (Ohkawa et al. 1979).
Determination of antioxidant enzyme activities
The tissues (liver and kidney) were homogenized in phosphate buffer (100 mM, pH 7.4) at 1:10 (1 g in 10 ml) ratio. The homogenate was centrifuged at 4 °C for 10 min at 10,000 g and the supernatant obtained was used for analysis of superoxide dismutase (McCord and Fridovich 1969), catalase (Luck 1965), glutathione peroxidase (Flohe et al. 1984) and glutathione-S-transferase (Habig and Jacoby 1981).
Statistical analysis
All observations and calculations were made separately. Values were presented as mean ± SD of n = 6 rats in all groups. Statistical analysis of data was performed using one-way analysis of variance (ANOVA) with a Tukey’s multiple comparison post-test and significance at *P < 0.05, **P < 0.01 and ***P < 0.001.
Results and discussion
Bioactive compounds and antioxidant properties of ethanol extract
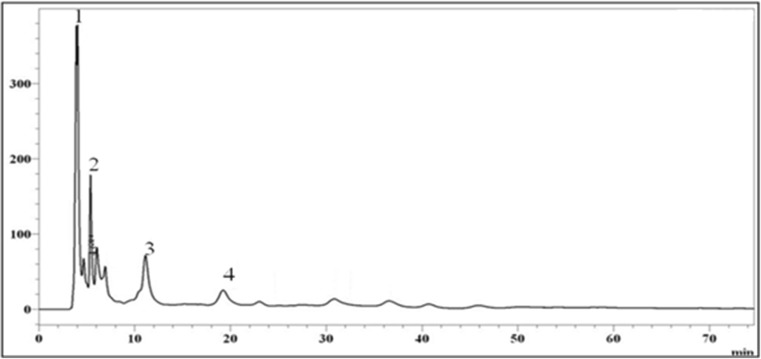
Aqueous methanol, acetone or ethanol was commonly used solvent for the extraction of bioactive compounds form different plant tissues. Of these solvents, ethanol is less toxic compared to the other solvents (Shi et al. 2005). As the major objective of the present study was to use the mango peel extract to treat the diabetic rats, aqueous ethanol was used for the extraction of bioactive compounds from the peel. The peel ethanol extract showed phenolic compounds, carotenoids, flavonoids and anthocyanins (Table 1). The peel extract was tested for its antioxidant properties using two different assays viz., DPPH radical scavenging activity and reducing power assay. Peel extract showed EC50 value of 4 μg GAE by DPPH radical scavenging method and is comparable to that of synthetic BHA (4.0 μg GAE). The extract showed better reducing power activity compared to BHA. For example, at a dose of 5 μg GAE, the absorption at 700 nm was found to be 0.412 for peel extract, while it was 0.189 for BHA. Gallic, protocatechuic, chlorogenic and ferulic acids were the phenolic acids identified in the peel ethanol extract by RP-HPLC. Gallic acid was found to be the major phenolic acid found in the mango peel ethanol extract (Table 1; Fig. 1). Recently, we have reported the presence of gallic, protocatechuic, and gentisic acids in the acetone extract of mango peel (Gondi et al. 2014). In the present study, we found the presence of ferulic acid and chlorogenic acid, which were not reported earlier in acetone extract of mango peel. The differences noticed in the present study compared to the earlier study may be due to the differences in the extractability of aqueous acetone and aqueous ethanol as their polarities are different, or due to the changes in agro-climatic conditions. Chlorogenic acid was described as a potential antidiabetic agent. It exerts its antidiabetic effects by attenuating intestinal glucose absorption (Bassoli et al. 2008). Gallic acid from Terminalia bellerica was reported to stimulate insulin secretion (insulin secretagogue) (Latha and Daisy 2011). Ferulic acid, as low as 0.01 % level in the basal diet, supressed blood glucose levels significantly in STZ-induced diabetic mice. Gallic acid and protocatechuic acid are reported to exhibit higher antioxidant activities compared to the most of the phenolic acids reported in the literature (Palafox-Carlos et al. 2012). Thus, it appears that ethanol extract of peel contains antidiabetic compounds which may exert antidiabetic compounds through different mechanisms.
Table 1.
Nutraceutical composition of mango peel ethanol extract
| Parameter | Content (mg/g) |
|---|---|
| Total polyphenol | 83.0 ± 1.5 |
| Flavonoids | 8.0 ± 0.7 |
| Anthocyanins | 10.5 ± 0.5 |
| Carotenoids | 3.9 ± 0.4 |
| Phenolic acid | |
| Gallic acid | 9.0 ± 0.21 |
| Protocatechuic acid | 1.7 ± 0.09 |
| Chlorogenic acid | 0.8 ± 0.01 |
| Ferulic acid | 0.9 ± 0.01 |
| DPPH radical scavenging activity, EC50 | 4.0 ± 0.08 μg GAE |
All data are the mean ± SD of three replicates
Fig. 1.
HPLC profile of phenolic acids in mango peel ethanol extract. 1. Gallic acid, 2. Protocathuic acid, 3. Chlorogenic acid, 4. Ferulic acid
Therefore, we have studied the antidiabetic properties of the ethanol extract by different mechanism such as i) in vitro carbohydrate hydrolysing enzyme inhibition which are involved at the small intestine ii) in vivo antidiabetic parameters as well as secretion of insulin and glucose tolerance test. As gallic acid is the major phenolic acid identified in mango peel extract, we used commercial gallic acid at 10 mg/kg body weight as well as metformin for comparison.
α-Amylase and α-glucosidase inhibition by mango peel extract
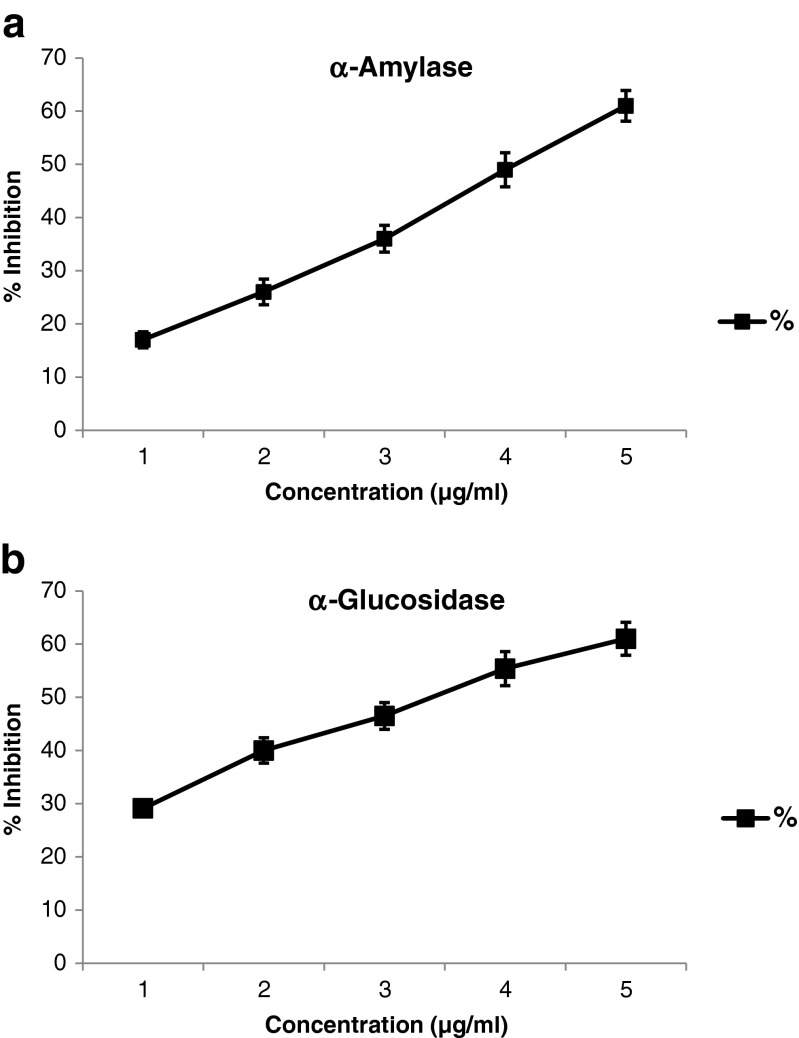
Diet containing high content of α-amylase and α-glucosidase inhibitors is one of the approaches to control postprandial glucose level. These enzyme inhibitors lower the rate of glucose absorption through delayed carbohydrate digestion and extended digestion time. As synthetic inhibitors exhibit side effects, inhibitors from plant sources offer an attractive therapeutic approach. Therefore, In vitro antidiabetic property of the extract was examined by determining the inhibition of α-amylase and α-glucosidase activities by the peel extract. The percentage inhibition of α-amylase was increased with an increase in peel extract concentration in a dose dependent manner (Fig. 2a) and the extracts showed 50 % of enzyme inhibition (IC50) at 4 μg/ml. The inhibition of α-glucosidase was also exhibited by the peel extract in a dose dependent manner (Fig. 2b). The extract showed α-glucosidase IC50 value of 3.5 μg/ml. The results suggest that the inhibitory potential of α-glucosidase was much higher than α-amylase. The IC50 values obtained in the present study, with mango peel extract are much lower than some of the reported values for other plant extracts including mango kernel. For example, seed extracts of mango (Mangifera indica) and Mucuna urens, the IC50 values for α-amylase were 0.71 and 1.13 mg/ml and for α-glucosidase were 0.34 and 0.52 mg/ml, respectively (Irondi et al. 2014), while for methanol extract of Citrus macroptera fruit had IC50 value of 3.68 mg/ml for α-amylase enzymes activity (Uddin et al. 2014). The low IC50 value for mango peel extract may be due to the presence of mangiferin and their derivatives, in addition to other phenolic acids, flavonoids and carotenoids like gallic, eallagic, protocatecuic acids, quercitin, rutin, ß-carotene etc (Ajila et al. 2010). One of the characteristic features of mango peel is that it contains mangiferin, which has many beneficial biological activities, including antidiabetic effect in diabetic rats (Sellamuthu et al. 2012). The inhibitory potential varies depending on the structural aspects of the phenolic acids, flavonoids and anthocyanins, and the inhibition potential by the compounds also varies depending on the source of enzyme (Tadera et al. 2006). Earlier reports indicate some of the phenolic acids like gallic acid, chlorogenic acid and ferulic acid, which are identified in mango peel ethanol extract, were shown to inhibit α-amylase and α-glucosidase activities. It has been reported that the gallic acid can competitively inhibit α-glucosidase by concentration dependent manner with IC50 value of 1.16 mM (Wan et al. 2012). Chlorogenic acid was reported to inhibit the α-amylase with an IC50 value of 9.10 μg/ml and α-glucosidase activity with an IC50 value of 9.24 μg/ml. (Oboh et al. 2014). Ferulic acid was also reported to inhibit intestinal α-glucosidase enzyme activity with an IC50 value of 0.45 mM (Adisakwattana et al. 2009).
Fig. 2.
The percentage inhibition of a α-amylase activity; b α-glucosidase activity by mango peel ethanol extract
Effect of mango peel extract on oral glucose tolerance
The oral glucose tolerance experiment indicates the body’s ability to utilize glucose. The effect of different doses of mango peel extracts on glucose tolerance was evaluated by measuring the blood glucose level at different time points of 30, 60, 90, 120 and 150 min after glucose challenge. Different doses of peel extracts, 100, 150 and 200 mg/kg body weight, showed significant reduction in glucose levels (P < 0.001) 15, 23 and 26 %, respectively compared to control (Table 2). Gallic acid (10 mg/kg) and metformin reduced the blood glucose levels by 13 and 29 %, respectively compared to control group. In all the treated groups, the reduction in blood glucose level was observed higher at 150 min. The results suggest that increased levels of glucose tolerance may be due to increased secretion of insulin. These results suggest that the ethanol extract of peel possess hypoglycemic effect.
Table 2.
Effect of mango peel ethanol extract on oral glucose tolerance test
| Groups | Time (Min) | |||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | |
| Glucose control | 86.5 ± 1.56 | 113.5 ± 1.30 | 110.2 ± 1.21 | 105.7 ± 1.83 | 101.4 ± 1.86 | 99.5 ± 1.24 |
| Glucose + MI (100 mg/kg) | 86.1 ± 0.93 | 102 ± 1.42* | 98.3 ± 1.18* | 95.2 ± 1.73* | 89.3 ± 1.943* | 85.4 ± 1.26** |
| Glucose + MI (150 mg/kg) | 85.4 ± 1.05 | 99.5 ± 1.34* | 95.5 ± 1.74* | 91.1 ± 1.41** | 85.2 ± 1.25** | 76.6 ± 1.705*** |
| Glucose + MI (200 mg/kg) | 85.3 ± 1.26 | 98.3 ± 1.56* | 93.7 ± 1.28** | 89.2 ± 1.61** | 83.3 ± 0.948* | 74.2 ± 1.28*** |
| Glucose + Metformin (10 mg/kg) | 85.5 ± 1.43 | 90.5 ± 1.64** | 88.3 ± 0.825** | 82.5 ± 1.29** | 80.5 ± 1.48** | 71.4 ± 1.95*** |
| Glucose + Gallic acid (100 mg/kg) | 85.6 ± 1.85 | 98.5 ± 1.32* | 95.9 ± 1.58* | 92.8 ± 1.97* | 89.8 ± 1.20* | 86.0 ± 1.85** |
All values represent mean ± SD *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, by Turkey’s multiple comparison test
Changes in diabetic parameters
Effect of different doses of mango peel extracts, gallic acid and metformin on the urine sugar, urine volume and body weight in STZ induced diabetic rats is shown in Table 3. At the end of 60 days treatment, STZ induced diabetic rats showed increase in urine sugar and urine volume when compared to normal rats. However, different doses of mango peel extracts, gallic acid and metformin treated diabetic rats showed significant decrease (P < 0.001) in urine sugar and urine volume levels, when compared to diabetic control rats. Body weight of normal rats increased and was 205 g at the end of the experiment. Diabetic control group continued to lose weight till the end of the study. Metformin, gallic acid and different doses of mango peel extract (100, 150 and 200 mg/kg) showed a significantly higher (P < 0.001) body weight compared to the diabetic control rats.
Table 3.
Effect of mango peel ethanol extract on various diabetic parameters in STZ-induced diabetic rats
| Parameters | SFC | SFD | SFDM-100 | SFDM-150 | SFDM-200 | SFDMet-10 | SFDGA-10 |
|---|---|---|---|---|---|---|---|
| Body weight (g) | 205 ± 7.9 | 146 ± 8.5a*** | 155 ± 8.9 | 174 ± 9.4b*** | 178 ± 10.8b*** | 181 ± 8.2b*** | 157 ± 8.5 |
| Urine volume (ml/24 h) | 16.8 ± 2.5 | 47.2 ± 3.7a*** | 42.6 ± 6.5b* | 34.0 ± 3.8b*** | 26.6 ± 2.7b*** | 28.4 ± 4.3b*** | 43.5 ± 5.7b* |
| Urine sugar (g/24 h) | 0.07 ± 0.01 | 7.1 ± 0.97a*** | 5.8 ± 0.68b* | 4.3 ± 0.86b*** | 3.8 ± 0.48b*** | 4.1 ± 0.47b*** | 5.9 ± 0.72b* |
| Microalbunuria (mg/24 h) | 0.64 ± 0.05 | 5.16 ± 0.39a*** | 3.98 ± 0.41b*** | 2.76 ± 0.30b*** | 2.46 ± 0.55b*** | 2.24 ± 0.51 b*** | 3.92 ± 0.4b*** |
| Glomular filtration rate (ml/min) | 0.91 ± 0.06 | 5.1 ± 0.93a*** | 4.0 ± 0.82b* | 3.12 ± 0.58b*** | 2.70 ± .46b*** | 2.48 ± 0.35b*** | 3.90 ± 0.45b* |
| Serum fructosamine (mmol/mg protein) | 0.23 ± 0.09 | 0.71 ± 0.09a*** | 0.51 ± 0.08b* | 0.45 ± 0.07b** | 0.38 ± 0.09b*** | 0.36 ± 0.08b*** | 0.54 ± 0.09b* |
| Glycated Heamoglobin (%) | 2.3 ± 0.48 | 10.9 ± 1.05a*** | 8.7 ± 0.94b** | 7.2 ± 0.89b*** | 6.5 ± 0.68b*** | 5.5 ± 0.91b*** | 9.70 ± 0.85b** |
| Fasting plasma Insulin (μU/mL) | 15.3 ± 1.50 | 5.4 ± 0.87a*** | 8.0 ± 0.89b*** | 11.9 ± 1.90b*** | 12.4 ± 1.93b*** | 12.0 ± 1.81 b*** | 8.1 ± 1.09b*** |
All values represent mean ± SD *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, by Turkey’s multiple comparison test
aCompared to normal control
bCompared to diabetic control
Effect of mango peel extract on the levels of fructosamine and glycated hemoglobin
Fructosamine and glycated hemoglobin are non-enzymatically glycated proteins and are frequently used marker glycated proteins for monitoring diabetic status. Fructosamine levels increased in STZ-induced diabetic rats compared to normal group (Table 3). Upon oral administration of different doses of mango peel extract as well as gallic acid and metformin, fructosamine levels in treated diabetic rats was significantly decreased. Effect of different doses of mango peel extracts on glycated hemoglobin (HbA1C) is presented in Table 3. In STZ induced diabetic rats, there was a significant increase in the levels of HbA1C compared to normal rats. In diabetic patients the incidence of myocardial infraction doubles as HbA1C levels increase from 5 to 10 % (Stratton et al. 2000). Oral administration of different doses of mango peel extract, significantly decreased (P < 0.001) the level of glycated hemoglobin. Among all the doses of mango peel extracts, 200 mg/kg body weight was more effective in decreasing the levels of fructosamine and HbA1C.
Effect of mango peel extract on plasma insulin and fasting blood glucose
Insulin is the most important factor in the regulation of plasma glucose homeostasis. In STZ induced diabetic rats, there was a significant decrease (P < 0.001) in the level of plasma insulin compared to normal rats. Oral administration of different doses of mango peel extracts, significantly increased (P < 0.001) the level of plasma insulin. Among all the doses of mango peel extracts, 200 mg/kg body weight was more effective in increasing the level of plasma insulin (Table 3).
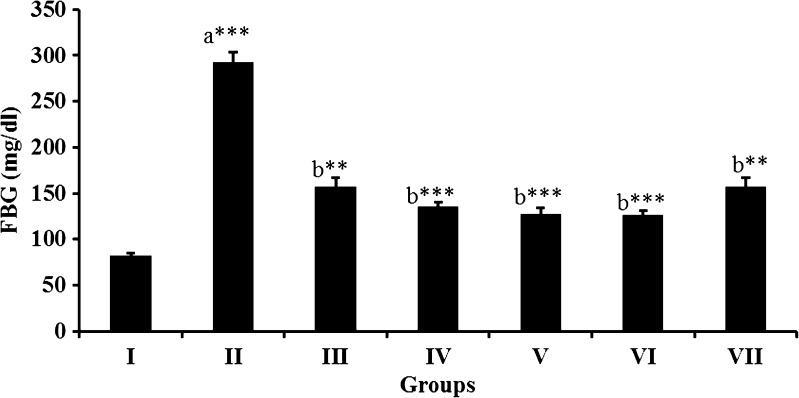
Administration of different doses of peel extracts such as 100, 150 and 200 mg/kg body weight, to STZ-induced diabetic rats resulted in significant (P < 0.001) decline in blood glucose levels in a dose dependent manner by 49, 59 and 66 %, respectively. On the other hand, metformin treatment showed 67 % decrease in blood glucose level, while gallic acid treatment decreased only 51 % compared to diabetic control group (Fig. 3). Mango peel extract at a dose of 200 mg/kg body weight ameliorated blood glucose level to the best, in STZ induced diabetic rats, when compared to the other group of rats received different doses of mango peel extract and it is comparable to metformin. Metformin is widely used drug for type 2 diabetes and it decreases blood glucose levels by making body cell more sensitive to insulin. However, It has been proposed that metformin decreases the blood glucose levels in STZ-induced diabetic rats via an increase of endorphin secretion from adrenal glands to stimulate opioid receptor linkage, which leads to an increase of GLUT-4 gene expression and an attenuation of hepatic phosphoenolpyruvate carboxykinase (PEPCK) gene expression (Cheng et al. 2006). It also activates 5′AMP-activated protein kinase (AMPK) in hepatocytes, thereby reduces the activity of acetyl-CoA carboxylase and lowers expression of a lipogenic transcription factor as well as inhibiting hepatic gluconeogenesis (Zhou et al. 2001; Lochhead et al. 2000).
Fig. 3.
Effect of mango peel extract on fasting blood glucose at different concentrations on STZ induced diabetic rats, compared to standard drug metformin. Group I – Starch fed control (SFC), Group II – Starch fed diabetic (SFD), Group III – Starch fed diabetic + 100 mg/kg (SFDM-100 mg/kg), Group IV – Starch fed diabetic + 150 mg/kg (SFDM-150 mg/kg), Group V – Starch fed diabetic + 200 mg/kg (SFDM-200 mg/kg), Group VI – Starch fed diabetic + Metformin 10mg/kg (SFDMet-10 mg/kg), Group VII – Starch fed diabetic + gallic acid 10mg/kg (SFDGA-10 mg/kg). All values represent mean ± SD *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, by Turkey’s multiple comparison test. a Compared to normal control. b Compared to diabetic control
Effect of mango peel extract on microalbunuria and glomular filtration rate (GFR)
The microalbunuria level was significantly higher in STZ-induced diabetic rats when compared to normal rats. Diabetic rats that have received mango peel extracts, gallic acid and metformin showed significant decline (P < 0.001) in the microalbunuria and at the dose of 200 mg/kg, the value was lowest among the diabetic rats (Table 3). Upon administration of different doses of mango peel extracts (100, 150 and 200 mg/kg) and gallic acid and metformin, decrease in the levels of GFR was observed in treated diabetic rats compared to diabetic control rats. Maximum beneficial effect on GFR in STZ induced treated diabetic rats was observed in diabetic rats treated with mango peel extract at a dose of 200 mg/kg body weight (Table 3).
Effect of mango peel extract on liver antioxidant enzymes and lipid peroxidation
In STZ induced diabetic rats, we observed a significant decrease (P < 0.001) in the activities of liver antioxidant enzymes, viz., catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-S-transferase (GST) and increase in the MDA (malonaldehyde) levels (Table 4). Upon treatment of diabetic rats with metformin, gallic acid and different doses of mango peel extracts, significant increase (p < 0.001) in the CAT, SOD, GPx and GST activities, and decrease in the level of MDA were observed. The results suggest that metformin, gallic acid and all the doses of mango peel extracts increased the enzyme activities of CAT, SOD, GPx and GST, but mango peel extract at a dosage of 200 mg/kg was more effective in increasing the antioxidant enzyme activities in diabetic rats, compared with other doses of mango peel extract. It has been reported that metformin exert its in vivo antioxidant activity by different pathways such as increasing the antioxidant enzyme activities, and decreasing the markers of lipid peroxidation (Tessier et al. 1999; Pavlovic et al. 2000; Tanaka et al. 1999). Earlier reports also indicate that metformin reduces the oxidative stress in STZ-induced diabetic animal models (Alhaider et al. 2011).
Table 4.
Effect of mango peel ethanol extract on liver antioxidant enzymes and lipid peroxidation
| Parameters | SFC | SFD | SFDM-100 | SFDM-150 | SFDM-200 | SFDMet-10 | SFDGA-10 |
|---|---|---|---|---|---|---|---|
| CAT (mmoles min−1 mg protein−1) | 82.15 ± 6.24 | 38.12 ± 5.81a*** | 45.10 ± 4.26b* | 55.23 ± 3.84b** | 61.34 ± 4.93b*** | 64.75 ± 3.52b*** | 46.38 ± 3.64b* |
| SOD (U min−1 mg protein−1) | 14.82 ± 1.56 | 9.82 ± 0.98a*** | 11.50 ± 0.84b* | 13.52 ± 0.79b** | 13.83 ± 1.05b** | 13.91 ± 0.93b** | 10.95 ± 0.86b* |
| GPx (mmoles min−1 mg protein−1) | 9.21 ± 0.83 | 3.45 ± 0.68a*** | 4.84 ± 0.82b** | 5.90 ± 0.95b*** | 6.54 ± 0.98 b*** | 7.41 ± 0.83b*** | 5.10 ± 0.97b** |
| GST (μmoles min−1 mg protein−1) | 9.86 ± 0.95 | 3.83 ± 0.21a*** | 4.91 ± 0.54b** | 7.40 ± 1.08b*** | 8.12 ± 1.04b*** | 8.53 ± 1.13b*** | 4.52 ± 0.95b** |
| LPO (mmoles min−1 mg protein−1) | 1.85 ± 0.15 | 5.20 ± 0.46a*** | 4.27 ± 0.92b** | 3.56 ± 0.85b** | 3.12 ± 0.71b** | 2.52 ± 0.97b*** | 4.31 ± 0.86b** |
All values represent mean ± SD *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, by Turkey’s multiple comparison test
aCompared to normal control
bCompared to diabetic control
Effect of mango peel extract on kidney antioxidant enzymes and lipid peroxidation
The activities of antioxidant enzymes, CAT, SOD, GPx, GST were significantly decreased (P < 0.001) in diabetic control group and MDA levels were significantly increased (Table 5). Diabetic rats treated with metformin, gallic acid and different doses of mango peel extracts (100, 150 and 200 mg/kg) showed significant increase (P < 0.001) in enzyme activity levels of CAT, SOD, GPx and GST and decrease in the MDA levels. Thus, the results suggest that metformin, gallic acid and all the doses of mango peel extract showed an increase in the level of CAT, SOD, GPx and GST, however, mango peel extract at a dosage of 200 mg/kg body weight was more effective in increasing the antioxidant enzyme activities in diabetic rats compared to other doses of mango peel extract.
Table 5.
Effect of mango peel ethanol extract on kidney antioxidant enzymes and lipid peroxidation
| Parameters | SFC | SFD | SFDM-100 | SFDM-150 | SFDM-200 | SFDMet-10 | SFDGA-10 |
|---|---|---|---|---|---|---|---|
| CAT (mmoles min−1 mg protein−1) | 56.75 ± 6.24 | 25.64 ± 3.52a*** | 34.80 ± 2.93b** | 41.95 ± 3.50b*** | 47.15 ± 2.81b*** | 50.86 ± 4.20b*** | 36.28 ± 2.63b** |
| SOD (U min−1 mg protein−1) | 20.92 ± 2.15 | 12.24 ± 1.75a*** | 14.28 ± 1.26b* | 17.63 ± 1.58b** | 18.92 ± 2.48b*** | 19.14 ± 1.69b*** | 14.85 ± 1.37b* |
| GPx (mmoles min−1 mg protein−1) | 10.53 ± 1.08 | 4.95 ± 0.83a*** | 6.37 ± 0.85b** | 7.88 ± 0.95b*** | 7.95 ± 1.07b*** | 8.53 ± 0.92b*** | 6.58 ± 0.82b** |
| GST (μmoles min−1 mg protein−1) | 7.85 ± 0.83 | 2.97 ± 0.47a*** | 3.51 ± 0.38b* | 4.91 ± 0.64b*** | 5.28 ± 0.75b*** | 6.63 ± 0.94b*** | 3.64 ± 0.86b* |
| LPO (mmoles min−1 mg protein−1) | 2.45 ± 0.62 | 6.74 ± 0.70a*** | 5.32 ± 0.64b* | 4.50 ± 0.59b*** | 4.2 ± 0.86b*** | 3.59 ± 0.94b*** | 5.51 ± 0.81b* |
All values represent mean ± SD *P < 0.05; **P < 0.01; ***P < 0.001; ANOVA, by Turkey’s multiple comparison test
aCompared to normal control
bCompared to diabetic control
Increased lipid peroxide concentration (MDA levels) was observed in liver and kidney of STZ induced diabetic rats, which may be due to increased free radicals and due to the depletion of antioxidant scavenger systems. SOD plays a pivotal role in oxygen defence metabolism by intercepting and reducing superoxide to hydrogen peroxide, which is easily reduced to water by CAT and GPx in mammals. Therefore, the free radical scavenging activity of SOD is effective, only when it is followed by the increase in CAT or GPx activity. Mango peel extract might have played a role in the recovery from diabetic condition possibly by scavenging free radicals and improving the antioxidant enzyme activities.
Conclusions
The present study reveals that mango peel extract has ability to ameliorate diabetes. The peel extract may protect both type 1 and type 2 diabetes and its complication by decreasing the glucose release by inhibition of carbohydrate hydrolysing enzymes, its absorption by the small intestine, through increased levels of plasma insulin, improved antioxidative status, protection against LPO. Peel extract is rich in phenolic acids, flavonoids and carotenoids, and these compounds may provide a protective effect against hyperglycemia-induced chronic diseases due to their antioxidant properties and inhibition of starch digestion by inhibiting its digestive enzyme activities. The present study demonstrates that the mango peel ethanol extract, which is rich in bioactive compounds, has the ability to ameliorate diabetes and its complications. Several studies reported that natural products obtained from plants are safe. However, some herbal extracts exhibit side effects due to active ingredients, contaminants, or interactions with drug (Bent 2008). Mango peel is an edible part of the fruit and can be consumed along with the fruit. Whole peel can be supplemented with food products to improve the health benefits and peel extract can be used as nutraceutical.
Acknowledgments
The first author is thankful to the Indian Council of Medical Research, New Delhi, for the award of a Senior Research Fellowship.
References
- Aderibigbe AO, Emudianughe TS, Lawal BA. Antihyperglycaemic effect of Mangifera indica in rat. Phytother Res. 1999;13:504–507. doi: 10.1002/(SICI)1099-1573(199909)13:6<504::AID-PTR533>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Adisakwattana S, Chantarasinlapin P, Thammarat H, Yibchok-Anun S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal alpha-glucosidase. J Enzyme Inhib Med Chem. 2009;24:1194–1200. doi: 10.1080/14756360902779326. [DOI] [PubMed] [Google Scholar]
- Ajila CM, Prasada Rao UJS. Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangifera indica L. peel extract. Food Chem Toxicol. 2008;46:303–309. doi: 10.1016/j.fct.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Ajila CM, Bhat SG, Prasada Rao UJS. Valuable components of raw and ripe peels from two Indian varieties. Food Chem. 2007;102:1006–1011. doi: 10.1016/j.foodchem.2006.06.036. [DOI] [Google Scholar]
- Ajila CM, Jaganmohan Rao L, Prasada Rao UJS. Characterization of bioactive compounds from raw and ripe Mangifera indica L. peel extracts. Food Chem Toxicol. 2010;48:3406–3411. doi: 10.1016/j.fct.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact. 2011;92:233–242. doi: 10.1016/j.cbi.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Arunachalam K, Parimelazhagan T. Antidiabetic activity of Ficusamplissima Smith. Bark extract in streptozotocin induced diabetic rats. J Ethnopharmacol. 2013;14:302–310. doi: 10.1016/j.jep.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Bassoli BK, Cassolla P, Borba-Murad GR, Constantin J, Salgueiro-Pagadigorria CL, Bazotte RB. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: effects on hepatic glucose release and glycaemia. Cell Biochem Funct. 2008;26(3):320–328. doi: 10.1002/cbf.1444. [DOI] [PubMed] [Google Scholar]
- Bastaki S. Diabetes mellitus and its treatment. Int J Diabetes Metab. 2005;13:111–134. [Google Scholar]
- Baynes JW. Role of oxidative stress in development of complication in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Bent S. Herbal medicine in the United States: review of efficacy, safety, and regulation. J Gen Intern Med. 2008;23:854–859. doi: 10.1007/s11606-008-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulis S, Wiley C, Selvin E, Wilson R, Bass EB, Brancati FL. Systematic review: comparative effectiveness and safety of oral medications fortype 2 diabetes mellitus. Ann Intern Med. 2007;147:386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- Bonner-weir S. Morphological evidence of pancreatic polarity of beta cells within islets of Langerhans. Diabetes. 1988;37:616–621. doi: 10.2337/diab.37.5.616. [DOI] [PubMed] [Google Scholar]
- Botting KJ, Young MM, Pearson AE, Harris PJ, Ferguson LR. Antimutagens in food plants eaten by Polynesians: micronutrients, phytochemicals and protection against bacterial mutagenicity of the heterocyclic amine 2-amino-3-methylimidazo(4,5-f) quinoline. Food Chem Toxicol. 1999;37:95–103. doi: 10.1016/S0278-6915(98)00121-5. [DOI] [PubMed] [Google Scholar]
- Bowers LD. Kinetic serum creatinine assays. The role of various factors in determining specificity. Clin Chem. 1980;26:551–554. [PubMed] [Google Scholar]
- Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea-a review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- Carrascosa M, Pascual F, Arest S. Acarbose-induced acute severe hepatotoxicity. Lancet. 1997;349:698–699. doi: 10.1016/S0140-6736(05)60134-1. [DOI] [PubMed] [Google Scholar]
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat Rev Endocrinol. 2012;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- Cheng JT, Huang CC, Liu IM, Tzeng TF, Chang CJ. Novel mechanism for plasma glucose-lowering action of metformin in streptozotocin-induced diabetic rats. Diabetes. 2006;55(3):819–825. doi: 10.2337/diabetes.55.03.06.db05-0934. [DOI] [PubMed] [Google Scholar]
- Feinglos MN, Bethel MA. Therapy of type 2 diabetes, cardiovascular death, and the UGDP. Am Heart J. 1999;138:346–352. doi: 10.1016/S0002-8703(99)70034-7. [DOI] [PubMed] [Google Scholar]
- Flohe L, Wolfgng A, Gunzler WA. Assay of glutathione peroxidase. Methods Enzymol. 1984;105:114–130. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Garrido G, Gonzalez D, Delporte C. Analgesic and anti-inflammatory effects of Mangifera indica extract (Vimang) Phytother Res. 2001;15:18–21. doi: 10.1002/1099-1573(200102)15:1<18::AID-PTR676>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Girish TK, Pratape VM, Prasada Rao UJS. Nutrient distribution, phenolic acid composition, antioxidant and alpha-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and its milled by-products. Food Res Int. 2012;46:370–377. doi: 10.1016/j.foodres.2011.12.026. [DOI] [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- Gondi M, Shaik AB, Jamuna JB, Salimath PV, Prasada Rao UJS. Antidiabetic effect of dietary mango (Mangifera indica L.) peel in streptozotocin-induced diabetic rats. J Sci Food Agric. 2014;95:991–999. doi: 10.1002/jsfa.6778. [DOI] [PubMed] [Google Scholar]
- Guevara Garcia M, Gonzalez Laime S, Alvarez A, et al. Ethnomedical uses of Mangifera indica L. stem bark extract in Cuba (spanish) Rev Cub Plant Med. 2004;9:27–32. [Google Scholar]
- Habig WH, Jacoby WB (1981) Assays for differentiation of glutathione S-transferase. Methods Enzymol 77:398– 405 [DOI] [PubMed]
- Hui H, Tang G, Go VL. Hypoglycemic herbs and their action mechanisms. Chin Med. 2009;4:11. doi: 10.1186/1749-8546-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irondi EA, Oboh G, Akindahunsi AA, Boligon AA, Athaydes ML. Phenolic composition and inhibitory activity of Mangifera indica and Mucuna urens seeds extracts against key enzymes linked to the pathology and complications of type 2 diabetes. Asian Pac J Trop Biomed. 2014;4(11):903–910. doi: 10.12980/APJTB.4.201414B364. [DOI] [Google Scholar]
- Iwai K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-A(y) mice. Plant Foods Hum Nutr. 2008;63:163–169. doi: 10.1007/s11130-008-0098-4. [DOI] [PubMed] [Google Scholar]
- Iwai K, Kim MY, Onodera A, Matsue H. Alpha-glucosidase inhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J Agric Food Chem. 2006;54:4588–4592. doi: 10.1021/jf0606353. [DOI] [PubMed] [Google Scholar]
- Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4(1):5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung EH, Kim SR, Hwang IK, Ha TY. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J Agric Food Chem. 2007;55:9800–9804. doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- Kelle DE. Effects of weight loss on glucose homeostasis in NIDDM. Diabetes Rev. 1995;3:366–377. [Google Scholar]
- Kessler MA, Meinitzer A, Petek W, et al. Microalbuminuria and borderline-increased albumin excretion determined with a centrifugal analyzer and the Albumin Blue 580 fluorescence assay. Clin Chem. 1997;43:996–1002. [PubMed] [Google Scholar]
- Kim JS, Kwon CS, Son KH. Inhibition of alpha glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000;64:2458–2461. doi: 10.1271/bbb.64.2458. [DOI] [PubMed] [Google Scholar]
- Kim H, Moon JY, Kim H, et al. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010;121:429–436. doi: 10.1016/j.foodchem.2009.12.060. [DOI] [Google Scholar]
- Kwon YI, Apostolidis E, Kim YC, Shetty K. Health benefits of traditional corn, beans and pumpkin: in vitro studies for hyperglycemia and hypertension management. J Med Food. 2007;10:266–275. doi: 10.1089/jmf.2006.234. [DOI] [PubMed] [Google Scholar]
- Latha RCR, Daisy P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin–induced diabetic rats. Chem Biol Interact. 2011;189:112–118. doi: 10.1016/j.cbi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- Luck H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic; 1965. pp. 885–894. [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Naveed T, Siddiqui JI, Ansari SH, Mukhtar HM. Immunomodulatory activity of mangifera indica. J Nat Remi. 2005;5:137–140. [Google Scholar]
- Oboh G, Agunloye OM, Adefegha SA, Akinyemi AJ, Ademiluyi AO. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): a comparative study. J Basic Clin Physiol Pharmacol. 2014;26:167–170. doi: 10.1515/jbcpp-2013-0141. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Orgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med. 2008;233:1066–1080. doi: 10.3181/0712-MR-347. [DOI] [PubMed] [Google Scholar]
- Palafox-Carlos H, Yahia EM, Gonzalez-Aguilar GA. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD–MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012;135:105–111. doi: 10.1016/j.foodchem.2012.04.103. [DOI] [Google Scholar]
- Pavlovic D, Kocic R, Kocic G, Jevtovic T, Radenkovic S, Mikic D, Stojanovic M, Djordjevic PB. Effect of four week metformin treatment on plasma and erythrocyte antioxidative defense enzymes in newly diagnosed obese patients with type 2 diabetes. Diabetes Obes Metab. 2000;2:251–256. doi: 10.1046/j.1463-1326.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- Percival SS, Talcott ST, Chin ST, Mallak AC, Lounds-Singleton A, Pettit-Moore J. Neoplastic transformation of BALB/3T3 cells and cell cycle of HL-60 cells are inhibited by mango (Mangifera indica L.) juice and mango juice extract. J Nutr. 2006;136:1300–1304. doi: 10.1093/jn/136.5.1300. [DOI] [PubMed] [Google Scholar]
- Perpetuo GF, Salgado JM. Effect of mango (Mangifera indica, L.) ingestion on blood glucose levels of normal and diabetic rats. J Plant Foods Hum Nutr. 2003;58:1–12. doi: 10.1023/B:QUAL.0000040336.38013.83. [DOI] [Google Scholar]
- Roger NJ. Fructosamine a new approach to the estimation of serum glycosyl protein; an index of diabetic control. Clin Chim Acta. 1982;127:87–95. doi: 10.1016/0009-8981(83)90078-5. [DOI] [PubMed] [Google Scholar]
- Saxena AK, Srivastava P, Kale RK, Baquer NZ. Impaired antioxidant status in diabetic rat liver. Effect of vanadate. Biochem Pharmacol. 1993;45(3):539–542. doi: 10.1016/0006-2952(93)90124-F. [DOI] [PubMed] [Google Scholar]
- Sellamuthu PA, Arulselvan P, Muniappan BP, Kandasamy M. Effect of mangiferin isolated from Salacia chinensis regulates the kidney carbohydrate metabolism in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2012;2:S1583–S1587. doi: 10.1016/S2221-1691(12)60457-2. [DOI] [Google Scholar]
- Sharma SR, Dwivedi SK, Swarup D. Hypoglycemic potential of Mangifera indica leaves in rats. Int J Pharmacol. 1997;35:130. doi: 10.1076/phbi.35.2.130.13276. [DOI] [Google Scholar]
- Sheehan MT. Current therapeutic opinion in type 2 diabetes mellitus: a practical approach. Clin Med Res. 2003;1:189–200. doi: 10.3121/cmr.1.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Nawaz H, Pohorly J, Mittal G, Kakuda Y, Jiang Y. Extraction of polyphenolics from plant material for functional foods-engineering and technology. Food Rev Int. 2005;21:139–166. doi: 10.1081/FRI-200040606. [DOI] [Google Scholar]
- Singleton UL, Rossi J. Colorimetry of total phenolics with phosphomolybdic-posphotungustic acid reagent. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. The metabolic effects of metformin in noninsulin-dependent diabetes mellitus. N Eng J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- Sutharson L, Hariharan RS, Vamsadhara C. Drug Utilization Study in Diabetology outpatient setting of a tertiary hospital. Indian J Pharmacol. 2003;35:237–240. [Google Scholar]
- Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol (Tokyo) 2006;52:149–153. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Uchino H, Shimizu T, Yoshii H, Njwa M, Ohmura C, Mitsuhashi N, Onuma T, Kawamori R. Effect of metformin on advanced glycation end product formation and peripheral nerve function in streptozocin-induced diabetic rats. Eur J Pharmacol. 1999;376:17–22. doi: 10.1016/S0014-2999(99)00342-8. [DOI] [PubMed] [Google Scholar]
- Tessier D, Maheux P, Khalil A, Fulop T. Effect of gliclazide versus metformin on the clinical profile and lipid peroxidation markers in type 2 diabetes. Metabolism. 1999;48:897–903. doi: 10.1016/S0026-0495(99)90226-3. [DOI] [PubMed] [Google Scholar]
- Uddin N, Hasan MR, Hossain MM, Sarker A, Hasan AH, Islam AF, Chowdhury MM, Rana MS. In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera. Montr Fruit Asian Pac J Trop Biomed. 2014;4(6):473–479. doi: 10.12980/APJTB.4.2014C1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnagri A, Kandhare AD, Chakravarty S, Ghosh P, Bodhankar SL. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharma Biol. 2014;52:814–828. doi: 10.3109/13880209.2013.870584. [DOI] [PubMed] [Google Scholar]
- Wan C, Yuan T, Li L, Kandhi V, Cech NB, Xie M, Seeram NP. Maplexins, new alpha-glucosidase inhibitors from red maple (acer rubrum) stems. Bioorg Med Chem Lett. 2012;22:597–600. doi: 10.1016/j.bmcl.2011.10.073. [DOI] [PubMed] [Google Scholar]
- WHO . Traditional medicine strategy 2002–2005. Geneva: World Health Organization; 2002. [Google Scholar]
- Wolfe K, Wu XZ, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- Xu BJ, Chang SKC (2007) A comparative study of phenolic profiles and antioxidant activity of legumes as affected by extraction solvents. J Food Sci 72:159–166 [DOI] [PubMed]
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- Yokozawa T, Chung HY, Qun HE. Effectiveness of green tea tannin on rats with chronic renal failure. Biosci Biotechnol Biochem. 1996;60:1000–1005. doi: 10.1271/bbb.60.1000. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino S. Type 2 diabetes and glycemic response to grapes or grape products. J Nutr. 2009;139:1794S–1800S. doi: 10.3945/jn.109.107631. [DOI] [PubMed] [Google Scholar]