Abstract
β-carotene, a potent antioxidant, has been encapsulated and slow release (SR) formulations were prepared using laboratory synthesized poly(ethylene glycols) (PEGs) based functionalized amphiphilic copolymers. Encapsulation efficiency and loading capacity of the developed formulations were determined which ranged from 22.60 to 28.08 % and 2.2 to 2.8 % respectively. The release kinetics of β-carotene from developed formulations in water revealed increased solubility and prolonged stability of β-carotene. The formulations were further subjected to different pH conditions (viz., 1.8, 6.8 and 7.8) corresponding to human gastrointestinal tract to study the effect of pH on the release of β-carotene. The diffusion exponent (n values) ranged from the 0.1540 to 0.2342 for developed formulation. The results showed that developed slow release formulations were unaffected by the highly acidic conditions referring to the gastric environment of human body. However, the release of β-carotene was high at pH 7.8 and slightly higher at pH 6.8.
Keywords: Amphiphilic copolymers, Slow release formulations, β-carotene, Encapsulation efficiency, Loading capacity, Release kinetics
Introduction
In recent years, the use of natural colorants has been steadily increasing, primarily because of changes in consumer preference toward more natural products known to exhibit specific functional properties (Ramoneda et al. 2011). The demand for β-carotene has increased due to reported anticancer (Giovannucci 1999; Nishino et al. 1999), free radical quencher, and other biological antioxidant activities (Delgado-Vargas et al. 2000; Bendich and Olson 1989). However, the high degree of unsaturation in the β-carotene structure renders it extremely susceptible to oxygen. The degradation appears to occur through direct oxidation without previous isomerisation, and the resulting oxidation products do not have coloring properties (Wagner and Warthesen 1995). Encapsulation has found numerous applications in the food industry for coating colorants, flavors and other sensitive functional food ingredients, in an effort to increase the shelf life and also for the development of novel food products containing bioactive ingredients (Shahidi and Han 1993; Brazel 1999; Qi and Xu 1999; Rodriguez-Huezo et al. 2004; Xin-Yuan and Tian-Wei 2002) and with helpful properties (nutraceutics) (Schrooyen et al. 2001).
However, β-carotene is insoluble in water and weakly soluble in oil at ambient temperature because of its crystalline form, thus making it difficult to be incorporated in food products (Ribeiro and Cruz 2005). Furthermore, β-carotene is sensitive to light, oxygen, and heat, which limits its applications in the food industry even more (Orset et al. 1999; Rodriguez-Huezo et al. 2004).
Nanotechnology renders a helping hand to overcome these problems (Moraru et al. 2003; Quintanilla-Carvajal et al. 2010). One important application of nanotechnology in food and nutrition is the design and development of novel functional food ingredients with improved water solubility, thermal stability, bioavailability, sensory attributes, and physiological performance. Encapsulation and controlled-release of active food ingredients are important applications that can be attained with nanotechnological approaches.
The unique properties of poly(ethylene glycol) (PEG), including its wide range of solubility, lack of toxicity, non-interference with enzymatic activities and conformations of polypeptides and ease of excretion from living organisms, make them ideal drug carriers. Copolymers derived from PEGs and diester and their use in drug delivery systems to encapsulate both hydrophilic and hydrophobic drugs have been reported in literature (Kumar et al. 2002 and Kumar et al. 2004; Shakil et al. 2010; Loha et al. 2011; Kaushik et al. 2013). This approach is based on the formation of nano-micelles by the self-assembly of amphiphilic copolymers in aqueous media (Shakil et al. 2010).
In continuation of our work on encapsulation of bioactive ingredients in the nanospheres of aggregated amphiphilic polymers, we herein report the encapsulation of β-carotene with functionalized PEG-based amphiphilic copolymers and determination of encapsulation efficiency. β-carotene, used in the study, was extracted from carrots and purified by column chromatography. The release kinetics, of developed slow release formulations, was also studied both in water and in different pH conditions.
Materials and methods
Materials
Fresh orange carrots (Daucus carota L., Nayanjyoti variety), grown under open field conditions of Indian Agricultural Research Institute (IARI), New Delhi, India were taken for the extraction of β-carotene. PEG-1500 and dimethyl 5-hydroxyisophthalate, which were used for the synthesis of amphiphilic polymers, were purchased from Sigma-Aldrich. 98 % conc. H2SO4 and silicon oil were locally procured. All other chemicals and solvents were of analytical grade and were used as received unless otherwise noted. β-carotene standard was also purchased from Sigma-Aldrich. The characterization of the polymers was done by 1 H and 13C-NMR spectra using Bruker NMR spectrometer (400 MHz). Silica gel-100 was used for Column Chromatography. Silica TLC plates were used for Thin Layer Chromatography. Waters e2695- Separations module HPLC system with Photodiode array detector (PDA-2998) was used for the chromatographic determination of β-carotene and release study. HPLC grade methanol and t-butyl methyl ether (TBME) were used as solvent.
Analysis of β-carotene by HPLC
β-carotene content was analyzed by using HPLC (Waters e2695 Separations Module) fitted with 2998 photodiode array detector (Waters Corp. Milford, Mass., USA) along with 25 cm × 4.6 mm × 5 μm RP-C18 column (LiChroCART®, Merck, Germany) and isocratic elution was carried out with methanol/TBME (70:30 v/v) mobile phase at a flow rate of 0.75 mL/min. The wavelength for detection of analyte was 451 nm as reported in literature (Britton 1995). The retention time (RT) for β-carotene was 12.07 min under these conditions.
Synthesis of amphiphilic copolymers
The monomers, dimethyl 5-hydroxyisophthalate (0.21 g) (1) and PEG-1500 (1.5 g) (2) were taken in equimolar quantities in two necked round bottom flask and kept on silicon oil bath at 80–90 °C (Shakil et al. 2010). The reaction was performed under vacuum with constant stirring. After proper mixing of both the reagents, one drop of concentrated H2SO4 (1 % with respect to monomers) was added to the flask. The reaction was allowed to proceed for 72 hrs and was monitored by thin layer chromatography (TLC). After completion of reaction, the reaction was quenched by adding chloroform and unreacted H2SO4 and neutralized by using NaOH solution. The organic solvent was then evaporated under vacuum and the residue was dialyzed using membrane (MWCO 10000) to give the product Poly [Poly-(Oxyethylene-1500)-oxy-5-hydroxyisophthaloyl] which was then freeze-dried and characterized with the help of 1 H and 13C Nuclear Magnetic Resonance (NMR) spectroscopy and its particle size was determined by Dynamic Light Scattering (DLS) using zetasizer.
General method of coupling of bromodecane with poly[poly(oxyethylene-1500)-Oxy-5-hydroxyisophthaloyl] (4)
Equimolar quantities of polymers (3) (1.7 g) and bromodecane (0.24 g) were dissolved in dry acetone (10 ml) and to the resultant solution was added equimolar amount of anhydrous potassium carbonate (0.13 g) (Shakil et al. 2010). The reaction mixture was refluxed at 60–70 °C and progress of the reaction was monitored by TLC using ethyl acetate in petroleum ether (30 %). After completion, the potassium carbonate was removed by filtration and the solvent was removed under vacuum to give the final product, Poly[Poly(Oxyethylene-1500)-Oxy-5-dodecanyloxyisophthaloyl] (4).
The 1 H and 13C NMR data of synthesized polymers are reproduced below (Shakil et al. 2010).
Poly [poly-(oxyethylene-1500)-oxy-5-hydroxyisophthaloyl](3)
The polymer was obtained as viscous oil after freeze-drying in 90 % yield.
1 H NMR Data (CDCl3): δ 3.64–3.68 (brs, methylene PEG protons on C-9 and C-10 carbons of the repeating units and on C-α and C-β), 3.82 (t, 2 H, C-8 H), 3.89 (s, 3 H, −COOCH3), 4.45 (t, 2 H, C-7 H), 7.45 (m, 2 H, C-4 H and C-6 H) and 8.19 (s, 1 H, C-2 H).
13C NMR Data (CDCl3): δ 52.69 (−OCH3 end group), δ 61.47 (C-α), 64.41 (C-β), 68.62 (C-8), 70.43 (repeating PEG units carbons), 72.89 (C-7), 119.66 (C-4 and C-6), 122.51 (C-2), 131.64 (C-1 and C-3), 159.20 (C-5), 165.61 (−COOCH2-) and 166.25 (−COOMe).
Poly[poly(oxyethylene-1500)-Oxy-5-dodecanyloxyisophthaloyl](4)
Functionalized copolymer was obtained as viscous oil in 80 % yield.
1 H NMR Data (CDCl3): δ 0.86–0.90 (brs,3 H, C-22 H), 1.26–1.35 (m,C-13 H to 22 H), 1.79–1.82 (m, 2 H, C-12 H), 3.60–3.67 (brs, methylene PEG protons on C-9 and C-10 carbons of repeating units and on C-α and C-β), 3.74 (t, 2 H, C-8 H), 3.94 (s, 3 H,-COOCH3 end group), 4.05 (t, 2 H, C-11 H), 4.51 (t, 2 H, C-7 H), 7.75 (m, 2 H, C-4 H and C-6 H) and 8.26 (s, 1 H, C-2 H).
13C NMR Data (CDCl3): δ 14.09 (C-22), 22.65–31.83 (C-12 to C-21), 52.36 (−OCH3 end group), 61.47 (C-α), 64.41 (C-β), 69.54 (C-8), 69.04 (C-11), 70.04 (repeating PEG units carbons), 72.69 (C-7), 120.04 (C-4 and C-6), 122.94 (C-2), 131.64 (C-1 and C-3), 159.20 (C-5), 165.47 (−COOCH2-) and 166.21 (−COOMe).
Particle size analysis
Particle size analyzer (Zetatrac™) is based on Dynamic Light Scattering (DLS) which detects the fluctuation of the scattering intensity due to the Brownian motion of macromolecules or particles in suspension. DLS measurements were performed at 25 °C and light scattering was detected at a fixed angle. 200 mg L−1 sample solution was prepared in distilled water. 5 ml of the sample solution was taken into a glass vial. Minimum quantity (50 μL) of chloroform was added to the polymer solution and the vial was sonicated for 5 minutes to form a proper emulsion. Dual optical probe technology was used for particle size analysis. Optical light sources were dual solid-state laser diodes in 780 nm (near-infrared) wavelength.
The amphiphilic copolymers when dissolved in water aggregated to form nano-micelles. The particle size and its distribution were determined by Dynamic Light Scattering (DLS) using zeta sizer (Zetatrac™). The radius of gyration (Rg) for the copolymer was found to be in nano range (less than 100 nm). These nano micelles were utilized for the encapsulation of bioactive molecules to develop slow release formulations.
Encapsulation of β-carotene in nanospheres
The solubility of the synthesized polymers and β-carotene was checked by different solvents and dichloromethane was selected, based on its low volatility. Two types of method were used to encapsulate β-carotene. In the first method (F-1), the amphiphilic polymers (1.0 g) and β-carotene (0.10 g, 95 % purity) were dissolved in dichloromethane separately (in 1:10 a.i./polymer w/w ratios) and mixed together in a round bottom flask at room temperature. Then, the solution was stirred for 4 hrs. After removal of the solvent, the residue was dissolved in water and left on stirring for the formation of nano-spheres for another 4 hrs. In this step, some percentage of β-carotene got encapsulated in the amphiphilic polymer and un-encapsulated/non-incorporated β-carotene precipitated out of the water. The second method (F-2) was almost same as the first, the only difference being that instead of stirring, the solution was mixed by using a high shear mixer (IKA-T18 basic, Ultra-Turrax) for 30 minutes in both steps. Hence, the second method consumed less time. The non-incorporated β-carotene was separated from the aqueous layer by filtration. The filtrate was lyophilized to get the encapsulated material.
TEM and SEM analysis
Transmission electron microscopy (TEM) measurements were performed on a JEM 1011(JEOL, Indonesia) instrument operated at an accelerating voltage of 80 kV. Sample for analysis was prepared on carbon-coated copper grids. Scanning Electron Microscopy (SEM) studies was done with EVOMA 10 (Zeiss, Germany) scanning electron microscope. Very small amount of material was mounted on template, which was air dried and subjected to palladium coating. The material was observed under SEM at 20KV and 10 Pa.
Loading capacity and encapsulation efficiency
Weight percent loading was calculated on the basis of the concentration of β-carotene detected in the formulation over the concentration of amphiphilic macromolecules which were added to the solution. It can be represented as:
Encapsulation efficiency was based on the calculation of concentration of β-carotene detected in the formulation over the initial concentration of β-carotene added to make the formulation.
Release kinetics of β-carotene from its encapsulated formulations in water
The release of β-carotene from the developed slow release formulations was determined in two ways, represented by two different experiments. In the first experiment, the release was determined in distilled water (DW). The second experiment represents the release rate study at different pH conditions using buffer solutions of different pH namely 1.8, 6.8 and 7.8. The different pH conditions signify the chemical environment of human gastrointestinal tract (Furr and Clark 1997). The term release has been taken to imply, as the amount of active ingredient recorded at a given time and has been taken as synonymous to release in this study for making different comparisons.
The analytical condition for estimation of β-carotene was standardized to avoid interference of polymers using the same HPLC system as mentioned before. The mobile phase was kept at such a rate that the active ingredient and polymer do not appear very close in the HPLC chromatogram, i.e., Methanol:TBME (70:30 v/v). The literature procedure was followed in both experiments (Shakil et al. 2010).
An accurately weighed quantity of developed formulations of encapsulated materials (each from both methods; about 100 mg) were taken and added to 10 ml water in stoppered volumetric flasks. Both methods here refer to the two different methods (F-1 and F-2) used for encapsulation as already discussed. A control sample containing pure β-carotene was also taken and added in water. Flasks were kept in a Biological Oxygen Demand (BOD) Incubator at 37 ± 1 °C. At different time intervals (0, 1, 3, 5, 7, 9, 23, 24, 26, 28, 30, 32, 48, 50, 52, 54, 70, 73 and 77 hours), aliquots of 1 ml were removed for determination of β-carotene by HPLC, and then unused sample portions were returned to the flasks. In another experiment, an accurately weighed quantity of developed formulations of encapsulated materials (about 50 mg) were taken and added to 5 ml buffer solutions of different pH namely 1.8, 6.8 and 7.8, each in stoppered volumetric flasks representing three different samples P-1, P-2 and P-3 respectively. Control samples C-1, C-2 and C-3 containing pure β-carotene were also taken and added to buffer solutions of different pH namely 1.8, 6.8 and 7.8 respectively. Flasks were kept in a BOD at 37 ± 1 °C. At different time intervals (0, 1, 3, 5, 6, 7, 9, 24, 26, 28, 30, 32, 46, 48, 50, and 52 hours), aliquots of 1 ml were removed for determination of β-carotene by HPLC, and then unused sample portions were returned to the flasks. A standard solution of β-carotene of 100 ppm was also injected in HPLC for quantitative determination for both the experiments.
Analysis of release data
In model-dependant approaches, release data were fitted to five kinetic models including the Baker-Lonsdale (Eq. 1) (Baker and Lonsdale 1974), Hixon–Crowell (Eq. 2) (Hixon and Crowell 1931), Higuchi matrix (Eq. 3) (Higuchi Higuch 1963), first order (Eq. 4) (Narashimhan et al. 1999) and Peppas–Korsmeyer (Eq. 5) (Ritger and Peppas 1987) release equations to find the equation with the best fit.
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
where Mt/Mo is the fraction of active ingredient released at time t, K1, K2, K3, K4 and K5 are the release rate constants of Baker-Lonsdale, Hixon-Crowell, Higuchi matrix, first-order and Peppas–Korsmeyer model, respectively. n is the release exponent applied to Peppas-Korsmeyer model. These models were earlier applied to drugs delivery from miceller matrices (Dash et al. 2010). In the present study, similar types of polymeric matrices were used for encapsulating β-carotene. Therefore, its release data were subjected to these models for understanding its release mechanism.
Determination of the diffusion exponents of the β-carotene in formulations
To describe the kinetics of β-carotene release from slow release formulations, release data were analyzed according to Ritger and Peppas (1987) equation as
| 6 |
where Mt/Mo is the fraction of active ingredient released at time t, K is a constant that incorporates characteristics (porosity, tortuosity) of the macro molecular network system and the active ingredients, and n, a diffusion parameter which is indicative of the transport mechanism. The model has been fitted by taking logarithm on both sides of Eq. 6:
| 7 |
The values of K5 and n were determined from β-carotene release data.
Results
Synthesis and characterization of amphiphilic copolymers
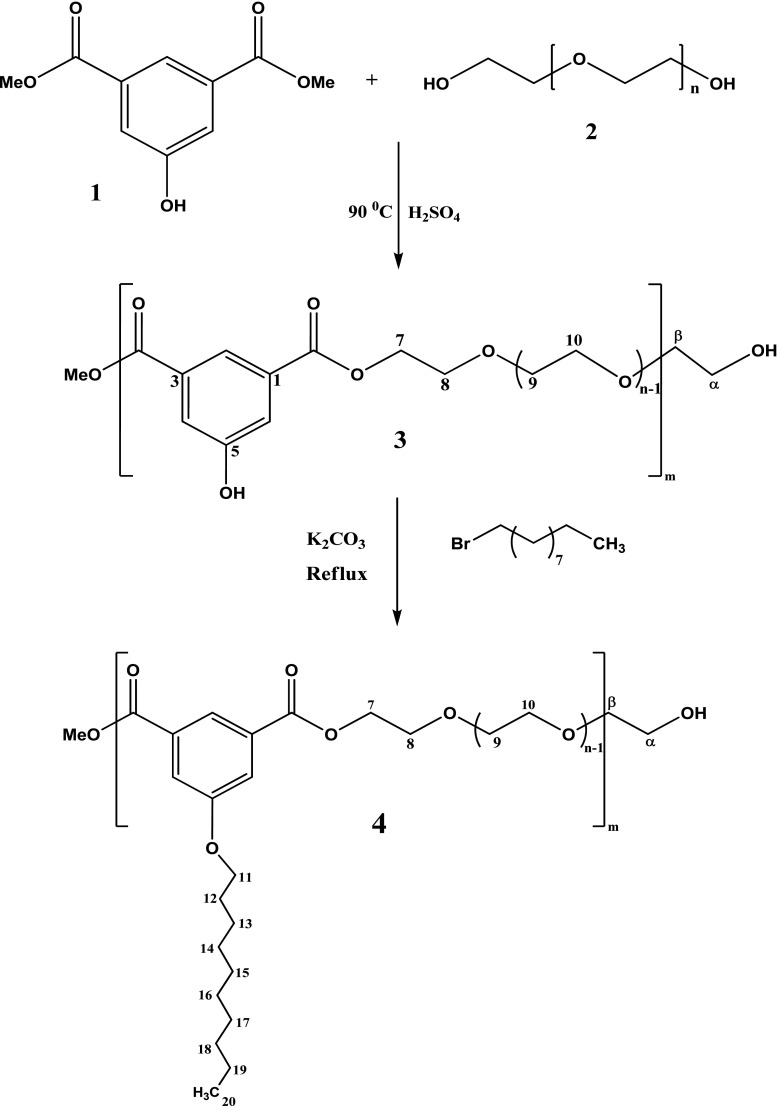
In the present study, functionalized amphiphilic polymers were synthesized using poly(ethylene glycol)-1500 as hydrophilic block and dimethyl 5-hydroxyisophthalate as hydrophobic block in presence of catalyst Conc. H2SO4 as reported by Shakil et al. (2010) (Fig. 1). The structure of the polymer (3) Poly[Poly(Oxyethylene-1500)-oxy-5-hydroxyisophthaloyl] was established from their 1 H and 13C NMR spectrum. Further, this polymer had been derivatized by attaching decanyl chain to phenolic hydroxyl group as reported by Shakil et al. 2010. The resulting amphiphilic polymeric system (4), Poly[Poly(Oxyethylene-1500)-oxy-5-decanylisophthaloyl] were also characterized by 1 H and 13C NMR spectra.
Fig. 1.
General method of polymerization and O-alkylation (functionalisation)
Encapsulation of β-carotene, loading capacity and encapsulation efficiency
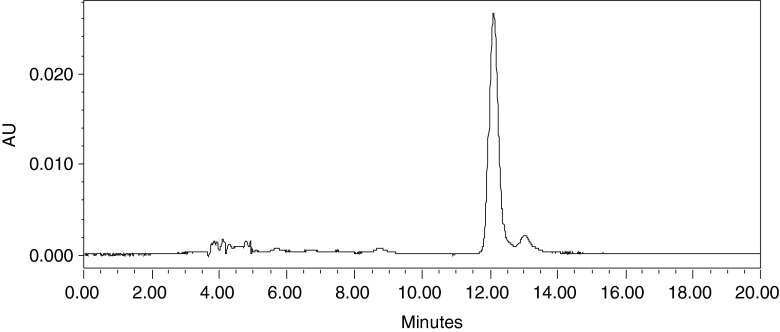
The encapsulation of β-carotene in nano micelles, formed by the self assembly of copolymers, was achieved and confirmed by the HPLC. The RT value along with the absorption maxima matched with the standard β-carotene. The HPLC chromatogram for the encapsulated β-carotene is represented in the Fig. 2. Loading capacity and encapsulation efficiencies were determined for all the formulations using the method as stated above. The loading capacity of the polymer ranged from 2.2 to 2.8 %. The encapsulation efficiencies for different formulations were in the range of 22.60 to 28.08 %.
Fig. 2.
HPLC Chromatogram of encapsulated β-carotene (λmax. 451 nm)
Release of β-carotene from the developed formulation
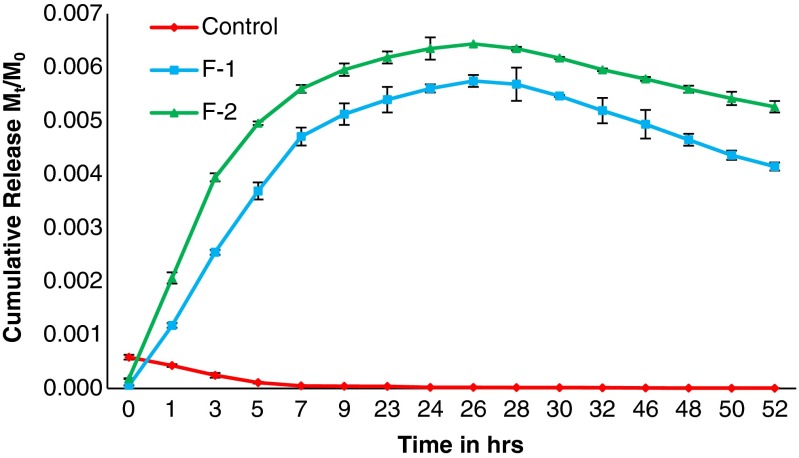
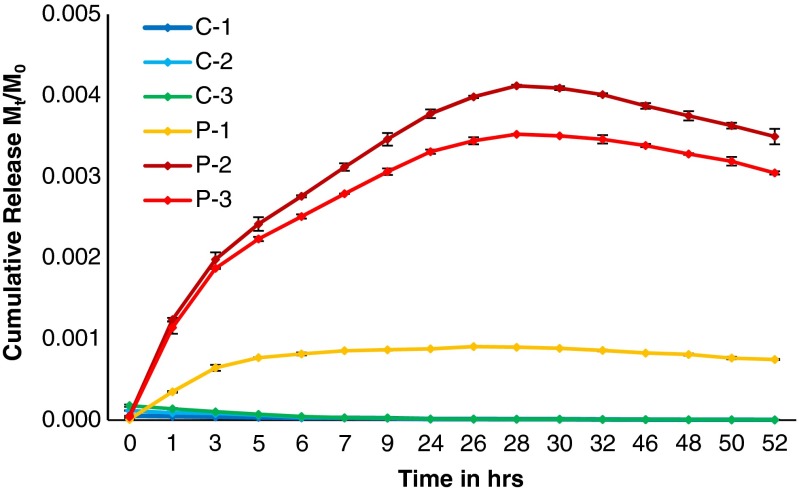
Cumulative release of β-carotene in water from the developed formulations is depicted in Fig. 3. Also, cumulative release of β-carotene from developed slow release formulations in water at different pH is given in Fig. 4. The experiment was done in three replicates and the standard deviation is also shown as error bar in the figures.
Fig. 3.
Cumulative release of β-carotene in water from developed slow release formulations
Fig. 4.
Cumulative release of β-carotene from developed slow release formulations in buffer solution at different pH
The rate of release from developed slow release (SR) formulations was slower, which revealed maximum release of active ingredient after which the content decreased. The maximum concentration of β-carotene was obtained at 26th hour for F-1 formulations and after that the active ingredient (a.i.) content decreased rapidly in water. Similarly in another formulation F-2, maximum release of β-carotene was obtained at 26th hour. At the 46th hour, decrease in concentration of β-carotene started in above formulations. The control sample release profile was such that content of β-carotene kept on decreasing, revealing its insolubility in water. Due to slow release of a.i. from the developed SR formulations, the a.i. was detected till the 52nd hour. After the maximum release of a.i. from all formulations, the decrease in water could be due to degradation.
In case of release in buffer at different pH, β-carotene was released slowly and steadily attaining the maximum concentration obtained at 28th hour for formulations P-2 and P-3 (i.e., at pH 6.8 and 7.8 respectively) and after that the a.i. content started decreasing in water. At the 46th hour, significant decrease in concentration of β-carotene was observed in above formulations. But in case of P-1 formulation at pH 1.8, very low and insignificant release was observed, which remain almost same throughout the experiment.
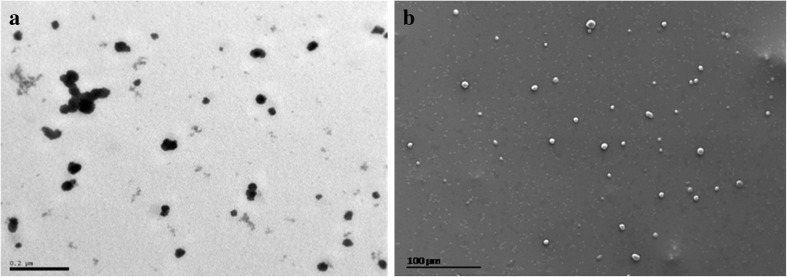
TEM and SEM analysis
TEM technique was used to examine the morphology of formulations prepared. Figure 5a showed individual as well as a number of aggregated spherical nanomicelles. The scanning electron microscope (SEM) images also show spherical and irregular shaped nanomicelles with different sizes (Fig. 5b). Also certain nanomicelles clusters were present which may be due to aggregation of nanomicelles formed during sample preparation, causing variation in a particle size.
Fig. 5.
TEM (a) and SEM (b) micrographs of encapsulated carotene formulation
Discussion
Synthesis and characterization of amphiphilic copolymers
In the polymer (3) Poly[Poly(Oxyethylene-1500)-oxy-5-hydroxyisophthaloyl], the protons of the repeating PEG units (C-9 & C-10) appeared as a broad singlet in the range δ 3.64–3.68, while the two protons of the C-8 methylene group of PEG chain appeared in the range of δ 3.79–3.82. The protons corresponding to the methoxy group appeared as a singlet, albeit with a much reduced intensity, in the range of δ 3.85–3.89 and the two protons of the C-7 methylene group of the PEG chain appeared in the range of δ 4.32–4.49. These methylene protons (C-7) appear downfield compared to the corresponding methylene protons of PEG thereby confirming the formation of the ester linkage (Shakil et al. 2010; Loha et al. 2011; Shuai et al. 2000; Seongbong et al. 2001). Also, in the resulting amphiphilic polymeric system (4), Poly[Poly(Oxyethylene-1500)-oxy-5-decanylisophthaloyl], the appearance of signal at δ 4.10 indicated the presence of a methylene group attached to phenoxy moiety of polymer (i.e., C-11 H), which was not present earlier in the 1 H NMR spectrum of polymers. This showed the coupling of decane chain to the polymer, which was further supported by the appearance of peaks corresponding to other protons of alkyl chain in 1 H NMR spectrum. The huge peak in the region δ 3.66–3.69 represents the PEG main chain protons, except the proton at C-7 H near the ester functionality (which appeared as a triplet at δ 4.51) and the C-8 H, which appeared at δ 3.87. Similarly, all other chemical shift values matched well and structure of alkylated polymers was unambiguously determined. The radius of gyration of the nano-micelles falls within the nano range for the funtionalised amphiphilic copolymers.
Encapsulation of β-carotene, loading capacity and encapsulation efficiency
The highest encapsulation efficiency and loading capacity were observed for the formulation F-2, whereas the lowest one was observed for the formulation F-1. Results show that encapsulation efficiency and loading capacity vary with difference in the process of encapsulation and homogenization was found to be more effective than stirring for encapsulation process. Hence, formulation F-2 was used for the next experiment.
Release of β-carotene from the developed formulation
In case of developed formulations, the a.i. was entrapped in nano micelles, formed by the aggregation of amphiphilic polymers, which protect the a.i. against attack by microbial and other environmental agents such as light, water, oxygen, hydrolysis, oxidation, reduction etc. Due to this, the a.i. release was optimum and for a longer duration. From the release study, it revealed that our slow release formulations was unaffected by the highly acidic conditions referring to the gastric environment of the human body.
To describe the kinetics of a.i. release from micelle, release data were analyzed according to different kinetic equations and regression coefficient values (R2) of all batches are shown in Tables 1 and 2. On analyzing regression coefficient values of all formulations, it was found that all the formulations followed Korsmeyer-peppas model, as it has regression coefficient nearer to one than other models. The parameters (K and n) obtained from β-carotene released in water and different pH conditions are also presented in Tables 1 and 2. There is a good correlation of the release of β-carotene from developed formulations with time up to maximum release level. According to correlation coefficients, we can deduce that the release profiles of β-carotene formulations fit well to the empirical equation. The n values were found in the range of 0.1540 to 0.2342 for slow release formulations, while the K values obtained from the equation ranged from 0.0005 to 0.0019. Values of n close to 0.43 are stated to indicate that the release is diffusion-controlled (Ritger and Peppas 1987). The release data obtained for control sample (pure β-carotene) were very low and so not fitted to any kinetic model, relating to the poor solubility of β-carotene in water.
Table 1.
The constants derived from fitting of the empirical equation Mt./Mo = Ktn to release data of β-carotene in water from slow release formulations
| Formulation | K | n | R2 |
|---|---|---|---|
| F-1 | 0.0019 | 0.2187 | 0.81 |
| F-2 | 0.0014 | 0.2342 | 0.87 |
Table 2.
The constants derived from fitting of the empirical equation Mt./Mo = Ktn to release data of β-carotene in water from slow release formulations at different pH
| Formulation | K | n | R2 |
|---|---|---|---|
| P-1(pH = 1.8) | 0.0005 | 0.154 | 0.60 |
| P-2 (pH = 6.8) | 0.0017 | 0.2299 | 0.83 |
| P-3 (pH = 7.8) | 0.0015 | 0.2272 | 0.86 |
Conclusion
The encapsulation of β-carotene in nano micelles, formed by the self assembly of copolymers, was achieved. Encapsulation conditions for β-carotene were standardized and homogenization was found to be more effective than stirring for encapsulation process.
Furthermore, its slow release formulations were developed successfully and release data revealed significant increase in the solubility and stability in aqueous medium. These results also opened the path for the use of these formulations targeting slow release of β-carotene in human body. Release of β-carotene in buffer of different pH was found to be quite promising, which coincided with the literature regarding the absorption pattern of β-carotene in human body. The results showed that developed slow release formulations were stable in highly acidic conditions referring to the gastric environment of the human body. Furthermore, the release of β-carotene was high at pH 7.8 (referring to human intestinal environment) and a slight increase in release was observed at pH 6.8 (compared to pH 7.8). Similar results were also reported in the literature (Furr and Clark 1997).
Acknowledgments
This work has been supported and funded by NAIP and Indian Council of Agricultural Research (ICAR), New Delhi.
Footnotes
Research Highlights • Slow release formulations of β-carotene, a sparingly soluble anti-oxidant, were developed.
• The encapsulation efficiency and loading capacity of the developed formulations was determined.
• The bioavailability of β-carotene in water increased in developed formulations.
• Release kinetics of developed formulations revealed prolonged stability of β-carotene in water.
• The highly acidic conditions did not have any effect on formulations.
References
- Baker RW, Lonsdale HS. Controlled release of biologically active agents. In: Lacey RE, editor. Tanquary AC. New York: Plenum Press; 1974. pp. 15–17. [Google Scholar]
- Bendich A, Olson JA. Biological actions of carotenoids. FASEB J. 1989;3:1927–1932. [PubMed] [Google Scholar]
- Brazel CS. Microencapsulation offering solutions for the food industry. Cereal Foods World. 1999;44:338–393. [Google Scholar]
- Britton G (1995) UV/visible spectroscopy. In: Britton G, Liaaen-Jensen S, & Pfander H (eds.) Carotenoids, Vol. 1B: Spectroscopy, Birkhauser, Verlag Basel, pp. 13–62
- Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery system. Acta Pol Pharm. 2010;67(3):217–223. [PubMed] [Google Scholar]
- Delgado-Vargas F, Jime’nez AR, Paredes-Lo’pez O. Natural pigments: carotenoids, anthocyanins, and betalains - characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci Nutr. 2000;40:173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- Furr HC, Clark RM. Intestinal absorption and tissue distribution of carotenoids. J Nutr Biochem. 1997;8:364–377. doi: 10.1016/S0955-2863(97)00060-0. [DOI] [Google Scholar]
- Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91(4):317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- Higuch T. Mechanism of sustained-action medication: theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- Hixon AW, Crowell JH. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. 1931;23:923–931. doi: 10.1021/ie50260a018. [DOI] [Google Scholar]
- Kaushik P, Shakil NA, Kumar J, Singh MK, Singh MK, Yadav SK. Development of controlled release formulations of thiram employing amphiphilic polymers and their bioefficacy evaluation in seed quality enhancement studies. J Environ Sci Health Part B. 2013;48(8):677–685. doi: 10.1080/03601234.2013.778614. [DOI] [PubMed] [Google Scholar]
- Kumar R, Shakil NA, Chen MH, Parmar VS, Samuelson LA, Kumar J, Watterson AC. Chemo-enzymatic synthesis and characterization of novel functionalised amphiphilic polymers. J Macromol Sci Pure Appl Chem. 2002;39:1137–1149. doi: 10.1081/MA-120014841. [DOI] [Google Scholar]
- Kumar R, Tyagi R, Parmar VS, Samuelson LA, Kumar J, Watterson AC. Biocatalytic "green" synthesis of PEG-based aromatic polyesters: optimization of the substrate and reaction conditions. Green Chem. 2004;6(10):516–520. doi: 10.1039/b407700h. [DOI] [Google Scholar]
- Loha KM, Shakil NA, Kumar J, Singh MK, Adak T, Jain S. Release kinetics of β-cyfluthrin from its encapsulated formulations in water. J Environ Sci Health Part B. 2011;46:201–206. doi: 10.1080/03601234.2011.540200. [DOI] [PubMed] [Google Scholar]
- Moraru CI, Panchapakesan CP, Huang Q, Takjistov P, Liu S, Kokini JI. Nanotechnology: a new frontier in food science. Food Technol. 2003;57:24–29. [Google Scholar]
- Narashimhan B, Mallapragada SK, Peppas NA. Release kinetics, data interpretation. In: Mathiowitz E., editor. Encyclopedia of controlled drug delivery. New York: Wiley; 1999. [Google Scholar]
- Nishino H, Tokuda H, Satomi Y, Masuda M, Bu P, Onozuka M, Yamaguchi S, Okuda Y, Takagasu J, Tusuruta J, Okuda M, Ichiishi E, Murakoshi M, Kato T, Misawa N, Narisawa T, Takasuka N, Yano M. Cancer prevention by carotenoids. Pure Appl Chem. 1999;71:2273–2278. doi: 10.1351/pac199971122273. [DOI] [Google Scholar]
- Orset S, Leach GC, Morais R, Young AJ. Spray-drying of the microalga dunaliellasalina: effects on beta-carotene content and isomer composition. J Agric Food Chem. 1999;47:4782–4790. doi: 10.1021/jf990571e. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Xu A. Starch-based ingredients for flavor encapsulation. Cereal Food World. 1999;44:460–465. [Google Scholar]
- Quintanilla-Carvajal MX, Camacho-Díaz BH, Meraz-Torres LS, Chanona-Pérez JJ, Alamilla-Beltrán L, Jimenéz-Aparicio A, Gutiérrez-López GF. Nanoencapsulation: a new trend in food engineering processing. Food Eng Rev. 2010;2:39–50. doi: 10.1007/s12393-009-9012-6. [DOI] [Google Scholar]
- Ribeiro HS, Cruz RCD. Highly concentrated carotenoid-containing emulsions. Eng Life Sci. 2005;5:84–88. doi: 10.1002/elsc.200403367. [DOI] [Google Scholar]
- Ramoneda XA, Ponce-Cevallos PA, Buera MDP, Elizalde BE. Degradation of β-carotene in amorphous polymer matrices. Effect of water sorption properties and physical state. J Sci Food Agric. 2011;91(14):2587–2593. doi: 10.1002/jsfa.4497. [DOI] [PubMed] [Google Scholar]
- Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-fickian release from non swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–36. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Huezo ME, Pedroza-Islas R, Prado-Barragan LA, Beristain CI, Vernon-Carter EJ. Microencapsulation by spray drying of multiple emulsions containing carotenoids. J Food Sci. 2004;69:351–359. doi: 10.1111/j.1365-2621.2004.tb13641.x. [DOI] [Google Scholar]
- Schrooyen PMM, van der Meer R, De Kruif CG. Microencapsulation: its application in nutrition. Proc Nutr Soc. 2001;60(4):475–479. doi: 10.1079/PNS2001112. [DOI] [PubMed] [Google Scholar]
- Seongbong J, Shin H, Shung AK, Fisher JP, Mikos AG. Synthesis and characterization of (oligo (polyethylene glycol) fumarate) macromer. Macromolecules. 2001;34:2839–2844. doi: 10.1021/ma001563y. [DOI] [Google Scholar]
- Shahidi F, Han XQ. Encapsulation of food ingredients. Crit Rev Food Sci Nutr. 1993;33(6):501–547. doi: 10.1080/10408399309527645. [DOI] [PubMed] [Google Scholar]
- Shakil NA, Singh MK, Pandey A, Kumar J, Pankaj Parmar VS, Singh MK, Pandey RP, Watterson AC. Development of poly(ethylene glycol) based amphiphilic copolymers for controlled release delivery of carbofuran. J Macromol Sci Pure Appl Chem. 2010;47:241–247. doi: 10.1080/10601320903527038. [DOI] [Google Scholar]
- Shuai X, Zbigniew Jedlinski, Luo Q, Farhod N. Synthesis of novel block copolymers of poly(3- hydroxybutyric acid) with poly(ethylene glycol) through anionic polymerization. Chinese J Polym Sci. 2000;18(1):19–23. [Google Scholar]
- Wagner LA, Warthesen JJ. Stability of spray-dried encapsulated carrot carotenes. J Food Sci. 1995;60(5):1048–1053. doi: 10.1111/j.1365-2621.1995.tb06290.x. [DOI] [Google Scholar]
- Xin-Yuan S, Tian-Wei T. Preparation of chitosan/ethylcellulose complex microcapsule and its application in controlled release of vitamin D2. Biomaterials. 2002;23:4469–4473. doi: 10.1016/S0142-9612(02)00165-5. [DOI] [PubMed] [Google Scholar]