Abstract
Enzyme browning is the main challenge in the preparation of fresh apple juice. The influence of sonication on browning, as well as polyphenols and antioxidant activity of fresh apple juice was investigated. It was found that ultrasound can inhibit the browning of fresh apple (Malus pumila Mill, cv. Red Fuji) juice, but decreased the contents of total phenolic content (TPC), total flavonoid content (TFC) and chlorogenic acid and reduced the antioxidant activity. On the whole, ultrasound technology cannot be used to the antibrowning of fresh apple (Malus pumila Mill, cv. Red Fuji) juice.
Keywords: Fresh apple juice, Ultrasound, Polyphenols, Antioxidant activity, Browning index, Polyphenol oxidase
Introduction
Fresh apple juice is one of the most popular fresh juices due to natural viridian, pleasant taste, fresh flavor, nutritional value and an important source of phenolics (e.g. epicatechin and chlorogenic acid). Fresh apple juice are usually processed by squeezing machines in restaurants and then directly served to consumers in a glass without preservatives, heat or refrigeration. The pleasant aroma and bioactive compounds of freshly-squeezed apple juice is noticeably better than thermally processed juices and reconstituted from concentrate when they are immediately dinked after squeezed.
However, we usually have the experience in a restaurant that the delicate color and uniform appearance of fresh apple juice had changed to brown and layers just in a few minutes. Enzyme browning is the main challenge of fresh apple juice. Therefore, development of effective strategies except for heat, preservatives to inhibit browning of fresh apple juice has been a critical part in the preparation process in the meantime keeping the fresh characteristics. Some literatures reported that addition of other foods or food compositions can prevent the browning of fresh apple juice. For example, it is usual in restaurants the addition of a little milk to apple juice and shake it, which can prevent the browning. Lozanodegonzalez et al. (1993) found that addition of a little pineapple prevented the browning of fresh apple juice, Suh et al. (2011) found that natural phytochemicals from Rumex crispus L. seed prevented the browning of apple juice; López-Nicolás et al. (2007) found that maltosyl-β –cyclodextrin prevented fresh apple juice enzymatic browning. However, the methods above mentioned altered the chemical composition of fresh juice, and the ingestion of fruit in association with milk impaired the antioxidant properties of fruit and reduced the absorption of phenolic compounds Serafini et al. (2009). Hence, to seek a physical method to prevent browning of fresh apple juice is highly desirable.
To date, various innovative technologies have been explored for improvement of nutritional and sensorial qualities of fruits juice. Among these technologies ultrasound treatment is considered to be inexpensive, simple, reliable, environmentally friendly and highly effective in achieving microbial decontamination (Arroyo et al. 2012; Gabriel 2012), inactivation of enzymes (Jang and Moon 2011; O’Donnell et al. 2010; De Gennaro et al. 1999), stability of juice cloud and improvement of viscosity (Vercet et al. 2002; Seshadri et al. 2003), improvement of bioactive compounds (Bhat et al. 2011). But there are no reports on the effects of ultrasound on anti-browning of fresh apple juice.
The aim of this work was to investigate the influence of ultrasound on the browning of fresh apple juice, explore the antibrowning mechanism of ultrasound, and further analyze the effects of ultrasound on polyphenols and antioxidant activities.
Materials and methods
Chemicals
Catechol, chlorogenicacid, Trolox (6-Hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid), gallic acid and rutin were purchased from Sigma Aldrich Co. (St. Louis, MO, USA). HPLC-grade acetonitrile and acetic acid was purchased from Merk (Darmstadt, Germany). Polyvinylpyrrolidone (PVPP), fluoresdein disodium salt, 2, 2-azobis (2-methylpropionamidine) dihydrochloride (AAPH), Folin–Ciocalteu phenol reagent and other reagents were analytical grade purchased from Aladdin Reagent Database Inc. (Shanghai, China).
Fruits
All of apple (Malus pumila Mill, cv. Red Fuji, Yantai, China) used in the experiments were purchased from Wal-Mart (Hangzhou, China) once, then stored at 4 °C freezer.
Preparation of fresh apple juice
Three apples were rapidly and mechanically cored, diced into about 2*2*2 cm cubes, then mixed and packaged with YS-DO-400 vacuum package machine (Youngsun Intelligent Equipment Co., Ltd., Hangzhou, China). All of the diced apples were stored at 4 °C refrigerator. Took a package each time, freshly squeezed with KP60DP centrifugal juicer (Kejialin Electrical Appliance MFG Co., LTD, Foshan, China) and got the apple juice (50 mL per time). The juice was dispensed into 2 glass tube (internal diameter was 2 cm and a depth of 9.5 cm), one of them was treated by ultrasound and the other was used as control.
Ultrasound treatments
Ultrasound treatments (US) were carried out with a probe ultrasonic processor (JY92-IIDN, Ningbo Scientz Biotechnology Co., Ningbo, China) with a 6 mm diameter probe. The highest output of ultrasound power is 900 W, frequency is 20 ~ 25 kHz. 20 mL juice prepared in section “Preparation of fresh apple juice” was added to glass tubes (2 cm diameter × 9.5 cm height), then the tubes with juice were immersed in low-temperature thermostatic bath (DC-1006, Safe Corporation, Ningbo, China) to maintain temperature constant. Apart from the single factor experiments of time and temperature, the general ultrasound conditions were: the treatment time of 10 min, ultrasonic intensity of 2.0 W/cm2 determined by calorimetric method, which is equal to 180w input power, temperature of 15 °C, pulsed mode of 2 s on and 2 s off. The main reason of using pulse mode of ultrasonic treatment is that the performance of the ultrasound transducer under continuous ultrasound decrease with time, but the performance of ultrasound transducer under pulsed ultrasound is the constant, which was found by Sun and Ye (2013). The probe was placed 1 cm from the top surface of apple juice. The sample at the same conditions except for ultrasound treatment was used as control check (CK).
Browning index measurements
The browning index (BI) of the fresh apple juice was determined by the spectrophotometric assay described by Tiwari et al. (2009). 5 mL of ethyl alcohol at 4 °C was added to 5 mL of sample, centrifuged for 10 min at 10,000 × g (10,900 rpm) (CF16RX, Hitachi Koki Co., LTD, Japan), then the absorbance of the supernatant was measured at 420 nm in UV-2550 spectrophotometer (Shimadzu Co., Kyoto, Japan). The browning index was described by absorbance, the bigger the absorbance is, the bigger browning index is.
Polyphenol oxidases (PPO) and peroxidase (POD) assay
Enzyme extract was obtained by the following method. 5 g apple juice prepared in section “Ultrasound treatments” was added to a 15 mL tapered plastic centrifuge tube containing 5 mL of phosphate buffer(50 mM, pH 6.5) with addition of 4 % PVPP(w/v), shook thoroughly. The buffer solution was cooled at 4 °C until used. And then the tubes were centrifuged for 10 min, at 10,000 × g (10,900 rpm), 4 °C, the supernatants were adjusted to 10 mL with cooled phosphate buffer. Enzymatic activity assays were conducted by measuring the rate of change in absorbance for 3 min at different wavelengths (variable for different enzymes) in UV-2550 spectrophotometer (Shimadzu Co., Kyoto, Japan). A unit of enzyme activity was defined as the increase of 0.01 per minute in the absorbance value under the conditions; specific activity of the enzyme was expressed as units per milligram protein. PPO activity was measured at 425 nm. The reaction mixture contained 1.0 mL of fresh 50 mM catechol, 1.0 mL phosphate buffer and 1.0 mL of the enzyme extract. Catechol solution and phosphate buffer was balanced at 30 °C before used. POD activity was measured at 470 nm. 1.0 mL of enzyme extract was mixed with substrate solution which contained 2 mL of phosphate buffer with 1.0 % (w/w) frozen guaiacol (bathed at 30 °C before used) and 150 μL of 0.15 %(w/w) hydrogen peroxide.
Total phenolic content (TPC) and total flavonoid content (TFC) analysis
The total phenolic content (TPC) was determined by the Folin–Ciocalteu method. Briefly, 5 g juice prepared in section “Ultrasound treatments” was mixed with 2 mL 20 % trichloroacetic acid (v/v), as an enzyme inactivation for the precipitation of proteins, centrifuged at 4000 × g for 20 min. The supernatant was transferred into the 10 mL volumetric flask and filled up to the mark with distilled water. This solution was marked as phenolic extract (PE). One milliliter of extract was added to a 25 mL colorimetric cylinder containing 0.5 mL of Folin-Ciocalteu reagent, shook thoroughly. After 5 min standing, 5 mL of 5 % Na2CO3 solution was added and mixed with a vortex shaker, using distilled water to make up the total volume of 25 mL. After 90 min, the absorbance at 750 nm was measured in UV-2550 spectrophotometer (Shimadzu Co., Kyoto, Japan) using distilled water as blank agent. A calibration curve was prepared by using a standard solution of gallic acid, and the results of total phenols were expressed as mg gallic acid equivalents (GAE) per 100 g of juice.
The total flavonoid content (TFC) was evaluated by the following method. Briefly, 1 mL PE was added to 10 mL volumetric flask containing 4.0 mL of dH2O, stirred well; Then mixed sufficiently with 5 % NaNO2(w/v), stood for 5 min; added 0.3 mL of 10 % Al(NO3)3, stood for another 5 min; added 4 mL 1 mol/L NaOH solution and added up to 10 mL with dH2O. After 10 min at room temperature, the absorbance was measured at 510 nm. The results were expressed in mg rutin equivalents per 100 g of fresh sample weight (mg RE/100 g) according a calibration curve using rutin as standard solution.
Chlorogenic acid analysis
The analysis of chlorogenic acid was performed on an Alliance 2695 separations module linked simultaneously to a PDA 2996 detector (Waters, Milford, MA) equipped with Agilent C18 analytical column (5 μm,4.6 mm × 250 mm) and a pre-column (5 μm, 4.6 mm × 30 mm, C18). 1 mL PE was filtered through 0.45 μm polyvinylidene fluoride microfiltration membrane (Shanghai xingya purification material Co., Shanghai, China), then stored at −18 °C for further HPLC analysis. The solvent system contained of 3 % acetic acid solution (A) 100 % acetonitrile (B), gradient elution was employed under the following conditions: 6–14 % B, 1.5–0.8 mL/min of flow rate from 0 to 5 min; 14–18 % B, 0.8–1.0 mL/min of flow rate from 5 to 8 min; 18–20 % B, 1.0–0.8 mL/min of flow rate from 8 to 10 min; held at 20 % B, 0.8 mL/min of flow rate from 10 to 15 min, 20–24 % B, 0.8–1.2 mL/min of flow rate from 15 to 20 min; followed by column equilibration from 21 to 30 min, oven temperature was set at 37 °C.
Oxygen radical absorption capacity assay (ORAC)
The ORAC assay (Ou et al. 2001; Prior et al. 2003) was carried out on a fluorescence micro-plate reader (Fluoroskan Ascent FL, Thermo Fisher Scientific Inc., MA, USA) with 96-well plates Thermal degradation of AAPH produces peroxyl radicals (ROO·), which oxidize fluorescer resulting in the loss of fluorescence. Antioxidants that can scavenge peroxyl radicals protect the fluorescent molecule from peroxyl-radical-induced oxidative reaction. The degree of inhibition of the oxidation was used to calculate antioxidant activity. Preliminary experiments were performed to determine concentration of sample as well as the ratio between sample and the dosage of AAHP, not only to optimize reproducibility and linearity between juice extract concentration and ORAC, but also to ensure reaction to be completed (fluorescer was oxidized completely) within 120 min.
The micro-plate reader was programmed to record the fluorescence of FL (25 μL, 504 nM, final concentration was 12.8 mM per hole) at 485 nm excitation and 538 nm emission every 2 min after addition of 150 μL AAPH (17.07 mM, and gave a final AAPH concentration of 12.8 mM) to start the reaction. The total volume of the reaction system was 200 mL, containing 25 μL phosphate buffer (75 mM, pH 7.4), and the reaction was carried out at 37 °C, all of regents were dissolved in the phosphate buffer. The areas under the average fluorescence-reaction time kinetic curve (AUC) for both blank and samples were integrated and final antioxidant activity results were calculated using a water soluble analogue of Trolox as a standard, expressed as micromole Trolox equivalents (TE) per 100 g of fresh apple juice.
Statistical analysis
Each treatment was replicated triplicate times. The results were expressed as mean ± SD. All the data were subjected to statistical analyses using SPSS Statistics versions 16.0. Mean values were considered significantly different when p < 0.05.
Results and discussion
Effects of ultrasound on the browning of fresh apple juice
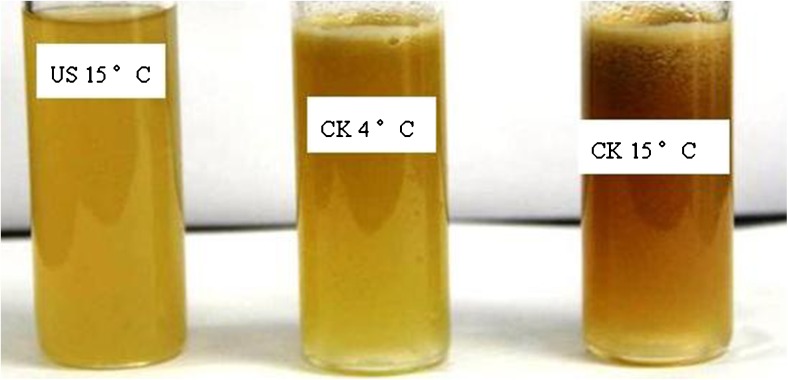
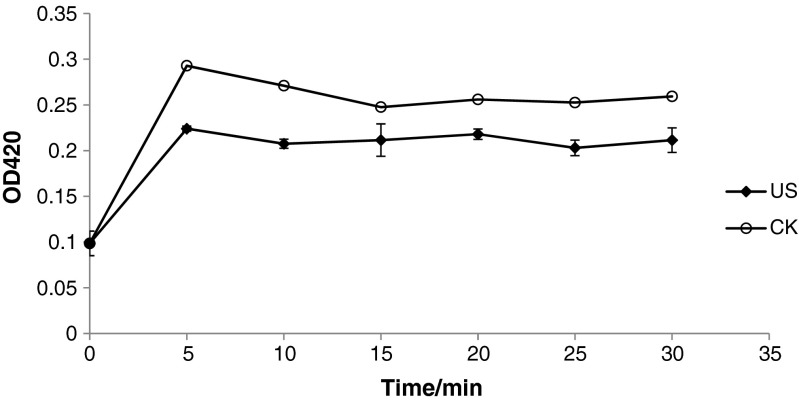
The fresh apple juices under ultrasound treatment at 15 °C, untreated at 4 °C, untreated at 15 °C were shown in Fig. 1. It was obviously observed that the appearance including the color and homogeneity of fresh apple juice treated by ultrasound was better than untreated at two temperatures, which had lower browning, no layers and foams. Figure 2 also indicated that the browning index of fresh apple juice was higher than untreated at15 °C. So ultrasound could inhibit the browning of the fresh apple juice. The studies reported in literatures on effects of ultrasound on fruits or fruits juices are only in initial level and the results in different literatures were not consistent. Jang et al. (2009), Jang and Moon (2011) found that ultrasound in combination with ascorbic acid showed a higher antibrowning rate, but ultrasound alone had a higher browning on fresh-cut apple. Baslar and Ertugay (2013) found that apple juice treated by ultrasound at 40 °C did not change the colour, but at 50 and 60 °C was increased the L value. Valdramidis et al. (2010) found that low temperatures and intermediate amplitude resulted in lower non-enzymatic browning (NEB), but the NEB rate significantly increased with processing temperature while at intermediate ultrasound amplitudes and the NEB rate appeared to be lower than at higher or lower amplitude levels for the same temperatures.
Fig. 1.
Fresh apple juice treated by ultrasound at 15 °C and untreated at 4 and 15 °C
Fig. 2.
Effects of ultrasound on the browing index of fresh apple juice
Effects of ultrasound on the activity of PPO and POD of fresh apple juice
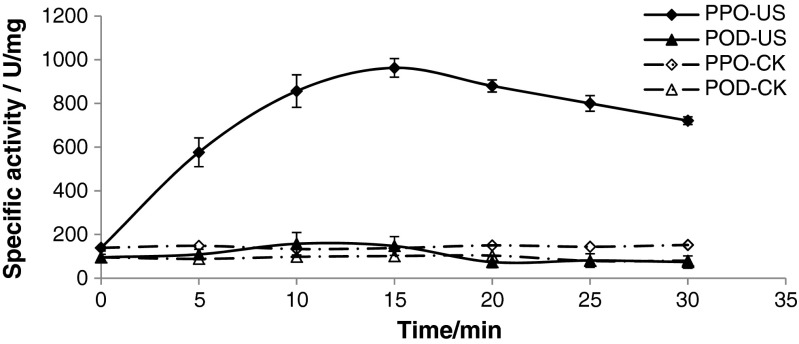
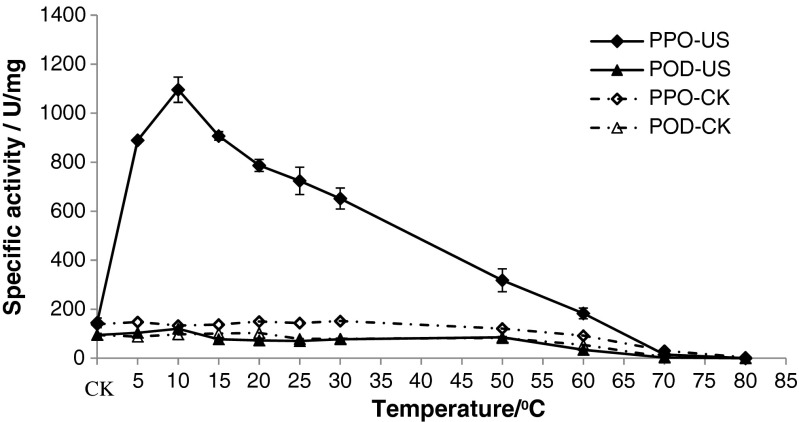
It is well known that the browning of apple juice is mainly caused by enzyme browning catalyzed by polyphenol oxidases (PPO) and peroxidase (POD). For further analysis the mechanism of antibrowning of ultrasound mentioned above, the specific activities of PPO and POD were investigated (shown in Fig. 3). The results were beyond our hypothesis, which indicated that the activity of PPO was increased and the activity of POD did not changed significantly (P < 0.01), and increasing rate of PPO activity decreased with temperature (Fig. 4). The activity of PPO and POD under ultrasound in different literatures was inconsistent. Fonteles et al. (2012) found that applying ultrasound power intensity of 376 W/cm2 for 10 min at 4 °C resulted in significant reduction of POD and PPO activities; De Gennaro et al. (1999) found that the action of ultrasounds in combination with conventional heat treatment at 80 °C is quite an active in deactivating peroxidase of horseradish. Ercan and Soysal (2011) found that as the ultrasonic power increased, inactivation rate increased. 100 % POD inactivation was observed at 50 % power for 150 s and at 75 % power for 90 s of ultrasonication at 63–67 °C. Lopez et al. (1994) found that the combined effects of heat and ultrasonic waves reduced enzyme (peroxidase, polyphenol oxidase, and lipoxygenase) resistance and the enzyme destruction efficiency of the combined process greatly increased with ultrasonic wave amplitude. However, Cheng et al. (2007) found that sonication at 20 °C gave rise to a greater PPO activity. Jang and Moon (2011) found that the combined treatment of ultrasound and ascorbic acid inactivated monophenolase, diphenolase, and peroxidase of fresh-cut apple, whilst the individual treatment of ultrasound or ascorbic acid had inverse and limited inhibitory effect on the enzymes. Baslar and Ertugay (2013) found that some levels of ultrasound increased the total PPO activity and some levels of ultrasound decreased the total PPO activity. The difference of the results depends upon several factors such as fruit juice composition, enzyme type, pH and processing parameters. The mechanism of activation of PPO maybe that a low ultrasound power level enhance the disruption of biological cell walls to facilitate the release of their contents and moreover, low power levels can induce stimulation of enzymes (O’Donnell et al. 2010), and the mechanism (Chandrapala et al. 2012) of inactivation of PPO maybe that defragmentation of the enzyme or formation into aggregates of monomeric enzymes, or polymeric enzymes tend to fragment into monomeric subunits. Which attributed to the cavitation effects generated by bubble collapse (mechanical, thermal, chemical), or the extreme agitation created by microstreaming could disrupt Van der Waals interactions and hydrogen bonds in the polypeptide or free radical mediated deactivation. But which conditions PPO and POD is activated or inactivated under ultrasound need further investigations.
Fig. 3.
Effects of ultrasound treatment time on the activity of PPO and POD of fresh apple juice
Fig. 4.
Effects of ultrasound treatment tempetature on the activity of PPO and POD of fresh apple juice
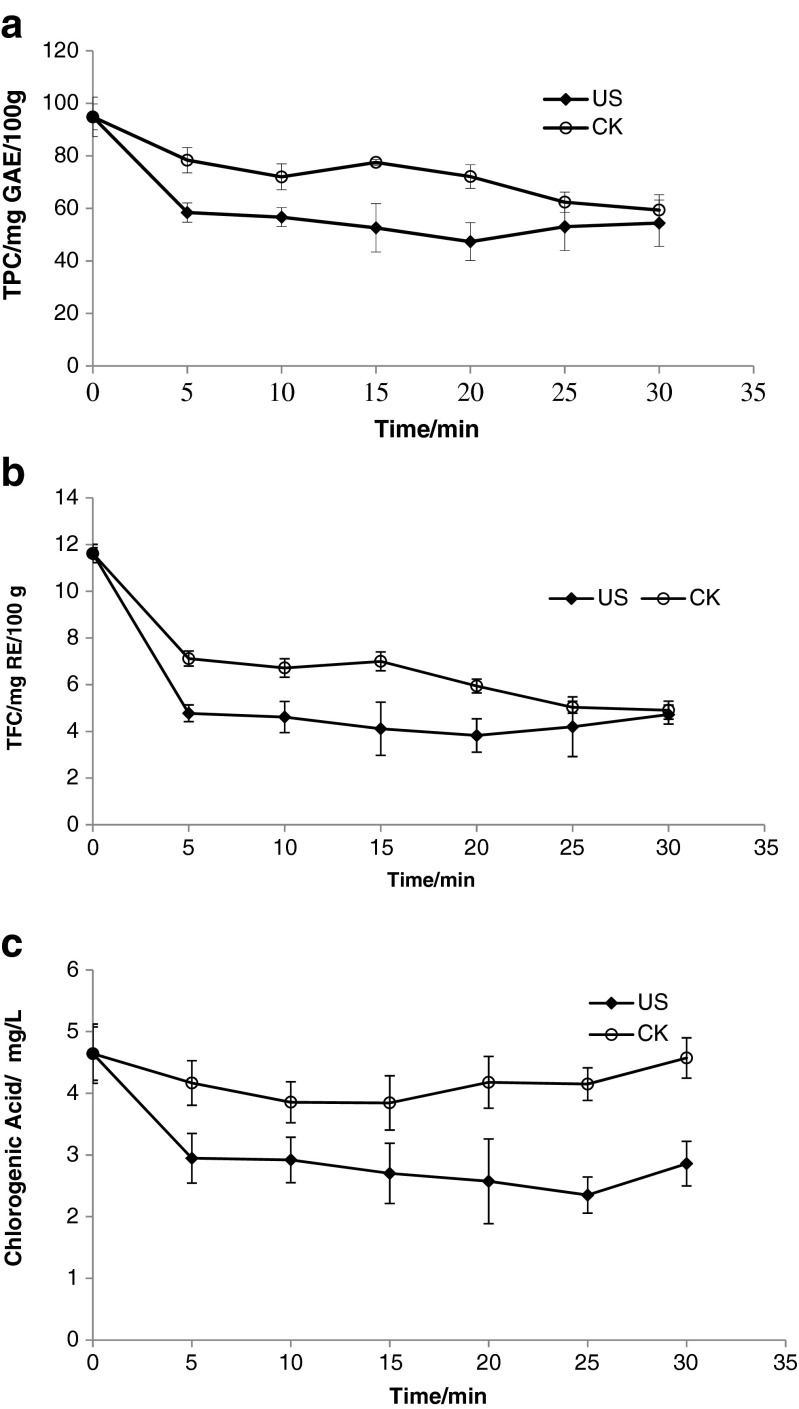
The effects of ultrasound on the total phenolic contents, total flavonoids contents and chlorogenic acid of fresh apple juice were shown in Fig. 5. It is shown that TPC, TFC and chlorogenic acid of fresh apple juice after ultrasound treatment was degraded higher than control. Figure 5a showed that the total phenolic content of fresh apple juice untreated decreased with time and the total phenolic content of fresh apple juice treated by ultrasound firstly decreased, then increased with time. The total flavonoids content and chorogenic acid of fresh apple juice untreated and treated had the similar variation trend (Fig.5-c). The results may be due to the combination of free radicals of ultrasound and extraction of ultrasound. Free radicals of ultrasound increased the degradation of polyphenol, and the polyphenol extraction of ultrasound brought more polyphenol. The results were different with literatures. Bhat et al. (2011) reported that sonication of kasturi lime (Citrus microcarpa) juice showed enhancement in total phenolics. Caminiti et al. (2011) found that ultrasound did not influence total phenolics of apple and cranberry juice blend. Tiwari et al. (2008) reported that anthocyanin of strawberry juice increased at low ultrasonic intensity and degradation at high ultrasonic intensity.
Fig. 5.
Effects of ultrasound on TPC (a), TFC (b), and chlorogenic acid (c) of fresh apple juice
Effects of ultrasound on antioxidant activity of fresh apple juice
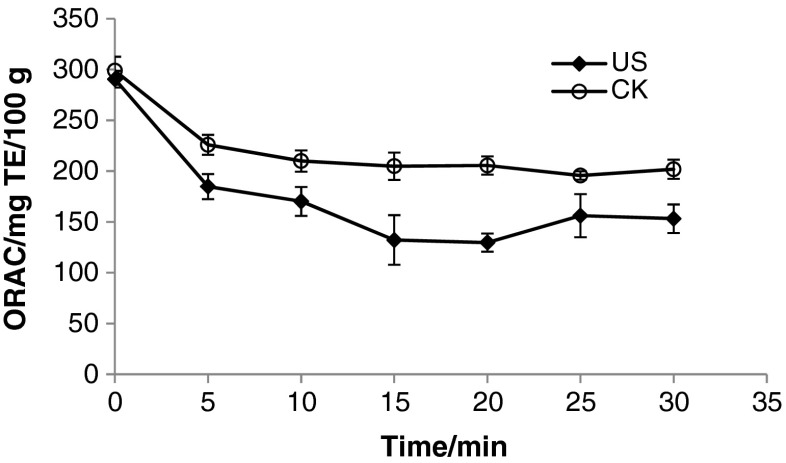
The effect of ultrasound on the antioxidant activity of fresh apple juice was also investigated. Antioxidant activity of fresh apple juice was evaluated by oxygen radical absorption capacity assay. It was found that the antioxidant activity of fresh apple juice after ultrasound treatment was reduced higher than untreated, and had similar variation trend as TPC, TFC and chlorogenic acid mentioned above (Fig. 6). The reason is obvious, which is caused by the decrease of TPC, TFC and chlorogenic acid. There are few studies on effects of ultrasound on antioxidant activity. Caminiti et al. (2012) found that ultrasound had no influence on antioxidant activity of orange and carrot juice blend. Bhat et al. (2011) found ultrasound increased antioxidant activity of kasturi lime (Citrus microcarpa) juice.
Fig. 6.
Effects of ultrasound on antioxidant activity of fresh apple juice
Based on above results, it is obviously that ultrasound can reduce browning rate, but increased the PPO activity, which looks like contradictory. Enzyme browning of fresh apple juice is depended on three parameters: PPO activity, oxygen contents, and phenolic compounds contents. Although the PPO activity increased but oxygen in solution and intercellular spaces of pulp particles during ultrasound treatment is removed, enzyme browning is still weak. The reduction of phenolic compounds and antioxidant activity of fresh apple juice may be not mainly caused by enzyme browning and may be degraded by the free radicals brought by acoustic cavitations. It can be concluded that ultrasound degassing impaired enzymatic browning and free radicals caused by the acoustic cavitations brought the reduction of phenolic compounds and antioxidant activity.
Conclusions
Ultrasound could inhibit the browning of the fresh apple (Malus pumila Mill, cv. Red Fuji) juice to a certain extent, but at the same time it accelerated the degradation of polyphenols and decrease of antioxidant activity of fresh apple (Malus pumila Mill, cv. Red Fuji) juice. So ultrasound cannot be applied to the antibrowning of fresh apple (Malus pumila Mill, cv. Red Fuji) juice.
Acknowledgments
This project was supported by National Natural Science Foundation of China (31401474 and 31471592) and National Key Technology R&D Program (2012BAD31B06).
References
- Arroyo C, Cebrián G, Pagán R, Condón S. Synergistic combination of heat and ultrasonic waves under pressure for cronobacter sakazakii inactivation in apple juice. Food Control. 2012;25:342–348. doi: 10.1016/j.foodcont.2011.10.056. [DOI] [Google Scholar]
- Baslar M, Ertugay MF. The effect of ultrasound and photosonication treatment on polyphenoloxidase (PPO) activity, total phenolic component and colour of apple juice. Int J Food Sci Technol. 2013;48:886–892. doi: 10.1111/ijfs.12015. [DOI] [Google Scholar]
- Bhat R, Kamaruddin NSBC, Liong MT, Karim AA. Sonication improves kasturi lime (citrus microcarpa) juice quality. Ultrason Sonochem. 2011;18:1295–1300. doi: 10.1016/j.ultsonch.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Caminiti IM, Noci F, Muñoz A, Whyte P, Morgan DJ, Cronin DA, Lyng JG. Impact of selected combinations of Non-thermal processing technologies on the quality of an apple and cranberry juice blend. Food Chem. 2011;124:1387–1392. doi: 10.1016/j.foodchem.2010.07.096. [DOI] [Google Scholar]
- Caminiti IM, Noci F, Morgan DJ, Cronin DJ, Lyng JG. The effect of pulsed electric fields, Ultraviolet Light or High Intensity Light Pulses in Combination with Manothermosonication on Selected Physico-Chemical and Sensory Attributes of an Orange and Carrot Juice Blend. Food Bioprod Process. 2012;90:442–448. doi: 10.1016/j.fbp.2011.11.006. [DOI] [Google Scholar]
- Chandrapala J, Oliver C, Kentish S, Ashokkuma M. Ultrasonics in food processing: food quality assurance and food safety. Trends Food Sci Technol. 2012;26:88–98. doi: 10.1016/j.tifs.2012.01.010. [DOI] [Google Scholar]
- Cheng LH, Soh CY, Liew SC. Effects of sonication and carbonation on guava juice quality. Food Chem. 2007;104:1396–1401. doi: 10.1016/j.foodchem.2007.02.001. [DOI] [Google Scholar]
- De Gennaro L, Cavella S, Romano R, Masi P. The use of ultrasound in food technology I: inactivation of peroxidase by thermosonication. J Food Eng. 1999;39:401–407. doi: 10.1016/S0260-8774(99)00028-X. [DOI] [Google Scholar]
- Ercan SS, Soysal C. Effect of ultrasound and temperature on tomato peroxidase. Ultrason Sonochem. 2011;18:689–695. doi: 10.1016/j.ultsonch.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Fonteles TV, Costa MGM, de Jesusde ALT, Miranda MRA, Fernandes FAN, Rodrigues S. Power ultrasound processing of cantaloupe melon juice: effects on quality parameters. Food Res Int. 2012;48:41–48. doi: 10.1016/j.foodres.2012.02.013. [DOI] [Google Scholar]
- Gabriel AA. Microbial inactivation in cloudy apple juice by multi-frequency dynashock power ultrasound. Ultrason Sonochem. 2012;19:346–351. doi: 10.1016/j.ultsonch.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Jang JH, Kim ST, Moon KD. Inhibitory effects of ultrasound in combination with ascorbic acid on browning and polyphenol oxidase activity of fresh-cut apples. Food Sci Biotechnol. 2009;18:1417–1422. [Google Scholar]
- Jang JH, Moon KD. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011;124:444–449. doi: 10.1016/j.foodchem.2010.06.052. [DOI] [Google Scholar]
- Lopez P, Sala FJ, de la Fuente JL, Condbn S, Raso J, Burgos J. Inactivation of peroxidase, lipoxygenase, and polyphenol oxidase by manothermosonication. J Agric Food Chem. 1994;42:252–256. doi: 10.1021/jf00038a005. [DOI] [Google Scholar]
- Lozanodegonzalez PG, Barrett DM, Wrolstad RE, Durst RW. Enzymatic browning inhibited in fresh and dried apple rings by pineapple juice. J Food Sci. 1993;58:399–404. doi: 10.1111/j.1365-2621.1993.tb04284.x. [DOI] [Google Scholar]
- López-Nicolás JM, Núñez-Delicado E, Pérez-López AJ, Sánchez-Ferrer Á, García-Carmona F. Reaction’s mechanism of fresh apple juice enzymatic browning in the presence of maltosyl-β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2007;57:219–222. doi: 10.1007/s10847-006-9191-1. [DOI] [Google Scholar]
- O’Donnell CP, Tiwari BK, Bourke P, Cullen PJ. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci Technol. 2010;21:358–367. doi: 10.1016/j.tifs.2010.04.007. [DOI] [Google Scholar]
- Serafini M, Testa MF, Villaño D, Pecorari M, van Wieren K, Azzini E, Brambilla A, Maiani G. Antioxidant activity of blueberry fruit is impaired by association with milk. Free Radic Biol Med. 2009;46:769–774. doi: 10.1016/j.freeradbiomed.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Seshadri R, Weiss J, Hulbert GJ, Mount GJ. Ultrasonic processing influences rheological and optical properties of high methoxyl pectin dispersions. Food Hydrocoll. 2003;17:191–197. doi: 10.1016/S0268-005X(02)00051-6. [DOI] [Google Scholar]
- Suh HJ, Park S, Park S. Inhibition of browning on fresh apple juices by natural phytochemicals from rumex crispus L. Seed J Korean Soc Appl Biol Chem. 2011;54:524–530. doi: 10.3839/jksabc.2011.080. [DOI] [Google Scholar]
- Sun YJ, Ye XQ. Enhancement or reduction of sonochemical activity of pulsed ultrasound compared to continuous ultrasound at 20 kHz? Molecules. 2013;18:4858–4867. doi: 10.3390/molecules18054858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari BK, Muthukumarappan K, O’Donnell CP, Cullen PJ. Inactivation kinetics of pectin methylesterase and cloud retention in sonicated orange juice. Innov Food Sci Emerg Technol. 2009;10:166–171. doi: 10.1016/j.ifset.2008.11.006. [DOI] [Google Scholar]
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4926. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang D, Ou B, Jacob R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC FL)) of plasma and other biological and food samples. J Agric Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Tiwari BK, O’Donnell CP, Patras A, Cullen PJ. Anthocyanin and ascorbic acid degradation in sonicated strawberry juice. J Agric Food Chem. 2008;56:10071–10077. doi: 10.1021/jf801824v. [DOI] [PubMed] [Google Scholar]
- Valdramidis VP, Cullen PJ, Tiwari BK, O’Donnell CP. Quantitative modelling approaches for ascorbic acid degradation and Non-enzymatic browning of orange juice during ultrasound processing. J Food Eng. 2010;96:449–454. doi: 10.1016/j.jfoodeng.2009.08.025. [DOI] [Google Scholar]
- Vercet A, Sanchez C, Burgos J, Montañés L, Buesa PL. The effects of manothermosonication on tomato pectic enzymes and tomato paste rheological properties. J Food Eng. 2002;53:273–278. doi: 10.1016/S0260-8774(01)00165-0. [DOI] [Google Scholar]