Abstract
Use of dimethyl sulfoxide (DMSO) as a suitable extraction solvent under the optimum conditions of microwave assisted extraction (MAE) prior to total phenolics determination and antioxidant activity assay was conducted. The MAE method was done with 0.05 g sample in 10 mL DMSO at 500 W within 5 min. The effects of DMSO on various antioxidant activities using DPPH·+, DMPD·+, ABTS·+ and FRAP, and Folin-Ciocalteu reagent were investigated. From the results, it is clearly demonstrated that the DMSO itself shows no effect on any of those antioxidant assays including total phenolics content. The DMSO extracts of 14 local chilli varieties gave their antioxidant activities in the following ranges: DPPH, 3.07–20.0; DMPD, 1.52–6.61; ABTS, 20.4–56.0; FRAP, 8.98–42.1 mg GA/g DW. Their total phenolics contents were found in the range of 53.7–200 mg GA/g DW. This study demonstrates that DMSO was found as the most suitable extraction solvent for antioxidants and phenolics from chilli. In addition, analysis of the data obtained among four antioxidant activity assays with respect to total phenolics shows a highly significant and positive regression coefficient (r > 0.92), indicating the total phenolics are primarily responsible for their antioxidant activity of the chilli extract.
Keywords: Dimethyl sulfoxide, Total phenolics, Antioxidant activity, Microwave assisted extraction
Introduction
Dimethyl sulfoxide ((CH3)2SO or DMSO), is polar aprotic solvent. It is fully solubilized in all solvents, many polar and apolar substances. It is a polyfunctional molecule with two hydrophobic CH3 groups and a highly polar S = O group. DMSO is well tolerated by human and presents only low or no toxicity. It exhibits a myriad of biological actions (Hollebeeck et al. 2011; Tjäderhane et al. 2013). DMSO was detected at a trace level in some beverages (coffee, beer, tea), fruits and vegetables (cabbage, corn, raspberry, tomato) (Pearson et al. 1981).
Chilli (the genus Capsicum) is an important vegetable industrial crop of Thailand’s economy. It is a crop of tropical and sub-tropical regions and requires a warm humid climate. Chilli can be grown in any variety of soils, especially in the Northeast of Thailand. Chilli is just an indispensable condiment of every Thai people household. It is daily consumed as common diets as either single ingredient or other food supplements. Additionally, as the chilli domestic consumption needs, the production of chilli and its products are exported to other countries. Thailand, India, China, Pakistan and Peru are the largest dried chilli producers in the world (Bhutia et al. 2015). In 2014 Thailand exported chilli with the commercial values of 15,449 tons. The world demand is expectedly increasing. Therefore, it is predicted that there is a vast scope for exporting of chilli. The different varieties of chilli are being used in taste, flavor or aroma and pungency for beverages, pharmaceuticals, and/or cosmetics industries (Awang et al. 2013). More recently, a number of biological properties and potential health benefits of consuming chillies have been investigated, e.g., powerful antioxidants (free radical scavengers) (Reddy et al. 2011), anti-inflammatory (Tag et al. 2014), anti-arthritic (Chinn et al. 2011), anti-neoplastic properties (Barbero et al. 2010), anti-cancer (Meghvansi et al. 2010) and antifungal (Singh and Chittenden 2008). Additionally, it also has a use as bird, animal and insect repellent as well as biochemical pesticides (Chinn et al. 2011). This reason makes them extremely important to pharmacological effects and medical care. Prior to the determination of the antioxidant activity and total phenolics content, the optimum conditions for extraction methods of the antioxidants are needed. Some typical solvents have been used for the extraction of antioxidants including methanol, ethanol, acetone, water, acetonitrile, tetrahydrofuran, hexane, ethyl acetate (Leccese et al. 2011; Sang et al. 2014; Nguyen et al. 2015). A large number of extraction techniques for the antioxidants have also been developed for analysis of the antioxidants including ultrasound assisted extraction (UAE), microwave assisted extraction (MAE), supercritical fluid extraction (SFE), pressurized fluid extraction (PFE), accelerated solvent extraction (ASE) and enzyme assisted extraction (Chen et al. 2015; Ranic et al. 2014). In order to overcome their drawbacks possibly present, an alternative extraction method has been studied. Microwave assisted extraction (MAE) is one of simple and rapid extraction sophisticated techniques, now very well known, and conveniently used. The major advantages of this technique is the considerable reduction in the use of solvents and acceleration of the extraction process. The MAE system rapidly generates heat and this characteristic results in a shorter extraction time and good quality extracts with better target compound recovery (Setyaningsih et al. 2015). Since the resulting data of antioxidant activity depends on the method used, the most suitable extraction method will give an accurate prediction of antioxidant capacity of the antioxidants (Arts et al. 2003; Rebiai and Lanez 2012).
This study was aimed to investigate the optimum conditions for MAE method including the effect of solvent type. Then DMSO was selected, since none has been reported as the potential use for determining both total phenolics content by Folin-Ciocalteu reagent and antioxidant activity by four comparative assays (DPPH, DMPD, ABTS, and FRAP) of three main species of the chilli extracts.
Materials and methods
Chilli samples
Fourteen varieties of chilli (Capsicum annuum, Capsicum chinense, and Capsicum frutescens) used in this study were collected from local breeding cultivars. Their common Thai names of the chilli samples are Phet Mo Dindaeng, Huai Si Thon Kanlapaphruek, Shima Sakon Nakhon, Homkhao, Yot Son Khem 80, Mokho 2, PBC932, Akkhani Phirot, Habanero White, Thapthim Mo Dindaeng, Habanero Orange, Ratchaburi, Som and Phirot. Most samples were experimentally cultivated in the practical fields belonging to the Department of Plant Science and Agricultural Resources, Faculty of Agriculture, Khon Kaen University. These samples were dried in an oven at 60 °C for 48 h. Samples were ground in a kitchen grinder (Philips, Indonesia) to pass a 100 mesh sieves and analyzed as soon as they arrived at the laboratory. Otherwise, they were stored in plastic bottles and kept in desiccator until analysis. Figure 1 summarizes the varieties used in this study.
Fig. 1.
Representative picture for the fourteen varieties of different chilli
Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), and 2,2’-azino-bis(3-ethylbenzothiazolin-6-sulfonic acid (ABTS) were obtained from Sigma-Aldrich (USA). N,N–Dimethylp-phenylenediamine dihydrochloride and gallic acid were obtained from Fluka (Switzerland). Folin-Ciocalteu reagent was acquired from Merck (USA). Sodium carbonate, methanol and ethyl acetate were purchased from Carlo Erba (Italy). Potassium persulfate, sodium acetate, ferric chloride, ethanol, acetone, acetonitrile, acetic acid and hydrochloric acid were available from QRecTM (New Zealand). Dimethyl sulfoxide (99.9 %) was acquired from RCI Labscan, Thailand. Tetrahydrofuran was purchased from UNILAB, Australia. All chemicals and solvents used were of analytical grade.
Extraction of antioxidants and total phenolics
A modified method was used to separate the antioxidants extract of chilli. (Shao et al. 2012). Briefly, 0.05 g of dried chilli powder was accurately weighed into a high pressure PTFE vessel and 10 mL of an extraction solvent was added. The vessel was closed and placed inside the microwave oven unit. It was then heated following a one-step extraction programmed at 500 W for 5 min by microwave assisted extraction (Anton Parr 3000, Austria) with 8 units of a high pressure PTFE vessel. The extract obtained after the extraction was cooled and transferred into centrifuge tube and centrifuged at 4000 rpm for 10 min. The supernatant was used for determination of total phenolics contents and antioxidant activities. Four experimental factors potentially affecting the extraction efficiency were studied using the same procedure as mentioned above. The experiments were carried out with various organic solvents (dimethyl sulfoxide, methanol, ethanol, acetone, acetonitrile, tetrahydrofuran and ethyl acetate) including water. The sample amounts were varied between 0.05 and 1 g of chilli dry powder, while 10 mL of the solvent volume was fixed. The electrical power was altered between 50 and 900 W. The extraction time was also varied between 1 and 20 min.
DPPH free radical scavenging activity assay
Radical scavenging activity of a sample extract was measured by slightly modified DPPH method (Vallverdú-Queralt et al. 2012). The stable DPPH radical in methanol or ethanol is dark purple in color. The compounds, against hydrogen atom or electron donating ability, are measured by bleaching of the purple colored solution of DPPH. The final concentration of DPPH in ethanol was 0.1 mM and 900 μL of the reaction volume was used, and 100 μL of various concentrations of each standard compound (gallic acid) or the sample extract was added. These solutions were vortexed thoroughly and then incubated for 30 min in the dark at room temperature and measured spectrophotometrically at 517 nm against a blank sample (Agilent Technologies Cary 60 UV–vis spectrophotometer, Germany). The percentage of an inhibition of the DPPH was calculated and plotted as a function of concentration of a gallic acid used as the reference. The final DPPH values were calculated using a regression equation between the gallic acid concentration and the percentage of DPPH inhibition, and the results were expressed as milligram of gallic acid per gram dry weight (mg GA/g DW). The percentage of an inhibition of DPPH free radical was calculated using the following equation:
| 1 |
where Ac is the absorbance of control reaction which contains all reagents except standard or sample and As is the absorbance in the presence of standard or sample.
DMPD radical scavenging activity assay
The method is based on the use of the radical DMPD·+ (purple colour) generated by a reaction of Fe (III) in acidic medium with DMPD. The antioxidant components do have the ability to transfer a hydrogen atom to the coloured radical DMPD·+ turning it into a colourless DMPD+. The DMPD·+ radical shows an absorbance peak at 505 nm (Sripakdee et al. 2015). Radical DMPD·+ was generated from the mixture of 1 mL of 100 mM DMPD, 100 mL of 0.1 M acetate buffer pH 5.25 and 0.4 mL of 0.05 M FeCl3. The standard antioxidant or chilli extract (100 μL) and 900 μL of the DMPD•+ solution was incubated at about 29 °C for 10 min under continuous stirring. The absorbance at 505 nm was measured. The percentage inhibition was calculated according to the equation:
| 2 |
where Ac is the absorbance of uninhibited radical cation and As is the absorbance measured after 10 min incubation followed by the addition of antioxidant sample.
ABTS radical cation decolorization assay
Radical cation scavenging capacity of the chilli extracts including a reference standard was examined against ABTS·+ with some modifications (Thaipong et al. 2006). The ABTS·+ was produced by the reaction of 7.4 mM ABTS in water with 2.6 mM K2S2O8, stored in the dark at room temperature for 12–16 h. Before use, the ABTS·+ solution was diluted with methanol to get the absorption between 0.7 and 0.9 AU at 734 nm. Briefly, 100 μL of the standard or antioxidant extract was mixed with 900 μL of ABTS·+ solution and kept in the dark at room temperature. The absorbance at 734 nm was read after 30 min, and the percentage inhibition of ABTS was calculated in the same manner as mentioned in the DPPH and DMPD assay, for each concentration against a blank absorbance. Gallic acid with concentrations from 1 to 20 mg/L was invoked as a standard curve. The free radical scavenging activity was expressed as mg GA/g DW. All determinations were performed in triplicate.
Ferric ion reducing antioxidant power (FRAP) assay
The ferric ion reducing antioxidant power (FRAP) assay was used to evaluate the decreasing antioxidant capacity of chilli extracts from different varieties. This method was run with slight modifications (Hossaina et al. 2008; Li et al. 2012). The FRAP method measures the ability of antioxidants to reduce ferric–tripyridyl-triazine (Fe3+-TPTZ) complex in the blue colored ferrous form which absorbs light at 593 nm. The ferric-TPTZ reagent was prepared by mixing 300 mM acetate buffer, pH 3.6, 10 mM TPTZ in 40 mM HCl and 20 mM FeCl3 in the ratio of 10:1:1 (v/v/v). The FRAP reagent was freshly prepared before each experiment. Briefly, 100 μL of different concentrations of the reference standard or the sample extract were mixed with 900 μL of the FRAP reagent and incubated at 37 °C for the duration of the reaction. Absorbance readings were taken at 593 nm after 30 min. The increasing absorbance of the reaction mixture indicates an increase of the reduction capability. Six concentrations of 0.5, 2, 4, 6, 8 and 10 mg/L were used to prepare the standard curve of gallic acid. The antioxidant activities of the chilli extracts were expressed as mg GA/g DW.
Determination of total phenolics
Total phenolic compounds of the chilli extracts were determined by a method adapted from Queiroz et al. (2009). Folin-Ciocalteu reagent and gallic acid used as a standard compound were employed. The sample extract (100 μL) was mixed with 500 μL of 10 % Folin-Ciocalteu reagent. After 3 min, 400 μL of 7.5 % Na2CO3 was added into the mixture solution. The mixture was allowed to stand in the dark for 30 min at room temperature. The absorbance was measured spectrophotometrically at 765 nm against the blank. The same procedure was repeated for all gallic acid standard solutions (20–90 mg/L). The total phenolics content is expressed as mg of gallic acid per gram of dry weight (mg GA/g DW).
Effect of DMSO on antioxidant activity and total phenolics methods
Various volumes of 99.9 % DMSO (0.1, 0.2, 0.3, 0.4 and 0.5 mL) were made up to 0.5 mL with different solvent depending on each assays; ethanol (DPPH), water (DMPD, FRAP, Folin-Ciocalteu reagent), methanol (ABTS). Then, 0.9 mL of each free radical or oxidant reagent (DPPH, DMPD, ABTS, FRAP) was added. For Folin-Ciocalteu reagent, each DMSO of 0.5 mL was mixed with 0.5 mL of 10 % Folin-Ciocalteu reagent and 0.4 mL of 7.5 % Na2CO3. The solution needed to be measured in the same manner as mentioned above in the each assay.
Data analysis
Data results are given as mean ± standard deviation (SD) of three measurements (n = 3). All graphs, linear regression and statistical analysis in this paper were analyzed by an Origin 8.1 Software for Windows.
Results and discussion
Optimum conditions for extraction of total phenolics and antioxidants
The extraction of total phenolics and antioxidants from different chilli varieties were conducted using the microwave assisted extraction. For the optimal extraction efficiency, some of the experimental parameters including the amount of sample, extraction solvents, extraction time and electrical power were examined in the detail.
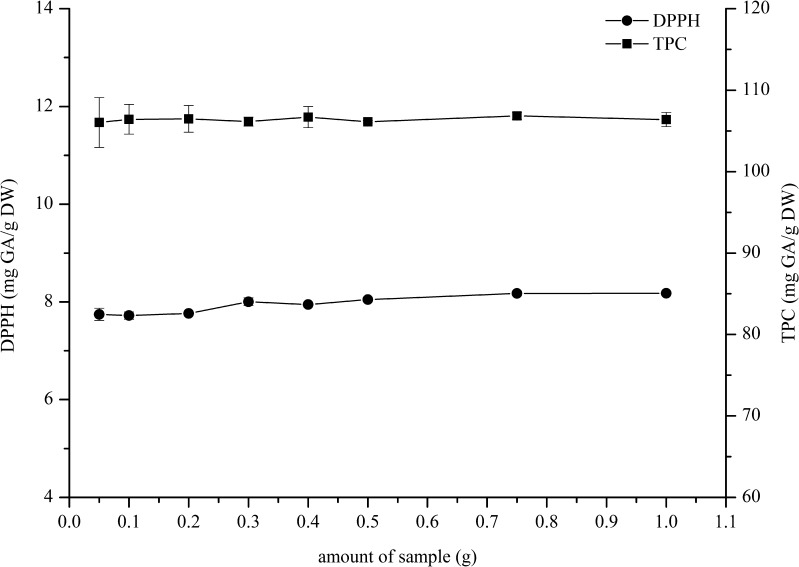
Effect of sample amount
The amount of sample concerns the contact area of solid and liquid, consequently influence the extraction efficiency. Contact area can reach to biggest when solid are saturated with liquid. In this study, the maintaining of the solvent volume was fixed constant of 10 mL while sample quantities of 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.75, and 1 g of chilli dry powder were employed. From the results, Fig. 2 shows that the trends in average DPPH antioxidant activity and TPC are not statistically different from using 0.05 to 1 g of chilli dry powder. Therefore, the sample amount of 0.05 g, which gave 7.75 ± 0.12 and 106.04 ± 3.06 mg GA/g DW for DPPH and TPC respectively, was used to be the optimum weight in the extraction process.
Fig. 2.
Effect of sample amount for the chilli extract on the DPPH antioxidant activity and total phenolics content
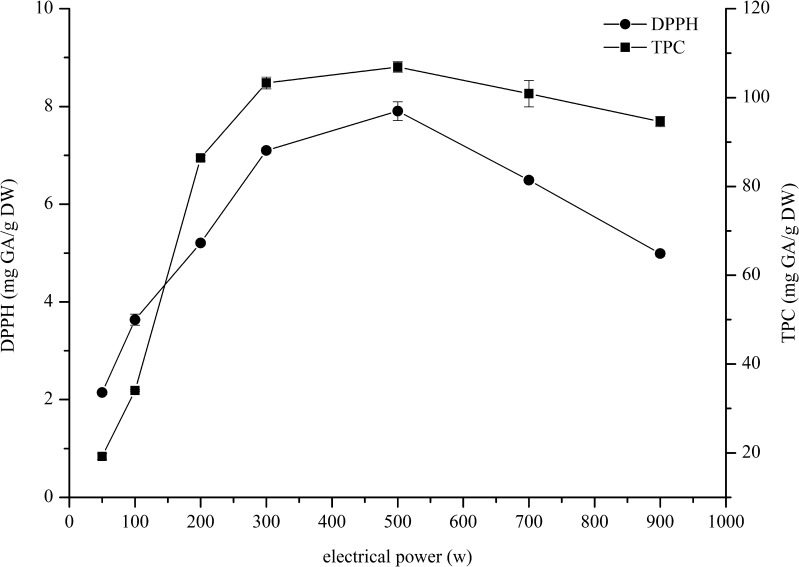
Effect of electrical power
The dried sample (0.05 g) was extracted in 10 mL of DMSO with different electrical powers (50, 100, 200, 300, 500, 700, 900 W) for 5 min to enhance the total phenolics and antioxidants extraction without disintegrating during the extraction. Figure 3 shows the total phenolics content and DPPH antioxidant activity obtained from the extraction. It can be noted that the use of electrical power in all range (50–900 W) gave similar tendency results of which DPPH and TPC increased with the electrical power from 50 to 500 W. At 500 W the highest TPC (106.89 ± 1.19 mgGA/g DW) and DPPH antioxidant activity (7.91 ± 0.19 mgGA/g DW) were obtained. After that their trend decreased with an increasing extraction power from 700 to 900 W. However, most likely degradation processes also increased due to elevated temperatures promoting the oxidation degradation reaction of the antioxidants. Therefore, 500 W was chosen at which was rather not so high power for the extraction method.
Fig. 3.
Effect of electrical power for the chilli extract on the DPPH antioxidant activity and total phenolics content
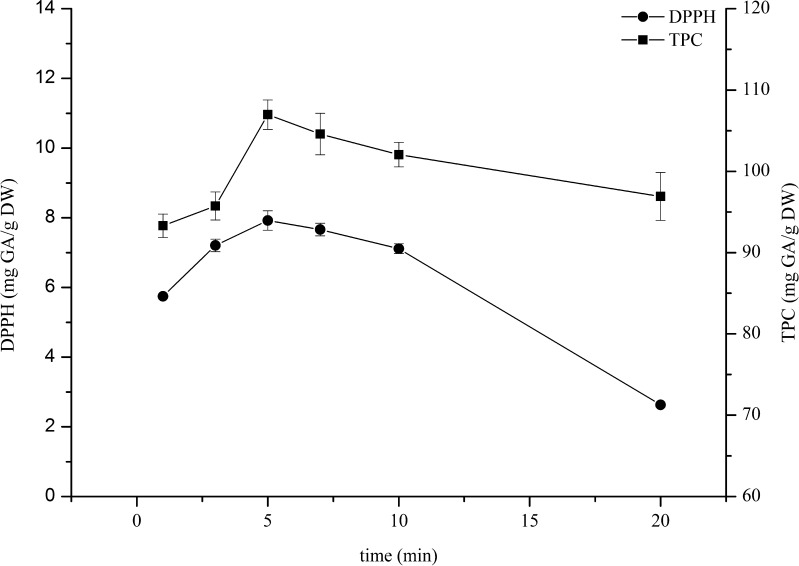
Effect of extraction time
The effect of heating time (1–20 min) of the microwave power (500 W) using DMSO as the solvent extraction was investigated. The obtained results are shown in Fig. 4. TPC and DPPH antioxidant activity slightly increased with the extraction time between 1 and 5 min, and then they decreased gradually upto 20 min. It could be explained that at longer time, the heat might be a cause of the antioxidants degradation. Thus, the heating time of about 5 min is reasonable to get the highest yield.
Fig. 4.
Effect of extraction time for the chilli extract on the DPPH antioxidant activity and total phenolics content
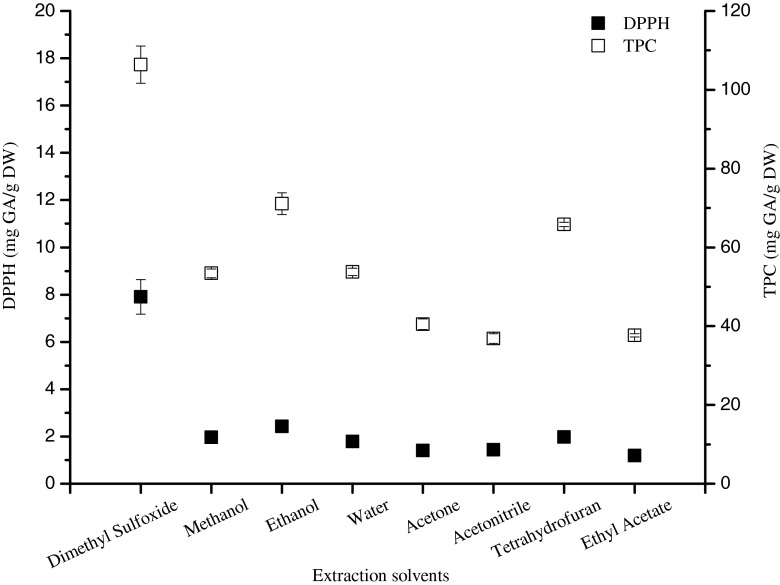
Effect of extraction solvents
To evaluate the effect of the solvent on the MAE, a series of extractions were performed with 8 different organic solvents (dimethyl sulfoxide, methanol, ethanol, acetone, acetonitrile, tetrahydrofuran, ethyl acetate) including water. While other parameters were fixed (5 min extraction time, 500 W electrical power and 0.05 g sample amount). The efficient extraction of antioxidants and total phenolics from chilli fruits depends on the type of solvents used. From the results (Fig. 5), TPC and DPPH antioxidant activity revealed that dimethyl sulfoxide provided the highest efficient extraction (TPC, 106.39 ± 4.70 mg GA/g DW and DPPH, 7.91 ± 0.73 mg GA/g DW). Thus, it was preferred to be good extraction solvent compared with other solvents, because the amount of co-extracted compounds from the chilli matrix from real samples decreased. The lowest values (TPC, 37.67 ± 0.43 mg GA/g DW and DPPH, 1.19 ± 0.09 mg GA/g DW) were obtained with ethyl acetate. However, the nature of extraction solvent affects strongly the extraction yields and the antioxidant capacities, due to the presence of different antioxidant compounds of varied polarities and chemical characteristics, which makes them soluble or not in a particular solvent. Therefore, in the case, DMSO was prominently used as the extraction solvent.
Fig. 5.
Effect of extraction solvents for the chilli extracts on the DPPH antioxidant activity and total phenolics content
Effect of DMSO on antioxidant activity and total phenolics methods
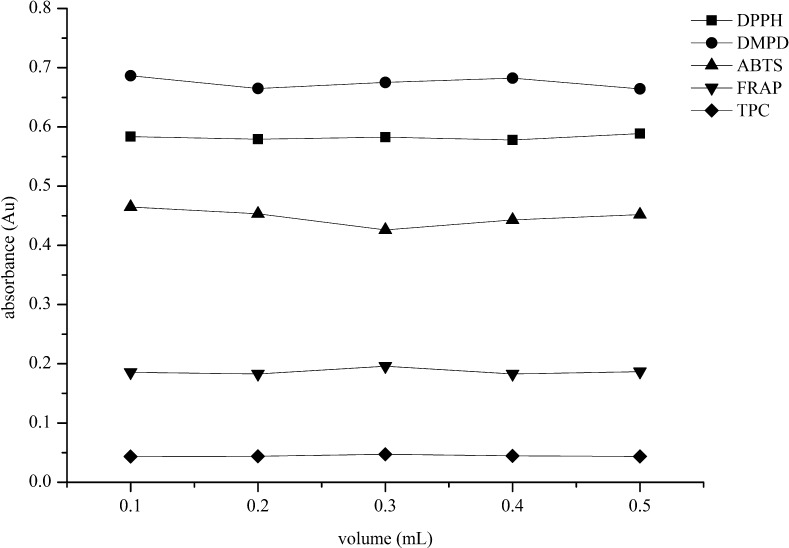
Dimethyl sulfoxide (CH3) 2SO), depicting one polar sulfinyl domain and two apolar methyl groups, is considered as an aprotic solvent that can solubilize many polar and apolar substances (Hollebeeck et al. 2011). To study the role of DMSO as an extraction solvent on the antioxidant capacity assays including DPPH, DMPD, ABTS, FRAP) and TPC method, various volumes of 99.9 % DMSO (0.1, 0.2, 0.3, 0.4 and 0.5 mL) were used for preliminary tests. Although DMSO is an antioxidant by scavenging hydroxyl free radicals (Bektasoglu et al. 2006), the obtained results (Fig. 6) showed that the use of DMSO up to 0.5 mL had no effect at all to any of the antioxidant assays including TPC. It is noted that small amount of DMSO can not obscure the absorbance interestingly. Thus, DMSO was found to be a better choice of the extraction solvent for the subsequent experiments.
Fig. 6.
Effect of DMSO solvent volume on DPPH, DMPD, ABTS, FRAP and TPC assays
Antioxidant activity of chilli samples
For alternative choice of a suitable common standard for the tested methods, different calibration standards and different expression ways of concentrations (dry weight, DW; fresh weight, FW; molar or mass unit) have been applied to express the results in the literature. These facts are held for reducing complicatedly by comparing the results from various sources. Generally applicable standard for all methods enables us to obtain a set of simple comparable results. It would strongly be prominent and applicable data. Therefore, the calibration data of gallic acid were used for the evaluation of antioxidant activity of 14 varieties of chilli samples, expressed as mg GA/g DW. The results are presented in Table 1, listing for the antioxidant activity measurements of each chilli sample evaluated by DPPH, DMPD, ABTS and FRAP assays. Different kinds of the chilli showed different antioxidant activity profiles. In DPPH assay, Phet Mo Dindaeng chilli gave the highest antioxidant activity (20.00 ± 0.30 mg GA/g DW), while Phirot chilli had the lowest antioxidant activity (3.07 ± 0.16 mg GA/g DW). Phet Mo Dindaeng chilli also showed the highest antioxidant activity in DMPD (6.61 ± 0.05 mg GA/g DW), ABTS (56.03 ± 0.28 mg GA/g DW) and FRAP (42.10 ± 0.42 mg GA/g DW) assays. Also, Phirot chilli gave the lowest ones measured with DMPD (1.52 ± 0.08 mg GA/g DW), ABTS (20.42 ± 0.32 mg GA/g DW) and FRAP (8.98 ± 0.57 mg GA/g DW) assays.
Table 1.
Antioxidant activities determined by four selected different assays and total phenolics contents of the chilli extracts
| Sample | Species | DPPH | DMPD | ABTS | FRAP | TPC |
|---|---|---|---|---|---|---|
| Phet Mo Dindaeng | C. chinense | 20.00 ± 0.30 | 6.61 ± 0.05 | 56.03 ± 0.28 | 42.10 ± 0.42 | 200.41 ± 4.49 |
| Huai Si Thon Kanlapaphruek | C. annuum | 17.96 ± 0.02 | 6.47 ± 0.07 | 48.43 ± 0.26 | 32.11 ± 0.91 | 193.49 ± 1.08 |
| Shima Sakon Nakhon | C. frutescens ± C. chinense | 12.51 ± 0.11 | 5.75 ± 0.09 | 47.40 ± 0.33 | 28.91 ± 0.60 | 185.67 ± 2.43 |
| Homkhao | C. chinense | 10.54 ± 0.04 | 5.12 ± 0.08 | 42.48 ± 0.20 | 27.02 ± 0.29 | 184.58 ± 5.27 |
| Yot Son Khem 80 | C. annuum | 10.03 ± 0.09 | 5.01 ± 0.05 | 42.43 ± 0.10 | 24.39 ± 0.86 | 139.96 ± 0.79 |
| Mokho 2 | C. annuum | 9.68 ± 0.08 | 4.98 ± 0.05 | 40.17 ± 0.23 | 23.30 ± 0.27 | 122.00 ± 2.05 |
| PBC932 | C. chinense | 8.78 ± 0.26 | 4.67 ± 0.03 | 38.67 ± 0.18 | 23.02 ± 0.40 | 111.21 ± 1.15 |
| Akkhani Phirot | C. chinense | 7.90 ± 0.05 | 4.08 ± 0.18 | 37.56 ± 0.21 | 22.36 ± 1.41 | 106.74 ± 2.00 |
| Habanero White | C. chinense | 6.17 ± 0.30 | 4.04 ± 0.05 | 36.43 ± 1.57 | 21.99 ± 0.16 | 106.28 ± 3.81 |
| Thapthim Mo Dindaeng | C. chinense | 4.69 ± 0.11 | 3.75 ± 0.06 | 34.27 ± 0.07 | 20.89 ± 0.11 | 94.96 ± 0.92 |
| Habanero Orange | C. chinense | 4.05 ± 0.02 | 3.10 ± 0.03 | 30.85 ± 0.26 | 13.45 ± 0.10 | 69.08 ± 1.46 |
| Ratchaburi | C. frutescens | 3.65 ± 0.38 | 1.85 ± 0.04 | 24.49 ± 0.13 | 12.86 ± 0.05 | 54.96 ± 1.08 |
| Som | C. chinense | 3.40 ± 0.03 | 1.85 ± 0.08 | 22.87 ± 0.19 | 10.16 ± 0.14 | 54.51 ± 0.59 |
| Phirot | C. chinense | 3.07 ± 0.16 | 1.52 ± 0.08 | 20.42 ± 0.32 | 8.98 ± 0.57 | 53.73 ± 0.41 |
DPPH, DMPD, ABTS, FRAP and TPC were expressed as mg GA/g DW. Data are given as the mean ± SD (n = 3)
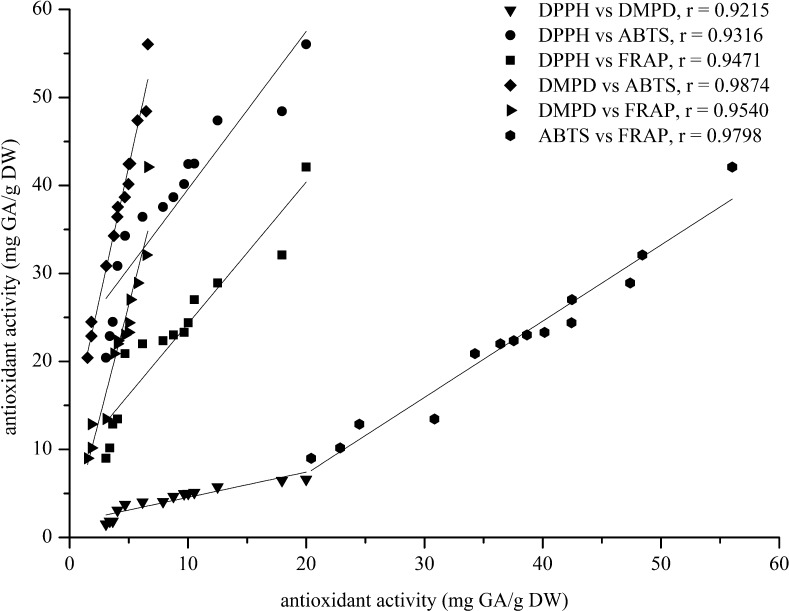
In this study, linear correlations among each of both antioxidant assays were found with their correlation coefficients (r) of 0.9215 for DPPH vs DMPD, 0.9316 for DPPH vs ABTS, 0.9471 for DPPH vs FRAP, 0.9874 for DMPD vs ABTS, 0.9540 for DMPD vs FRAP and 0.9798 for ABTS vs FRAP as shown in Fig. 7 It is indicating that when all 14 varieties of these chilli samples were comparatively analyzed statistically, the correlation coefficients among their antioxidant activities based on DPPH, DMPD, ABTS, and FRAP assays were positively high and highly significant relationship.
Fig. 7.
The linear correlation coefficients given among their antioxidant activities (DPPH, DMPD, ABTS and FRAP assays) from 14 chilli varieties
Total phenolics content
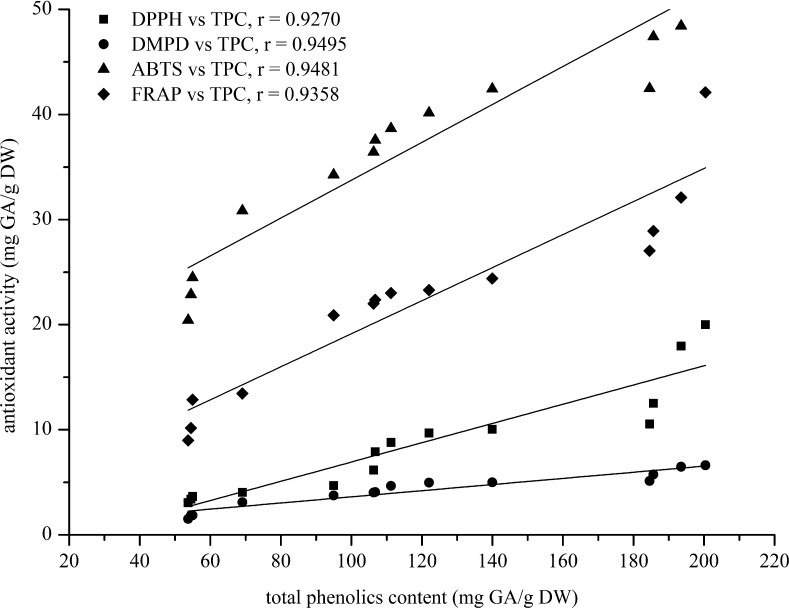
Since the role of phenolic compounds as natural antioxidants attributing for the quality of chillies, total phenolics was determined by the Folin-Ciocalteu reagent. The calibration equation for total phenolics was constructed by plotting the absorbance against the gallic acid concentrations (in triplicate). The absorbance obtained over a concentration range of 20–90 mg/L was linear (y = 0.0086x + 0.0432) with a regression coefficient (R2) of 0.9984 (data not shown). Table 1 also illustrates that the contents of total phenolics in the chilli samples of 14 varieties ranging from 53.73 ± 0.41 to 200.41 ± 4.49 mg GA/g DW. The chilli of Phet Mo Dindaeng gave the highest amount of TPC (200.41 ± 4.49 mg GA/g DW) and was comparable with that of Huai Si Thon Kanlapaphruek (193.49 ± 1.08 mg GA/g DW), while that of Phirot variety was the lowest (53.73 ± 0.41 mg GA/g DW). This is in agreement with Scalzo et al. (2005), who highlights the importance of the plant genotype (species and variety within species) for determination of total phenolics content. The antioxidant capacity depends on the antioxidant contents of the extract. The total phenolics content does not represent all antioxidant compounds of the extract but its mainly due, which can be attributed for the clear relationship between the antioxidant activity and the total phenolics content. The correlations in between each assay of DPPH, DMPD, ABTS and FRAP were compared with their TPC subjecting for each of the correlation coefficients of 0.9270, 0.9495, 0.9481 and 0.9358 for DPPH, DMPD, ABTS and FRAP, respectively (Fig. 8). The results indicate that when all 14 chilli varieties were comparatively analyzed statistically, there were a positive and highly significant trend, indicating that total polyphenols are mainly responsible for the antioxidant activity of the chilli.
Fig. 8.
Correlation plots between each of antioxidant activity (DPPH, DMPD, ABTS and FRAP assays) and total phenolics content of 14 chilli varieties
Conclusion
The study reported the first time that DMSO was employed and optimised for microwave assisted extraction of total phenolic compounds from chilli samples and for their antioxidant assay. The spectrophotometric method for analysis of both antioxidant activity and total phenolics were conducted. According to the data obtained from the present study, these chilli varieties were found to be effective antioxidant sources as demonstrated by numerous in vitro assays, including DPPH, DMPD, ABTS and FRAP. These methods were used for the determination of antioxidant activity. The common one (Folin-Ciocalteu reagent) was used for the determination of total phenolics content, applying for the same sets of the chilli extracts using identical calibration procedures with gallic acid standard that permitted better comparison of the results. The DMSO extract shows significantly the higest value of in vitro antioxidant activity compared with other common solvent used. Quantification of the total phenolic compounds are thus helpful in a total evaluation of their antioxidant activity. However, neither single compound nor group of compounds sufficiently defines the total antioxidant capacity, since other antioxidant nutrients present in the chilli would give a synergistic effect on the total antioxidant activity.
Acknowledgments
The authors thank the Higher Education Research Promotion and National Research University (NRU) Project of Thailand, Office of the Higher Education Commission, through the Food and Functional Food Research Cluster of Khon Kaen University (KKU), and the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education and Ministry of Education, for financial support.
Footnotes
Highlights
• This is the first report on dimethyl sulfoxide for optimization microwave assisted extraction
• DMSO gave the best performance of total phenolics and antioxidants
• The comparison between five antioxidant methods were high correlation.
• Total phenolics are major deals of antioxidant activity.
• Green and efficient extraction of antioxidants from chilli was obtained.
References
- Arts MJTJ, Sebastiaan DJ, Voss HP, Haenen GRMM, Bast A. A critical appraisal of the use of the antioxidant capacity (TEAC) assay in defining optimal antioxidant structures. Food Chem. 2003;80:409–414. doi: 10.1016/S0308-8146(02)00468-5. [DOI] [Google Scholar]
- Awang NA, Islama MR, Ismail MR, Zulkarami B, Omar D. Effectiveness of different elicitors in inducing resistance in chilli (Capsicum annuum L.) against pathogen infection. Sci Hortic. 2013;164:461–465. doi: 10.1016/j.scienta.2013.08.038. [DOI] [Google Scholar]
- Barbero G, Molinillo JMG, Varela RM, Palma M, Macias FA, Barroso CG. Application of Hansch’s model to capsaicinoids and capsinoids: a study using the quantitative structure - activity relationship. A novel method for the synthesis of capsinoids. J Agric Food Chem. 2010;58:3342–3349. doi: 10.1021/jf9035029. [DOI] [PubMed] [Google Scholar]
- Bektasoglu B, Esin Celik S, Ozyurek M, Guclu K, Apak R. Novel hydroxyl radical scavenging antioxidant activity assay for water-soluble antioxidants using a modified CUPRAC method. Biochem Biophys Res Commun. 2006;345:1194–1200. doi: 10.1016/j.bbrc.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Bhutia ND, Seth T, Shende VD, Dutta S. Chattopadhyay A, estimation of heterosis, dominance effect and genetic control of freshfruit yield, quality and leaf curl disease severity traits of chilli pepper (Capsicum annuum L.) Sci Hortic. 2015;182:47–55. doi: 10.1016/j.scienta.2014.11.017. [DOI] [Google Scholar]
- Chen M, Zhao Y, Yu S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015;172:543–550. doi: 10.1016/j.foodchem.2014.09.110. [DOI] [PubMed] [Google Scholar]
- Chinn MS, Sharma-Shivappa RR, Cotter JL. Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod Process. 2011;89:340–345. doi: 10.1016/j.fbp.2010.08.003. [DOI] [Google Scholar]
- Hollebeeck S, Raas T, Piront N, Schneider Y, Toussaint O, Larondelle Y, During A. Dimethyl sulfoxide (DMSO) attenuates the inflammatory response in the in vitro intestinal Caco-2 cell model. Toxicol Lett. 2011;206:268–275. doi: 10.1016/j.toxlet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Hossaina MB, Bruntonb NP, Barry-Ryana C, Martin-Dianaa AB, Wilkinsonc M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J Chem. 2008;1:751–756. [Google Scholar]
- Leccese A, Viti R, Bartolini S. The effect of solvent extraction on antioxidant properties of apricot fruit. Cent Eur J Biol. 2011;6:199–204. [Google Scholar]
- Li H, Deng Z, Wu T, Liu R, Loewen S, Tsao R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012;130:928–936. doi: 10.1016/j.foodchem.2011.08.019. [DOI] [Google Scholar]
- Meghvansi MK, Siddiqui S, Khan MH, Gupta VK, Vairale MG, Gogoi HK, Singh L. Naga chilli: a potential source of capsaicinoids with broad-spectrum ethnopharmacological applications. J Ethnopharmacol. 2010;132:1–14. doi: 10.1016/j.jep.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Bowyer MC, Vuong QV, Altena IAV, Scarlett CJ. Phytochemicals and antioxidant capacity of Xao tam phan (Paramignya trimera) root as affected by various solvents and extraction methods. Ind Crop Prod. 2015;67:192–200. doi: 10.1016/j.indcrop.2015.01.051. [DOI] [Google Scholar]
- Pearson TW, Dawson HJ, Lackey HB. Natural occurring levels of dimethyl sulfoxide in selected fruits, vegetables, grains, and beverages. J Agric Food Chem. 1981;29:1089–1091. doi: 10.1021/jf00107a049. [DOI] [PubMed] [Google Scholar]
- Queiroz YS, Ishimoto EY, Bastos DHM, Sampaio GR, Torres EAFS. Garlic (Allium sativum L.) and ready-to-eat garlic products: in vitro antioxidant activity. Food Chem. 2009;115:371–374. doi: 10.1016/j.foodchem.2008.11.105. [DOI] [Google Scholar]
- Ranic M, Nikolic M, Pavlovic M, Buntic A, Siler-Marinkovic S, Dimitrijevic-Brankovic S. Optimization of microwave-assisted extraction of natural antioxidants from spent espresso coffee grounds by response surface methodology. J Clean Prod. 2014;80:69–79. doi: 10.1016/j.jclepro.2014.05.060. [DOI] [Google Scholar]
- Rebiai A, Lanez T. Chemical composition and antioxidant activity of Apis Mellifera bee pollen from Northwest Algeria. J Fundam Appl Sci. 2012;4:26–35. [Google Scholar]
- Reddy KK, Ravinder T, Prasad RBN, Kanjilal S. Evaluation of the antioxidant activity of capsiate analogues in polar, nonpolar, and micellar media. J Agric Food Chem. 2011;59:564–569. doi: 10.1021/jf104244m. [DOI] [PubMed] [Google Scholar]
- Sang SY, Jamharee F, Prasad KN, Azlan A, Maliki N. Influence of drying treatments on antioxidant capacity of forage legume leaves. J Food Sci Technol. 2014;51:988–993. doi: 10.1007/s13197-011-0596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo J, Politi A, Pellegrini N, Mezzetti B, Battino M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition. 2005;21:207–213. doi: 10.1016/j.nut.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Setyaningsih W, Saputro IE, Palma M, Barroso CG. Optimisation and validation of the microwave-assisted extraction of phenolic compounds from rice grains. Food Chem. 2015;169:141–149. doi: 10.1016/j.foodchem.2014.07.128. [DOI] [PubMed] [Google Scholar]
- Shao P, He J, Sun P, Zhao P. Analysis of conditions for microwave-assisted extraction of total water-soluble flavonoids from Perilla Frutescens leaves. J Food Sci Technol. 2012;49:66–73. doi: 10.1007/s13197-011-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Chittenden C. In-vitro antifungal activity of chilli extracts in combination with Lactobacillus casei against common sapstain fungi. Int Biodeterior Biodegrad. 2008;62:364–367. doi: 10.1016/j.ibiod.2007.10.009. [DOI] [Google Scholar]
- Sripakdee T, Sriwicha A, Jansam N, Mahachai R, Chanthai S. Determination of total phenolics and ascorbic acid related to an antioxidant activity and thermal stability of the Mao fruit juice. Int Food Res J. 2015;22:618–624. [Google Scholar]
- Tag HM, Kelany OE, Tantawy HM, Fahmy AA. Potential anti-inflammatory effect of lemon and hot pepper extracts on adjuvant-inducedarthritis in mice. J Basic Appl Zool. 2014;67:149–157. doi: 10.1016/j.jobaz.2014.01.003. [DOI] [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Tjäderhane L, Mehtälä P, Scaffa P, Vidal C, Pääkkönen V, Breschi L, Hebling J, Tay FR, Nascimento FD, Pashley DH, Carrilho MR. The effect of dimethyl sulfoxide (DMSO) on dentin bonding and nanoleakage of etch-and-rinse adhesives. Dent Mater. 2013;29:1055–1062. doi: 10.1016/j.dental.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Vallverdú-Queralt A, Medina-Remón A, Casals-Ribes I, Lamuela-Raventos RM. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012;130:222–227. doi: 10.1016/j.foodchem.2011.07.017. [DOI] [Google Scholar]